Abstract
Effects of plant diversity on grassland productivity, or overyielding, are found to be robust to nutrient enrichment. However, the impact of cumulative nitrogen (N) addition (total N added over time) on overyielding and its drivers are underexplored. Synthesizing data from 15 multi-year grassland biodiversity experiments with N addition, we found that N addition decreases complementarity effects and increases selection effects proportionately, resulting in no overall change in overyielding regardless of N addition rate. However, we observed a convex relationship between overyielding and cumulative N addition, driven by a shift from complementarity to selection effects. This shift suggests diminishing positive interactions and an increasing contribution of a few dominant species with increasing N accumulation. Recognizing the importance of cumulative N addition is vital for understanding its impacts on grassland overyielding, contributing essential insights for biodiversity conservation and ecosystem resilience in the face of increasing N deposition.
Subject terms: Biodiversity, Community ecology, Ecosystem services
15 multi-year grassland biodiversity experiments suggest that with increasing cumulative nitrogen addition, the effect of diversity on productivity becomes increasingly reliant on a small number of dominant species rather than on overall species richness.
Introduction
Humans are enriching the environment with nitrogen (N) at an unprecedented rate1, and profoundly altering Earth’s ecosystems2–4. In grasslands, plant diversity5,6 and productivity7,8 change, as N accumulates over time9,10. Nitrogen enrichment, whether from experimental addition or atmospheric deposition, usually increases primary productivity by alleviating N limitation8. However, it reduces plant diversity by increasing competition for light11–13, acidifying soil14–16, reducing belowground niche dimensionality17, as well as accelerating the loss of rare species5,18,19 or even of common species20. Nitrogen addition may also alter the relationship between diversity and productivity20–22. If N addition weakens the positive effects of diversity on productivity23,24, this would have profound consequences for ecosystem management and our understanding of biodiversity-ecosystem functioning relationships. However, there is no consensus on how N affects biodiversity-ecosystem functioning relationships because the underlying mechanisms remain largely unexplored.
Nitrogen addition could alter how biodiversity affects productivity20,25,26. The effects of biodiversity on productivity can be quantified through net diversity effects, that is the extent to which species mixtures differ from the productivity expected from their constituent monocultures. Net diversity effects can be partitioned into two components: complementarity and selection effects27. Complementarity effects occur when species perform better in mixtures than expected from monocultures28,29. This can occur via several underlying mechanisms: 1) resource partitioning, where species exploit resources more completely in mixtures30–32; 2) greater facilitation in diverse mixtures33–37; or 3) reduced impacts of consumers, pathogens, or other natural enemies in mixtures38,39. Such mechanisms often operate more effectively in more diverse communities, leading to an increase in complementarity effects with species richness21. Nitrogen addition can decrease complementarity effects by decreasing positive interactions between legumes and other plants32,35, or by decreasing resource partitioning through a reduction in niche dimensionality and belowground nutrient trade-offs31,40,41. Alternatively, positive selection effects occur when species with a high productivity in monoculture increase their productivity in mixtures, while negative selection effects occur if the opposite happens. Nitrogen enrichment may enhance selection effects by increasing the dominance of some species and decreasing evenness, because alleviating N limitation may result in stronger competition for other resources, such as light or water11,42,43. Thus, N enrichment may either weaken or strengthen the effects of biodiversity on productivity, depending on whether it primarily affects complementarity or selection effects. Some empirical evidence suggests that N addition leads to a decrease in complementarity effects and an increase in the selection effects26,32,44.
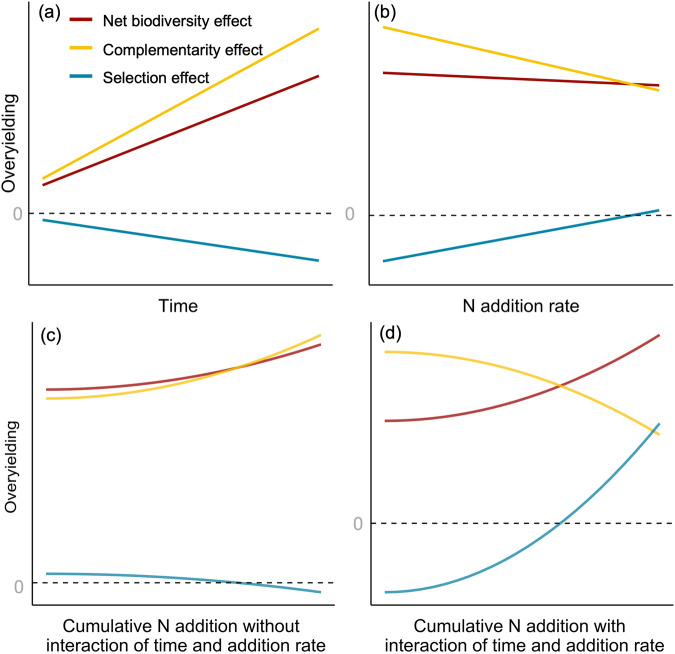
The effects of N addition on complementarity and selection effects may also change over time. Complementarity effects typically increase over time, leading to increased overyielding as plant communities mature, while selection effects decrease45–47 (Fig. 1a). Under N addition, complementarity effects may decline linearly, while selection effects may increase linearly with N addition (Fig. 1b). If these two effects (Fig. 1a, b) are additive, then the combination of an increase with time and a decrease with N addition rate will result in a convex relationship between cumulative N addition and net biodiversity effects (Fig. 1c). This pattern may be driven by a convex relationship between cumulative N addition and complementarity effects, which is partly counteracted by a concave relationship between cumulative N addition and selection effects. In this case, nutrient enrichment would gradually erode the positive effects of biodiversity on ecosystem functioning. This erosion would likely occur even when nutrient enrichment increases the strength of selection effects, if selection effects remain a small fraction of the total effects of biodiversity on productivity. However, it is also possible that N addition interacts with time, leading to multiplicative effects of cumulative N addition. An interaction would occur if effects of N addition on complementarity and selection effects strengthen over time. For example, increasing N enrichment could cause a decrease in species richness due to the recycling of N through litter48 and soil acidification6, thereby preventing the increase in complementarity effects over time49. Alternatively, selection effects could increase more over time44 due to gradual changes in the soil microbial community and abiotic environment50. Selection effects may therefore contribute a larger fraction of biodiversity effects than would be the case without long term N addition (Fig. 1d). Overall, the impacts of nitrogen enrichment on the relationship between plant diversity and productivity can be complex, and its effects may vary depending on the N addition rate and the duration of nitrogen addition. To our knowledge, no previous study has quantified the impacts of cumulative nitrogen addition on overyielding and its underlying processes, nor has any experiment explored the interaction of N addition rate and duration in a full factorial design. This research gap results in an incomplete understanding of the effects of N addition on net biodiversity effects over time. Understanding these relationships is crucial for predicting how long-term eutrophication may alter the effects of biodiversity on ecosystem functioning in the future.
