Abstract
Background
There is limited knowledge regarding whether an elevated triglyceride glucose (TyG) index can serve as a prognostic marker for mortality and cardiovascular outcomes, independent of diabetes mellitus (DM) and plaque burden, in patients with chronic coronary syndrome (CCS).
Methods
Patients with CCS (n = 684) were categorized into subgroups based on the presence of DM, and patients without DM were further divided into two groups based on presence or absence of an elevation of TyG index >8.8. Coronary plaque burden was evaluated using coronary computed tomography angiography. Major cardiovascular adverse event (MACE) was defined as a composite event of nonfatal myocardial infarction, unstable angina or unplanned coronary revascularization, stroke, non-cardiovascular mortality and cardiovascular mortality.
Results
Patients without DM exhibited significantly greater plaque and epicardial adipose tissue volumes than those with DM. Multivariable Cox proportional hazards models demonstrated that DM and an elevated TyG index >8.8 were independently associated with the risk of MACE after adjusting for age, sex, and plaque volume. Patients with DM (hazard ratio, 3.74; 95% confidence interval, 1.97–7.08; p < 0.001) and patients without DM with an elevated TyG index (hazard ratio, 1.99; 95% confidence interval, 1.01–3.91; p = 0.045) had an increased risk of MACE.
Conclusion
This study indicates that DM and an elevated TyG index are predictors of MACE, independent of plaque volume, in patients with CCS.
Keywords: Atherosclerosis, Coronary computed tomography angiography, Coronary artery disease, Diabetes, Insulin resistance, Mortality
1. Background
Chronic coronary syndrome (CCS) is a spectrum of progressive diseases involving nonobstructive and obstructive coronary artery disease (CAD) [1]. Traditional risk factors that accelerate atherosclerosis, such as hypertension and hyperlipidemia, provide only a limited explanation for the pathogenesis of CAD [2]. Diabetes mellitus (DM) is a predictor of atherosclerotic cardiovascular disease (ASCVD) and carries nearly two-fold risk of vascular diseases [3]. In addition, the identification of patients with pre-DM can help prevent progression to DM and subsequent ASCVD events through lifestyle modification and optimized medical therapy [4].
Insulin resistance (IR) plays a pivotal role in the development of DM and CAD [5]. IR has been shown to be an independent predictor of vascular events in patients with and without DM [6]. In addition, meta-analyses have consistently found that the homeostatic model assessment for insulin resistance (HOMA-IR) is associated with an increased risk of incident cardiovascular disease and all-cause mortality in individuals without DM [7,8]. More recently, the triglyceride glucose (TyG) index, which is calculated on the basis of fasting plasma glucose and triglyceride levels, has emerged as a marker of IR [9]. The TyG index, which is reportedly associated with coronary calcification, metabolic syndrome, and acute coronary syndrome [[10], [11], [12]], serves as a marker of ASCVD events [13,14].
Previous studies using intracoronary imaging have showed that DM and IR are associated with plaque vulnerability, lipid-rich plaques, and calcification [15,16]. Coronary computed tomography angiography (CCTA) is a first-line defense for the management of patients with CCS that enables noninvasive assessment of the entire coronary artery plaque burden together with luminal stenosis [[17], [18], [19]]. However, it remains unclear whether elevated TyG index serves as a marker of mortality and cardiovascular outcomes in dependent of DM and plaque burden. We aimed to investigate the association of DM, elevated TyG index, and coronary atherosclerotic disease burden with mortality and cardiovascular events in patients with CCS who underwent CCTA.
