Abstract
Background
Atopic dermatitis is one of the most common skin disorders. Evidence has suggested an association between skin disorders, such as atopic dermatitis, and Parkinson's disease (PD). However, whether atopic dermatitis has a causal effect on PD remains unknown.
Methods
The study aimed to determine whether their association between atopic dermatitis and PD is causal, using a bidirectional two‐sample Mendelian randomization method. Genetic variants from the public genome‐wide association studies for atopic dermatitis (n = 10788 cases and 30047 controls) were selected to evaluate their causal effects on the risk of PD (33,674 cases and 449,056 controls). The inverse variance weighted (IVW) method was used as the primary analysis.
Results
The IVW results indicated that atopic dermatitis was associated with decreased risk of PD {fixed effects: odds ratio [OR] [95% confidence interval (CI)]: .905 [.832–.986], p = .022; OR [95% CI]: .905 [.827–.991], p = .032}. However, we failed to detect the causal effects of PD on risk of atopic dermatitis in the reverse causation analysis.
Conclusion
This study indicated causal association of genetically proxied atopic dermatitis with the risk of PD. Future studies are warranted to explore the underlying mechanism and investigate the targeting effect of atopic dermatitis on PD.
Keywords: atopic dermatitis, causal association, Mendelian randomization, Parkinson's disease
1. INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative disorder, which is characterized by progressive degeneration of dopamine neurons in brain (Jankovic, 2008; Vernier et al., 2004), and it affects a great number of people around the world. In recent years, the skin presentations of PD are attracting increasing attentions. For example, seborrheic face is recognized as a featured manifestation of PD (Niemann et al., 2021). Besides, Tomic et al. (2022) reported that seborrheic dermatitis was associated with the severity of motor symptoms of PD. Therefore, it is important to investigate the relationship between PD and skin disorders, which helps better understanding of PD.
Atopic dermatitis is the most common chronic, inflammatory, relapsing skin disorder, with a prevalence of 7%–14% among adult population (Bylund et al., 2020). It mainly presents with symptoms, including severe itching and recurring eczematous lesions (Li et al., 2021), and imposes a great burden to patients and their family (Drucker, 2017). Emerging evidence has suggested that atopic dermatitis is associated with depression and anxiety (Baurecht et al., 2021), which are common symptoms of PD (Pontone & Mills, 2021), pointing out the potential link between atopic dermatitis and PD.
Their association is likely to involve the inflammatory nature of the two disorders. Many studies have indicated the association of atopic dermatitis with an immune response of the cell community through the human body, including Type 1 T helper (Th1), Type 2 T helper (Th2), and Regulatory T (Treg) cells (Auriemma et al., 2013). It is believed that an alteration of Th1‐ and Th2‐mediated immune responses and IgE‐regulated hypersensitivity reactions is responsible for the development and progression of the disorder. For PD, systemic inflammation helps explain the onset, development, and progression of the disorder (Tansey & Romero‐Ramos, 2019). Thus, atopic dermatitis might have a distinct role in the pathology of PD. However, a cohort study by Nam et al. (2024) did not reveal a significant increased risk of PD in patients with atopic dermatitis. Then, it is crucial to investigate whether there is a causal relationship between atopic dermatitis and PD remains unclear.
Mendelian randomization (MR) analysis provides a method to estimate the causal effect of a certain risk factor on an outcome of interest (such as a disease), using genetic variants as instrumental variables (IVs) (Skrivankova et al., 2021). MR has been increasingly applied in epidemiology and clinical studies, for its less susceptibility to unmeasured confounding factors and potential reverse causation (Burgess et al., 2021). In this study, we used the MR method to explore the causality between atopic dermatitis and risk of PD.
2. MATERIALS AND METHODS
2.1. Data sources
Publicly available data were used in this study, including genome‐wide association studies (GWAS) data for atopic dermatitis and PD. Informed patients consents and ethical approvals were available in each data center. Summary genetic association estimates for PD were extracted from the International PD Genomics Consortium (IPDGC) (Nalls et al., 2019), including 33,674 cases and 449,056 controls of European ancestry. Summary level statistics for atopic dermatitis were from the study by Paternoster et al. (2015), including 10,788 cases and 30,047 controls from 20 studies of European ancestry, excluding 23andMe study.
