Summary
In the past two decades, the treatment of metastatic non-small cell lung cancer (NSCLC), has undergone significant changes due to the introduction of targeted therapies and immunotherapy. These advancements have led to the need for predictive molecular tests to identify patients eligible for targeted therapy. This review provides an overview of the development and current application of targeted therapies and predictive biomarker testing in European patients with advanced stage NSCLC. Using data from eleven European countries, we conclude that recommendations for predictive testing are incorporated in national guidelines across Europe, although there are differences in their comprehensiveness. Moreover, the availability of recently EMA-approved targeted therapies varies between European countries. Unfortunately, routine assessment of national/regional molecular testing rates is limited. As a result, it remains uncertain which proportion of patients with metastatic NSCLC in Europe receive adequate predictive biomarker testing. Lastly, Molecular Tumor Boards (MTBs) for discussion of molecular test results are widely implemented, but national guidelines for their composition and functioning are lacking. The establishment of MTB guidelines can provide a framework for interpreting rare or complex mutations, facilitating appropriate treatment decision-making, and ensuring quality control.
Keywords: Predictive biomarker testing, Targeted therapy, Non-small cell lung cancer, Europe
Key messages.
-
•
The use of targeted therapies contributes to optimal treatment of patients with advanced stage non-small cell lung cancer (NSCLC);
-
•
The number of European Medicine Agency (EMA)-approved targeted therapies for patients with advanced stage NSCLC is increasing rapidly;
-
•
Predictive biomarker testing is required for the identification of the appropriate molecular aberrations that are actionable with specific targeted therapies;
-
•
The availability of European Medicine Agency-approved targeted therapies for patients with advanced stage NSCLC varies across European countries;
-
•
National guidelines of European countries contain recommendations for predictive biomarker testing in patients with NSCLC but only partially align with current ESMO guidelines;
-
•
Evidence for high predictive biomarker testing rates in patients with NSCLC in Europe is limited due to the lack of (publicly) available data of national testing rates;
-
•
National guidelines for the functioning of Molecular Tumor Boards are lacking in most European countries and should be established to provide a framework for multidisciplinary interpretation of rare or complex mutations in patients with NSCLC.
Introduction
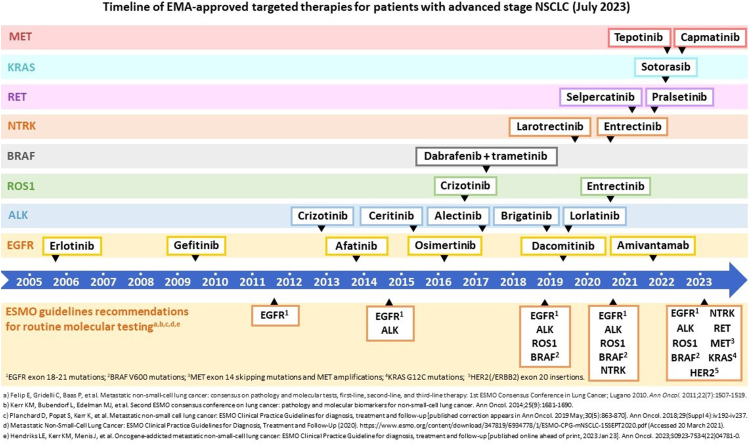
Lung cancer remains the leading cause of cancer-related mortality in Europe, resulting in a considerable number of deaths.1 Projections for 2023 indicate that lung cancer will account for a combined total of 275,956 fatalities in the European Union and the United Kingdom.2 Within the realm of lung cancer treatment, significant advancements have occurred over the past two decades, particularly in the management of non-small cell lung cancer (NSCLC), comprising approximately 85% of all lung cancer cases. These developments encompass various modalities such as minimally invasive surgery techniques and stereotactic body radiotherapy for localized disease, as well as targeted therapies and immunotherapy as systemic therapeutic options.3 As roughly 55–70% of NSCLC patients are diagnosed with metastatic disease at time of presentation, targeted therapies such as EGFR and ALK tyrosine kinase inhibitors are thought to have contributed significantly to the observed increase in overall survival rates between 2010 and 2016.4 Due to the expanding repertoire of European Medicines Agency (EMA) approved targeted therapies for advanced-stage NSCLC, it has become imperative to perform molecular testing to identify actionable molecular aberrations and determine the appropriate patient population for targeted treatment (Fig. 1).
Fig. 1.
Timeline of EMA-approved targeted therapies and the ESMO-recommendations for predictive biomarker testing associated with these targeted drugs for patients with advanced stage NSCLC (as of July 2023).
The current Clinical Practice Guidelines of the European Society for Medical Oncology (ESMO) for oncogene-addicted advanced-stage NSCLC (2023) recommend molecular testing for nine predictive biomarkers.5 However, previous studies have described suboptimal NSCLC testing rates for targetable molecular aberrations in various European countries, suggesting that routine predictive biomarker testing is not keeping pace with the approval of targeted therapies.6, 7, 8, 9 Notwithstanding the presence of European guidelines, discrepancies have also been observed between national guidelines for molecular testing in NSCLC and the European consensus, potentially contributing to variation in testing and treatment practices across individual European countries.6,8 Moreover, recent studies have described delayed uptake of next-generation sequencing (NGS) for oncology patients across Europe.9,10 Additionally, discrepancies were reported in the availability and reimbursement of precision medicines between European countries.9 The latter two studies9,10 provided a broad overview of the European application of NGS and/or precision medicines for all (solid tumor) oncology patients, while the previously mentioned studies on NSCLC6, 7, 8 predominantly focused on biomarker testing rather than targeted therapy availability and by now, are from several years ago. The aim of this review is to provide an overview of the timeline, developments, and current application of targeted therapies and predictive biomarker testing in patients with advanced stage NSCLC in Europe in 2023.
The first part of this review highlights the major, recent developments in targeted therapy treatment in advanced-stage NSCLC in Europe (Section EMA-approved targeted therapies) and the changes in European guidelines for predictive biomarker testing (Section European guidelines for predictive biomarker testing). The second part provides an overview of national targeted therapy availability (Section Availability of targeted therapies), national guidelines for predictive biomarker testing (Section National guidelines for predictive biomarker testing), and molecular testing rates (Section Predictive biomarker testing rates), in a selection of European countries including Austria, Belgium, the Czech Republic, England, France, Germany, the Netherlands, Portugal, Slovenia, Spain, and Sweden. To accomplish this, one (molecular) pathologist or clinical scientist in molecular pathology and one (pulmonary) oncologist of these countries, with extensive expertise with regard to targeted treatment and molecular testing in patients with NSCLC, sought to obtain up-to-date information on their national guidelines, availabilities of targeted therapies, and molecular testing rates in patients with advanced stage non-squamous NSCLC, using a detailed questionnaire (see Supplementary File 1). Participants also provided information regarding molecular test types and molecular test result interpretation specific to their affiliated institutes (Sections Interpretation of molecular test results and Barriers for off-label use, compassionate use programs and trial participation). Collection of information from all countries was completed between March and September of 2023. The objective of this approach is to identify potential differences in predictive biomarker testing rates and targeted therapy availability for patients with advanced stage NSCLC among European countries that are linked by international collaboration and common legislation. In the corresponding viewpoint paper in this Clinical Series, a future perspective of the application of molecular testing in advanced stage NSCLC is described.11
Search strategy and selection criteria.
References for this Review were identified through EMA European public assessments reports (EPAR) of EMA-authorized targeted therapies for non-small cell lung cancer (see Supplementary File 2 for references and last date of access), subsequent scrutinization of references of included articles and through searches of the authors’ own files. References of national guidelines and (national) biomarker testing rates were provided by participants from each country, if available. There was no selection based on language or date of publication.
EMA-approved targeted therapies
Between 2005 and 2018, the EMA granted approval for eight targeted therapies in advanced stage NSCLC for specific molecular aberrations in four genes: EGFR, ALK, ROS1, and BRAF (Fig. 1). These therapies included erlotinib, gefitinib, afatinib, and osimertinib for EGFR mutations, crizotinib, ceritinib, and alectinib for ALK fusions, crizotinib for ROS1 fusions, and the combination treatment of dabrafenib and trametinib in BRAF-mutated advanced stage NSCLC.
