Summary
The treatment landscape of resectable early-stage non-small cell lung cancer (NSCLC) is set to change significantly due to encouraging results from randomized trials evaluating neoadjuvant and adjuvant immunotherapy, as well as adjuvant targeted therapy. As of January 2024, marketing authorization has been granted for four new indications in Europe, and regulatory approvals for other study regimens are expected. Because cost-effectiveness and reimbursement criteria for novel treatments often differ between European countries, access to emerging developments may lead to inequalities due to variations in recommended and available lung cancer care throughout Europe. This Series paper (i) highlights the clinical studies reshaping the treatment landscape in resectable early-stage NSCLC, (ii) compares and contrasts approaches taken by the European Medicines Agency (EMA) for drug approval to that taken by the United States Food and Drug Administration (FDA), and (iii) evaluates the differences in access to emerging treatments from an availability perspective across European countries.
Keywords: Non-small-cell lung cancer, Lung neoplasms, Immune checkpoint inhibitors, Protein kinase inhibitors, Disease-free survival, Humans, Europe, United States, United States Food and Drug Administration, Access to primary care, Adjuvant chemotherapy, Biomarkers, Surgery
Search strategy and selection criteria.
We searched PubMed and Embase up to August 17, 2023, using search terms “non-small cell lung cancer”, “adjuvant”, “postoperative”, “neoadjuvant”, “preoperative”, and “perioperative”, with no restrictions by language. Only papers reporting on randomized phase II and III clinical trials with results were included. A search with similar search terms was conducted on ClinicalTrials.gov and WHO ICTRP up to August 17, 2023. Only ongoing randomized phase III clinical trials were included. Articles were also identified through searches of the authors’ own files. The final reference list was generated on the basis of relevance to the scope of this Series paper.
Introduction
Non-small cell lung cancer (NSCLC) is one of the most frequently diagnosed cancer types and remains a leading cause of cancer-related death.1 Recent systemic treatments, including immune checkpoint inhibitors (ICIs) and targeted therapies, have significantly improved survival and health-related quality of life (HRQOL) in metastatic NSCLC.2 For instance, the Netherlands Cancer Registry showed that 5-year survival rates rose from 12% (1995–2004) to 25% (2015–2021).3 These advancements have led to interest in using these treatments in early-stage NSCLC, where traditionally only chemotherapy showed survival benefits.4, 5, 6 As of January 2024, the European Medicines Agency (EMA) has approved adjuvant osimertinib, adjuvant atezolizumab, adjuvant pembrolizumab, and neoadjuvant nivolumab plus chemotherapy for resectable early-stage NSCLC, with more decisions pending.7, 8, 9, 10
The United States Food and Drug Administration (US FDA) and the EMA are central in regulating medical products in the US and the European Union (EU).11,12 Both engage in processes for drug approval, including pre-authorization, detailed assessment of clinical trial data, and post-marketing surveillance. However, they have distinct regulatory frameworks: the FDA's unified federal system ensures a streamlined process, while the EMA operates within the European Medicines Regulatory Network, a closely-coordinated framework of national competent authorities in the Member States of the European Economic Area working together with the EMA and the European Commission (EC).
Substantial differences in healthcare systems and drug reimbursement across Europe may further amplify existing disparities in lung cancer care. This Series paper provides an overview of studies influencing the upcoming changes in resectable early-stage (stages I-IIIA) NSCLC, discusses the approach for drug approval by the EMA compared to that of the FDA, and evaluates access to innovative therapies from an availability perspective across EU countries.13
Background
For decades, early-stage NSCLC treatment remained largely unchanged. Operable stages I and II NSCLC, and selected IIIA cases, typically undergo an anatomical resection (segmentectomy, (bi)lobectomy, or pneumonectomy) with lymph node dissection.14 Stereotactic ablative radiotherapy (SABR) is an effective alternative when patients with stage I disease are unwilling or unable to undergo surgery, as shown in the revised STARS study.15 Platinum-based chemotherapy in stage II-IIIA NSCLC and selected stage IB cases improved survival by approximately 5% in both neoadjuvant and adjuvant settings.4, 5, 6 In case of positive surgical margins, postoperative radiotherapy is considered.14 For patients with resectable stage III NSCLC with PD-L1 expression ≥1% who cannot undergo surgery, the preferred treatment is definitive chemoradiotherapy followed by durvalumab.13
Despite curative intent treatments, 5-year survival rates for early-stage NSCLC vary from 92% in stage IA to 36% in stage IIIA.16 This modest long-term survival is largely due to distant tumor relapse, which is reported to occur up to three times more frequently than local recurrences.17 This highlights the need for more effective systemic treatments.
Novel adjuvant and neoadjuvant treatment approaches
Summary of landmark trials
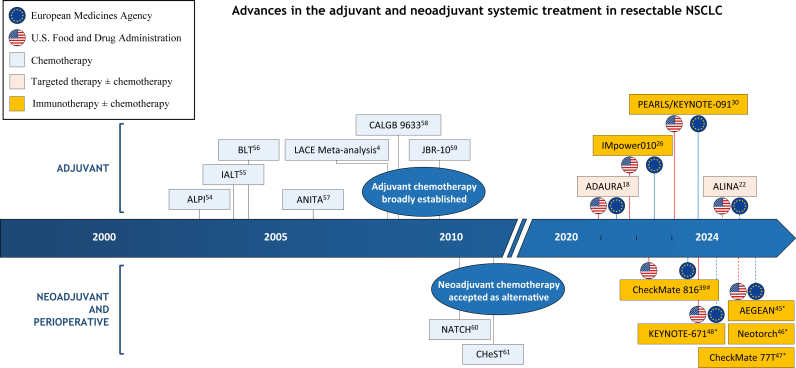
Fig. 1 shows a timeline with relevant landmark trials that have impacted the systemic treatment in resectable NSCLC. Published phase II and III randomized trials and ongoing phase III randomized trials in resectable NSCLC are summarized in Tables 1 and 2, respectively.
Fig. 1.
Timeline of key studies influencing the treatment of resectable NSCLC.
Dashed red and blue lines indicate that marketing approval is pending. (#) Neoadjuvant treatment only; (∗) perioperative treatment. Abbreviation: NSCLC, non-small cell lung cancer.
Table 1.
Randomized phase II and III clinical trials with reported results in resectable NSCLC.
| Name/NCT identifier | Phase | No. of patients | Population | Treatment | Primary endpoint(s) | Primary or key result(s) |
|---|---|---|---|---|---|---|
| Adjuvant immunotherapy (with CT or optional CT) | ||||||
| CANOPY-A NCT03447769 |
III | 1382 | Stage II-IIIB | Canakinumab vs placebo (optional adjuvant CT) | DFS | HR 0.94 (95% CI 0.78–1.14) |
| IMpower010 NCT02486718 |
III | 1280 | Stage IB-IIIA–II: 47%–IIIA: 41% | Sequential CT + atezolizumab vs sequential CT + BSC | DFS | Stage II-IIIA PD-L1 ≥1%: HR 0.66 (95% CI 0.50–0.88) Stage II-IIIA: HR 0.79 (95% CI 0.64–0.96) Stage IB-IIIA: HR 0.81 (95% CI 0.67–0.99) |
| PEARLS/KEYNOTE-091 NCT02504372 |
III | 1177 | Stage IB-IIIA–II: 57%–IIIA: 29% | Pembrolizumab vs placebo (optional adjuvant CT) | DFS | Stage IB-IIIA: HR 0.76 (95% CI 0.63–0.91) PD-L1 ≥50%: HR 0.82 (95% CI 0.57–1.18) |
| Neoadjuvant immunotherapy (with or without CT) | ||||||
| CANOPY-N NCT03968419 |
II | 88 | Stage IB-IIIA | Canakinumab, pembrolizumab, or combined | MPR | Canakinumab: MPR 2.9% Pembrolizumab: MPR 11.1% Combined: MPR 17.1% |
| NeoCOAST NCT03794544 |
II | 84 | Stage I-IIIA | Durvalumab, durvalumab + oleclumab, durvalumab + monalizumab, or durvalumab + danvatirsen | MPR | Durvalumab: MPR 11.1% Oleclumab: MPR 19.0% Monalizumab: MPR 30.0% Danvatirsen: MPR 31.3% |
| NEOSTAR NCT03158129 |
II | 44 | Stage I-IIIA | Nivolumab vs nivolumab + ipilimumab | MPR | Nivolumab: MPR 22% (5/23) Nivolumab + ipilimumab: MPR 38% (8/21) |
| TD-FOREKNOW NCT04338620 |
II | 88 | Stage IIIA-IIIB | CT + camrelizumab vs CT | pCR | Camrelizumab: pCR 32.6% (14/43) CT: pCR 8.9% (4/45) |
| CheckMate 816 NCT02998528 |
III | 358 | Stage IB-IIIA–IB/II: 36%–IIIA: 64% | CT + nivolumab vs CT | EFS, pCR | HR 0.68 (95% CI 0.49–0.93) Nivolumab: pCR 24.0% (43/179), MPR 36.9% CT: pCR 2.2% (4/179), MPR 8.9% |
| Neoadjuvant immunotherapy (with CT) followed by surgery and adjuvant immunotherapy (with or without CT) | ||||||
| NADIM II NCT03838159 |
II | 86 | Stage IIIA-IIIB | CT + nivolumab vs neoadjuvant CT alone | pCR | Nivolumab: pCR 37% (21/57) CT: pCR 7% (2/29) |
| neoSCORE NCT04459611 |
II | 60 | Stage IB-IIIA | Neoadjuvant CT + sintilimab (2 cycles) then adjuvant CT (2 cycles) + sintilimab vs neoadjuvant CT + sintilimab (3 cycles) then adjuvant CT (1 cycle) + sintilimab | MPR | 2 cycles: MPR 26.9% (7/26) 3 cycles: MPR 41.4% (12/29) |
| AEGEAN NCT03800134 |
III | 740 | Stage II-IIIB–IIIA/B: 71% | CT + durvalumab vs CT + placebo | EFS, pCR | HR 0.68 (95% CI 0.53–0.88) Durvalumab: pCR 17.2% (63/366), MPR 33.3% Placebo: pCR 4.3% (16/374), MPR 12.3% |
| CheckMate 77T NCT04025879 |
