Abstract
Effective exudate management is key for optimal ulcer healing. Superabsorbent dressings are designed to have high fluid handling capacity, reduced risk of exudate leakage, fluid retention under compression, and to sequester harmful exudate components. This study aimed to systematically identify existing evidence for the clinical efficacy and cost‐effectiveness of superabsorbent dressings for the treatment of moderate‐to‐highly exudating chronic ulcers of various etiologies. The aim is focused on examining the ‘class’ effect of all superabsorbers, not any particular dressing. Clinical and cost effectiveness systematic reviews were conducted, searching Embase, MEDLINE, the Cochrane Library, and the Cumulative Index to Nursing and Allied Health Literature. The Cost Effectiveness Analysis Registry and Econ papers were also searched for the economic review. Outcomes of interest included ulcer closure, dressing properties, hospital‐ and infection‐related outcomes, safety, and economic outcomes. Fourteen studies were included in the clinical systematic review. Eleven were case series, with one randomised controlled trial, one retrospective matched observational study, and one retrospective cohort study. The studies investigated eight superabsorbent dressings and were heterogeneous in their patient population and outcomes. Superabsorbent dressings may result in favourable outcomes, including reductions in frequency of dressing change and pain scores. As most studies were case series, drawing firm conclusions was difficult due to absence of a comparator arm. The economic systematic review identified seven studies, five of which were cost‐utility analyses. These suggested superabsorbent dressings are a more cost‐effective option for the treatment of chronic ulcers compared with standard dressings. However, the small number and low quality of studies identified in both reviews highlights the need for future research.
Keywords: chronic ulcers, clinical effectiveness, cost‐effectiveness, superabsorbent wound dressings, systematic review
List of Abbreviations
- AE
adverse event
- ALU
arterial leg ulcer
- CINAHL
cumulative index to nursing and allied health literature
- CMC
carboxymethylcellulose
- CRD
centre for reviews and dissemination
- DFU
diabetic foot ulcer
- ECM
extracellular matrix
- EUnetHTA
European Network for Health Technology Assessment
- HAS
Haute Autorité de Santé
- HTA
health technology assessment
- MMP
matrix metalloproteinase
- NFLU
non‐contact low frequency ultrasound
- NHS
national Health Service
- NICE
National Institute for Health and Care Excellence
- NPWT
negative pressure wound therapy
- PI
pressure injury
- PICOS
population, Intervention
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- PROSPERO
Prospective Register of Systematic reviews
- PU
pressure ulcer
- PUSH
Pressure Ulcer Scale for Healing
- QoL
quality of life
- RCT
randomised controlled trial
- SD
standard deviation
- SLR
systematic literature review
- TIMP
tissue inhibitor of metalloproteinase
- UK
United Kingdom
- USA
United States of America
- VAC
vacuum‐assisted closure
- VAS
visual analogue scale
- VLU
venous leg ulcer
1. INTRODUCTION
Chronic (or non‐healing) ulcers are classified based on their causative etiologies, including pressure ulcers/injuries (PUs/PIs), diabetic foot ulcers (DFU), and venous leg ulcers (VLUs)/arterial leg ulcers (ALUs). While healing can be divided into four overlapping phases: haemostasis, inflammation, proliferation and maturation/remodelling, 1 chronic ulcers do not progress through these stages in an orderly or timely fashion. 2 Often with a prolonged inflammation phase, 3 they can take several months, or sometimes years, to heal completely. 4
Ulcer exudate (i.e., the fluid leaking from ulcer) is produced as a natural part of the healing process and supports it in several ways. 5 However, its overproduction or changes to the normal composition can impair ulcer healing, and there are fundamental differences between acute and chronic ulcer exudate. 5 The latter contains higher levels of pro‐inflammatory macrophages, 6 with reduced clearance capacity, creating a strong inflammatory environment. Increased levels of matrix metalloproteinases (MMP‐2 and MMP‐9), 7 , 8 , 9 released by macrophages, and lower levels of their inhibitors (tissue inhibitors of metalloproteinases [TIMPs]), 7 leads to deregulated and excessive degradation of the skin's extracellular matrix (ECM). Elevated levels of proteinases in chronic ulcers, including MMPs, have also been linked to the degradation of growth factors that are important for healing. 10 Chronic ulcers are also susceptible to the formation of biofilms, where the bacteria further stimulate the production of pro‐inflammatory cytokines, growth factors and proteinases (including MMPs). 11 , 12
Chronic ulcers and high volumes of exudate have a significant impact on a patient's quality of life (QoL), affecting their physical and mental wellbeing. 13 , 14 , 15 They also present a significant economic burden, with an estimated 1%–3% of the total healthcare expenditure going towards the treatment of chronic ulcers in developed countries. 16 Poor exudate management may contribute to these costs, and lead to increased demands being placed on clinician time and resources. 15
The effective management of exudate is key for optimal ulcer healing 5 ; it may reduce the time‐to‐healing and problems associated with excess exudate. 17 Several dressings have been developed for moderate‐to‐high exudating ulcers of various etiologies. 5 , 18 Superabsorbent dressings are designed to have a higher fluid handling capacity than other standard dressings, reducing the risk of exudate leakage, and to retain fluid under compression. 19 They also sequester exudate components, such as bacteria 20 and MMPs 21 , 22 into the dressing core, reducing the risk of infection and maceration.
To our knowledge, no study has been undertaken to systematically identify existing evidence for the clinical efficacy and cost‐effectiveness of superabsorbent wound dressings for the treatment of moderate to highly exudating chronic ulcers of various etiologies. Such a review is important to inform both clinical decision making and future research. Therefore, clinical and cost‐effectiveness systematic literature reviews (SLRs) were conducted to identify interventional, observational, and cost‐effectiveness studies of superabsorbent wound dressings in patients with chronic ulcers with aim to estimate ‘class’ effect of all superabsorbers, not any particular dressing.
2. MATERIALS AND METHODS
The SLRs were performed in accordance with the following guidelines: Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 23 ; National Institute for Health and Care Excellence (NICE) methodology checklist for systematic reviews and meta‐analyses 24 ; the Centre for Reviews and Dissemination's (CRD) guidance for undertaking systematic reviews in health care 25 ; the Cochrane Handbook for Systematic Reviews of Interventions 26 ; and the European Network for Health Technology Assessment (EUnetHTA) methodological guidelines. 27 This systematic review is registered at the International Prospective Register of Systematic reviews (PROSPERO) (CRD42021286124). The only deviation from the protocol was concerning the population eligibility criterion. A population consisting of >80% of patients with chronic ulcers was initially proposed in the protocol, however, this was relaxed to >50% of patients with chronic ulcers, to include a wider range of studies.
2.1. Literature sources and searches
For both SLRs, the Ovid platform was used to search the following electronic databases: Embase, MEDLINE, the Cochrane Library (2 December 2021), and the EBSCO platform was used to search the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 9 December 2021). For the economic SLR, additional databases (The Cost Effectiveness Analysis Registry, and Econ papers) were searched on 14 March 2022. The database search strings identified all relevant publications indexed in Embase and were modified for performing searches in Medline and the Cochrane Library to account for differences in syntax and thesaurus headings. The complete search strings are provided in Tables S1–S7.