Over the past five years (2018–2023), the EMA has authorized the use of targeted therapies for four other molecular biomarkers in advanced-stage NSCLC. These include NTRK fusions targeted by larotrectinib and entrectinib, RET fusions targeted by selpercatinib and pralsetinib, KRAS G12C mutations targeted by sotorasib, and MET exon 14 skipping mutations targeted by tepotinib and capmatinib. Furthermore, entrectinib was also approved for the treatment of ROS1 fusion-positive NSCLC, brigatinib and lorlatinib were approved as ALK inhibitors, and the group of EGFR-targeting drugs was enriched with the approval of amivantamab for EGFR exon 20 insertion mutations. Lastly, the treatment indication of osimertinib was recently extended to include non-metastatic NSCLC patients (see also early-stage NSCLC review).12
The following paragraphs provide an overview of the key characteristics and recent developments associated with each targeted therapy, subdivided by their respective biomarker. For a comprehensive overview of all EMA-approved indications for targeted therapy use in patients with NSCLC, including references, see Supplementary File 2.
EGFR-targeted treatment
Activating mutations in EGFR have a prevalence of approximately 12–15% among European patients with NSCLC, although they are more common in Asian populations.13 These mutations are generally divided into three groups: (1) common EGFR mutations (exon 19 deletions and L858R mutations), (2) EGFR exon 20 insertion mutations, and (3) uncommon/rare EGFR mutations.14 First- (erlotinib, gefitinib) and second-generation (afatinib) EGFR tyrosine kinase inhibitors (TKI) have been available for over a decade and were primarily developed for the treatment of common EGFR mutations. Osimertinib, a third-generation EGFR-TKI, was initially approved as second-line treatment for patients with the acquired T790M resistance mutation. However, in 2018, the EMA expanded the approval of osimertinib to include first-line treatment of EGFR mutation-positive NSCLC. This decision was based on the FLAURA study, which demonstrated improved progression-free survival, overall survival, and treatment of brain metastasis compared to first-generation EGFR-TKIs.15
Patients with EGFR exon 20 insertion mutations have generally shown poor response to treatment with first-, second-, and third-generation EGFR-TKIs.14,16 Newly developed EGFR-targeting therapies, including amivantamab and mobocertinib, have shown promising treatment results in patients with EGFR exon 20 insertion mutations.17, 18, 19, 20, 21 In 2021, amivantamab, a bispecific anti-EGFR and anti-MET antibody, was approved by the EMA as a second-line treatment option for advanced-stage NSCLC patients with EGFR exon 20 insertion mutations, based on the results of the phase I CHRYSALIS trial.19
The group of uncommon/rare EGFR mutations encompasses all remaining EGFR mutations and the actionability of these mutations may vary. In Dutch patients with advanced stage NSCLC, 18.7% of all EGFR mutations detected by multi-gene assays were uncommon/rare EGFR mutations, forming a significant subgroup of EGFR-mutated NSCLC.14 Better clinical outcomes have typically been reported with afatinib compared to osimertinib, in particular in uncommon EGFR mutations with relatively high prevalence, such as G719X.16,22 While there is limited clinical data comparing the treatment outcome of TKI in rare EGFR mutations, pre-clinical studies have demonstrated significant differences in the efficacy of different EGFR-TKIs, using codon-based and/or structure-based classifications to explain and predict efficacy.23
In 2021, the phase III ADAURA trial results led to the approval of osimertinib as an adjuvant therapy following complete tumor resection (with or without adjuvant chemotherapy) in patients with stage IB–IIIA NSCLC harboring either an EGFR exon 19 deletion or L858R mutation.24 This marks the first-approved targeted therapy for use in adjuvant setting in NSCLC, opening up possibilities for potential benefits of other targeted therapies in an adjuvant treatment setting.12,25
ALK-targeted treatment
Fusions involving anaplastic lymphoma kinase (ALK) are found in approximately 2–5% of patients with NSCLC.13 The introduction of crizotinib, a first-generation ALK inhibitor, revolutionized the treatment landscape for ALK fusion-positive NSCLC patients. However, in the past five years, there have been significant advancements with the approval of second-generation (alectinib, ceritinib and brigatinib) and third-generation (lorlatinib) ALK inhibitors by the EMA for their use in patients with ALK fusion-positive metastatic NSCLC. These newly approved ALK inhibitors not only provide additional options for first-line ALK-targeted treatment options, but they also demonstrate improved clinical efficacy in the central-nervous system (CNS) and in cases where certain ALK mutations may arise as resistance mechanisms to ALK inhibitors.26 The development of resistance mutations is a common challenge in targeted therapies, and the emergence of these mutations can limit the effectiveness of initial treatment. However, brigatinib and lorlatinib have shown promising effects in overcoming resistance and maintaining therapeutic response in patients with specific ALK mutations.26
The development of new ALK inhibitors and their sequential use in routine clinical practice has prolonged the lives of patients with ALK fusion-positive NSCLC up to five to seven years.27 The continuous development and approval of next-generation ALK inhibitors highlight the importance of ongoing research and innovation in the field of targeted therapies for NSCLC.28
ROS1-targeted treatment
A phase I trial of crizotinib demonstrated a significant overall response rate (ORR) of 72% (36 out of 50 patients) in individuals with ROS1-rearranged NSCLC.29 Subsequently, in 2016, the EMA extended the treatment indication of crizotinib to include patients with advanced stage ROS1 fusion-positive NSCLC. An integrated analysis of three ongoing separate trials with entrectinib, a combined pan-TRK, ROS1 and ALK-inhibitor, demonstrated an ORR of 77% (41 out of 53 patients) with entrectinib.30 Notably, the response rates to entrectinib were similar between patients with and without CNS metastases at baseline, whereas the efficacy of crizotinib may be compromised in these patients due to its limited ability to cross the blood-brain barrier and reduced intracerebral drug activity.31 In 2020, entrectinib received simultaneous approval as a treatment option for patients with advanced stage ROS1 fusion-positive NSCLC, and as pan-tumor therapy in NTRK fusion-positive tumors (see Section NTRK-targeted treatment. NTRK fusions). However, it is important to note that entrectinib is indicated for patients not previously treated with ROS1 inhibitors as its effectiveness may be limited to treatment-naïve patients.
BRAF-targeted treatment
Mutations in V-raf murine sarcoma viral oncogene homolog B1 (BRAF) occur in approximately 2–4% of patients with NSCLC.13,32,33 Among these mutations, missense mutations at codon 600 of BRAF (known as V600 mutations) account for around 40% of all BRAF mutations in NSCLC. These BRAF V600 mutations are classified as class I BRAF mutations, as they lead to increased BRAF kinase activity that is not dependent on BRAF dimerization or activation of RAS.32 In 2017, the EMA approved the use of dabrafenib in combination with trametinib for the treatment of advanced NSCLC patients with V600 mutations in BRAF.33 However, it is important to note that there is currently no EMA approval for the use of combined BRAF and MEK inhibition in NSCLC with class II (RAS-independent dimerization) or class III (RAS-dependent dimerization with CRAF) BRAF driver mutations. These different classes of BRAF mutations have distinct molecular characteristics and response profiles to targeted therapies. A recent meta-analysis described that MAPK-targeted therapies, including BRAF and MEK inhibitors, have demonstrated clinical activity in some tumors with non-V600 BRAF mutations, in particular those with class II mutations.34 However, clinical trials regarding the efficacy of MAPK-targeted therapies in patients with NSCLC with class II or class III BRAF mutations are currently lacking. This is primarily due to the heterogeneity of mutations within each class. Certain non-V600 BRAF mutations may still be targetable in NSCLC using existing BRAF and/or MEK inhibitors, although further research is needed to determine their effectiveness.
NTRK-targeted treatment
Fusions involving the neurotrophic tropomyosin receptor kinase genes (NTRK1, NTRK2, and NTRK3) are known oncogenic drivers in various types of malignancies, including NSCLC, and have a combined prevalence of <1% in NSCLC.13,35 In 2019, the EMA approved larotrectinib, a selective pan-TRK (TRKA, TKRB, TRKC) inhibitor, followed in 2020 by the approval of entrectinib, a combined pan-TRK, ROS1 and ALK inhibitor, based on two studies with combined results of multiple phase I/II trials of these therapies in solid tumors.36,37 A clinical study later showed the efficacy of larotrectinib in previously treated patients with lung cancer harboring an NTRK1/2/3 fusion. This study demonstrated an ORR of 73% and a median progression-free survival (PFS) of 33.9 months among evaluable patients (11 out of 15 patients).38 Entrectinib showed a comparable ORR of 70% in patients with NTRK1/2/3 fusion-positive NSCLC (7 out of 10 patients).39 These findings suggest that targeted therapy directed at NTRK1/2/3 fusion-positive NSCLC can provide significant and durable clinical benefits in metastatic NSCLC, despite representing a small subset of patients.
