Summary
Background
Use of the high-dose levonorgestrel-releasing intrauterine system (LNG-IUS) has been associated with increased risk of incident depression. Evidence is lacking on the influence of use of two recently marketed low-dose LNG-IUS on risk of depression. This study aims to examine associations between use of different doses of LNG-IUS and risk of depression.
Methods
We conducted a nationwide prospective cohort study involving all first-time users of an LNG-IUS among all Danish nulliparous women aged 15–34 years with no medical history of depression, major psychiatric diseases, endometriosis, heavy menstrual bleeding, polyp, myoma, dysmenorrhoea, iron supplement use, abortion, and infertility treatment.
Findings
A total of 46,565 first-time users of LNG-IUS were followed for 80,516 person-years with 1,531 incident initiations of antidepressant use observed during follow-up. Use of the high-dose LNG-IUS containing 52 mg levonorgestrel was initiated by 9,902 (21%) women, while 20,665 (44%), and 15,998 (34%) initiated use of the low-dose LNG-IUS containing 19·5 mg and 13·5 mg levonorgestrel, respectively.
The age-, calendar-time-, and education-standardised incidence rates of first-time depression per 1,000 person-years at full LNG-IUS duration were 30.8 (95% CI 23·6–39·5) for the 52 mg LNG-IUS, 19·8 (95% CI 16·1; 24·0) for the 19·5 mg LNG-IUS, and 17·7 (95% CI 14·4–21·5) for the 13·5 mg LNG-IUS-. Compared to the high-dose 52 mg LNG-IUS, the adjusted number of avoided depressions per 1,000 person-years were 11·0 (95% CI 7·1–14·9) for the 19·5 mg LNG-IUS and 13·1 (95% CI 9·6–16·6) for the 13·5 mg LNG-IUS. The corresponding adjusted rate ratios were 0·77 (95% CI 0·68; 0·88) and 0·85 (95% CI 0·75–0·96). The reduced risk of depression with low-dose LNG-IUS compared to high-dose LNG-IUS was observable throughout duration of use.
Interpretation
Use of low-dose LNG-IUS containing 19·5 mg and 13·5 mg levonorgestrel, respectively, were associated with a reduced risk of incident depression compared to use of the high-dose 52 mg LNG-IUS. The study suggests that low-dose LNG-IUS should be preferred over the high-dose LNG-IUS for contraceptive purpose.
Funding
Sygeforsikringen “Danmark” grant: 2021–0128.
Keywords: Antidepressant, Depression, Hormonal contraception, Levonorgestrel-releasing intrauterine device
Research in context.
Evidence before this study
Depression is a recognised common adverse effect of LNG-IUS use according to the summaries of product characteristics of the three marketed LNG-IUS containing 52 mg, 19·5 mg, and 13·5 mg levonorgestrel, respectively. Pre-marketing trials have not been sufficiently powered to detect a potential difference in risk of mood changes between different doses of LNG-IUS, and post marketing safety studies are lacking. A recent observational study from France reported a slightly higher risk of antidepressant use among women using the 52 mg LNG-IUS compared to those using the 19·5 mg LNG-IUS. To our knowledge, no studies have investigated the 13·5 mg LNG-IUS as of the search date 08/08/2023.
Added value of this study
Use of low-dose 13·5 mg and 19·5 mg LNG-IUS was associated with significantly lower risk of first-time depression compared to use of the high-dose 52 mg LNG-IUS. The depression risk was similar for the two low-dose LNG-IUS.
Implications of all the available evidence
The study supports use of low-dose LNG-IUS rather than high-dose LNG-IUS when used for contraceptive purpose only.
