Abstract
Stereodefined vinylboron compounds are important organic synthons. The synthesis of E−1-vinylboron compounds typically involves the addition of a B-H bond to terminal alkynes. The selective generation of the thermodynamically unfavorable Z-isomers remains challenging, necessitating improved methods. Here, such a proficient and cost-effective catalytic system is introduced, comprising a cobalt salt and a readily accessible air-stable CNC pincer ligand. This system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with excellent Z-selectivity. High turnover numbers (>1,600) and turnover frequencies (>132,000 h−1) are achieved at room temperature, and the reaction can be scaled up to 30 mmol smoothly. Kinetic studies reveal a formal second-order dependence on cobalt concentration. Mechanistic investigations indicate that the alkynes exhibit a higher affinity for the catalyst than the alkene products, resulting in exceptional Z-selective performance. Furthermore, a rare time-dependent stereoselectivity is observed, allowing for quantitative conversion of Z-vinylboronate esters to the E-isomers.
Subject terms: Homogeneous catalysis, Synthetic chemistry methodology, Catalytic mechanisms
Stereodefined vinylboron compounds are important organic synthons, but the selective generation of the thermodynamically unfavorable Z-isomers remains challenging. Here, the authors develop a cobalt catalytic system, enabling the transformation of terminal alkynes with excellent Z-selectivity, even in the presence of bulky substituents.
Introduction
Organoboron compounds are remarkable versatile building blocks for organic synthesis due to their availability and exceptional tolerance toward various functional groups1–6. Among these compounds, alkenylboron compounds with a defined stereochemistry play a crucial role in a wide range of cross-coupling reactions. These reactions lead to the generation of functionalized alkenes possessing specific configurations, which are important for the synthesis of bioactive compounds and natural products7–20. The hydroboration of terminal alkynes represents a straightforward and economically efficient method to generate vinylboron compounds. The anti-Markovnikov addition of the B-H group to the C≡C bond of terminal alkynes primarily affords E-1-alkenylboron compounds as the major products8–32. Conversely, achieving the production of Z-alkenylborones from terminal alkynes via hydroboration presents a more challenging task, primarily due to the fact that the E-isomers are thermodynamically more stable. Consequently, only a dozen of catalytic reactions leading selectively to the Z-isomers through hydroboration of terminal alkynes have been reported31–44. Notably, these studies predominantly employ noble metal catalysts, including rhodium34,35,44, iridium34, ruthenium36,42, and palladium37,40. Among these catalytic systems, the ruthenium pincer catalyst reported by the Leitner group stands out as the most versatile and active one, displaying moderate turnover numbers (TON) of up to 920 and turnover frequencies (TOF) of up to 38 h−1 at −15 °C36. To date, the highest TON of 9800 was achieved by Saito et al. using a ruthenium complex with an N-heterocyclic carbene and PCy3 ligand but this reaction necessitated a long reaction time of 6 days and heating42.
In the pursuit of developing more sustainable catalysts, the exploration of complexes with earth-abundant metals has been in the focus of recent research45–54, which includes the investigation of Z-selective hydroboration of terminal alkynes38,39,41,43. To date, three pincer Co and Fe complexes have been developed to facilitate this transformation with favorable Z-selectivity (Fig. 1a). Pioneering work by Chirik et al. demonstrated the possibility to perform the Z-selective hydroboration of alkyl and aryl alkynes at room temperature using a bis(imino)pyridine cobalt catalyst38. Kirchner et al. developed an iron polyhydride catalyst [Fe(PNP)(H)2(η2-H2)] with a non-classical hydrogen ligand for the hydroboration reaction in C6D6, demonstrating moderate TONs of up to 245 and TOFs of up to 82 h−141. Furthermore, de Ruiter et al. recently reported the use of a related iron complex [Fe(PCNHCP)(H)2(N2)] with a labile dinitrogen ligand as catalyst precursor for performing this type of reaction43.
Fig. 1. Selective hydroboration of terminal alkynes catalyzed by earth-abundant metals.
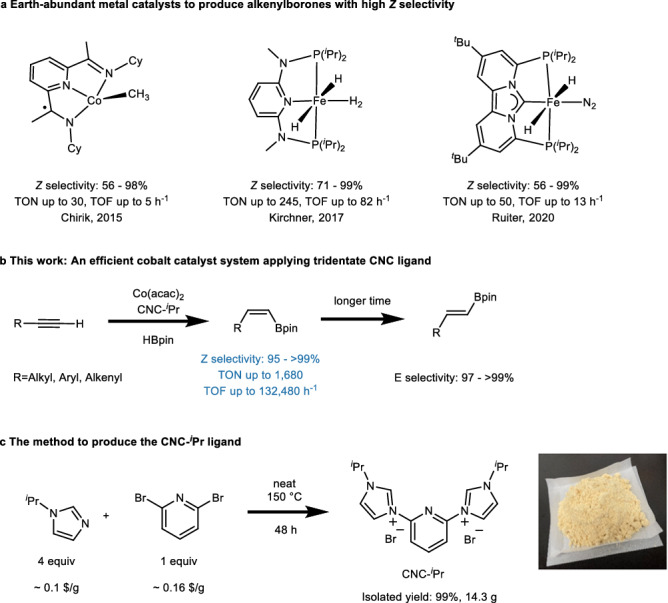
a Comparison of three reported earth-abundant metal catalysts for achieving highly Z-selective hydroboration of terminal alkynes. The Z selectivity, highest turnover numbers (TONs), and turnover frequencies (TOFs) of each system are shown. b This work: an efficient and general cobalt catalyst system for hydroboration of terminal alkynes with time-dependent stereoselectivity. c Easy large-scale production of CNC-iPr from inexpensive commercially available compounds with high isolated yields. Prices listed are sourced from bidepharm.com.
Despite the existence of successful catalytic examples demonstrating highly Z-selective hydroboration of terminal alkynes, the catalytic systems reported so far often suffer from limitations in terms of substrate scope, stereoselectivity, and reactivity toward sterically hindered alkyl and/or aryl alkyne substrates. For instance, arylacetylenes with substituents at ortho or even meta positions on aryls proved to be challenging substrates. Achieving good Z-selectivity typically requires the use of ligands and complexes synthesized in multiple-steps, which hinders their practical application in syntheses31–44. Typically, bulky pincer ligands with flexible arms are employed to stabilize the complexes and improve catalytic performance. However, the use of such bulky complexes inevitably leads to low efficiencies in catalytic reactions with sterically encumbered substrates. Given the limitations of the current methods on one side but the broad use of vinylboronate esters on the other, there is a high need for the development of a general, cost-effective, highly-efficient, and practical synthetic route for the stereo-selective hydroboration of terminal alkynes.