2.2. Selection of SNPs
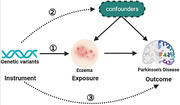
Under the design of MR (Figure 1), single nucleotide polymorphisms (SNPs) for exposure were selected as IVs when they met three key assumptions. Based on these principals, we did the following processes to select suitable IVs. First, SNPs should passed the genome‐wide significance criteria (p < 5e − 08). Then they were pruned based on the linkage disequilibrium (r 2 = .001, clumping distance = 10,000 kb) to ensure the independence of each SNP. Moreover, we calculated the F statistic to ensure the strength of the exposures, and F statistic > 10 indicated that the screening IVs were strongly correlated with the exposure. The R 2 and F statistics of each SNP were calculated based on the formulas: R 2 = 2 × EAF × (1 − EAF) × β 2 and F statistics = R 2 × (N − 2)/(1 − R 2). After that, the instrument SNPs were extracted from the outcome GWAS dataset. Finally, variants and their alleles from the exposure and outcome data were harmonized to ensure that the beta coefficients and standard errors for the exposure and outcome correspond to the same allele.
FIGURE 1.
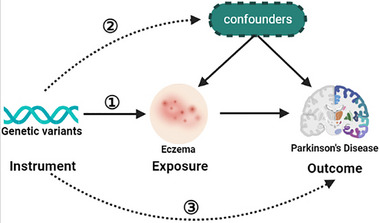
Graphic representation of the Mendelian randomization (MR) design. Three principal assumptions should be fulfilled in the MR study: (1) genetic instrumental variants should be stably associated with eczema; (2) genetic instrumental variants should not be associated with any confounding factors; and (3) genetic instrumental variants should only influence the risk of Parkinson's disease (PD).
2.3. MR analysis
We carried out two‐sample MR analysis to explore the causal effects of eczema on risk of PD, using the “TwoSampleMR” package of R software. Both fixed‐ and random‐effects inverse variance weighted (IVW) models were conducted as the primary analysis. The IVW model assumes that all SNPs included for the estimation of causal effects are valid (Mounier & Kutalik, 2023). Alternative MR methods were conducted for supplements, including simple median, MR‐Egger, and maximum likelihood methods (Bowden et al., 2017; Burgess & Thompson, 2017; Hemani et al., 2018). Cochran's Q test in IVW model was performed to detect the heterogeneity among SNPs in the respective analysis. The MR‐Egger regression intercept was applied to detect the presence of horizontal pleiotropy, and a p intercept less than .05 was considered significant for the presence of horizontal pleiotropy. Furthermore, we used the MR pleiotropy residual sum and outlier (MR‐PRESSO) global test to detect and correct for horizontal pleiotropic outliers (Verbanck et al., 2018). Leave‐one‐out test was done to detect the effect of one single SNP on the outcome.
3. RESULTS
3.1. Genetic variants as instrumental variables
A total of 12 SNPs were identified as IVs for atopic dermatitis. The F statistics of them were all above 10, ranging from 174.062 to 419.088. The detailed information of SNPs associated with atopic dermatitis and PD are shown in Tables S1 and S2, respectively.
3.2. Two‐sample MR of atopic dermatitis and PD
The complete sets of MR results are displayed in Table 1 and Figure 2. The results of both fixed‐ and random‐effect IVW analyses showed a causal effect of atopic dermatitis on risk of PD (odds ratio [OR] = .905, 95% confidence interval [CI]: .832–.986, p = .022; OR = .905, .827–.991, p = .032). Similar results were observed in the models of simple median, maximum likelihood, and IVW radial. Unfortunately, the MR‐Egger regression model results did not show statistically significance. No heterogeneity was detected by Cochran's Q test among the IVs (Q = 12.541,p = .324) (Table 2). Moreover, no horizontal pleiotropy was detected using the MR‐Egger intercept (p intercept = .150) (Table 2). The MR‐PRESSO analysis did not detect any instrumental outlier at the significance level of .05 (Table 2). A leave‐one‐out analysis was done to test the conformity of the included SNPs, and it indicated that no specific SNP significantly affected the overall MR results. The forest plot, funnel plot, and leave‐one‐out analysis results are presented in Figure 3.
