Abstract
Background
Immune checkpoint inhibitors (ICIs) can be commonly associated with the occurrence of immune-related adverse drug reactions (irADRs), which can involve any tissue and organ. ICI-induced skin toxicities are common irADRs and they can be a consequence of a rheumatologic ADR, such as in the case of scleroderma. A recent literature review reported that scleroderma and scleroderma mimics represent a group of disorders with significant morbidity that have been described during ICIs’ use.
Objective and Methods
Considering the clinical significance of scleroderma cases, the present study aimed to analyze the occurrence of these events in patients receiving ICIs by describing data from individual case safety reports (ICSRs) retrieved from the European spontaneous reporting system, EudraVigilance (EV).
Results
Until February 2023, 70 ICSRs with at least one ICI as the suspected drug and at least one preferred term (PT) related to scleroderma cases were retrieved from the EV. Pembrolizumab was reported as suspected in 41 ICSRs, nivolumab in 25 ICSRs, ipilimumab in 8 ICSRs and atezolizumab in 3 ICSRs. Patients who experienced scleroderma cases were adults, and no differences were found in terms of sex distribution. Scleroderma cases were mainly classified as serious, while the outcome was mainly reported as favorable. The most reported PTs were scleroderma and morphea.
Conclusions
Considering the seriousness of ICI-induced scleroderma cases and the recent marketing authorization of some ICIs, we believe that further high-quality clinical studies should be conducted on this topic to better estimate the impact of these events in patients with cancer.
Key Points
| ICIs can induce dermatologic reactions, like scleroderma. |
| In the Eudravigilance we found 70 cases of scleroderma associated with ICIs. |
| Further high-quality clinical studies are strongly needed to estimate the real impact of these events in patients with cancer. |
Introduction
Immune checkpoint inhibitors (ICIs), which currently include one CTLA-4 inhibitor (ipilimumab), three PD-1 inhibitors (nivolumab, pembrolizumab and cemiplimab) and three PD-L1 inhibitors (atezolizumab, avelumab and durvalumab), represent one of the most recent breakthrough therapies to be approved for the treatment of cancer. For these drugs the innovation lies in their mechanism of action. Indeed, the role of the immune system in recognizing and attacking foreign cells, such as cancer cells, is well recognized thanks to the presence of checkpoint proteins on immune cells [1]. The main role of immune checkpoints is the prevention of an immune response that destroys healthy cells in the body. Immune checkpoints engage when T cells bind to partner proteins on other cells, such as some tumor cells [2]. When the checkpoint and partner proteins bind together, they send an “off” signal to the T cells. This can prevent the immune system from destroying the cancer. Thus, ICIs block checkpoint proteins from binding with their partner proteins, allowing the T cells to kill cancer cells [3].
The efficacy of these drugs has been extensively demonstrated by several clinical trials carried out in patients diagnosed with different types of cancer. For instance, as reported by Jácome AA et al. in a meta-analysis of three studies (1657 patients, of whom 985 were treated with ICIs and 672 received standard treatment), ICIs are associated with superior efficacy and safety compared with standard therapies in patients with unresectable hepatocellular carcinoma [significantly improved overall survival (OS) (hazard ratio 0.75; 95% CI 0.62–0.92), progression-free survival (PFS) (hazard ratio 0.74; 95% CI 0.56–0.97), and overall response rate (odds ratio 2.82; 95% CI 2.02–3.93)] [4]. Other researchers reported that the addition of ICIs to standard of care benefits a greater number of patients, prolonging survival with a manageable toxicity profile [5]. Data from a recent systematic review and meta-analysis highlighted that although the addition of ICIs to standard chemotherapy result in significant improvements in PFS and OS for patients with lung cancer, a worse safety profile can be expected [more adverse drug reactions (ADRs) observed] [6]. Finally, their efficacy was also demonstrated in the treatment of some rare tumors, such as Merkel cell carcinoma, squamous cell carcinoma of the skin, thymic epithelial tumors, and gestational trophoblastic neoplasia, as recently highlighted by Guven et al. [7].