RET-targeted treatment
Rearranged during transfection (RET) fusions have a prevalence of 1–3% in patients with NSCLC.13 In 2021, two selective RET kinase-inhibitors, selpercatinib and pralsetinib, were approved by the EMA for treatment of patients with advanced stage RET fusion-positive NSCLC, including application as first-line therapy. The phase I/II LIBRETTO-001 trial evaluating the efficacy of selpercatinib in previously treated patients with RET fusions showed promising results, demonstrating an ORR of 64% (67 out of 105 patients) and a median PFS of 17.5 months.40 In previously untreated patients, selpercatinib as first-line systemic therapy resulted in an ORR of 85% (33 out of 39 patients).40 Similarly, pralsetinib has demonstrated favorable response rates in patients with RET fusion-positive NSCLC. In patients previously treated with platinum-based chemotherapy, pralsetinib treatment resulted in an ORR of 61% (53 out of 87 patients), while treatment naïve patients showed an ORR of 70% (19 out of 27 patients).41 These results suggest that selpercatinib and pralsetinib are an effective therapeutic option for both previously treated and treatment-naïve NSCLC patients with RET fusions. Ongoing clinical trials will further refine the understanding of RET fusions and optimize the use of RET kinase inhibitors compared to currently standard first-line chemo-immunotherapy in the management of NSCLC.
KRASG12C-targeted treatment
Somatic mutations in rat sarcoma virus (RAS) genes, including KRAS, NRAS, and HRAS, are the most commonly observed oncogenic aberrations in human cancers, occurring in approximately 25% of all cancers.42 Among the RAS genes, driver mutations in the Kirsten rat sarcoma virus (KRAS) gene account for about 85% of all RAS-driven malignancies. In NSCLC, the prevalence of KRAS mutations ranges from 26 to 41%.42, 43, 44, 45 Directly targeting mutated KRAS has posed a significant challenge due to the high affinity of KRAS for GDP/GTP and the absence of identified allosteric regulatory binding sites.46 However, in 2013, the discovery of the switch-II pocket in KRASG12C provided a breakthrough for the development of clinically effective KRASG12C inhibitors.47,48 Sotorasib has shown promising results in pretreated NSCLC patients with an ORR of 37% (46 out of 124 patients) and disease control rate (DCR) of 81% (100 out of 124 patients).49 Moreover, in the phase III CodeBreaK 200 trial, sotorasib improved PFS compared to docetaxel treatment (5.6 months versus 4.5 months) with more favorable safety profile, in patients pretreated with chemo-immunotherapy.50 As KRAS G12C mutations account for more than a third of all KRAS mutations in patients with NSCLC (overall prevalence of 12–17%), KRASG12C inhibitors such as sotorasib hold the potential to improve clinical outcome in a significant group of patients with advanced stage NSCLC.13,44,45 In 2021, the EMA approved the use of sotorasib for patients with advanced stage KRASG12C-mutated NSCLC, who have received at least one prior line of systemic therapy. The development of KRASG12C inhibitors represents a significant advancement in the field of targeted therapies for NSCLC and offers new hope for improving treatment outcomes in patients with KRASG12C-mutated tumors. Ongoing research and clinical trials will further explore the potential of KRAS-targeted therapies and their combination with other treatment modalities in the management of NSCLC.
MET-targeted treatment
Intron 13 and splice-site mutations of mesenchymal-epithelial transition (MET) gene result in loss of transcription of exon 14 lead to increased activation of MET signaling, due to the loss of the Y1003 binding site of CBL on the juxtamembrane domain of MET.51 These MET exon 14 skipping mutations occur in 1–3% of patients with NSCLC.52 Unlike crizotinib, a type 1a MET inhibitor, capmatinib and tepotinib are selective MET kinase domain inhibitors (type 1b MET inhibitor).53 In the phase II GEOMETRY mono-1 study, capmatinib has demonstrated response rates of 41% in pretreated patients (28 out of 69 patients) and 68% in treatment-naïve patients (19 out of 28 patients) with NSCLC harboring a MET exon 14 skipping mutation.54 With simultaneously published results, in the phase II VISION study, tepotinib demonstrated an ORR of 46% in patients with advanced stage NSCLC (46 out of 99 patients), with similar response rates observed in both pretreated and treatment-naïve patients.55
Both capmatinib and tepotinib were approved by the EMA in 2021 for the treatment of advanced stage NSCLC patients harboring MET exon 14 skipping mutations who require systemic therapy after treatment with immunotherapy and/or platinum-based chemotherapy. Though capmatinib and tepotinib have demonstrated comparable response rates, the clinical trial evaluating capmatinib reported a higher occurrence of grade III or IV adverse events compared to the tepotinib trial.54,55 The safety and tolerability profiles of these drugs should be considered when making treatment decisions for patients with MET exon 14 skipping-mutated NSCLC.
European guidelines for predictive biomarker testing
The ESMO Clinical Practice Guidelines for patients with metastatic NSCLC, as of 2018, recommended molecular testing for detection of EGFR mutations in exons 18–21, BRAF V600 mutations, ALK fusions, and ROS1 fusions (Table 1).56 In the 2020 guideline update, NTRK fusions were added to the previously recommended biomarkers. In the current ESMO guidelines (2023), this list of recommended predictive biomarkers has been expanded by the addition of MET exon 14 skipping mutations, MET amplifications, KRASG12C mutations, RET fusions, and HER2/ERBB2 mutations.5 Generally it takes one to two years for the ESMO guidelines to include recommendations for molecular biomarker testing after EMA-approval of corresponding targeted therapies (Fig. 1). However, the inclusion of ERBB2 exon 20 insertions as a recommended biomarker in the current ESMO guidelines is an exception. Although ERBB2-targeted therapies such as trastuzumab-deruxtecan and pyrotinib have been approved by the FDA, these targeted therapies have not yet received approval from the EMA.5 Nevertheless, in many European countries patients can still be treated with ERBB2-targeted treatment in clinical trials (see also Section Availability of targeted therapies).
Table 1.
Availability of targeted therapies (EMA- and/or FDA-approved) for advanced stage NSCLC in a selection of European countries.
 |
Future guidelines are likely to incorporate additional biomarkers that have predictive and/or prognostic value for targeted therapies (e.g., TP53 mutations) and/or for non-targeted therapies (e.g., tumor-mutation burden, STK11 mutations, KEAP1 mutations). The ongoing development of novel targeted therapies as well as availability of tumor-agnostic therapies already approved in other cancers, and the necessary expansion of biomarkers associated with these novel therapies will continue to shape the landscape of predictive biomarker testing in advanced-stage NSCLC. For more detailed information and a future perspective of predictive biomarker testing in advanced-stage NSCLC, the corresponding viewpoint in this Clinical Series should be read.11
Availability of targeted therapies
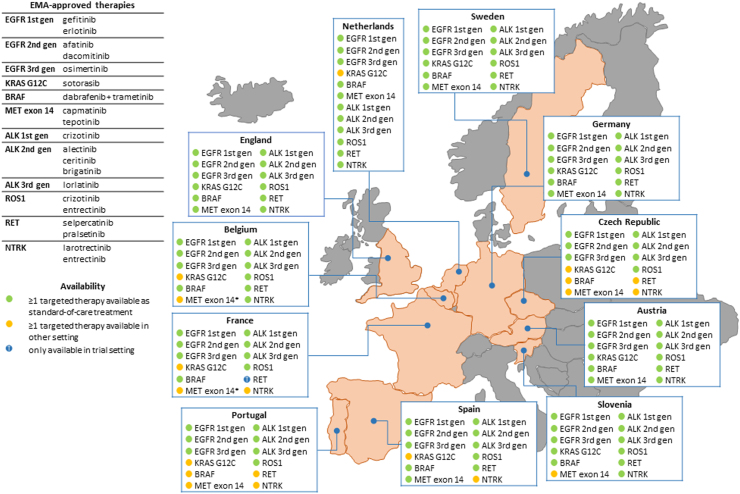
Using a questionnaire sent to all participants (see Supplemental File 1), we collected present-day information on the availability of targeted therapies in eleven European countries (summarized in Fig. 2; Table 1). Any targeted therapy with a treatment indication approval for patients with advanced stage NSCLC by either the EMA and/or the FDA, were included in the questionnaire to form a comprehensive overview of available targeted treatments. Overall, not all EMA-approved therapies are available in every participating European country. First-, second-, and third-generation EGFR TKIs, first-, second- and third-generation ALK inhibitors, and ROS1 inhibitors are available as standard-of-care treatment in all European countries participating in this study (Fig. 2; Table 1). Amivantamab is only available as standard-of-care treatment in four out of eleven countries. Targeted therapy as standard-of-care treatment is not available in all countries for patients with advanced stage NSCLC with an NTRK fusion (available as standard-of-care in seven out of eleven countries), RET fusion (8/11 countries), BRAF V600 mutation (9/11 countries), MET exon 14 skipping mutation (6/11 countries), or KRAS G12C mutation (5/11 countries). It should be noted that in some countries the only available MET-targeted treatment is crizotinib, which is FDA-approved but not EMA-approved for its application in patients with advanced stage NSCLC harboring an MET exon 14 skipping mutation.