Hand‐searching was used as a supplementary measure to ensure all relevant studies were included. The following sources were searched: Health Technology Assessment (HTA) Database of the International Network of Agencies for HTA (https://www.inahta.org/); NICE (including published guidance, guidance in development and Medtech innovation briefings); Scottish Medicines Consortium; All Wales Medicines Strategy Group; Canadian Agency for Drugs and Technologies in Health; Pharmaceutical Benefits Advisory Committee; Haute Autorité de Santé (HAS); German Institute for Quality and Efficiency in Health Care; Gemeinsamer Bundesausschuss (The Federal Joint Committee). Bibliographic reference lists of included publications and relevant SLRs were also screened. Additionally, a citation search was undertaken using the web application ‘Connected Papers’ (www.connectedpapers.com) to screen for publications potentially missed by other methods.
2.2. Study selection criteria
The population, intervention, comparator(s), outcomes, and study designs (PICOS) elements used to assess study eligibility for the clinical and economic SLRs are presented in Tables S8 and S9, respectively. The clinical SLR search was restricted to English language studies published between the 1st January 2000 and the search date, while the economic SLR search had the same date restriction, but was not restricted by language.
2.3. Screening and extraction
Two independent and blinded reviewers screened citations in Covidence against the pre‐defined eligibility criteria at the title/abstract and full‐text screening stage. Any conflicts were resolved by a third, more senior investigator. At the full‐text screening stage, a record was kept of papers excluded, along with a clear justification for their exclusion. Data from the included publications were extracted by one reviewer into standardised, piloted data extraction tables in Microsoft® Excel; this information was checked and validated by conducting an independent internal data check once all required data had been entered.
2.4. Quality assessment
Quality assessment of the included studies was performed using the Cochrane RoB 2 28 checklist for randomised controlled trials (RCTs), the Cochrane ROBINS‐I 29 checklist for non‐randomised studies, the JBI checklist 30 for case series, and The Drummond 31 checklist for cost‐effectiveness studies.
2.5. Statistical analysis
Studies identified from the SLR were reviewed for their suitability to be included in a meta‐analysis or indirect treatment comparison. This included a review of study design, baseline characteristics across studies, and review of which data were available for synthesis.
3. RESULTS
In the clinical SLR, the electronic database search identified 8508 citations. On removal of 1157 duplicates and 7279 citations at the title/abstract screening stage, 72 records were sought for retrieval, three of which were not available. Of the 69 full‐text publications assessed for eligibility, 13 publications were considered relevant for inclusion. One further publication was identified by hand‐searching/other grey literature searches, resulting in a total of 14 publications being included in the clinical SLR (Figure 1). In the cost‐effectiveness SLR, the electronic database search identified 1648 citations. Following removal of 49 duplicates and 1583 citations at the title/abstract screening stage, 16 were sought for retrieval. A total of six publications were considered relevant for inclusion. One further publication was identified by hand‐searching/other grey literature searches, resulting in a total of seven publications being included in the cost‐effectiveness SLR (Figure 2).
FIGURE 1.
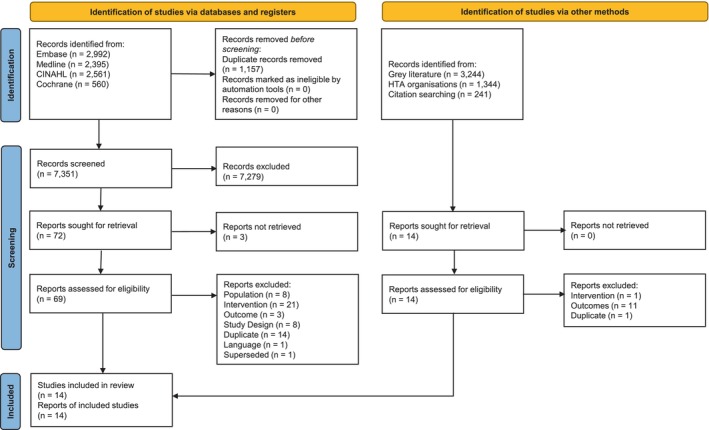
Clinical SLR PRISMA flow diagram. HTA, Health technology assessment; SLR, systematic literature review.
FIGURE 2.
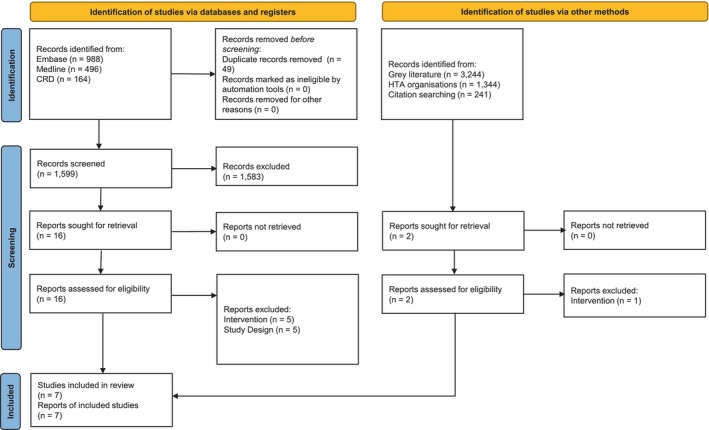
Cost‐effectiveness SLR PRISMA flow diagram. HTA, Health technology assessment; SLR, systematic literature review.
3.1. Characteristics of the included studies
3.1.1. Clinical studies
Of the 14 publications identified in the clinical SLR, the majority were case series (n = 11), 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 with one RCT, 43 one retrospective matched observational cohort study, 44 and one retrospective cohort study 45 (Table 1). The most common country of origin was the United Kingdom (UK) (n = 8). 32 , 33 , 34 , 35 , 37 , 39 , 41 , 44 The 14 studies investigated nine different superabsorbent dressings: three investigated Sorbion sachet S, 40 , 44 , 45 three DryMax Extra, 32 , 36 , 44 three Zetuvit Plus, 33 , 34 , 35 three Flivasorb, 39 , 41 , 44 two Vliwasorb, 38 , 42 one Eclypse Adherent Sacral, 37 one Kerramax, 44 and Curea P1 Duo Active. 43 Only four studies investigated more than one intervention. 40 , 43 , 44 , 45 Most of the studies included patients with ulcers of various etiologies (n = 11) 33 , 34 , 35 , 36 , 38 , 39 , 40 , 41 , 42 , 43 , 45 ; the others included patients with leg ulcers (LUs), 32 PUs, 37 or VLUs. 44 The number of patients included in the analyses ranged from 9 37 to 439. 44 Baseline patient and ulcer characteristics of the studies identified in the clinical SLR are presented in Table 2.
TABLE 1.