2. Methods
2.1. Study participants
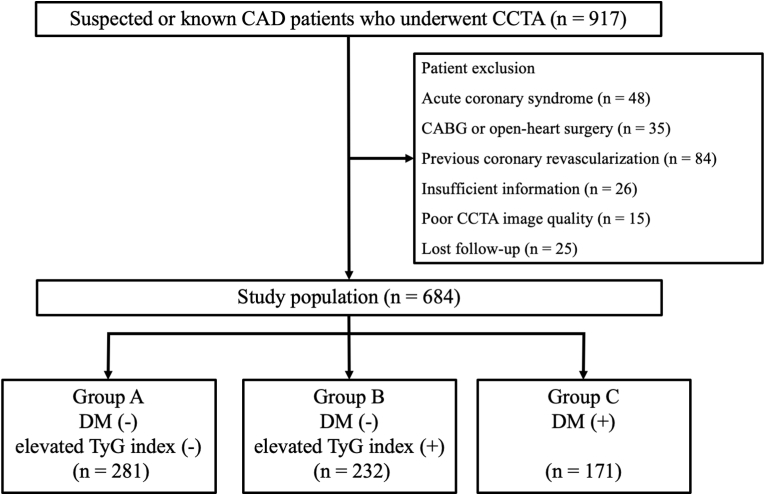
In this single-center retrospective observational study, we screened consecutive patients with CCS who underwent CCTA to assess CAD between December 2017 and September 2021 at Kashibaseiki Hospital. CCS was defined on the basis of the current guideline of the European Society of Cardiology [1]. The exclusion criteria were as follows: (1) acute coronary syndrome, (2) coronary artery bypass graft or open-heart surgery, (3) history of percutaneous coronary intervention, (4) insufficient patient information, (5) poor image quality, and (6) loss to follow-up (Fig. 1). The Kashibaseiki Hospital Institutional Review Board approved the pooled data analysis (No. 2023-G). All participants provided written informed consent for the use of de-identified data, including clinical information, laboratory test results, and CCTA imaging results.
Fig. 1.
Flow chart of study patients. CAD, coronary artery disease; CCTA, coronary computed tomography angiography; DM, diabetes mellitus; TyG, triglyceride glucose; CABG, coronary artery bypass graft surgery.
The study participants were categorized into subgroups based on the presence of DM. Patients without DM were further divided into two groups according to the TyG index (IR) (Fig. 1). The median value of the TyG index in the study population was defined as an increase in IR (>8.8), where the TyG index was calculated as Ln (fasting plasma glucose [FPG] × triglyceride/2) using a fasting blood sample test before the CCTA examination [9]. According to the Japanese Clinical Practice Guidelines for Diabetes 2019 [20], type 2 DM was defined as having any of the following criteria: (1) an elevated FPG level of ≥126 mg/dL or casual plasma glucose level of ≥200 mg/dL on at least two different visits, (2) glycated hemoglobin (HbA1c) level of ≥6.5% and either FPG level of ≥126 mg/dL or casual blood glucose level of ≥200 mg/dL, or (3) a history of a prior diagnosis of DM.
2.2. CCTA image analysis
CCTA was performed using a 320-row multidetector computed tomography (CT) scanner with an electrocardiogram-triggered prospective gating method (Aquilion ONE/NATURE Edition; Canon Medical Systems, Inc., Japan). The scan parameters included a detector collimation of 0.5 × 320 mm, gantry rotation time of 350 ms, tube voltage of 120 kV, and tube current of 130–600 mA. The Agatston method was used to assess the coronary artery calcium (CAC) scores at a fixed thickness of 3 mm. The Agatston scores were categorized as 0, 1–100, 101–400, and >400 Agatston units using SYNAPSE VINCENT version 4.6 (Fujifilm Inc., Tokyo, Japan).
The images were reconstructed using a forward-projected model-based iterative reconstruction solution for coronary artery analysis, with a cross-sectional thickness of 0.5 mm and a reconstruction increment of 0.25 mm. For coronary plaque analysis, coronary artery centerlines were identified semi-automatically; the proximal and distal portions of the coronary plaque lesions were manually defined; and the vessel wall, lumen, and plaque components were autosegmented, followed by manual correction of the segmentation. The plaque volume was calculated as the plaque volume divided by the vessel volume [18].