Results
In this work, we present a catalytic system utilizing a cobalt salt and a CNC-iPr ligand as precursors to generate stereoselective hydroboration catalysts (Fig. 1b). The CNC-iPr ligand was synthesized following a procedure previously reported by Herrmann with modifications55. By employing commercially inexpensive compounds, we successfully synthesized the air-stable salt CNC-iPr in one-step with an isolated yield of 99% (Fig. 1c). The flat structure of the CNC-pincer ligand—obtained from the salt in situ by double deprotonation—with two isopropyl groups in the periphery pointing away from the metal center should allow to coordinate a wide range of terminal alkynes, including those with sterically demanding substituents that were previously challenging to functionalize selectively. Indeed, this catalytic system exhibits an exceptional TON of up to 1680, surpassing the performance of noble metal catalysts at room temperature. Additionally, TOFs of up to 132,480 h−1 were reached. To the best of our knowledge, this represents the fastest transition metal-catalyzed hydroboration reaction reported to date. Also, a rare time-dependent stereoselectivity was observed which allows to prepare either the Z-isomer or E-isomer only by variation of the reaction time.
The initial investigation focused on utilizing different NHC-pincer ligands in conjunction with CoCl2 to achieve Z-selective hydroboration of phenylacetylene (1a) in DMF at room temperature (Table 1). A significant influence of the structure of the ligands on the reaction outcome was observed (entries 1–6). Notably, the use of the CNC-iPr ligand resulted in the formation of styrylboronate ester (1b) with a remarkable 99% yield and high E/Z selectivity of 98:2 (entry 4). However, when ligands with smaller (entries 1–3) or bulkier N-substituents (entries 5, 6) were employed, the reactions gave lower or no yields and also lower E/Z selectivities. To our surprise, we discovered that reducing the reaction time to just 10 min led to the formation of Z-styrylboronate ester in 99% yield with an inverse Z/E selectivity of 96:4 (entry 7). This result suggests the possibility of a Z/E isomerization process during the reaction and indicates that the stereoselectivity of hydroboration may be kinetically controlled (entry 7 vs. 4). Interestingly, reducing the amount of pinacol borane, HBpin, to 1.5 equivalents significantly suppressed the isomerization process (entry 8 vs. 4). Furthermore, substituting CoCl2 (entry 9) with Co(acac)2 proved to give a more efficient catalyst in combination with CNC-iPr as ligand (entry 10). After further optimization (Supplementary Tables 9–12), the reaction could be successfully carried out with excellent Z-selectivity, achieving a 90% isolated yield at a low catalyst loading of 0.1 mol% (entry 11). Notably, reducing the amount of HBpin did not affect the performance of the reaction (entry 12).
Table 1.
Optimization study
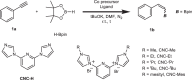 | ||||
|---|---|---|---|---|
| Entry | Precursor | Ligand | Yield (%)a | Z/E ratiob |
| 1 | CoCl2 | CNC-H | 0 | – |
| 2 | CoCl2 | CNC-Me | 71% | 3:97 |
| 3 | CoCl2 | CNC-Et | 78% | 36:64 |
| 4 | CoCl2 | CNC-iPr | 99% | 2:98 |
| 5 | CoCl2 | CNC-tBu | 7% | <1:99 |
| 6 | CoCl2 | CNC-Mes | 0 | – |
| 7c | CoCl2 | CNC-iPr | 99% | 96:4 |
| 8d | CoCl2 | CNC-iPr | 70% | >99:1 |
| 9e | CoCl2 | CNC-iPr | 45% | 97:3 |
| 10e | Co(acac)2 | CNC-iPr | 98% | 51:49 |
| 11f | Co(acac)2 | CNC-iPr | 99% (90%)g | 98:2 |
| 12f,h | Co(acac)2 | CNC-iPr | 98% (90%)g | >99:1 |
Reaction conditions: CoCl2 (5.0 mol%), 1a (0.4 mmol, 1.0 equiv), ligand (5.0 mol%),
tBuOK (20.0 mol%), HBpin (3.0 equiv), DMF (2 ml), room temperature (r.t.), N2 atmosphere, 12 h.
aNMR yield determined using methylene bromide as the internal standard.
bZ/E ratio based on 1H NMR analysis.
cReacted for 10 min.
dHBpin used in 1.5 equiv., 24 h.
eCo precursor (0.2 mol%), ligand (0.22 mol%),
tBuOK(0.8 mol%), DMF (1 ml).
fCo(acac)2 (0.1 mol%), ligand (0.14 mol%),
tBuOK(0.56 mol%), DMF (0.5 ml).
gIsolated yield.
hHBpin (1.3 equiv.).
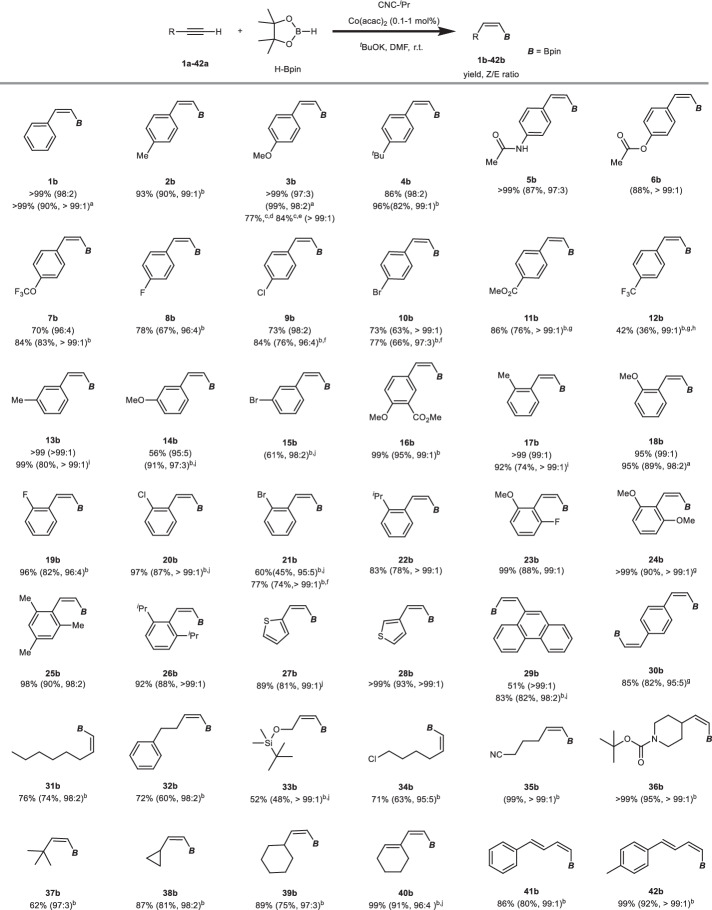
Subsequently, we investigated the applicability of our catalytic system to various terminal alkynes for Z-selective hydroboration (Fig. 2). Delightfully, very high selectivity and moderate to excellent yields for all the tested substrates were observed (1a–42a). Arylacetylenes bearing electron-donating groups on the para-position of the benzene ring (1a–6a vs. 8a–12a) generally exhibited higher yields of Z-styrylboronate esters (1b–6b) compared to those with electron-withdrawing groups (8b–12b). Aryl alkynes (13a–16a) with meta-substituted phenyl groups displayed no diminished reactivity or selectivity. Ortho-substituted Z-styrylboronate esters (17b–22b) could be obtained in high yields with excellent Z-selectivity from the corresponding arylacetylenes, which are typically challenging substrates due to steric hindrance. Surprisingly, even sterically highly demanding phenylacetylenes with two ortho-substituents on the phenyl ring, such as 2,6-diisopropylphenylacetylene (26a), exhibit excellent Z-selectivity and yields of Z-styrylboronate esters (23b–26b) are high (>80%). These results highlight the excellent compatibility of the Co(acac)2/CNC-iPr catalytic system with sterically hindered substrates. Further exploration of arylacetylene substrates revealed good tolerance toward thienyl (27b, 28b) and polycyclic aryl (29b) groups. Interestingly, even a substrate bearing two ethynyl groups smoothly underwent the reaction, leading to the target product with an impressive 82% isolated yield and very high ZZ selectivity (30b). Importantly, we also examined the possibility of conducting reactions with a low catalyst loading of 0.05 mol% and with p-methoxystyrene 3a we achieved yields of 77% (TON = 1540) and 84% (TON = 1680) in 24 and 48 h, respectively (3b).