Study characteristics (clinical SLR).
| Publication (year) | Country | Sample size | Study design | Intervention(s) | Study population | Outcomes of interest reported |
|---|---|---|---|---|---|---|
| Allymamod (2011) 32 | UK | 16 | Case series |
|
LUs |
|
| Atkin (2020) 33 | UK | 49 | Case series |
|
Mixed aetiology ulcers, VLUs, and DFUs |
|
| Barrett (2018) 34 | UK | 50 | Case series |
|
VLUs, PUs, and DFUs |
|
| Barrett (2020) 35 | UK | 52 | Case series |
|
VLUs, PUs, DFUs, and malignant wounds |
|
| Hindhede (2012) 36 | Belgium | 30 | Case series |
|
VLUs, PUs, arterial ulcer, hematoma ulcer, postoperative wound, lymphatic leak caused by traumatic leg wound, lymphatic ulcer, mixed aetiology LU, posttraumatic wound/venous hypertension, skin tear, ulcer caused by herpes zoster |
|
| Lloyd‐Jones (2011) 37 | UK | 9 | Case series |
|
PUs |
|
| Münter (2018) 38 | Germany | 171 | Prospective case series |
|
VLUs, PUs, DFUs, acute post‐surgical healing by secondary intention, post‐trauma, incised abscess, wound at risk for infection, superficial burn grade II, other |
|
| Panca (2013) 44 | UK | 439 | Retrospective matched observational cohort |
|
VLUs |
Complete wound closure Time to complete wound closure
|
| Probst (2022) 43 | Switzerland | 77 | RCT, open‐label |
|
VLUs, DFUs, arterial leg ulcers, mixed LUs |
|
| Tickle (2013) 39 | UK | 12 | Case series |
|
Sinus ulcers, LUs, DFUs, traumatic ulcers |
|
| van Leen (2014) 40 | UK, Netherlands | 29 | Case series |
|
LUs, PUs |
|
| Verrall (2010) 41 | UK | 19 | Case series |
|
VLUs, PUs, arterial ulcer, chest wound |
|
| Faucher (2012) 42 | France | 15 | Prospective case series |
|
PUs, VLUs, mixed ulcers, DFUs, tumour ulcers, acute surgical wounds and other wounds |
|
| Hermans (2015) 45 | USA | 38 | Retrospective cohort |
|
VLUs, PUS, surgical wounds |
|
Abbreviations: AE, adverse event; CMC, carboxymethylcellulose; DFU, diabetic foot ulcer; LU, leg ulcer; NPWT, negative pressure wound therapy; PU, pressure ulcer; RCT, randomised controlled trial; SLR, systematic literature review; UK, United Kingdom; USA, United States of America; VAC vacuum‐assisted closure, VLU, venous leg ulcer.
TABLE 2.
Baseline patient and ulcer characteristics (clinical SLR).
| Publication (year) | Age (years), mean (SD) | Sex, n (%) | Ulcer duration, mean (SD) | Ulcer size (cm2), mean (SD) |
|---|---|---|---|---|
| Allymamod (2011) 32 | – |
|
>6 weeks | 30–50 |
| Atkin (2020) 33 |
Male: 73.6 (9.5) Female: 78.2 (12.4) |
|
>1 week | – |
| Barrett (2018) 34 |
Male: 74.71 (15.47) Female: 78 (14.78) |
|
– | – |
| Hindhede (2012) 36 | 69 (16.2) | – | – | – |
| Lloyd‐Jones (2011) 37 | – |
|
– | – |
| Münter (2018) 38 | 69 (35) |
|
13 (8.89) | 44.96 (126.84) |
| Panca (2013) 44 |
DryMax Extra: 71.7 (95% CI: 67.1, 76.3) Flivasorb: 74.9 (95% CI: 72.6, 77.3) Kerramax: 70.3 (95% CI: 67.1, 73.4) Sorbion Sachet S: 74.3 (95% CI: 71.6, 77.1) CMC: 74.3 (95% CI: 71.5, 77) |
Male:
|
|
|
| Probst (2022) 43 | 77.5 (12.6) |
|
≥3 months | – |
| Tickle (2013) 39 | – | – | – | – |
| Van Leen (2014) 40 |
Patients with PUs: 64.6 (NR) Patients with LUs: 71.7 (NR) |
Patients with PUs:
Patients with LUs:
|
– | – |
| Verrall (2010) 41 | 66.5 (NR) |
|
1.5 years (NR) | – |
| Faucher (2012) 42 | 69.7 (10.36) |
|
– | – |
| Hermans (2015) 45 |
|
|
– |
|
Abbreviations: CI, confidence interval; CMC, carboxymethylcellulose; LU, leg ulcer; NPWT, negative pressure wound therapy; NR, not reported; PU, pressure ulcer; SD, standard deviation; SLR, systematic literature review.
3.1.2. Cost‐effectiveness studies
Of the seven studies identified in the cost‐effectiveness SLR, five were cost‐utility analyses, 44 , 46 , 47 , 48 , 49 one cost‐description 34 and one cost‐comparison analyses (Table 3). 45 The countries of interest included the UK (n = 4), 34 , 44 , 46 , 48 Germany (n = 1), 47 the United States of America (USA; n = 1) 45 and France (n = 1). 49 The study populations comprised patients with LUs (n = 3), 46 , 47 , 49 VLUs (n = 2), 44 , 48 and ulcers of various etiologies (n = 2). 34 , 45 Four of the cost‐utility analyses used a Markov model (all with a cycle length of 1 week), 46 , 47 , 48 , 49 and one used a decision tree. 44 Perspectives of the analyses were the UK National Health Service (NHS) (n = 3), 44 , 46 , 48 HAS, 49 payer and societal, 47 and hospital. 45 One study did not report the perspective. 34 The time horizons ranged from 2 weeks 34 to 1 year, 48 with the majority of studies stating a time horizon of 6 months (n = 4). 44 , 46 , 47 , 49
TABLE 3.
Study characteristics (economic SLR).
| Publication (year) | Country (perspective) | Type of economic analysis | Intervention | Comparator(s) | Study population | No. health states | Cycle length | Time horizon | Discounting |
|---|---|---|---|---|---|---|---|---|---|
| Barrett (2018) 34 | UK (NR) | Cost‐comparison, before‐after model |
|
– | VLUs, PUs, arterial wound, chest wound, wet legs, surgical wound | NR | NR | 2 weeks | NA |
| Panca (2013) 44 | UK (UK NHS) | Cost‐utility, decision‐tree model |
|
|
VLUs | 5 | NR | 6 months | NR |
| Veličković (2020) 46 | UK (UK NHS) | Cost‐utility, Markov model |
|
SoC:
|
LUs | 6 | 1 week (half‐cycle correction) | 6 months | None |
| Veličković (2021) 47 | Germany (Payer & societal) | Cost‐utility, Markov model |
|
SoC:
|
LUs | 6 | 1 week | 6 months | NR |
| Walzer (2018) 48 | UK (UK NHS) | Cost‐utility, Markov model |
|
|
VLUs | 5 | 1 week | 1 year | NR |
| Hermans (2015) 45 | USA (Hospital) | Cost‐comparison, simple‐comparison model |
|
|
VLUs, PUs, DFUs and surgical wounds | NR | NR | NR | NA |
| Veličković (2022) 49 | France (HAS national payer) | Cost‐utility, Markov model |
|
Mix of foam dressings:
|
LU | 6 | 1 week | 6 months | None |
Abbreviations: DFU, diabetic foot ulcer; HAS, Haute Autorité de Santé; LU, leg ulcer; NA, not applicable; NHS, National Health Service; NPWT, negative pressure wound therapy; NR, not reported; PU, pressure ulcer; SLR, systematic literature review; SoC, standard of care; UK, United Kingdom; USA, United States of America; VAC, vacuum‐assisted therapy; VLU, venous leg ulcer.