Fig. 2.
Availability of targeted therapies for patients with metastatic NSCLC across eleven European countries. For each country, the displayed information was provided by a detailed questionnaire (Supplementary File 1) that was completed by a (molecular) pathologist, clinical scientist in molecular pathology and/or (pulmonary) oncologist with expertise in the field of NSCLC. ∗Presented availability concerns crizotinib (FDA-approved), due to its better availability compared to tepotinib and capmatinib (EMA-approved) (see also Table 2).
National guidelines for predictive biomarker testing
The representatives of all eleven participating European countries reported current national guidelines with recommendations regarding predictive biomarkers testing in patients with (advanced stage) NSCLC. Table 2 summarizes the specific details on the recommendations of each participating country regarding biomarker testing as reported in the national guidelines of these countries. The interpretation of what constitutes valid, applicable national guidelines is not always clear-cut. For example, England does not have tumor-specific, detailed guidelines on molecular testing requirements, and Portugal has limited national guidelines supplemented by national expert consensus recommendations.57, 58, 59 National guidelines largely align with the biomarkers listed in the ESMO guidelines including EGFR, ALK, and ROS1, which were among the first biomarkers for which targeted therapies were available. Testing for these biomarkers is recommended in all participating countries.57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68 For the other biomarkers, however, the representation of testing recommendations in national guidelines varies, and the introduction of these biomarkers into the guidelines has occurred over a broader range of time compared to EGFR, ALK, and ROS1. As other biomarkers each belong to more recently EMA-approved targeted therapies (see Fig. 1), a plausible explanation is that these differences are caused by delays in the updating of national guidelines.
Table 2.
Biomarker recommendations and year of introduction in national guidelines of European countries for molecular testing of patients with metastatic NSCLC.
 |
Some countries have included NRG1 testing as either mandatory (the Netherlands) or as recommendation (Austria, France) in their national guidelines. This inclusion is due to the relatively poor prognosis of patients with NRG1 fusion-positive NSCLC, the lack of response to treatment with either chemotherapy or immunotherapy, and the potential inclusion of these patients in ongoing clinical trials (NCT02912949).69
Predictive biomarker testing rates
Real-world data of predictive biomarker testing rates in patients with NSCLC is limited, and the available information is often outdated or grouped with older data (Table 3).7,44,70,72,76 However, recent studies from England, Spain, the Netherlands, and Norway have demonstrated high testing rates for EGFR and ALK.71,74,75,77 It should be noted that testing rates of predictive biomarkers introduced after EGFR and ALK tend to be lower, but should be interpreted with caution, due to the often limited reporting of these biomarkers and potential variations in testing methodologies over time. Observational studies have also highlighted substantial variation in molecular testing practices for NSCLC between institutions within a country, both in European countries and the United States.78, 79, 80 This suggests that access to predictive biomarker testing may be influenced by factors such as the location of the hospital where patients receive their diagnosis and treatment, accessibility of technology for comprehensive biomarker testing, reimbursement issues and/or lack of knowledge. In some countries, efforts have been made to actively improve access and overall functioning of molecular testing for NSCLC patients through dedicated projects and policies.81, 82, 83, 84 With regard to England, it should be noted that a comprehensive overview of national predictive biomarker testing rates is currently being established by the National Disease Registration Service. In the Netherlands, molecular testing results are registered nationwide in the Dutch Pathology Registry (Palga), which enables researchers to examine these reports of patients diagnosed with NSCLC within a specified time period. Using this registry, reliable national testing rates of EGFR, ALK, ROS, MET, RET, BRAF, and ERBB2 in 2017, and KRAS in 2013–2017 have previously been determined (see Table 3).44,74 Importantly, for other countries, national testing rates are not yet available and (publicly) available testing rates are limited to regional or multi-institutional patient populations, thereby potentially limiting their representativeness for the entire country.
Table 3.
Published molecular testing rates for patients with metastatic NSCLC across participating European countries.
| Austria70 | Belgiuma | Czech Republicc | England71 | France72 | Germany73 | Netherlands44,74 | Portugal | Slovenia61 | Spain75 | Swedend | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EGFR | 77.1% | 51% | 79.3% | 92% | 53% | 72.5% | ∼60–82%b | No data | 91% | 91.4% | No data (authors estimate: ∼90%) |
| Year(s) of data | (2013–2015) | (2011) | (2011) | (2017) | (2015–2018) | (2015–2019) | (2017) | (Single center, 2020) | (2018–2019) | ||
| ALK | 62.5% | No data | 82.7% | 80% | 46% | 74.5% | ∼35%–55%b,e | No data | 87% | 80.1% | No data (authors estimate: ∼90%) |
| Year(s) of data | (2013–2015) | (2013) | (2017) | (2015–2018) | (2015–2019) | (2017) | (Single center, 2018) | (2018–2019) | |||
| ROS1 | No data | No data | 82.7% | No data | 34% | 66.1% | ∼28–38%b,f | No data | 86% | 56.2% | No data (authors estimate: ∼90%) |
| Year(s) of data | (2015) | (Mutation and/or fusion, 2015–2018) | (2015–2019) | (2017, total) | (Single center, 2018) | (2018–2019) | |||||
| BRAF | No data | No data | No data | No data | 38% | 53.0% | ∼60–78%b | No data | No data | No data | No data (authors estimate: ∼70%) |
| Year(s) of data | (2015–2018) | (2015–2019) | (2017) | ||||||||
| RET | No data | No data | No data | No data | No data | 26.9% | ∼18–19%b,g | No data | No data | No data | No data (authors estimate: ∼70%) |
| Year(s) of data | (2015–2019) | (2017, in total) | |||||||||
| MET mut. | No data | No data | No data | No data | 12% | 35.4% | ∼55–68%b | No data | No data | No data | No data (authors estimate: ∼70%) |
| Year(s) of data | (Mutation and/or fusion, 2015–2018) | (2015–2019) | (2017) | ||||||||
| MET amp. | No data | No data | No data | No data | See ‘MET mut.’ | No data | No data | No data | No data | No data | No data |
| Year(s) of data | |||||||||||
| KRAS | No data | No data | No data | No data | 45% | 44.9% | 82.0% | No data | No data | No data | No data (authors estimate: ∼70%) |
| Year(s) of data | (2015–2018) | (2015–2019) | (2017) | ||||||||
| NTRK | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data (authors estimate: ∼50%) |
| Year(s) of data | |||||||||||
| HER2/ERBB2 | No data | No data | No data | No data | 30% | 15.2% | ∼55–76%b | No data | No data | No data | No data (authors estimate: ∼50%) |
| Year(s) of data | (2015–2018) | (2015–2019) | (2017) | ||||||||
| NRG1 | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data | No data (authors estimate: ∼20%) |
| Year(s) of data | |||||||||||
| PD-L1 | No data | No data | 89.5% | 87% | 17% | 66.2% | No data | No data | 74–91% | 58.1% | No data (authors estimate: ∼90%) |
| Year(s) of data | (2016) | (2017) | (2015–2018) | (2015–2019) | (Single center, 2018) | (2019–2020) |
Recent data not publicly available, but could be requested at RIZIV and Belgian Cancer registration.
Higher rate is applicable to adenocarcinoma, lower rate is applicable to NSCLC-NOS.