Fig. 2. Scope of Z-selective hydroboration of terminal alkynes.
Reaction conditions: terminal alkyne (1.0 equiv, 0.4 mmol), HBpin (1.3 equiv), Co(acac)2 ([Co], 0.5 mol%), CNC-iPr (1.4 equiv to [Co]), tBuOK (5.6 equiv to [Co]) in DMF (0.5 ml) at room temperature (r.t.). See Supplementary Information for experimental details. 1H NMR yields are shown with methylene bromide or mesitylene as the internal standard. Isolated yields and Z/E ratios are provided in parenthesis. a0.1 mmol% Co(acac)2, 0.8 mmol scale. b1 mmol% Co(acac)2. c0.05 mmol% Co(acac)2, yield determined by GC-MS. d24 h. e48 h. f2.5 equiv. HBpin. g3 equiv. HBpin. hWith 10 mol% diphenylacetylene, i0.2 mmol% Co(acac)2. j2 equiv. HBpin.
The compatibility of the Co(acac)2/CNC-iPr catalytic system with alkyl (31a–39a) and alkenyl alkynes (40a–42a) was investigated. Similar to the sterically hindered arylacetylene substrates, tert-butyl and cyclic alkyl/alkenyl functionalized terminal alkynes were also efficiently converted into the desired vinylboronate esters (36b–40b) with excellent Z-selectivity. This unique capability of the catalytic system to hydroborate both aryl and alkyl substituted terminal alkynes, particularly those containing bulky substituents with overall very high Z-selectivity (Z:E > 95:5), is not found in the literature. Importantly, the reaction exhibits a broad substrate scope, accommodating various important functional groups, thereby providing flexibility for subsequent transformations.
To assess the practicality of our procedure, we conducted large-scale reactions under an inert atmosphere (nitrogen). Initially, an ice bath was employed to regulate the temperature due to the exothermic nature of the reaction. Figure 3 illustrates the results obtained from reactions on the 30 mmol scale, which proceeded smoothly and rapidly, yielding various Z-alkenylboronate esters 1b, 3b, 13b, 22b, 28b, 31b, and 38b with good to excellent isolated yields and selectivity. Most reactions at the 30 mmol scale gave even better results when compared to the small-scale reactions. Significantly, the utilization of compound 3b has been demonstrated in the synthesis of the natural product Nyasol19. This methodology holds promise for the efficient large-scale production of valuable Z-alkenylboronate esters8–19,31,32.
Fig. 3. Large scale reaction.
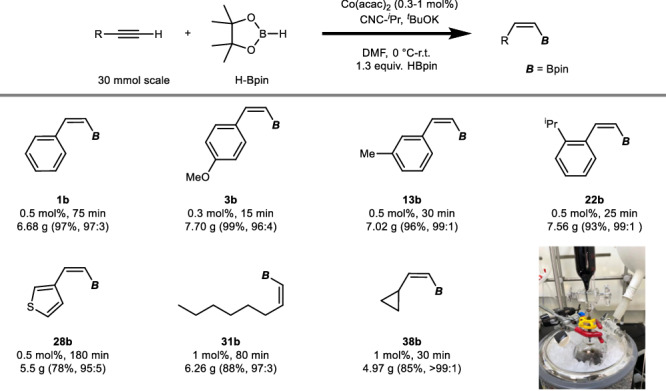
Reaction conditions: terminal alkyne (1.0 equiv, 30 mmol), HBpin (1.3 equiv), Co(acac)2 ([Co], as presented), CNC-iPr (1.4 equiv to [Co]), tBuOK (5.6 equiv to [Co]) in DMF (35 ml) at 0 °C–room temperature (r.t.).
Discussion
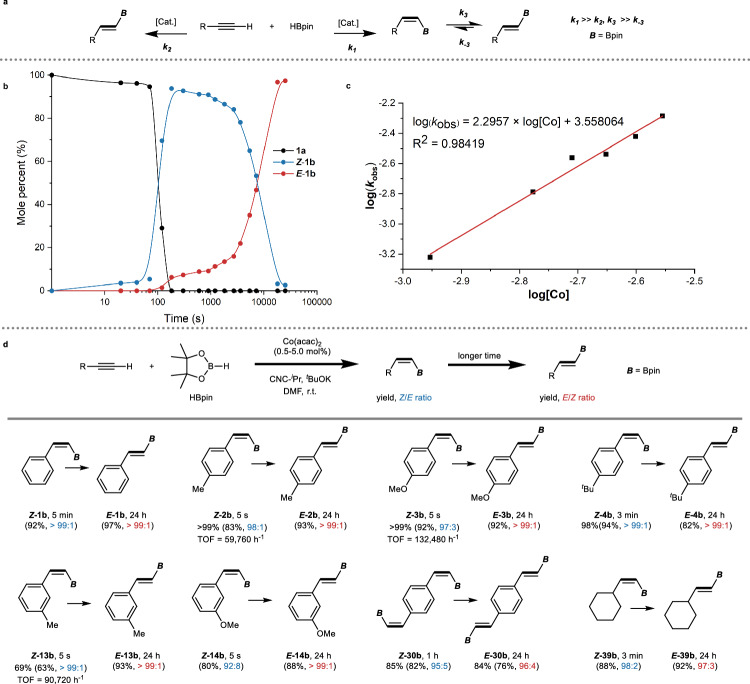
The performance of the Co(acac)2/CNC-iPr catalytic system prompted us to revisit the optimization study (vide supra) with the aim to better understand under which conditions Z or E selectivity can be achieved (Table 1, entries 4, 7). Typically, obtaining good Z/E stereoselectivity in the synthesis of alkenylboron compounds requires the use of two different catalyst systems, each favoring either a kinetic or thermodynamically controlled process. However, with the Co(acac)2/CNC-iPr catalyzed reactions it is possible to produce both isomers selectively using the same catalyst system at room temperature but at different reaction times. This finding indicates a significantly faster rate for the formation of the Z isomer compared to the E isomer (Fig. 4a, k1 » k2, k3). Additionally, we observed a relatively slow but nearly irreversible Z/E isomerization process (k3 » k−3), leading predominantly to the E-configured product after a longer reaction time. To gain further insights into the dynamic progress of the reaction, we monitored the amounts of 1a, Z-1b, and E-1b over time (Fig. 4b). Using 0.5 mol% catalyst and 2 equivalents of HBpin, the reaction exhibited an induction period of ~70 s, indicating the formation of the catalytically active species. Subsequently, there was a rapid consumption of 1a within 90 s, leading to an almost quantitative formation of Z-1b and only negligible amounts of E-1b were observed. Over the next 5 h, Z-1b gradually transformed into E-1b at a nearly constant rate (see Supplementary Fig. 1). This phenomenon suggests that the increasing concentration of E-1b does not affect the transformation rate, which differs from traditional reversible isomerization processes. Importantly, it is this feature of the Co(acac)2/CNC-iPr catalytic system that explains the formation of E-1b with an excellent E-stereoselectivity (large E/Z ratio).