The source publication uses the Resposorb brand name.
The cost‐description and cost‐comparison analyses were primary studies, which used their own efficacy data. 34 , 45 One study 46 sourced efficacy data from Atkin et al. 33 two studies sourced efficacy data 47 , 49 from merged cohorts from Atkin et al. 33 and Barrett et al. 35 and three studies 44 , 46 , 49 sourced utility data from Clegg et al. 50
All five of the cost‐utility studies performed sensitivity analyses. 44 , 46 , 47 , 48 , 49 Four studies completed both deterministic and probabilistic sensitivity analyses (PSA). 44 , 46 , 47 , 49 Of the studies reporting PSA, three used a Monte‐Carlo methodology 46 , 47 , 49 and one study used bootstrapping to identify ranges for PSA. 44
3.2. Quality assessment
In the clinical SLR, the only RCT identified was considered to have an overall high risk of bias using the Cochrane RoB 2 checklist 28 (Table S10). Of the two cohort studies included, one was considered to have a low risk of bias, 44 and the other, a serious risk ,45 using the Cochrane ROBINS‐I checklist 29 (Table S11). Using the JBI checklist, 30 results for the case series were highly variable for domains 3, 5, 6 and 9 (Table S12). Most of the case series scored ‘yes’ for domain 1 (7/10 studies), domain 7 (8/10 studies), domain 8 (7/10 studies) and domain 10 (7/10 studies), while most scored ‘no’ for domain 4 (8/10 studies), and ‘unclear’ for domain 2 (8/10 studies) (note, the conference abstract 39 was not quality assessed). Of the individual studies, van Leen et al. 40 was the highest quality, scoring mostly ‘yes’ (8/10 domains), while Lloyd‐Jones et al. 37 and Faucher et al. 42 were the lowest, scoring ‘yes’ for only 3/10 domains. The remaining studies scored ‘yes’ for 4 32 , 33 , 36 or 5 34 , 35 , 38 , 41 out of the 10 domains.
For the cost‐effectiveness SLR, results for the Drummond checklist were heterogeneous across the various domains. However, most studies were of fairly high quality, scoring mostly ‘yes’ for the 36 questions (ranging between 23 48 and 30 46 answers of ‘yes’). Barrett 34 and Hermans et al. 45 both scored lower, with only 12 and 8 answers of ‘yes’ and 10, and 13 answers of ‘no’, respectively. However, for these studies, several questions were scored as ‘not applicable’ (10 and 13, respectively) (Table S13).
3.3. Efficacy outcomes
3.3.1. Ulcer closure
Only three studies identified in the SLR included outcomes related to ulcer closure. 36 , 40 , 44 The identified studies provide an overview of the evolution of ulcer closure parameters following the use of superabsorbent dressings on various chronic ulcers. One case series, investigating a superabsorbent dressing for patients with exuding ulcers of various etiologies, reported the rate of partial ulcer closure (defined in the study as ‘almost healed’) to be 43%, and complete ulcer closure to be 20% at the end of the study. 36 Complete wound closure rates were reported for six wound types (haematoma ulcer, lymphatic leak caused by traumatic leg wound, lymphatic ulcer, postoperative ulcer, skin tear, VLU). 36 Another, retrospective cohort study, investigating several superabsorbent dressings for the treatment of VLUs, reported higher rates for complete ulcer closure (from 39% to 56%) over a period of 6 months. 44 The mean time to complete ulcer closure ranged from 2.1 to 3.3 months. Another case series investigating the effect of superabsorbent dressings on non‐healing ulcers reported that of the 11 patients with a PU, one healed in Week 4, one in Week 5 and two in Week 6. 40 The study did not report on complete healing for the 16 patients with LUs. 40
3.3.2. Dressing properties
A total of 10 studies reported outcomes related to dressing change. One study evaluating 16 patients with various chronic wounds (12 VLUs, 2 chronic ulcers associated with lymphoedema, 1 PU, 1 deep dermal burn) treated with a superabsorbent dressing up to 4 weeks found that patients required 15 fewer dressing changes with the superabsorbent dressing versus baseline at Week 2, which increased to 44 fewer changes at Week 4. 32 Verrall et al, 2010, an observational evaluation of 19 patients with highly exuding ulcers (16 VLUs, 1 PU, 1 arterial ulcer, 1 chest ulcer), reported that throughout the 4‐week observation period, the average number of dressing changes reduced from 3.2 per week before the study to 1.8 per week after the introduction of the superabsorbent dressing. 41 Faucher et al. 42 reported that a reduction in dressing change frequency from once daily to twice weekly was obtained in 12 (80%) cases after only 3 days of treatment with a superabsorbent dressing. Another study reported that using a superabsorbent dressing reduced the frequency of dressing changes in all 12 patients, sometimes from daily to twice per week (daily: n = 5; every other day n = 4; every 2–3 days: n = 3). 39
Hermans et al. 45 reported the average number of dressing changes was 13 in the superabsorbent dressing group (23 patients with 26 ulcers) and 12 in the negative pressure wound therapy (NPWT) group (15 patients with 16 ulcers). Most ulcers evaluated in the retrospective study were either PUs or postsurgical ulcers. This average was lower in the subpopulation of patients without non‐contact, low‐frequency ultrasound (NFLU) adjunct (10 vs. 6 in each group, respectively). Panca et al. 44 found that among 439 patients with highly exuding chronic VLUs of ≥3 months of age, over 6 months, patients' dressings were changed every 2–4 days on average.