Introduction
The levonorgestrel-releasing intrauterine system (LNG-IUS) is an effective long-acting reversible contraceptive growing in popularity and availability worldwide. In 2015, the World Health Organization added the LNG-IUS to its Essential Medicines List,1 and the LNG-IUS Working Group, a consortium of governments, manufacturers, researchers, service delivery groups, and donors, was established to support expanded access to this contraceptive method.2 Whereas use of combined hormonal contraceptives has been declining, recent trends of increasing use of the LNG-IUS have been observed globally, including in teenagers.3, 4, 5, 6
The first-marketed LNG-IUS containing 52 mg levonorgestrel has been associated with an increased risk of depression compared with non-use and compared with use of combined oral contraceptives also containing levonorgestrel.7, 8, 9 This association has been reported strongest in young women aged 15–19.7,10
The influence of use of the two recently marketed low-dose LNG-IUS on depression risk remains uncertain. They contain a hormone reservoir of 13·5 mg and 19·5 mg levonorgestrel, respectively, and are physically smaller than the high-dose 52 mg LNG-IUS, which is why they have been recommended to nulliparous for easier insertion.11 Use of the low-dose LNG-IUS yield a smaller rise in serum-levels of levonorgestrel compared to use of the high-dose 52 mg LNG-IUS,12 but evidence is lacking on the whether this reduces the risk of experiencing depression as an adverse effect of LNG-IUS use.13
Pre-marketing randomised controlled trials have been too small to study a potential difference in depression risk between the different doses of LNG-IUS.14, 15, 16 A recent post-marketing observational study from France among women with a mean age of 32 years found a small increased risk of antidepressant use in women using the 52 mg LNG-IUS compared with users of the 19·5 mg LNG-IUS (adjusted odds ratio [aOR] of 1·13 [1·05–1·21]), with a higher corresponding risk observed in women below 25 years of age (aOR of 1·37 [0·97–1·93]). The women were matched on gravidity and only 18% were nulliparous, increasing the risk of dilution of the effect by including women in risk of postpartum depression.13 To our knowledge, no post-marketing studies have compared the depression risk with use of the 13·5 mg LNG-IUS with that of the other marketed LNG-IUS.
Considering the worldwide increasing use of LNG-IUS,3, 4, 5, 6 especially among teenagers, and the emerging evidence on the impact of hormonal contraception on mental health,7,8,10,13 there is an urging need of evidence on the influence of LNG-IUS use on depression risk in young reproductive-aged women and the impact of dose on this risk.
We report a nationwide cohort study on the depression risk among nulliparous first-time users of LNG-IUS according to the three marketed doses of LNG-IUS.
Methods
Study design
We conducted a nationwide cohort study using the following Danish national registries: 1)The National Registry of Medicinal Product Statistics,17 which includes information on all redeemed prescriptions at Danish pharmacies since January 1995, 2)The Danish Psychiatric Central Research Register,18 which includes information on all patients treated a psychiatric departments in Denmark since 1995, 3) the National Registry of Patients,19 which comprises information on discharge diagnoses and surgical codes on all somatic hospitalizations since 1977, 4) the Danish National Birth Registry,20 which holds information on all live and death births since 1973, and 5) Statistics Denmark, which provides, daily information on deaths and emigration/immigration, and yearly update on education status of all Danish citizens.
A personal identification number given to all Danish citizens at birth or immigration was used to reliably link the registries.
The nationwide cohort study included all Danish women aged 15–34 years in the period 1 January 2001–30 June 2021. The included women had no history of depression, major psychiatric disease, antidepressant use, any pregnancy, endometriosis, heavy or irregular menstrual bleeding, endometrial polyp, myoma, dysmenorrhoea, use of iron supplement, infertility treatment, or prior use of LNG-IUS. Exact definitions of the exclusion criteria are outlined in Table S1 (Supplementary Appendix).
Included women were followed from the date of first prescription redemption of an LNG-IUS, their 15th birthday, or January 1, 2001, whichever came last, until the end of the maximum approved duration of LNG-IUS use (five years for the 52 mg and 19·5 mg LNG-IUS and three years for the 13·5 mg LNG-IUS), June 30th 2021, the event of interest, death, emigration, their 35th birthday, any pregnancy, major psychiatric disease, endometriosis, heavy or irregular menstrual bleeding, endometrial polyp, myoma, dysmenorrhoea, iron supplement use, infertility treatment, or if they switched to another hormonal contraceptive.
To ensure full prescription history on all included subjects, women immigrating to Denmark following the year 1994 were excluded from the study.