Fig. 4. Kinetic investigation.
a Proposed equilibrium equation of the hydroboration of terminal alkynes. b Kinetic profile of the hydroboration of phenylacetylene (1a). Reaction conditions: 1a (1.0 equiv, 0.4 mmol), HBpin (2.0 equiv), Co(acac)2 ([Co], 0.5 mol%), CNC-iPr (1.4 equiv to [Co]), tBuOK (5.6 equiv to [Co]) in DMF (0.5 ml) at room temperature (r.t.). c Kinetic analysis of the formal reaction order based on the concentration of catalyst ([Co]). d Time-dependent stereoselective hydroboration of terminal alkynes. Reaction conditions: terminal alkyne (1.0 equiv, 0.4 mmol), HBpin (3.0 equiv), Co(acac)2 ([Co], 0.5–5 mol%), CNC-iPr (1.4 equiv to [Co]), tBuOK (5.6 equiv to [Co]) in DMF (0.5 ml) at room temperature (r.t.). See Supplementary Information for experimental details. 1H NMR yields are shown with methylene bromide as the internal standard. Isolated yields and Z/E ratios in parenthesis. The reaction time for the transformation of the E isomers was not optimized.
Further analysis revealed that the reaction exhibited a pronounced kinetic response to both the catalyst concentration and the amount of HBpin. Therefore, the increased presence of HBpin can significantly promote the reaction process due to kinetic effects (Supplementary Table 4, see also Fig. 2). Variation of the catalyst concentration has a more significant influence. To investigate the kinetics of the reaction, we examined the apparent reaction rate constants (kobs) at different catalyst concentrations ([Co]) (Fig. 4c and Supplementary Fig. 2). Remarkably, plotting log(kobs) against log[Co] yielded a straight line with a slope of 2.30, indicating an approximately second-order dependence of the reaction on the catalyst concentration ([Co]). This result is in agreement with the experiments that showed larger reaction rates at higher catalyst concentrations.
Based on the kinetic studies, a time-dependent method for the stereoselective hydroboration of terminal alkynes was successfully developed (Fig. 4d). Apart from substrate 1a, numerous alkyne substrates were effectively converted into their corresponding Z- and E-alkenylboronate esters selectively within varying reaction times, utilizing the same reaction setup. Notably, both isomers exhibited very high stereoselectivity. Note that the catalyst derived from Co(acac)2/CNC-iPr shows the fastest rates ever reported for Z-selective hydroboration reactions, with certain reactions completing instantaneously upon mixing the reactants and catalyst, leading to impressive TOFs for the production of some Z-alkenylboronate esters (see Z-2b, Z-3b, Z-13b).
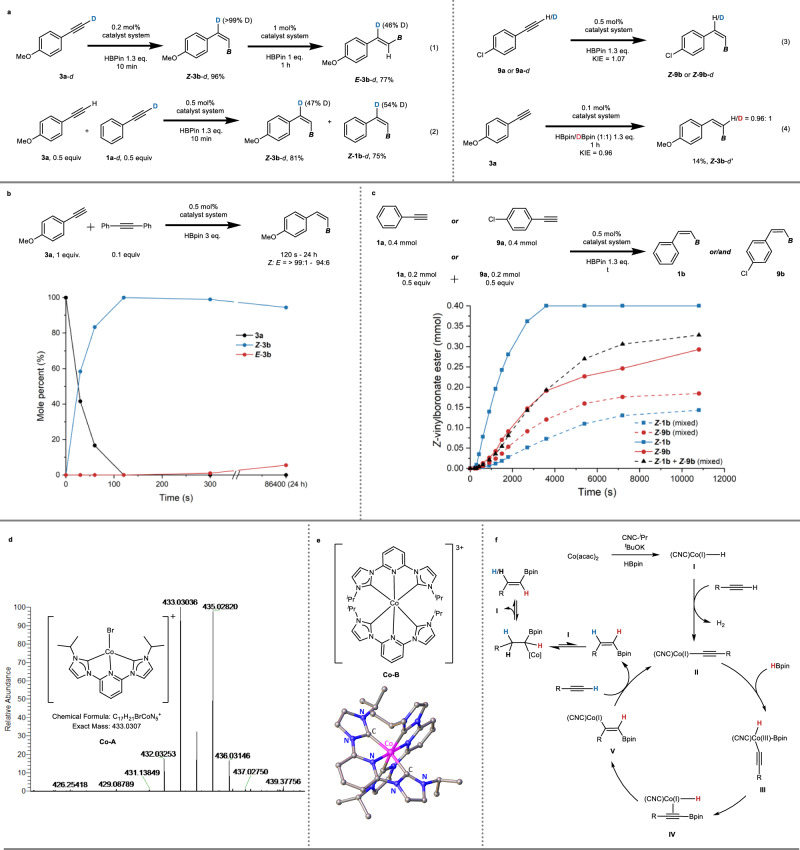
Deuterium labeling experiments were performed in order to gain a deeper understanding of a possible reaction mechanism. The hydroboration of 1-(4-methoxyphenyl) acetylene-2-d (3a-d) produced Z-3b-d with 99% deuterium retention at the beta position of the Bpin group (Fig. 5a, eq 1 left and Supplementary Fig. 3). This result is consistent with the 1,1-hydroboration process observed in the catalytic hydroboration of alkynes by noble metals (Rh, Ir, Ru)34–36 and earth-abundant metals (Co, Fe)38,41 as previously reported. With 1 mol% Co(acac)2/CNC-iPr and 1 equiv of HBpin, isomerization of Z-3b-d to E-3b-d occurred, resulting in a reduced deuteration percentage (from >99% to 46%) at the beta-position of the Bpin group (Fig. 5a, eq 1 right and Supplementary Fig. 4). Prolonging the reaction time did not alter this deuteration ratio, and no isotopic labeling was observed geminal to the BPin group. These findings suggest that a Co-H insertion followed by a β-H elimination occurs during the isomerization process56. An appropriate excess of HBpin can ensure the generation of a sufficient concentration of the Co-H complex and provide a reductive environment to stabilize it. Similar reactions applying 3a and DBpin were also performed, leading to the same conclusion as Fig. 5a, eq 1 (Supplementary Figs. 5–7). This observation is distinct from the reactions performed by Chirik et al.38. When a 1:1 mixture of 1-phenylacetylene-2-d (1a-d) and 4-methoxy-phenylacetylene (3a) was employed, both corresponding hydroboration products exhibited ~50% deuterium exchange, indicating intermolecular hydrogen transfer of the C(sp)-H bonds (Fig. 5a, eq 2 and see Supplementary Figs. 8 and 9). We also conducted kinetic isotope effect experiments on the C(sp)-H bond and B-H bond, and observed similar KIE values (1.07 and 0.96, respectively, see Supplementary Figs. 10 and 11), suggesting that activation of either bond is not the rate-determining step (Fig. 5a, eq 3 and 4). Furthermore, the different deuterium-labeled Z-alkenylboronate esters clearly demonstrate that the hydrogen atom adjacent to the Bpin group originates from HBpin, while the hydrogen at the beta position is transferred from the alkynyl hydrogen, in accordance with eq 1 (Fig. 5a, eq 3 and 4).