National testing rates from national cancer registry, calculated from cases eligible for molecular testing (e.g., molecular testing for NSCLC NOS and adenocarcinoma, PD-L1 for all NSCLC).
No public data available on testing rates, displayed numbers are the Swedish authors’ estimations based on their expected national adherence to guidelines, personal communication of authors, degree of sequential testing.
∼70–80% if EGFR/KRAS/BRAF/ERBB2/MET wildtype.
∼60–70% if EGFR/KRAS/BRAF/ERBB2/MET wildtype.
∼42–44% if EGFR/KRAS/BRAF/ERBB2/MET wildtype.
Interpretation of molecular test results
Among the participating centers, there is a significant overlap in the elements provided in molecular pathology reports for NSCLC. These include information on the genes tested, the specific variants detected, variant allele frequency, and tumor cell percentage (Table 4). However, differences arise in the translation of test outcome to report conclusion, and the interpretation of reports by healthcare professionals for patient care. While most participating centers provide general treatment advice based on test results, only a few centers offer mutation-specific recommendations. International guidelines on reporting molecular results recommend a general treatment advice in cases with pathogenic, actionable molecular aberrations.85 However, in some countries, national guidelines state that such treatment recommendations are not allowed to be stated in the pathology report.
Table 4.
Reporting of molecular testing results for patients with metastatic NSCLC across participating centers (of clinical scientist in molecular pathology/(molecular) pathologist).
 |
We also inquired among the participating centers what methodology was used for discussing and advising on complex molecular results of patients with metastatic NSCLC (Table 5). The establishment of dedicated Molecular Tumor Boards (MTBs) for the interpretation of complex molecular testing results is common among the centers. However, only a few countries, including Germany and the Netherlands, have national guidelines in place that outline the composition and functioning of these treatment advisory boards.65,86,87 The variation in the translation and interpretation of complex or rare molecular testing results highlights the need for both to use and report following existing guidelines as well as further standardized guidelines and recommendations to ensure a consistent and accurate understanding of complex/rare molecular testing results among healthcare professionals.85 These guidelines should help to guide appropriate treatment decision-making and patient care optimization in the context of NSCLC.
Table 5.
Methodology of discussing complex molecular results of patients with metastatic NSCLC across participating centers (of clinical scientist in molecular pathology/(molecular) pathologist).
 |
Barriers for off-label use, compassionate use programs and trial participation
As stated in Section Availability of targeted therapies, the availability of targeted therapies for patients with NSCLC varies among European countries. Some targeted therapies have not (yet) received approval from the EMA, or they may not be registered or covered by national health insurance systems. In cases where targeted therapy is not standardly available, there are alternative options for patients to access these treatments. These options include participation in clinical trials, off-label targeted therapy use, and compassionate use or patient-named programs. Off-label use refers to the use of a drug for a purpose not (yet) specifically approved by regulatory authorities. Compassionate use programs allow patients with serious or life-threatening conditions to access experimental therapies outside of clinical trials.
Though these alternative options for targeted drug access are valuable for patients who would otherwise not receive (potentially) effective targeted therapy treatment, various authors of this review have encountered issues when attempting to utilize these opportunities for their patients. These issues include the lack of up-to-date and user-friendly databases for clinical trials and compassionate use programs, limited access to ongoing (international) clinical studies, challenges related to ineligibility criteria such as brain metastases, and compulsory individual negotiations with health insurances providers for off-label use, which may vary depending of the treating center. In some cases, university hospitals may have better chances of obtaining approval for off-label use due to their expertise and resources. Overall, navigating access to targeted therapies outside of standard availability can be complex and may require close collaboration between healthcare providers, patients, and regulatory bodies to ensure the best possible available treatment options for individual patients. In addition, we should aim for equal access to all presently available innovative systemic treatments (i.e., targeted therapies, immune checkpoints inhibitors, antibody-drug conjugates) for all patients with metastatic NSCLC.
Conclusions
The availability of targeted therapies for advanced stage NSCLC varies across European countries, with high availability of EGFR- (except amivantamab), ALK-, ROS1-, and BRAF V600-targeted therapies. However, there is considerable variability in the availability of other targeted therapies, and not all national guidelines align with European guidelines or reflect the availability of specific targeted therapies in each country. Recent real-world data studies in Norway, Spain, the Netherlands, and England have reported encouraging EGFR testing rates, though recent data are not publicly available for other participating countries. Data on testing rates for other predictive markers are often either outdated or not widely reported. Therefore, it remains uncertain what proportion of European patients with advanced stage NSCLC receive adequate molecular testing. All centers contributing to this review have the capacity to perform DNA- and RNA-based NGS for recommended biomarkers (Table 6), and nearly all have established MTBs to discuss complex/rare molecular testing results. Molecular test reports of participating centers were largely overlapping in their content, with the exception of the comprehensiveness of treatment recommendations. While providing an overview of the characteristics of this select group of high-expertise, often university-affiliated centers, the overall real-world implementation of (large-panel) NGS and use of MTB may be suboptimal in other laboratories performing molecular testing within these European countries, as indicated in recent literature.88,89 Importantly, there is still a lack of international guidelines that define the role, criteria for patient enrollment, and composition of MTBs. Developing such consensus guidelines will provide a framework for interpretation of rare or complex mutations, guide appropriate treatment-decision making, and ensure quality control within MTBs. A future perspective for molecular testing in advanced stage NSCLC is described in the separate viewpoint paper.11
Table 6.
Available biomarker test types for patients with metastatic NSCLC in participating centers (of clinical scientist in molecular pathology/(molecular) pathologist).
 |
Contributors
VdJ contributed to the conceptualization, data curation, methodology, visualization, writing–original draft, writing–review & editing; WT contributed to writing–original draft, writing–review & editing; AB, JB, LB, RB, GF, LH, MH, PH, AJ, MJ, LvK, IK, FLR, ML, JCM, KM, LPA, AR, PT, and JW contributed to the investigation/data acquisition, writing–review & editing; ES and AvdW contributed to the conceptualization, methodology, supervision, writing–original draft, writing–review & editing.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