III | 461 | Stage II-IIIB–IIIA/B: 64% | CT + nivolumab vs CT + placebo | EFS | HR 0.58 (97.36% CI 0.42–0.81) Nivolumab: pCR 25.3%, MPR 35.4% Placebo: pCR 4.7%, MPR 12.1% |
| KEYNOTE-671 NCT03425643 |
III | 797 | Stage II-IIIB–IIIA/B: 70% | CT + pembrolizumab vs CT + placebo | EFS, OS | OS: HR 0.72 (95% CI 0.56–0.93) EFS: HR 0.59 (95% CI 0.48–0.72) Pembrolizumab: pCR 18.1%, MPR 30.2% Placebo: pCR 4.0%, MPR 11.0% |
| Neotorch NCT04158440 |
III | 404a | Stage II-III | Neoadjuvant CT + toripalimab (3 cycles) then adjuvant CT (1 cycle) + toripalimab vs neoadjuvant CT + placebo (3 cycles) then adjuvant CT (1 cycle) + placebo | ÈFS, MPR | HR 0.40 (95% CI 0.28–0.57) Toripalimab: MPR 48.5% (98/202), pCR 24.8% Placebo: MPR 8.4% (17/202), pCR 1.0% |
| RATIONALE-315 NCT04379635 |
III | 453 | Stage II-IIIA–IIIA: 58% | CT + tislelizumab vs CT + placebo | ÈFS, MPR | Tislelizumab: MPR 56.2% (127/226), pCR 40.7% Placebo: MPR 15.0% (34/227), pCR 5.7% |
| Adjuvant targeted therapy (with or without CT) | ||||||
| CORIN NCT02264210 |
II | 128 | Stage IB, EGFRm | Icotinib vs observation | 3-year DFS | 3-year DFS: Icotinib 96.1% vs observation 84.0% HR: 0.23 (95% CI 0.07–0.81) |
| EVAN NCT01683175 |
II | 102 | Stage IIIA, EGFRm | Erlotinib vs CT | 2-year DFS | 2-year DFS: Erlotinib 81.4% vs CT 44.6% HR 0.27 (95% CI 0.14–0.53) |
| ICOMPARE NCT01929200 |
II | 109 | Stage II-IIIA, EGFRm | 2-year icotinib vs 1-year icotinib | DFS | HR 0.51 (95% CI 0.28–0.94) |
| IFCT-0703 NCT00775307 |
II/III | 142 | Stage I | Pazopanib vs placebo | Compliance | RFS: HR 1.3 (95% CI 0.6–2.7) OS: HR 1.8 (95% CI 0.6–5.5) |
| ADAURA NCT02511106 |
III | 682 | Stage IB-IIIA, EGFRm | Osimertinib vs placebo (optional adjuvant CT) | DFS | Stage II-IIIA: DFS HR 0.23 (95% CI 0.18–0.30), OS HR 0.49 (95.03% CI 0.33–0.73) Stage IB-IIIA: DFS HR 0.27 (95% CI 0.21–0.34), OS HR 0.49 (95.03% CI 0.34–0.70) |
| ALINA NCT03456076 |
III | 257 | Stage IB-IIIA, ALK+ | Alectinib vs CT | DFS | Stage II-IIIA: HR 0.24 (95% CI 0.13–0.45) Stage IB-IIIA: HR 0.24 (95% CI 0.13–0.43) |
| BR19 NCT00049543 |
III | 503 | Stage IB-IIIA | Gefitinib vs placebo (optional adjuvant CT) | OS | HR 1.24 (95% CI 0.94–1.64) |
| CTONG1104 NCT01405079 |
III | 222 | Stage II-IIIA, EGFRm | Gefitinib vs CT | DFS | HR 0.56 (95% CI 0.40–0.79) |
| ECOG-E1505 NCT00324805 |
III | 1501 | Stage IB-IIIA | CT + bevacizumab vs CT | OS | HR 0.99 (95% CI 0.82–1.19) |
| EVIDENCE NCT02448797 |
III | 322 | Stage II-IIIA, EGFRm | Icotinib vs CT | DFS | HR 0.36 (95% CI 0.24–0.55) |
| IMPACT UMIN000006252 |
III | 234 | Stage II-IIIB, EGFRm | Gefitinib vs CT | DFS | HR 0.92 (95% CI 0.67–1.28) |
| RADIANT NCT00373425 |
III | 1252 | Stage IB-IIIA, EGFRm | Erlotinib vs placebo (optional adjuvant CT) | DFS | HR 0.90 (95% CI 0.74–1.10) |
| Neoadjuvant targeted therapy | ||||||
| CTONG1103 NCT01407822 | II | 72 | Stage IIIA, EGFRm | Erlotinib (both neoadjuvant and adjuvant) vs CT (both neoadjuvant and adjuvant) | ORR | Erlotinib: 54.1% CT: 34.3% |
Abbreviations: NSCLC, non-small cell lung cancer; NCT, national clinical trial; CT, chemotherapy; DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; BSC, best supportive care; PD-L1, programmed cell death ligand 1; MPR, major pathological response; pCR, pathological complete response; EFS, event-free survival; OS, overall survival; EGFRm, activating epidermal growth factor receptor mutation; RFS, recurrence-free survival; ALK+, anaplastic lymphoma kinase alteration; UMIN, university hospital medical information network; ORR, objective response rate.
Based on the stage III interim analysis.
Table 2.
Ongoing randomized phase III clinical trials in resectable NSCLC.
| Name/NCT identifier | No. of patients | Key eligibility criteria | Experimental arm | Control arm | Primary endpoint(s) |
|---|---|---|---|---|---|
| Adjuvant immunotherapy (no CT or optional CT) | |||||
| ALCHEMIST-ANVIL NCT02595944 |
903a | Stage IB(≥4 cm) or II-IIIAb NSCLC (R0), no EGFR/ALK mutation | Sequential CT + nivolumab | CT followed by observation | DFS, OS |
| ALCHEMIST-Chemo-IO NCT04267848 |
1210a | Stage IIA-IIIB(N2)c NSCLC (R0), no EGFR/ALK mutation | Sequential CT + pembrolizumab or CT + pembrolizumab then pembrolizumab | CT followed by observation | DFS |
| BR31 NCT02273375 |
1415d | Stage IB-IIIAb NSCLC (R0) | Durvalumab | Placebo | DFS |
| LungMate-008 NCT04772287 |
341a | Stage II-IIIB(N2)c NSCLC (R0), no EGFR/ALK mutation | CT + toripalimab | CT + placebo | DFS |
| MERMAID-1 NCT04385368 |
89d | Stage II-IIIe NSCLC (R0), no EGFR/ALK mutation, MRD positive post-surgery | CT + durvalumab | CT + placebo | DFS |
| MERMAID-2 NCT04642469 |
30d | Stage II-IIIf NSCLC (R0), became MRD-positive in a 96-week surveillance period, no EGFR/ALK mutation | Durvalumab (optional neoadjuvant and/or adjuvant CT) | Placebo (optional neoadjuvant and/or adjuvant CT) | DFS |
| NADIM-ADJUVANT NCT04564157 |
210a | Stage IB-IIIAe NSCLC (R0), no EGFR/ALK/STK11/KEAP1 mutation | CT + nivolumab then nivolumab maintenance | CT followed by observation | DFS |
| NCT05487391 | 632a | Stage II-IIIBc NSCLC (R0), no EGFR/ALK mutation | CT + QL1706g | CT + placebo | DFS |
| Neoadjuvant CT + immunotherapy followed by surgery and adjuvant immunotherapy | |||||
| CIBI308G301 NCT05116462 |
800a | Stage IIB(>4 cm), IIIA or IIIB(N2)c resectable NSCLC | CT + sintilimab | CT + placebo | EFS, pCR |
| IMpower030 NCT03456063 |
453d | Stage II-IIIB(T3N2)c resectable NSCLC, no EGFR/ALK mutation | CT + atezolizumab | CT + placebo | EFS |
| NCT05157776 | 72a | Stage IIIAc resectable NSCLC, no EGFR/ALK mutation | CT + sintilimab (4 neoadjuvant cycles) | CT + sintilimab (2 neoadjuvant cycles + 2 optional adjuvant cycles) | pCR |
| neoSCORE II NCT05429463 |
250a | Stage II-IIIBc resectable, squamous NSCLC | CT + sintilimab (4 neoadjuvant cycles) | CT + sintilimab (3 neoadjuvant cycles) | MPR |
| RATIONALE-315 NCT04379635 |
453d | Stage II-IIIAf resectable NSCLC, no EGFR/ALK mutation | CT + tislelizumab | CT + placebo | EFS, MPR |
| SHR-1316-III-303 NCT04316364 |
537a | Stage II-IIIBc resectable NSCLC | CT + adebrelimab | CT + placebo | EFS, MPR |
| Adjuvant targeted therapy (with or without CT) | |||||
| ADAURA2 NCT05120349 |
380a | Stage IA2 or IA3c NSCLC (R0), EGFR mutation-positive | Osimertinib | Placebo | DFS |
| ALCHEMIST-A081105 NCT02193282 |
450a | Stage IB(≥4 cm) or II-IIIAb NSCLC (R0), EGFR mutation-positive | Erlotinib (double-blind or open-label) | Placebo (vs erlotinib double-blind) or observation (vs erlotinib open-label) | OS |
| ALCHEMIST-E4512 NCT02201992 |
168a | Stage IB(≥4 cm) or II-IIIAb NSCLC (R0), ALK translocation/inversion | Crizotinib | Observation | OS |
| APEX NCT04762459 |
606a | Stage II-IIIAf NSCLC (R0), EGFR mutation-positive | Aumolertinib or aumolertinib + CT | CT | DFS |
| FORWARD NCT04853342 |
318a | Stage IB-IIIAf NSCLC (R0), EGFR mutation-positive | Furmonertinib (optional adjuvant CT) | Placebo (optional adjuvant CT) | DFS |
| HS-10296-302 NCT04687241 |
192a | Stage II-IIIB(T3N2)f NSCLC (R0), EGFR mutation-positive | Aumolertinib | Placebo | DFS |
| ICTAN NCT01996098 |
318a | Stage II-IIIAf NSCLC (R0), EGFR mutation-positive | Icotinib (6 months) or icotinib (12 months) | No intervention after adjuvant CT | DFS |
| ICWIP NCT02125240 |
124a | Stage II-IIIAf NSCLC, EGFR mutation-positive | Icotinib | Placebo | DFS |
| LIBRETTO-432 NCT04819100 |
170a | Stage IB-IIIAf NSCLC, previously treated with definitive surgery or RT, activating RET gene fusion | Selpercatinib (after CT or durvalumab if suitable) | Placebo (after CT or durvalumab if suitable) | EFS |
| NCT03381066 | 225a | Stage II-IIIB(N2)e NSCLC (R0) EGFR mutation-positive | CT + gefitinib | CT | DFS |
| NCT05341583 | 202a | Stage II-IIIB(T3N2)f NSCLC (R0), ALK mutation-positive | Ensartinib (optional adjuvant CT) | Placebo (optional adjuvant CT) | DFS |
| NFEC-2019-077 NCT03983811 |
174a | Stage IIB-IIIA (N1-2)f NSCLC (R0), EGFR mutation-positive | CT + icotinib | CT + placebo | DFS |
| W-TONG002 NCT02518802 |
220a | Stage II-IIIA (N1-2)f NSCLC (R0), EGFR mutation-positive | CT + gefitinib | CT | DFS |
| Neoadjuvant targeted therapy | |||||
| NeoADAURA NCT04351555 |
328a | Stage II-IIIB(N2)e resectable NSCLC, EGFR mutation-positive | Osimertinib or CT + osimertinib | CT + placebo | MPR |
Abbreviations: NSCLC, non-small cell lung cancer; NCT, national clinical trial; CT, chemotherapy; R0, completely resected; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; DFS, disease-free survival; OS, overall survival; MRD, minimal residual disease; STK11, Serine/threonine kinase 11; KEAP1, Kelch-like ECH-associated protein 1; EFS, event-free survival; pCR, pathological complete response; MPR, major pathological response; RT, radiotherapy; RET, rearranged during transfection.
