Summary
Background
Each year, five million people are left disabled after stroke. Upper-extremity (UE) dysfunction is a leading problem. Neuroplasticity can be enhanced by non-invasive brain stimulation (NIBS) but evidence from large, randomized multicenter trials is lacking. We aimed at demonstrating efficacy of NIBS to enhance motor recovery after ischemic stroke.
Methods
We randomly assigned patients to receive anodal transcranial direct current (tDCS, 1 mA, 20 min) or placebo stimulation (‘control’) over the primary motor cortex of the lesioned hemisphere in addition to standardized rehabilitative training over ten days in the subacute phase after stroke. The original study was planned to enrol 250 but, following a blinded interim analysis, ended with 123 participants. The primary outcome parameter was UE impairment, measured by UE-Fugl-Meyer-Assessment (UEFMA), one to seven days after the end of the treatment intervention (ClinicalTrials.gov, NCT00909714).
Findings
From 2009 to 2019, 123 patients were included, with 119 entering intention-to-treat analysis (ITT). The control group (N = 61) improved 8.9 (SD 7.7) UEFMA points, the tDCS group (N = 58) improved 9.0 (8.8) points. ITT was neutral with respect to the primary efficacy endpoint (p = 0.820). We found no difference in UEFMA change between active tDCS and control. The safety profile of tDCS was favorable. In particular, there were no seizures.
Interpretation
In patients with ischemic stroke, anodal tDCS applied to the motor cortex of the lesioned hemisphere over 10 days in the subacute phase was safe but did not improve the recovery of upper extremity function compared with placebo stimulation.
Funding
Deutsche Forschungsgemeinschaft (GE 844/4-1).
Keywords: Stroke, Recovery, Brain stimulation, Neuroplasticity, Neurorehabilitation, Clinical trial
Research in context.
Evidence before this study
We searched PubMed using the term “tDCS direct-current stroke recovery”. 494 results were obtained between 2005 and 2023 (as of September 8, 2023). These results were screened for original studies, systematic reviews, and meta-analyses focusing on non-invasive brain stimulation by means of transcranial direct current stimulation (tDCS) to improve recovery after stroke. Proof-of-principle trials have suggested that especially anodal tDCS could improve sensorimotor functions, and have raised expectations in patients, relatives, and therapists. However, the translation into clinical application is still pending. Publication biases concern small sample sizes, monocenter designs, and variability in outcomes. A Cochrane database analysis of 67 studies involving a total of 1729 patients after stroke found only very low to moderate evidence of any effectiveness of tDCS to improve functional outcomes. Sufficiently controlled and powered prospective, randomized multicenter trials in the acute or subacute phase after stroke are not available.
Added value of this study
The results of this randomized clinical trial show that anodal tDCS (1 mA) applied to the ipsilesional motor cortex in subacute stroke patients, combined with standardized rehabilitative training over 10 days, is a safe intervention. However, the primary outcome, an improvement in the Upper-Extremity-Fugl-Meyer-Assessment, 1–7 days after the end of the treatment intervention, was not different between the intervention and control groups.
Implications of all the available evidence
This trial clarifies that 1 mA of anodal tDCS, combined with intensive training, is not effective for improving functional recovery of the upper limb in subacute stroke patients. The results do not preclude that higher stimulation intensities, stimulation in different time windows relative to the stroke, stimulation of more severely impaired patients, or stimulation adapted to individual patient characteristics might be effective.
Introduction
Stroke is a major cause of death and one of the leading causes of impairment worldwide. Motor impairment occurs in approximately 80% of all stroke patients and is associated with persistent disability and dependence in more than 30% of these cases.1 Accordingly, stroke causes a majority of disability-adjusted life years, which will continue to be a global burden due to an aging society.2
Ischemic lesions induce changes of brain metabolism, functional activation, neuronal excitability, and structure. The amount of this spontaneous reorganization of neuronal circuits and brain areas often fails to enable functional recovery.3 Lesion-function analyses after stroke have pointed to areas that seem to be particularly important in the recovery process. For upper-extremity (UE) motor function, these areas include, among others, the primary motor cortex (M1) of the lesioned hemisphere.4,5
The core concept of the Neuroregeneration Enhanced by Transcranial direct current stimulation in Stroke (NETS) trial was to enhance neuroplasticity in the M1 of the stroke hemisphere by anodal transcranial direct current stimulation (tDCS), a non-invasive brain stimulation (NIBS) method which is simple to apply and potentially feasible for routine use in neurorehabilitation. Proof-of-principle trials have suggested that NIBS, especially anodal tDCS, could improve sensorimotor function.6 An encouraging meta-analysis of those studies suggested a small but significant effect for tDCS on motor function when applied to patients after stroke but also provided evidence for publication bias.7 Sufficiently controlled and powered randomized multicenter trials in the acute or subacute phase after stroke are not available.
The NETS trial aimed to determine if recovery of UE dysfunction can be improved by anodal tDCS to the M1 of the lesioned hemisphere in the subacute phase after stroke.
Methods
Study design
NETS was an investigator-initiated, multicenter, randomized, double-blind, placebo-controlled clinical trial of patients with ischemic stroke. The trial was conducted in 11 study centers, including nine centers in Germany and one each in Austria and Italy. Sites were selected if they were experienced in stroke research or rehabilitation. The trial was approved by national or local ethics committees or institutional review boards. The trial protocol was published previously.8
The trial was overseen by a steering committee and an independent Data and Safety Monitoring Board (DSMB; see Supplementary Online Material, SOM). NETS would have been stopped if there had been a medically relevant increase in major, unexpected adverse events (such as seizures) with anodal tDCS compared with placebo stimulation. There was no industry involvement in any aspect of the trial.
Patients
Patients aged ≥18 years whose first clinically overt ischemic non-hemorrhagic stroke occurred five to 45 days ago, i.e., subacute stage, were eligible. Subcortical and cortical strokes could be included. Eligible inpatients were informed about the NETS trial by the local study team. If there was UE hemiparesis, defined as Upper-Extremity-Fugl-Meyer-Assessment (UEFMA) 20–58 (inclusive), active wrist extension of at least 5° or the ability to perform repetitive grasping, and written informed consent was obtained, patients could be included. Patients were allocated to sex categories male or female, no further information regarding gender was collected.
Patients with pre-existing lesions of >1.5 cm (maximum diameter) in a brain area belonging to the anatomically defined sensorimotor system or completely lesioned hand-knob area of M1 were excluded. Exclusion criteria further comprised presence of bilateral motor impairment, alcohol and/or drug abuse, severe psychiatric illness (e.g., schizophrenia), severe language impairment preventing informed consent or adequate evaluation. So were tumor disease with a life expectancy <1 year, increased intracranial pressure, polyneuropathy and/or ischemic peripheral disease (if UE sensorimotor function was impaired in a clinically relevant way), severe cognitive deficits (Mini-Mental State Examination (MMSE), ≤23), pregnancy, or contraindication to MRI (e.g., metallic implants) or to transcranial magnetic stimulation (e.g., epilepsy).
Randomization and masking
A web-based randomization procedure with center-wise block stratification and variable block size was used to allocate patients with a ratio of 1:1 to receive active tDCS (‘intervention’) or placebo stimulation (‘control’). The randomization sequence generation was done by a statistician who was not involved in any other part of the study. Patients, therapists, caregivers, and outcome assessors were blinded to the intervention. The therapists were asked to which group the patient was assigned after the intervention in order to monitor effective blinding (of 119 delivered stimulations, 104 answers were available, of which 58 were true and 46 false, indicating that blinding was successful).