With regard to their safety profile, as our research group had previously reported [8, 9], ICIs can be commonly associated with immune-related ADRs (irADRs), a new spectrum of events that are different from the classical chemotherapy-related toxicities whose frequency depends on the ICI used, the exposure time, and the administered dose apart from the patient’s intrinsic risk factors. Given the nature of these reactions, ICI-induced irADRs can involve any tissue and organ and can occur anytime [10]. For instance, cutaneous toxicities are common irADRs and they have a variety of clinical presentations, including eczematous, morbilliform, and lichenoid dermatoses, vitiligo, and pruritus. In some cases, the cutaneous manifestation is a consequence of a rheumatologic ADR, such as in the case of scleroderma, dermatomyositis or cutaneous lupus erythematosus. These reactions usually develop 3–6 months after the initiation of the therapy and improve or resolve within 3 months, at least those amenable to treatment i.e., dermatomyositis and cutaneous lupus erythematosus [11, 12]. Due to the risk for inducing scleroderma renal crisis, corticosteroids are not recommended for the treatment of scleroderma [13]. Indeed, the choice of the treatment represents a great challenge for the clinician considering that the disease’s cause is unknown and thus therapies are directed to improve peripheral blood circulation using, for example, vasodilators, to prevent the release of cytokines with immunosuppressant drugs, and to reduce fibrosis with agents that reduce collagen synthesis [14]. A recent literature review reported that scleroderma and scleroderma mimics represent a group of disorders with significant morbidity that have been described during ICIs’ use. Scleroderma, also known as systemic sclerosis, is a rare and complex autoimmune connective tissue disease. The early recognition of the disease and its accurate diagnosis is essential to obtain resolution of signs and symptoms [15]. Indeed, early diagnosis of scleroderma can be difficult because the early clinical stages of the disease are similar to that of other autoimmune conditions. In addition, the disease is associated with major organ manifestations, including interstitial lung disease, pulmonary hypertension, renal involvement and cardiac disease. Thus, as reported by McMahan et al., the screening for major organ manifestations is a priority since the early intervention might prevent disease progression [16].
Available data seem to suggest that scleroderma cases tend to be more common during PD-1i/PD-L1i therapy compared with CTLA-4i. In addition, an association between these irADRs and malignancy and other common comorbidities seems to exist [17]. Considering the clinical significance of ICI-related scleroderma, the present study aims to evaluate the occurrence of these events (in terms of type of irADRs, seriousness, outcome) after treatment with ICIs by describing data from individual case safety reports (ICSRs) retrieved from the European spontaneous reporting system, EudraVigilance (EV).
Methods
Study Design
This is a pharmacovigilance study based on the analysis of ICSRs retrieved from the EV website.
Exposure Definition and Measurement
By using the line listing function of the EV database, ICSRs reporting an ICI as the suspected drug and scleroderma cases were retrieved from the date of marketing authorization granted by the EMA for each ICI to February, 2023. The ICIs considered were ipilimumab (authorized by the EMA in 2011), nivolumab (2015), pembrolizumab (2015), atezolizumab (2017), durvalumab (2018), avelumab (2017), and cemiplimab (2019).
Outcome Definition and Measurement
According to the Medical Dictionary for Regulatory Activities (MedDRA—version 26.0, March 2023), cases of scleroderma were defined as any ICSR that reported one of the following preferred terms (PTs): scleroderma, scleroderma renal crisis, scleroderma-like reaction, systemic scleroderma, scleroderma associated digital ulcer, systemic sclerosis pulmonary, CREST syndrome, or morphea.
Data Source and Data Mining
The data source was the EV database that is managed by the European Medicines Agency (EMA) and is used for the collection, management, and analyses of ICSRs related to both medicines or vaccines, which are authorized or are being studied in clinical trials in the European Economic Area. These data are publicly available for transparency through the EMA website (www.adrreports.eu). According to data recently shared by the EMA [18], the EV database currently holds over 25.3 million ICSRs relating to 14.8 million unique suspected ADR case reports, being one of the largest pharmacovigilance databases in the world.
Search on EV was made on 28 February 2023. Ipilimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, avelumab, and cemiplimab were considered as suspected drugs and “scleroderma”, “scleroderma renal crisis”, “scleroderma-like reaction”, “systemic scleroderma”, “scleroderma associated digital ulcer”, “systemic sclerosis pulmonary”, “CREST syndrome”, and “morphea” as PTs reaction terms.
Study Population
All ICSRs retrieved from the EV were included in the analysis, including cases reported from both EU and non-EU countries and pediatric cases.
Data Analyses
Information on patient characteristics [age group (<18 years, 18–64 years and 65–85 years) and sex], adverse event (type, outcome and seriousness), therapeutic indication, primary source qualification, primary source country for regulatory purposes, number of suspected drugs other than ICIs, and number of concomitant drugs was provided for all.
ICSRs with two or more ICIs as suspected drugs were described separately. The time-to-event (TTE) was calculated only for ICSRs that reported both the duration of the therapy and the drug withdrawal as action taken after the occurrence of the ADR.
According with the International Council on Harmonization E2D guidelines, a case is defined as “serious” if it is life threatening, results in death, requires or prolongs a hospitalization, results in persistent or significant disability/incapacity, determines a congenital anomaly/birth defect, or results in some other clinically important conditions. The outcome was classified as favorable (“recovered/resolved” and “recovering/resolving”), unfavorable (“recovered/resolved with sequelae”, “not recovered/not resolved”, or “fatal”) and not reported (“unknown”). The outcome with the lower level of resolution was chosen for classification whether an ICSR reported two or more PTs with different outcomes. ICSRs were classified as fatal if death occurred.
Data were analyzed using Microsoft Office Excel and STATA v.17 programs.
Ethical Standards
Safety data extracted from the spontaneous reporting system comply with ethical standards and are anonymous. Therefore, no further ethical measures were required.