TABLE 1.
Mendelian randomization (MR) results of the association between atopic dermatitis and Parkinson's disease (PD).
| Exposure | Outcome | Model | No. of SNPs | β | SE | OR [95% CI] | p Value |
|---|---|---|---|---|---|---|---|
| Eczema | PD | IVW (fixed effects) | 12 | −.099 | .043 | .905 [.832–.986] | .022 |
| IVW (random effects) | 12 | −.099 | .046 | .905 [.827–.991] | .032 | ||
| IVW radial | 12 | −.099 | .046 | .905 [.827–.991] | .032 | ||
| MR Egger | 12 | .187 | .189 | 1.205 [.833–1.744] | .347 | ||
| Simple median | 12 | −.139 | .065 | .871 [.767–.989] | .032 | ||
| Maximum likelihood | 12 | −.100 | .044 | .905 [.830–.987] | .024 |
Abbreviations: CI, confidence interval; IVW, inverse variance weighted; OR, odds ratio; SE, standard error; SNP, single nucleotide polymorphisms.
FIGURE 2.
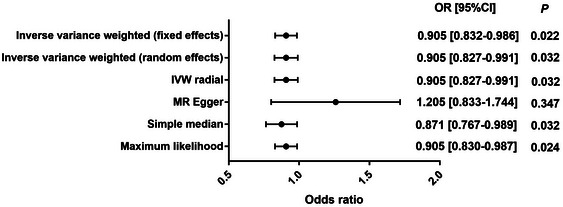
Mendelian randomization (MR) results of the association between atopic dermatitis and Parkinson's disease (PD). CI, confidence interval; OR, odds ratio.
TABLE 2.
Heterogeneity and horizontal pleiotropy analysis between atopic dermatitis and Parkinson's disease (PD).
| Heterogeneity | Horizontal pleiotropy | MR‐PRESSO | ||||
|---|---|---|---|---|---|---|
| Q | df` | p Value | Egger intercept | SE | p Value | p Value |
| 12.541 | 11 | .324 | −.040 | .026 | .150 | .354 |
Abbreviation: SE, standard error.
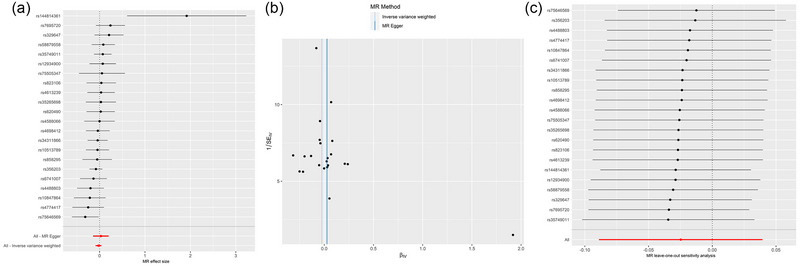
FIGURE 3.
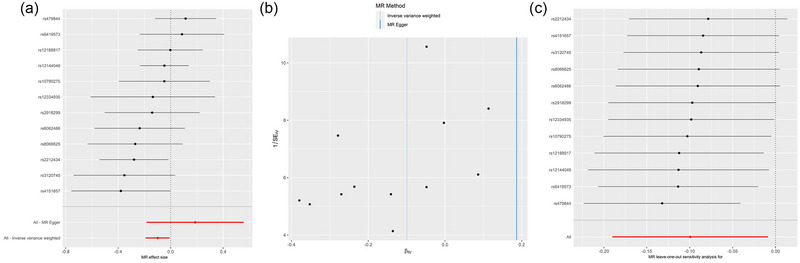
Forest plot (A), scatter plot (B), and leave‐one‐out analysis (C) of the causal effect of atopic dermatitis on Parkinson's disease (PD).
Reverse MR analysis of the effects of PD on risk of atopic dermatitis was done and indicated no causality between the two disorders (Table S3). The heterogeneity and horizontal pleiotropy analysis are shown in Table S4. The scatter plot, forest plot, funnel plot, and leave‐one‐out analysis results are presented in Figure 4.