Randomization was stratified by age (<70 years/≥70 years) and lesion type (subcortical/cortical). Both groups (intervention and control) received standardized UE training according to the study protocol. Concomitant treatment was performed according to standard of care.
Procedures
The active intervention consisted in anodal tDCS of 1 mA that was delivered for 20 min through 35 cm2 (5 cm × 7 cm) sponge-electrodes soaked with sodium-chloride solution leading to a current density of 0.03 mA/cm2 (Eldith, Neuroconn, Germany). At the time of designing the study, this stimulation intensity could already be considered safe.6 The anode was centered at C3/4 of the international 10/20 system of EEG electrode placement, near the hand representation area in the M1. This approach had been applied previously and had exerted reliable and durable effects on M1 excitability6,9 as well as some behavioral effects in chronic stroke patients6,10 (see also Supplementary Online Material). The cathode was located over the contralateral supraorbital region. The electrical current was applied with an 8 s fade-in and fade-out interval to attenuate itching sensations. For the placebo condition, anodal tDCS was limited to 40 s duration, a procedure demonstrated to warrant successful blinding.11
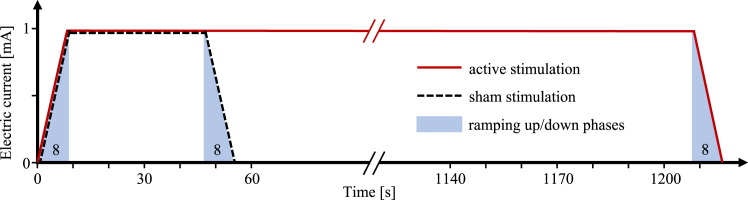
Active or placebo stimulation was applied once daily over two weeks (ten working days) in addition to 45 min of standardized UE function rehabilitative training. Each training session started with onset of tDCS/placebo stimulation so that both treatments were given concurrently for 20 min in the active tDCS and 40 s in control condition (see Fig. 1). The contents of rehabilitative training were described and illustrated in a detailed manual (Supplementary Online Material). Briefly, the therapy content can be described as follows: the 45 min per therapy session were divided into three areas: pre-functional, functional, and activities of daily living (ADL). The duration of each area (or the number of exercises from one area) was allocated according to the individual, functional level of the patient, so most of the therapy session was spent on active practice of functional tasks. This means that the time spent on pre-functional activities at the beginning of the intervention phase is minimized as soon as possible to focus on functional activities with increasing complexity. All therapists and investigators were trained by the Hamburg study team on (i) standardized rehabilitative training, (ii) application of tDCS, and (iii) assessment of outcome measures. Investigators were trained in standardized score collection. After successful completion of the training, the standardization of the recording of the primary outcome was also verified by a video analysis of at least two test runs per rater. Therapists received training on the delivery of standardized therapy and the application of tDCS. They were required to be either physical or occupational therapists. After mounting the electrodes, tDCS was started by entering a code and thus initiating a pre-set, masked program on the stimulator (the respective code was obtained in the randomization procedure).
Fig. 1.
Schematic of the intervention. For both conditions (active tDCS, solid red line, and placebo stimulation, dashed black line, the electrical current was ramped up over 8 s to 1 mA (blue shaded areas indicate ramps). Active tDCS remained at 1 mA for 20 min followed by fade-out over 8 s from 1 mA to 0 mA. Placebo stimulation remained at 1 mA for only 40 s before fading out over 8 s.
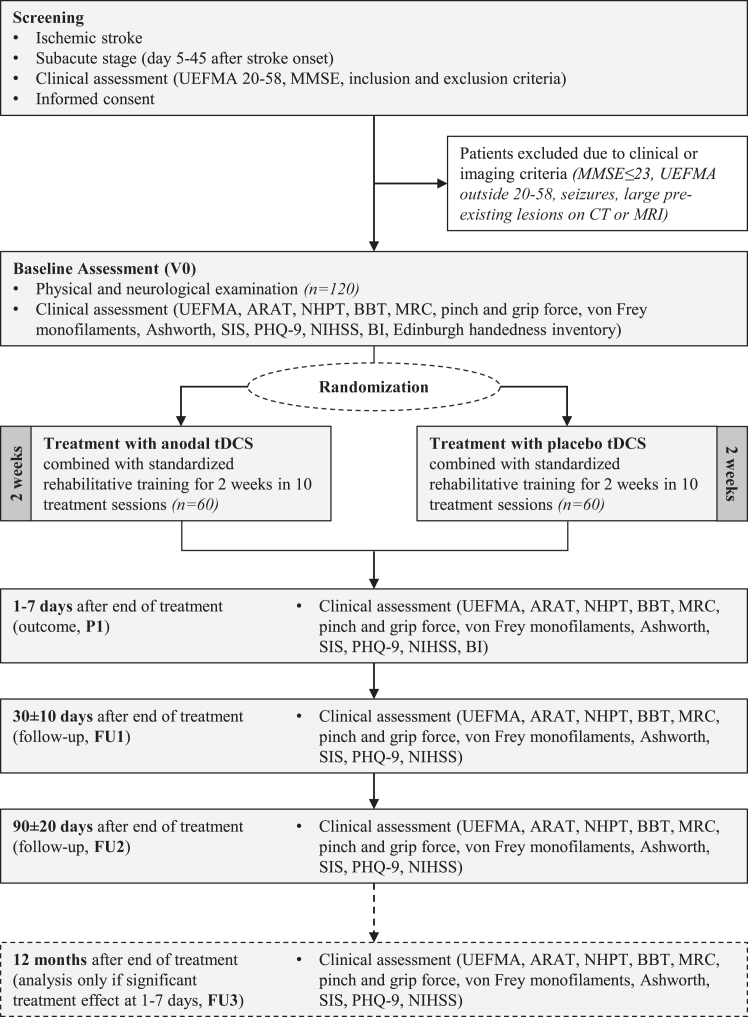
Clinical assessments were performed at baseline (V0), 1–7 days after the end of the treatment intervention (primary outcome, P1), 30 ± 10 days (Follow-Up 1, FU1), and 90 ± 20 days (FU2) after randomization. They comprised standard assessments of demographic characteristics, medical history, neurological and physical examination including Edinburgh handedness inventory, MMSE, National Institutes of Health Stroke Scale (NIHSS) score, Barthel index (BI), UEFMA, action research arm test (ARAT), nine-hole-peg test (NHPT), box-and-block test (BBT), and muscle strength according to Medical Research Council (MRC). Pinch and grip force were measured with a dynamometer, sensory function with von-Frey monofilaments, spasticity of shoulder, elbow, wrist, and finger flexors and extensors were assessed by the Ashworth scale. Further, the Stroke impact scale (SIS) and the short version of the patient health questionnaire (PHQ-9) were used.
In addition, these clinical measures were acquired 12 ± 1 months after randomization (FU3) but with the pre-specified strategy only to analyze them if there were a significant treatment effect at P1, to test for long-term sustainability of potential benefits. For study design and assessments see Fig. 2.
Fig. 2.
Trial design and assessment flow chart. ARAT = action research arm test; Ashworth = Ashworth spasticity scale; BBT = box-and-block test; BI = Barthel Index; CT = computed tomography scan; MMSE = mini-mental state examination; MRC = Medical Research Council; MRI = magnetic resonance image; NHPT = nine-hole peg test; NIHSS = National Institutes of Health stroke scale; PHQ-9 = patient health questionnaire; SIS = stroke impact scale; tDCS = transcranial direct current stimulation; UEFMA = upper-extremity Fugl-Meyer assessment.