Results
During the study period, 70 ICSRs, covering 158 PTs (including scleroderma cases and nonscleroderma cases) with at least one ICI as the suspected drug were retrieved from the EV (Tables 1 and 2). Regarding suspected ICIs, PD-1 inhibitors were those most commonly reported as suspected (n = 63), followed by ICIs combination (n = 8), PD-L1 inhibitors (n = 3), and CTLA-4 inhibitor (n = 1) (Table 3). Specifically, among PD-1 inhibitors, pembrolizumab was reported as suspected in 41 ICSRs and nivolumab in 25 ICSRs (data not shown).
Table 1.
Demographic characteristics and distribution for seriousness, outcomes, primary source, primary source country for regulatory purposes, number of suspected drugs other than immune checkpoint inhibitors (ICIs), and number of concomitant drugs of Individual Case Safety Reports (ICSRs) reporting at least one event related to scleroderma and having one ICI as suspected drug among those reported in the EudraVigilance database from the date of marketing authorization to 28 February 2023
| Variable | Level | All ICSRs (n = 70), n (%) |
|---|---|---|
| Age group | 18–64 years | 32 (45.7) |
| 65–85 years | 31 (44.3) | |
| Not specified | 7 (10) | |
| Gender | F | 33 (47.2) |
| M | 36 (51.4) | |
| Missing | 1 (1.4) | |
| Seriousness | Caused/prolonged hospitalization | 14 (20) |
| Disabling | 2 (2.9) | |
| Other medically important conditions | 44 (62.8) | |
| Results in death* | 1 (1.4) | |
| Not reported | 9 (12.9) | |
| Outcome | Recovered/resolved | 7 (10) |
| Recovering/resolving | 18 (25.7) | |
| Not recovered/not resolved | 14 (20) | |
| Fatal* | 1 (1.4) | |
| Unknown | 30 (42.9) | |
| Primary source country for regulatory purposes | European economic area | 40 (57.1) |
| Non-European economic area | 30 (42.9) | |
| Suspected drug(s) other than ICIs | 0 | 63 (90) |
| 1 | 4 (5.7) | |
| 2 | 2 (2.9) | |
| >2 | 1 (1.4) | |
| Concomitant drug(s) | 0 | 58 (82.9) |
| 1 | 1 (1.4) | |
| 2 | 5 (7.1) | |
| 3 | 1 (1.4) | |
| 4 | 2 (2.9) | |
| ≥ 5 | 3 (4.3) |
*This ICSR described a case of scleroderma, immune-mediated dermatitis, immune-mediated lung disease, and tubulointerstitial nephritis that led to patient’s death
Table 2.
List of preferred terms (PTs) reported in retrieved individual case safety reports (ICSRs)
| List of PTs reported in retrieved ICSRs | |
|---|---|
| Morphea | 23 (14.6%) |
| Scleroderma | 20 (12.7%) |
| Systemic scleroderma | 18 (11.4%) |
| Scleroderma-like reaction | 12 (7.6%) |
| Hypothyroidism | 5 (3.2%) |
| Malignant neoplasm progression | 4 (2.5%) |
| Immune-mediated adverse reaction | 4 (2.5%) |
| Eosinophilia fasciitis | 4 (2.5%) |
| Vitiligo | 3 (2%) |
| Scleroderma renal crisis | 2 (1.2%) |
| Erythema | 2 (1.2%) |
| Generalized edema | 2 (1.2%) |
| Edema | 2 (1.2%) |
| Edema peripheral | 2 (1.2%) |
| Immune thrombocytopenia | 2 (1.2%) |
| Diarrhea | 2 (1.2%) |
| Pneumonitis | 2 (1.2%) |
| Rash | 2 (1.2%) |
| Tubulointerstitial nephritis | 2 (1.2%) |
| Other* | 45 (28.5%) |
| Total PTs (scleroderma and non-scleroderma cases) | 158 (100%) |
*Each one of the following PTs were reported once in retrieved ICSRs: aphasia, arthralgia, autoimmune disorder, autoimmune nephritis, capillary leak syndrome, colitis, collagen disorder, condition aggravated, coronavirus disease 2019 (COVID-19), diffuse large B cell lymphoma recurrent, disease recurrence, dysphagia, encephalitis, fatigue, hepatitis, hypophysitis, immune-mediated dermatitis, immune-mediated hypothyroidism, immune-mediated lung disease, joint swelling, juvenile idiopathic arthritis, lymphedema, lichen sclerosus, neuritis, myositis, musculoskeletal disorder, neuropathy peripheral, neutropenia, pain, pemphigoid, peripheral swelling, pleural effusion, pruritus, Raynaud’s phenomenon, rheumatoid arthritis, skin atrophy, skin hypertrophy, skin plaque, skin tightness, skin lesion, thyrotoxic crisis, thyroiditis, total lung capacity decreased, tumor hyperprogression, tumor pseudoprogression
Table 3.