Fig. 5. Mechanistic study.
a Deuterium labeling experiments. b Inhibition of Z/E isomerization with an inert alkyne. c Kinetic trace of Z-selective products for reactions employing 1a, 9a, and mixture of 1a and 9a, respectively. Products of the mixed system have been marked in the profile. Reaction conditions: terminal alkyne (1.0 equiv, 0.4 mmol), HBpin (1.3 equiv), Co(acac)2 ([Co], 0.5 mol%), CNC-iPr (1.4 equiv to [Co]), tBuOK (5.6 equiv to [Co]) in DMF (0.5 ml) at room temperature (r.t.). d High Resolution Mass Spectrometry (HRMS) of [Co(II)(CNC-iPr)Br]+. e X-ray structure of [Co(III) (CNC-iPr)2]Br3. Hydrogen, bromine, and solvent atoms are omitted for clarity. See Supplementary Fig. 14 and Supplementary Tables 13–15 for details. f Proposed mechanism. The positions of the colored hydrogen atoms have been traced by the deuterium labeling experiments (a).
Subsequently, a mixture of 3a and 0.1 equiv of 1,2-diphenylethyne, which remained inert at room temperature, was prepared using a 0.5 mol% catalyst system with 3 equiv of HBpin to investigate the influence of an alkyne on the Z/E isomerization process (Fig. 5b). In the absence of 1,2-diphenylethyne, complete conversion of 3a to Z-3b was achieved within 5 s, followed by subsequent Z/E isomerization (Fig. 4d). But the presence of 1,2-diphenylethyne slowed down the hydroboration procedure, leading to a significantly longer reaction time of 120 s for the full conversion of 3a to Z-3b. Notably, already a small amount of 1,2-diphenylethyne strongly inhibited the isomerization process. Even after 24 h, Z-3b was still observed in high yield and high Z/E ratio. This inhibition of the Z/E isomerization process can be attributed to the higher affinity of the alkyne (in this case 1,2-diphenylethyne) to the cobalt catalyst compared to Z-3b56. This phenomenon partially explains the unique kinetic behavior of the reaction and provides a rationale for the excellent Z selectivity, indicating that the E isomer can only form after nearly complete consumption of the alkyne substrate (Fig. 4b, d). The influence of the alkyne was further investigated using a combination of the terminal alkynes phenylacetylene (1a) and 4-chlorophenylacetylene (9a). Separately, 9a exhibited lower reactivity compared to 1a. However, when they were mixed together, a reversal in the transformation rates of the two alkynes was observed, with 9a undergoing a more rapid transformation (Fig. 5c and Supplementary Fig. 12). This observation suggests that the reaction may be influenced by the acidity of the alkynyl hydrogen in the alkyne substrate (although the KIE experiment indicated that activation of the sp C-H bond is not the rate-determining step; Fig. 5a, eq 3). In comparison to 9a, phenylacetylene possesses a lower C(sp)-H acidity, resulting in inferior reactivity in the mixed system.
Based on the aforementioned experiments and relevant studies in the literature, a mechanistic pathway consistent with the observed results can be proposed. It is postulated that an in situ formed [Co(II)(CNC-iPr)] (CNC-iPr = 2,6-bis(3-isopropylimidazol-2-ylidene)pyridine) complex serves as the catalyst precursor. Supporting evidence for the formation of this complex (Co-A, see Fig. 5d and Supplementary Fig. 13) was obtained by analyzing the high-resolution mass spectrometry (HRMS) data from a solution containing CoBr2, CNC-iPr, and KOtBu. Unfortunately, we were not able to isolate any stable [Co(II)(CNC-iPr)] complex because these undergo rather rapidly a disproportionation reaction. As a result, a single crystal of a [Co(III)(CNC-iPr)2]3+ complex (Co-B) was obtained (see Fig. 5e, Supplementary Figs. 14 and 15 and Supplementary Tables 13–15). An X-ray diffraction (XRD) study using a single crystal of Co-B confirms, however, the suggested coordination mode of the ligand and double deprotonation of the bis(imidazolium salt) CNC-iPr. The [Co(III)(CNC-iPr)2]3+ complex is presumed to represent the resting state of the active catalyst and using Co-B as catalyst precursor likewise results in significant catalytic activity (see Supplementary Fig. 16).
Upon reduction of the Co(II) precursor (Fig. 5f), an active Co-H species (I) was formed, which was observed by HRMS (Supplementary Fig. 17). Similar Co(I) species have been proposed in numerous hydrogenation56–58 and hydroboration38,59–62 reactions, and can be readily generated from Co(II)38,56–61,63 or Co(III)64 pincer complexes with suitable reductants. Subsequently, a reaction between I and a terminal alkyne leads to the deliberation of dihydrogen (H2 was detected in course of the reaction, Supplementary Fig. 18) resulting in the formation of an alkynylcobalt complex (II). This complex then reacts with pinacolborane under oxidative addition of the B-H bond to yield an intermediate Co(III) species (III) which rearranges under reductive elimination to a Co(I) alkynylboronate complex (IV). Sequentially, a stereoselective syn-hydrocobaltation takes place, generating a vinylcobalt intermediate (V). After protonation and ligand exchange with a terminal alkyne, V ultimately leads to the formation of the Z-vinylboronate ester product and the regeneration of the alkynylcobalt complex II. When the alkyne substrate is (nearly) fully consumed, an insertion of the Z-vinylboronate ester into the Co-H bond may occur resulting in the generation of the corresponding E-vinylboronate ester through a β-H elimination step56,60,61,65,66. The mechanism is in accord with the labeling experiments and the different hydrogen atoms transferred during the reaction are visualized in Fig. 5f in blue, red, and black.
In summary, we have developed a highly efficient method for the stereoselective hydroboration of terminal alkynes, providing access to a wide range of vinylboronate ester compounds with excellent stereoselectivity. This transformation is achieved using a simple and cost-effective cobalt catalyst system generated in situ from a readily available CNC pincer ligand and Co(acac)2. The catalyst system can be applied to a wide range of substrates and is especially suited for the conversion of terminal alkynes with sterically demanding substituents using commercial pinacol borane. This synthetic protocol exhibits excellent scalability, making it suitable for large-scale reactions without compromising neither the efficiency nor the selectivity. Impressive TONs and TOFs were achieved, with the highest hydroboration rate reported so far for the production of Z-vinylboronate esters. Kinetic investigations allow to propose a mechanism in which alkynes exhibit a higher affinity toward the cobalt catalyst compared to the Z-vinylboronate ester products. This preferential binding inhibits a fast Z/E isomerization process, which can only occur after almost complete consumption of the alkyne substrate to Z-vinylboronate ester. It is this special feature of the catalytic system that leads to a time-dependent stereoselectivity.