WT has received consulting fees from Merck Sharp and Dohme, Bristol-Myers Squibb, and Altana (fees to institution), is board member of Dutch Society of Pathology and member of Council for Research and Innovation of the Federation of Medical Specialists (FMS); AB has received consulting fees from Sanofi (OncoCollective advisory board), payments or honoraria from Roche (oral presentation), support for attending ASCO 2023 from Pfizer; JB has received research grants from Amgen and Bristol-Myers Squibb, lecture honoraria from AstraZeneca, Merck Sharp and Dohme, Roche, Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, Novartis, GSK, Eli Lilly, Amgen, and Sanofi, and support for attending meetings and/or travel from Amgen; LB has received grants or contract from Takeda, Roche, AstraZeneca, and Bristol-Myers Squibb, payments or honoraria from Invitae, Eli Lilly, AstraZeneca, Roche, Merck Sharp and Dohme, Merck, Bristol-Myers Squibb, Pfizer, Novartis, Takeda, and Janssen, has participated in data safety monitoring boards or advisory boards for Invitae, Eli Lilly, AstraZeneca, Roche, Merck Sharp and Dohme, Merck, Bristol-Myers Squibb, Pfizer, Novartis, Takeda, and Janssen, is Int. Secretary of the Austrian Society of Pathology, PPS Membership and Awards Committee, and is Member of the Mesothelioma Committee of IASLC; RB has received payments or honoraria for lectures and advisory boards for Abbvie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Illumina, Janssen, Lilly, Merck-Serono, Merck Sharp and Dohme, Novartis, Qiagen, Pfizer, Roche, and Targos MP Inc., has received payments for expert testimony from Merck Sharp and Dohme, is co-founder and co-owner of Gnothis Inc. (SE), Timer Therapeutics Inc. (GE); GF has received fees for advisory boards participation, meetings, or as invited speaker, from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Janssen, Merck Sharp and Dohme, Pfizer, Roche Farmacêutica Química Lda, and Takeda; MH has received payments or honoraria from Merck Sharp and Dohme, Roche, Lilly, AstraZeneca, and Takeda; PH has received consulting fees from AstraZeneca, Roche, Abbvie, Qiagen, and Pfizer, has received payments or honoraria from Thermofisher Scientist, Novartis, Janssen, AstraZeneca, and Biocartis, has received support for attending meetings and/or travel from Thermofisher Scientist, Novartis, Janssen, AstraZeneca, and Biocartis, has participated on a Data Safety Monitoring Board or Advisory Board for Thermofisher Scientist, Novartis, Janssen, AstraZeneca, Biocartis, Bristol-Myers Squibb, Sanofi, Roche, and Abbvie; LvK has received institutional grants or contract from Amgen, AstraZeneca, Bayer, Janssen-Cilag, Merck, Roche, and Servier, has received institutional payments or honoraria from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Eli Lilly, Novartis, Pfizer, and Roche, has received institutional support for attending meeting and/or travel from Roche, has participated on a Data Safety Monitoring Board or Advisory Board from Janssen-Cilag, Merck, and Roche, has a leadership or fiduciary role in the Commission Personalized Medicine—Belgium (unpaid), has stock or stock options in Cyclomics (institutional/personal); JCM has received payments or honoraria from Lilly Portugal, Novartis Portugal, Roche Portugal, Fresco Produções, Janssen-Cilag Portugal, Pierre Fabre Portugal, and Servier Portugal; has participated on a Data Safety Monitoring Board or Advisory Board of Roche Portugal; KM has received payments or honoraria from Takeda, Janssen, Pfizer, Merck Sharp and Dohme, Bristol-Myers Squibb, Roche, Amgen, Novartis, and Eli Lilly, has participated on a Data Safety Monitoring Board or Advisory Board of BMS, Takeda, AstraZeneca, Amgen, Roche, Boehringer Ingelheim, and Janssen; SP has received consulting fees (personal fees) from Amgen, AstraZeneca, Bayer, Blueprint, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, GSK, Guardant Health, Incyte, Janssen, Lilly, Merck Serono, Merck Sharp and Dohme, Novartis, Roche, Takeda, Pfizer, Seattle Genetics, Turning Point Therapeutics, and EQRx, has received payments or honoraria (personal fees) from AstraZeneca, Bayer, Guardant Health, Janssen, Merck Serono, Roche, and Takeda, has received payments for expert testimony (personal fees) from Roche, and Merck Serono, has received reimbursement of travel expenses from Janssen, and Roche, has participated on a Data Safety Monitoring Board or Advisory Board as per consulting fees, has a leadership role or fiduciary role (all unpaid) in the British Thoracic Oncology Group, ALK Positive UK, Lung Cancer Europe, Ruth Strauss Foundation, Mesothelioma Applied Research Foundation, and ETOP-IBCSG Partners Foundation Board; AR has received consulting fees for participation on advisory boards of AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Amgen, Merck Sharp and Dohme, Gilead, and Sanofi, has received honoraria for lectures and presentations from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Amgen, Merck Sharp and Dohme, Gilead, Roche, Merck-Serono, has received support for attendings meetings and travel from Sanofi, Gilead, Roche, and Bristol-Myers Squibb; JW has received payments or honoraria, has received support for attendings meetings and travel, and has participated in a Data Safety Monitoring Board or Advisory Board, from Amgen, AstraZeneca, Bayer, Blueprint, BMS, Boehringer-Ingelheim, Chugai, Daiichi Sankyo, Janssen, Lilly, Loxo, Merck, Mirati, MSD, Novartis, Nuvalent, Pfizer, Pierre-Fabre, Roche, Seattle Genetics, Takeda, and Turning Point; ES has received unrestricted grants (all paid to UMCG institution) from Abbott, Biocartis, AstraZeneca, Invitae/Archer, Bayer, Bio-Rad, Roche, Agena Bioscience, CC Diagnostics, MSD/MERCK, and Boehringer Ingelheim, has received consulting fees (all paid to UMCG institution) from MSD/Merck, AstraZeneca, Roche, Novartis, Bayer, BMS, Lilly, Amgen, Illumina, Agena Bioscience, CC Diagnostics, Janssen Cilag (Johnson & Johnson), Astellas Pharma, GSK, Sinnovisionlab, and Sysmex, has received payments or honoraria (all paid to UMCG institution) from Bio-Rad, Seracare, Roche, Biocartis, Lilly, Agena Bioscience, and Illumina, has received support for attending meetings and/or travel from BioRad, Biocartis, Ageno Sciences, and Illumina, is a board member for the Dutch Society of Pathology (unpaid), European Society of Pathology (unpaid), European Liquid Biopsy Society (unpaid), is a secretary/member of the advisory committee for assessment of molecular diagnostics (cieBOD) (honoraria paid to UMCG institution), is committee member of national guideline advisory (honoraria paid to UMCG institution); AvdW, has received grants or contracts from AstraZeneca, Boehringer-Ingelheim, Pfizer, Roche, and Takeda, has received consulting fees from AstraZeneca, Janssen, Lilly, Roche, and Takeda, has received payments or honoraria from AstraZeneca, BMS, Lilly, Pfizer, and Roche, has a leadership or fiduciary role in the oncology section NVALT, guideline committee NSCLC and CUP, dure geneesmiddelen committee NVALT and FMS. All other authors declare no competing interests.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.100838.
Contributor Information
Ed Schuuring, Email: e.schuuring@umcg.nl.
Anthonie J. van der Wekken, Email: a.j.van.der.wekken@umcg.nl.
Appendix A. Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Malvezzi M., Santucci C., Boffetta P., et al. European cancer mortality predictions for the year 2023 with focus on lung cancer. Ann Oncol. 2023;34(4):410–419. doi: 10.1016/j.annonc.2023.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Gregor A., Inage T., Hwangbo B., Yasufuku K. Lung cancer staging: state of the art in the era of ablative therapies and surgical segmentectomy. Respirology. 2020;25(9):924–932. doi: 10.1111/resp.13827. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N., Forjaz G., Mooradian M.J., et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendriks L.E., Kerr K.M., Menis J., et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):339–357. doi: 10.1016/j.annonc.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Thunnissen E., Weynand B., Udovicic-Gagula D., et al. Lung cancer biomarker testing: perspective from Europe. Transl Lung Cancer Res. 2020;9(3):887–897. doi: 10.21037/tlcr.2020.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryska A., Berzinec P., Brcic L., et al. NSCLC molecular testing in Central and Eastern European countries. BMC Cancer. 2018;18(1):269. doi: 10.1186/s12885-018-4023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerr K.M., Bibeau F., Thunnissen E., et al. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer. 2021;154:161–175. doi: 10.1016/j.lungcan.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Normanno N., Apostolidis K., Wolf A., et al. Access and quality of biomarker testing for precision oncology in Europe. Eur J Cancer. 2022;176:70–77. doi: 10.1016/j.ejca.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Bayle A., Bonastre J., Chaltiel D., et al. ESMO study on the availability and accessibility of biomolecular technologies in oncology in Europe. Ann Oncol. 2023;34(10):934–945. doi: 10.1016/j.annonc.2023.06.011. [DOI] [PubMed] [Google Scholar]
- 11.de Jager V.D., Timens W., Bayle A., et al. Future perspective for the application of predictive biomarker testing in advanced stage non-small cell lung cancer. Lancet Reg Health Eur. 2024;38:100839. doi: 10.1016/j.lanepe.2024.100839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houda I., Dickhoff C., Uyl-de Groot C.A., et al. New systemic treatment paradigms in resectable non-small cell lung cancer and variations in patient access across Europe. Lancet Reg Health Eur. 2024;38:100840. doi: 10.1016/j.lanepe.2024.100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adib E., Nassar A.H., Abou Alaiwi S., et al. Variation in targetable genomic alterations in non-small cell lung cancer by genetic ancestry, sex, smoking history, and histology. Genome Med. 2022;14(1):39. doi: 10.1186/s13073-022-01041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koopman B., Cajiao Garcia B.N., Kuijpers C., et al. A nationwide study on the impact of routine testing for EGFR mutations in advanced NSCLC reveals distinct survival patterns based on EGFR mutation subclasses. Cancers. 2021;13(14):3641. doi: 10.3390/cancers13143641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soria J.C., Ohe Y., Vansteenkiste J., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 16.Yang J.C., Sequist L.V., Geater S.L., et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830–838. doi: 10.1016/S1470-2045(15)00026-1. [DOI] [PubMed] [Google Scholar]
