Abstract
Background
Despite multiple antibiotics being available to manage dental infections (DI), there is lack of data comparing commonly prescribed antibiotics in India.
Objectives
The aim of this study was to evaluate the real-world effectiveness and tolerability of cephalexin-clavulanic acid fixed-dose combination (cephalexin CV FDC) in contrast with amoxicillin-clavulanic acid (co-amoxiclav FDC) and cefuroxime among patients with dental infections (odontogenic) in India.
Methods
This retrospective, multi-centric, observational, real-world electronic medical record (EMR)-based study was conducted between January 2022 and December 2022. The EMRs of 355 adults with DI receiving oral cephalexin CV, co-amoxiclav, or cefuroxime were categorized into two distinct groups: Group I (Test Group) with patients prescribed cephalexin extended release 375/750 mg along with clavulanic acid 125 mg; and Group II (Comparator Group) with patients prescribed co-amoxiclav 625 mg (500 mg amoxicillin + 125 mg clavulanic acid) or cefuroxime (250 mg/500 mg).
Results
Toothache was the most common complaint, reported by 95.5% of patients, followed by swelling (46.8%), tooth sensitivity (35.5%), pus discharge (33.0%), redness and halitosis (30.4% each). Dental caries was observed in 81.1% of patients. Clinical improvement, defined as improvement/partial resolution of infection-related clinical signs and symptoms (composite measure of pain, swelling, fever, requirement of additional antimicrobial therapy) as per dentists’ judgment, was recorded in 98.3% of patients with cephalexin CV, 96.8% of patients with co-amoxiclav, and 98.9% of patients treated with cefuroxime within 10 days. Time (days) to clinical improvement was numerically lesser among patients receiving cephalexin CV (4.6 ± 2.0) compared with cefuroxime (4.9 ± 2.1) and co-amoxiclav (5.0 ± 2.6). All treatments were well tolerated.
Conclusion
Cephalexin CV was as effective as co-amoxiclav and cefuroxime, with faster clinical improvement and better resolution of certain symptoms.
Key Points
| In a real-world, retrospective study, cephalexin CV demonstrated comparable effectiveness to cefuroxime and co-amoxiclav in achieving clinical improvement among patients with dental infections. |
| A similar mean time to clinical improvement was observed in patients receiving cephalexin CV compared with cefuroxime and co-amoxiclav, indicating its potential for faster relief. |
| Cephalexin CV was more effective in reducing fever, redness, and bleeding gums compared with cefuroxime and in decreasing complications like trismus compared with co-amoxiclav. |
| All the antibiotics used in the study were well tolerated by patients without any major adverse effects, highlighting their safety in dental infection management. |
Introduction
Dental infections, or odontogenic infections, are the infections originating from teeth and their supporting structures, typically arising due to pulpal (advanced caries), periodontal, or peri-coronal etiology [1]. The majority of dental infections are minor; however, they may progress to severe maxillofacial involvement, requiring hospitalization, and may develop life-threatening complications such as airway obstruction, necrotizing fasciitis, brain infection, mediastinitis, or sepsis [2, 3]. Dental caries, a bacterial infection of the teeth, is one of the most prevalent diseases worldwide [4]. Deep dental caries cause symptoms such as sensitivity to hot and cold food items or dental pain, eventually leading to the destruction of periapical tissue, occasionally accompanied by pus formation, called as periapical abscess [5]. Periodontitis, another common cause of dental infections, presents as gingival recession, periodontal pocket formation, and/or tooth mobility. Patients with gingivitis and periodontitis often present with symptoms of halitosis, bleeding on brushing, gingival swelling, and pain [1, 5, 6].
Oral cavity infection has been found to have a significant impact on overall human health. Studies have reported an association between dental infections and comorbidities such as cardiovascular diseases, type 2 diabetes mellitus, respiratory diseases, stroke, adverse pregnancy outcomes, osteoporosis, renal diseases, and gastrointestinal diseases. Consequently, these infections need to be treated promptly to reduce local symptoms and prevent them from spreading to distant sites [7, 8].
More than 700 species of bacteria live in the oral cavity and are adapted to essentially distinct ecological niches. Some of the normal oral flora belong to families such as Enterococcus, Peptostreptococcus, Streptococcus, Staphylococcus, Actinomyces, Corynebacterium, Eubacterium, Lactobacillus, Bacteroides, Campylobacter, Leptotrichia, Porphyromonas, Treponema, Fusobacterium, etc. [9]. The most common pathogens in the oral cavity of healthy people are Streptococcus, Granulicatella, and Veillonella, which cause dental infections like caries, periodontitis, endodontic infections, and tonsillitis. In addition to these there are other pathogens, like Staphylococcus and Candida, causing various dental infections [10].
In an Indian study, Staphylococcus (40.3%) was the most commonly found microorganism causing dental infection, followed by Streptococcus (14.5%), Escherichia coli (8.1%), Klebsiella (9.7%), Acinetobacter (1.6%), and Enterococcus (8.1%) [11]. Staphylococcus aureus can cause many infections, including oral diseases such as angular cheilitis, mucositis, periodontitis, and dentistry implant-related infections [12].
Antibiotics are generally used in dental procedures to treat odontogenic, non-odontogenic, local, and focal infections [13]. There is a lack of up-to-date comprehensive standard treatment guidelines for dental infections in India, and various antibiotics such as amoxicillin, clindamycin, azithromycin, cephalexin, clarithromycin, doxycycline, spiramycin, erythromycin, ciprofloxacin, cefadroxil, minocycline, and cefuroxime are being used in dental practice for treating dental infections [13–16].
Cephalosporins are broad-spectrum antibiotics used to manage a wide range of bacterial infections and, hence, are commonly prescribed in dental practice [15]. Cephalexin and cefazolin (first-generation cephalosporins) and cefuroxime (second-generation cephalosporin) are among the most prescribed cephalosporins in dental practice [13, 16]. Besides the gram-negative bacteria that are covered by first-generation cephalosporins, second-generation cephalosporins are also effective against H. influenzae, Enterobacter aerogenes, Neisseria species, and Serratia marcescens [16, 17]. Amoxicillin is another antibiotic reported to be preferred by dentists to control odontogenic infections [13]. These drugs are usually prescribed in combination with clavulanic acid, which inhibits the beta lactamase enzyme and enhances the antibacterial effect of antibiotics [18, 19]. Metronidazole is often added along with broad-spectrum antibiotics for the management of anaerobic infections [20].
Although there are multiple antibiotics available to manage dental infections, co-amoxiclav and cephalosporins like cephalexin CV and cefuroxime are the most commonly used. However, there is a lack of relevant clinical studies in the Indian context to draw comparison between these drugs in the context of their real-world effectiveness and tolerability. Hence, this study was planned to evaluate the real-world effectiveness and tolerability of cephalexin-clavulanic acid fixed-dose combination (cephalexin CV FDC) compared with amoxicillin-clavulanic acid (co-amoxiclav FDC) and cefuroxime in patients with dental infections (odontogenic) in India.
Methods
This was a retrospective, multi-centric, observational study conducted in patients aged 18 years or above with dental infections to assess effectiveness and tolerability of cephalexin CV FDC, cefuroxime, and co-amoxiclav FDC. Patients from ten dental clinics across eight cities (Mumbai, Kolkata, Delhi, Ajmer, Nagpur, Asansol, Pune, and Bengaluru) in India who were prescribed any of the study antibiotics for a period between January 2022 and December 2022 and had complete electronic medical records (EMRs) were selected. Patients with severe infections requiring parenteral antibiotics, and those with allergy/contraindication to the study medication were excluded from the study. The records of 355 patients included in the study were divided into the following two groups:
Group I (Test): Patients diagnosed with dental infection who received an FDC of cephalexin extended release 375/750 mg and clavulanic acid 125 mg orally as directed by the treating dentist.
Group II (Comparator): Patients diagnosed with dental infection who received an FDC of co-amoxiclav 625 mg (500 mg + 125 mg), or cefuroxime (250 mg/500 mg) orally as directed by the treating dentist.
The reported clinical efficacy of cephalosporins was observed to be between 81.0% and 93.0%. Three hundred and forty-six subjects were required to demonstrate non-inferiority between the two groups (test and comparator- [cefuroxime + co-amoxiclav]) considering 10% non-inferiority margin, 80% power and two-sided α error of 0.05.
The study was approved by the Royal Pune Independent Ethics Committee (RPIEC), located in Pune, India (Ethics committee registration number: ECR/45/Indt/MH/2013/RR-19, Ethics Approval Number: RPIEC090223; dated 7th Feb 2023).