LNG-IUS use
A woman was considered a user of an LNG-IUS from date of prescription redemption of such. This information was provided by the Danish Prescription Registry. Prescriptions of LNG-IUS were identified using the Anatomical Therapeutic Chemical (ATC) code G02BA03, and the registry holds information on dose of LNG-IUS purchased, allowing the distinction between LNG-IUS containing 13·5 mg, 19·5 mg, and 52 mg levonorgestrel, respectively.
In Denmark, the 52 mg LNG-IUS was available throughout the entire study period, while the 13·5 mg LNG-IUS was introduced in 2014 (late 2013) and the 19·5 mg LNG-IUS in 2017, respectively.
Depression
An incident depression was defined as a first redeemed prescription of an antidepressant from the Danish Prescription Registry (ATC code N06A∗ except N06AX12).
Statistical methods
Poisson regression models were used to estimate rates of incident depression according to the different doses of LNG-IUS. All estimates were adjusted for age (1-year intervals), calendar time (1-year intervals), and educational level (elementary school, secondary school, skilled worker, theoretical education).
To compare the depression rates across different LNG-IUS with different distributions of age, calendar time, and educational level, we have calculated the standardized incidence rates and rate differences of depression per 1,000 person-years using direct standardization. We standardized to the distribution of age, calendar year, and educational level in the entire cohort by reweighting the stratum-specific rates of depression such that the exposure groups become comparable with respect to age, calendar time, and educational level. The standardized estimates can be interpreted as the expected depression rates in the subgroups if the distribution of age, calendar time, and educational level in the subgroups had followed the distribution of these variables in the entire cohort. Standardised incidence rate differences are reported as number of avoided depressions per 1,000 person-years.
Other than at time of maximum duration of use, incidence rate ratios and differences were also estimated at 6 months, 1 year, 2 years, and 3 years following prescription redemption to account for the influence of time since initiation of use and to minimise the effect of potential unmeasured early discontinuation.
Analyses were repeated in the subpopulation of users aged 15–19 years and in the subpopulation of users in 2014–2021 (two LNG-IUS available in the entire study period) and 2017–2021 (all three LNG-IUS available in the entire study period) to study the effect of different availability of the different LNG-IUS throughout the study period.
Sensitivity analyses were conducted, in which the depression outcome was defined at time of the second and third prescription redemption of antidepressants with the condition that all the prescriptions should be filled within two years.
Previous use of other types of hormonal contraceptives was adjusted for in a sensitivity analysis.
To ensure further interchangeability between high-dose LNG-IUS users and users of the low-dose LNG-IUS, an analysis was conducted in which users of the high-dose LNG-IUS were only included in the analysis, if they had been prescribed the LNG-IUS prior to the launch of the low-dose LNG-IUS, ensuring the absence of a conscious deselection of the low-dose LNG-IUS among high-dose LNG-IUS users.
Finally, a quasi-Poisson was run in a sensitivity analysis to account for potential overdispersion.
All sensitivity analyses were conducted for 1-year use, in which 1) continuation of LNG-IUS use and 2) the difference in hormonal reservoir between the LNG-IUS are highest.
R version 3·6 was used for statistical analysis.16
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
A total of 46,565 nulliparous women initiated a first use of LNG-IUS and were followed for 80,516 years (flowchart in Supplementary Material, Figure S1). The mean age at entry were 22·2 (SD 3·7) and the average follow-up were 1·7 (SD 1·4) years. During follow-up, 1,531 events of a first antidepressant use were detected. Of the 46,565 LNG-IUS users, 9,902 (21%) initiated use of the high-dose 52 mg LNG-IUS, while 20,665 (44%), and 15,998 (34%) initiated use of the low-dose LNG-IUS containing 19·5 mg and 13·5 mg levonorgestrel, respectively. Number of events was 504 (5%), 492 (2%), and 535 (3%) for the 52 mg, 19·5 mg, and 13·5 mg LNG-IUS, respectively.
Characteristics of the women initiating the different types of LNG-IUS are shown in Table 1.
Table 1.