In an amendment 2017, some changes regarding endpoint assessment were decided: While the BI was originally planned to be assessed during all follow-ups, the assessment was reduced to V0 and P1 because no relevant information was expected to be gained in later follow-ups. Moreover, at the beginning of the trial there was a further follow-up examination planned at six months. This follow-up was removed to simplify the conduct of the study and facilitate recruitment. It was also specified that the Jebsen-Taylor-hand-function-test would be removed from the test battery due to missing baseline data and to simplify the inclusion process. This amendment was reviewed and approved by the responsible ethics committee.
All data were stored in an electronic Case Report Form. Monitoring was conducted by the Clinical Trial Centre North (Hamburg, Germany) and in compliance with E6 ICH GCP guideline.
Outcomes
The primary efficacy endpoint was the UEFMA at P1 compared with V0 measured at the respective study center.
Secondary efficacy endpoints included UEFMA at 30 ± 10 days and 90 ± 20 days after intervention as well as passive joint motion, ARAT, NHPT, BBT, MRC, pinch and grip force, sensory function, Ashworth spasticity scale of the affected side, SIS, PHQ-9 and the NIHSS at days 1–7, 30 ± 10, and 90 ± 20 after intervention, BI at days 1–7. Moreover, two predefined responder analyses: (a) ‘clinically relevant response’, defined as number of patients exhibiting an UEFMA improvement of ≥5 points (P1 minus V0),12 and (b) ‘compound score response’, defined as UEFMA ≥5 and/or NHPT time improvement of at least 32 s in the affected UE13,14 and/or whole-hand grip-strength improvement ≥5.7 N (always P1 minus V0).15,16
Primary safety endpoint was the incidence of epileptic seizures during the intervention period.
For more details, see Supplementary Online Material.
Statistical analysis
NETS was designed to show superiority of the active tDCS intervention over control. To detect a clinically relevant difference of 5 points in the UEFMA12 with an expected SD of 12.5 UEFMA points,17 a power of 80% and α = 5% with a two-sample, two-sided t-test (calculated with PASS 2008) an effective sample size of 100 patients per group was initially considered necessary. The planned sample size was increased by 25% to adjust for an early drop-out rate of 20%, resulting in a cohort of 250. In the first version of the study protocol, blinded re-assessment of sample size was planned after 80% of the patients had been recruited or if cessation of funding before completion of recruitment could have been anticipated. Due to slow recruitment the blinded re-assessment was already conducted after inclusion of 83 patients of whom 76 already had participated in the first follow-up examination and provided a valid measurement of the primary outcome. The result was a residual variance of 67.8 (61.5 after last-observation-carried forward (LOCF) for missing outcomes in seven patients, as defined in the statistical analysis plan), corresponding to a standard deviation of 8.2, rather than the initially assumed 12.5 points of UEFMA. Based on this information, the sample size was adapted to 2 × 40 patients (80 complete data sets). Considering drop-outs and potentially incomplete data sets, the final sample size was then set to 120. The first version of the statistical analysis plan was prepared in May 2019, and the final version was approved on February 18, 2021 (123 patients randomized). The full history of protocol changes is available at https://clinicaltrials.gov/ct2/history/NCT00909714 and in the protocol paper.8
The primary efficacy endpoint was the UEFMA. The intention to treat (ITT) population consisted of all patients who received at least one session of active or placebo stimulation. All endpoints were analyzed in the respective full analysis set (FAS), which is as close as possible to the ITT population.18 While for missing follow-up measurements, a LOCF procedure was pre-specified (the treatment policy strategy was defined as the estimand strategy used for the intercurrent events of lost to follow up), the FAS for the primary efficacy endpoint consisted of the ITT population after exclusion of two patients in the intervention arm because of missing baseline UEFMA measurement. For the analysis of the primary endpoint an ANCOVA model was calculated using the difference of P1 to V0 UEFMA as response variable, treatment group and type of stroke as factors, and baseline UEFMA, age, and time interval between index event and baseline as covariates. To estimate the treatment effect, the contrast of the mean difference between treatment groups was estimated with a 95% confidence interval (CI). Secondary endpoints were analyzed likewise. Since these analyses were explorative, no adjustment for multiplicity was provided. LOCF was applied for patients lost to follow-up for all endpoints. Additionally, a multiple imputation procedure was conducted as a sensitivity analysis for the primary endpoint. An imputation model following previously published recommendations19,20 was set up with ten repetitions.
Additional pre-specified analyses included a further adjustment of the primary analysis model for ‘severity of stroke’ as measured by the baseline NIHSS score as well as the following subgroup analyses: (i) subcortical stroke vs. stroke involving cortex, (ii) younger vs. older patients (<67 vs. ≥67 years, where 67 years was the median age of the study population), (iii) male vs. female, (iv) mild vs. moderate and severe stroke (NIHSS <5 vs. ≥5), (v) mild vs. moderate and moderately severe UE dysfunction (UEFMA ≥43 vs. UEFMA <43),21 and (vi) smoker vs. non-smoker. Moreover, the primary analysis model was extended to include an interaction term between treatment group and time interval between index event and baseline to determine whether the treatment effect is different when the treatment starts early. To analyze the recovery over time (time trend analysis until the FU at 90 days) a linear mixed model was fitted, adjusted for the same variables that were used in the primary analysis model and further including the FU time point (P1, 30 and 90 days) as well as the interaction between treatment group and FU time point, which was supposed to be removed if the interaction has a p > 0.05. A random intercept for patient was included to adjust for the cluster structure induced by multiple measurements per patient and a random slope for the FU time point.
The same analyses were repeated for the per-protocol (PP) population, excluding all patients with major protocol violations (e.g., <9 of 10 stimulations applied or violation of inclusion or exclusion criteria). Like in the ITT population, the analyses were applied within the respective FAS.
Safety outcomes were analyzed descriptively. For the safety population all patients who received any amount of stimulation were analyzed according to the ITT principle.
All analyses were performed using STATA 17 (StataCorp., 2021).
This trial was registered with ClinicalTrials.gov, NCT00909714.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
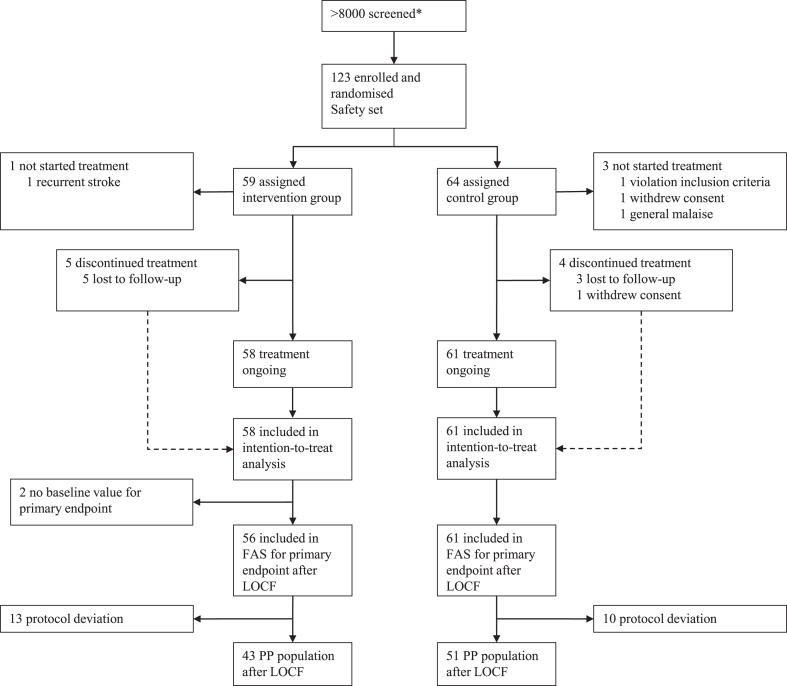
From November 18, 2009 to September 02, 2019, a total of 123 patients were enrolled in the trial. Of those 123 patients, 119 patients had received at least one stimulation (ITT population) and 94 patients had no protocol violations (PP population). All 123 patients were included in the safety analysis. In the ITT population, 61 patients were randomly assigned to control, and 58 patients to intervention. Data analysts first accessed the unblinded data on February 19, 2021. In two patients of the intervention arm, baseline UEFMA scores were missing so that the final statistical analysis of the primary endpoint included 56 patients in the intervention group (FAS). In the PP population, 51 patients received placebo stimulation, and 43 patients received active tDCS. The two patients in whom baseline UEFMA was missing were also part of the PP population leading to 41 patients in the intervention arm for the final statistical model of the primary endpoint in the PP population (Fig. 3).