Overall, data extracted from the EMRs was used for statistical analysis. Categorical variables were expressed as count and percentages and Chi-square (or Fisher exact test as appropriate) was used to detect differences between treatment groups. Continuous variables were represented as mean ± SD; paired T-test was used for within-group analysis and analysis of variance (ANOVA) was used for between-group analysis. Statistical significance was considered at p < 0.05.
Results
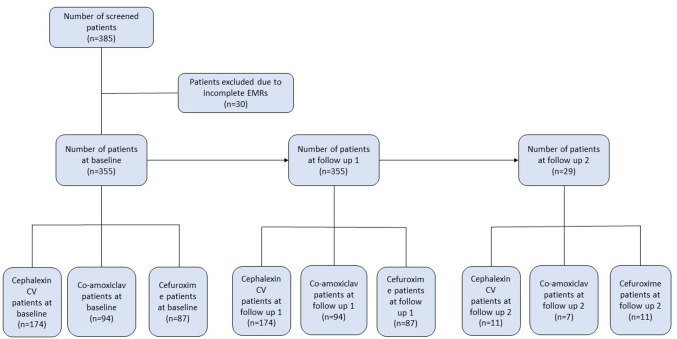
After screening, EMRs of 355 out of 385 patients were found to be complete and hence were included in the study (Fig. 1). The duration from baseline to follow-up 1 was 1–20 days and from follow up 1 to follow up 2 was 3–7 days.
Fig. 1.
Eligible patients’ disposition at baseline, follow-up 1 and follow-up 2. CV clavulanic acid, co-amoxiclav amoxicillin–clavulanic acid, n number of patients
Mean age of the patients was 39.1 ± 14.5 years, with almost equal distribution of males (49.9%) and females (50.1%). Tooth pain was the most common complaint at baseline, reported by 95.5% of patients (94.3%, 97.7%, and 95.7% in cephalexin CV, cefuroxime, and co-amoxiclav groups, respectively), followed by swelling (46.8%), tooth sensitivity (35.5%), pus discharge (33.0%), redness and halitosis (30.4% each), periodontal pockets (27.6%), bleeding gums (23.7%), fever (20.0%), and trismus (12.4%) (Table 1).
Table 1.
Demographic characteristics, symptoms, definitive diagnosis, and complications at baseline
| Parameters | Total (N = 355) |
Group I | Group II | |
|---|---|---|---|---|
| Cephalexin CV (n = 174) |
Cefuroxime (n = 87) |
Co-amoxiclav (n = 94) |
||
| Age, years | ||||
| Mean ± SD | 39.1 ± 14.5 | 39.6 ± 15.3 | 39.6 ± 13.6 | 37.7 ± 13.6 |
| Median (range) | 37 (18–83) | 38 (18–82) | 37 (18–71) | 35 (18–83) |
| Gender; n (%) | ||||
| Female | 178 (50.1) | 88 (50.6) | 37 (42.5) | 53 (56.4) |
| Male | 177 (49.9) | 86 (49.4) | 50 (57.5) | 41 (43.6) |
| Chief complaints, n (%) | ||||
| Tooth pain | 339 (95.5) | 164 (94.3) | 85 (97.7) | 90 (95.7) |
| Swelling | 166 (46.8) | 85 (48.9) | 51 (58.6) | 30 (31.9) |
| Tooth sensitivity | 126 (35.5) | 55 (31.6) | 35 (40.2) | 36 (38.3) |
| Pus discharge | 117 (33.0) | 61 (35.1) | 45 (51.7) | 11 (11.7) |
| Redness | 108 (30.4) | 58 (33.3) | 42 (48.3) | 8 (8.5) |
| Halitosis | 108 (30.4) | 49 (28.2) | 46 (52.9) | 13 (13.8) |
| Periodontal pocket | 98 (27.6) | 49 (28.2) | 39 (44.8) | 10 (10.6) |
| Bleeding gums | 84 (23.7) | 40 (23.0) | 35 (40.2) | 9 (9.6) |
| Fever | 71 (20.0) | 32 (18.4) | 35 (40.2) | 4 (4.3) |
| Trismus | 44 (12.4) | 19 (10.9) | 15 (17.2) | 10 (10.6) |
| Definitive diagnosis, n (%) | ||||
| Dental caries | 288 (81.1) | 141 (81.0) | 68 (78.2) | 79 (84.0) |
| Periapical infection | 200 (56.3) | 96 (55.2) | 68 (78.2) | 36 (38.3) |
| Gingivitis | 92 (25.9) | 44 (25.3) | 31 (35.6) | 17 (18.1) |
| Pericoronitis | 59 (16.6) | 27 (15.5) | 13 (14.9) | 19 (20.2) |
| Periodontitis | 48 (13.5) | 25 (14.4) | 19 (21.8) | 4 (4.3) |
| Others | 21 (5.9) | 10 (5.8) | 9 (10.3) | 2 (2.1) |
| Complications, n (%) | ||||
| Abscess | 135 (38.0) | 68 (39.1) | 58 (66.7) | 9 (9.6) |
| Pericoronitis | 56 (15.8) | 26 (14.9) | 14 (16.1) | 16 (17.0) |
| Trismus | 44 (12.4) | 19 (10.9) | 15 (17.2) | 10 (10.6) |
| Dry socket | 4 (1.1) | 2 (1.2) | 0 (0.0) | 2 (2.1) |
| Re-suturing | 2 (0.6) | 1 (0.6) | 0 (0.0) | 1 (1.1) |
| Dose, mg; n (%) | ||||
| 250 | 0 (0.0) | 3 (3.5) | 0 (0.0) | |
| 375 | 32 (18.4) | 0 (0.0) | 0 (0.0) | |
| 500 | 0 (0.0) | 84 (96.6) | 0 (0.0) | |
| 625 | 0 (0.0) | 0 (0.0) | 94 (100.0) | |
| 750 | 142 (81.6) | 0 (0.0) | 0 (0.0) | |
| Frequency | ||||
| OD | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| BD | 158 (90.8) | 87 (100.0) | 63 (67.0) | |
| TDS | 16 (9.2) | 0 (0.0) | 31 (33.0) | |
| Duration, days | ||||
| Mean ± SD | 4.7 ± 1.5 | 4.7 ± 1.1 | 4.8 ± 1.3 | |
| Median | 5 | 5 | 5 | |
| Range | 1–13 | 3–7 | 3–10 | |
BD bis in die (twice a day), Co-amoxiclav amoxicillin-clavulanic acid, CV clavulanic acid, mg milligrams, n number of patients, N total patients, OD omne in die (once daily), SD standard deviation, TDS ter die sumendum (three times a day)
Dental caries was the most common cause of infection, reported in 81.1% patients (81.0%, 78.2%, and 84.0% in cephalexin CV, cefuroxime, and co-amoxiclav groups, respectively), followed by periapical infection (56.3%), gingivitis (25.9%), pericoronitis (16.6%), and periodontitis (13.5%) (Table 1).
Abscess was the most common complication seen in 38.0% patients (39.1%, 66.7%, 9.6% in cephalexin CV, cefuroxime, and co-amoxiclav groups, respectively) followed by pericoronitis (15.8%), trismus (12.4%), dry socket (1.1%), and re-suturing and gaping (0.6% each) (Table 1). At baseline, cephalexin CV was prescribed to 81.6% of patients at a dosage of 750/125 mg, with 90.8% being administered twice daily for a mean duration of 5 days. In the cefuroxime group, most patients were prescribed cefuroxime 500 mg (96.6%) twice daily for a mean duration of 5 days. All patients in the co-amoxiclav group were prescribed a dosage of 625 mg twice (67.0%) or thrice (33.0%) daily, for a mean duration of 5 days. A similar prescription pattern was observed at follow-up visits 1 and 2 across all the antibiotic groups (Table 1).