FIGURE 4.
Forest plot (A), scatter plot (B), and leave‐one‐out analysis (C) of the causal effect of Parkinson's disease (PD) on atopic dermatitis.
4. DISCUSSION
In the current study, we leveraged the effects of atopic dermatitis on risk of PD by identifying genetic variants as IVs for the analysis of their associations. This MR study indicated that atopic dermatitis was negatively associated with risk of PD. No evidence in the reverse MR analysis was observed. These provided a better understanding of the role of atopic dermatitis in PD.
Dermatological diseases, such as seborrheic dermatitis, pemphigoid, and melanoma, are recognized as common non‐motor symptoms of PD (Niemann et al., 2021). Cumulative studies have indicated an increased prevalence of skin disorders among patients with PD (Niemann et al., 2021), even before the presentation of motor symptoms (Pont‐Sunyer et al., 2015). In a previous study by Tanner et al. (2012), the authors demonstrated that seborrheic dermatitis might represent a premotor feature of PD, ascribable to the dysregulation of autonomic nervous system, and suggested that this skin disorder could serve as an early disease indicator. The explanation for the association between dermatological disorders and PD remains unclear, but various hypotheses have been proposed. Among them, a potential genetic link was raised by researchers. For instance, the association of seborrheic dermatitis with PD may originate from the shared genetic polymorphisms of GBA, LRRK2, and PINK1 (Trinh & Farrer, 2013), which play a role in immune changes, lipid metabolism, and homeostasis of brain (Dzamko et al., 2015; Magnusen et al., 2021). Then, it is important to investigate the underlying genetic link between skin diseases and PD.
MR study helps understand the causality between certain exposures and outcomes, by applying genetic variants as IVs (Skrivankova et al., 2021). The causality between skin disorder and PD has also been established in a previous MR study focusing on psoriasis (Li et al., 2022), which indicated that higher psoriasis risk was nominally associated with progression of PD. The associations may originate from the immune activities in psoriasis (Afonina et al., 2021). Psoriasis is a common skin disease, which represents marked inflammatory changes, including keratinocyte hyperproliferation and altered differentiation and infiltration of leukocytes in skin tissues (Eberle et al., 2016; Ortiz‐Lopez et al., 2022). Patients with psoriasis are likely to represent chronic periphery inflammation (Reich, 2012), which could have an essential role in the development of PD (Williams et al., 2022). It is believed that systemic inflammation is responsible the activation of microglia, the production of reactive oxygen species, and neuronal damages (Hoogland et al., 2015). These changes are of particular importance for the degeneration of substantia nigra, and other parts in PD brains (Marinova‐Mutafchieva et al., 2009). Moreover, the inflammatory components could increase the accumulation of α‐synuclein, which in turn causes activation of immune cells and the production of inflammatory factors (Allen Reish & Standaert, 2015).
Interestingly, our study indicated that atopic dermatitis is negatively associated with risk of PD through an MR framework. Results were confirmed in both fixed‐ and random‐effects IVW models, as well as most MR models. This was not consistent with the associative trend between psoriasis, which also shares an immune cause with atopic dermatitis and PD. Atopic dermatitis is a chronic inflammatory skin disease with clinical manifestations, including intense pruritus and eczematous lesions (Tamagawa‐Mineoka & Katoh, 2020). Intensive immune activities can also be found in atopic dermatitis (Novak, 2003). Studies have indicated that atopic dermatitis is induced by the immune response in human body, including Th1, Th2, and Treg cells, as well as the related immune alterations (Auriemma et al., 2013). Both atopic dermatitis and psoriasis are common T‐cell mediated inflammatory diseases of the skin, and they can be treated by specific cytokine antagonists or immunosuppressive agents. In addition, they are quite similar in the alterations of epidermal keratinocytes when responding to T‐cell produced cytokines. However, the two skin disorders display varied T‐cell polarity and different spectrums of cytokines (Guttman‐Yassky & Krueger, 2017). Specially, psoriasis is driven by a single polar immune pathway mainly associated with Th17 T‐cells and activation of IL‐17, whereas atopic dermatitis is driven by multiple polar immune pathways. For instance, Th2 and Th22 are two major T‐cell subsets commonly present across the subtypes of atopic dermatitis. Therefore, the opposite causal trends of atopic dermatitis and psoriasis with PD may be originated from the participation of different T‐cell subsets, and other unknown immune components.