Characteristics of nulliparous women at study entry for different LNG-IUS types.
| Characteristic | 13·5 mg LNG-IUS | 19·5 mg LNG-IUS | 52 mg LNG-IUS |
|---|---|---|---|
| Number of women initiatingLNG-IUSuse: | |||
| Total period 2001–2021 (row %) | 15,998 (34%) | 20,665 (44%) | 9,902 (21%) |
| Period 2001–2013 (row %) | 7 (0·2%) | – | 3,636 (99·8%) |
| Period 2014–2016 (row %) | 7,205 (74·5%) | – | 2,470 (25·5%) |
| Period 2017–2021 (row %) | 8,786 (26·4%) | 20,665 (62·2%) | 3,796 (11·4%) |
| Person-years: | |||
| Max duration | 30,883 | 25,619 | 24,014 |
| 6 months duration | 8,315 | 9,732 | 4,856 |
| One year duration | 14,273 | 15,236 | 8,359 |
| Two year duration | 24,029 | 21,951 | 14,226 |
| Three year duration | 30,883 | 24,885 | 18,694 |
| Meanperson-yearsfollowed withLNG-IUSuse (SD) | 1.9 (SD1·1) | 1.2 (SD1·0) | 2.4 (SD1·8) |
| Number of first antidepressant use: | |||
| Max duration | 535 | 492 | 504 |
| 6 months duration | 110 | 166 | 115 |
| One year duration | 208 | 269 | 203 |
| Two year duration | 403 | 406 | 321 |
| Three year duration | 535 | 472 | 410 |
| Mean age at initiation (SD) | 21.9 (SD3·6) | 22.0 (SD3·5) | 23.0 (SD4·1) |
| Number of previous users of other types of hormonal contraceptives (column %) | 13,941 (87·1%) | 18,027 (87·2%) | 9,003 (90·9%) |
| Highest completed education: | |||
| Elementary school only (column %) | 4,691 (29·3%) | 5,885 (28·5%) | 2,627 (26·5%) |
| Secondary school only (column %) | 7,089 (44·3%) | 10,100 (48·9%) | 3,926 (39·6%) |
| Skilled worker (column %) | 1,245 (7·8%) | 1,254 (6·1%) | 1,344 (13·6%) |
| Theoretical education (column %) | 2,973 (18·6%) | 3,426 (16·6%) | 2,005 (20·2%) |
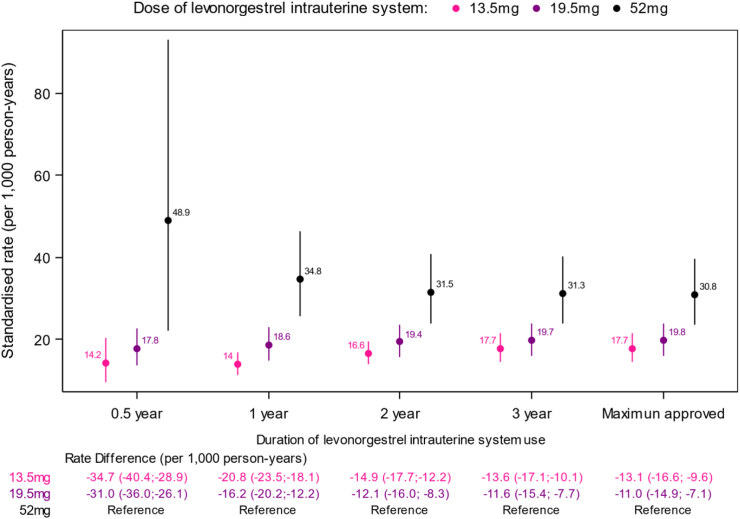
Across the maximum duration of use of the high-dose 52 mg LNG-IUS, the standardised number of depressions was 30·8 (95% CI 23·6–39·5) per 1,000 person-years. For the low-dose LNG-IUS, the corresponding number was 19·8 (95% CI 16·1–24·0) per 1,000 person-years for the 19·5 mg LNG-IUS and 17·7 (95% CI 14·4–21·5) per 1,000 person-years for the low-dose 13·5 mg LNG-IUS, respectively. The incidence rates of depression for each dose of LNG-IUS according to duration of LNG-IUS use are illustrated in Fig. 1.
Fig. 1.
Standardised∗ rates and rate differences of a first antidepressant use according to different doses and durations of LNG-IUS use. ∗Standardised according to the distribution of age (1-year intervals), calendar time (1-year intervals), and educational status (elementary school, secondary school, skilled worker, theoretical education) in the entire cohort.