Overall Clinical Improvement
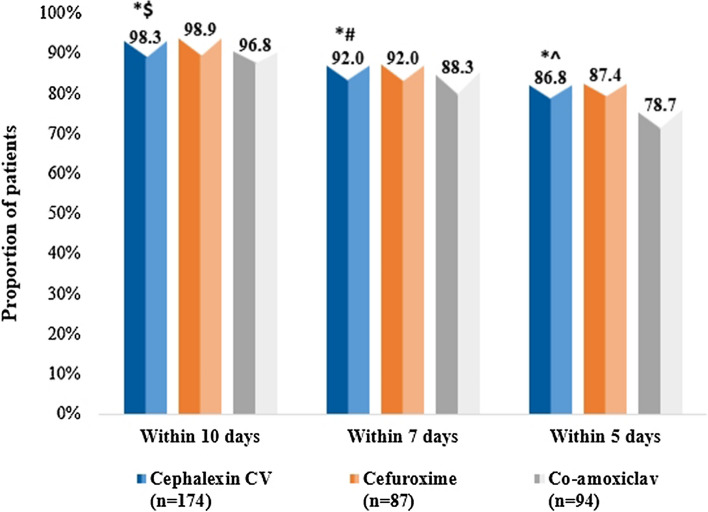
Overall clinical improvement (defined as improvement or partial resolution of infection-related clinical signs and symptoms [a composite measure of pain, swelling, fever, and requirement of additional antimicrobial therapy] as per the dentist’s judgment) within 10 days of the treatment period, was seen in similar proportions of patients in cephalexin CV (98.3%), co-amoxiclav (96.8%), and cefuroxime groups (98.9%). These differences were not statistically significant. A similar trend was observed within 5 and 7 days of treatment (Fig. 2).
Fig. 2.
Overall clinical improvement in patients within 10 days, 7 days, and 5 days among cephalexin CV, cefuroxime, and co-amoxiclav groups. * p = 1.000 for cephalexin CV in comparison with cefuroxime within 5, 7 and 10 days of treatment; $ p = 0.426 in comparison with co-amoxiclav within 10 days of treatment; # p = 0.446 in comparison with co-amoxiclav within 7 days of treatment; ^ p = 0.123 in comparison with co-amoxiclav within 5 days of treatment. CV clavulanic acid, co-amoxiclav amoxicillin–clavulanic acid, n number of patients
Improvement in Pain, Swelling, and Fever
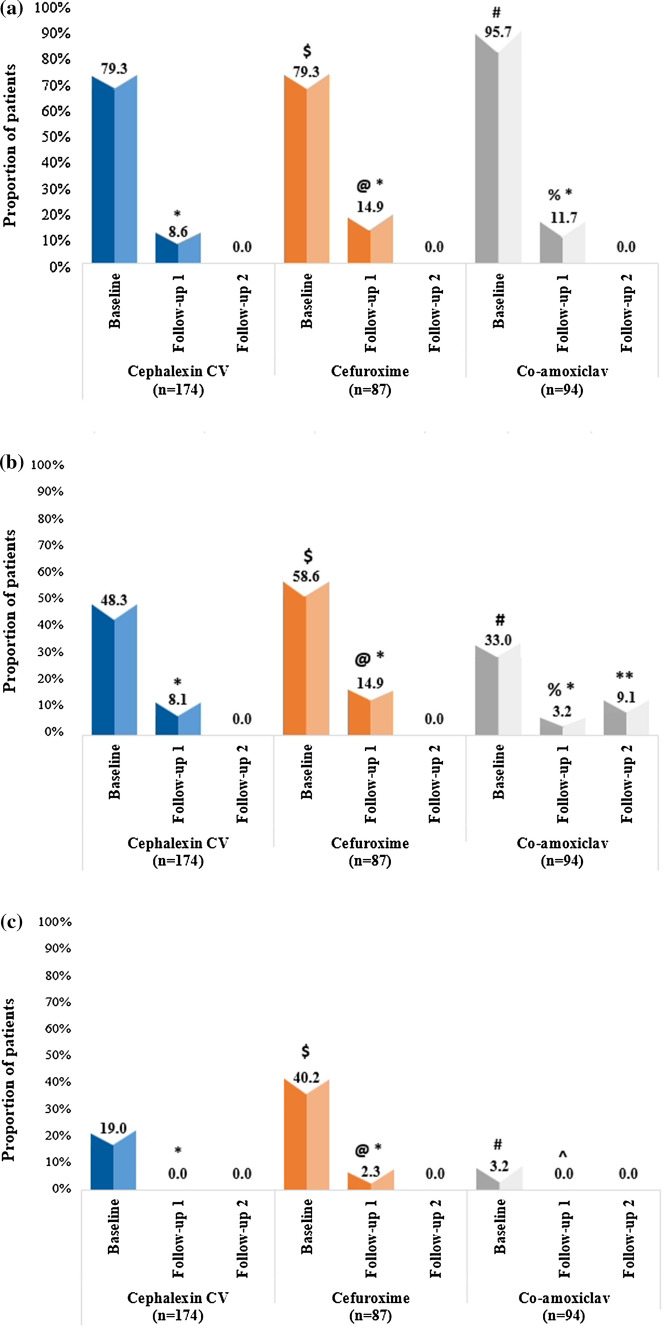
Patients in all antibiotic groups showed a significant improvement in pain at follow-up visit 1 compared with baseline (p < 0.001). At follow-up 1, the percentage of patients with pain was numerically lower in the cephalexin CV group (8.6%) than in cefuroxime (14.9%) and co-amoxiclav (11.7%) groups at follow-up visit 1; however, the results were not statistically significant (p = 0.179 and p = 0.551, respectively, for cephalexin CV in comparison with cefuroxime and co-amoxiclav at follow-up 1) (Fig. 3a).
Fig. 3.
a Presence of pain at baseline, follow-up 1 and 2 visits among cephalexin CV, cefuroxime, and co-amoxiclav groups. * p < 0.001 for cephalexin CV, cefuroxime, and co-amoxiclav at follow-up 1 from baseline in respective antibiotic groups; $ p = 1.000 at baseline for cephalexin CV in comparison with cefuroxime; # p < 0.001 at baseline for cephalexin CV in comparison with co-amoxiclav; @ p = 0.179 at follow-up 1 for cephalexin CV in comparison with cefuroxime, % p = 0.551 at follow-up 1 for cephalexin CV in comparison with co-amoxiclav. CV clavulanic acid, co-amoxiclav amoxicillin–clavulanic acid, n number of patients. b Presence of swelling at baseline, follow-up 1 and 2 visits among cephalexin CV, cefuroxime, and co-amoxiclav groups. * p < 0.001 for cephalexin CV, cefuroxime, and co-amoxiclav at follow-up 1 from baseline in respective antibiotic groups; $ p = 0.148 at baseline for cephalexin CV in comparison with cefuroxime; # p = 0.022 at baseline for cephalexin CV in comparison with co-amoxiclav; @ p = 0.131 at follow-up 1 for cephalexin CV in comparison with cefuroxime; % p = 0.196 at follow-up 1 for cephalexin CV in comparison with co-amoxiclav; ** p = 1.000 at follow-up 2 for cephalexin CV in comparison with co-amoxiclav. CV clavulanic acid, co-amoxiclav amoxicillin–clavulanic acid, n number of patients. c Presence of fever at baseline, follow-up 1 and 2 visits among cephalexin CV, cefuroxime, and co-amoxiclav groups. * p < 0.001 for cephalexin CV and cefuroxime from baseline to follow-up 1 in respective antibiotic groups; ^ p = 0.081 for co-amoxiclav from baseline to follow-up 1; $ p < 0.001 for cephalexin CV in comparison with cefuroxime at baseline; # p < 0.001 for cephalexin CV in comparison with co-amoxiclav at baseline, @ p = 0.045 for cephalexin CV in comparison with cefuroxime at follow-up 1. CV clavulanic acid, co-amoxiclav amoxicillin–clavulanic acid, n number of patients
Similarly, a significant improvement in swelling was observed in all antibiotic groups (p < 0.001) at follow-up visit 1 compared with baseline. At follow-up 1, there was no significant difference among groups in the proportion of patients with swelling (p = 0.131 and p = 0.196, for cephalexin CV in comparison with cefuroxime and co-amoxiclav, respectively) (Fig. 3b).
Patients with fever were significantly reduced at follow-up 1 in cephalexin CV and cefuroxime groups (p < 0.001 for both in comparison with baseline). In the cephalexin CV group, no patients had fever at follow-up 1, and there was a significant difference in comparison with cefuroxime (p = 0.045 for cephalexin CV in comparison with cefuroxime at follow-up 1) (Fig. 3c).