Estimated enrollment.
Seventh edition of the American Joint Committee on Cancer (AJCC) TNM classification.
AJCC eighth edition.
Actual enrollment.
Eighth edition of the International Association for the Study of Lung Cancer TNM classification.
Staging criteria not specified.
Bifunctional antibody (anti-PD-1 IgG4 and anti-CTLA-4 IgG1).
Adjuvant targeted therapy
ADAURA is the first phase III trial to demonstrate improved overall survival (OS) in resectable NSCLC using targeted therapy.18,19 In this double-blind, phase III trial, 682 patients with completely resected epidermal growth factor receptor (EGFR exon 19 deletion or exon 21 L858R mutation) mutation-positive stage IB-IIIA NSCLC (according to the 7th edition of the Tumor, Node, Metastasis (TNM) classification system) were randomized to receive up to three years of adjuvant osimertinib (80 mg orally once daily), a third-generation EGFR tyrosine kinase inhibitor, or placebo. Adjuvant platinum-based chemotherapy was allowed. After a median follow-up of 22.1 months in the experimental arm and 14.9 months in the placebo arm, ADAURA met its primary endpoint with improved disease-free survival (DFS) in favor of the experimental arm in both the stage II-IIIA population (median not reached (NR) vs 19.6 months with placebo; hazard ratio (HR) 0.17, 99.06% confidence interval (CI) 0.11–0.26; p < 0.001) and overall population (median NR vs 27.5 months with placebo; HR 0.20, 99.12% CI 0.14–0.30; p < 0.001). Interestingly, patients showed comparable benefits from osimertinib regardless of prior adjuvant chemotherapy. Moreover, fewer central nervous system (CNS) recurrences (HR 0.18, 95% CI 0.10–0.33) and maintained HRQOL were noted with osimertinib.20 Based on these results without mature OS data, osimertinib was approved by the FDA (December 2020) and EMA (May 2021) for patients with completely resected stage IB–IIIA EGFR mutation-positive NSCLC.7,21 The final OS analysis in mid-2023 reported a significant OS benefit, with 5-year OS rates of 85% vs 73% with placebo in the stage II-IIIA population (HR 0.49, 95.03% CI 0.33–0.73; p < 0.001), and, in the overall population, 88% vs 78% with placebo (HR 0.49, 95.03% CI 0.34–0.70; p < 0.001).19
The open-label, phase III ALINA trial investigated adjuvant alectinib, an anaplastic lymphoma kinase (ALK) inhibitor, in 257 patients with completely resected stage IB(≥4 cm)-IIIA NSCLC (7th edition) with an ALK alteration who were randomized to receive either adjuvant alectinib (600 mg orally twice daily) for up to 24 months, or up to four cycles of platinum-based chemotherapy.22 An improvement in investigator-assessed DFS was demonstrated favoring the alectinib arm in both stage II–IIIA (median follow-up duration 27.8 months; median NR vs 44.4 months with chemotherapy; HR 0.24, 95% CI 0.13–0.45; p < 0.0001) and overall populations (median NR vs 41.3 months with chemotherapy; HR 0.24, 95% CI 0.13–0.43; p < 0.0001). CNS-DFS advantages in the overall population were also observed (HR 0.22, 95% CI 0.08–0.58). While OS data were immature, no new safety signals were identified. As of January 2024, adjuvant alectinib has not received EMA or FDA approval.
Adjuvant immunotherapy
Immunotherapy has revolutionized NSCLC treatment in both advanced and locally advanced stages and is now being explored in early stages. Immunotherapy offers better outcomes for patients, with fewer side effects than traditional chemotherapy.2 The EMA's 2015 approval of nivolumab, a monoclonal antibody against PD-1, for advanced NSCLC post-chemotherapy was a milestone, followed by various treatment approvals in advanced and locally advanced stage, including the anti-PD-L1 durvalumab post-chemoradiotherapy for unresectable stage III tumors.13,23 These advances were driven by discoveries in cancer immunology, notably PD-1 and CTLA-4, leading to the 2018 Nobel Prize in Medicine, awarded to Tasuku Honjo (PD-1) and James Allison (CTLA-4).24
Adjuvant immunotherapy shows promise in eradicating micrometastatic disease, reversing the immunosuppressive post-surgical microenvironment, and targeting circulating tumor cells.25 It may enhance the elimination of minimal residual disease, particularly when combined with adjuvant chemotherapy, which alters neoantigen exposure patterns and may augment ICI efficacy. Adjuvant atezolizumab, a monoclonal antibody targeting PD-L1, was investigated in IMpower010 and is now the first EU-approved immunotherapy for resectable NSCLC.26 In this open-label, phase III trial, 1005 patients with completely resected stage IB(≥4 cm)-IIIA NSCLC (7th edition) were randomized between atezolizumab 1200 mg every three weeks for 16 cycles or one year, or best supportive care (BSC). To be eligible, patients should have received at least one cycle of platinum-based adjuvant chemotherapy, and postoperative radiotherapy was not permitted. The primary endpoint of investigator-assessed DFS was evaluated first in the stage II-IIIA population with PD-L1 ≥1% tumors, followed by all patients with stage II-IIIA disease, and finally in the overall population. After a median follow-up of 32.2 months, the first DFS analysis showed improved DFS with atezolizumab in patients with stage II-IIIA tumors and PD-L1 expression ≥1% (median NR vs 35.3 months with BSC; HR 0.66, 95% CI 0.50–0.88; p = 0.0039) as well as in the stage II-IIIA population (median 42.3 vs 35.3 months with BSC; HR 0.79, 95% CI 0.64–0.96; p = 0.020) but not in the overall population. The most pronounced DFS benefit was observed in stage II-IIIA patients with PD-L1 expression ≥50% (HR 0.43, 95% CI 0.27–0.68). No DFS benefit was observed in the stage II-IIIA population with PD-L1 negative tumors, never-smokers, and those with EGFR or ALK alterations. Based on these DFS results, the FDA approved adjuvant atezolizumab in October 2021 for completely resected stage II-IIIA EGFR wild-type and ALK-negative NSCLC with PD-L1 expression ≥1%, following completion of adjuvant platinum-based chemotherapy.27 In June 2022, the EMA approved atezolizumab only for those patients with PD-L1 ≥50% tumors, after a blinded independent central review (BICR).8,28 This step was crucial as the EMA did not accept the open-label design and investigator-assessed DFS of the study. The BICR specifically confirmed the DFS benefit in this group. Also, the first prespecified interim OS analysis showed OS benefits with atezolizumab in patients with PD-L1 ≥50% tumors and without EGFR or ALK alterations (5-year OS 85% vs 68% with BSC; HR 0.42, 95% CI 0.23–0.78).29
The triple-blind, phase III PEARLS/KEYNOTE-091 trial randomly assigned 1177 patients with stage IB(≥4 cm)-IIIA NSCLC (7th edition) to receive either the anti-PD-1 pembrolizumab 200 mg or placebo after complete surgical resection, both administered every three weeks for up to 18 cycles.30 Unlike IMpower010, adjuvant chemotherapy was optional but encouraged for stage II-IIIA patients. Study co-primary endpoints were DFS in the overall population and in those with PD-L1 ≥50% tumors. The planned second interim analysis was driven by the DFS events that occurred in the latter group. After a median follow-up of 35.6 months, improved DFS was observed in the overall population with pembrolizumab (median 53.6 vs 42.0 months with placebo; HR 0.76, 95% CI 0.63–0.91; p = 0.0014) but, interestingly, not in the PD-L1 ≥50% population (both groups, median NR; HR 0.82, 95% CI 0.57–1.18). Notably, patients with PD-L1 1%–49% tumors showed improved DFS (HR 0.67, 95% CI 0.48–0.92). Contrary to IMpower010, never-smokers and patients with EGFR-mutation positive tumors did seem to benefit.31 However, these subgroup analyses should be interpreted with caution due to their exploratory nature and differences in trial design. Other factors that may have contributed to the differences in efficacy results are the overperformance of the placebo group with PD-L1 ≥50% tumors in PEARLS/KEYNOTE-091 and the differences in enrolled populations and PD-L1 assays used. Both trials reported comparable safety profiles with around 20% of patients experiencing grade ≥3 treatment-related adverse events.26,30 Adjuvant pembrolizumab treatment was completed by 52% of patients, in contrast to a completion rate of 65% for adjuvant atezolizumab. As of January 2024, OS data are pending. The FDA approved adjuvant pembrolizumab in January 2023 for stage IB(≥4 cm)-IIIA NSCLC regardless of PD-L1 expression following complete surgical resection and adjuvant platinum-based chemotherapy.32 In October 2023, the EMA granted a similar approval.10
Neoadjuvant immunotherapy
Previous phase II studies of neoadjuvant nivolumab alone or with chemotherapy showed promising results.33, 34, 35 Neoadjuvant therapy has several advantages over adjuvant treatment, such as more reliable treatment delivery and early eradication of micrometastatic disease. Treating tumors with high neoantigen burden while still present may facilitate tumor conditioning and expand tumor-specific memory T-cells.36, 37, 38 This approach also allows for direct assessment of treatment effects and the identification of potential biomarkers of efficacy in resection specimens, supporting the development of predictive models for ICI efficacy.