Distribution of individual case safety reports (ICSRs) by immune checkpoint inhibitors (ICIs) and preferred terms (PTs)
| CTLA-4 inhibitor | ICIs combination | PD-1 inhibitors | PD-L1 inhibitors | |
|---|---|---|---|---|
| Morphea, n (%) | 1 (100) | 4 (50) | 18 (29) | – |
| Scleroderma, n (%) | – | – | 19 (30) | 1 (33) |
| Systemic scleroderma, n (%) | – | 4 (50) | 14 (22) | |
| Scleroderma-like reaction, n (%) | – | – | 10 (16) | 2 (67) |
| Scleroderma renal crisis, n (%) | – | – | 2 (3) | – |
| Total ICSRs/ICI* | 1 (100) | 8 (100) | 63 (100) | 3 (100) |
*The total number of ICSRs/ICIs and the total number of PTs exceed the total number of ICSRs retrieved from the EV (n = 70) since more than one ICI and PT can be reported in a single ICSR
As reported in Table 1, a similar number of patients who experienced scleroderma cases following ICI treatment belonged to the age group 18–64 years and 65–85 years, and no differences were found regarding the distribution of patients by sex. Among ICSRs for which the seriousness degree was reported (n = 61), 100% were classified as serious (mainly reported as “other medically important condition”). Among ICSRs for which the outcome was reported (n = 40), 63% (n = 25) had a favorable outcome (reported as “recovered” or “recovering”). In the majority of ICSRs (90%), ICIs were the only suspected drugs and in almost 83% of ICSRs concomitant drugs were not reported (Table 1). For all ICSRs the source was the healthcare professional (data not shown), and 158 PTs were listed in retrieved ICSRs (Tables 2, 3 and 4). Morphea was the most commonly reported PT (23 total cases, of which 18 related to PD-1 inhibitors), followed by scleroderma (20 cases), systemic scleroderma (18 cases), scleroderma-like reaction (12 cases), and scleroderma renal crisis (2 cases). As reported in Table 4, cases of morphea and scleroderma mainly had a favorable outcome; on the contrary, cases of scleroderma-like reaction and systemic scleroderma mainly had an unfavorable outcome.
Table 4.
Distribution of individual case safety reports (ICSRs) by preferred terms (PTs) and outcome
| Morphea | Scleroderma | Scleroderma renal crisis | Scleroderma-like reaction | Systemic scleroderma | |
|---|---|---|---|---|---|
| Favorable, n (%) | 12 (52) | 6 (30) | 1 (50) | 3 (25) | 6 (33) |
| Unfavorable, n (%) | 3 (13) | 4 (20) | – | 5 (42) | 7 (39) |
| Not available, n (%) | 8 (35) | 10 (50) | 1 (50) | 4 (33) | 5 (28) |
| Total ICSRs/PTs | 23 (100) | 20 (100) | 2 (100) | 12 (100) | 18 (100) |
Favorable outcome: recovering/resolving; unfavorable outcome: not recovered/not resolved; death
The most commonly reported therapeutic indications were malignant melanoma (n = 29) and lung cancer (n = 24). In six ICSRs, ICI therapeutic indications were not reported (data not shown).
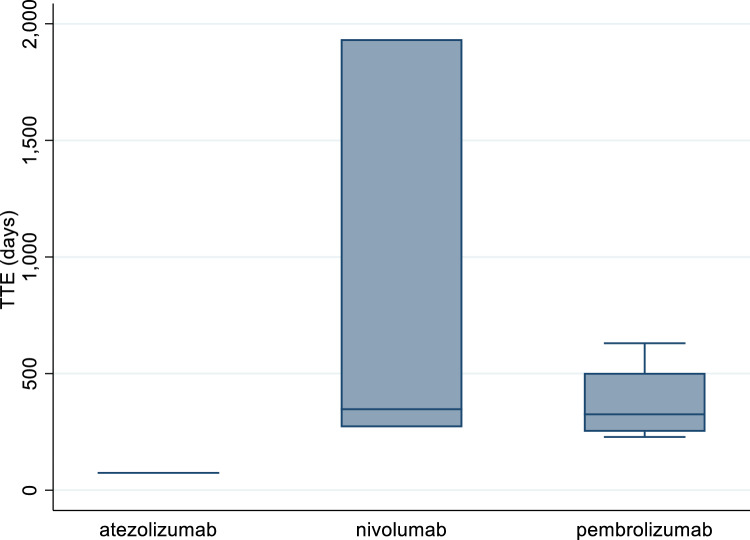
Overall, eight ICSRs reported both the duration of the therapy and the drug withdrawal as action taken after the occurrence of the ADR (four related to pembrolizumab, three to nivolumab, and one to atezolizumab); thus, for these ICSRs, the TTE in days was calculated (Fig. 1). The median TTE was 325 days (IQR: 262.5–439.5) for pembrolizumab and 347 days (IQR: 308.5–1140.5) for nivolumab. The only TTE available for atezolizumab was 74 days.