Fig. 1. Conceptual figure about potential changes of overyielding and its components with cumulative nitrogen addition.
Potential changes in net biodiversity effect, complementarity effect and selection effect with (a) time (without N addition; unit-year); (b) N addition rate (unit-kg/ha/year); (c) cumulative N addition (the total amount of N added across years; unit-kg/ha), when the effects of time and N addition rates are additive, these patterns without an interaction between the two effects were derived by multiplying the fitted trends in Fig. 1a, b; and (d) cumulative N addition, when the effects of time and N addition rates are multiplicative, these patterns with interaction between the two effects were derived by the fitted trend of multiplying data in Fig. 1a, b. Note that the x-axes are on the log scale for comparison with the results presented in this study.
Here, we use a meta-level synthesis to determine the main effects of plant species richness and N addition on productivity, using multi-year experiments that manipulated both factors (Supplementary Table S1). We evaluate the impacts of N addition rate, time and cumulative N addition (over time), on net biodiversity, complementarity and selection effects. Our hypotheses are (see Supplementary Table S2 for fully detailed hypotheses and mechanisms):
H1 Nitrogen addition:
H1a: Nitrogen addition treatment (binary): complementarity effects decrease, and selection effects increase with N addition, resulting in no overall change in net biodiversity effects. Effects of N addition on complementarity and selection effects are more pronounced at higher species richness.
H1b: Nitrogen addition rates: Effects of N addition on complementarity and selection effects are more pronounced at higher rates of N addition (Fig. 1b).
H2 Nitrogen addition treatment * Time: under ambient conditions, complementarity effects increase, and selection effects decrease with time (year). We expect larger increases in complementarity effects than decreases in selection effects, leading to increase in net biodiversity effects over time (Fig. 1a). The effects of N addition counteract the effects of time on complementarity effects and selection effects.
H3 Cumulative N addition: Additive effects between N addition rate and time (year) will lead to a convex relationship of complementarity effects and a concave relationship of selection effects with increasing cumulative N addition (Fig. 1c). If there is an interaction between N addition rate and time (year), complementarity effects may decrease and selection effects increase more rapidly at higher levels of cumulative N addition. The shift from complementarity to selection effects will lead to a convex relationship between net biodiversity effects and cumulative N addition (Fig. 1d).
Results
Impacts of the nitrogen addition treatment
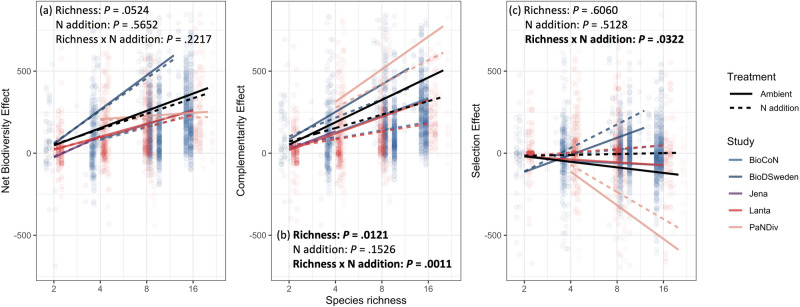
We found a marginally significant increase in net biodiversity effects with species richness (Fig. 2a; Supplementary Table S3). This increase was largely due to an increase in complementarity effects with species richness, as there was no significant change in selection effects. Nitrogen addition interacted with species richness and strongly reduced the complementarity effects (Fig. 2b) and reduced the negative selection effects (Fig. 2c) at high species richness. Despite these changes in complementarity and selection effects, the relationship between species richness and net biodiversity effects remained unchanged by N addition, because the opposing effects of nitrogen addition on complementarity and selection effects canceled each other out.
Fig. 2. The impacts of nitrogen addition treatment on the relationships between species richness and overyielding.
The impacts of N addition on the relationships of species richness with net biodiversity effects (a), complementarity effects (b) and selection effects (c). Black lines indicate fixed effects, and colored lines indicate random effects. Note that the x-axes are on the log-2 scale, the y-axes are on the original scale with unit g/m2/year.
Impacts of nitrogen addition rate
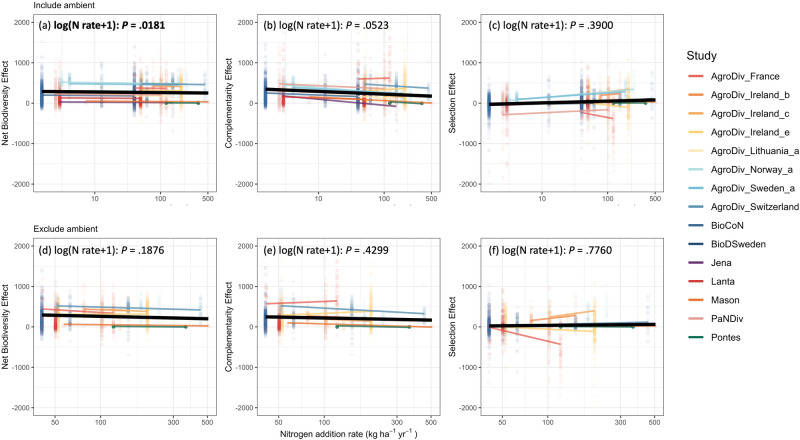
We found that higher N addition rates marginally reduced complementarity effects but did not affect selection effects (Fig. 3b, c; Supplementary Table S3), leading to reduced net biodiversity effects with increasing N addition rate (Fig. 3a). However, these relationships were mainly driven by the difference between ambient and fertilized plots (with experimental N addition), i.e., the significant and marginally significant relationships with ambient and fertilized plots became non-significant when ambient plots were removed (Fig. 3d, e; Supplementary Table S3). As a result, experimental N addition decreased complementarity effects (Supplementary Fig. S1b) but increased selection effects (Supplementary Fig. S1c), regardless of the annual rate of nutrient enrichment. These opposite but proportional responses led to no effect of experimental N addition on the net biodiversity effects (Supplementary Fig. S1a).
Fig. 3. The impacts of nitrogen addition rate on overyielding.
The impacts of N addition rate (from both experimental addition and atmospheric deposition) on net biodiversity effects (a, d), complementarity effects (b, e) and selection effects (c, f) including (a–c) or excluding (d–f) the ambient plots. Black lines indicate fixed effect, colored lines indicate random effect based on individual study. The X-axes are on the log scale with unit kg/ha/year, the y-axes are on the original scale with unit g/m2/year.