In a prospective, non‐comparative clinical study on exuding ulcers, Atkin et al., 2020 reported a mean (standard deviation [SD]) time between the superabsorbent dressing change of 3.7 (2.2) days and median (range) of 3 (1–21) days. 33 The wear time distribution was <4 days, 58.1%; 4–5 days, 30.6%; and >5 days, 11.2%. Another prospective, non‐comparative clinical study investigating the use of a superabsorbent polymer wound dressing for exudate management reported that over the evaluation period, no patients had their dressings changed twice or several times per day, while 8% had their dressing changed once per day, 36% every 2 days, 42% every 3 days, 4% every 4 days, 4% every 5 days, 2% weekly and 2% other. 34 The dressing change frequency with treatments used prior to study inclusion was several times a day (6%), twice daily (4%), once daily (48%), every 2 days (18%), every third day (20%), with 0 every 4, 5 or 7 days. 34 The median time between dressing change was 3 days (within a range of 1.5–11 days). 34 In Barrett 2020, the mean frequency of dressing change over the evaluation period was several times daily, 0%; twice daily, 2%; daily, 12%; every 2 days, 30%; every 3 days, 44%; every 4 days, 12%. 35 The wear time distribution was <4 days, 70.2%; 4–5 days, 21.1%; and >5 days, 8.7%. Before study inclusion, 2% of patients had their dressing changed several times daily, 12% twice daily, 15% daily, 27% every 2 days, 44% every 3 days and 0 every 4 days. When using a superabsorbent wound dressing with a silicone adhesive interface, there was a shift to longer wearing time, with 72% of patients changing their dressing every third day or longer. 35
3.3.3. Hospital‐related outcomes
None of the identified studies reported length of stay, readmissions, skin grafts or surgical debridement.
3.3.4. Infection‐related outcomes
Four publications, 32 , 34 , 41 , 43 including the only identified RCT, investigated colonisation with antimicrobial resistant pathogens or clinical signs and symptoms of infection during the treatment period; all reported that zero patients had clinical signs of ulcer infection. Verral et al., 2010 reported that during the 4‐week treatment period with a superabsorbent dressing there was no incidence of clinical infection among 19 patients with VLUs, PUs, arterial ulcers, and chest ulcers. 41 Clinical signs of colonisation were assessed visually in the ulcers. Barrett 34 reported that 40.6% of subjective assessments of included ulcers noted ulcer odour, 28.4% an infection and 34.2% critical colonisation. Nearly half of the included ulcers presented some kind of infection‐related signs: redness (23.2%), edema (14.9%) or friable tissue (10.2%). Over an observation period of 2 weeks, odour was eliminated in 22% of patients and infection parameters in 10% of patients.
Only one study evaluating patient outcomes related to the use of different superabsorbent dressings reported antibiotic use, which decreased for all groups of patients using superabsorbent dressings over the study period; the mean change from baseline ranged from −19% to −32%. 44 At the time of inclusion, all patients were using antibiotics to support ulcer treatment.
3.3.5. Other clinical efficacy endpoints
The primary endpoint in the only identified RCT 43 was the reduction in ulcer area, which was not included in the PICOS. In total, 77 patients were randomised either to treatment with a superabsorbent dressing or a non‐adhesive hydrocellular foam dressing. In the superabsorbent dressing group the mean ulcer area reduction was 1.96 cm2 versus 0.76 cm2 in the foam dressing group (mean difference of −1.19 cm2 [95% CI: –1.68, −0.72]; p < 0.001, equating to a mean percentage difference of 29.8% [95% CI: 22.0, 37.6]; p < 0.001). 43 The mean reduction was faster with the superabsorbent dressing (0.45 cm2 per day) compared with the foam dressing (0.2 cm2 per day; p = not significant). Three other studies also reported this endpoint. In an observational study of 19 patients with highly exuding ulcers treated with a superabsorbent dressing, a mean ulcer size reduction of 7.92 cm 2 was observed over a 4‐week period. 41 Hermans et al. 45 reported a relative ulcer size reduction of 42% and 33% from baseline with a superabsorbent dressing and NPWT, respectively. Panca et al. 44 reported the change from baseline in the size of unhealed ulcers at 6 months; in the carbomethylcellulose (CMC) dressing group the size of unhealed ulcers increased by 43%, while all superabsorbent dressings groups had a reduction ranging from −22% to −53%. A clear downward trend in an ulcer surface area reduction was observed in patients with PUs (11 patients) as well as LUs (20 patients). Mean surface area of PUs decreased from 15.27 cm2 in Week 0 to 7.63 cm2 in Week 8, while mean surface area of LUs decreased from 19.43 cm2 in Week 0 to 7.19 cm2 in Week 8.
In one multicenter case series assessing the application and progression of ulcer healing in patients receiving a superabsorbent dressing, a Pressure Ulcer Scale for Healing (PUSH) was evaluated over the 8‐week observation period. 40 The PUSH score categorises the ulcers and generates a result with reference to: ulcer surface area (length × width), level of exudate (none, light, moderate, heavy), and type of tissue present in the ulcer (closed, epithelial tissue, granulation tissue, exudate, necrotic tissue). The mean PUSH scores decreased from 11.05 in week 0 to 5.0 in week 8 following the application of the absorbent dressings to all patients with PUs. 40
3.4. Safety outcomes
Only three publications reported on adverse events (AEs). Faucher et al. 42 investigating a superabsorbent dressing in a prospective non‐comparative study, reported zero all‐cause AEs during the test period. Münter et al. 38 investigating another superabsorbent dressing in a clinician's survey evaluation of 171 patients noted zero dressing‐related AEs during the test period. Another retrospective study of 439 patients with highly exuding chronic VLUs of ≥3 months of age which evaluated outcomes of treatment with one CMC and four superabsorbent dressings, reported mortality rates ranging from 1% to 5% within the patient groups. 44
In total, seven studies reported outcomes related to pain (Table 4), of which six used a visual analogue scale (VAS) as their outcome metric. Of the publications reporting the change from baseline in VAS pain score, the mean score was reduced in all three studies, 33 , 40 , 45 with the largest reduction reported by Hermans et al. 45 Patients in the superabsorbent dressing group experienced a reduction in average pain level of –3.1 from baseline, versus –0.5 in the vacuum‐assisted closure (VAC) therapy group. 45 In van Leen et al. 40 a case series investigating the influence of superabosrbent dressings on non‐healing ulcers, patients with PUs experienced a reduction in background pain of –1.77 and –3.02 at 4 and 8 weeks from baseline, respectively. They also experienced a reduction in pain at dressing change (4 weeks: –1.85; 8 weeks: –2.48). In patients with LUs, the change from baseline in mean VAS score for background pain was –0.75 (4 weeks) and –1.55 (8 weeks), and was reduced by –0.85 (4 weeks) and –2.1 (8 weeks) at dressing change. In Atkin et al 33 the mean change from baseline in VAS pain score with a superabsorbent dressing, at or between dressing changes was −1.1 and −0.5, respectively.
TABLE 4.