Fig. 1.
Time-to-event (TTE) for cases of atezolizumab-, nivolumab-, and pembrolizumab-induced scleroderma
Discussion
The present study described cases of ICI-induced scleroderma using data from the EV database. ICIs are commonly associated with the occurrence of dermatologic irADRs, such as rash, dermatitis, photosensitivity, toxic epidermal necrolysis. On the other hand, they are also able to induce the occurrence of rheumatic and musculoskeletal irADRs, such as arthralgia and myalgia [19, 20]. Being a chronic autoimmune disease associated with skin and organ fibrosis, scleroderma is characterized by high morbidity and mortality [21]. Although the pathophysiologic mechanism of the ICI-induced cutaneous sclerosis is unknown, it is possible to postulate that widespread inflammation induced by PD-1 inhibition in patients with melanoma could lead to a profibrotic cascade resulting in a scleroderma phenotype [22]. According to literature data, many drugs can be responsible of the occurrence of scleroderma, including bleomycin, taxanes, gemcitabine, and ICIs [23]. Scleroderma-like lesions derived from a process of inflammation, vasculopathy, and fibrosis affecting the skin and multiple organs. On the other hand, morphea, also known as localized scleroderma, affects the skin and the subcutaneous tissues leading to collagen deposition and subsequent fibrosis [24, 25].
In our study, the majority of ICSRs were related to nivolumab and pembrolizumab. In our opinion, the higher number of ICSRs reporting these ICIs as suspected might depend on their earlier marketing approval [26]. In line with this, many case reports and series on the associations nivolumab/scleroderma and pembrolizumab/scleroderma can be found in the published literature [27–30]. For instance, Barbosa et al. described the occurrence of scleroderma in two patients who were receiving pembrolizumab for metastatic melanoma. Authors suggested that the prompt recognition and treatment of irADRs may contribute to a better understanding of the manifesting autoimmune disease [29]. DeMaio A et al. reported a case of nivolumab-induced scleroderma-like syndrome in a 69-year-old woman with metastatic small-cell lung cancer on nivolumab for 2.5 years. The patient failed to respond to steroid therapy but her condition improved with intravenous immunoglobulin [30].
Among ICSRs retrieved for this study the main therapeutic indications were lung and skin cancers. Again, these data are not surprising if we consider that ICIs are mainly indicated for the treatment of melanoma, renal cell carcinoma, bladder cancer, head and neck cancers, and lung cancers [31].
The clinical manifestations of scleroderma are often serious, being associated with significant morbidity and mortality compared with other rheumatic diseases [25]. With regard to the outcome, we found one case with a fatal outcome deriving from the occurrence of a combined manifestation of scleroderma, immune-mediated dermatitis, immune-mediated lung disease and tubulointerstitial nephritis. Fatal outcomes are usually reported in case of scleroderma deriving organ complications, such as lung fibrosis, pulmonary artery hypertension, and scleroderma renal crisis [25]. In the majority of ICSRs retrieved from the EV, the outcome was favorable. In line with this, many of the published case reports and series described favorable outcomes associated with scleroderma cases. For instance, Tjarks et al. reported a case of scleroderma in a 61-year-old man who was treated with nivolumab for oligometastatic renal cell carcinoma. Drug discontinuation and initiation of steroid therapy led to the patient’s symptoms improvement [28]. Another case concerned an 81-year-old patient diagnosed with NSCLC who developed an eosinophilic fasciitis-like disorder after 1 year and half the beginning of pembrolizumab therapy. The patient received methylprednisolone and symptoms improved after a 2.5 months [32]. Similarly, the discontinuation of pembrolizumab and the treatment with phototherapy, corticosteroids, and topical calcineurin inhibitors in an 82-year-old patient diagnosed with NSCLC who experienced generalized morphea led to a good therapeutic response [33]. On the other hand, another case report concerned a 61-year-old female patient who developed skin changes on the abdomen, breasts, and limbs 10 months after the starting of the combined therapy ipilimumab and nivolumab. Staging examinations revealed progressive melanoma brain metastases; the patient died almost 2 years after the development of the scleroderma-like skin changes [34]. Thus, the clinical outcome of scleroderma seems to widely vary from person to person. Although the course of the disease can be unpredictable, when scleroderma is associated with organ involvement, especially the lungs, heart, and kidneys, it is more severe [25].
Lastly, only 8 out of 70 ICSRs reported both the duration of the therapy and the drug withdrawal as action taken after the occurrence of the ADR, and consequently the TTE in days was calculated only for these cases, resulting in 325 days for pembrolizumab and 347 days for nivolumab. The results of a retrospective study reported the occurrence of grade III flares after a median of 10 months of therapy with pembrolizumab [35]. The review carried out by Suarez-Almazor and Abdel-Wahab [36] described the main characteristics of diffuse scleroderma-like syndromes that occurred in four male patients receiving an anti-PD-1 therapy [28, 37]. According to data reported, the median TTE of skin manifestations was 8 (3.8–9.8) months. Two patients with melanoma developed eosinophilic fasciitis, one of them a month after completing 18 months of pembrolizumab [38].