The open-label, phase III CheckMate 816 trial randomized 358 patients with resectable stage IB-IIIA NSCLC (7th edition) without known EGFR or ALK alterations to receive either neoadjuvant nivolumab 360 mg every three weeks for three cycles with platinum-based chemotherapy or chemotherapy-alone.39 No adjuvant immunotherapy was planned. In the unplanned interim analysis and after a median follow-up of 29.5 months, an improvement in EFS favoring the combination arm was observed (median 31.6 vs 20.8 months with chemotherapy-alone; HR 0.63, 97.38% CI 0.43–0.91; p = 0.005). EFS was better across most subgroups, especially in patients with stage IIIA disease, non-squamous histology, and those with PD-L1 ≥1% tumors (HR 0.41, 95% CI 0.24–0.70). No benefit was observed in the PD-L1 negative group (HR 0.85, 95% CI 0.54–1.32). The other co-primary endpoint pathological complete response (pCR: defined as 0% residual viable tumor cells in either the primary tumor or the sampled lymph nodes) also favored the combination arm (24% vs 2.2%; p < 0.001). The major pathological response rate (MPR: defined as ≤10% residual viable tumor cells in the resection specimen) was higher as well (36.9% vs 8.9%). These pathological responses are generally in line with the pCR and MPR rates of 20–25% and 30–40%, respectively, observed in the perioperative immunotherapy trials (Table 1). The feasibility of surgery was not compromised, with a comparable safety profile and no detrimental impact on HRQOL.40 A 3-year trial update indicated maintained EFS benefits and a promising OS trend with nivolumab (OS HR 0.62, 99.34% CI 0.36–1.05).41,42 At three years, 78% of patients were alive in the combination arm, compared to 64% in the chemotherapy-alone arm. Based on the first EFS results, the FDA approved neoadjuvant nivolumab with platinum-doublet chemotherapy for resectable stage IB(≥4 cm)-IIIA NSCLC regardless of PD-L1 expression in March 2022, a first in the neoadjuvant ICI setting.43 In June 2023, the EMA approved this regimen for resectable stage II-IIIA NSCLC with PD-L1 expression ≥1%.9,44
The perioperative approach
Phase III trials have explored the addition of immunotherapy in both neoadjuvant and adjuvant treatment phases, a so-called perioperative strategy. This approach combines ICIs with chemotherapy pre-surgery to maximize tumor reduction and systemic control, followed by ICI monotherapy post-surgery to maintain surgical outcomes and target residual micrometastatic disease. Perioperative trials like AEGEAN, Neotorch, Checkmate 77T, and KEYNOTE-671 have reported positive results for immunotherapy.45, 46, 47, 48 Notably, KEYNOTE-671 is the only one among these to have investigated OS as a primary endpoint.
In AEGEAN, 802 patients with resectable stage II-IIIB (N2 node stage) NSCLC (8th edition) were randomized to receive either neoadjuvant durvalumab or placebo with platinum-based chemotherapy for four cycles every three weeks followed by adjuvant durvalumab, or placebo for 12 cycles.45 Patients planned for a pneumonectomy were excluded, as were patients staged with T4 tumors for any reason other than size (>7 cm). The trial met both of its co-primary endpoints with improved EFS (median follow-up duration 11.7 months among patients without an event; median NR vs 25.9 months with placebo; HR 0.68, 95% CI 0.53–0.88; p = 0.004) and pCR favoring the durvalumab arm. The improvement in EFS was seen across disease stages, PD-L1 expressions, and types of platinum agents used.
Neotorch aimed to randomize 500 patients with resectable stage II-III NSCLC (8th edition) to receive either neoadjuvant anti-PD-1 toripalimab or placebo with platinum-based chemotherapy for three cycles and one cycle postoperatively followed by adjuvant toripalimab or placebo monotherapy for 13 cycles.46 In the first planned interim analysis of stage III patients, 404 patients were included. With a median follow-up of 18.3 months, an improvement in EFS with toripalimab was observed (median NR vs 15.1 months with placebo; HR 0.40, 95% CI 0.28–0.57; p < 0.0001), with consistent effect across subgroups. Both MPR, the co-primary endpoint, and pCR rates were higher with toripalimab. Immature OS results also indicated a trend favoring toripalimab. Since this study was conducted in the Chinese population, it could impact potential approval by the EMA or FDA. For example, a typical rule of thumb cited by experts for a treatment to be considered for FDA approval is that at least 20% of the supporting clinical data should be from US based patients.49 However, the FDA has permitted acceptance of clinical studies based on solely high-quality foreign data before and has regulations under which marketing approval may be granted.
CheckMate 77T enrolled 461 patients with resectable stage II-IIIB (N2) NSCLC (8th edition) who received either neoadjuvant nivolumab plus platinum-based chemotherapy followed by adjuvant nivolumab or chemotherapy plus placebo followed by adjuvant placebo for one year.47 In the first prespecified interim analysis, better EFS was demonstrated in favor of the nivolumab arm (median follow-up duration 25.4 months; median NR vs 18.4 months with placebo; HR 0.58, 97.36% CI 0.42–0.81; p = 0.00025). Additionally, pCR and MPR rates were higher with nivolumab.
KEYNOTE-671 compared four cycles of neoadjuvant pembrolizumab with placebo, both administered with platinum-based chemotherapy, followed by postoperative pembrolizumab or placebo every three weeks for up to 13 cycles in 797 patients with resectable stage II-IIIB (N2) NSCLC (8th edition).48 At the second planned interim analysis, the pembrolizumab group showed a maintained EFS benefit (median follow-up duration 36.6 months; median 47.2 vs 18.3 months with placebo; HR 0.59, 95% CI 0.48–0.72) and a significant OS improvement (median NR vs 52.4 months with placebo; HR 0.72, 95% CI 0.56–0.93; p = 0.00517). These results, consistent across most subgroups and without new safety concerns, led to FDA approval in October 2023 of neoadjuvant pembrolizumab with platinum-based chemotherapy followed by adjuvant pembrolizumab for resectable NSCLC (tumors ≥4 cm or node positive).50 As of January 2024, KEYNOTE-671's regimen is the only perioperative treatment with FDA approval, with EMA approval pending.
Immunotherapy and SABR
The randomized phase II I-SABR trial demonstrated promising EFS outcomes and manageable toxicity when 4 cycles of nivolumab were added to SABR, suggesting a new combined treatment strategy for medically inoperable patients with stage I or II NSCLC (8th edition).51 Interestingly, 20% of the study population were potentially operable, indicating a need for further exploration in future trials.
Identifying study endpoints
In adjuvant and neoadjuvant studies, OS is widely recognized as the most reliable and valuable parameter for drug approvals and guideline recommendations.52,53 However, in the curative setting, OS data often need a long time to mature, as could be seen by the long period needed to establish adjuvant platinum-based chemotherapy as a standard of care (Fig. 1).54, 55, 56, 57, 58, 59, 60, 61 Consequently, many recent studies use surrogate endpoints, often lacking statistical power to show significant OS differences. Based on surrogate endpoints, accelerated approval for oncology drugs can be granted, although these may be temporary while awaiting mature OS data.52,62 DFS and EFS are often used as such endpoints. DFS is defined as the time from randomization until disease recurrence or death from any cause and is typically used in adjuvant trials. This measure is usually applied to patients who have undergone surgery and are considered fit postoperatively. By contrast, EFS is used in neoadjuvant trials and is defined as the time from randomization to progression of disease that precludes surgery, disease recurrence after surgery, or death from any cause. The EFS population mainly consists of preoperative patients who are fit but still require surgery. These patients have a higher chance of experiencing disease worsening before or because of surgery. Therefore, it is important to note that the thresholds for determining significant benefits in EFS should not be directly compared with those used in DFS, due to the different patient populations and circumstances in which these measures are used. Given these differences, it may even be argued that EFS cutoffs should be less stringent compared to those for DFS, as the EFS population faces a higher likelihood of adverse events during the preoperative and surgical periods. Table 3 summarizes the merits and limitations of selected endpoints.52,63,64
Table 3.
Summary of advantages and disadvantages of key clinical endpoints used in adjuvant and neoadjuvant trials.
| Clinical endpoint | Advantages | Disadvantages |
|---|---|---|
| Adjuvant setting | ||
| DFS |
|
|
| ctDNA MRD |
|
|
| Neoadjuvant setting | ||
| EFS |
|
|
| pCR |
|
|
| MPR |
|
|
| ctDNA clearance |
|
|
| ORR |
|
|
| Adjuvant and neoadjuvant setting | ||
| OS |
|
|
| HRQOL |
|
|
Abbreviations: DFS, disease-free survival; OS, overall survival; ctDNA, circulating tumor DNA; MRD, minimal residual disease; EFS, event-free survival; pCR, pathological complete response; MPR, major pathological response; ORR, objective response rate; HRQOL, health-related quality of life.
pCR and MPR can be potential surrogate endpoints in neoadjuvant trials.65 The FDA and EMA take a cautious, context-specific stance on using pCR and MPR as endpoints in oncology trials. For instance, the EMA accepts approval based on pCR in high-risk early-stage breast cancer when part of a well-established regimen with significant pCR increase and minimal toxicity.66 This is due to the longer time needed for DFS data to mature in breast cancer. However, the shorter DFS in lung cancer reduces the urgency to use pCR as a surrogate endpoint. Neither FDA nor EMA has fully endorsed pCR and MPR as definitive endpoints for drug approval in NSCLC. The agencies typically require more data demonstrating a direct correlation between these endpoints and long-term patient outcomes before considering them for approval.52 Therefore, while pCR and MPR are valuable for assessing immediate treatment response, their role in predicting long-term benefits in early-stage NSCLC is still being evaluated.
To determine the clinical significance of a surrogate endpoint, it is essential to establish appropriate thresholds. In the absence of conclusive endpoints such as OS or when awaiting OS data to mature, surrogate endpoints could be considered using thresholds that are expected to align with the magnitude of benefit that could be expected from the conclusive endpoints. Such thresholds often come about through consensus. Tools like the European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS) provide a more objective approach for evaluating clinical benefit, albeit using arbitrary rules.53 For instance, on the ESMO-MCBS scale, a 95% CI lower limit of the DFS hazard ratio below 0.65 is scored as grade A, and therefore deemed most beneficial, as was the case in ADAURA, IMpower010, PEARLS/KEYNOTE-091, and CheckMate 816.