Improvement in Redness, Bleeding Gums, Bleeding on Probing, Halitosis, Pus Discharge
At baseline, 27.6%, 35.6%, and 36.2% of patients reported a moderate level of pain and 29.3%, 29.9%, and 23.4% of patients reported slight swelling in cephalexin CV, cefuroxime, and co-amoxiclav groups, respectively. At baseline, pus discharge was reported in 34.5%, 51.7%, and 12.8% of patients, bleeding on probing in 32.2%, 57.5%, and 9.6% of patients, and halitosis in 28.7%, 52.9%, and 12.8% of patients in cephalexin CV, cefuroxime, and co-amoxiclav groups, respectively (Table 2).
Table 2.
Clinical improvement status of symptoms in all three antibiotic groups
| Parameters | Group I | Group II |
p value (cephalexin CV vs cefuroxime [at f/up 1]) |
p value (cephalexin CV vs co-amoxiclav [at f/up 1]) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cephalexin CV |
p-Value (baseline to f/up 1) |
Cefuroxime |
p value (baseline to f/up 1) |
Co-amoxiclav |
p value (baseline to f/up 1) |
|||||||||
| Baseline n = 174 |
Follow-up visit 1 n = 174 |
Follow-up visit 2 n = 11 |
Baseline n = 87 |
Follow-up visit 1 n = 87 |
Follow-up visit 2 n = 7 |
Baseline n = 94 |
Follow-up visit 1 n = 94 |
Follow-up visit 2 n = 11 |
||||||
| Pain; n (%) | ||||||||||||||
| No pain (0) | 36 (20.7) | 159 (91.4) | 11 (100.0) | <0.001 | 18 (20.7) | 74 (85.1) | 7 (100.0) | <0.001 | 4 (4.3) | 83 (88.3) | 11 (100.0) | <0.001 | 0.267 | 0.307 |
| Mild pain (2) | 44 (25.3) | 13 (7.5) | 0 (0.0) | 7 (8.1) | 11 (12.6) | 0 (0.0) | 29 (30.9) | 11 (11.7) | 0 (0.0) | |||||
| Moderate pain (4) | 48 (27.6) | 2 (1.2) | 0 (0.0) | 31 (35.6) | 1 (1.2) | 0 (0.0) | 34 (36.2) | 0 (0.0) | 0 (0.0) | |||||
| Severe pain (6) | 27 (15.5) | 0 (0.0) | 0 (0.0) | 20 (23.0) | 0 (0.0) | 0 (0.0) | 18 (19.2) | 0 (0.0) | 0 (0.0) | |||||
| Very severe pain (8) | 13 (7.5) | 0 (0.0) | 0 (0.0) | 11 (12.6) | 1 (1.2) | 0 (0.0) | 5 (5.3) | 0 (0.0) | 0 (0.0) | |||||
| Worst possible pain (10) | 6 (3.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (4.3) | 0 (0.0) | 0 (0.0) | |||||
| Swelling; n (%) | ||||||||||||||
| None | 90 (51.7) | 160 (92.0) | 11 (100.0) | <0.001 | 36 (41.4) | 74 (85.1) | 7 (100.0) | <0.001 | 63 (67.0) | 91 (96.8) | 10 (90.9) | <0.001 | 0.061 | 0.120 |
| Slight | 51 (29.3) | 14 (8.1) | 0 (0.0) | 26 (29.9) | 11 (12.6) | 0 (0.0) | 22 (23.4) | 3 (3.2) | 1 (9.1) | |||||
| Average | 26 (14.9) | 0 (0.0) | 0 (0.0) | 21 (24.1) | 2 (2.3) | 0 (0.0) | 9 (9.6) | 0 (0.0) | 0 (0.0) | |||||
| Much | 7 (4.0) | 0 (0.0) | 0 (0.0) | 4 (4.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| Redness; n (%) | ||||||||||||||
| Absence | 116 (66.7) | 174 (100.0) | 11 (100.0) | <0.001 | 45 (51.7) | 81 (93.1) | 7 (100.0) | <0.001 | 86 (91.5) | 93 (98.9) | 11 (100.0) | 0.017 | <0.001 | 0.173 |
| Presence | 58 (33.3) | 0 (0.0) | 0 (0.0) | 42 (48.3) | 6 (6.9) | 0 (0.0) | 8 (8.5) | 1 (1.1) | 0 (0.0) | |||||
| Duration; days | ||||||||||||||
| Mean ± SD | 4.2 ± 3.5 | 4.8 ± 3.4 | 2.9 ± 1.4 | |||||||||||
| Bleeding gums; n (%) | ||||||||||||||
| Absence | 134 (77.0) | 167 (96.0) | 10 (90.9) | <0.001 | 52 (59.8) | 75 (86.2) | 7 (100.0) | <0.001 | 85 (90.4) | 91 (96.8) | 10 (90.9) | 0.073 | 0.004 | 0.732 |
| Presence | 40 (23.0) | 7 (4.0) | 1 (9.1) | 35 (40.2) | 12 (13.8) | 0 (0.0) | 9 (9.6) | 3 (3.2) | 1 (9.1) | |||||
| Duration; days | ||||||||||||||
| Mean ± SD | 4.4 ± 2.4 | 5.6 ± 4.8 | 14.4 ± 28.5 | |||||||||||
| Bleeding on probing; n (%) | ||||||||||||||
| Absence | 118 (67.8) | 160 (92.0) | 10 (90.9) | <0.001 | 37 (42.5) | 74 (85.1) | 7 (100.0) | <0.001 | 85 (90.4) | 91 (96.8) | 10 (90.9) | 0.073 | 0.085 | 0.120 |
| Presence | 56 (32.2) | 14 (8.1) | 1 (9.1) | 50 (57.5) | 13 (14.9) | 0 (0.0) | 9 (9.6) | 3 (3.2) | 1 (9.1) | |||||
| Duration; days | ||||||||||||||
| Mean ± SD | 5.5 ± 11.8 | - | 4.8 ± 4.6 | 15.0 ± 28.3 | ||||||||||
| Halitosis; n (%) | ||||||||||||||
| Absence | 124 (71.3) | 168 (96.6) | 9 (81.8) | <0.001 | 41 (47.1) | 81 (93.1) | 7 (100.0) | <0.001 | 82 (87.2) | 92 (97.9) | 10 (90.9) | 0.005 | 0.201 | 0.544 |
| Presence | 50 (28.7) | 6 (3.5) | 2 (18.2) | 46 (52.9) | 6 (6.9) | 0 (0.0) | 12 (12.8) | 2 (2.1) | 1 (9.1) | |||||
| Duration; days | ||||||||||||||
| Mean ± SD | 8.8 ± 24.9 | 10.8 ± 15.8 | 15.2 ± 24.8 | |||||||||||
| Fever; n (%) | ||||||||||||||
| Absence | 141 (81.0) | 174 (100.0) | 11 (100.0) | <0.001 | 52 (59.8) | 85 (97.7) | 7 (100.0) | <0.001 | 91 (96.8) | 94 (100.0) | 11 (100.0) | 0.081 | 0.045 | |
| Presence | 33 (19.0) | 0 (0.0) | 0 (0.0) | 35 (40.2) | 2 (2.3) | 0 (0.0) | 3 (3.2) | 0 (0.0) | 0 (0.0) | |||||
| Duration; days | ||||||||||||||
| Mean ± SD | 2.3 ± 0.9 | 3.4 ± 3.1 | 2.0 ± 0.0 | |||||||||||
| Pus discharge; n (%) | ||||||||||||||
| Absence | 114 (65.5) | 168 (96.6) | 11 (100.0) | <0.001 | 42 (48.3) | 82 (94.3) | 7 (100.0) | <0.001 | 82 (87.2) | 92 (97.9) | 11 (100.0) | 0.005 | 0.384 | 0.544 |
| Presence | 60 (34.5) | 6 (3.5) | 0 (0.0) | 45 (51.7) | 5 (5.8) | 0 (0.0) | 12 (12.8) | 2 (2.1) | 0 (0.0) | |||||
| Duration; days | ||||||||||||||
| Mean ± SD | 5.3 ± 12.5 | 4.9 ± 4.5 | 7.3 ± 16.7 | |||||||||||
Co-amoxiclav amoxicillin–clavulanic acid, CV clavulanic acid, f/up follow-up, mg milligrams, n number of patients, SD standard deviation
*Fisher Exact Test or Chi Square Test was used to compare the distribution of the categories of the variables for Group I with Group II at baseline/follow-up visits
At follow-up visit 1, redness was resolved significantly compared with baseline in all patients receiving cephalexin CV (p < 0.001 in comparison with baseline). While comparing cephalexin CV with cefuroxime, a significant improvement was observed in redness (p < 0.001) and bleeding gums (p = 0.004) in the cephalexin CV group (Table 2).