Strengths and Limitations
A descriptive analysis of ICSRs retrieved from the EV database has been carried out. The spontaneous reporting system represents the main tool for the collection and analysis of safety data related to medicines and vaccines used in real life conditions. Indeed, contrary to what happen in the premarketing phase, specific events, including rare and serious ones, can be easily identified through the analysis of spontaneous reports. Furthermore, the spontaneous reporting system involves ICSRs related to a frail population, such as patients with comorbid conditions and those receiving multiple pharmacological treatments, the elderly, pregnant women, and children, which are usually excluded by the premarketing clinical trials [39–42].
On the other hand, the spontaneous reporting system carries some intrinsic limitations, such as the under-reporting and the poor quality of information listed in each ICSR. Indeed, the limited number of ICSRs retrieved from the EV represents the major limitation of this study. In addition, seriousness and outcome degrees were not reported in all ICSRs and we were able to compute the TTE only for a very limited number of ICSRs (8/70 ICSRs) considering that information on ICI withdrawal and therapy duration were missing in the majority of retrieved ICSRs. Thus, this result should be interpreted with caution. Considering these limitations, we are aware that the real rheumatic and cutaneous safety profile of ICIs cannot be fully established, but needs to be confirmed by the results obtained from ad hoc studies. Furthermore, almost 17% of ICSRs reported concomitant medications, whose role in the occurrence of scleroderma cases should be considered together with the role of the neoplastic disease for which ICIs were used. Indeed, the role of the cancer itself in the occurrence of irADRs evaluated in this study cannot be excluded considering that patients with scleroderma have an increased risk of cancer compared with the general population as a consequence of chronic inflammation and tissue damage, malignant transformation provoked by immunosuppressive therapies, or a common inciting factor [43]. Considering all these limitations and all multiple factors that may have contributed to the occurrence of scleroderma, we believe that the evaluation of the causality assessment between the treatment with ICI and the occurrence of scleroderma, whether calculated, would have resulted as mainly doubtful or possible, but not probable neither definite.
Conclusions
Using the EV database, we collected ICSRs describing cases of ICI-induced scleroderma. Based on the data retrieved from the EV showing that all scleroderma cases were classified as serious and considering the recent marketing authorization of ICIs, we believe that further pharmacovigilance studies evaluating the rheumatic and cutaneous safety profile of ICIs are strongly needed. In addition, considering that irADRs represent a new spectrum of events that can involve any tissue and organ and that the clinical manifestations of scleroderma are often serious as a result of organ complications, we believe that further high-quality clinical studies should be conducted on this topic to better estimate the impact of these events in patients with cancer, who already represent a frail population. In the meantime, the regular follow-up with a rheumatologist or specialist experienced in scleroderma is essential to manage the disease and its complications effectively.
Author Contributions
M.M.N. and C.S.: conceptualization and writing original draft. M.M.N., E.C., V.C., C.R., M.R.C., D.R., P.M.B., G.D. and C.S.: writing—review and editing. C.R., D.R. and C.S.: formal analysis and validation. M.M.N., E.C., V.C., C.R., M.R.C., D.R., P.M.B., G.D. and C.S. contributed to the article and approved the submitted version.
Funding
This research received no external funding.
Data Availability
Data analyzed for this article were retrieved from the EV database that is publicly accessible.
Code Availability
Not applicable
Declarations
Conflict of Interest
M.M.N., E.C., V.C., C.R., M.R.C., D.R., P.M.B., G.D. and C.S. declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable considering that study participants could not be identified by the data presented.
Footnotes
Giovanni Docimo and Cristina Scavone are co-authors.
References
- 1.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Institute. Immune Checkpoint Inhibitors. Available at: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/checkpoint-inhibitors#:~:text=Immunotherapy%20drugs%20called%20immune%20checkpoint,checkpoint%20protein%20called%20CTLA%2D4 (Accessed on 04 Nov 2023).