The availability and accessibility of new therapies in Europe
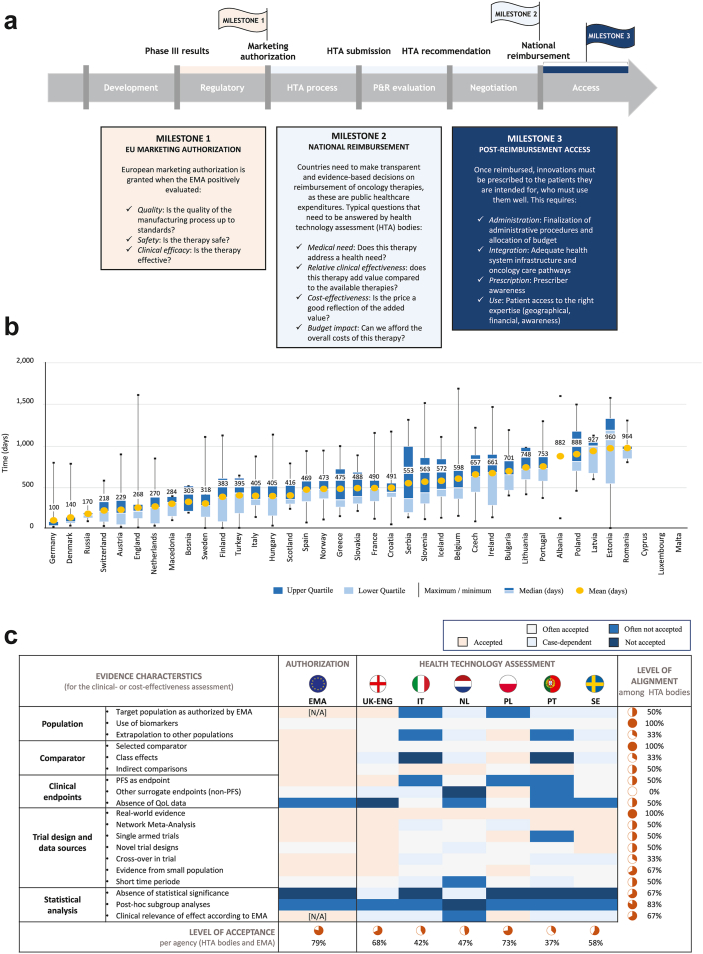
Three milestones must be achieved before patients can gain access to novel adjuvant and neoadjuvant immunotherapies and targeted therapies: (1) marketing authorization, (2) national reimbursement, and (3) post-reimbursement access (Fig. 2a).67
Fig. 2.
Inequalities in patient access in Europe: (a) the path for novel therapies, (b) assessing delays, and (c) evidence requirements for patient access.
(a) Thee milestones must be achieved before patients have access to new therapies. (b) The median time to availability in days in European countries (2017–2020), assessed from the date of marketing authorization to, for most countries, the date of acceptance on the reimbursement list. (c) Different evidence requirements are used across Europe, delaying the time to medicine access (Figure 2a adapted and modified from the European Federation of Pharmaceutical Industries and Associations (EFPIA), figure 2b adapted and modified from IQVIA, and figure 2c adapted from EFPIA67, 83). Abbreviations: EU, European Union; EMA, European Medicines Agency; HTA, health technology assessment; P&R, pricing and reimbursement; UK-ENG, United Kingdom-England; IT, Italy; NL, the Netherlands; PL, Poland; PT, Portugal; SE, Sweden; PFS, progression-free survival; QoL, quality of life.
Marketing authorization
After the authorization application to EMA, the EMA performs a single EU-wide assessment to evaluate the safety, efficacy, and quality of a product and provides a recommendation to the EC on whether to grant a marketing authorization.12 Once granted by the EC, the marketing authorization is automatically valid in all EU Member States.
Discrepancies between EMA and FDA
Studies have highlighted discrepancies between EMA and FDA in oncology drug approvals. In general, the FDA tends to grant approvals earlier than EMA. Uyl-de Groot and colleagues found that drugs take an average of 403 days (range 17–1187 days) to reach the EU market, 242 days later than the US.68 Also, while half of the drug approvals and label wordings are similar between the agencies, about 20% are approved by only one, and 28% have different labeling.69 Often, the second agency to review a drug chooses a more restrictive indication. Furthermore, when comparing the special regulatory pathways of both agencies, such as the FDA's Accelerated Approval and the EMA's Conditional Marketing Authorization, there are frequent discrepancies in decision-making and pathway usage, despite using the same pivotal trials.70 Both agencies often approve drugs amidst significant uncertainty, underscoring the need for further post-marketing studies. The delay in fulfilling post-marketing obligations raises concerns about approval standards.
Recent experiences in the field of resectable NSCLC have also shown delays in drug approval by the EMA. For example, adjuvant atezolizumab received EMA approval 235 days after the FDA, totaling 344 days from application.8,27,28 Adjuvant osimertinib was approved 154 days later in Europe, taking 268 days.7,21,71 The FDA approved neoadjuvant nivolumab three days before application in the EU, with EMA taking 476 days to approve.9,43,72 The EMA recently approved adjuvant pembrolizumab 259 days after the FDA, 554 days post-application.10,32,73 Additionally, there are notable differences in labeling. For instance, the FDA approved adjuvant atezolizumab for stage II-IIIA NSCLC with PD-L1 expression ≥1%, whereas the EMA approved it only for patients with PD-L1 ≥50% tumors. For neoadjuvant chemo-nivolumab, the EMA's approval is for stage II-IIIA NSCLC with PD-L1 expression ≥1%, contrasting with FDA's approval for stage IB-IIIA regardless of PD-L1 expression.
Speeding up review times
Excluding clock stops (pauses in the review process), both FDA and EMA have similar review durations.74 However, when these pauses are included, the FDA's process is notably faster. Over the last decade, both agencies have slightly reduced their mean review times, with the FDA often receiving new drug applications before EMA. This trend is due to factors like international cooperation and initiatives like Project Orbis, aimed at expediting drug approvals. In April 2023, the EC proposed policy changes to reduce the EMA's review times, including pre-submission scientific support to applicants, a practice already implemented by the FDA.75 This policy change could reduce the review time gap between the two agencies.
Following Brexit, the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom (UK) approved adjuvant osimertinib for EGFR-mutated resectable NSCLC in May 2021, the first authorization issued by the MHRA under Project Orbis.76 This project is a global collaborative review program of seven regulatory partners including the UK and Switzerland. This initiative, led by the FDA, aims to accelerate patient access to new cancer drugs internationally through parallel submission and reviews, while maintaining independent regulatory decision by each partner.77,78 Under Project Orbis, the UK often approves drugs faster than the EMA, but typically after FDA approval.79 For instance, adjuvant atezolizumab and neoadjuvant nivolumab were approved 131 and 314 days earlier, respectively, than the EMA.8,9,76 While the FDA's operations are partly supported by industry sources, this does not necessarily influence Orbis' operations.80 Each country independently reviews the FDA's dossiers, as exemplified by the different PD-L1 expression cutoffs for adjuvant atezolizumab approval. For instance, while the FDA set a cutoff at 1%, the UK MHRA and SwissMedic chose the higher cutoff of 50%. Aligning with the FDA's processes, Project Orbis facilitates rapid approval of innovative medicines, particularly beneficial for smaller regulatory agencies, and represents a concerted effort to reduce global inequalities in cancer treatment access, although its primary impact is currently seen in high-income countries alone.81
National reimbursement differences per country
In the EU, post-marketing authorization involves several steps before patients can access novel drugs, including regulatory procedures, price regulations, and health technology assessments (HTA) to decide on reimbursement through national health services or via insurance schemes. While the EMA grants approval at the EU level, individual Member States control the coverage and reimbursement of EMA-approved drugs. In contrast, the US uses a more centralized system through the Centers for Medicare & Medicaid Services, which incorporates reimbursement for FDA-approved therapies into the National and Local Coverage Determination.82 Although this process can be lengthy, it ensures consistent reimbursement policies across the US.
Reimbursement delays
In Europe, national reimbursement decisions involve a multiple-stage decision-making process, and authorities at various levels, including national, regional, and local hospital settings, may employ different processes and requirements, leading to delays and inequalities in patient access.67 The 2021 ‘Patient W.A.I.T. indicator’ survey revealed the average reimbursement time of 545 days for novel oncology therapies in Europe, ranging from 100 days in Germany to over 964 days in Romania (Fig. 2b).83 These delays are attributed to factors like late submissions, non-adherence to maximum timelines, and complex decision-making layers.67 Additionally, varying reimbursement criteria, unclear national requirements, and differences in value and price assessments contribute to these delays.
Health technology assessment
EU HTA bodies evaluate clinical trial evidence to determine the acceptability of new treatments, but their criteria may vary greatly (Fig. 2c).67 Notably, there is a lack of consensus on surrogate endpoints, crucial in most adjuvant and neoadjuvant studies. For instance, surrogate endpoints are accepted in Poland, often in Sweden, but infrequently in Portugal. England and Italy make decisions on a case-by-case basis. The absence of mature OS data complicates predicting long-term survival benefits, crucial for assessing cost-effectiveness. This leads to hesitancy in adopting therapies based on surrogate endpoints, causing delays in access times and regional inequalities. For example, France did not reimburse adjuvant atezolizumab, while Germany accepted it for all patients meeting the EMA label, and the Netherlands partially, for only a subgroup of patients with non-N2 stage III disease or those with an unforeseen postoperative N2 (Dutch Medicines Z-index, G-standaard February 2023).84,85 To address these discrepancies, the new Regulation (EU) 2021/2282 on Health Technology Assessment (HTAR), effective from January 2025, aims to harmonize HTA processes by performing a EU clinical assessment within a permanent framework for joint work including Member States, to remove the fragmentation of the internal market, reduce redundant assessments, and enhance transparency in evaluations, potentially speeding up patient access to new treatments.86
Post-reimbursement access
Post-reimbursement patient access to new therapies varies considerably across EU countries.67 Despite a relatively short time to reimbursement (234 days on average), only 20% of patients in the Netherlands receive a novel cancer therapy within 12 months after national reimbursement. Poland has a longer delay (891 days) before reimbursement, and 24% of patients have access to novel cancer therapies within 12 months following definitive decision. France, with a delay of 579 days for reimbursement after EMA approval, achieves an 80% access rate within the first year. Germany stands out for its short delay to market access (134 days), facilitated by a temporary period of free pricing for EMA-authorized therapies, with a patient access rate of 50%.