Fig. 3.
CONSORT flow diagram. FAS = full analysis set (according to EMA guideline); ITT = intention-to-treat; LOCF = last observation carried forward; NA = not available; PP = per-protocol. ∗The number of patients screened was estimated post-hoc based on the clinical diagnosis lists provided by the principal study center where stroke patients are generally screened for eligibility to participate in mechanistic or clinical studies.
Baseline demographic and clinical characteristics were not different between groups (Table 1, ITT population; see SOM Supplementary Table S3 for demographic variables of the PP population). The mean (±SD) age was 65.6 ± 12.4 years in the intervention and 67.1 ± 11.6 years in the control group. Thirty-seven percent were female. On average, patients were included in the trial 20.0 ± 11.7 days after stroke. The mean NIHSS score was 4.1 ± 1.7 for the intervention group and 3.6 ± 2.2 for the control. The mean UEFMA score at baseline was 37.0 ± 11.0 for the intervention and 39.8 ± 11.4 for the control group. Numerically, there were more left-hemispheric lesions in the intervention group (53.5% vs. 39.3% in control). Diabetes mellitus was numerically more frequent in control (26.2% vs. 15.5% in intervention).
Table 1.
Baseline demographic and clinical characteristics of subjects by treatment group—ITT population.
| Random group |
Total (N = 119) | ||
|---|---|---|---|
| Intervention (N = 58) | Control (N = 61) | ||
| Age (years) | 67 (58–74) | 68 (59–75) | 67 (58–75) |
| Sex | |||
| Male | 38 (66%) | 37 (61%) | 75 (63%) |
| Female | 20 (34%) | 24 (39%) | 44 (37%) |
| Time between stroke and BL (days) | 21 (10–30) | 19 (9–27) | 20 (10–28) |
| Type of stroke | |||
| Cortical | 19 (33%) | 22 (36%) | 41 (34%) |
| Subcortical | 39 (67%) | 39 (64%) | 78 (66%) |
| Lesion side | |||
| Left | 31 (53%) | 24 (39%) | 55 (46%) |
| Right | 27 (47%) | 37 (61%) | 64 (54%) |
| Mini mental status test | 29 (26–30)a | 29 (28–30)a | 29 (27–30)b |
| UEFMA | 36 (28–45) | 39 (30–50)c | 39 (29–47)c |
| NIHSS | 4.1 (1.7) | 3.6 (2.2) | 3.8 (2.0) |
| Barthel Index | 63.6 (22.6)d | 68.8 (25.2) | 66.3 (24.0) |
| Intravenous thrombolysis | |||
| Yes | 17/58 (29%) | 18/60 (30%) | 35 (30%) |
| No | 41/58 (71%) | 42/60 (70%) | 83 (70%) |
| Edinburgh | |||
| Ambidextrous | 11/54 (20%) | 13/56 (23%) | 24/110 (22%) |
| Left handed | 1/54 (2%) | 0/56 (0%) | 1/110 (1%) |
| Right handed | 42/54 (78%) | 43/56 (77%) | 85/110 (77%) |
| Risk factors | |||
| Diabetes mellitus | 9 (16%) | 16 (26%) | 25 (21%) |
| Arterial hypertension | 42 (72%) | 49 (80%) | 91 (77%) |
| Hyperlipidemia | 26/58 (45%) | 30/59 (51%) | 56/117 (48%) |
| Nicotine | 14 (24%) | 16 (26%) | 30 (25%) |
| Atrial fibrillation | 6/58 (10%) | 6/60 (10%) | 12/118 (10%) |
Data are n (%), median (IQR), mean (SD), or n/N (%).
BL = baseline; UEFMA = Upper-Extremity-Fugl-Meyer-Assessment; NIHSS = National Institutes of Health Stroke Scale.
Data is missing for five patients.
Data is missing for ten patients.
Data is missing for two patients.
Data is missing for one patient.
In the ITT population, UE function as measured by the UEFMA improved from baseline to P1 by 9.0 ± 8.8 points in the intervention and by 8.9 ± 7.7 points in the control group (unadjusted). After adjustment for baseline UEFMA, type of stroke (cortical, subcortical), age, and time between stroke and baseline examination, patients improved by 8.8 (95% CI 6.9–10.7) in the intervention and by 9.1 (95% CI 7.2–10.9) in the control group. Primary endpoint analysis revealed no significant difference between treatment arms (difference, −0.3; 95% CI −3.0 to 2.4; p = 0.820) (Table 2). Additional adjustment for baseline stroke severity (based on the NIHSS score) did not change this result. The sensitivity analysis based on multiple imputation of missing values further confirmed these results (intervention group, 8.9-point improvement (95% CI 6.6–11.2) vs. 10.2 (95% CI 8.2–12.2) in the control group; p = 0.417).
Table 2.
Analysis of primary endpoint in FAS and PP population.
| N |
Baseline |
P1 |
Mean change (unadj.) |
Mean change (adj.) |
Int.-Ctrl. |
p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Int. | Ctrl. | Int. | Ctrl. | Int. | Ctrl. | Int. | Ctrl. | Int. | Ctrl. | Diff (95% CI) | ||
| FAS | 56 | 61 | 36.96 ± 11.02 | 39.80 ± 11.44 | 46.27 ± 12.08 | 49.18 ± 11.38 | 9.00 ± 8.76 | 8.85 ± 7.73 | 8.76 (6.86; 10.67) | 9.07 (7.24; 10.90) | −0.31 (−2.97; 2.35) | 0.820 |
| PP | 41 | 51 | 35.66 ± 10.79 | 38.80 ± 11.32 | 46.36 ± 11.25 | 48.75 ± 11.43 | 10.59 ± 8.46 | 9.94 ± 7.74 | 10.20 (8.00; 12.40) | 10.25 (8.28; 12.22) | −0.05 (−3.03; 2.93) | 0.972 |
Mean UEFMA values at baseline and P1, mean change from baseline to P1: unadjusted, as mean ± SD and adjusted as mean with (95% CI) as well as the difference in change between the groups with (95% CI). Adjusted means and mean difference resulting from an ANCOVA model adjusted for baseline UEFMA, type of stroke (cortical, subcortical), age and time between stroke and baseline examination. All values shown are based on data imputed by the LOCF approach.
Int. = intervention group; Ctrl. = control group; UEFMA = Upper-Extremity-Fugl-Meyer-Assessment.