- 4.Jácome AA, Castro ACG, Vasconcelos JPS, Silva MHCR, Lessa MAO, Moraes ED, Andrade AC, Lima FMT, Farias JPF, Gil RA, Prolla G, Garicochea B. Efficacy and safety associated with immune checkpoint inhibitors in unresectable hepatocellular carcinoma: a meta-analysis. JAMA Netw Open. 2021;4(12):e2136128. doi: 10.1001/jamanetworkopen.2021.36128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carretero-González A, Otero I, Lora D, Carril-Ajuria L, Castellano D, de Velasco G. Efficacy and safety of anti-PD-1/PD-L1 combinations versus standard of care in cancer: a systematic review and meta-analysis. Oncoimmunology. 2021;10(1):1878599. doi: 10.1080/2162402X.2021.1878599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Tian P, Zhong J, Fan X. Efficacy and safety of immune checkpoint inhibitors combined with chemotherapy in patients with extensive-stage small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Front Oncol. 2023;19(13):1151769. doi: 10.3389/fonc.2023.1151769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guven DC, Stephen B, Sahin TK, Cakir IY, Erul E, Aksoy S. The efficacy of immune checkpoint inhibitors in rare tumors: a systematic review of published clinical trials. Crit Rev Oncol Hematol. 2022;174:103700. doi: 10.1016/j.critrevonc.2022.103700. [DOI] [PubMed] [Google Scholar]
- 8.Ruggiero R, Fraenza F, Scavone C, di Mauro G, Piscitelli R, Mascolo A, Ferrajolo C, Rafaniello C, Sportiello L, Rossi F, Capuano A. Immune checkpoint inhibitors and immune-related adverse drug reactions: data from Italian pharmacovigilance database. Front Pharmacol. 2020;9(11):830. doi: 10.3389/fphar.2020.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mascolo A, Scavone C, Ferrajolo C, Rafaniello C, Danesi R, Del Re M, Russo A, Coscioni E, Rossi F, Alfano R, Capuano A. Immune checkpoint inhibitors and cardiotoxicity: an analysis of spontaneous reports in eudravigilance. Drug Saf. 2021;44(9):957–971. doi: 10.1007/s40264-021-01086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Quach HT, Johnson DB, LeBoeuf NR, Zwerner JP, Dewan AK. Cutaneous adverse events caused by immune checkpoint inhibitors. J Am Acad Dermatol. 2021;85(4):956–966. doi: 10.1016/j.jaad.2020.09.054. [DOI] [PubMed] [Google Scholar]
- 12.Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postolova A, Chen JK, Chung L. Corticosteroids in myositis and scleroderma. Rheum Dis Clin North Am. 2016;42(1):103–18. doi: 10.1016/j.rdc.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sapadin AN, Fleischmajer R. Treatment of scleroderma. Arch Dermatol. 2002;138(1):99–105. doi: 10.1001/archderm.138.1.99. [DOI] [PubMed] [Google Scholar]
- 15.Volkmann ER, Andréasson K, Smith V. Systemic sclerosis. Lancet. 2023;401(10373):304–318. doi: 10.1016/S0140-6736(22)01692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahan ZH, Hummers LK. Systemic sclerosis—challenges for clinical practice. Nat Rev Rheumatol. 2013;9(2):90–100. doi: 10.1038/nrrheum.2012.191. [DOI] [PubMed] [Google Scholar]
- 17.Macklin M, Yadav S, Jan R, Reid P. Checkpoint inhibitor-associated scleroderma and scleroderma mimics. Pharmaceuticals (Basel) 2023;16(2):259. doi: 10.3390/ph16020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Medicines Agency. 2022 Annual Report on EudraVigilance for the European Parliament, the Council and the Commission. Available at: https://www.ema.europa.eu/en/documents/report/2022-annual-report-eudravigilance-european-parliament-council-commission_en.pdf (Accessed on 04 Nov 2023)
- 19.Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7–18. doi: 10.1016/j.ctrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Cappelli LC, Gutierrez AK, Bingham CO, III, Shah AA. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systemic review of the literature [published online ahead of print December 20, 2016] Arthritis Care Res (Hoboken) 2016 doi: 10.1002/acr.23177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Distler O, Cozzio A. Systemic sclerosis and localized scleroderma—current concepts and novel targets for therapy. Semin Immunopathol. 2016;38(1):87–95. doi: 10.1007/s00281-015-0551-z. [DOI] [PubMed] [Google Scholar]
- 22.Lafyatis R. Transforming growth factor β-- the centre of systemic sclerosis. Nat Rev Rheumatol. 2014;10(12):706–719. doi: 10.1038/nrrheum.2014.137. [DOI] [PubMed] [Google Scholar]
- 23.Hamaguchi Y. Drug-induced scleroderma-like lesion. Allergol Int. 2022;71(2):163–168. doi: 10.1016/j.alit.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Hamaguchi Y. Drug-induced scleroderma-like lesion. Allergol Int. 2022;71(2):163–168. doi: 10.1016/j.alit.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhao M, Wu J, Wu H, Sawalha AH, Lu Q. Clinical treatment options in scleroderma: recommendations and comprehensive review. Clin Rev Allergy Immunol. 2022;62(2):273–291. doi: 10.1007/s12016-020-08831-4. [DOI] [PubMed] [Google Scholar]