These disparities arise from different healthcare decision-making approaches in Europe.87 Some countries, like Iceland and Croatia, centralize pricing processes and budget allocation at the national level. Others like Italy have mixed national and regional systems with budget allocations, managed by healthcare insurers or at the hospital level, leading to significant variability in treatment accessibility and timeliness. In Italy, the time between national authorization and regional availability of a drug can range from 29 to 293 days due to the need for the drug to pass through 20 distinct local processes across Italy's regions, from Lombardy in the north to Sicily in the south, even after a national price is set. Delays are also common between the reimbursement decision and their official publications in national gazettes, as seen in countries like Belgium, Italy, and Hungary. In Bulgaria, the reimbursement list is updated annually, potentially delaying access by up to a year. Additionally, outdated clinical guidelines can lead to delays in incorporating new therapies into treatment pathways and hinder the adoption of new treatments by prescribers.
Budgetary impact on healthcare systems
EU healthcare spending has risen notably over the past decade.88 For example, the Netherlands saw a rise from €56 billion to €79.1 billion in 2020.89 Despite a slight decrease in the total care budget from 8.9% to 8.3%, medicine spending increased to €6.6 billion (excluding pharmacy fees). The projected direct mean costs of the new adjuvant and neoadjuvant treatments for Dutch patients diagnosed with stage IB-IIIA resectable NSCLC over one year could range from €39.9 million to €57.1 million (Table 4; Supplementary Tables S1–S3). These costs come on top of the current direct costs. For example, in Italy, the current average direct costs per NSCLC patient in the first year post-diagnosis, in stages I, II, and III were €16,291, €19,530, and €21,938, respectively.90 Surgery seems to be the primary driver of costs in stage I (58.9%), decreasing to 45.9% and 15.0% in stage II and stage III, respectively.91 In France, the average costs of surgery are €9474 for video-assisted thoracoscopic surgery and €10,418 for thoracotomy.92 These new adjuvant and neoadjuvant therapies, despite their potential DFS or EFS benefits and savings from reducing relapses, will significantly raise healthcare costs, considering their direct medicine costs but also indirect costs such as molecular testing, day treatment units, staff, and general healthcare expenses.
Table 4.
An overview of cost estimates of the novel adjuvant and neoadjuvant treatments based on the NSCLC incidence in the Netherlands.
| Drug | Trial | Treatment costs (€a) | EMA indication | Least expensive scenario (estimated minimum total costs, €) |
Most expensive scenario (estimated maximum total costs, €) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Minimumb | Maximumc | Mean | Minimumb | Maximumc | ||||
| Osimertinib | ADAURA | 224,486 | Three years of adjuvant treatment (80 mg once daily) in stage IB-IIIA, EGFR mutation-positive (ex19del or ex21L858R) NSCLC | 19,754,768 | 15,714,020 | 23,795,516 | 19,754,768 | 15,714,020 | 23,795,516 |
| Atezolizumab | IMpower010 | 64,528 | One year of adjuvant treatment (1200 mg every three weeks) following chemotherapy in stage II-IIIA, EGFR wildtype, and ALK-negative NSCLC with PD-L1 ≥50% | Not includedd | N/A | N/A | Not includede | N/A | N/A |
| Nivolumab | CheckMate-816 | 11,920 | Three cycles (360 mg every three weeks) of neoadjuvant treatment combined with chemotherapy in stage II-IIIA NSCLC with PD-L1 ≥1% | 6,007,680 | 5,816,960 | 6,198,400 | 2,622,400 | 2,538,960 | 2,705,840 |
| Pembrolizumab | PEALRS/KEYNOTE-091 | 102,981 | One year of adjuvant treatment (200 mg every three weeks) following chemotherapy in stage IB(≥4 cm)-IIIA NSCLC regardless of PD-L1 expression | 14,211,378 | 9,680,214 | 18,742,542 | 34,704,597 | 23,685,630 | 45,723,564 |
| Total | 39,973,826 | 31,211,194 | 48,736,458 | 57,081,765 | 41,938,610 | 72,224,920 | |||
Patients receive only one treatment. TNM staging is according to the seventh edition of the TNM classification system.
Abbreviations: EMA, European Medicines Agency; NSCLC, non-small cell lung cancer.
Based on the list prices in the Netherlands including VAT.
Based on the proportion of patients who completed treatment in each trial.
Based on a 100% treatment completion rate.
Atezolizumab is not included in this scenario because, theoretically, nivolumab may also be indicated in the same population but is less expensive.
Atezolizumab is not included in this scenario because, theoretically, pembrolizumab may also be indicated in the same population but is more expensive.
The financial impact of incorporating these treatments varies across countries, reflecting their GDP allocations and healthcare policies. In resectable NSCLC, for example, where the conclusive benefits of adjuvant immunotherapy following neoadjuvant chemoimmunotherapy are not fully established, particularly without mature survival data, countries must balance innovative care with budgetary constraints. Ultimately, the integration of these therapies depends on each country's financial capacity and healthcare approach, especially when the clinical value of these treatments is yet to be fully recognized.
Discussion
This Series paper examines the evolving neoadjuvant and adjuvant treatment approaches for resectable early-stage NSCLC, focusing on key phase III study findings and the journey of a treatment from its initial phase III results to its availability for patients. Our paper identifies disparities in patient care across EU countries. The accompanying Viewpoint on resectable NSCLC delves deeper into the challenges and unanswered questions presented by current studies and discusses the necessary measures to tackle issues of access inequalities from a clinician's perspective.93
The path of novel therapies from development to patient access involves several phases, each with unique challenges that need to be addressed.
Marketing authorization
During the marketing authorization phase, lengthy delays in the regulatory review and approval processes are a significant issue.87 Improving processes and reducing current timelines during this phase are crucial. Additionally, in certain situations, early access to life-saving medicines prior to formal approval is critical. Therefore, expanding compassionate use programs and early access schemes is vital, enabling patients to access essential treatments before they receive official approval.
Value assessment procedures
Value assessment procedures during HTA may suffer from variable and misaligned evidence requirements, leading to inefficiencies and delays in accessing new treatments.87 Harmonizing value assessment frameworks through initiatives such as the upcoming HTAR will establish uniform standards, vital to streamline drug evaluations and approvals. Additionally, it is essential to robustly acknowledge drug differentiation in value assessments, ensuring that the unique benefits and distinct advantages of new, innovative therapies are fully recognized and factored into healthcare decisions.
Pricing and reimbursement procedures
In the context of initiating pricing and reimbursement procedures after marketing authorization, substantial delays often occur in starting price negotiations, hindering timely access to medications.87 Beginning these negotiations immediately post-approval and streamlining national decision-making processes for pricing and reimbursement are essential to improve timely patient access to new treatments.
Allocating budget and improving infrastructure
Budgetary constraints and insufficient funding within healthcare systems often impede the adoption of new therapies.87 It is crucial to allocate sufficient funds and resources, where feasible, to ensure the timely availability and implementation of these innovative treatments within the healthcare framework. Additionally, investing in infrastructural improvements, including upgrading diagnostic tools, infrastructure, multidisciplinary tumor boards, and updating clinical guidelines, is key to enhancing access to new therapies.
The disparities across EU countries underline the importance of unified strategies and collaborative efforts to ensure equal patient access. As this landscape continuously evolves, both researchers and policymakers must prioritize patients’ needs, ensuring that novel breakthrough treatments not only represent research victories but also offer real benefits to individuals.
Conclusions
Treatment outcomes for patients with resectable early-stage NSCLC may be expected to improve in the near future due to the approval of new neoadjuvant and adjuvant systemic options as outcomes of recent trials have demonstrated encouraging results. Patient access to these innovative therapies varies among countries, and this disparity is expected to worsen. Steps to reduce inequalities in patient access should have higher priority.
Contributors
I.H. contributed with conceptualization, data curation, formal analysis, methodology, project administration, resources, visualization, writing—original draft, writing—review & editing. N.R., M.P., A.L., R.D., C.P., M.D.M., M.T., A.B., and S.P. contributed with data curation, investigation, resources, validation, writing—review & editing. C.D. contributed with conceptualization, data curation, formal analysis, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review & editing. C.A.G. contributed with data curation, formal analysis, resources, supervision, validation, visualization, writing—review & editing. S.S. contributed with conceptualization, investigation, methodology, project administration, resources, supervision, validation, writing—original draft, writing—review & editing. I.B. contributed with conceptualization, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review & editing.