Impacts of nitrogen addition over time
By analyzing the long-term BioCoN experiment we found that N addition treatment interacted with time, influencing both complementarity effects and selection effects (Supplementary Fig. S2). Specifically, N addition reduced the increase of complementarity effects with time (Supplementary Fig. S2b) and offset the decrease in selection effects with time (Supplementary Fig. S2c). These opposing effects of time and nitrogen addition led to decreasing net biodiversity effects over time (Supplementary Fig. S2a).
Impacts of cumulative nitrogen addition
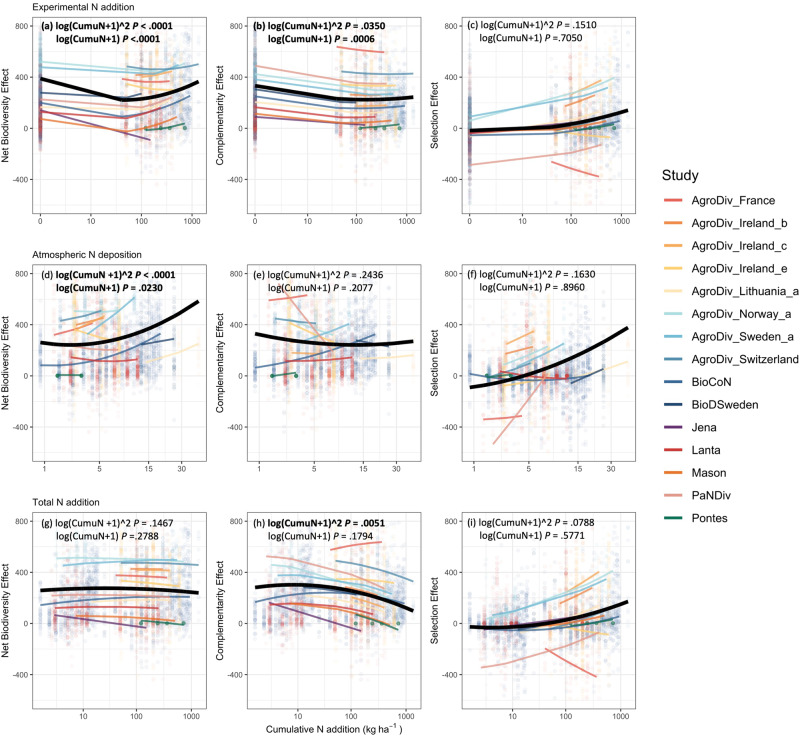
Across all experiments, we found a convex relationship between cumulative experimental N addition and net biodiversity effects (Fig. 4a; Supplementary Table S3). Complementarity effects decreased first and then levelled off with increasing cumulative experimental N addition (Fig. 4b), while selection effects increased continuously (Fig. 4c). As expected, similar trends were found with atmospheric N deposition (Fig. 4d–f). However, when combining inputs from both experimental N addition and atmospheric N deposition, complementarity effects decreased more rapidly (Fig. 4h), while selection effects increased more rapidly (Fig. 4i) at higher levels of cumulative N addition. These counteracting effects were proportional, leading to a non-significant relationship between net biodiversity effects and total amount of cumulative N addition from both experimental addition and atmospheric deposition (Fig. 4g).
Fig. 4. The impacts of cumulative nitrogen addition on overyielding.
The impacts of cumulative N addition on net biodiversity effects (a, d, g), complementarity effects (b, e, h) and selection effects (c, f, i). Cumulative N addition includes inputs from experimental addition (a–c), atmospheric deposition (d–f) and both (g–i). Black lines indicate fixed effect, colored lines indicate random effect. The x-axes are on the log scale with unit kg/ha, the y-axes are on the original scale with unit g/m2/year.
Discussion
Our study reveals that time and N addition rate interactively affect overyielding and its drivers. Low levels of cumulative experimental N addition decrease net biodiversity effects and complementarity effects, while high levels of cumulative N addition increase net biodiversity effects and selection effects. This finding highlights that cumulative N addition alters overyielding by modulating the relative contributions of complementary and selection effects.
Our synthesis of 15 grassland experiments is consistent with previous findings that overyielding is robust to nutrient enrichment21. However, our results indicate that this lack of effect occurs due to contrasting effects on the different components of net biodiversity effects, with a decrease of complementarity effects and a proportional increase in selection effects with nutrient addition32 (Fig. 2). Nitrogen addition reduces complementarity effects more strongly at higher levels of species richness, potentially due to changes in underlying ecological mechanisms. One potential explanation is that with increasing N, plant species may facilitate each other less29. Weaker facilitation may be partially attributes to a lower abundance or lower N2 fixation rate of legumes and, therefore, reducing N fixation36,51–53. However, this is likely not the only explanation for the observed decrease in complementarity effects, as our dataset includes experiments without legumes54 (PaNDiv experiment). Another potential explanation is that N addition may modify the community of beneficial belowground mycorrhizal fungi or rhizobacteria, thereby reducing positive interactions mediated by microbes55–57. Moreover, N enrichment may cause the loss of plant species by alleviating N limitation and promoting interspecific competition11,13,58,59. These effects are especially pronounced in species-rich communities16, as the increased resources reduce the opportunity for different species to partition resource utilization in space, time, or form, leading to larger decreases in plant species richness, complementarity effects and thereby productivity in diverse communities20.
We also find that N addition decreases the negative effect of species richness on selection effects. This finding is in contrast with previous studies reporting non-significant interactions between N and richness21,60. This difference may be explained by the fact that our analysis includes studies that added relatively high amounts of N (e.g., 500 kg/ha/year32 or 450 kg/ha/year61), cover larger species richness gradients (e.g., for 1–20 species in PaNDiv experiment54), and use an agricultural-based species pool instead of a broader species pool61. The larger increase in selection effects with N addition at higher diversity (Fig. 2c) may occur because species mixtures with higher diversity have a greater chance of including species that are more sensitive to the change in N availability. By growing faster and taller, these species are able to capture more light and shade the other species, leading to an increased competitive ability and, therefore, increased selection effects with N addition62–65.
Our study reveals that the responses of net biodiversity, complementarity and selection effects to N enrichment are independent of the annual rate of experimental N addition (Fig. 3d–f). The lack of effect of N addition rate on overyielding could be due to the complementary utilization of N by plants with different functional traits, which maintains ecosystem productivity along a N addition rate gradient66. However, it is important to note that any threshold of annual N addition rate may be too low to be detected with the addition rates used in current experiments (Fig. 3; Supplementary Table S3). In addition, a large proportion of the impact of different N addition rates depends on variation of annual N addition rates among experiments; other factors that vary among the studies could also obscure the effects of N addition rate, including the form of N added, soil type, and how much N was initially available at the site67 (Supplementary Table S1). More experiments fully crossing diversity with multiple levels of N addition would be needed to fully test this idea.