Methods
General procedure for catalytic Z-selective hydroboration
In a nitrogen atmosphere, a vail was charged with Co(acac)2 (4.1 mg, 0.016 mmol), CNC-iPr (10.2 mg, 0.022 mmol), tBuOK (10.1 mg, 0.09 mmol) in dry DMF (2 ml) and was stirred for 5 min. The freshly prepared stock solution of the in situ prepared active catalyst ([Co] = 8.0 mM in DMF) was added via a micro-syringe (50–500 µl, 0.0004–0.004 mmol, 0.1–1 mol%, as noted) to a vial charged with HBpin (75 µl, 0.52 mmol, 1.3 equiv., unless otherwise noted) and DMF([alkyne] = 0.8 M). The mixture was stirred for 5 min. Alkyne (0.4 mmol, unless otherwise noted) was added rapidly and the resulting mixture was stirred for 12 h. The reaction was then quenched by adding water. Twenty ml EtOAc was added and the organic phase was washed with 10 ml brine twice to remove most of DMF. Pure product was isolated by column chromatography over silica gel deactivated with 2% NEt3 in petroleum ether using petroleum/EtOAc as the eluent. For some specific substrates, the reaction conditions were slightly changed, the detailed information of which can be found in the SI.
General procedure for large scale catalytic Z-selective hydroboration of terminal alkynes
In nitrogen atmosphere, a 100 ml Schlenk vial was charged with alkyne (30 mmol) and dry DMF (20 ml), the alkyne solution was placed in an ice path. In another vial charged with Co(acac)2 (38.6 mg, 0.15 mmol), CNC-iPr (96.0 mg, 0.21 mmol), tBuOK (94.3 mg, 0.84 mmol), dry DMF (15 ml) was stirred for 5 min and HBpin (5.6 ml, 39 mmol) was added and stirred for a further 5 min. The catalyst solution was added dropwise into the alkyne solution under ice bath during 10 min. After that, ice bath was removed and the mixture was stirred at room temperature for a certain time. The reaction was then quenched by adding water. Three hundred ml EtOAc was added and the organic phase was washed with 3*100 ml water and 2*100 ml brine. the organic phase was passed through a short pad of silica gel. Pure product was obtained after removing volatiles under reduced pressure.
Supplementary information
Acknowledgements
P.H. thank Guangdong Provincial Department of Science and Technology (nos. 2019QN01L151, 2023A0505050137, 2022A1515011215), Guangzhou Municipal Science and Technology Bureau (no. 202201011797), GBRCE for Functional Molecular Engineering, Sun Yat-sen University, and State Key Laboratory of Structural Chemistry (FJIRSM, CAS) for support. We would like to express our gratitude to Prof. David Milstein (Weizmann Institute of Science) for reviewing the article and providing valuable suggestions.
Author contributions
P.H. designed and directed the project. J.W. carried out catalytic experiments. Y.H. and Y.Z. discussed and shared ideas regarding the design of the experiments. J.W., H.G., and P.H. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks Tarun Kanti Panda and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. The X-ray crystallographic coordinate for the structure reported (Co-B) has been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 2288260. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk. All data are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-46550-y.
References
- 1.Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. [DOI] [Google Scholar]
- 2.Fu GC. The development of versatile methods for palladium-catalyzed coupling reactions of aryl electrophiles through the use of P(t-Bu)3 and PCy3 as ligands. Acc. Chem. Res. 2008;41:1555–1564. doi: 10.1021/ar800148f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jana R, Pathak TP, Sigman MS. Advances in transition metal (Pd, Ni, Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 2011;111:1417–1492. doi: 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennox AJJ, Lloyd-Jones GC. Selection of boron reagents for Suzuki–Miyaura coupling. Chem. Soc. Rev. 2014;43:412–443. doi: 10.1039/C3CS60197H. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida H. Borylation of alkynes under base/coinage metal catalysis: some recent developments. ACS Catal. 2016;6:1799–1811. doi: 10.1021/acscatal.5b02973. [DOI] [Google Scholar]
- 6.Mlynarski SN, Schuster CH, Morken JP. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature. 2014;505:386–390. doi: 10.1038/nature12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundararaju B, Fürstner A. A trans-selective hydroboration of internal alkynes. Angew. Chem. Int. Ed. 2013;52:14050–14054. doi: 10.1002/anie.201307584. [DOI] [PubMed] [Google Scholar]
- 8.Reddy YK, Falck JR. Asymmetric total synthesis of (+)-Fostriecin. Org. Lett. 2002;6:969–971. doi: 10.1021/ol025537r. [DOI] [PubMed] [Google Scholar]
- 9.López S, Montenegro J, Saá C. Highly convergent, stereospecific synthesis of 11-cis-retinoids by metal-catalyzed cross-coupling reactions of (Z)-1-alkenylmetals. J. Org. Chem. 2007;72:9572–9581. doi: 10.1021/jo701664r. [DOI] [PubMed] [Google Scholar]
- 10.Robles O, McDonald FE. Convergent synthesis of fostriecin via selective alkene couplings and regioselective asymmetric dihydroxylation. Org. Lett. 2009;11:5498–5501. doi: 10.1021/ol902365n. [DOI] [PubMed] [Google Scholar]
- 11.Gao D, O’Doherty GA. Total synthesis of fostriecin: via a regioand stereoselective polyene hydration, oxidation, and hydroboration sequence. Org. Lett. 2010;12:3752–3755. doi: 10.1021/ol101340n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim HN, Parker KA. Total synthesis of kingianin A. Org. Lett. 2013;15:398–401. doi: 10.1021/ol303388k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuwa H, Mizunuma K, Sasaki M, Suzukib T, Kubo H. Total synthesis and biological evaluation of (−)-exiguolide analogues: importance of the macrocyclic backbone. Org. Biomol. Chem. 2013;11:3442–3450. doi: 10.1039/c3ob40131f. [DOI] [PubMed] [Google Scholar]
- 14.Moore JC, Davies ES, Walsh DA, Sharma P, Moses JE. Formal synthesis of kingianin A based upon a novel electrochemically-induced radical cation Diels–Alder reaction. Chem. Commun. 2014;50:12523–12525. doi: 10.1039/C4CC05906A. [DOI] [PubMed] [Google Scholar]