- 17.Elamin Y.Y., Robichaux J.P., Carter B.W., et al. Poziotinib for EGFR exon 20-mutant NSCLC: clinical efficacy, resistance mechanisms, and impact of insertion location on drug sensitivity. Cancer Cell. 2022;40(7):754–767.e6. doi: 10.1016/j.ccell.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou C., Ramalingam S.S., Kim T.M., et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR Exon 20 insertion-positive metastatic non-small cell lung cancer: a phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol. 2021;7(12) doi: 10.1001/jamaoncol.2021.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park K., Haura E.B., Leighl N.B., et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391–3402. doi: 10.1200/JCO.21.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duke E.S., Stapleford L., Drezner N., et al. FDA approval summary: mobocertinib for metastatic non-small cell lung cancer with EGFR exon 20 insertion mutations. Clin Cancer Res. 2023;29(3):508–512. doi: 10.1158/1078-0432.CCR-22-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chon K., Larkins E., Chatterjee S., et al. FDA approval summary: amivantamab for the treatment of patients with non-small cell lung cancer with EGFR exon 20 insertion mutations. Clin Cancer Res. 2023;29(17):3262–3266. doi: 10.1158/1078-0432.CCR-22-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C., Zhao K., Hu S., Dong W., Gong Y., Xie C. Clinical outcomes of afatinib versus osimertinib in patients with non-small cell lung cancer with uncommon EGFR mutations: a pooled analysis. Oncologist. 2023;28(6):e397–e405. doi: 10.1093/oncolo/oyad111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robichaux J.P., Le X., Vijayan R.S.K., et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature. 2021;597(7878):732–737. doi: 10.1038/s41586-021-03898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y.L., Tsuboi M., He J., et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 25.Houda I., Dickhoff C., Uyl-de Groot C.A., et al. Challenges and controversies in resectable non-small cell lung cancer: a clinician’s perspective. Lancet Reg Health Eur. 2024;38:100841. doi: 10.1016/j.lanepe.2024.100841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koopman B., Groen H.J.M., Schuuring E., et al. Actionability of on-target ALK resistance mutations in patients with non-small cell lung cancer: local experience and review of the literature. Clin Lung Cancer. 2022;23(2):e104–e115. doi: 10.1016/j.cllc.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Ariyasu R., Kakuto S., Miyadera K., et al. Real-world outcome analysis of patients with stage IV NSCLC treated with tyrosine kinase and immune checkpoint inhibitors. JTO Clin Res Rep. 2023;4(6) doi: 10.1016/j.jtocrr.2023.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ou S.I., Nagasaka M., Brazel D., Hou Y., Zhu V.W. Will the clinical development of 4th-generation "double mutant active" ALK TKIs (TPX-0131 and NVL-655) change the future treatment paradigm of ALK+NSCLC? Transl Oncol. 2021;14(11) doi: 10.1016/j.tranon.2021.101191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw A.T., Ou S.H., Bang Y.J., et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drilon A., Siena S., Dziadziuszko R., et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou S.I., Zhu V.W. CNS metastasis in ROS1+ NSCLC: an urgent call to action, to understand, and to overcome. Lung Cancer. 2019;130:201–207. doi: 10.1016/j.lungcan.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 32.Dankner M., Rose A.A.N., Rajkumar S., Siegel P.M., Watson I.R. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37(24):3183–3199. doi: 10.1038/s41388-018-0171-x. [DOI] [PubMed] [Google Scholar]
- 33.Lim G.H.T., Balbi K.J., Poskitt B., Bennett P., Moore D.A. Prevalence and breakdown of non-small cell lung cancer BRAF driver mutations in a large UK cohort. Lung Cancer. 2022;173:71–74. doi: 10.1016/j.lungcan.2022.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Dankner M., Wang Y., Fazelzad R., et al. Clinical activity of mitogen-activated protein kinase-targeted therapies in patients with non-V600 BRAF-mutant tumors. JCO Precis Oncol. 2022;6 doi: 10.1200/PO.22.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forsythe A., Zhang W., Phillip Strauss U., Fellous M., Korei M., Keating K. A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920975613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong D.S., DuBois S.G., Kummar S., et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531–540. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drilon A., Siena S., Ou S.I., et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1) Cancer Discov. 2017;7(4):400–409. doi: 10.1158/2159-8290.CD-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drilon A., Tan D.S.W., Lassen U.N., et al. Efficacy and safety of larotrectinib in patients with tropomyosin receptor kinase fusion-positive lung cancers. JCO Precis Oncol. 2022;6 doi: 10.1200/PO.21.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doebele R.C., Drilon A., Paz-Ares L., et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21(2):271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drilon A., Oxnard G.R., Tan D.S.W., et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med. 2020;383(9):813–824. doi: 10.1056/NEJMoa2005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gainor J.F., Curigliano G., Kim D.W., et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22(7):959–969. doi: 10.1016/S1470-2045(21)00247-3. [DOI] [PubMed] [Google Scholar]
- 42.Hobbs G.A., Der C.J., Rossman K.L. RAS isoforms and mutations in cancer at a glance. J Cell Sci. 2016;129(7):1287–1292. doi: 10.1242/jcs.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terrenato I., Ercolani C., Di Benedetto A., et al. A real-world systematic analysis of driver mutations' prevalence in early- and advanced-stage NSCLC: implications for targeted therapies in the adjuvant setting. Cancers. 2022;14(12):2971. doi: 10.3390/cancers14122971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia B.N.C., van Kempen L.C., Kuijpers C., Schuuring E., Willems S.M., van der Wekken A.J. Prevalence of KRAS p.(G12C) in stage IV NSCLC patients in the Netherlands; a nation-wide retrospective cohort study. Lung Cancer. 2022;167:1–7. doi: 10.1016/j.lungcan.2022.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Isaksson J., Berglund A., Louie K., et al. KRAS G12C mutant non-small cell lung cancer linked to female sex and high risk of CNS metastasis: population-based demographics and survival data from the National Swedish Lung Cancer Registry. Clin Lung Cancer. 2023;24(6):507–518. doi: 10.1016/j.cllc.2023.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Cox A.D., Fesik S.W., Kimmelman A.C., Luo J., Der C.J. Drugging the undruggable RAS: mission possible? Nat Rev Drug Discov. 2014;13(11):828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostrem J.M., Peters U., Sos M.L., Wells J.A., Shokat K.M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanman B.A., Allen J.R., Allen J.G., et al. Discovery of a covalent inhibitor of KRAS(G12C) (AMG 510) for the treatment of solid tumors. J Med Chem. 2020;63(1):52–65. doi: 10.1021/acs.jmedchem.9b01180. [DOI] [PubMed] [Google Scholar]
- 49.Skoulidis F., Li B.T., Dy G.K., et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Langen A.J., Johnson M.L., Mazieres J., et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet. 2023;401(10378):733–746. doi: 10.1016/S0140-6736(23)00221-0. [DOI] [PubMed] [Google Scholar]
- 51.Fujino T., Suda K., Mitsudomi T. Lung cancer with MET exon 14 skipping mutation: genetic feature, current treatments, and future challenges. Lung Cancer. 2021;12:35–50. doi: 10.2147/LCTT.S269307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Awad M.M., Oxnard G.R., Jackman D.M., et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-met overexpression. J Clin Oncol. 2016;34(7):721–730. doi: 10.1200/JCO.2015.63.4600. [DOI] [PubMed] [Google Scholar]
- 53.Remon J., Hendriks L.E.L., Mountzios G., et al. MET alterations in NSCLC-current perspectives and future challenges. J Thorac Oncol. 2023;18(4):419–435. doi: 10.1016/j.jtho.2022.10.015. [DOI] [PubMed] [Google Scholar]