Studies reporting outcomes related to pain (clinical SLR).
| Publication (year) | Population | Intervention | Timepoint/cut‐off | Pain outcome metric | Total n (%) | Mean score (SD) | Median (lower range, upper range) | Mean CFB |
|---|---|---|---|---|---|---|---|---|
| Allymamod (2011) 32 | LUs | DryMax Extra | 4 weeks | Numeral rating scale of 1 (no pain)–5 (excruciating pain) at dressing changes at Weeks 1–4 |
Score of 1: 3 (NR) Score of 2: 11 (NR) Score NR: 2 (NR) |
– | NR (1, 2) | – |
| Atkin (2020) 33 | Mixed aetiology ulcers, VLUs, and DFUs | Zetuvit Plus Silicone | 2 weeks | VAS pain score at dressing change (scale NR) | – | 1.4 (2) | – | –1.1 |
| VAS pain score between dressing changes (scale NR) | – | 1.4 (1.7) | – | –0.5 | ||||
| Barrett (2018) 35 | VLUs, PUs, and DFUs | Zetuvit Plus | 2 weeks | % of patients with the same VAS pain score at dressing change | NR (56%) | – | – | – |
| % of patients with reduced VAS pain score at dressing change | NR (38%) | – | – | – | ||||
| % of patients with increased VAS pain score at dressing change | NR (4%) | – | – | – | ||||
| % of patients with the same VAS pain score between dressing changes | NR (60%) | – | – | – | ||||
| % of patients with reduced VAS pain score between dressing changes | NR (32%) | – | – | – | ||||
| % of patients with increased VAS pain score between dressing changes | NR (8%) | – | – | – | ||||
| Hindhede (2012) 36 | VLUs, PUs, AU, hematoma ulcer, postoperative wound, lymphatic leak caused by traumatic leg wound, lymphatic ulcer, mixed aetiology LU, posttraumatic wound/venous hypertension, skin tear, ulcer caused by herpes zoster | DryMax Extra | – | Dressing removal pain | 1 (NR) | – | – | –10 a |
| Lloyd‐Jones (2011) 37 | PUs | Eclypse Adherent Sacral | – | VAS pain score, 0–10 scale | – | – | NR (0, 3) | – |
| van Leen (2014) 40 | PUs | Sorbion sachet S, Sorbion Sana | 4 weeks | Background pain (VAS pain score, 0–10 scale) | – | 1.92 | – | −1.77 |
| 8 weeks | Background pain (VAS pain score, 0–10 scale) | – | 0.67 | – | −3.02 | |||
| 4 weeks | Pain at dressing change (VAS pain score, 0–10 scale) | – | 1.38 | – | −1.85 | |||
| 8 weeks | Pain at dressing change (VAS pain score, 0–10 scale) | – | 0.75 | – | −2.48 | |||
| LUs | Sorbion sachet S, Sorbion Sana | 4 weeks | Background pain (VAS pain score, 0–10 scale) | – | 2.7 | – | −0.75 | |
| 8 weeks | Background pain (VAS pain score, 0–10 scale) | – | 1.9 | – | −1.55 | |||
| 4 weeks | Pain at dressing change (VAS pain score, 0–10 scale) | – | 2.55 | – | −0.85 | |||
| 8 weeks | Pain at dressing change (VAS pain score, 0–10 scale) | – | 1.3 | – | −2.1 | |||
| Hermans (2015) 45 | VLUs, PUS, DFUs and surgical wounds |
Sorbion sachet S Sorbion Sana |
Days, average (range): 29.2 (7–104) | VAS pain score, 0–10 scale | – | 0.6 | – | −3.1 |
| VAC therapy NPWT | Days, average (range): 27.3 (8–55) | – | 0.2 (NR) | – | −0.5 |
Abbreviations: AU, arterial ulcer; CFB, change from baseline; DFU, diabetic foot ulcer; LU, leg ulcer; NPWT, negative pressure wound therapy; NR, not reported; PU, pressure ulcer; SD, standard deviation; VAC, vacuum‐assisted closure; VAS, visual analogue scale; VLU, venous leg ulcer.
Total, n (median).
3.5. Economic outcomes
In three cost‐utility analyses for the UK, it has been shown that superabsorbent dressings lead to more favourable health outcomes in terms of healing rates and QoL. 44 , 46 , 48 Simultaneously, the cost of care is reduced compared with a mix of other dressings. In a cost‐description analysis from the UK, Barrett 2018, found that treating 10 patients for 2 weeks with a superabsorbent dressing led to cost savings of £1179, compared with patients' total costs in the 2 weeks before the start of the study. 34
Similarly, a cost‐utility analysis for Germany demonstrated that when compared with a mix of dressings for moderate to high exuding leg ulcers, treatment with superabsorbent dressings leads to more favourable health outcomes and reduced cost of care. 47
In a cost‐utility analysis investigating patients with VLUs in a French setting, a superabsorbent dressing was compared with foam dressings and demonstrated dominance (better health outcomes with reduced cost of care). 49
In the only cost‐comparison analysis identified for the USA, Hermans et al. 45 demonstrated that the cost savings from using superabsorbent over NPWT were $44.13 per percentage surface area reduction and $20.79 per volume reduction.
In the studies reporting dominant treatment strategies, the results of the sensitivity analyses demonstrated that the base‐case results were maintained in all scenarios. 44 , 46 , 47 , 48 , 49
4. DISCUSSION
This manuscript is, to our knowledge, the first reporting the results of SLRs undertaken to identify clinical and cost‐effectiveness evidence for superabsorbent wound dressings for the treatment of chronic ulcers.
4.1. Discussion of clinical SLR results
SLR identified eight different superabsorbent dressings Zetuvit Plus (Silicone Border), Sorbion sachet S, DryMax Extra, Flivasorb, Vliwasorb, Eclypse Adherent Sacral and Kerramax. However, the aim was focused on examining the ‘class’ effect of all superabsorbers, not any particular dressing, and there was no possibility to have a reasonable indirect treatment comparison.
In the clinical SLR, only one RCT was identified, comparing a superabsorbent charcoal dressing with a silver foam dressing. 43 Most of the other identified studies are case series (n = 11), making it difficult to draw any firm conclusions due to the absence of a comparator arm. In addition, the studies are heterogeneous in their intervention(s), patient population and the outcomes reported. Overall, the majority of existing clinical evidence is of low quality; the only RCT, 43 and one of the two identified cohort studies, 45 were considered to be at high or serious risk of bias, respectively. Quality assessment of the case series revealed that it was unclear for most studies whether the conditions were measured in a standard or reliable way, and most studies did not use consecutive sampling. Results were also highly variable between studies for whether valid methods for identification of the condition were used, whether there was complete inclusion of participants, and whether the participant and presenting site(s)/clinic(s) demographics were clearly reported. van Leen et al. 40 was the highest quality case series, scoring ‘yes’ for most checklist domains (8/10), while Lloyd‐Jones et al., 37 and Faucher et al., 42 scored mostly ‘no’ or ‘unclear’, with only 3 answers of ‘yes’. The remaining case series were also of low quality, only scoring ‘yes’ for 4 or 5 out of the 10 domains.
As the evidence base identified from the SLR was comprised of only one RCT and one retrospective comparative analysis, there were insufficient studies of sufficient quantity and quality to be included in a formal statistical analysis. Case series are not suitable for inclusion in quantitative analysis as they are often associated with weak inferences and high likelihood of bias. In addition, non‐comparative case series would not provide a measure of relative treatment effect, such as odds ratios or relative risks.