For symptoms such as bleeding on probing, halitosis, and pus discharge, a numerically higher proportion of patients showed improvement in the cephalexin CV group as compared with the cefuroxime group and similar improvement in the co-amoxiclav group; however, the differences amongst the groups were not statistically significant (p > 0.05) (Table 2).
Effect on Complications
A majority of patients across treatment groups showed a statistically significant improvement at the first follow-up visit compared with baseline for complications such as abscess, pericoronitis, and trismus (p < 0.05 compared with baseline). Further, 94.7% of patients receiving cephalexin CV demonstrated significant resolution of trismus (p < 0.001) compared with the co-amoxiclav group (20.0%) (Table 3).
Table 3.
Improvement in dental infection complications in all three antibiotic groups
| Parameter | Cephalexin CV N = 87 |
Cefuroxime N = 94 |
Co-amoxiclav N = 174 |
p-Value (cephalexin CV vs cefuroxime) |
p-Value (cephalexin CV vs co-amoxiclav) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n = 174) |
Follow-up visit 1 (n = 174) |
p-Value (baseline to follow-up 1) | Baseline (n = 87) |
Follow-up visit 1 (n = 87) |
p-Value (baseline to follow-up 1) | Baseline (n = 94) |
Follow-up visit 1 (n = 94) |
p-Value (baseline to follow-up 1) | |||
| Abscess; n (%) | |||||||||||
| Yes | 69 (100.0) | <0.001 | 58 (100.0) | <0.001 | 8 (100.0) | <0.001 | 0.763 | 1.000 | |||
| Improved | 64 (92.8) | 52 (89.7) | 8 (100.0) | ||||||||
| No change | 5 (7.3) | 6 (10.3) | 0 (0.0) | ||||||||
| Pericoronitis; n (%) | |||||||||||
| Yes | 27 (100.0) | <0.001 | 14 (100.0) | <0.001 | 15 (100.0) | <0.001 | 1.000 | 1.000 | |||
| Improved | 24 (88.9) | 12 (85.7) | 13 (86.7) | ||||||||
| No change | 3 (11.1) | 2 (14.3) | 2 (13.3) | ||||||||
| Re-exploration; n (%) | |||||||||||
| Yes | NA | NA | NA | NA | NA | ||||||
| Improved | |||||||||||
| No change | |||||||||||
| Dry socket; n (%) | |||||||||||
| Yes | 2 (100.0) | 0.333 | NA | 2 (100.0) | 1.000 | 1.000 | 1.000 | ||||
| Improved | 2 (100.0) | 1 (50.0) | |||||||||
| No change | 0 (0.0) | 1 (50.0) | |||||||||
| Trismus; n (%) | |||||||||||
| Yes | 19 (100.0) | <0.001 | 15 (100.0) | <0.001 | 10 (100.0) | 0.474 | 0.571 | <0.001 | |||
| Improved | 18 (94.7) | 13 (86.7) | 2 (20.0) | ||||||||
| No change | 1 (5.3) | 2 (13.3) | 8 (80.0) | ||||||||
| Others; n (%) | |||||||||||
| No | 174 (100.0) | 174 (100.0) | 1.000 | 87 (100.0) | 87 (100.0) | 1.000 | 94 (100.0) | 94 (100.0) | 1.000 | 1.000 | 1.000 |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
Co-amoxiclav amoxicillin–clavulanic acid, CV clavulanic acid, n number of patients, N total patients, NA not applicable
Time to Clinical Improvement
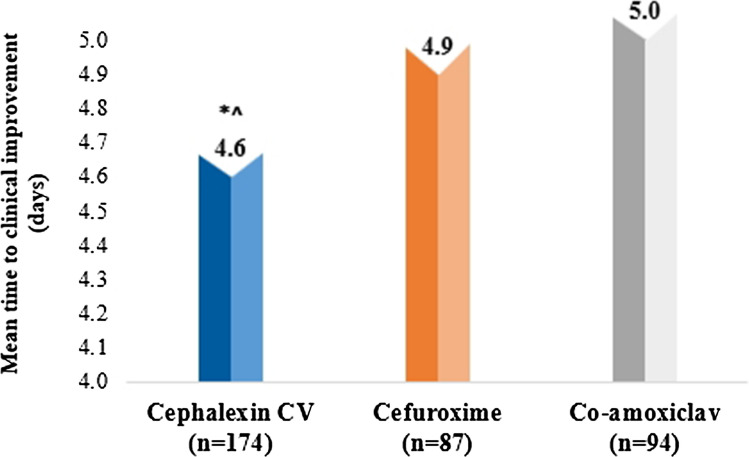
Mean time to clinical improvement was similar among patients receiving cephalexin CV (4.6 ± 2.0 days) compared with patients receiving cefuroxime (4.9 ± 2.1 days) and patients receiving co-amoxiclav (5.0 ± 2.6 days) (p = 0.071 for cephalexin CV vs cefuroxime and p = 0.484 for cephalexin CV vs co-amoxiclav at follow-up 1) (Fig. 4).
Fig. 4.
Mean time to clinical improvement across all the antibiotic groups. * p = 0.071 in comparison with cefuroxime at follow-up 1; ^ p = 0.484 in comparison with co-amoxiclav at follow-up 1. CV clavulanic acid, co-amoxiclav amoxicillin–clavulanic acid, n number of patients
Analgesic Usage in Patients
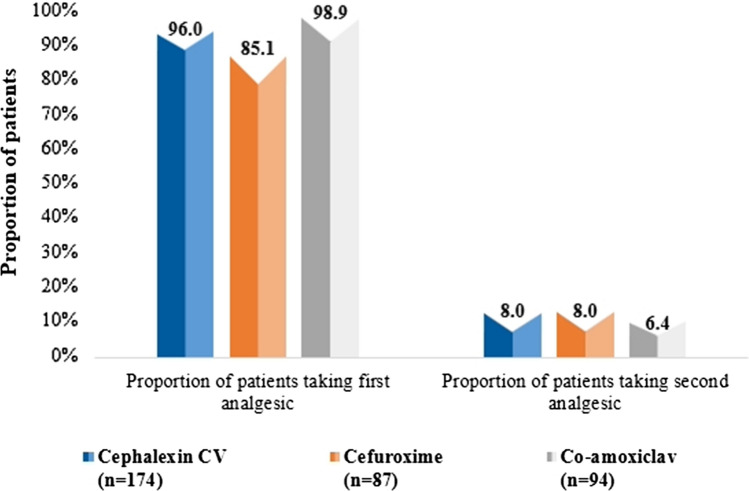
The proportion of patients taking first analgesic during the study period in the cephalexin CV group (96.0%) was numerically lesser compared with the co-amoxiclav group (98.9%) but higher in comparison with the cefuroxime group (85.1%) (Fig. 5). The overall duration of first analgesic use was numerically lesser among patients in the cephalexin CV group (5.8 ± 2.5 days) than in the cefuroxime (6.0 ± 2.7 days) and co-amoxiclav (6.1 ± 2.5 days) groups. Further, the proportion of patients taking a second analgesic in the cephalexin CV and cefuroxime groups was 8.0% whereas in the co-amoxiclav group it was 6.0%. The overall duration of second analgesic use was numerically shorter among patients in the cephalexin CV group (4.8 ± 0.5 days) compared with the cefuroxime group (5.0 ± 0.0 days), and similar to the co-amoxiclav (4.8 ± 2.2 days) group (Table 4).
Fig. 5.
Proportion of patients requiring additional analgesic/NSAID. CV clavulanic acid, co-amoxiclav amoxicillin–clavulanic acid, n number of patients, NSAID nonsteroidal anti-inflammatory drug
Table 4.
Patients requiring the addition of analgesic/NSAID
| Parameters | Group I | Group II | |
|---|---|---|---|
| Cephalexin CV n = 174 |
Cefuroxime n = 87 |
Co-amoxiclav n = 94 |
|
| Proportion of patients using first analgesic | 167 (95.9%) | 74 (85.1%) | 93 (98.9%) |
| Duration of (first) analgesic/NSAID use; days | |||
| Mean ± SD | 5.8 ± 2.5 | 6.0 ± 2.7 | 6.1 ± 2.5 |
| Median (range) | 5 (2–19) | 5 (2–16) | 5 (3–16) |
| Proportion of patients using second analgesic | 14 (8.1%) | 7 (8.1%) | 6 (6.4%) |
| Duration of (second) analgesic/NSAID use; days | |||
| Mean ± SD | 4.8 ± 0.5 | 5.0. ± 0.0 | 4.8 ± 2.2 |
| Median (range) | 5 (3–5) | 5 (5–5) | 4 (3–8) |
CV clavulanic acid, co-amoxiclav amoxicillin–clavulanic acid, n number of patients, NSAID nonsteroidal anti-inflammatory drug, SD standard deviation
Safety Outcomes
Overall, adverse events reported by patients during the treatment period included diarrhea in 14/355 (3.9%), alteration of taste, hyperacidity, and nausea in 2/355 (0.5%), and skin rashes in 1/355 (0.3%) patients. There were no serious adverse events reported during the study period.