- 54.Wolf J., Seto T., Han J.Y., et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383(10):944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 55.Paik P.K., Felip E., Veillon R., et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Planchard D., Popat S., Kerr K., et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 57.NHS England National genomic test directory (version June 2023) https://www.england.nhs.uk/publication/national-genomic-test-directories/
- 58.Lung cancer: diagnosis and management (NICE guideline [NG122]) 2023. https://www.nice.org.uk/guidance/ng122 [PubMed] [Google Scholar]
- 59.Teixeira M.E., Oliveira J., Borralho P., et al. Portuguese consensus recommendations for next-generation sequencing of lung cancer, rare tumors, and cancers of unknown primary origin in clinical practice. Acta Med Port. 2022;35(9):677–690. doi: 10.20344/amp.17680. [DOI] [PubMed] [Google Scholar]
- 60.Popper H.H., Gruber-Mosenbacher U., Pall G., et al. The 2020 update of the recommendations of the Austrian working group on lung pathology and oncology for the diagnostic workup of non-small cell lung cancer with focus on predictive biomarkers. Memo. 2020;13(1):11–26. [Google Scholar]
- 61.Janžič U. 2022. POROČILO BOLNIŠNIČNEGA REGISTRA TUMORJEV PRSNEGA KOŠA KLINIKE GOLNIK ZA LETA 2010 – 2020.https://www.klinika-golnik.si/dejavnost-klinike/onkoloska-dejavnost/klinicni-register-bolnikov-z-rakom-pljuc [Google Scholar]
- 62.Oncologie: Terugbetaling van de moleculair biologische testen met “next generation sequencing” (NGS) https://www.riziv.fgov.be/nl/professionals/verzorgingsinstellingen/laboratoria/Paginas/oncologie-terugbetaling-moleculair-biologische-ngs.aspx
- 63.Griesinger F., Absenger G., Bleckmann A., et al. 2022. Lungenkarzinom, nicht-kleinzellig (NSCLC)https://www.onkopedia.com/de/onkopedia/guidelines/lungenkarzinom-nicht-kleinzellig-nsclc/@@guideline/html/index.html [Google Scholar]
- 64.Patients atteints d'un cancer bronchique non à petites cellules: indications des tests moléculaires en vue de la prescription de traitements de précision. 2023. https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Patients-atteints-d-un-cancer-bronchique-non-a-petites-cellules-indications-des-tests-moleculaires-en-vue-de-la-prescription-de-traitements-de-precision [Google Scholar]
- 65.Federatie Medisch Specialisten (FMS) - Richtlijn Niet-kleincellig longcarcinoom. 2020. https://richtlijnendatabase.nl/richtlijn/niet_kleincellig_longcarcinoom/startpagina_-_niet-kleincellig_longcarcinoom.html [Google Scholar]
- 66.Lungcancer - Nationellt vårdprogram (v7.0) 2023. https://kunskapsbanken.cancercentrum.se/globalassets/cancerdiagnoser/lunga-och-lungsack/vardprogram/nationellt-vardprogram-lungcancer.pdf [Google Scholar]
- 67.Isla D., Lozano M.D., Paz-Ares L., et al. [New update to the guidelines on testing predictive biomarkers in non-small-cell lung cancer: a National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology] Rev Esp Patol. 2023;56(2):97–112. doi: 10.1016/j.patol.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Direção-Geral da Saúde (DGS) - Diagnóstico e Tratamento do Carcinoma de Não Pequenas Células do Pulmão. 2013. https://normas.dgs.min-saude.pt/wp-content/uploads/2019/09/diagnostico-e-tratamento-do-carcinoma-de-nao-pequenas-celulas-do-pulmao.pdf [Google Scholar]
- 69.Drilon A., Duruisseaux M., Han J.Y., et al. Clinicopathologic features and response to therapy of NRG1 fusion-driven lung cancers: the eNRGy1 global multicenter registry. J Clin Oncol. 2021;39(25):2791–2802. doi: 10.1200/JCO.20.03307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burghuber O.C., Kirchbacher K., Mohn-Staudner A., et al. Results of the Austrian national lung cancer audit. Clin Med Insights Oncol. 2020;14 doi: 10.1177/1179554920950548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adizie J.B., Tweedie J., Khakwani A., et al. Biomarker testing for people with advanced lung cancer in England. JTO Clin Res Rep. 2021;2(6) doi: 10.1016/j.jtocrr.2021.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lamy T., Cabarrou B., Planchard D., et al. Biomarker testing in older patients treated for an advanced or metastatic non-squamous non-small-cell lung cancer: the French ESME real-life multicenter cohort experience. Cancers. 2021;14(1):92. doi: 10.3390/cancers14010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griesinger F., Eberhardt W., Nusch A., et al. Biomarker testing in non-small cell lung cancer in routine care: analysis of the first 3,717 patients in the German prospective, observational, nation-wide CRISP registry (AIO-TRK-0315) Lung Cancer. 2021;152:174–184. doi: 10.1016/j.lungcan.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 74.Steeghs E.M.P., Groen H.J.M., Schuuring E., et al. Mutation-tailored treatment selection in non-small cell lung cancer patients in daily clinical practice. Lung Cancer. 2022;167:87–97. doi: 10.1016/j.lungcan.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 75.Salas C., Martin-Lopez J., Martinez-Pozo A., et al. Real-world biomarker testing rate and positivity rate in NSCLC in Spain: prospective central lung cancer biomarker testing registry (LungPath) from the Spanish Society of Pathology (SEAP) J Clin Pathol. 2022;75(3):193–200. doi: 10.1136/jclinpath-2020-207280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuijpers C., van den Heuvel M.M., Overbeek L.I.H., et al. [National variation in molecular diagnostics in metastatic lung cancer] Ned Tijdschr Geneeskd. 2018;162 [PubMed] [Google Scholar]
- 77.Eide I.J.Z., Nilssen Y., Stensland E.M., Brustugun O.T. Real-world data on EGFR and ALK testing and TKI usage in Norway-a nation-wide population study. Cancers. 2023;15(5):1505. doi: 10.3390/cancers15051505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baijal S., Crosbie P., Fenemore J., Desai K. Variations in genomic testing in non-small cell lung carcinoma: a healthcare professional survey of current practices in the UK. Oncologist. 2023;28(8):e699–e702. doi: 10.1093/oncolo/oyad134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fox A.H., Jett J.R., Roy U.B., et al. Knowledge and practice patterns among pulmonologists for molecular biomarker testing in advanced non-small cell lung cancer. Chest. 2021;160(6):2293–2303. doi: 10.1016/j.chest.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roberts T.J., Kehl K.L., Brooks G.A., et al. Practice-level variation in molecular testing and use of targeted therapy for patients with non-small cell lung cancer and colorectal cancer. JAMA Netw Open. 2023;6(4) doi: 10.1001/jamanetworkopen.2023.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cox S., Powell C., Morgan S. Implementing genomic testing for lung cancer into routine clinical practice - the Welsh experience. Clin Oncol. 2022;34(11):716–723. doi: 10.1016/j.clon.2022.08.025. [DOI] [PubMed] [Google Scholar]
- 82.de Jager V.D., Cajiao Garcia B.N., ter Elst A., et al. Optimalisatie van de zorgketen moleculaire diagnostiek bij niet-kleincellig longcarcinoom in Noord-Nederland. Ned Tijdschr Oncol. 2023;20:70–76. [Google Scholar]
- 83.Ossowski S., Neeman E., Borden C., et al. Improving time to molecular testing results in patients with newly diagnosed, metastatic non-small-cell lung cancer. JCO Oncol Pract. 2022;18(11):e1874–e1884. doi: 10.1200/OP.22.00260. [DOI] [PubMed] [Google Scholar]
- 84.Navani N., Butler R., Ibrahimo S., et al. Optimising tissue acquisition and the molecular testing pathway for patients with non-small cell lung cancer: a UK expert consensus statement. Lung Cancer. 2022;172:142–153. doi: 10.1016/j.lungcan.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Deans Z.C., Costa J.L., Cree I., et al. Integration of next-generation sequencing in clinical diagnostic molecular pathology laboratories for analysis of solid tumours; an expert opinion on behalf of IQN Path ASBL. Virchows Arch. 2017;470(1):5–20. doi: 10.1007/s00428-016-2025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koopman B., van der Wekken A.J., Ter Elst A., et al. Relevance and effectiveness of molecular tumor board recommendations for patients with non-small-cell lung cancer with rare or complex mutational profiles. JCO Precis Oncol. 2020;4:393–410. doi: 10.1200/PO.20.00008. [DOI] [PubMed] [Google Scholar]
- 87.Koopman B., Groen H.J.M., Ligtenberg M.J.L., et al. Multicenter comparison of molecular tumor boards in the Netherlands: definition, composition, methods, and targeted therapy recommendations. Oncologist. 2021;26(8):e1347–e1358. doi: 10.1002/onco.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stenzinger A., Moltzen E.K., Winkler E., et al. Implementation of precision medicine in healthcare-a European perspective. J Intern Med. 2023;294(4):437–454. doi: 10.1111/joim.13698. [DOI] [PubMed] [Google Scholar]
- 89.Hofman P., Calabrese F., Kern I., et al. Real-world EGFR testing practices for non-small-cell lung cancer by thoracic pathology laboratories across Europe. ESMO Open. 2023;8(5) doi: 10.1016/j.esmoop.2023.101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.