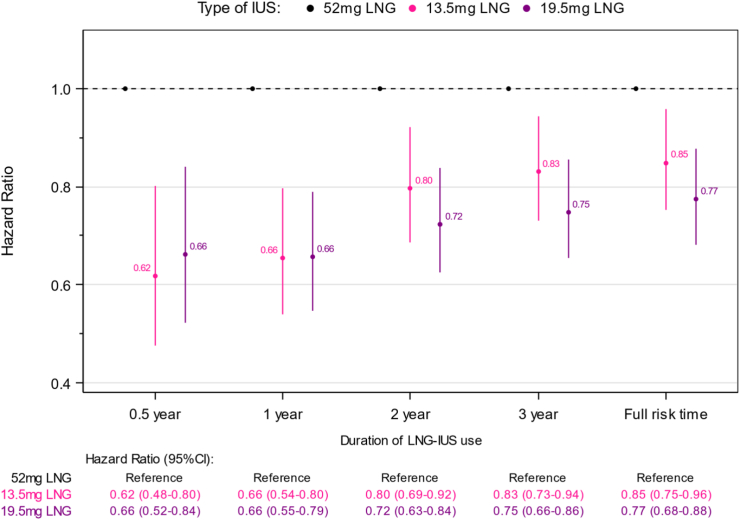
Compared to the high-dose 52 mg LNG-IUS, the estimated number of avoided depressions with use of the 19·5 mg LNG-IUS was 11·0 (95% CI 7·1–14·9) per 1,000 person-years when women were considered using the LNG-IUS for the maximum approved duration of use (Fig. 1). The corresponding adjusted relative rate ratio was 0·77 (95% CI 0·68–0·88) (Fig. 2). For the 13·5 mg LNG-IUS, the corresponding number of avoided depressions was 13·1 (95% CI 9·6–16·6) per 1,000 person-years compared to use of the high-dose 52 mg LNG-IUS (Fig. 1), yielding a relative rate ratio of 0·85 (95% CI 0·75–0·96, Fig. 2).
Fig. 2.
Adjusted∗ incidence rate ratios of first antidepressant use according to different doses and durations of LNG-IUS use. ∗Adjusted for: age (1-year intervals), calendar time (1-year intervals), and educational status (elementary school, Secondary school, skilled worker, Theoretical education).
The reduction in depression risk with the two low-dose LNG-IUS compared to the high-dose LNG-IUS persisted, albeit decreased, with increasing duration of use (Fig. 2).
No significant difference in depression risk was observed between the two low-dose LNG-IUS (Figure S2).
All findings persisted in sub-populations of women aged 15–19 years old (relative rate ratio 0·60 (95% CI 0·42–0·85) for 19·5 mg LNG-IUS for one year follow up), when adjusting for previous use of other hormonal contraceptives (relative rate ratio 0·67 (95% CI 0·56–0·81) for 19·5 mg LNG-IUS for one year follow up), in different times of the study period, and when considering the second and third antidepressant prescription redemption (Table S2). Finding also persisted when comparing users of low-dose LNG-IUS exclusively high-dose LNG-IUS users initiating use prior to the launch of the low-dose LNG-IUS (Table S2). The quasi-Poisson estimate also showed statistical significance (relative rate ratio 0·66 (95% CI 0·46–0·94) for 19·5 mg LNG-IUS for one year follow up) Table S2.
Discussion
Statement of principal findings of the study
Use of low-dose 13·5 mg and 19·5 mg LNG-IUS was associated with significantly lower risk of first-time antidepressant use compared to use of the high-dose 52 mg LNG-IUS in this Danish nationwide cohort of nulliparous women. No difference in depression risk was observed between the 13·5 mg low-dose LNG-IUS and the 19·5 mg low-dose LNG-IUS.
Strengths and weaknesses of the study
To our knowledge, this nationwide cohort study is the first post-authorisation safety study on depression risk with LNG-IUS use including all three currently marketed types.