Among 14 patients reporting diarrhea, 3/174 (1.7%) patients were receiving cephalexin CV, 1/87 (1.2%) cefuroxime, and 10/94 (10.6%) co-amoxiclav. The majority of patients reported mild severity diarrhea (2 [66.7%] in cephalexin CV, 1 [100.0%] in cefuroxime, and 3 [30.0%] in the co-amoxiclav group). Moderate severity was reported only in the co-amoxiclav group (6 [60.0%]) (Table 5).
Table 5.
Adverse events
| Adverse events | Total (N = 355) |
Group I | Group II | |
|---|---|---|---|---|
| Cephalexin CV (n = 174) |
Cefuroxime (n = 87) |
Co-amoxiclav (n = 94) |
||
| Alteration of taste | ||||
| Number of patients (%) | 2 (0.6) | 0 (0.0) | 1 (1.2) | 1 (1.5) |
| Duration | ||||
| Mean ± SD | 4.5 ± 0.7 | 4.0 ± 0.0 | 5.0 ± 0.0 | |
| Median | 5 | 4 | 5 | |
| Range | 4–5 | 4–4 | 5–5 | |
| Ongoing; n (%) | 2 (100.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| Severity; n (%) | ||||
| Mild | 1 (50.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) |
| Moderate | 1 (50.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Diarrhea | ||||
| Number of patients (%) | 14 (3.9) | 3 (1.7) | 1 (1.2) | 10 (10.6) |
| Duration | ||||
| Mean ± SD | 5.5 ± 1.9 | 5.0 ± 1.7 | 7.0 ± 0.0 | 5.6 ± 2.1 |
| Median | 5 | 4 | 7 | 5 |
| Range | 3–9 | 4–7 | 7–7 | 3–9 |
| Ongoing; n (%) | 4 (28.6) | 0 (0.0) | 0 (0.0) | 4 (40.0) |
| Severity; n (%) | ||||
| Mild | 6 (42.8) | 2 (66.7) | 1 (100.0) | 3 (30.0) |
| Moderate | 6 (42.8) | 0 (0.0) | 0 (0.0) | 6 (60.0) |
| Severe | 2 (14.3) | 1 (33.3) | 0 (0.0) | 1 (10.0) |
| Hyperacidity | ||||
| Number of patients (%) | 2 (0.5) | 2 (1.2) | 0 (0.0) | 0 (0.0) |
| Duration | ||||
| Mean ± SD | 3.0 ± 1.4 | 3.0 ± 1.4 | ||
| Median | 3 | 3 | ||
| Range | 2–4 | 2–4 | ||
| Ongoing; n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severity; n (%) | ||||
| Mild | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Moderate | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) |
| Severe | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) |
| Nausea | ||||
| Number of patients (%) | 2 (0.5) | 2 (1.2) | 0 (0.0) | 0 (0.0) |
| Duration | ||||
| Mean ± SD | 2.5 ± 0.7 | 2.5 ± 0.7 | ||
| Median | 3 | 3 | ||
| Range | 2–3 | 2–3 | ||
| Ongoing; n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severity; n (%) | ||||
| Mild | 2 (100.0) | 2 (100.0) | 0 (0.0) | 0 (0.0) |
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Skin rashes | ||||
| Number of patients (%) | 1 (0.3) | 1 (0.6) | 0 (0.0) | 0 (0.0) |
| Duration | ||||
| Mean ± SD | 3.0 ± 0.0 | 3.0 ± 0.0 | ||
| Median | 3 | 3 | ||
| Range | 3–3 | 3–3 | ||
| Ongoing; n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severity; n (%) | ||||
| Mild | 1 (100.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Co-amoxiclav amoxicillin–clavulanic acid, CV clavulanic acid, n number of patients, N total patients, SD standard deviation
Discussion
According to the 2022 World Health Organization (WHO) global oral health report, over 3.5 billion people worldwide are estimated to be affected by oral diseases, with middle-income nations accounting for three out of every four of these cases [21]. Dental caries and periodontal diseases are historically known as the top oral health burden in both developing and developed nations, affecting around 20.0%–50.0% of the population globally [22]. In general, dental infections account for 60.0% of all consultation visits to the dentist [23, 24].
Antibiotics are often prescribed in dental practice for the management of dental infections as well as for prophylaxis against systemic infections [7]. There are multiple antibiotic alternatives available to manage dental infections; with co-amoxiclav and cephalosporins being the among the most prescribed antibiotics. This retrospective, multi-centric, observational study was conducted to provide real-world evidence for the effectiveness and tolerability of cephalexin CV as compared with co-amoxiclav and cefuroxime in patients with dental infections (odontogenic) in India.
In the current study, the average age of patients reporting dental infections was about 39 years, similar to multiple studies reporting dental infections and their severity increasing with patients’ age [25–27]. Additionally, this study indicated an equal distribution of both genders (49.9% male and 50.1% female); comparable to the study by Hunt et al. [28], where both genders were equally affected by odontogenic infections.
Untreated dental caries in permanent teeth is the most common health condition according to the Global Burden of Disease 2019, and the pooled prevalence of dental caries in India was reported to be 54.2% [29]. The most common cause of dental infections in the current study was also found to be dental caries (81.1%); similar findings were observed by Shakya et al. in 100 patients with odontogenic infections, with caries (65.0%) as the most common cause of dental infection [30]. A persistent high intake of free sugars, inadequate exposure to fluoride, plaque, and a lack of dental hygiene are a few aspects behind dental caries, which cause dental infection [31].
In the present study, tooth pain was the most common complaint reported by 95.5% of patients, followed by swelling, which was consistent with other studies across various regions [32–34]. Abscess was the most reported complication in this study. In a recently conducted retrospective cross-sectional study in India by Labh et al., abscess was reported as the third most common dental crisis [35]. Abscesses typically arise secondary to dental caries, trauma, or failed dental root canal treatment, and if untreated, they can cause severe pain and risk of spreading to deep neck space or intracranial sinuses.
Detecting, treating, and educating patients about dental infections will provide symptomatic relief, help eradicate infections, and prevent complications [3, 5, 36]. Antibiotics are indicated in dental practice when there are evident signs of systemic infection and/or when the infection progresses rapidly [37]. Various dose regimens are used for the amoxicillin-CV combination, ranging from 625 mg thrice daily to 1–2 g twice daily [38, 39]. For the dose regimen of cephalexin, the current American Dental Association (ADA) recommendation in acute dental infections is 500 mg, four times a day for 3–7 days [40]. Sustained release (SR) preparations have the advantage of a reduced frequency of doses as compared with the normal preparations [41]. In the present study, dose and duration of the antibiotics prescribed were as per the recommended clinical guidelines.
In the current study, it was observed that within 10 days of the treatment period, clinical improvement was noted in all antibiotic groups. In accordance with the current study findings, Matijević et al. showed that the signs and symptoms of infection last on average for almost the same time in amoxicillin and cephalexin groups, however, the antibiotic susceptibility of isolated bacteria to cefalexin was higher than that of amoxicillin (89.2% and 76.6%, respectively) [42]. Hence, clinical improvement in dental infections can be achieved with the optimum dose and duration of treatment.
Further, the current study reported 96.0% of patients taking analgesics during the study period in the cephalexin CV group, 85.0% in the cefuroxime group, and 98.9% in the co-amoxiclav group. Comparable findings were observed by Buttar et al., whereby the proportion of patients taking analgesics along with antibiotics was 95.0% [43]. In a survey by Maslamani et al., amoxicillin was recommended in combination with analgesics (most commonly diclofenac and ibuprofen) to 41.0% of patients for acute pain relief [44]. Analgesics and antibiotics are often prescribed as adjuncts or as definitive treatments for common dental diseases and are useful and cost effective when prescribed appropriately [45]. The most common reason for adding analgesics to antibiotics is usually sustained pain or fever, comparable to this study. The overall duration of first analgesic use was numerically shorter among patients in the cephalexin CV group, which could be attributed to a better symptom resolution as compared with other groups.