The main advantage of the study is that a bidirectional MR design was applied to avoid reverse causality and confounding factors as much as possible. In addition, no evidence is available of the reverse association analysis between eczema and PD, ensuring the stability of the results. More studies should be done to explore the potential mechanisms.
4.1. Limitation
However, our study also has limitations. First, MR study might be influenced by the ethnic population sources. A recent study by Nam et al. (2024) analyzed the associations of allergic diseases, such as allergic rhinitis, asthma, and atopic dermatitis with PD, and they did not find a significant increase in the risk of PD among patients with atopic dermatitis. The participants in that study were included from the Korean NHIS database, which covered approximately 98% of the population in South Korean. This is quite different from our study that the summary‐level GWAS datasets used in our study were mainly from people of European ancestry. Therefore, our findings should be applied with caution when generalized to other ancestry groups. Second, a major limitation for MR analysis is the potential pleiotropic effects. Further studies with larger sample size should help solve it. Third, MR‐Egger model referred to a nonsignificant relationship between atopic dermatitis and PD. Although MR‐Egger is desirable as sensitivity analysis as it allows all genetic variants to violate the instrumental variable assumptions to assess the robustness of statistical results, it requires all genetic variants to satisfy an alternative assumption (Burgess & Thompson, 2017). Therefore, MR‐Egger is far from the only solution for sensitivity analysis in MR.
5. CONCLUSION
The current project identified genetic variants to instrument the effects of eczema and employed them in the MR framework to generate genetic evidence supporting the causal effects of decreased incidence of eczema on risk of PD.
AUTHOR CONTRIBUTIONS
Conceptualization; formal analysis; writing—original draft; writing—review and editing; supervision: Taofeng Zhou. Methodology; validation; formal analysis; writing—original draft: Baohao Wei. Methodology; data curation: Yachun Hu. Data curation; validation: Xiaoming Zhou. Methodology; validation: Xiaoying Cai. Writing—review and editing; writing—original draft; conceptualization; supervision; funding acquisition: Xiaolei Shi.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.3468.
Supporting information
Table S1 The SNPs associated with atopic dermatitis. Chr, chromosome; EAF, effect allele frequency; SE, standard error; SNP, single nucleotide polymorphism.
Table S2 The SNPs associated with PD. Chr, chromosome; EAF, effect allele frequency; PD, Parkinson's disease; SE, standard error; SNP, single nucleotide polymorphism.
Table S3 The reverse causation analysis of the effects of PD on atopic dermatitis. CI, confidence interval; IVW, inverse variance weighted; MR, Mendelian randomization; OR, odds ratio; PD, Parkinson's disease; SE, standard error.
Table S4 Heterogeneity and horizontal pleiotropy analysis between PD and atopic dermatitis. PD, Parkinson's disease; SE, standard error.
ACKNOWLEDGMENTS
The study was supported by Basic and Applied Basic Research Foundation of Guangdong Province (Grant No: 2022A1515220119, 2019A1515110087), Guangzhou Municipal Science and Technology Project (Grant No: 2023A03J0438), Guangzhou Municipal Key Discipline in Medicine (2021–2023), Guangzhou High‐level Clinical Key Specialty, and Guangzhou Research‐oriented Hospital.
Zhou, T. , Wei, B. , Hu, Y. , Zhou, X. , Cai, X. , & Shi, X. (2024). Causal association between atopic dermatitis and Parkinson's disease: A bidirectional Mendelian randomization study. Brain and Behavior, 14, e3468. 10.1002/brb3.3468
DATA AVAILABILITY STATEMENT
Data used in the study were from the IEU GWAS database (https://gwas.mrcieu.ac.uk/).