In the PP population, the UEFMA improved from baseline to P1 by 10.6 ± 8.5 points in the intervention and by 9.9 ± 7.7 points in the control group (unadjusted). After adjustment, the corresponding values were 10.2 (95% CI 8.0–12.4) in the intervention and 10.3 (95% CI 8.3–12.2) in the control group (p = 0.972) (Table 2). Additional adjustment for baseline stroke severity (based on the NIHSS score) did not change this result. A further sensitivity analysis based on multiple imputation of missing values confirmed these results (intervention 10.1-point difference (95% CI 7.8–12.3), control 10.2 (95% CI 8.2–12.1); p = 0.949).
Pre-specified subgroup analyses of the primary endpoint provided consistent results to the main primary endpoint analysis across patients with mild vs. moderate or severe stroke, patients with cortical or subcortical stroke, younger or older patients, smokers or non-smokers (Table 3).
Table 3.
Subgroup analysis of primary endpoint in FAS population.
| N (%) | Mean change P1-BL |
Effect estimate (Int.-Ctrl.) | p value (Interaction term) | ||
|---|---|---|---|---|---|
| Int. | Ctrl. | ||||
| Baseline UEFMA categorized | |||||
| Mild | 47 (40%) | 6.30 ± 6.19 | 7.15 ± 5.63 | −1.51 [−5.76; 2.74] | 0.481 |
| Moderate/severe | 70 (60%) | 10.50 ± 9.66 | 10.21 ± 8.91 | 0.44 [−2.99; 3.87] | |
| Type of stroke | |||||
| Cortical | 40 (34%) | 9.78 ± 6.57 | 9.00 ± 8.12 | −1.45 [−6.06; 3.15] | 0.547 |
| Subcortical | 77 (66%) | 8.63 ± 9.69 | 8.77 ± 7.61 | 0.27 [−3.00; 3.53] | |
| Age group | |||||
| Young (≤67) | 59 (50%) | 10.00 ± 8.16 | 9.90 ± 8.12 | 0.39 [−3.35; 4.14] | 0.594 |
| Old (>67) | 58 (50%) | 7.93 ± 9.39 | 7.84 ± 7.32 | −1.04 [−4.84; 2.77] | |
| Sex | |||||
| Male | 75 (64%) | 8.00 ± 7.91 | 10.35 ± 7.97 | −2.96 [−6.20; 0.27] | 0.007 |
| Female | 42 (36%) | 11.11 ± 10.25 | 6.54 ± 6.88 | 4.59 [0.21; 8.97] | |
| Baseline NIHSS categorized | |||||
| NIHSS <5 | 78 (67%) | 8.52 ± 7.81 | 7.42 ± 6.42 | 0.56 [−2.74; 3.86] | 0.307 |
| NIHSS ≥5 | 39 (33%) | 9.70 ± 10.11 | 12.88 ± 9.73 | −2.44 [−7.16; 2.28] | |
| Nicotine | |||||
| No | 88 (75%) | 8.19 ± 8.52 | 8.33 ± 7.06 | −0.82 [−3.91; 2.26] | 0.488 |
| Yes | 29 (25%) | 11.69 ± 9.35 | 10.31 ± 9.46 | 1.33 [−4.01; 6.68] | |
| All patients | 117 | 9.00 ± 8.76 | 8.85 ± 7.73 | −0.31 [−2.97; 2.35] | 0.820 |
Subgroup analysis for primary endpoint based on interaction tests of the respective subgroup with treatment group within the primary analysis model. Number of patients within the subgroup are shown, unadjusted means ± SD of change in the UEFMA between P1 and Baseline, the contrast estimate within the respective subgroup (difference in mean UEFMA change between intervention and control group) and the p-value of the interaction term. All values shown are based on data imputed by the LOCF approach.
Int. = intervention group; Ctrl. = control group; UEFMA = Upper-Extremity-Fugl-Meyer-Assessment; NIHSS = National Institutes of Health Stroke Scale.
There was a different pattern for male and female patients (p = 0.007). While in men, there was numerically less improvement in the intervention (8.0 ± 7.9) than control arm (10.4 ± 8.0), the opposite was true for women (intervention 11.1 ± 10.3, control 6.5 ± 6.9). There were only 42 women included in the entire NETS trial and there was no a priori hypothesis in relation to sex differences. Caution is further advised when interpreting this finding due to sample size reduction after interim analysis.
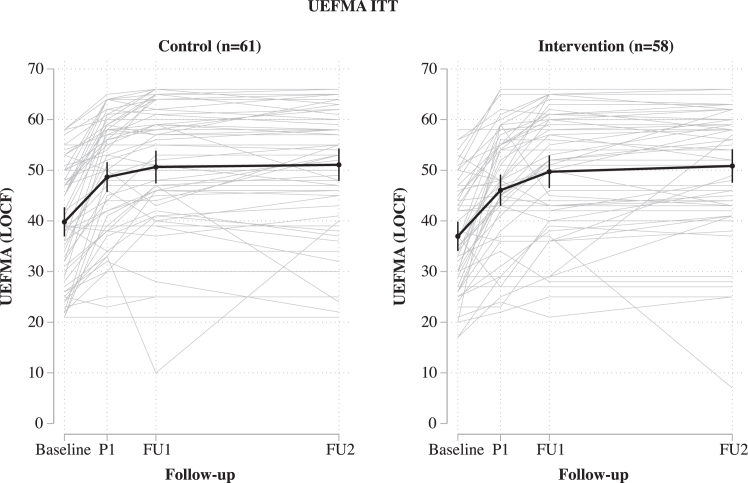
A pre-specified extension of the ANCOVA model included an interaction term between treatment group and time interval between index event and baseline to determine whether the treatment effect is different when the intervention is applied early. For both groups, there was an association between the time interval and a change in the UEFMA score (the more time passed by since the index event, the less pronounced was the improvement in the UEFMA score: mean change in the difference P1-V0 with every day −0.21 [95% CI −0.38 to −0.04] for the control group and −0.33 [95% CI −0.48 to −0.17] for the intervention group). We did not observe a difference between groups (p = 0.319) (Fig. 4).
Fig. 4.
Individual recovery curves for every patient (light grey) by group as well as the mean together with the 95% CI (black line and error bars). All values shown are based on data imputed by the LOCF approach. P1 = 1–7 days after the end of the treatment intervention (primary outcome); FU1 = follow-up 1, 30 ± 10 days after randomization; FU2 = follow-up 2, 90 ± 20 days after randomization; LOCF = last observation carried forward; UEFMA = upper-extremity Fugl-Meyer assessment.
In the PP population, subgroup analyses of the primary endpoint provided consistent results across patients with mild vs. moderate stroke, patients with cortical or subcortical stroke, younger or older patients, smokers or non-smokers (see Supplementary Table S4 in the Supplementary Online Material). Like in the ITT population, the pattern for male and female patients differed (p = 0.007). While in men, there was numerically less improvement in the intervention (9.9 ± 7.0) than control arm (11.8 ± 7.9), the opposite was true for women (intervention 11.7 ± 10.8, control 7.3 ± 7.0). There were only 36 women in the PP population, rendering this observation inconclusive.
Also in the PP population, the pre-specified extension of the ANCOVA model with an interaction term between treatment group and time interval between index event and baseline did not show an interaction.
Pre-specified analyses of secondary endpoints (LOCF, baseline to P1) did not reveal relevant differences between treatment arms (Table 4). There was a marginal difference in the SIS item ‘communication’, but given the otherwise neutral results on SIS, we did not consider this clinically relevant.