In the present study, adverse events were captured in only 0.6% of the study population during the study period, which included alteration of taste, diarrhea, hyperacidity, nausea, and skin rashes. Similar side effects were observed in the studies by Sasaki et al. and Horii et al., in which gastrointestinal symptoms, diarrhea, rash, and itching was seen in 0.8%–1.0% patients [41, 46].
Previous studies have demonstrated cephalosporins to have better antimicrobial activity as compared with other antibiotics [47, 48]. However, there is a paucity of literature evaluating the usage and dose regimen of the cephalexin–clavulanic acid combination in dental infections. These study results provide evidence that a 125–750 mg dose of SR cephalexin CV, when taken twice daily for a period of 5 days, provides a sufficient clinical improvement in symptoms of dental infections.
The study had some limitations. As this retrospective EMR study represents real-world data, baseline values for signs and symptoms were not matching for all the groups. Treatment with cephalexin CV was significantly better in terms of improvement in certain baseline signs and symptoms like fever, redness, and bleeding gums in comparison with cefuroxime, and resolution of trismus as a baseline complication in comparison with co-amoxiclav. However, baseline data for these signs and symptoms and for trismus did not match as this was retrospective data from EMRs. The data from EMRs was not recorded systematically under strictly controlled conditions but represents real-world in-clinic scenarios. Additionally, EMR data lacks comprehensive information on patient history, treatments and adverse effects, and patient adherence to medication. The findings of our research predominantly center around odontogenic dental infections and are not applicable to other dental infections. In retrospective studies, the absence of randomization and a control group can introduce selection bias. A randomized controlled trial could be considered to give more insights on the comparative efficacy of antibiotics.
Conclusions
Dental infections commonly present with symptoms of pain, fever, and swelling. Antibiotic therapy is crucial to control dental infections. In this real-world, retrospective EMR study, cephalexin CV was as effective as cefuroxime and co-amoxiclav in providing clinical improvement. Mean time to clinical improvement was nearly similar among patients receiving cephalexin CV compared with cefuroxime and co-amoxiclav. Cephalexin CV was significantly better in comparison with cefuroxime in reducing fever, redness, and bleeding gums. Cephalexin CV was also significantly better in comparison with co-amoxiclav in resolving trismus, which was one of the complications. All the antibiotics were well tolerated without any major adverse effects.
Acknowledgements
The author would like to thank Dr Farah Iram, Dr Manjeeta Gupta, and Ms Rupali Jangid from THB c/o Sekhmet Technologies Pvt. Ltd. for their contribution to manuscript writing and Mr Raman Gupta for statistical analysis.
Author Contributions
The authors KB, AK, KAS, DB, PM, NMP, PP, SPA, AC, and RNM have contributed to formal analysis, investigation, resources, and data curation. The author ASK has contributed to methodology, resources, data curation, writing—original draft preparation, review and editing, visualization, supervision. The author NM has contributed to methodology, writing—review and editing, visualization, funding acquisition. The author SD has contributed to writing—original draft preparation, writing—review and editing. The author CK has contributed to methodology, formal analysis, resources, data curation, writing—original draft preparation, review and editing, visualization, supervision, project administration. The author AN has contributed to formal analysis, resources, writing—review and editing, visualization, supervision. The author SM has contributed to methodology, resources, writing—review and editing, visualization, supervision. The author SJ has contributed to methodology, visualization, supervision. All authors read and approved the final version.
Funding
Sun Pharmaceutical Industries Ltd., Mumbai, India.
Data Availability
The data substantiating the results of this real-world study can be obtained from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Declarations
Conflict of Interest
The authors KB, AK, KAS, DB, PM, NMP, PP, SPA, AC, and RNM declare no conflict of interest. The authors ASK, NM, SD, CK, AM, SM, and SJ are employees of Sun Pharmaceutical Industries Ltd., Mumbai, India, who sponsored the study. Author, SJ was affiliated with Sun Pharmaceutical Industries Ltd., Mumbai, India, as Senior Vice President and Head- Global Medical Affairs and Clinical Research during the study execution.
Ethical Approval
The written and dated approval from the institutional ethics committee (IEC) was taken as per Sekhmet’s (THB) ethical committee submission standard operating procedure (SOP). The study was approved by the Royal Pune Independent Ethics Committee (RPIEC) located in Pune, India (Ethics Approval Number: RPIEC090223; dated 7th Feb 2023).
Consent to Participate
This retrospective real-world study was conducted using anonymized data (existing medical records that are available as of the date of IEC submission) without any additional prospective components for research purposes. Hence, the process did not necessitate obtaining informed consent since the study did not involve identifiable individuals. Accordingly, permission for the ICF waiver was obtained as per the ICMR Guidelines for Biomedical Research 2017 from RPIEC before initiating the data collection process for this study.
Consent for Publication
Not applicable.
References
- 1.Ortiz R, Espinoza V. Odontogenic infection. Review of the pathogenesis, diagnosis, complications and treatment. Res Rep Oral Maxillofac Surg. 2021;5:055. [Google Scholar]
- 2.Neal TW, Schlieve T. Complications of severe odontogenic infections: a review. Biology (Basel) 2022 doi: 10.3390/biology11121784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogle OE. Odontogenic infections. Dent Clin North Am. 2017;61:235–252. doi: 10.1016/j.cden.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Bernabe E, Marcenes W, Hernandez CR, Bailey J, Abreu LG, Alipour V, Amini S, Arabloo J, Arefi Z, Arora A, et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J Dent Res. 2020;99:362–373. doi: 10.1177/0022034520908533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erazo D, Whetstone DR. Dental infections. StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 6.Gonçalves L, Lauriti L, Yamamoto MK, Luz JG. Characteristics and management of patients requiring hospitalization for treatment of odontogenic infections. J Craniofac Surg. 2013;24:e458–462. doi: 10.1097/SCS.0b013e3182902e95. [DOI] [PubMed] [Google Scholar]
- 7.Patil R, Gondivkar SM, Gadbail AR, Yuwanati M, Mankar M, Likhitkar M, Sarode S, Sarode G, Patil S. Role of oral foci in systemic diseases: an update. Int J Contemp Dent Med Rev. 2017 doi: 10.15713/ins.ijcdmr.115. [DOI] [Google Scholar]
- 8.Seymour RA. Is gum disease killing your patient? Br Dent J. 2009;206:551–552. doi: 10.1038/sj.bdj.2009.472. [DOI] [PubMed] [Google Scholar]
- 9.Donkor ES, Kotey FC. Methicillin-resistant Staphylococcus aureus in the oral cavity: implications for antibiotic prophylaxis and surveillance. Infect Dis. 2020;13:1178633720976581. doi: 10.1177/1178633720976581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meinen A, Reuss A, Willrich N, Feig M, Noll I, Eckmanns T, Al-Nawas B, Markwart R. Antimicrobial resistance and the spectrum of pathogens in dental and oral-maxillofacial infections in hospitals and dental practices in Germany. Front Microbiol. 2021;12:676108. doi: 10.3389/fmicb.2021.676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garbacz K, Jarzembowski T, Kwapisz E, Daca A, Witkowski J. Do the oral Staphylococcus aureus strains from denture wearers have a greater pathogenicity potential? J Oral Microbiol. 2018;11:1536193. doi: 10.1080/20002297.2018.1536193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar N, Khare VV, Jamdade A, Aggarwal A, Rathore NS, Yadav S. Antibiotic susceptibility of the bacteria causing odontogenic infections: an observational study. J Indian Acad Oral Med Radiol. 2022;34:442–446. doi: 10.4103/jiaomr.jiaomr_194_22. [DOI] [Google Scholar]
- 13.Ahmadi H, Ebrahimi A, Ahmadi F. Antibiotic therapy in dentistry. Int J Dent. 2021;2021:6667624. doi: 10.1155/2021/6667624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MoHFW. Standard Treatment guidelines for Oral Health. 2010. Available from: https://main.mohfw.gov.in/sites/default/files/N_56820_1613385504626.pdf. Accessed 12 June 2023.