REFERENCES
- Afonina, I. S. , Van Nuffel, E. , & Beyaert, R. (2021). Immune responses and therapeutic options in psoriasis. Cellular and Molecular Life Sciences, 78, 2709–2727. 10.1007/s00018-020-03726-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen Reish, H. E. , & Standaert, D. G. (2015). Role of α‐synuclein in inducing innate and adaptive immunity in Parkinson disease. Journal of Parkinson's Disease, 5(1), 1–19. 10.3233/JPD-140491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriemma, M. , Vianale, G. , Amerio, P. , & Reale, M. (2013). Cytokines and T cells in atopic dermatitis. European Cytokine Network, 24(1), 37–44. 10.1684/ecn.2013.0333 [DOI] [PubMed] [Google Scholar]
- Baurecht, H. , Welker, C. , Baumeister, S.‐E. , Weidnger, S. , Meisinger, C. , Leitzmann, M. F. , & Emmert, H. (2021). Relationship between atopic dermatitis, depression and anxiety: A two‐sample Mendelian randomization study. British Journal of Dermatology, 185(4), 781–786. 10.1111/bjd.20092 [DOI] [PubMed] [Google Scholar]
- Bowden, J. , Del Greco M, F. , Minelli, C. , Davey Smith, G. , Sheehan, N. , & Thompson, J. (2017). A framework for the investigation of pleiotropy in two‐sample summary data Mendelian randomization. Statistics in Medicine, 36(11), 1783–1802. 10.1002/sim.7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, S. , Swanson, S. A. , & Labrecque, J. A. (2021). Are Mendelian randomization investigations immune from bias due to reverse causation? European Journal of Epidemiology, 36, 253–257. 10.1007/s10654-021-00726-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, S. , & Thompson, S. G. (2017). Interpreting findings from Mendelian randomization using the MR‐Egger method. European Journal of Epidemiology, 32(5), 377–389. 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund, S. , Kobyletzki, L. , Svalstedt, M. , & Svensson, Å. (2020). Prevalence and incidence of atopic dermatitis: A systematic review. Acta Dermato‐Venereologica, 100(12), adv00160. 10.2340/00015555-3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker, A. M. (2017). Atopic dermatitis: Burden of illness, quality of life, and associated complications. Allergy and Asthma Proceedings, 38(1), 3–8. 10.2500/aap.2017.38.4005 [DOI] [PubMed] [Google Scholar]
- Dzamko, N. , Geczy, C. L. , & Halliday, G. M. (2015). Inflammation is genetically implicated in Parkinson's disease. Neuroscience, 302, 89–102. 10.1016/j.neuroscience.2014.10.028 [DOI] [PubMed] [Google Scholar]
- Eberle, F. C. , Brück, J. , Holstein, J. , Hirahara, K. , & Ghoreschi, K. (2016). Recent advances in understanding psoriasis. F1000Research, 5, 770. 10.12688/f1000research.7927.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman‐Yassky, E. , & Krueger, J. G. (2017). Atopic dermatitis and psoriasis: Two different immune diseases or one spectrum? Current Opinion in Immunology, 48, 68–73. 10.1016/j.coi.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Hemani, G. , Zheng, J. , Elsworth, B. , Wade, K. H. , Haberland, V. , Baird, D. , Laurin, C. , Burgess, S. , Bowden, J. , Langdon, R. , Tan, V. Y. , Yarmolinsky, J. , Shihab, H. A. , Timpson, N. J. , Evans, D. M. , Relton, C. , Martin, R. M. , Davey Smith, G. , Gaunt, T. R. , & Haycock, P. C. (2018). The MR‐base platform supports systematic causal inference across the human phenome. Elife, 7, e34408. 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland, I. C. M. , Houbolt, C. , Van Westerloo, D. J. , Van Gool, W. A. , & Van De Beek, D. (2015). Systemic inflammation and microglial activation: Systematic review of animal experiments. Journal of Neuroinflammation, 12, 1–13. 10.1186/s12974-015-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic, J. (2008). Parkinson's disease: Clinical features and diagnosis. Journal of Neurology Neurosurgery and Psychiatry, 79(4), 368–376. 10.1136/jnnp.2007.131045 [DOI] [PubMed] [Google Scholar]