Table 4.
Analysis of secondary endpoints in the ITT population.
| N |
Mean change P1-BL |
Difference in change between groups [95% CI] | p value | |||
|---|---|---|---|---|---|---|
| Int. | Ctrl. | Int. | Ctrl. | |||
| UEFMA passive joint motion/pain | 57 | 61 | −0.32 | −0.25 | −0.00 [−0.80; 0.79] | 0.990 |
| Action Research Arm Test | 57 | 61 | 9.81 | 12.08 | −1.67 [−5.17; 1.83] | 0.347 |
| Nine hole peg test–non affected hand (Test) | 58 | 61 | 0.03 | 0.01 | 0.01 [−0.02; 0.03] | 0.494 |
| Nine hole peg test–non affected hand (Mean of training & test) | 58 | 61 | 0.02 | 0.02 | −0.01 [−0.03; 0.02] | 0.556 |
| Nine hole peg test–affected hand (Test) | 58 | 61 | 0.07 | 0.08 | −0.01 [−0.04; 0.02] | 0.608 |
| Nine hole peg test–affected hand (Mean of training & test) | 58 | 61 | 0.07 | 0.07 | −0.00 [−0.03; 0.03] | 0.762 |
| Box and block test | 57 | 60 | 10.32 | 10.75 | −0.70 [−4.10; 2.71] | 0.686 |
| Muscle strength, affected side (MRC) | 58 | 61 | 0.42 | 0.45 | −0.02 [−0.20; 0.16] | 0.850 |
| Grip pinch force–whole hand power grip | 58 | 61 | 0.08 | 0.10 | −0.03 [−0.08; 0.03] | 0.374 |
| Grip pinch force–pincer grasp | 57 | 60 | 0.15 | 0.18 | −0.02 [−0.10; 0.07] | 0.669 |
| Grip pinch force–key grip | 58 | 61 | 0.12 | 0.13 | −0.00 [−0.08; 0.07] | 0.924 |
| Grip pinch force–thumb opposition | 56 | 58 | 0.09 | 0.11 | −0.01 [−0.09; 0.07] | 0.851 |
| Frey somatosensory (non-affected hand) | 54 | 58 | −0.07 | 0.17 | −0.18 [−0.40; 0.04] | 0.107 |
| Frey somatosensory (affected hand) | 51 | 55 | 0.30 | 0.34 | −0.09 [−0.35; 0.18] | 0.524 |
| Ashworth spasticity scale, affected side | 58 | 61 | 0.00 | −0.02 | 0.03 [−0.05; 0.10] | 0.487 |
| Stroke impact scale strength | 56 | 59 | 11.50 | 11.33 | −0.21 [−5.58; 5.17] | 0.940 |
| Stroke impact scale memory | 56 | 59 | 5.93 | 4.11 | −1.60 [−5.08; 1.89] | 0.367 |
| Stroke impact scale emotion | 56 | 59 | 1.64 | 6.21 | −3.88 [−8.61; 0.86] | 0.107 |
| Stroke impact scale communication | 56 | 58 | 2.17 | 3.61 | −3.65 [−6.97; −0.33] | 0.031 |
| Stroke impact scale activities of daily living | 56 | 58 | 14.44 | 12.79 | −0.14 [−5.31; 5.03] | 0.958 |
| Stroke impact scale mobility | 55 | 59 | 16.48 | 15.64 | −0.11 [−6.45; 6.24] | 0.974 |
| Stroke impact scale hand function | 53 | 59 | 17.95 | 25.73 | −7.79 [−16.27; 0.69] | 0.071 |
| Stroke impact scale social participation | 50 | 55 | 7.46 | 3.09 | −1.89 [−11.22; 7.45] | 0.689 |
| Stroke impact scale physical domain | 53 | 58 | 15.19 | 16.14 | −1.63 [−6.52; 3.26] | 0.511 |
| Stroke impact scale stroke recovery | 53 | 59 | 13.81 | 16.07 | −1.56 [−7.39; 4.28] | 0.598 |
| Patient Health Questionnaire 9 | 54 | 58 | −1.35 | −1.83 | 1.14 [−0.13; 2.40] | 0.077 |
| NIHSS | 58 | 61 | −0.93 | −1.16 | 0.31 [−0.30; 0.92] | 0.316 |
| Barthel Index | 58 | 60 | 12.84 | 11.75 | −1.01 [−6.93; 4.90] | 0.735 |
Mean change from baseline to P1 shown unadjusted (as mean ± SD) and adjusted (as mean with [95% CI]) as well as the adjusted difference in change between groups (difference with [95% CI]). Adjusted means and mean difference resulting from an ANCOVA model adjusted for the respective baseline measurement, type of stroke (cortical, subcortical), age and time between stroke and baseline examination. All values shown are based on data imputed by the LOCF approach.
Int. = intervention group; Ctrl. = control group; UEFMA = Upper-Extremity-Fugl-Meyer-Assessment; NIHSS = National Institutes of Health Stroke Scale.
A responder analysis confirmed the results of the ITT analysis of the primary endpoint. In the intervention arm, 35/56 patients (62.5%) had a clinically relevant response; in the control arm, this was true for 43/61 patients (70.5%) (p = 0.282). The same held true for the compound score response with 38/48 (79.2%, intervention) vs. 46/54 (85.2%, control) patients (p = 0.190) (Table 5).
Table 5.
Response analysis in FAS population.
| Int. | Ctrl. | OR (Int. vs. Ctrl.) [95% CI] | p value | |
|---|---|---|---|---|
| Clinically relevant response | 35/56 (62.5%) | 43/61 (70.5%) | 0.63 [0.27; 1.47] | 0.282 |
| Compound score response | 38/48 (79.2%) | 46/54 (85.2%) | 0.47 [0.15; 1.46] | 0.190 |
Odds Ratio (OR) with 95% CI and p-values are resulting from logistic regression adjusted for type of stroke (cortical, subcortical), age and time between stroke and baseline examination. The model for clinically relevant response was furthermore adjusted for baseline UEFMA measurement and the model for compound score response for the NIHSS at baseline. All values shown are based on data imputed by the LOCF approach.
Int. = intervention group; Ctrl. = control group.
Fig. 4 shows recovery curves as measured by the UEFMA until FU2 in both groups. The recovery curve was expectedly steepest from baseline to P1 and approached a steady state between FU1 and FU2. The model revealed no interaction between FU time and treatment group (p = 0.177). Independent of treatment group recovery increases with time (30 days FU vs. P1 +2.7 (95% CI 1.7–3.6), and 90 days FU vs. P1 +3.5 (95% CI 2.2–4.8); model without interaction). The difference in change from baseline UEFMA between FU2 and FU1 was 0.4 ± 6.0 in the control and 1.2 ± 6.7 in the intervention group (unadjusted).
Also in the PP population, corresponding pre-specified analyses of secondary endpoints did not reveal any differences between treatment arms (see Supplementary Table S5 of the Supplementary Online Material).
The responder analysis of the PP population confirmed the results of the ITT analyses (see Supplementary Table S6 of the Supplementary Online Material). In the intervention arm, 29/41 patients (70.7%) had a clinically relevant response; in the control arm, this was true for 40/51 patients (78.4%) (p = 0.445). The same held true for the compound score response with 31/40 (77.5%, intervention) vs. 42/50 (84.0%, control) patients (p = 0.284).
The safety profile of tDCS was favorable. A total of 67 severe adverse events (SAEs) in 40 patients were reported, 17/40 patients in the intervention group, 23/40 in control. There were no epileptic seizures during the intervention period in either treatment group.