- 15.Kulesza U, Plum LA, DeLuca HF, Mouriño A, Sicinski RR. Novel 9‑alkyl- and 9‑alkylidene-substituted 1α,25-dihydroxyvitamin D3 analogues: synthesis and biological examinations. J. Med. Chem. 2015;58:6237–6247. doi: 10.1021/acs.jmedchem.5b00795. [DOI] [PubMed] [Google Scholar]
- 16.Dong G, Li B, O’Doherty GA. Total and formal syntheses of fostriecin. Org. Chem. Front. 2020;7:3608–3615. doi: 10.1039/D0QO01121E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng T, Liu B, Wu R, Zhu S. Cu-catalyzed carboboration of acetylene with Michael acceptors. Chem. Sci. 2022;13:7604–7609. doi: 10.1039/D2SC02306G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Speed AWH, Mann TJ, O’Brien RV, Schrock RR, Hoveyda AH. Catalytic Z‑selective cross-metathesis in complex molecule synthesis: a convergent stereoselective route to Disorazole C1. J. Am. Chem. Soc. 2014;136:16136–16139. doi: 10.1021/ja509973r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao F, Carr JL, Hoveyda AH. A broadly applicable NHC−Cu-catalyzed approach for efficient, site‑, and enantioselective coupling of readily accessible (pinacolato)alkenylboron compounds to allylic phosphates and applications to natural product synthesis. J. Am. Chem. Soc. 2014;136:2149–2161. doi: 10.1021/ja4126565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Chen Y, Tian Q, Wang B, Cheng G. Palladium-catalyzed multicomponent assembly of (Z)-alkenylborons via carbopalladation/boronation/retro-diels–alder cascade reaction. J. Org. Chem. 2023;88:11793–11800. doi: 10.1021/acs.joc.3c01084. [DOI] [PubMed] [Google Scholar]
- 21.Brown HC, Gupta SK. Catecholborane (1,3,2-benzodioxaorole) as a new, general monohydroboration reagent for alkynes. Convenient synthesis of alkeneboronic esters and acids from alkynes via hydroboration. J. Am. Chem. Soc. 1972;94:4370–4371. doi: 10.1021/ja00767a072. [DOI] [Google Scholar]
- 22.Tucker CE, Davidson J, Knochel P. Mild and stereoselective hydroborations of functionalized alkynes and alkenes using pinacolborane. J. Org. Chem. 1992;57:3482–3485. doi: 10.1021/jo00038a044. [DOI] [Google Scholar]
- 23.He X, Hartwig JF. True metal-catalyzed hydroboration with titanium. J. Am. Chem. Soc. 1996;118:1696–1702. doi: 10.1021/ja9516773. [DOI] [Google Scholar]
- 24.Pereira S, Srebnik M. A study of hydroboration of alkenes and alkynes with pinacolborane catalyzed by transition metals. Tetrahedron Lett. 1996;37:3283–3286. doi: 10.1016/0040-4039(96)00576-X. [DOI] [Google Scholar]
- 25.Greenhalgh MD, Thomas SP. Chemo-, regio-, and stereoselective iron-catalysed hydroboration of alkenes and alkynes. Chem. Commun. 2013;49:11230–11232. doi: 10.1039/c3cc46727a. [DOI] [PubMed] [Google Scholar]
- 26.Bismuto A, Thomas SP, Cowley MJ. Aluminum hydride catalyzed hydroboration of alkynes. Angew. Chem. Int. Ed. 2016;55:15356–15359. doi: 10.1002/anie.201609690. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z, et al. An aluminum dihydride working as a catalyst in hydroboration and dehydrocoupling. J. Am. Chem. Soc. 2016;138:2548–2551. doi: 10.1021/jacs.6b00032. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima K, Kato T, Nishibayashi Y. Hydroboration of alkynes catalyzed by pyrrolide-based PNP pincer-iron complexes. Org. Lett. 2017;19:4323–4326. doi: 10.1021/acs.orglett.7b01995. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Daat H, et al. Hydroboration of alkynes and nitriles using an α-diimine cobalt hydride catalyst. Chem. Commun. 2017;53:7333–7336. doi: 10.1039/C7CC02281F. [DOI] [PubMed] [Google Scholar]
- 30.Magre M, Maity B, Falconnet A, Cavallo L, Rueping M. Magnesium-catalyzed hydroboration of terminal and internal alkynes. Angew. Chem. Int. Ed. 2019;58:7025–7029. doi: 10.1002/anie.201902188. [DOI] [PubMed] [Google Scholar]
- 31.Rej S, Das A, Panda TK. Overview of regioselective and stereoselective catalytic hydroboration of alkynes. Adv. Synth. Catal. 2021;363:4818–4840. doi: 10.1002/adsc.202100950. [DOI] [Google Scholar]
- 32.Carreras J, Caballero A, Pérez PJ. Alkenyl boronates: synthesis and applications. Chem. Asian J. 2019;14:329–343. doi: 10.1002/asia.201801559. [DOI] [PubMed] [Google Scholar]
- 33.McGough JS, Butler SM, Cade IA, Ingleson MJ. Highly selective catalytic transhydroboration of alkynes mediated by borenium cations and B(C6F5)3. Chem. Sci. 2016;7:3384–3389. doi: 10.1039/C5SC04798F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohmura T, Yamamoto Y, Miyaura N. Rhodium- or iridium-catalyzed trans-hydroboration of terminal alkynes, giving (Z)-1-alkenylboron compounds. J. Am. Chem. Soc. 2000;122:4990–4991. doi: 10.1021/ja0002823. [DOI] [Google Scholar]
- 35.Cid J, Carbo JJ, Fernandez E. Catalytic non-conventional trans-hydroboration: a theoretical and experimental perspective. Chem. Eur. J. 2012;18:1512–1521. doi: 10.1002/chem.201102729. [DOI] [PubMed] [Google Scholar]
- 36.Gunanathan C, Holscher M, Pan F, Leitner W. Ruthenium catalyzed hydroboration of terminal alkynes to Z vinylboronates. J. Am. Chem. Soc. 2012;134:14349–14352. doi: 10.1021/ja307233p. [DOI] [PubMed] [Google Scholar]
- 37.Xu S, Haeffner F, Li B, Zakharov LN, Liu S. Monobenzofused 1,4-azaborines: synthesis, characterization, and discovery of a unique coordination mode. Angew. Chem. Int. Ed. 2014;53:6795–6799. doi: 10.1002/anie.201403903. [DOI] [PubMed] [Google Scholar]
- 38.Obligacion JV, Neely JM, Yazdani AN, Pappas I, Chirik PJ. Cobalt catalyzed Z selective hydroboration of terminal alkynes and elucidation of the origin of selectivity. J. Am. Chem. Soc. 2015;137:5855–5858. doi: 10.1021/jacs.5b00936. [DOI] [PubMed] [Google Scholar]
- 39.Jang WJ, Lee WL, Moon JH, Lee JY, Yun J. Copper-catalyzed trans-hydroboration of terminal aryl alkynes: stereodivergent synthesis of alkenylboron compounds. Org. Lett. 2016;18:1390–1393. doi: 10.1021/acs.orglett.6b00325. [DOI] [PubMed] [Google Scholar]