Overyielding may be regulated more by cumulative N addition over time than by the annual rate of N added. In our data, some studies apply a relatively low amount of N annually for a relatively long period of time (e.g., 40 kg/ha/year for 23 years)20,68,69, while others apply a higher amount of N annually for a shorter period of time (e.g., 360 kg/ha/year for 3 years)70. Confirming previous results46,47, we find that complementarity and selection effects changed over 23 years in BioCoN (Supplementary Fig. S2). Nitrogen addition also interacts with time to affect overyielding and reduce the increase of complementarity effects with time, while shifting selection effects from negative to positive over time (Supplementary Fig. S2b, c). Considering cumulative N addition over time, we find a faster decrease in complementarity effects at low levels of cumulative N addition (Fig. 4b, e). This may indicate a higher sensitivity of biotic interactions to low levels of cumulative N addition. Our results suggest that net biodiversity effects may level off or even bounce back in the long run under cumulative N addition, due to increases in overyielding following increased species dominance4,45. However, this may result in the community behaving as a functional monoculture despite a positive net biodiversity effect. Specifically, the consistent increase in selection effects with cumulative N addition may overwhelm the decrease in complementarity effects, resulting in a convex relationship between net biodiversity effects and cumulative N addition (from either experimental addition or atmospheric deposition; Figs. 1d and 4a, d). This result contrasts with the expected concave relationship based on the null hypothesis that the effects of N addition rate and time are independent (Fig. 1c). Instead, this convex relationship suggests a strong interaction between the impact of N addition rate and the impact of time, further indicating a shift in the relative importance of biodiversity effects, from complementarity to selection effects, under N addition. This shift in relative importance occurs regardless of the pathway of N addition, i.e., atmospheric N deposition or fertilization. Similar convex relationships between net biodiversity effects and cumulative N addition are found from experimental addition (at higher levels, generally 40–400 kg/ha/year) or atmospheric deposition (at lower levels, generally 0–40 kg/ha/year) alone. These two convex relationships may result in a non-significant relationship between net biodiversity effects and cumulative N addition when combining both pathways of N inputs.
The shift in the relative importance of complementarity and selection effects under increasing cumulative N addition may be due to changes in community structure, i.e., changes in evenness. That is, the reduction of N-limitation over extended periods may favor large and fast-growing species, leading to an increase in selection effects36,71,72. Additionally, N addition may lead to asymmetric competition for light in mixtures. This increased asymmetric competition may simultaneously reduce opportunities for species complementarity in resource use and intensify the effects of competitive hierarchies on species relative abundances47,65,73. Furthermore, communities dominated by highly productive species are usually more susceptible to climate change, leading to higher variation through time3,74. However, our analysis does not account for potential indirect effects of N addition through species composition on overyielding75,76. We also note that our estimates of cumulative N addition do not account for N losses due to N leaching or biomass removal. Some experiments included in the present study, e.g., the Jena Experiment and the PaNDiv experiment, remove all aboveground biomass annually, while others do not, e.g., BioCON. Removal of biomass and the associated nitrogen may lead to a decrease in soil N that is accessible to plants over time, which would have otherwise been recycled within the system48,77,78. Additionally, biodiversity effects on soil N mineralization rates also have been found to shift from negative to positive over time, indicating that species rich communities could have higher N retention than species poor communities with increasing N addition duration79. The interaction between N addition and species richness found in our study implies that future studies should consider the cumulative amount of N when assessing the impacts of N addition on overyielding. This involves incorporating N rates and duration in a full factorial design and measuring N content in litter, in removed biomass, or within the plant-soil system.
To sum up, our study reveals that cumulative N addition influences the ecological mechanisms underlying overyielding, thereby expanding our understanding of how global change affects biodiversity-ecosystem functioning relationships across grasslands. Specifically, with increased cumulative N addition, we observe a shift in the relative importance of the components of net biodiversity effects from complementarity to selection effects. While cumulative N addition boosts selection effects, it does not generate a net impact on overyielding due to the diminishing role of complementarity effects in high diversity communities. Our results suggest that the effect of biodiversity on productivity becomes increasingly reliant on a small number of dominant species rather than on overall species richness38, thereby amplifying ecosystem susceptibility to environmental fluctuations associated with global change, such as disease outbreaks80–82, climate variability74,83,84, and disturbance65.
Methods
Data collection
We conducted a meta-level synthesis to explore the impact of N addition on overyielding in grassland ecosystems. We had three requirements for datasets to be included in this study: 1) the experiments needed to cross a gradient of sown plant species richness with a N addition treatment; 2) the experiments needed to measure species-level biomass (g/m2) at the plot scale for each plant community, including monocultures; 3) biomass should be measured at earliest in the second year after the establishment of experiments establishment. For studies that collected biomass in the same location more than once a year (No. 4, 5, 7–15 in Supplementary Table S1), we summed biomass from multiple harvests per year as a proxy for aboveground annual productivity (g/m2/year), to enable comparison across studies. In total, 15 grassland studies met our criteria, with observations from 1504 plots. The selected studies were distributed across ten countries, with the richness of sown species ranging from 1 to 20; the number of years for which we had biomass data ranging from 1 to 23 years; and N addition rate ranging from 0 to 500 kg/ha/year.
We then tested our hypotheses using the studies meeting our selection criteria (Supplementary Table S1). To test the impacts of N addition as a binary factor (H1a), we used studies that included both unmanipulated ambient plots and N addition plots (studies No. 1–5, 7, 12–14); to test whether overyielding varied with species richness under N addition, we used studies with more than two species richness levels, in addition to monocultures (studies No. 1–3, 5, 7); H2); to test the interaction between the effects of N addition (binary) and the effects of time (H2), data from the plots with N but not CO2 enrichment at BioCoN experiment (study No. 1) was used since it has run continuously for 23 years, while other studies lasted less than 5 years (studies No. 2–15); and to test the effects of N addition rate (H1b) and cumulative N addition (H3) on overyielding, the full dataset was used (studies No. 1–15).
Diversity effects calculation
Relative yield of species i and the total relative yield of the mixture were calculated as in Harper (1977)85:
where and are the observed yield of species i in mixture and monoculture, respectively.
The change in the relative yield , net biodiversity effect, complementarity effect and selection effect were calculated as in Loreau and Hector27:
where is the sown proportion of species i, is the mean above-ground productivity (g/m2/year) in a monoculture of each sown species and n is sown species richness. Note that we added 1 (the 1.25% left tail of distribution in our full dataset) to all the monoculture yields in our analysis, since relative yield approaches infinity with small monoculture yield. Complementarity and selection effects were calculated via the partitionBEFsp package with corrected covariance86.