- Li, C. , Li, X. , Lin, J. , Cui, Y. , & Shang, H. (2022). Psoriasis and progression of Parkinson's disease: A Mendelian randomization study. Journal of the European Academy of Dermatology and Venereology, 36(12), 2401–2405. 10.1111/jdv.18459 [DOI] [PubMed] [Google Scholar]
- Li, H. , Zhang, Z. , Zhang, H. , Guo, Y. , & Yao, Z. (2021). Update on the pathogenesis and therapy of atopic dermatitis. Clinical Reviews in Allergy & Immunology, 61(3), 324–338. 10.1007/s12016-021-08880-3 [DOI] [PubMed] [Google Scholar]
- Magnusen, A. F. , Hatton, S. L. , Rani, R. , & Pandey, M. K. (2021). Genetic defects and pro‐inflammatory cytokines in Parkinson's disease. Front Neurol, 12, 636139. 10.3389/fneur.2021.636139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinova‐Mutafchieva, L. , Sadeghian, M. , Broom, L. , Davis, J. B. , Medhurst, A. D. , & Dexter, D. T. (2009). Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: A time course study in a 6‐hydroxydopamine model of Parkinson's disease. Journal of Neurochemistry, 110(3), 966–975. 10.1111/j.1471-4159.2009.06189.x [DOI] [PubMed] [Google Scholar]
- Mounier, N. , & Kutalik, Z. (2023). Bias correction for inverse variance weighting Mendelian randomization. Genetic Epidemiology, 47(4), 314–331. 10.1002/gepi.22522 [DOI] [PubMed] [Google Scholar]
- Nalls, M. A. , Blauwendraat, C. , Vallerga, C. L. , Heilbron, K. , Bandres‐Ciga, S. , Chang, D. , Tan, M. , Kia, D. A. , Noyce, A. J. , Xue, A. , Bras, J. , Young, E. , Von Coelln, R. , Simón‐Sánchez, J. , Schulte, C. , Sharma, M. , Krohn, L. , Pihlstrøm, L. , Siitonen, A. , … Zhang, F. (2019). Identification of novel risk loci, causal insights, and heritable risk for Parkinson's disease: A meta‐analysis of genome‐wide association studies. Lancet Neurology, 18(12), 1091–1102. 10.1016/S1474-4422(19)30320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, J. Y. , Park, S. J. , Song, J. , Jeong, S. , Choi, S. , & Park, S. M. (2024). Association of allergic disease with Parkinson's disease: A nationally representative retrospective cohort study. Allergology International, 73(1), 107–114. 10.1016/j.alit.2023.07.005 [DOI] [PubMed] [Google Scholar]
- Niemann, N. , Billnitzer, A. , & Jankovic, J. (2021). Parkinson's disease and skin. Parkinsonism & Related Disorders, 82, 61–76. 10.1016/j.parkreldis.2020.11.017 [DOI] [PubMed] [Google Scholar]
- Novak, N. (2003). Immune mechanisms leading to atopic dermatitis. Journal of Allergy and Clinical Immunology, 112, (Suppl 6), S128–S139. 10.1016/j.jaci.2003.09.032 [DOI] [PubMed] [Google Scholar]
- Ortiz‐Lopez, L. I. , Choudhary, V. , & Bollag, W. B. (2022). Updated perspectives on keratinocytes and psoriasis: Keratinocytes are more than innocent bystanders. Psoriasis (Auckland), 12, 73–87. 10.2147/PTT.S327310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternoster, L. , Standl, M. , Waage, J. , Baurecht, H. , Hotze, M. , Strachan, D. P. , & Weidinger, S. (2015). Multi‐ancestry genome‐wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nature Genetics, 47(12), 1449–1456. 10.1038/ng.3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontone, G. M. , & Mills, K. A. (2021). Optimal treatment of depression and anxiety in Parkinson's disease. The American Journal of Geriatric Psychiatry, 29(6), 530–540. 10.1016/j.jagp.2021.02.037 [DOI] [PubMed] [Google Scholar]
- Pont‐Sunyer, C. , Hotter, A. , Gaig, C. , Seppi, K. , Compta, Y. , Katzenschlager, R. , Mas, N. , Hofeneder, D. , Brücke, T. , Bayés, A. , Wenzel, K. , Infante, J. , Zach, H. , Pirker, W. , Posada, I. J. , Álvarez, R. , Ispierto, L. , De Fàbregues, O. , Callén, A. , … Tolosa, E. (2015). The onset of nonmotor symptoms in Parkinson's disease (The ONSET PD study). Movement Disorders, 30(2), 229–237. 10.1002/mds.26077 [DOI] [PubMed] [Google Scholar]