There was one patient reporting pain or other sensations twice during stimulation in the intervention group, and another patient reporting pain or other sensations once in the control group.
Supplementary Table S7 in the Supplementary Online Material lists all observed SAEs.
Discussion
In this randomized, blinded, placebo-controlled multicenter trial, tDCS applied over the motor cortex of the affected hemisphere and combined with standardized rehabilitative training did not improve UE motor function of the impaired arm after ischemic stroke. In this group of stroke patients, after ten days of intervention, the UEFMA increased by 9 points between baseline and first follow-up examination in both study arms, active tDCS, and placebo stimulation. Transcranial DC stimulation was well tolerated. Most importantly, there was no increased risk of seizures in relation to excitatory anodal tDCS.
Our trial enrolled stroke patients with subacute ischemic stroke (20.0 ± 11.7 days after stroke) and relevant but not severe UE motor deficit (UEFMA, 38.4 ± 11.3 points). Data from previous smaller studies suggest a beneficial effect of anodal tDCS over the M1 of the affected hemisphere in stroke patients, including the use of tDCS in subacute stroke.6,22 Animal data have provided compelling evidence that excitatory stimulation of the lesioned hemisphere can promote plastic reorganization in perilesional tissue and enhance recovery of motor function. The common underlying mechanism is assumed to involve augmentation of neuronal excitability and neuronal plasticity.23, 24, 25 In line with this, excitability-enhancing tDCS induces long-term synaptic potentiation, enhances the secretion of brain-derived neurotrophic factor, activates tyrosine receptor kinase B,26 and induces the expression of plasticity-related genes. In addition to promising experimental and preclinical data, a recent meta-analysis of preclinical and small clinical studies (46 studies included, median sample size, N = 21) suggested that anodal tDCS might be capable of adding clinically relevant effects for motor recovery after stroke, with effect sizes up to 1.33.7
Despite this encouraging evidence from animal data and human preclinical studies, the result of NETS was neutral. The numerical difference in the ITT analysis was 0.3 points, which is unequivocally out of the range of clinical relevance in UEFMA differences. There are several possible explanations for the neutral outcome besides anodal tDCS being generally ineffective in this setting. (1) The mean NIHSS score of the patients studied in NETS was four. It is possible that anodal tDCS is more effective in patients with more severe deficits and larger recovery potential. We consider this explanation rather unlikely because the positive initial studies on chronic stroke patients6 also focused on mild to moderate strokes. (2) Our patients were in the subacute phase, similar to the Bornheim and colleagues study.22 Based on animal data and the physiological consideration that the highest potential of plastic reorganization occurs in temporal vicinity of the damage, an earlier intervention after stroke could be more effective. On the other hand, positive proof-of-principle studies on tDCS were conducted in chronic stroke patients. Furthermore, excitation potentially reaches a ceiling which could limit the additive effect of anodal tDCS on the already upregulated system very early after stroke. (3) To keep confounding factors related to variations in the training schemes at a minimum, we designed an extensive standardized rehabilitation protocol for both arms of the study. The intensity of this program exceeded common practice in rehabilitation centers. We cannot exclude that this program drove the recovery dynamics and left no room for additional improvement by anodal tDCS. If this interpretation is correct, anodal tDCS does not have additive effects over and beyond very intense training. Likewise, regarding the rehabilitative training, two additional arms with usual care control group were not included in the trial. Hence, the effect of the intensive training itself cannot be quantified. (4) Based on previous studies6 and still consistent with more recent observations10 we chose 1 mA as stimulation intensity, also because at the time of designing the NETS trial, this could be considered safe. When moving as close as five days to the event, safety had highest priority. The present data confirm that 1 mA anodal tDCS to the lesioned hemisphere in subacute stroke patients is feasible and safe. However, more recent studies have safely used higher currents (e.g., 2–4 mA)7,27 and we cannot exclude that anodal tDCS of higher intensities might have been effective in our cohort of patients. (5) The study recruited 123 patients rather than the originally planned 250. This negative result could therefore be due to a lack of power. However, an interim analysis of blinded re-assessment of residual variance has justified reducing the sample size, and the results with numerically less improvement in the active than in the placebo group clearly show that increasing the sample size would be futile. (6) All patients in the intervention group received anodal tDCS with identical parameters. This one-fits-all approach could be too coarse given the heterogeneity of individual anatomy and structural damage after stroke. It might be necessary to personalize stimulation parameters based on individual patterns of lesions to critical brain regions, measures of individual connectomes,28 and neurotransmitter characteristics.29
The challenge of conducting and completing a large-scale tDCS trial in stroke patients has been highlighted recently30 and is in line with our own experiences. Learmonth and colleagues recruited 24 patients over 29 months (0.8 patients/month), in NETS the corresponding numbers are 123 patients in 119 months (1.0 patients/month). These numbers should be kept in mind when planning subsequent trials in this field. We have no systematic information on the patients considered not suitable for NETS regarding the inclusion and exclusion criteria in the trial centers. Organization of this investigator-initiated trial and amount of (public) funding did not allow for a valid screening log across all recruiting sites and over the time span of nearly ten years. Hence, a selection bias cannot be fully ruled out.
In summary, NETS provides evidence that in mildly to moderately affected subacute stroke patients, anodal tDCS (1 mA, 20 min, ten sessions) applied to the primary motor cortex of the lesioned hemisphere, combined with intense standardized rehabilitation training, is not superior to placebo stimulation in improving UE motor function.
Contributors
Christian Gerloff (CG), Kirstin-Friederike Heise (KFH), and Friedhelm C. Hummel (FCH) designed the NETS trial and acquired funding. CG was the study chair. CG wrote the first draft of the manuscript, with input and substantial revisions from KFH, FCH, Robert Schulz (RS), and Silke Wolf (SW). Adverse events were adjudicated by CG and SW. Antonia Zapf (AZ), Linda Krause, Anna Suling, and Karl Wegscheider were responsible for calculating the sample size, developing the statistical plan and statistical analysis. Three of the authors in the writing committee (CG, SW, and AZ) had full access to all underlying data. All other contributors were local investigators or co-investigators and recruited patients and collected data.
Writing committee
CG (Dept. Neurology, University Medical Center Hamburg-Eppendorf (UKE), Martinistr. 52, 20246 Hamburg, Germany), KFH (Dept. Health Sciences and Research, College of Health Professions, Medical University of South Carolina, 77 President Street, MSC 700, Charleston SC 29425, USA), FCH (Neuro-X Institute (INX) and Brain Mind Institute (BMI), Ecole polytechnique fédérale de Lausanne (EPFL), Campus Biotech, 1202 Geneva, Switzerland; INX and BMI, Ecole polytechnique fédérale de Lausanne Valais (EPFL Valais), Clinic Romande de Readaptation (CRR), 1951 Sion, Switzerland, and Clinical Neuroscience, University Medical School of Geneva (HUG), 1202 Geneva, CH), Robert Schulz (Dept. Neurology, UKE, Martinistr. 52, 20246 Hamburg, Germany), Silke Wolf (Dept. Neurology, UKE, Martinistr. 52, 20246 Hamburg, Germany), Antonia Zapf (Inst. Medical Biometry and Epidemiology, UKE, Martinistr. 52, 20246 Hamburg, Germany). The members of the writing committee were responsible for the decision to submit the manuscript. All authors approved the final version of the manuscript.
Data sharing statement
The anonymized, individual data available for this publication can be obtained from the corresponding author on reasonable request as long as the data are not part of an ongoing or planned regulatory submission.