Statistics and reproducibility
We fitted separate mixed effect models to assess the effect of N addition on net biodiversity, complementarity, and selection effects. We partitioned the net biodiversity, complementarity, and selection effects following Loreau and Hector27, to capture both overyielding and underyielding. For the general impact of N addition treatment (H1a), we included the N addition treatment, experimental site (represented by different studies), and their interaction as fixed effects, and study specific plot ID nested in year as random effects. We also accounted for repeated measurements on the same plot via a first-order autoregressive temporal autocorrelation structure. After fitting the model, we calculated the estimated mean response under ambient or N addition treatments using the emmeans package87. We also tested how biodiversity effects changed with species richness under N addition by including N addition treatment (0, ambient; 1, N addition), sown species richness and their interaction as fixed effects, and species richness nested in study ID as a random effect. To be consistent with the design of the diversity gradients, we used log2-transformed sown species richness to represent species richness. To explore the impacts of N addition rate (H1b), we included N addition rate as a fixed effect and allowed random intercepts and slopes among different studies. To better meet the assumptions of our model, we used log-transformed N addition amount per year to represent annual N addition rate. To disentangle the impacts of N addition treatment (binary) and the impacts of N addition rate, we explored two conditions: including plots both with and without N experimental addition (Include ambient), or only plots with N addition (Exclude ambient). To represent the actual N addition per plot (including on control plots) and to account for spatial variation of N deposition rate, the annual N addition rate was the sum of N addition from experimental addition and atmospheric deposition. The total atmospheric deposition (NO− + NH+, both wet and dry) rate was extracted according to the location of each study site88,89. A static deposition rate was used here to cover spatial variation of deposition, not its temporal variation.
To explore the interaction between impacts of N addition treatment and the impacts of time (H2), we included N addition treatment, year and their interaction as fixed effects, and species richness as a random effect.
To explore the effect of cumulative N addition (H3), we multiplied N addition (experimental addition + atmospheric deposition, in kg/ha/year) by the number of years over which inputs occurred. We also compared models with cumulative N addition to those with an interaction of time and N addition rate, and models with cumulative N addition performed better based on Akaike information criterion (Supplementary Table S4). Based on our hypothesis of an interaction between the effects of time and nitrogen addition rates, we added a second order polynomial term for the impacts of cumulative N addition and assessed its goodness of fit based on the Akaike information criterion (Supplementary Table S5). The effects of evenness change with cumulative nitrogen addition and time was tested (Supplementary Table S6). Based on the goodness of fit, we set both the first and second order terms of cumulative N addition as fixed effects and allowed random intercepts and slopes among different studies (see Supplementary Table S3 for fully detailed model settings). In addition, we explored whether the impacts of cumulative N addition were regulated by different inputs: only experimental addition, only atmospheric deposition or both combined, due to the cascading effects of N from these inputs at different rates. All of the analyses were conducted using R version 4.0.590, within RStudio IDE91. The following packages were used: AICcmodavg92, dplyr93, emmeans87, itsadug94, lme495, lmerTest96, magrittr97, MuMIn98, nlme99, optimx100,101.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank two anonymous reviewers for their valuable comment. We thank all the people that contribute to experimental data collecting and processing. We are grateful to members in Ecology and Biodiversity group at Utrecht University, for great discussions which make this study in a better shape; and especially thanks to Ir. P.T.M. (Peter) Veenhuizen and G.P. (Betty) Verduyn for creating a supportive working environment. E.A., S.L.C. and N.A.P were supported by funding from the Swiss National Science Foundation (31003A_160212). J.D. was supported by the Czech Science Foundation (24-11954 S). F.I. was supported by the US National Science Foundation (DEB-2224852, DEB-1831944). C.P. continued the Swedish BIODEPTH experiment supported by the European Commission within the Framework IV Environment and Climate programme (ENV-CT95-0008); and her research was supported by Kempestiftelserna (JCK 99-09) and Skogs- och jordbrukets forskningsråd (SJFR 33.0388/00 731).
Author contributions
M.H., Y.H., K.E.B. and M.B.S conceived and designed the analysis; E.A., S.L.C., D.C., J.D., F.I., V.L., J.L., N.M., C.P., N.A.P., L.P., P.B.R. and C.R. contributed data; M.H and Y.H. performed the analysis; M.H., Y.H., K.E.B. drafted the paper; E.A., M.L. proofread the draft; all the coauthors reviewed and edited the manuscript.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Luke R. Grinham.
Data availability
The original data sets used in this data synthesis are available from data repositories of included studies, or upon request to data owners. The detailed information of included studies was documented on Table S1.
Code availability
All code used in this study available at the figshare repository with: 10.6084/m9.figshare.25153151.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-05999-9.
References
- 1.Food and Agriculture Organization, FAO statistical databases, Rome, available at http://faostat.fao.org/default.aspx.(2006).