Only three studies reported outcomes related to ulcer closure, 36 , 40 , 44 one of which investigated a CMC and several different superabsorbent wound dressings for patients with VLUs. The percentage of healed ulcers at 6 months ranged from 39% to 56%. The distribution of ulcer healing rates between the dressings was similar: 56% for the CMC dressing and between 39% and 55% for superabsorbent dressings. 44 This study also reported other outcomes of interest, including the number of mean dressing changes, the mean time between dressing changes, the mean reduction in antibiotic use, and mortality. Interestingly, for all of these outcomes, the difference between groups was not statistically significant (except for the reduction in antibiotic use [DryMax Extra (superabsorbent dressings) vs. other groups, p < 0.005]). Interestingly, although CMC had the highest percentage of healed ulcers (56%), unhealed ulcers increased in size with CMC (by 43%), while the size of unhealed ulcers reduced with all other interventions with superabsorbent dressings (ranging from –53% to –20%; CMC vs. other groups, p < 0.001).
Eleven studies reported outcomes related to dressing change, four of which reported that the number of dressing changes were reduced when using a superabsorbent dressing versus baseline. 32 , 39 , 41 , 42 In addition, Barrett, 34 reported most patients (78%) required their dressings to be changed only once every 2 or 3 days, while 95% of the dressing changes were scheduled changes, and only 4.5% were unscheduled changes due to ulcer observations, exudate handling or strikethrough. Barrett et al., 35 reported a shift to extended wear time with use of the superabsorbent wound dressing, with 72% of dressing changes being every third day or longer. In both studies, wear times appeared to increase during the evaluation period compared with those observed before studies commenced.
Several studies reported other clinical outcomes of interest including infection‐related outcomes, 32 , 41 , 43 ulcer area reduction, 41 , 43 , 44 , 45 AEs 38 , 42 , 44 and pain‐related outcomes. 32 , 33 , 40 , 45
In two publications, the clinical signs and symptoms of infection were evaluated visually by subjective judgement of the treating clinician. 32 , 41
All identified studies that evaluated the ulcer surface area reduction parameter reported a reduction in ulcer surface area when using superabsorbent dressings. The ulcer area reduction parameters were reported either as absolute changes or as relative changes. The studies evaluating outcomes related to pain reported reduction in mean VAS pain score for background pain 40 , 45 or pain related to the dressing change. 33 , 40
4.2. Discussion of cost‐effectiveness SLR results
All cost‐effectiveness studies were evaluated using the Drummond checklist. In general, the cost‐utility analyses were very well reported, particularly compared with the cost‐comparison analyses, although some questions in the checklist are not relevant to cost‐comparison analyses.
In some of the studies, results were reported as cost per percentage surface area reduction and per volume reduction. The average of the reduction alone does not have any clinical significance, as it does not differentiate between a clinically successful reduction and clinically stable disease without referring to the total expenditure or the daily expenditure.
5. CONCLUSIONS
The small number and low quality of studies identified in both the clinical SLR and cost‐effective SLR highlights an obvious need for future research on the clinical and cost‐effectiveness of superabsorbent wound dressings for the treatment of chronic ulcers. The clinical SLR demonstrates a complete gap in the literature for studies investigating outcomes related to hospital length of stay, readmissions, skin grafts, and surgical debridement, while there was a paucity of evidence for ulcer closure and safety outcomes. The low quality of the available evidence meant that it was not possible to perform any statistical analyses of the extracted data.
Evidence from case series indicates that the use of superabsorbent wound dressings may result in favourable outcomes, including reductions in both the frequency of dressing changes and pain scores. Furthermore, cost‐utility studies indicate superabsorbent wound dressings are a more cost‐effective option for the treatment of chronic ulcers compared with standard dressings.
These SLRs were conducted to a high standard, with a comprehensive search strategy that included validated published search filters and incorporated a wide range of medical databases and grey literature sources. The clear, robust methodology used forms a reliable basis for future SLR updates.
CONFLICT OF INTEREST STATEMENT
Dr Szijártó Attila, Dr Dávid Bárdos, Dr YunNan Lin, Dr HaoYu Chiao disclose no conflict of interest in relation to this research.
Supporting information
Data S1. Supporting information.
M. Veličković V, Macmillan T, Lones E, et al. Systematic review and quality assessment of clinical and economic evidence for superabsorbent wound dressings in a population with chronic ulcers. Int Wound J. 2024;21(3):e14750. doi: 10.1111/iwj.14750
Tom Macmillan, Isobel Munro, Neil Webb, and Dr Emma Lones are employees of Source Health Economics which received consultancy fees for the systematic literature review. Amy Crompton was an employee of Source Health Economics at the time the systematic literature review was conducted. Dr Vladica M. Veličković, Dr Viviane Fernandes Carvalho, and Yana Arlouskaya are employees of HARTMANN GROUP. Pablo Arija Prieto was an intern student at HARTMANN GROUP at the time the systematic literature review was conducted. Dr Sebastian Probst is part of the HARTMANN GROUP advisory board for development of digital health applications.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Schultz GS, Chin GA, Moldawer L, Diegelmann RF. Principles of wound healing. In: Fitridge R, Thompson M, eds. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists. Adelaide; 2011. [Google Scholar]
- 2. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9):560‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17(12):2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Docking R, Boateng J, Catanzano O, Schofield P. A preliminary study of pain relieving dressings for older adults with chronic leg ulcers from the provider's perspective: a qualitative study. J Pain Palliat Care Pharmacother. 2018;32(2–3):71‐81. [DOI] [PubMed] [Google Scholar]
- 5. World Union of Wound Healing Societies (WUWHS) . Wound Exudate, Effective Assessment and Management. Wounds International; 2019. Accessed July 18, 2023. https://www.woundsinternational.com/resources/details/wuwhs‐consensus‐document‐wound‐exudate‐effective‐assessment‐and‐management [Google Scholar]
- 6. Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro‐wound healing phenotypes. Front Physiol. 2018;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7(6):442‐452. [DOI] [PubMed] [Google Scholar]
- 8. Wysocki AB, Staiano‐Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP‐2 and MMP‐9. J Invest Dermatol. 1993;101(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 9. Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107(5):743‐748. [DOI] [PubMed] [Google Scholar]
- 10. Gibson D, Cullen B, Legerstee R, Harding KG, Schultz G. MMPs made easy. 2009. Accessed July 18, 2023. https://www.woundsinternational.com/resources/details/mmps-made-easy
- 11. Wu YK, Cheng NC, Cheng CM. Biofilms in chronic wounds: pathogenesis and diagnosis. Trends Biotechnol. 2019;37(5):505‐517. [DOI] [PubMed] [Google Scholar]
- 12. Demidova‐Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care. 2012;25(7):304‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan R, Yu F, Strandlund K, Han J, Lei N, Song Y. Analyzing factors affecting quality of life in patients hospitalized with chronic wound. Wound Repair Regen. 2021;29(1):70‐78. [DOI] [PubMed] [Google Scholar]
- 14. Tickle J. Wound exudate: a survey of current understanding and clinical competency. 2016. [DOI] [PubMed]
- 15. Tickle J. Living day‐to‐day with a heavily exuding wound: recommendations for practice. Wound Essentials. 2013;8(1):77‐81. [Google Scholar]
- 16. Olsson M, Jarbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen. 2019;27(1):114‐125. [DOI] [PubMed] [Google Scholar]
- 17. Romanelli M, Vowden K, Weir K. Exudate Management Made Easy. Wounds International; 2010. Accessed February 2023. http://www.woundsinternational.com [Google Scholar]
- 18. Wiegand C, Tittelbach J, Hipler U, Elsner P. Clinical efficacy of dressings for treatment of heavily exuding chronic wounds. 2015.