Declaration of interests
I.H., C.D., N.R., and I.B. declare no competing interests. C.A.G. has received grants or contracts from Boehringer Ingelheim, Astellas, Celgene, Sanofi, Janssen-Cilag, Bayer, Amgen, Genzyme, Merck, Gilead, Novartis, AstraZeneca, Roche, NIH, and ASCERTAIN, all payments were made to the institute, outside the submitted work. M.P. has received research funding from MSD, AstraZeneca, Roche, Boehringer Ingelheim, and Takeda, outside the submitted work; consulting fees from Bristol-Meyers, Roche, MSD, AstraZeneca, Takeda, Eli Lilly, F Hoffman-La Roche, Janssen, Pfizer, and Takeda, outside the submitted work; honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bristol-Meyers, Roche, MSD, AstraZeneca, Takeda, Eli Lilly, F Hoffman-La Roche, Janssen, and Pfizer, outside the submitted work; and support for attending meetings and/or travel from AstraZeneca, Boehringer Ingelheim, Bristol-Meyers Eli Lilly, F Hoffman-La Roche, Phierre Fabre Pharmaceuticals, and Takeda, outside the submitted work. A.L. has received grants for academic research from PharMamar, Beigene, Roche, AstraZeneca, and Amgen, outside the submitted work. R.D. has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Roche, AstraZeneca, Takeda, Novartis, BMS, MSD, Pfizer, and Amgen, outside the submitted work; support for attending meetings and/or travel from Pfizer, outside the submitted work; drug samples from Novartis, outside the submitted work; and participated on a Data Safety Monitoring Board or Advisory Board of GlaxoSmithKline, outside the submitted work. C.P. has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, outside the submitted work. M.D.M. has received institutional research funding from Tesaro/GlaxoSmithKline and institutional funding for work in clinical trials/contracted research from Beigene, Exelixis, MSD, Pfizer and Roche, outside the submitted work; and personal fees for consultancy or participation to advisory boards from AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme (MSD), Novartis, Pfizer, Roche, GlaxoSmithKline, Amgen, Merck, and Takeda, outside the submitted work. M.T. has received institutional research funding from AstraZeneca, BMS, MSD, Roche, and Takeda, outside the submitted work; payment or honoraria (personal) for speakers bureaus from Amgen, AstraZeneca, Beigene, BMS, Boehringer Ingelheim, Celgene, Chugai, Daiichi Sankyo, GlaxoSmithKline, Janssen Oncology, Lilly, MSD, Novartis, Pfizer, Roche, Sanofi, and Takeda, outside the submitted work; and support for attending meetings and/or travel from AstraZeneca, BMS, Boehringer Ingelheim, Daiichi Sankyo, Janssen Oncology, Lilly, Merck, MSD, Novartis, Pfizer, Roche, Sanofi, and Takeda, outside the submitted work. A.B. has received consulting fees (personal) from AstraZeneca, BMS, MSD, and Roche, outside the submitted work; and payment or honoraria (personal) for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, BMS, MSD, and Roche, outside the submitted work. S.P. has received consulting fees (personal) from Amgen, AstraZeneca, Bayer, Blueprint, BMS, Boehringer Ingelheim, Daiichi Sankyo, GSK, Guardant Health, Incyte, Janssen, Lilly, Merck Serono, MSD, Novartis, Roche, Takeda, Pfizer, Seattle Genetics, Turning Point Therapeutics, and EQRx, outside the submitted work; payment or honoraria (personal) for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Bayer, Guardant Health, Janssen, Merck Serono, Roche and Takeda, outside the submitted work; payment for expert testimony from Roche and Merck Serono, outside the submitted work; support for travel from Janssen and Roche, outside the submitted work; consulting fees for participation on an Advisory Board, outside the submitted work; unpaid leadership role in the British Thoracic Oncology Group, ALK Positive UK, Lung Cancer Europe, Ruth Strauss Foundation, Mesothelioma Applied Research Foundation, and ETOP-IBCSG Partners Foundation Board, outside the submitted work. S.S. has received research grants (institution) from AstraZeneca and personal honoraria for participating in the trial steering committee for immunotherapy in small cell lung cancer from AstraZeneca, outside the submitted work; consulting fees from AstraZeneca and BMS, outside the submitted work; speaker honoraria (self) from AstraZeneca, outside the submitted work; participates on a Data Safety Monitoring Board as a review panel member for lung toxicity adjudication with immunotherapy (MSD), outside the submitted work; and has a leadership role as an ETOP member of the scientific committee for lung cancer, outside the submitted work.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.100840.
Appendix A. Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Hendriks L.E., Kerr K.M., Menis J., et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):339–357. doi: 10.1016/j.annonc.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Netherlands Cancer Registry https://iknl.nl/kankersoorten/longkanker/registratie/overleving
- 4.Pignon J.P., Tribodet H., Scagliotti G.V., et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 5.Lim E., Harris G., Patel A., Adachi I., Edmonds L., Song F. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol. 2009;4(11):1380–1388. doi: 10.1097/JTO.0b013e3181b9ecca. [DOI] [PubMed] [Google Scholar]
- 6.NSCLC Meta-analysis Collaborative Group Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383(9928):1561–1571. doi: 10.1016/S0140-6736(13)62159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency www.ema.europa.eu/en/documents/procedural-steps-after/tagrisso-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf Accessed November 1, 2023.
- 8.European Medicines Agency https://www.ema.europa.eu/en/documents/procedural-steps-after/tecentriq-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf Accessed November 1, 2023.
- 9.European Medicines Agency https://www.ema.europa.eu/en/documents/procedural-steps-after/opdivo-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf Accessed November 1, 2023.
- 10.European Medicines Agency https://www.ema.europa.eu/en/documents/procedural-steps-after/keytruda-epar-procedural-steps-taken-and-scientific-information-after-authorisation_en.pdf Accessed November 1, 2023.
- 11.U.S. Food and Drug Administration. https://www.fda.gov/drugs/development-approval-process-drugs Accessed November 1, 2023.
- 12.European Medicines Agency https://www.ema.europa.eu/en/about-us/what-we-do/authorisation-medicines
- 13.Remon J., Soria J.C., Peters S., Comm E.G. Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32(12):1637–1642. doi: 10.1016/j.annonc.2021.08.1994. [DOI] [PubMed] [Google Scholar]
- 14.Postmus P.E., Kerr K.M., Oudkerk M., et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 15.Chang J.Y., Mehran R.J., Feng L., et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021;22(10):1448–1457. doi: 10.1016/S1470-2045(21)00401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstraw P., Chansky K., Crowley J., et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Demicheli R., Fornili M., Ambrogi F., et al. Recurrence dynamics for non–small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol. 2012;7(4):723–730. doi: 10.1097/JTO.0b013e31824a9022. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y.L., Tsuboi M., He J., et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 19.Tsuboi M., Herbst R.S., John T., et al. Overall survival with osimertinib in resected EGFR-mutated NSCLC. N Engl J Med. 2023;389(2):137–147. doi: 10.1056/NEJMoa2304594. [DOI] [PubMed] [Google Scholar]
- 20.Majem M., Goldman J.W., John T., et al. Health-related quality of life outcomes in patients with resected epidermal growth factor receptor-mutated non-small cell lung cancer who received adjuvant osimertinib in the phase III ADAURA trial. Clin Cancer Res. 2022;28(11):2286–2296. doi: 10.1158/1078-0432.CCR-21-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Food and Drug Administration 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-osimertinib-adjuvant-therapy-non-small-cell-lung-cancer-egfr-mutations
- 22.Solomon B.J., Ahn J.S., Dziadziuszko R., et al. LBA2 ALINA: efficacy and safety of adjuvant alectinib versus chemotherapy in patients with early-stage ALK+ non-small cell lung cancer (NSCLC) Ann Oncol. 2023;34:S1295–S1296. [Google Scholar]
- 23.Shields M.D., Marin-Acevedo J.A., Pellini B. Immunotherapy for advanced non–small cell lung cancer: a decade of progress. Am Soc Clin Oncol Educ Book. 2021;41:e105–e127. doi: 10.1200/EDBK_321483. [DOI] [PubMed] [Google Scholar]
- 24.Huang P.-W., Chang J.W.-C. Immune checkpoint inhibitors win the 2018 Nobel Prize. Biomed J. 2019;42(5):299–306. doi: 10.1016/j.bj.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matzner P., Sandbank E., Neeman E., Zmora O., Gottumukkala V., Ben-Eliyahu S. Harnessing cancer immunotherapy during the unexploited immediate perioperative period. Nat Rev Clin Oncol. 2020;17(5):313–326. doi: 10.1038/s41571-019-0319-9. [DOI] [PubMed] [Google Scholar]
- 26.Felip E., Altorki N., Zhou C., et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344–1357. doi: 10.1016/S0140-6736(21)02098-5. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-adjuvant-treatment-non-small-cell-lung-cancer
- 28.European Medicines Agency https://www.ema.europa.eu/en/documents/variation-report/tecentriq-h-c-h004143-ii-0064-epar-assessment-report-variation_en.pdf
- 29.Felip E., Altorki N., Zhou C., et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase III trial. Ann Oncol. 2023;34(10):907–919. doi: 10.1016/j.annonc.2023.07.001. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien M., Paz-Ares L., Marreaud S., et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274–1286. doi: 10.1016/S1470-2045(22)00518-6. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien M.E.R., Paz-Ares L., Jha N., et al. EORTC-1416-LCG/ETOP 8-15 – PEARLS/KEYNOTE-091 study of pembrolizumab versus placebo for completely resected early-stage non-small cell lung cancer (NSCLC): outcomes in subgroups related to surgery, disease burden, and adjuvant chemotherapy use. J Clin Oncol. 2022;40(16_suppl):8512. [Google Scholar]
- 32.U.S. Food and Drug Administration 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-adjuvant-treatment-non-small-cell-lung-cancer
- 33.Forde P.M., Chaft J.E., Smith K.N., et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Provencio M., Nadal E., González-Larriba J.L., et al. Perioperative nivolumab and chemotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2023;389(6):504–513. doi: 10.1056/NEJMoa2215530. [DOI] [PubMed] [Google Scholar]
- 35.Cascone T., William W.N., Jr., Weissferdt A., et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021;27(3):504–514. doi: 10.1038/s41591-020-01224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGranahan N., Furness A.J.S., Rosenthal R., et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Blake S.J., Yong M.C.R., et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6(12):1382–1399. doi: 10.1158/2159-8290.CD-16-0577. [DOI] [PubMed] [Google Scholar]
- 38.Martín-Ruiz A., Fiuza-Luces C., Martínez-Martínez E., et al. Effects of anti-PD-1 immunotherapy on tumor regression: insights from a patient-derived xenograft model. Sci Rep. 2020;10(1):7078. doi: 10.1038/s41598-020-63796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forde P.M., Spicer J., Lu S., et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felip E., Wang C., Ciuleanu T.E., et al. 932MO Nivolumab (NIVO) plus platinum-doublet chemotherapy (chemo) versus chemo as neoadjuvant treatment for resectable non-small cell lung cancer (NSCLC): health-related quality of life (HRQoL) outcomes from CheckMate 816. Ann Oncol. 2022;33:S973–S974. [Google Scholar]
- 41.Forde P.M., Spicer J., Girard N., et al. Neoadjuvant nivolumab (N) plus platinum-doublet chemotherapy (C) for resectable NSCLC: 3-y update from CheckMate 816. J Thorac Oncol. 2023;18(4):S89–S90. [Google Scholar]
- 42.Provencio Pulla M., Forde P.M., Spicer J.D., et al. LBA57 Neoadjuvant nivolumab (N) + chemotherapy (C) in the phase III CheckMate 816 study: 3-y results by tumor PD-L1 expression. Ann Oncol. 2023;34:S1298–S1299. [Google Scholar]
- 43.U.S. Food and Drug Administration 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neoadjuvant-nivolumab-and-platinum-doublet-chemotherapy-early-stage-non-small-cell-lung
- 44.European Medicines Agency https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf
- 45.Heymach J.V., Harpole D., Mitsudomi T., et al. Perioperative durvalumab for resectable non–small-cell lung cancer. N Engl J Med. 2023;389(18):1672–1684. doi: 10.1056/NEJMoa2304875. [DOI] [PubMed] [Google Scholar]
- 46.Wu L., Zhang W., Zhang P., et al. Perioperative toripalimab + platinum-doublet chemotherapy vs chemotherapy in resectable stage II/III non-small cell lung cancer (NSCLC): interim event-free survival (EFS) analysis of the phase III Neotorch study. J Clin Oncol. 2023;41(36_suppl) [Google Scholar]
- 47.Cascone T., Awad M.M., Spicer J.D., et al. LBA1 CheckMate 77T: phase III study comparing neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) vs neoadjuvant placebo plus chemo followed by surgery and adjuvant NIVO or placebo for previously untreated, resectable stage II–IIIb NSCLC. Ann Oncol. 2023;34:S1295. [Google Scholar]
- 48.Wakelee H., Liberman M., Kato T., et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491–503. doi: 10.1056/NEJMoa2302983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.IQVIA 2019. https://www.iqvia.com/library/white-papers/global-approaches-to-drug-development
- 50.U.S. Food and Drug Administration. 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neoadjuvant-adjuvant-pembrolizumab-resectable-non-small-cell-lung-cancer Accessed November 1, 2023.