- 2.Stevens CJ, Dise NB, Mountford JO, Gowing DJ. Impact of Nitrogen Deposition on the Species Richness of Grasslands. Science. 2004;303:1876–1879. doi: 10.1126/science.1094678. [DOI] [PubMed] [Google Scholar]
- 3.Hautier Y, et al. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature. 2014;508:521–525. doi: 10.1038/nature13014. [DOI] [PubMed] [Google Scholar]
- 4.Storkey J, et al. Grassland biodiversity bounces back from long-term nitrogen addition. Nature. 2015;528:401–404. doi: 10.1038/nature16444. [DOI] [PubMed] [Google Scholar]
- 5.Suding KN, et al. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl Acad. Sci. USA. 2005;102:4387–4392. doi: 10.1073/pnas.0408648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang Y, Xun F, Bai W, Zhang W, Li L. Long-Term Nitrogen Addition Leads to Loss of Species Richness Due to Litter Accumulation and Soil Acidification in a Temperate Steppe. PLOS One. 2012;7:e47369. doi: 10.1371/journal.pone.0047369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fay PA, et al. Grassland productivity limited by multiple nutrients. Nat. Plants. 2015;1:15080. doi: 10.1038/nplants.2015.80. [DOI] [PubMed] [Google Scholar]
- 8.Duran BEL, Duncan DS, Oates LG, Kucharik CJ, Jackson RD. Nitrogen fertilization effects on productivity and nitrogen loss in three grass-based perennial bioenergy cropping systems. PLoS One. 2016;11:e0151919. doi: 10.1371/journal.pone.0151919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark CM, Tilman D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature. 2008;451:712–715. doi: 10.1038/nature06503. [DOI] [PubMed] [Google Scholar]
- 10.Seabloom EW, et al. Increasing effects of chronic nutrient enrichment on plant diversity loss and ecosystem productivity over time. Ecology. 2021;102:e03218. doi: 10.1002/ecy.3218. [DOI] [PubMed] [Google Scholar]
- 11.Hautier Y, Niklaus PA, Hector A. Competition for light causes plant biodiversity loss after eutrophication. Science. 2009;324:636–638. doi: 10.1126/science.1169640. [DOI] [PubMed] [Google Scholar]
- 12.DeMalach N, Zaady E, Kadmon R. Light asymmetry explains the effect of nutrient enrichment on grassland diversity. Ecol. Lett. 2017;20:60–69. doi: 10.1111/ele.12706. [DOI] [PubMed] [Google Scholar]
- 13.Eskelinen A, Harpole WS, Jessen M-T, Virtanen R, Hautier Y. Light competition drives herbivore and nutrient effects on plant diversity. Nature. 2022;611:301–305. doi: 10.1038/s41586-022-05383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawley MJ, et al. Determinants of Species Richness in the Park Grass Experiment. Am. Nat. 2005;165:179–192. doi: 10.1086/427270. [DOI] [PubMed] [Google Scholar]
- 15.Silvertown J, et al. The Park Grass Experiment 1856–2006: its contribution to ecology. J. Ecol. 2006;94:801–814. doi: 10.1111/j.1365-2745.2006.01145.x. [DOI] [Google Scholar]
- 16.Kimmel K, et al. Diversity-dependent soil acidification under nitrogen enrichment constrains biomass productivity. Glob. Change Biol. 2020;26:6594–6603. doi: 10.1111/gcb.15329. [DOI] [PubMed] [Google Scholar]
- 17.Harpole WS, et al. Nutrient co‐limitation of primary producer communities. Wiley Online Libr. 2011;14:852–862. doi: 10.1111/j.1461-0248.2011.01651.x. [DOI] [PubMed] [Google Scholar]
- 18.Band N, Kadmon R, Mandel M, DeMalach N. Assessing the roles of nitrogen, biomass, and niche dimensionality as drivers of species loss in grassland communities. Proc. Natl Acad. Sci. 2022;119:e2112010119. doi: 10.1073/pnas.2112010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Hautier Y, Borer ET, Zhang C, Du G. Abundance- and functional-based mechanisms of plant diversity loss with fertilization in the presence and absence of herbivores. Oecologia. 2015;179:261–270. doi: 10.1007/s00442-015-3313-7. [DOI] [PubMed] [Google Scholar]
- 20.Isbell F, et al. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc. Natl Acad. Sci. USA. 2013;110:11911–11916. doi: 10.1073/pnas.1310880110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craven D, et al. Plant diversity effects on grassland productivity are robust to both nutrient enrichment and drought. Philos. Trans. R. Soc. B Biol. Sci. 2016;371:20150277. doi: 10.1098/rstb.2015.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prager CM, et al. A gradient of nutrient enrichment reveals nonlinear impacts of fertilization on Arctic plant diversity and ecosystem function. Ecol. Evol. 2017;7:2449–2460. doi: 10.1002/ece3.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atwater DZ, Callaway RM. Testing the mechanisms of diversity-dependent overyielding in a grass species. Ecology. 2015;96:3332–3342. doi: 10.1890/15-0889.1. [DOI] [PubMed] [Google Scholar]
- 24.Eskelinen A, et al. Resource-enhancing global changes drive a whole-ecosystem shift to faster cycling but decrease diversity. Ecology. 2020;101:1–12. doi: 10.1002/ecy.3178. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Chen HYH. Plant mixture balances terrestrial ecosystem C:N:P stoichiometry. Nat. Commun. 2021;12:1–9. doi: 10.1038/s41467-021-24889-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He K, et al. Response of aboveground biomass and diversity to nitrogen addition – a five-year experiment in semi-arid grassland of Inner Mongolia, China. Sci. Rep. 2016;6:31919. doi: 10.1038/srep31919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loreau M, Hector A. Partitioning selection and complementarity in biodiversity experiments. Nature. 2001;412:72–76. doi: 10.1038/35083573. [DOI] [PubMed] [Google Scholar]
- 28.Hector A, et al. Plant diversity and productivity experiments in European grasslands. Science. 1999;286:1123–1127. doi: 10.1126/science.286.5442.1123. [DOI] [PubMed] [Google Scholar]
- 29.Barry KE, et al. The Future of Complementarity: Disentangling Causes from Consequences. Trends Ecol. Evol. 2019;34:167–180. doi: 10.1016/j.tree.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Tilman D, Lehman CL, Thomson KT. Plant diversity and ecosystem productivity: Theoretical considerations. Proc. Natl Acad. Sci. 1997;94:1857–1861. doi: 10.1073/pnas.94.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahmen A, Renker C, Unsicker SB, Buchmann N. Niche complementarity for nitrogen: an explanation for the biodiversity and ecosystem functioning relationship? Ecology. 2006;87:1244–1255. doi: 10.1890/0012-9658(2006)87[1244:NCFNAE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Mason NWH, et al. Resource-use efficiency drives overyielding via enhanced complementarity. Oecologia. 2020;193:995–1010. doi: 10.1007/s00442-020-04732-7. [DOI] [PubMed] [Google Scholar]
- 33.Finn JA, et al. Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: A 3-year continental-scale field experiment. J. Appl. Ecol. 2013;50:365–375. doi: 10.1111/1365-2664.12041. [DOI] [Google Scholar]