- 19. Browning P, White R, Rowell T. Comparative evaluation of the functional properties of superabsorbent dressings and their effect on exudate management. 2016. [DOI] [PubMed]
- 20. Wiegand C, Abel M, Muldoon J, Ruth P, Hipler U. SAP‐containing dressings exhibit sustained antimicrobial effects over 7 days in vitro. 2013. [DOI] [PubMed]
- 21. Wiegand C, Hipler U. A superabsorbent polymer‐containing wound dressing efficiently sequesters MMPs and inhibits collagenase activity in vitro. 2013. [DOI] [PMC free article] [PubMed]
- 22. Eming S, Smola H, Hartmann B, et al. The inhibition of matrix metalloproteinase activity in chronic wounds by a polyacrylate superabsorber. Biomaterials. 2008;29(19):2932‐2940. [DOI] [PubMed] [Google Scholar]
- 23. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Institute for Health and Care Excellence . The guidelines manual: appendices B‐I. 2012. [PubMed]
- 25. Centre for Reviews and Dissemination . Systematic reviews: CRDs guidance for undertaking reviews in health care. 2009.
- 26. Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. www.training.cochrane.org/handbook
- 27. EUnetHTA . Methodological Guidelines: Process of Information Retrieval for Systematic Reviews and Health Technology Assessments on Clinical Effectiveness. Version 2.0. 2019.
- 28. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 29. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132‐140. [DOI] [PubMed] [Google Scholar]
- 31. Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313(7052):275‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allymamod A. Evaluation of a 16‐patient study using DryMax extra in four leg ulcer clinics. Wounds UK. 2011;7(4):57‐61. [Google Scholar]
- 33. Atkin L, Barrett S, Chadwick P, et al. Evaluation of a superabsorbent wound dressing, patient and clinician perspective: a case series. 2020. [DOI] [PubMed]
- 34. Barrett S. An observational study of a superabsorbent polymer dressing evaluated by clinicians and patients. J Wound Care. 2018;27(2):91‐100. [DOI] [PubMed] [Google Scholar]
- 35. Barrett S, Rippon M, Rogers A. Treatment of 52 patients with a self‐adhesive siliconised superabsorbent dressing: a multicentre observational study. 2020. [DOI] [PubMed]
- 36. Hindhede A, Meuleneire F. A clinical case‐series evaluation of a superabsorbent dressing on exuding wounds. J Wound Care. 2012;21(11):574‐582. [DOI] [PubMed] [Google Scholar]
- 37. Lloyd‐Jones M. The role of Eclypse Adherent Sacral® in managing sacral pressure ulcers. Br J Community Nurs. 2011;16:S38‐S42. [Google Scholar]
- 38. Münter K‐C, De Lange S, Eberlein T, Andriessen A, Abel M. Handling properties of a superabsorbent dressing in the management of patients with moderate‐to‐very high exuding wounds. J Wound Care. 2018;27(4):246‐253. [DOI] [PubMed] [Google Scholar]
- 39. Tickle J, Gregory L. Case series: the management of moderate to high exudate in chronic wounds. Br J Nurs. 2013;22:P19. [Google Scholar]
- 40. van Leen M, Rondas A, Neyens J, Cutting K, Schols JM. Influence of superabsorbent dressings on non‐healing ulcers: a multicentre case series from The Netherlands and the UK. J Wound Care. 2014;23(11):543‐550. [DOI] [PubMed] [Google Scholar]
- 41. Verrall D, Coulborn A, Bree‐Aslan C. Evaluating a super absorbent dressing (Flivasorb) in highly exuding wounds. Br J Nurs. 2010;19(7):449‐453. [DOI] [PubMed] [Google Scholar]
- 42. Faucher N, Safar H, Baret M, Philippe A, Farid R. Superabsorbent dressings for copiously exuding wounds. Br J Nurs. 2012;21(12 Suppl):S22‐S28. [DOI] [PubMed] [Google Scholar]
- 43. Probst S, Saini C, Rosset C, Skinner MB. Superabsorbent charcoal dressing versus silver foam dressing in wound area reduction: a randomised controlled trial. J Wound Care. 2022;31(2):140‐146. [DOI] [PubMed] [Google Scholar]
- 44. Panca M, Cutting K, Guest JF. Clinical and cost‐effectiveness of absorbent dressings in the treatment of highly exuding VLUs. J Wound Care. 2013;22(3):109‐118. [DOI] [PubMed] [Google Scholar]
- 45. Hermans MH, Kwon Lee S, Ragan MR, Laudi P. Results of a retrospective comparative study: material cost for managing a series of large wounds in subjects with serious morbidity with a hydrokinetic fiber dressing or negative pressure wound therapy. Wounds. 2015;27(3):73‐82. [PubMed] [Google Scholar]
- 46. Veličković VM, Chadwick P, Rippon MG, et al. Cost‐effectiveness of superabsorbent wound dressing versus standard of care in patients with moderate‐to‐highly exuding leg ulcers. J Wound Care. 2020;29(4):235‐246. [DOI] [PubMed] [Google Scholar]
- 47. Veličković VM, Szilcz M, Milosevic Z, Godfrey T, Siebert U. Cost‐effectiveness analysis of superabsorbent wound dressings in patients with moderate‐to‐highly exuding leg ulcers in Germany. Int Wound J. 2021;19:447‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walzer S, Droschel D, Vollmer L, Atkin L, Ousey K. A cost‐effectiveness analysis of a hydration response technology dressing in the treatment of venous leg ulcers in the UK. J Wound Care. 2018;27(3):166‐172. [DOI] [PubMed] [Google Scholar]
- 49. Veličković VM, Prieto PA, Krga M, Jorge AM. Superabsorbent wound dressings versus foams dressings for the management of moderate‐to‐highly exuding venous leg ulcers in French settings: an early stage model‐based economic evaluation. J Tissue Viability. 2022;31(3):523‐530. [DOI] [PubMed] [Google Scholar]
- 50. Clegg JP, Guest JF. Modelling the cost‐utility of bio‐electric stimulation therapy compared to standard care in the treatment of elderly patients with chronic non‐healing wounds in the UK. Curr Med Res Opin. 2007;23(4):871‐883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