- 26.Garon-Czmil J, Petitpain N, Rouby F, Sassier M, Babai S, Yéléhé-Okouma M, et al. Immune check point inhibitors-induced hypophysitis: a retrospective analysis of the French pharmacovigilance database. Sci Rep. 2019;9:1–5. doi: 10.1038/s41598-019-56026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jedlickova H, Durčanská V, Vašků V. Paraneoplastic scleroderma: are there any clues? Acta Dermatovenerol Croat. 2016;24(1):78–80. [PubMed] [Google Scholar]
- 28.Tjarks BJ, Kerkvliet AM, Jassim AD, Bleeker JS. Scleroderma-like skin changes induced by checkpoint inhibitor therapy. J Cutan Pathol. 2018;45(8):615–618. doi: 10.1111/cup.13273. [DOI] [PubMed] [Google Scholar]
- 29.Barbosa NS, Wetter DA, Wieland CN, Shenoy NK, Markovic SN, Thanarajasingam U. Scleroderma induced by pembrolizumab: a case series. Mayo Clin Proc. 2017;92(7):1158–1163. doi: 10.1016/j.mayocp.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 30.DeMaio A, Hashemi KB, Avery A, Metcalf JS, Winterfield LS. A case of nivolumab-induced scleroderma-like syndrome successfully treated with intravenous immunoglobulin. JAAD Case Rep. 2022;22(31):76–79. doi: 10.1016/j.jdcr.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shivaji UN, Jeffery L, Gui X, Smith SCLL, Ahmad OF, Akbar A, et al. Immune checkpoint inhibitor-associated gastrointestinal and hepatic adverse events and their management. Ther Adv Gastroenterol. 2019;12:175628481988419. doi: 10.1177/1756284819884196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salamaliki C, Solomou EE, Liossis SC. Immune checkpoint inhibitor-associated scleroderma-like syndrome: a report of a pembrolizumab-induced “eosinophilic fasciitis-like” case and a review of the literature. Rheumatol Ther. 2020;7(4):1045–1052. doi: 10.1007/s40744-020-00246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fattore D, Battista T, De Lucia M, Annunziata MC, Fabbrocini G. Scleroderma-like syndrome in the setting of pembrolizumab therapy for non-small cell lung cancer: diagnosis and dermatologic management. Case Rep Dermatol. 2022;14(2):225–229. doi: 10.1159/000525887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langan EA, Budner K, Zillikens D, Terheyden P. Generalized morphoea in the setting of combined immune checkpoint inhibitor therapy for metastatic melanoma: a case report. Medicine (Baltimore) 2021;100(16):e25513. doi: 10.1097/MD.0000000000025513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panhaleux M, Espitia O, Terrier B, Manson G, Maria A, Humbert S, Godbert B, Perrin J, Achille A, Arrondeau J, et al. Anti–programmed death ligand 1 immunotherapies in cancer patients with pre-existing systemic sclerosis: a postmarketed phase IV safety assessment study. Eur J Cancer. 2022;160:134–139. doi: 10.1016/j.ejca.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Wahab N, Suarez-Almazor ME. Frequency and distribution of various rheumatic disorders associated with checkpoint inhibitor therapy. Rheumatology. 2019 doi: 10.1093/rheumatology/kez297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richter MD, Crowson C, Kottschade LA, et al. Rheumatic syndromes associated with immune checkpoint inhibitors: a single-center cohort of sixty-one patients. Arthritis Rheumatol. 2019;71:468–475. doi: 10.1002/art.40745. [DOI] [PubMed] [Google Scholar]
- 38.Lidar M, Giat E, Garelick D, et al. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17:284–9. doi: 10.1016/j.autrev.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Scavone C, Di Mauro C, Ruggiero R, Bernardi FF, Trama U, Aiezza ML, Rafaniello C, Capuano A. Severe cutaneous adverse drug reactions associated with allopurinol: an analysis of spontaneous reporting system in southern Italy. Drugs Real World Outcomes. 2020;7(1):41–51. doi: 10.1007/s40801-019-00174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sessa M, Rafaniello C, Sportiello L, Mascolo A, Scavone C, Maccariello A, Iannaccone T, Fabrazzo M, Berrino L, Rossi F, Capuano A. Campania Region (Italy) spontaneous reporting system and preventability assessment through a case-by-case approach: a pilot study on psychotropic drugs. Expert Opin Drug Saf. 2016;15(sup2):9–15. doi: 10.1080/14740338.2016.1221397. [DOI] [PubMed] [Google Scholar]
- 41.Lombardi N, Crescioli G, Bettiol A, Tuccori M, Capuano A, Bonaiuti R, Mugelli A, Venegoni M, Vighi GD, Vannacci A, MEREAFaPS Study Group Italian emergency department visits and hospitalizations for outpatients’ adverse drug events: 12-year active pharmacovigilance surveillance (the MEREAFaPS study) Front Pharmacol. 2020;11:412. doi: 10.3389/fphar.2020.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crescioli G, Bonaiuti R, Corradetti R, Mannaioni G, Vannacci A, Lombardi N. Pharmacovigilance and pharmacoepidemiology as a guarantee of patient safety: the role of the clinical pharmacologist. J Clin Med. 2022;11(12):3552. doi: 10.3390/jcm11123552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weeding E, Casciola-Rosen L, Shah AA. Cancer and scleroderma. Rheum Dis Clin North Am. 2020;46(3):551–564. doi: 10.1016/j.rdc.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed for this article were retrieved from the EV database that is publicly accessible.
Not applicable