- 34.Grange G, Finn JA, Brophy C. Plant diversity enhanced yield and mitigated drought impacts in intensively managed grassland communities. J. Appl. Ecol. 2021;58:1864–1875. doi: 10.1111/1365-2664.13894. [DOI] [Google Scholar]
- 35.Li L, Tilman D, Lambers H, Zhang FS. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture. N. Phytol. 2014;203:63–69. doi: 10.1111/nph.12778. [DOI] [PubMed] [Google Scholar]
- 36.Nyfeler D, Huguenin-Elie O, Suter M, Frossard E, Lüscher A. Grass-legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric. Ecosyst. Environ. 2011;140:155–163. doi: 10.1016/j.agee.2010.11.022. [DOI] [Google Scholar]
- 37.Wright AJ, Wardle DA, Callaway R, Gaxiola A. The Overlooked Role of Facilitation in Biodiversity Experiments. Trends Ecol. Evol. 2017;32:383–390. doi: 10.1016/j.tree.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Cappelli SL, Pichon NA, Mannall T, Allan E. Partitioning the effects of plant diversity on ecosystem functions at different trophic levels. Ecol. Monogr. 2022;92:e1521. doi: 10.1002/ecm.1521. [DOI] [Google Scholar]
- 39.Granjel RR, Allan E, Godoy O. Nitrogen enrichment and foliar fungal pathogens affect the mechanisms of multispecies plant coexistence. N. Phytol. 2023;237:2332–2346. doi: 10.1111/nph.18689. [DOI] [PubMed] [Google Scholar]
- 40.Harpole WS, et al. Addition of multiple limiting resources reduces grassland diversity. Nature. 2016;537:93–96. doi: 10.1038/nature19324. [DOI] [PubMed] [Google Scholar]
- 41.Hoekstra NJ, Suter M, Finn JA, Husse S, Lüscher A. Do belowground vertical niche differences between deep- and shallow-rooted species enhance resource uptake and drought resistance in grassland mixtures? Plant Soil. 2015;394:21–34. doi: 10.1007/s11104-014-2352-x. [DOI] [Google Scholar]
- 42.Kirwan L, et al. Evenness drives consistent diversity effects in intensive grassland systems across 28 European sites. Wiley Online Libr. 2007;95:530–539. [Google Scholar]
- 43.Siebenkäs A, Roscher C. Functional composition rather than species richness determines root characteristics of experimental grasslands grown at different light and nutrient availability. Plant Soil. 2016;404:399–412. doi: 10.1007/s11104-016-2853-x. [DOI] [Google Scholar]
- 44.Song L, et al. Nitrogen enrichment enhances the dominance of grasses over forbs in a temperate steppe ecosystem. Biogeosciences. 2011;8:2341–2350. doi: 10.5194/bg-8-2341-2011. [DOI] [Google Scholar]
- 45.Fargione J, et al. From selection to complementarity: Shifts in the causes of biodiversity-productivity relationships in a long-term biodiversity experiment. Proc. R. Soc. B Biol. Sci. 2007;274:871–876. doi: 10.1098/rspb.2006.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reich PB, et al. Impacts of biodiversity loss escalate through time as redundancy fades. Science. 2012;336:589–592. doi: 10.1126/science.1217909. [DOI] [PubMed] [Google Scholar]
- 47.Wagg C, et al. Biodiversity–stability relationships strengthen over time in a long-term grassland experiment. Nat. Commun. 2022;13:7752. doi: 10.1038/s41467-022-35189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tilman D, Isbell F. Recovery as nitrogen declines. Nature. 2015;528:336–337. doi: 10.1038/nature16320. [DOI] [PubMed] [Google Scholar]
- 49.Isbell F, Tilman D, Polasky S, Binder S, Hawthorne P. Low biodiversity state persists two decades after cessation of nutrient enrichment. Ecol. Lett. 2013;16:454–460. doi: 10.1111/ele.12066. [DOI] [PubMed] [Google Scholar]
- 50.Silva LCR, Lambers H. Soil-plant-atmosphere interactions: structure, function, and predictive scaling for climate change mitigation. Plant Soil. 2020;461:5–27. doi: 10.1007/s11104-020-04427-1. [DOI] [Google Scholar]
- 51.DeLuca TH, Zackrisson O, Gundale MJ, Nilsson MC. Ecosystem feedbacks and nitrogen fixation in boreal forests. Science. 2008;320:1181. doi: 10.1126/science.1154836. [DOI] [PubMed] [Google Scholar]
- 52.Spehn EM, et al. The role of legumes as a component of biodiversity in a cross-European study of grassland biomass nitrogen. Oikos. 2002;98:205–218. doi: 10.1034/j.1600-0706.2002.980203.x. [DOI] [Google Scholar]
- 53.Vázquez E, et al. Nitrogen but not phosphorus addition affects symbiotic N2 fixation by legumes in natural and semi-natural grasslands located on four continents. Plant Soil. 2022 doi: 10.1007/s11104-022-05498-y. [DOI] [Google Scholar]
- 54.Pichon NA, et al. Decomposition disentangled: A test of the multiple mechanisms by which nitrogen enrichment alters litter decomposition. Funct. Ecol. 2020;34:1485–1496. doi: 10.1111/1365-2435.13560. [DOI] [Google Scholar]
- 55.Lambers H, et al. How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant Soil. 2018;424:11–33. doi: 10.1007/s11104-017-3427-2. [DOI] [Google Scholar]
- 56.Mommer L, et al. Lost in diversity: the interactions between soil-borne fungi, biodiversity and plant productivity. N. Phytol. 2018;218:542–553. doi: 10.1111/nph.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagg C, Bender SF, Widmer F, Van Der Heijden MGA. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl Acad. Sci. USA. 2014;111:5266–5270. doi: 10.1073/pnas.1320054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren Z, et al. Effects of resource additions on species richness and ANPP in an alpine meadow community. J. Plant Ecol. 2010;3:25–31. doi: 10.1093/jpe/rtp034. [DOI] [Google Scholar]
- 59.Wright A, Schnitzer SA, Reich PB. Daily environmental conditions determine the competition-facilitation balance for plant water status. J. Ecol. 2015;103:648–656. doi: 10.1111/1365-2745.12397. [DOI] [Google Scholar]
- 60.Roscher C, Schmid B, Kolle O, Schulze ED. Complementarity among four highly productive grassland species depends on resource availability. Oecologia. 2016;181:571–582. doi: 10.1007/s00442-016-3587-4. [DOI] [PubMed] [Google Scholar]
- 61.Kirwan L. The Agrodiversity Experiment three years of data from a multisite study in intensively managed grasslands. Ecology. 2014;95:2680. doi: 10.1890/14-0170.1. [DOI] [Google Scholar]
- 62.Hautier Y, Vojtech E, Hector A. The importance of competition for light depends on productivity and disturbance. Ecol. Evol. 2018;8:10655–10661. doi: 10.1002/ece3.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siebenkäs A, Schumacher J, Roscher C. Resource availability alters biodiversity effects in experimental grass-forb mixtures. PLoS ONE. 2016;11:e0158110. doi: 10.1371/journal.pone.0158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson SD, Tilman D. Component of Plant Competition Along an Experimental Gradient of Nitrogen Availability. Ecology. 1991;72:1050–1065. doi: 10.2307/1940605. [DOI] [Google Scholar]
- 65.Wilson SD, Tilman D. Plant Competition and Resource Availability in Response to Disturbance and Fertilization. Ecology. 1993;74:599–611. doi: 10.2307/1939319. [DOI] [Google Scholar]