- 51.Chang J.Y., Lin S.H., Dong W., et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: an open-label, randomised, phase 2 trial. Lancet. 2023;402(10405):871–881. doi: 10.1016/S0140-6736(23)01384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.U.S. Food and Drug Administration 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-endpoints-approval-cancer-drugs-and-biologics
- 53.Cherny N.I., Dafni U., Bogaerts J., et al. ESMO-magnitude of clinical benefit scale version 1.1. Ann Oncol. 2017;28(10):2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 54.Scagliotti G.V., Fossati R., Torri V., et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst. 2003;95(19):1453–1461. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 55.Arriagada R., Bergman B., Dunant A., Le Chevalier T., Pignon J.P., Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 56.Waller D., Peake M.D., Stephens R.J., et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardio Thorac Surg. 2004;26(1):173–182. doi: 10.1016/j.ejcts.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 57.Douillard J.Y., Rosell R., De Lena M., et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 58.Strauss G.M., Herndon J.E., 2nd, Maddaus M.A., et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the cancer and leukemia group B, radiation therapy oncology group, and north central cancer treatment group study groups. J Clin Oncol. 2008;26(31):5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butts C.A., Ding K., Seymour L., et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol. 2010;28(1):29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Felip E., Rosell R., Maestre J.A., et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non–small-cell lung cancer. J Clin Oncol. 2010;28(19):3138–3145. doi: 10.1200/JCO.2009.27.6204. [DOI] [PubMed] [Google Scholar]
- 61.Scagliotti G.V., Pastorino U., Vansteenkiste J.F., et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non–small-cell lung cancer. J Clin Oncol. 2012;30(2):172–178. doi: 10.1200/JCO.2010.33.7089. [DOI] [PubMed] [Google Scholar]
- 62.Gyawali B., Hwang T.J., Vokinger K.N., Booth C.M., Amir E., Tibau A. Patient-centered cancer drug development: clinical trials, regulatory approval, and value assessment. Am Soc Clin Oncol Educ Book. 2019;39:374–387. doi: 10.1200/EDBK_242229. [DOI] [PubMed] [Google Scholar]
- 63.Bestvina C.M., Garassino M.C., Neal J.W., Wakelee H.A., Diehn M., Vokes E.E. Early-stage lung cancer: using circulating tumor DNA to get personal. J Clin Oncol. 2023;41:JCO2300258. doi: 10.1200/JCO.23.00258. [DOI] [PubMed] [Google Scholar]
- 64.Blakely C.M., Weder W., Bubendorf L., et al. Primary endpoints to assess the efficacy of novel therapeutic approaches in epidermal growth factor receptor-mutated, surgically resectable non-small cell lung cancer: a review. Lung Cancer. 2023;177:59–72. doi: 10.1016/j.lungcan.2023.01.002. [DOI] [PubMed] [Google Scholar]
- 65.International Association for the Study of Lung Cancer (IASLC) https://www.iaslc.org/pathologic-response-project
- 66.European Medicines Agency https://www.ema.europa.eu/en/appendix-4-guideline-evaluation-anticancer-medicinal-products-man-condition-specific-guidance-scientific-guideline
- 67.Vintura 2020. https://www.efpia.eu/media/578013/every-day-counts.pdf
- 68.Uyl-de Groot C.A., Heine R., Krol M., Verweij J. Unequal access to newly registered cancer drugs leads to potential loss of life-years in Europe. Cancers (Basel) 2020;12:2313. doi: 10.3390/cancers12082313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trotta F., Leufkens H.G.M., Schellens J.H.M., Laing R., Tafuri G. Evaluation of oncology drugs at the European medicines agency and US Food and drug administration: when differences have an impact on clinical practice. J Clin Oncol. 2011;29(16):2266–2272. doi: 10.1200/JCO.2010.34.1248. [DOI] [PubMed] [Google Scholar]
- 70.Salcher-Konrad M., Naci H., Davis C. Approval of cancer drugs with uncertain therapeutic value: a comparison of regulatory decisions in Europe and the United States. Milbank Q. 2020;98(4):1219–1256. doi: 10.1111/1468-0009.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.European Medicines Agency https://www.ema.europa.eu/en/documents/variation-report/tagrisso-h-c-004124-ii-0039-g-epar-assessment-report-variation_en.pdf
- 72.European Medicines Agency https://www.ema.europa.eu/en/documents/variation-report/opdivo-h-c-003985-ii-0117-epar-assessment-report-variation_en.pdf
- 73.European Medicines Agency https://www.ema.europa.eu/en/documents/variation-report/keytruda-h-c-003820-ii-0121-epar-assessment-report-variation_en.pdf
- 74.Vokinger K.N., Serra-Burriel M., Glaus C.E.G., et al. Regulatory review duration and differences in submission times of drugs in the United States and Europe, 2011 to 2020. Ann Intern Med. 2023;176(10):1413–1418. doi: 10.7326/M23-0623. [DOI] [PubMed] [Google Scholar]
- 75.European Medicines Agency https://health.ec.europa.eu/medicinal-products/pharmaceutical-strategy-europe/reform-eu-pharmaceutical-legislation_en
- 76.Medicines And Healthcare Products Regulatory Agency https://www.gov.uk/guidance/guidance-on-project-orbis
- 77.de Claro R.A., Spillman D., Hotaki L.T., et al. Project Orbis: global collaborative review program. Clin Cancer Res. 2020;26(24):6412–6416. doi: 10.1158/1078-0432.CCR-20-3292. [DOI] [PubMed] [Google Scholar]
- 78.U.S. Food and Drug Administration https://www.fda.gov/about-fda/oncology-center-excellence/project-orbis
- 79.Lythgoe M.P., Krell J., Bower M., et al. From the European Medicines Agency to Project Orbis: new activities and challenges to facilitate UK oncology drug approval following Brexit. Lancet Oncol. 2023;24(4):e150–e160. doi: 10.1016/S1470-2045(22)00701-X. [DOI] [PubMed] [Google Scholar]
- 80.U.S. Food and Drug Administration. https://www.fda.gov/industry/fda-user-fee-programs/fda-user-fees-explained Accessed November 1, 2023.
- 81.Leighl N.B., Nirmalakumar S., Ezeife D.A., Gyawali B. An arm and a leg: the rising cost of cancer drugs and impact on access. Am Soc Clin Oncol Educ Book. 2021;41:e1–e12. doi: 10.1200/EDBK_100028. [DOI] [PubMed] [Google Scholar]
- 82.U.S. Food and Drug Administration. https://www.fda.gov/about-fda/domestic-mous/mou-225-10-0010 Accessed November 1, 2023.
- 83.IQVIA 2022. https://www.efpia.eu/media/676539/efpia-patient-wait-indicator_update-july-2022_final.pdf
- 84.Haute Autorité de Santé 2022. https://www.has-sante.fr/jcms/p_3390617/en/tecentriq-atezolizumab-cancer-du-poumon
- 85.Gemeinsamer Bundesausschuss 2023. https://www.g-ba.de/downloads/40-268-9303/2023-01-05_AM-RL-XII_Atezolizumab_D-828_ZD.pdf
- 86.European Commission https://health.ec.europa.eu/health-technology-assessment/regulation-health-technology-assessment_en
- 87.European Federation of Pharmaceutical Industries and Associations (EFPIA) 2023. https://www.efpia.eu/media/677292/cra-efpia-root-causes-unavailability-delay-080423-final.pdf [DOI] [PubMed]
- 88.Hofmarcher T., Szilagyiova P., Gustafsson A., et al. Access to novel cancer medicines in four countries in Central and Eastern Europe in relation to clinical benefit. ESMO Open. 2023;8(4) doi: 10.1016/j.esmoop.2023.101593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.2022. Vereniging Innovatieve Geneesmiddelen: Medicijnen voor de Samenleving.https://www.vereniginginnovatievegeneesmiddelen.nl/actueel/publicaties Accessed November 1, 2023. [Google Scholar]
- 90.Buja A., Rivera M., De Polo A., et al. Estimated direct costs of non-small cell lung cancer by stage at diagnosis and disease management phase: a whole-disease model. Thorac Cancer. 2021;12(1):13–20. doi: 10.1111/1759-7714.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Corral J., Espinas J.A., Cots F., et al. Estimation of lung cancer diagnosis and treatment costs based on a patient-level analysis in Catalonia (Spain) BMC Health Serv Res. 2015;15:70. doi: 10.1186/s12913-015-0725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bouabdallah I., Pauly V., Viprey M., et al. Unplanned readmission and survival after video-assisted thoracic surgery and open thoracotomy in patients with non-small-cell lung cancer: a 12-month nationwide cohort study. Eur J Cardio Thorac Surg. 2021;59(5):987–995. doi: 10.1093/ejcts/ezaa421. [DOI] [PubMed] [Google Scholar]
- 93.Houda I., Dickhoff C., Uyl-de Groot C.A., et al. Challenges and controversies in resectable non-small cell lung cancer: a clinician’s perspective. Lancet Reg Health Eur. 2024;38:100841. doi: 10.1016/j.lanepe.2024.100841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.