- 40.Xu S, Zhang Y, Li B, Liu S. Site-selective and stereoselective trans-hydroboration of 1,3-enynes catalyzed by 1,4-azaborine-based phosphine–Pd complex. J. Am. Chem. Soc. 2016;138:14566–14569. doi: 10.1021/jacs.6b09759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorgas N, et al. Stable, yet highly reactive nonclassical iron(II) polyhydride pincer complexes: Z‑selective dimerization and hydroboration of terminal alkynes. J. Am. Chem. Soc. 2017;139:8130–8133. doi: 10.1021/jacs.7b05051. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto K, Mohara Y, Mutoh Y, Saito S. Ruthenium-catalyzed (Z)‑selective hydroboration of terminal alkynes with naphthalene-1,8-diaminatoborane. J. Am. Chem. Soc. 2019;141:17042–17047. doi: 10.1021/jacs.9b06910. [DOI] [PubMed] [Google Scholar]
- 43.Garhwal S, Fridman N, de Ruiter G. Z‑selective alkyne functionalization catalyzed by a trans-dihydride N‑heterocyclic carbene (NHC) iron complex. Inorg. Chem. 2020;59:13817–13821. doi: 10.1021/acs.inorgchem.0c02057. [DOI] [PubMed] [Google Scholar]
- 44.Lyu Y, Toriumi N, Iwasawa N. (Z)‑Selective hydroboration of terminal alkynes catalyzed by a PSP–pincer rhodium complex. Org. Lett. 2021;23:9262–9266. doi: 10.1021/acs.orglett.1c03606. [DOI] [PubMed] [Google Scholar]
- 45.Obligacion JV, Chirik PJ. Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration. Nat. Rev. Chem. 2018;2:15–34. doi: 10.1038/s41570-018-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris RH. Exploiting metal–ligand bifunctional reactions in the design of iron asymmetric hydrogenation catalysts. Acc. Chem. Res. 2015;48:1494–1502. doi: 10.1021/acs.accounts.5b00045. [DOI] [PubMed] [Google Scholar]
- 47.Gorgas N, Kirchner K. Isoelectronic manganese and iron hydrogenation/dehydrogenation catalysts: similarities and divergences. Acc. Chem. Res. 2018;51:1558–1569. doi: 10.1021/acs.accounts.8b00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alig L, Fritz M, Schneider S. First-row transition metal (de)hydrogenation catalysis based on functional pincer ligands. Chem. Rev. 2019;119:2681–2751. doi: 10.1021/acs.chemrev.8b00555. [DOI] [PubMed] [Google Scholar]
- 49.Junge K, Papa V, Beller M. Cobalt–pincer complexes in catalysis. Chem. Eur. J. 2019;25:122–143. doi: 10.1002/chem.201803016. [DOI] [PubMed] [Google Scholar]
- 50.Ai W, Zhong R, Liu X, Liu Q. Hydride transfer reactions catalyzed by cobalt complexes. Chem. Rev. 2019;119:2876–2953. doi: 10.1021/acs.chemrev.8b00404. [DOI] [PubMed] [Google Scholar]
- 51.Mukherjee A, Milstein D. Homogeneous catalysis by cobalt and manganese pincer complexes. ACS Catalysis. 2018;8:11435–11469. doi: 10.1021/acscatal.8b02869. [DOI] [Google Scholar]
- 52.Irrgang T, Kempe R. 3d-metal catalyzed N- and C-alkylation reactions via borrowing hydrogen or hydrogen autotransfer. Chem. Rev. 2019;119:2524–2549. doi: 10.1021/acs.chemrev.8b00306. [DOI] [PubMed] [Google Scholar]
- 53.Wen H, Liu G, Huang Z. Recent advances in tridentate iron and cobalt complexes for alkene and alkyne hydrofunctionalizations. Coord. Chem. Rev. 2019;386:138–153. doi: 10.1016/j.ccr.2019.01.024. [DOI] [Google Scholar]
- 54.Chen J, Guo J, Lu Z. Recent advances in hydrometallation of alkenes and alkynes via the first row transition metal catalysis. Chin. J. Chem. 2018;36:1075–1109. doi: 10.1002/cjoc.201800314. [DOI] [Google Scholar]
- 55.Scherg T, et al. Bridged imidazolium salts used as precursors for chelating carbene complexes of palladium in the Mizoroki-Heck reaction. Synlett. 2006;18:2894–2907. [Google Scholar]
- 56.Fu S, et al. Ligand-controlled cobalt-catalyzed transfer hydrogenation of alkynes: stereodivergent synthesis of Z- and E-alkenes. J. Am. Chem. Soc. 2016;138:8588–8594. doi: 10.1021/jacs.6b04271. [DOI] [PubMed] [Google Scholar]
- 57.Srimani D, et al. Cobalt-catalyzed hydrogenation of esters to alcohols: unexpected reactivity trend indicates ester enolate intermediacy. Angew. Chem. Int. Ed. 2015;54:12357–12360. doi: 10.1002/anie.201502418. [DOI] [PubMed] [Google Scholar]
- 58.Lu P, Wang H, Mao Y, Hong X, Lu Z. Cobalt-catalyzed enantioconvergent hydrogenation of minimally functionalized isomeric olefins. J. Am. Chem. Soc. 2022;144:17359–17364. doi: 10.1021/jacs.2c08525. [DOI] [PubMed] [Google Scholar]
- 59.Li C, et al. Cobalt-catalyzed regio- and stereoselective hydroboration of allenes. Angew. Chem. Int. Ed. 2020;59:6278–6283. doi: 10.1002/anie.201915716. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, Huang Z. Synthesis of 1,1,1-tris(boronates) from vinylarenes by Co-catalyzed dehydrogenative borylations−hydroboration. J. Am. Chem. Soc. 2015;137:15600–15603. doi: 10.1021/jacs.5b11366. [DOI] [PubMed] [Google Scholar]
- 61.Guo J, Cheng B, Shen X, Lu Z. Cobalt-catalyzed asymmetric sequential hydroboration/hydrogenation of internal alkynes. J. Am. Chem. Soc. 2017;139:15316–15319. doi: 10.1021/jacs.7b09832. [DOI] [PubMed] [Google Scholar]
- 62.Ghosh P, Schoch R, Bauer M, von Wangelin AJ. Selective benzylic CH-borylations by tandem cobalt catalysis. Angew. Chem. Int. Ed. 2022;61:e202110821. doi: 10.1002/anie.202110821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Semproni SP, Milsmann C, Chirik PJ. Four-coordinate cobalt pincer complexes: electronic structure studies and ligand modification by homolytic and heterolytic pathways. J. Am. Chem. Soc. 2014;136:9211–9224. doi: 10.1021/ja504334a. [DOI] [PubMed] [Google Scholar]
- 64.Tokmic K, Jackson BJ, Salazar A, Woods TJ, Fout AR. Cobalt-catalyzed and Lewis acid-assisted nitrile hydrogenation to primary amines: a combined effort. J. Am. Chem. Soc. 2017;139:13554–13561. doi: 10.1021/jacs.7b07368. [DOI] [PubMed] [Google Scholar]
- 65.Chen C, Dugan TR, Brennessel WW, Weix DJ, Holland PL. Z‑selective alkene isomerization by high-spin cobalt(II) complexes. J. Am. Chem. Soc. 2014;136:945–955. doi: 10.1021/ja408238n. [DOI] [PubMed] [Google Scholar]
- 66.Tokmic K, Fout AR. Alkyne semihydrogenation with a well-defined nonclassical Co–H2 catalyst: a H2 spin on isomerization and E-selectivity. J. Am. Chem. Soc. 2016;138:13700–13705. doi: 10.1021/jacs.6b08128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the paper and its Supplementary Information. The X-ray crystallographic coordinate for the structure reported (Co-B) has been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 2288260. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk. All data are available from the corresponding author upon request.