The vast majority of Danish patients with depression are diagnosed and treated in primary care. All treatments with antidepressants in Denmark are provided through prescription. Consequently, all use of such is registered in the national prescription registry.17 Women diagnosed with depression in a hospital setting will also receive treatment through prescriptions detectable in the prescription registry. Thus, defining the outcome of interest through prescription redemption of antidepressants allowed the identification of all treated depressions in Denmark. An inclusion of some cases of anxiety treated with antidepressants cannot be ruled out. However, we still considered use of antidepressants as a clinically relevant outcome to address.
During the study period, around 90% of total sale of LNG-IUS in Denmark occurred in the primary sector of which around 95% was registered on individual level in the national prescription registry, allowing the inclusion of the majority of eligible subjects with minimal risk of selection bias.21
LNG-IUS was introduced to the Danish market after the establishment of the national prescription registry, thereby allowing a new-user design and the avoidance of healthy user bias.
The study had limitations. Being registry-based, the exposure was not randomised, leaving the possibility of residual confounding. Although the primary indication for use of any LNG-IUS is contraception, the high-dose LNG-IUS is also approved for management of heavy menstrual bleeding and may be favoured in women with severe dysmenorrhea such as endometriosis patients. Women with heavy menstrual bleeding or endometriosis have increased risk of depression (confounding by indication) and may be more closely followed by health professionals (detection bias). To account for these potential confounding factors, we excluded/censored women with a hospital diagnosis of heavy menstrual bleeding or dysmenorrhea, including endometriosis. However, we consider the influence of the potential differential distribution of heavy menstrual bleeding and dysmenorrhea across the different LNG-IUS users to be negligeable. Each type of LNG-IUS overtook the market upon its introduction, indicative of the fact that the choice of LNG-IUS primarily was driven by the newest option available, not non-contraception indications for the 52 mg LNG-IUS use. Nevertheless, to confirm further the interchangeability between women exposed to the high-dose 52 mg LNG-IUS and women exposed to the low-dose LNG-IUS, we conducted a sensitivity analysis in which high-dose LNG-IUS users were only included if purchasing the LNG-IUS prior to the launch of the low-dose LNG-IUS. This was to ensure that the use of the high-dose LNG-IUS was not based on a deselection of the low-dose LNG-IUS, thereby increasing the likelihood of the high-dose LNG-IUS being used for contraceptive purposes similar to the low-dose LNG-IUS. The increased risk of depression with the high-dose LNG-IUS compared to the low-dose LNG-IUS persisted in this analysis, reducing the risk of confounding by indication.
Some clinicians may have recommended the low-dose LNG-IUS over the high-dose LNG-IUS to vulnerable women at increased risk of depression because of the smaller hormonal reservoir and thereby the plausible decreased risk of systemic adverse events. This could have led to an underestimation of the difference in depression risk between use of high- and low-dose LNG-IUS. Still, the reduced depression risk in low-dose users versus high-dose users remained observable when comparing use following the launch of all three LNG-IUS.
Some of the mechanisms of the effect of progestin and progesterone on the brain in general and on mood in particular has been established.22,23
The increased risk of depression with the high-dose LNG-IUS compared to the low-dose LNG-IUS could be a reflection of the increased serum concentration of progestin with the 52 mg LNG-IUS compared to the 13·5 mg and 19·5 mg LNG-IUS. The finding of no difference in depression risk between the two low-dose LNG-IUS corresponds with the similar serum concentrations observed following 7 days of use.14, 15, 16 The decline in difference in depression risk over time between the high-dose and low-dose LNG-IUS could reflect the declining difference in hormonal reservoir between the high- and low-dose LNG-IUS over time. However, healthy user selection over time of women tolerating LNG-IUS could also contribute to the observed decline in difference in depression risk between high- and low-dose LNG-IUS over time.
Strengths and weaknesses in relation to other studies
A French nationwide study found use of the high-dose 52 mg LNG-IUS to be associated with a small and not clinically relevant increased risk of depression compared to use of the low-dose 19·5 mg LNG-IUS with an adjusted odds ratio of 1·13 (95% CI 1·05–1·21).13 Contrary to our study, the French study included older and parous women with a risk of postpartum depression, thereby potentially causing a dilution of the association between dose of LNG-IUS and depression risk. This is somewhat confirmed by the larger estimate observed in the subgroup of women aged <25 years, where the risk of depression with the high-dose LNG-IUS compared to the 19·5 mg LNG-IUS was found to be 1·37 (0·97–1·93), which is close to the estimate of the current study. In our study, we only included nulliparous women, thereby younger and not in risk of postpartum depression.