- 15.Ramu C, Padmanabhan TV. Indications of antibiotic prophylaxis in dental practice—review. Asian Pac J Trop Biomed. 2012;2:749–754. doi: 10.1016/s2221-1691(12)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bui T, Preuss CV. Cephalosporins. StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 17.Tartaglione TA, Polk RE. Review of the new second-generation cephalosporins: cefonicid, ceforanide, and cefuroxime. Drug Intell Clin Pharm. 1985;19:188–198. doi: 10.1177/106002808501900304. [DOI] [PubMed] [Google Scholar]
- 18.Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev. 2010;23:160–201. doi: 10.1128/cmr.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobottka I, Cachovan G, Stürenburg E, Ahlers MO, Laufs R, Platzer U, Mack D. In vitro activity of moxifloxacin against bacteria isolated from odontogenic abscesses. Antimicrob Agents Chemother. 2002;46:4019–4021. doi: 10.1128/aac.46.12.4019-4021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar KP, Kaushik M, Kumar PU, Reddy MS, Prashar N. Antibiotic prescribing habits of dental surgeons in hyderabad city, India, for pulpal and periapical pathologies: a survey. Adv Pharmacol Sci. 2013;2013:537385. doi: 10.1155/2013/537385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO global oral health report. 2022. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/odi.14516. Accessed 22 June 2023.
- 22.Haque M, Sartelli M, Haque SZ. Dental infection and resistance-global health consequences. Dent J (Basel) 2019 doi: 10.3390/dj7010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monasteri R, Henriquez SG. Prevalence and characterization of Odontogenic Infections in the Public Assistance Emergency Hospital between the Months of July to September of the Year 2015: Prospective Study. EC Dental Science. 2018;17:880–891. [Google Scholar]
- 24.Topazian RG, Goldberg MH, Hupp JR. Oral and maxillofacial infections. Saunders; 2002. [Google Scholar]
- 25.Seppänen L, Rautemaa R, Lindqvist C, Lauhio A. Changing clinical features of odontogenic maxillofacial infections. Clin Oral Investig. 2010;14:459–465. doi: 10.1007/s00784-009-0281-5. [DOI] [PubMed] [Google Scholar]
- 26.Mahmoodi B, Weusmann J, Azaripour A, Braun B, Walter C, Willershausen B. Odontogenic infections: a 1-year retrospective study. J Contemp Dent Pract. 2015;16:253–258. doi: 10.5005/jp-journals-10024-1671. [DOI] [PubMed] [Google Scholar]
- 27.Tadjoedin FM, Fitri AH, Kuswandani SO, Sulijaya B, Soeroso Y. The correlation between age and periodontal diseases. J Int Dental Med Res. 2017;10:327. [Google Scholar]
- 28.Hunt DE, King TJ, Fuller GE. Antibiotic suseptibility of bacteria isolated from oral infections. J Oral Surg. 1978;36:527–529. [PubMed] [Google Scholar]
- 29.Pandey P, Nandkeoliar T, Tikku AP, Singh D, Singh MK. Prevalence of dental caries in the Indian population: a systematic review and meta-analysis. J Int Soc Prev Community Dent. 2021;11:256–265. doi: 10.4103/jispcd.JISPCD_42_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shakya N, Sharma D, Newaskar V, Agrawal D, Shrivastava S, Yadav R. Epidemiology, microbiology and antibiotic sensitivity of odontogenic space infections in Central India. J Maxillofac Oral Surg. 2018;17:324–331. doi: 10.1007/s12663-017-1014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO. Oral health. 2023. Available from: https://cdn.who.int/media/docs/default-source/ncds/mnd/oral-health/eb152-draft-global-oral-health-action-plan-2023-2030-en.pdf. Accessed 22 June 2023.
- 32.Maheswaran T, Ramesh V, Krishnan A, Joseph J. Common chief complaints of patients seeking treatment in the government dental institution of Puducherry, India. J Indian Acad Dent Spec Res. 2015;2:55–58. doi: 10.4103/2229-3019.177921. [DOI] [Google Scholar]
- 33.Abdullah BA, Al-Tuhafi AA. Chief complaints of patients attending college of Dentistry at Mosul University. Al-Rafidain Dental J. 2007;7:201–205. doi: 10.33899/rden.2007.39756. [DOI] [Google Scholar]
- 34.Masiga M. Presenting chief complaints and clinical characteristics among patients attending the Department of Paediatric Dentistry Clinic at the University of Nairobi Dental Hospital. East Afr Med J. 2005;82:652–655. doi: 10.4314/eamj.v82i12.9372. [DOI] [PubMed] [Google Scholar]
- 35.Labh AK, Ramani P. Prevalence of periodontal abscess among patients visiting a private dental college and hospital in Chennai, India. Indian J Forensic Med Toxicol. 2020;14:5081–5092. [Google Scholar]
- 36.Sanders JL, Houck RC. Dental abscess. Treasure Island: StatPearls Publishing LLC; 2023. [PubMed] [Google Scholar]
- 37.Henry M, Reader A, Beck M. Effect of penicillin on postoperative endodontic pain and swelling in symptomatic necrotic teeth. Journal of endodontics. 2001;27:117–123. doi: 10.1097/00004770-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Tancawan AL, Pato MN, Abidin KZ, Asari AS, Thong TX, Kochhar P, Muganurmath C, Twynholm M, Barker K. Amoxicillin/clavulanic acid for the treatment of odontogenic infections: a randomised study comparing efficacy and tolerability versus clindamycin. Int J Dent. 2015;2015:472470. doi: 10.1155/2015/472470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumari S, Mohanty S, Sharma P, Dabas J, Kohli S, Diana C. Is the routine practice of antibiotic prescription and microbial culture and antibiotic sensitivity testing justified in primary maxillofacial space infection patients? A prospective, randomized clinical study. J Craniomaxillofac Surg. 2018;46:446–452. doi: 10.1016/j.jcms.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Lockhart PB, Tampi MP, Abt E, Aminoshariae A, Durkin MJ, Fouad AF, Gopal P, Hatten BW, Kennedy E, Lang MS, et al. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: a report from the American Dental Association. J Am Dent Assoc. 2019;150:906–921.e912. doi: 10.1016/j.adaj.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horii M, Morinaga T, Shimada S, Takeuchi T, Yamanaka H, Nishimura T, Noshi Y, Okada T, Sasaki T, Ikeda S, et al. Double-blind comparison of L-keflex and cephalexin (Keflex) in dental infections (author’s transl) Jpn J Antibiot. 1980;33:1194–1214. [PubMed] [Google Scholar]
- 42.Matijević S, Lazić Z, Kuljić-Kapulica N, Nonković Z. Empirical antimicrobial therapy of acute dentoalveolar abscess. Vojnosanit Pregl. 2009;66:544–550. doi: 10.2298/vsp0907544m. [DOI] [PubMed] [Google Scholar]
- 43.Buttar R, Aleksejūnienė J, Coil J. Antibiotic and opioid analgesic prescribing patterns of dentists in Vancouver and endodontic specialists in British Columbia. J Can Dent Assoc. 2017;83:h8. [PubMed] [Google Scholar]
- 44.Maslamani M, Sedeqi F. Antibiotic and analgesic prescription patterns among dentists or management of dental pain and infection during endodontic treatment. Med Princ Pract. 2018;27:66–72. doi: 10.1159/000486416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dana R, Azarpazhooh A, Laghapour N, Suda KJ, Okunseri C. Role of dentists in prescribing opioid analgesics and antibiotics: an overview. Dent Clin North Am. 2018;62:279–294. doi: 10.1016/j.cden.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki I, Morihana T, Kaneko A, Michi K, Takahashi K, Fukuoka F, Satoh T, Yoshinari N, Ohne M, Hara H, et al. Clinical evaluation of cefuroxime axetil in acute dental infections. Double blind comparative study vs. cefaclor. Jpn J Antibiot. 1990;43:2035–2068. [PubMed] [Google Scholar]
- 47.Kuriyama T, Karasawa T, Nakagawa K, Nakamura S, Yamamoto E. Antimicrobial susceptibility of major pathogens of orofacial odontogenic infections to 11 beta-lactam antibiotics. Oral Microbiol Immunol. 2002;17:285–289. doi: 10.1034/j.1399-302x.2002.170504.x. [DOI] [PubMed] [Google Scholar]
- 48.Al-Qamachi LH, Aga H, McMahon J, Leanord A, Hammersley N. Microbiology of odontogenic infections in deep neck spaces: a retrospective study. Br J Oral Maxillofac Surg. 2010;48:37–39. doi: 10.1016/j.bjoms.2008.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data substantiating the results of this real-world study can be obtained from the corresponding author upon reasonable request.
Not applicable.