Declaration of interests
CG declares, independent of the presented study, grants from Deutsche Forschungsgemeinschaft (DFG), Deutsches Zentrum f. Luft-und Raumfahrt (DLR), Hertie Foundation, Wegener Foundation, Schilling Foundation, Werner Otto Foundation, Merz Pharmaceuticals, Allergan, European Union; CG declares consulting fees from AlphaSights Ltd., and Life Science Praxis S.L., honoraria (for lectures, presentations) from AstraZeneca GmbH, Elements Communications Ltd., Boehringer Ingelheim, Streamedup GmbH, Abbott Medical, Bayer AG; CG declares participation in the DSMB of RESSTORE1, work as an editor of INFO Neurologie & Psychiatrie, Therapie und Verlauf neurologischer Erkrankungen (Textbook), and membership of the presidium of the German Neurological Society (DGN). FCH declares, independent of the presented study, grants from EU, PHRT, SNSF, Bertarelli Foundation, Defitech Foundation, Wyss Center for Bio and Neuroengineering; FCH declares board membership of Novartis Foundation. KFH, SW, RS, and AZ declare no competing interests.
Acknowledgements
NETS received funding from the Deutsche Forschungsgemeinschaft (DFG) under the grant agreement Ge 844/4-1.
We thank the patients and their families for participating in the trial; the members of the Data and Safety Monitoring Board (DSMB; Michael Hennerici, Michael Nitsche) for their advice; Annina Riener for supporting the trial organization; the DFG for generously extending the funding period.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100825.
Contributor Information
The NETS Trial Collaboration Group:
Diana Cordes, Christian Gerloff, Kirstin-Friederike Heise, Friedhelm C. Hummel, Robert Schulz, Silke Wolf, Kerstin Haevernick, Heike Krüger, Linda Krause, Anna Suling, Karl Wegscheider, Antonia Zapf, Jürgen Dressnandt, Barbara Schäpers, Christoph Schrödl, Björn Hauptmann, Anja Kirchner, Anna Brault, Alexander Gutschalk, Constanze Richter, Dennis A. Nowak, Jitka Veldema, Giacomo Koch, Michele Maiella, Christian Dohle, Katrin Jettkowski, Mario Pilz, Farsin Hamzei, Lydia Olischer, Caroline Renner, Marcus Groß, Michael Jöbges, and Bernhard Voller
Appendix A. Supplementary data
References
- 1.Langhorne P., Coupar F., Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Vliet R., Selles R.W., Andrinopoulou E.-R., et al. Predicting upper limb motor impairment recovery after stroke: a mixture model. Ann Neurol. 2020;87:383–393. doi: 10.1002/ana.25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krakauer J.W. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 5.Ward N.S. The neural substrates of motor recovery after focal damage to the central nervous system. Arch Phys Med Rehabil. 2006;87:S30–S35. doi: 10.1016/j.apmr.2006.08.334. [DOI] [PubMed] [Google Scholar]
- 6.Hummel F., Celnik P., Giraux P., et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 7.Bornheim S., Thibaut A., Beaudart C., Maquet P., Croisier J.-L., Kaux J.-F. Evaluating the effects of tDCS in stroke patients using functional outcomes: a systematic review. Disabil Rehabil. 2022;44:13–23. doi: 10.1080/09638288.2020.1759703. [DOI] [PubMed] [Google Scholar]
- 8.NETS Trial Collaboration Group A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of transcranial direct current stimulation to the motor cortex after stroke (NETS): study protocol. Neurol Res Pract. 2022;4:14. doi: 10.1186/s42466-022-00171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nitsche M.A., Seeber A., Frommann K., et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allman C., Amadi U., Winkler A.M., et al. Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aad5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandiga P.C., Hummel F.C., Cohen L.G. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Page S.J., Fulk G.D., Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. 2012;92:791–798. doi: 10.2522/ptj.20110009. [DOI] [PubMed] [Google Scholar]
- 13.Chen H.-M., Chen C.C., Hsueh I.-P., Huang S.-L., Hsieh C.-L. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil Neural Repair. 2009;23:435–440. doi: 10.1177/1545968308331146. [DOI] [PubMed] [Google Scholar]
- 14.Sivan M., O'Connor R.J., Makower S., Levesley M., Bhakta B. Systematic review of outcome measures used in the evaluation of robot-assisted upper limb exercise in stroke. J Rehabil Med. 2011;43:181–189. doi: 10.2340/16501977-0674. [DOI] [PubMed] [Google Scholar]
- 15.Bohannon R.W. Grip strength impairments among older adults receiving physical therapy in a home-care setting. Percept Mot Skills. 2010;111:761–764. doi: 10.2466/03.10.15.PMS.111.6.761-764. [DOI] [PubMed] [Google Scholar]
- 16.Lang C.E., Edwards D.F., Birkenmeier R.L., Dromerick A.W. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89:1693–1700. doi: 10.1016/j.apmr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladstone D.J., Danells C.J., Armesto A., et al. Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: a randomized, double-blind, placebo-controlled trial. Stroke. 2006;37:179–185. doi: 10.1161/01.STR.0000195169.42447.78. [DOI] [PubMed] [Google Scholar]
- 18.EMA . (EMEA) EMA; 1998. ICH Topic E 9: statistical principles for clinical trials. [Google Scholar]
- 19.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 20.Hardt J., Herke M., Leonhart R. Auxiliary variables in multiple imputation in regression with missing X: a warning against including too many in small sample research. BMC Med Res Methodol. 2012;12:184. doi: 10.1186/1471-2288-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woytowicz E.J., Rietschel J.C., Goodman R.N., et al. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch Phys Med Rehabil. 2017;98:456–462. doi: 10.1016/j.apmr.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bornheim S., Croisier J.-L., Maquet P., Kaux J.-F. Transcranial direct current stimulation associated with physical-therapy in acute stroke patients - a randomized, triple blind, sham-controlled study. Brain Stimul. 2020;13:329–336. doi: 10.1016/j.brs.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Adkins D.L., Hsu J.E., Jones T.A. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp Neurol. 2008;212:14–28. doi: 10.1016/j.expneurol.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plautz E.J., Barbay S., Frost S.B., et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25:801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 25.Koo H., Kim M.S., Han S.W., et al. After-effects of anodal transcranial direct current stimulation on the excitability of the motor cortex in rats. Restor Neurol Neurosci. 2016;34:859–868. doi: 10.3233/RNN-160664. [DOI] [PubMed] [Google Scholar]
- 26.Fritsch B., Reis J., Martinowich K., et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chhatbar P.Y., Chen R., Deardorff R., et al. Safety and tolerability of transcranial direct current stimulation to stroke patients – a phase I current escalation study. Brain Stimul. 2017;10:553–559. doi: 10.1016/j.brs.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch P.J., Park C.-H., Girard G., et al. The structural connectome and motor recovery after stroke: predicting natural recovery. Brain. 2021;144:2107–2119. doi: 10.1093/brain/awab082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stagg C.J., Bestmann S., Constantinescu A.O., et al. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol. 2011;589:5845–5855. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Learmonth G., Benwell C.S.Y., Märker G., et al. Non-invasive brain stimulation in Stroke patients (NIBS): a prospective randomized open blinded end-point (PROBE) feasibility trial using transcranial direct current stimulation (tDCS) in post-stroke hemispatial neglect. Neuropsychol Rehabil. 2021;31:1163–1189. doi: 10.1080/09602011.2020.1767161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.