Conclusion
Use of low-dose 13·5 mg and 19·5 mg LNG-IUS was associated with significantly lower risk of incident antidepressant use compared to use of the high-dose 52 mg LNG-IUS. The risk of antidepressant use was similar for the two low-dose LNG-IUS. If these findings indicate causality, women, choosing an LNG-IUS as their contraceptive method for exclusively contraceptive purpose, should be advised to get a low-dose LNG-IUS instead of a high-dose LNG-IUS. Since use of the 13·5 mg LNG-IUS has been linked to an increased risk of ectopic pregnancy compared to the 19·5 mg LNG-IUS,24 the 19·5 mg LNG-IUS would be the safest choice based on currently available evidence on drug safety of the different marketed LNG-IUS. The high-dose LNG-IUS may still be LNG-IUS of choice in women suffering from heavy menstrual bleeding or severe dysmenorrhea.
Contributors
Dr. Skovlund and Dr. Meaidi had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Skovlund, Mørch, Meaidi. Interpretation of data: all authors, Drafting of the manuscript: Skovlund, Mørch, Meaidi. Critical revision of the manuscript for important intellectual content: all authors. Dr Skovlund were responsible for the decision to submit the manuscript. Dr. Mørch and Dr. Meaidi contributed equally as co-last authors.
Data sharing statement
All data were de-identified, linked, and accessed through a secure server at Statistics Denmark. The authors do not have permission to share the data.
Ethical statement
In Denmark, ethical approval is not required for studies that are entirely register-based.
Declaration of interests
Drs Skovlund, Møller, and Meaidi report no conflicts of interest. Professor Torp-Pedersen declare that he had received grants from Novo Nordisk and Beyer to other studies. Dr Mørch has received grant from Sygesikringen Danmark.
Acknowledgements
This study was funded by Sygeforsikringen “Danmark” grant: 2021–0128. The funder had no role in the data collection or interpretation of the findings.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100813.
Appendix A. Supplementary data
References
- 1.Web Annex A. The selection and use of essential medicines 2023: executive summary of the report of the 24th WHO expert committee on the selection and use of essential medicines. World Health Organization; Geneva: 2023. World health organization model list of essential medicines – 23rd list, 2023. (WHO/MHP/HPS/EML/2023.02). Licence: CC BY-NC-SA 3.0 IGO.; 2023. [Google Scholar]
- 2.Rademacher K.H., Sripipatana T., Pfitzer A., et al. A global learning agenda for the levonorgestrel intrauterine system (LNG IUS): addressing challenges and opportunities to increase access. Glob Health Sci Pract. 2018;6(4):635–643. doi: 10.9745/GHSP-D-18-00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasvol T.J., Macgregor E.A., Rait G., Horsfall L. Time trends in contraceptive prescribing in UK primary care 2000–2018: a repeated cross-sectional study. BMJ Sex Reprod Health. 2022;48(3):193–198. doi: 10.1136/bmjsrh-2021-201260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King L.A., Michels K.A., Graubard B.I., Trabert B. Trends in oral contraceptive and intrauterine device use among reproductive-aged women in the US from 1999 to 2017. Cancer Causes Control. 2021;32(6):587–595. doi: 10.1007/s10552-021-01410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindh I., Skjeldestad F.E., Gemzell-Danielsson K., et al. Contraceptive use in the Nordic countries. Acta Obstet Gynecol Scand. 2017;96(1):19–28. doi: 10.1111/aogs.13055. [DOI] [PubMed] [Google Scholar]
- 6.Hognert H., Skjeldestad F.E., Gemzell-Danielsson K., et al. Ecological study on the use of hormonal contraception, abortions and births among teenagers in the Nordic countries. BMJ Open. 2018;8(10) doi: 10.1136/bmjopen-2018-022473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skovlund C.W., Mørch L.S., Kessing L.V., Lidegaard Ø. Association of hormonal contraception with depression. JAMA Psychiatry. 2016;73(11):1154–1162. doi: 10.1001/jamapsychiatry.2016.2387. [DOI] [PubMed] [Google Scholar]