- Reich, K. (2012). The concept of psoriasis as a systemic inflammation: Implications for disease management. Journal of the European Academy of Dermatology and Venereology, 26, 3–11. 10.1111/j.1468-3083.2011.04410.x [DOI] [PubMed] [Google Scholar]
- Skrivankova, V. W. , Richmond, R. C. , Woolf, B. A. R. , Yarmolinsky, J. , Davies, N. M. , Swanson, S. A. , Vanderweele, T. J. , Higgins, J. P. T. , Timpson, N. J. , Dimou, N. , Langenberg, C. , Golub, R. M. , Loder, E. W. , Gallo, V. , Tybjaerg‐Hansen, A. , Davey Smith, G. , Egger, M. , & Richards, J. B. (2021). Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE‐MR statement. JAMA, 326(16), 1614–1621. 10.1001/jama.2021.18236 [DOI] [PubMed] [Google Scholar]
- Tamagawa‐Mineoka, R. , & Katoh, N. (2020). Atopic dermatitis: Identification and management of complicating factors. International Journal of Molecular Sciences, 21(8), 2671. 10.3390/ijms21082671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner, C. , Albers, K. , Goldman, S. , Fross, R. , Leimpeter, A. , Klingman, J. , & Van Den Eeden, S. (2012). Seborrheic dermatitis and risk of future Parkinson's disease (PD) (S42. 001). Neurology, 78, S42.001. [Google Scholar]
- Tansey, M. G. , & Romero‐Ramos, M. (2019). Immune system responses in Parkinson's disease: Early and dynamic. European Journal of Neuroscience, 49(3), 364–383. 10.1111/ejn.14290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic, S. , Kuric, I. , Kuric, T. G. , Popovic, Z. , Kragujevic, J. , Zubonja, T. M. , Rajkovaca, I. , & Matosa, S. (2022). Seborrheic dermatitis is related to motor symptoms in Parkinson's disease. Journal of Clinical Neurology, 18(6), 628–634. 10.3988/jcn.2022.18.6.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh, J. , & Farrer, M. (2013). Advances in the genetics of Parkinson disease. Nature Reviews Neurology, 9(8), 445–454. 10.1038/nrneurol.2013.132 [DOI] [PubMed] [Google Scholar]
- Verbanck, M. , Chen, C.‐Y. , Neale, B. , & Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nature Genetics, 50(5), 693–698. 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernier, P. , Moret, F. , Callier, S. , Snapyan, M. , Wersinger, C. , & Sidhu, A. (2004). The degeneration of dopamine neurons in Parkinson's disease: Insights from embryology and evolution of the mesostriatocortical system. Annals of the New York Academy of Sciences, 1035, 231–249. 10.1196/annals.1332.015 [DOI] [PubMed] [Google Scholar]
- Williams, G. P. , Schonhoff, A. M. , Sette, A. , & Lindestam Arlehamn, C. S. (2022). Central and peripheral inflammation: Connecting the immune responses of Parkinson's disease. Journal of Parkinson's Disease, 12(s1), S129–S136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 The SNPs associated with atopic dermatitis. Chr, chromosome; EAF, effect allele frequency; SE, standard error; SNP, single nucleotide polymorphism.
Table S2 The SNPs associated with PD. Chr, chromosome; EAF, effect allele frequency; PD, Parkinson's disease; SE, standard error; SNP, single nucleotide polymorphism.
Table S3 The reverse causation analysis of the effects of PD on atopic dermatitis. CI, confidence interval; IVW, inverse variance weighted; MR, Mendelian randomization; OR, odds ratio; PD, Parkinson's disease; SE, standard error.
Table S4 Heterogeneity and horizontal pleiotropy analysis between PD and atopic dermatitis. PD, Parkinson's disease; SE, standard error.
Data Availability Statement
Data used in the study were from the IEU GWAS database (https://gwas.mrcieu.ac.uk/).