- 66.Hu Y, et al. Multi-trait functional diversity predicts ecosystem multifunctionality under nitrogen addition in a desert steppe. Plant Soil. 2022 doi: 10.1007/s11104-022-05731-8. [DOI] [Google Scholar]
- 67.Lange M, Eisenhauer N, Chen H. & Gerd Gleixner. Increased soil carbon storage through plant diversity strengthens with time and extends into the subsoil. Glob. Change Biol. 2023;29:2627–2639. doi: 10.1111/gcb.16641. [DOI] [PubMed] [Google Scholar]
- 68.Reich PB. Elevated CO2 reduces losses of plant diversity caused by nitrogen deposition. Science. 2009;326:1399–1402. doi: 10.1126/science.1178820. [DOI] [PubMed] [Google Scholar]
- 69.Reich PB, et al. Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature. 2001;410:809–812. doi: 10.1038/35071062. [DOI] [PubMed] [Google Scholar]
- 70.Pontes L, et al. Impacts of species interactions on grass community productivity under contrasting management regimes. Oecologia. 2012;168:761–771. doi: 10.1007/s00442-011-2129-3. [DOI] [PubMed] [Google Scholar]
- 71.Connolly J, Wayne P. Asymmetric competition between plant species. Oecologia. 1996;108:311–320. doi: 10.1007/BF00334656. [DOI] [PubMed] [Google Scholar]
- 72.Slate ML, Matallana-Mejia N, Aromin A, Callaway RM. Nitrogen addition, but not pulse frequency, shifts competitive interactions in favor of exotic invasive plant species. Biol. Invasions. 2022 doi: 10.1007/s10530-022-02833-3. [DOI] [Google Scholar]
- 73.DeMalach N, Zaady E, Weiner J, Kadmon R. Size asymmetry of resource competition and the structure of plant communities. J. Ecol. 2016;104:899–910. doi: 10.1111/1365-2745.12557. [DOI] [Google Scholar]
- 74.De Boeck HJ, et al. Patterns and drivers of biodiversity–stability relationships under climate extremes. J. Ecol. 2018;106:890–902. doi: 10.1111/1365-2745.12897. [DOI] [Google Scholar]
- 75.Marquard E, et al. Plant species richness and functional composition drive overyielding in a six-year grassland experiment. Ecology. 2009;90:3290–3302. doi: 10.1890/09-0069.1. [DOI] [PubMed] [Google Scholar]
- 76.van Paassen JG, et al. Legacy effects of nitrogen and phosphorus additions on vegetation and carbon stocks of upland heaths. N. Phytol. 2020;228:226–237. doi: 10.1111/nph.16671. [DOI] [PubMed] [Google Scholar]
- 77.Clark CM, Hobbie SE, Venterea R, Tilman D. Long-lasting effects on nitrogen cycling 12 years after treatments cease despite minimal long-term nitrogen retention. Glob. Change Biol. 2009;15:1755–1766. doi: 10.1111/j.1365-2486.2008.01811.x. [DOI] [Google Scholar]
- 78.Guiz J, et al. Long-term effects of plant diversity and composition on plant stoichiometry. Oikos. 2016;125:613–621. doi: 10.1111/oik.02504. [DOI] [Google Scholar]
- 79.Chen X, Chen HYH, Searle EB, Chen C, Reich PB. Negative to positive shifts in diversity effects on soil nitrogen over time. Nat. Sustain. 2021;4:225–232. doi: 10.1038/s41893-020-00641-y. [DOI] [Google Scholar]
- 80.Civitello DJ, et al. Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc. Natl Acad. Sci. 2015;112:8667–8671. doi: 10.1073/pnas.1506279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitchell CE, Tilman D, Groth JV. Effects of Grassland Plant Species Diversity, Abundance, and Composition on Foliar Fungal Disease. Ecology. 2002;83:1713–1726. doi: 10.1890/0012-9658(2002)083[1713:EOGPSD]2.0.CO;2. [DOI] [Google Scholar]
- 82.Rottstock T, Joshi J, Kummer V, Fischer M. Higher plant diversity promotes higher diversity of fungal pathogens, while it decreases pathogen infection per plant. Ecology. 2014;95:1907–1917. doi: 10.1890/13-2317.1. [DOI] [PubMed] [Google Scholar]
- 83.Isbell F, et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature. 2015;526:574–577. doi: 10.1038/nature15374. [DOI] [PubMed] [Google Scholar]
- 84.Van Sundert K, et al. Fertilized graminoids intensify negative drought effects on grassland productivity. Glob. Change Biol. 2021;27:2441–2457. doi: 10.1111/gcb.15583. [DOI] [PubMed] [Google Scholar]
- 85.Harper, J. L. Population biology of plants (The Blackburn Press, 1977).
- 86.Clark AT, et al. How to estimate complementarity and selection effects from an incomplete sample of species. Methods Ecol. Evol. 2019;10:2141–2152. doi: 10.1111/2041-210X.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lenth, R. V. et al. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.7.4-1, https://CRAN.R-project.org/package=emmeans (2022)
- 88.Simpson D, et al. The EMEP MSC-W chemical transport model – technical description. Atmos. Chem. Phys. 2012;12:7825–7865. doi: 10.5194/acp-12-7825-2012. [DOI] [Google Scholar]
- 89.Parfitt RL, Baisden WT, Schipper LA, Mackay AD. Nitrogen inputs and outputs for New Zealand at national and regional scales: Past, present and future scenarios. J. R. Soc. N. Z. 2008;38:71–87. doi: 10.1080/03014220809510547. [DOI] [Google Scholar]
- 90.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. (2021).
- 91.RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/. (2020).
- 92.Mazerolle, M. J. AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.3-1. https://cran.r-project.org/package=AICcmodavg. (2020).
- 93.Wickham, H., François, R., Henry, L., Müller, K. dplyr: A Grammar of Data Manipulation. https://dplyr.tidyverse.org, https://github.com/tidyverse/dplyr. (2022).
- 94.van Rij, J., Wieling, M., Baayen, R. H. & van Rijn, H. itsadug: Interpreting Time Series and Autocorrelated Data Using GAMMs. R package version 2.4.1 (2022)
- 95.Bates, D., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 67, 1–48 (2014).
- 96.Kuznetsova A, Brockhoff PB, Christensen RH. B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017;82:1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 97.Bache, S. M., & Wickham, H. magrittr: a forward-pipe operator for R. R package version 2.0.3. https://CRAN.R-project.org/package=magrittr. (2014).
- 98.Bartoń, K. MuMIn: Multi-Model Inference. R package version 1.46.0. https://CRAN.R-project.org/package=MuMIn. (2022).
- 99.Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., & Team, R. C. Linear and nonlinear mixed effects models. R package version. https://CRAN.R-project.org/package=nlme. (2007).
- 100.Nash, J. C. On Best Practice Optimization Methods in R. J. Stat. Softw. 60, 1–14 10.18637/jss.v060.i02. (2014)
- 101.Nash JC, Varadhan R. Unifying Optimization Algorithms to Aid Software System Users: optimx for R. J. Stat. Softw. 2011;43:1–14. doi: 10.18637/jss.v043.i09. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data sets used in this data synthesis are available from data repositories of included studies, or upon request to data owners. The detailed information of included studies was documented on Table S1.
All code used in this study available at the figshare repository with: 10.6084/m9.figshare.25153151.