- 8.Elsayed M., Dardeer K.T., Khehra N., et al. The potential association between psychiatric symptoms and the use of levonorgestrel intrauterine devices (LNG-IUDs): a systematic review. World J Biol Psychiatry. 2023;24(6):457–475. doi: 10.1080/15622975.2022.2145354. [DOI] [PubMed] [Google Scholar]
- 9.Lundin C., Wikman A., Lampa E., et al. There is no association between combined oral hormonal contraceptives and depression: a Swedish register-based cohort study. BJOG. 2022;129(6):917–925. doi: 10.1111/1471-0528.17028. [DOI] [PubMed] [Google Scholar]
- 10.Skovlund C.W., Mørch L.S., Kessing L.V., Lange T., Lidegaard Ø. Association of hormonal contraception with suicide attempts and suicides. Am J Psychiatry. 2018;175(4):336–342. doi: 10.1176/appi.ajp.2017.17060616. [DOI] [PubMed] [Google Scholar]
- 11.Gemzell-Danielsson K., Schellschmidt I., Apter D. A randomized, phase II study describing the efficacy, bleeding profile, and safety of two low-dose levonorgestrel-releasing intrauterine contraceptive systems and Mirena. Fertil Steril. 2012;97(3):616–622.e3. doi: 10.1016/j.fertnstert.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Seeber B., Ziehr S.C., et al. Quantitative levonorgestrel plasma level measurements in patients with regular and prolonged use of the levonorgestrel-releasing intrauterine system. Contraception. 2012;86(4):345–349. doi: 10.1016/j.contraception.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Roland N., Baricault B., Weill A., et al. Association between doses of levonorgestrel intrauterine systems and subsequent use of psychotropic drugs in France. JAMA. 2023;329(3):257. doi: 10.1001/jama.2022.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Product monograph Pr JAYDESS® levonorgestrel-releasing intrauterine system (13.5 mg) progestogen. Bayer inc. 2920 matheson boulevard east, mississauga, ontario, L4W 5R6. 2018. www.bayer.ca
- 15.Product monograph including patient medication information Pr KYLEENA® levonorgestrel-releasing intrauterine system (19.5 mg) progestogen bayer inc. 2920 matheson blvd east mississauga, ontario L4W 5R6. 2021. www.bayer.ca
- 16.Product monograph PrMIRENA® levonorgestrel-releasing intrauterine system (52 mg) to deliver up to 20 mcg levonorgestrel per day progestogen bayer inc. 2920 matheson blvd east mississauga, ontario L4W 5R6 Canada. 2018. www.bayer.ca
- 17.Wallach Kildemoes H., Toft Sørensen H., Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7_suppl):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 18.Munk-Jørgensen P., Mortensen P.B. The Danish psychiatric central register. Dan Med Bull. 1997;44(1):82–84. [PubMed] [Google Scholar]
- 19.Lynge E., Sandegaard J.L., Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39(7_suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 20.Knudsen L.B., Olsen J. The Danish medical birth registry. Dan Med Bull. 1998;45(3):320–323. [PubMed] [Google Scholar]
- 21.Medstat. 2023. https://medstat.dk/
- 22.Smith K., Nayyar S., Rana T., Archibong A., Looney K., Nayyar T. Do progestin-only contraceptives contribute to the risk of developing depression as implied by beta-arrestin 1 levels in leukocytes? A pilot study. Int J Environ Res Public Health. 2018;15(9):1966. doi: 10.3390/ijerph15091966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frokjaer V.G., Pinborg A., Holst K.K., et al. Role of serotonin transporter changes in depressive responses to sex-steroid hormone manipulation: a positron emission tomography study. Biol Psychiatry. 2015;78(8):534–543. doi: 10.1016/j.biopsych.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Meaidi A., Torp-Pedersen C., Lidegaard Ø., Mørch L.S. Ectopic pregnancy risk in users of levonorgestrel-releasing intrauterine systems with 52, 19.5, and 13.5 mg of hormone. JAMA. 2023;329(11):935. doi: 10.1001/jama.2023.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.