Volumetric measurements of epicardial adipose tissue (EAT) were performed on axial views with a 0.5-mm slice thickness on contrast-enhanced CT images [18,21]. The upper limit of the slice was set at the bifurcation of the pulmonary artery trunk, whereas the lower limit was set at the last slice containing any heart structure. In each plane, the SYNAPSE VINCENT software auto-detected a smooth, closed pericardial contour as the region of interest, where adipose tissue was identified with CT attenuation values ranging from −250 to −30 HU within the pericardial sac. Finally, EAT volume was calculated as the sum of the EAT areas in each slice.
2.3. Endpoints
The primary endpoint was major cardiovascular adverse event (MACE) defined as a composite event of nonfatal myocardial infarction, unstable angina or unplanned coronary revascularization, stroke, non-cardiovascular mortality and cardiovascular mortality. ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction were defined by the American College of Cardiology/American Heart Association guidelines [22]. Stroke was defined as the sudden onset of neurological signs or symptoms within focal or multifocal vascular territories, based on brain magnetic resonance imaging, that persisted for ≥24 h or until death. Clinical follow-up was performed by interviewing patients at each hospital visit. If two or more events occurred, only the first event was included in the analysis.
2.4. Statistical analysis
Categorical variables are presented as absolute and relative frequencies. Continuous variables are expressed as mean (standard deviation) for normally distributed variables and as median (interquartile range) for non-normally distributed variables. Patient characteristics were compared between the three groups using one-way analysis of variance. A logarithmic transformation was performed for the non-normally distributed variables. The effects of DM and the TyG index >8.8 on outcomes were investigated using Cox regression analysis with a proportional hazards model. The variables entered into model 1 were age, sex, plaque volume, DM, and TyG index >8.8; model 2 included age, sex, CAC score >400, DM, and TyG index >8.8; and model 3 included age, sex, obstructive CAD, DM, and TyG index >8.8. In addition, the hazard ratios (HR) for Group B and C were analyzed using the Cox proportional hazards model by adjusting for age and sex. Kaplan–Meier curves and log-rank tests were used to depict and assess the differences in cumulative event rates among the three groups. The analyses were initiated at the time of CCTA imaging and terminated at the earliest occurrence of the primary endpoint. Analyses were censored at the time of the last follow-up, nonfatal myocardial infarction, unstable angina or unplanned coronary revascularization, stroke, non-cardiovascular mortality and cardiovascular mortality, whichever occurred earlier. All statistical analyses were performed using the SPSS software (version 24; IBM Corp., Armonk, NY, USA). Statistical significance was set at p < 0.05 (two-sided).
3. Results
3.1. Baseline patient characteristics
The mean age of the study patients was 65 ± 11 years, and 416 (61%) were men. The median TyG index was 8.8 (8.4–9.2). The baseline characteristics stratified by the presence of DM and TyG index >8.8 are presented in Table 1. A total of 171 (25%) patients had DM (Group C), 232 (34%) had an elevated TyG index >8.8 but without DM (Group B), and the remaining patients (281, 41%) were without DM and an elevated TyG index (Group A) (Fig. 1). The highest TyG index was observed in Group B, followed by Group C and Group A. The body mass index was higher in Group B and Group C than in Group A. Patients in Group C were older and more likely to be men than in the other groups. Group B had higher cholesterol profiles, particularly triglyceride levels, than the other groups. In contrast, the highest FPG level was observed in Group C. Group C was more likely to receive antihypertensive drugs than Group A and Group B.
Table 1.
Patient characteristics.
| Group A DM (−) elevated TyG index (−) n = 281 |
Group B DM (−) elevated TyG index (+) n = 232 |
Group C DM (+) n = 171 |
p-value | |
|---|---|---|---|---|
| Age, years | 65 (12) | 64 (12) | 67 (10) | 0.064 |
| Male, n (%) | 150 (65%) | 143 (62%) | 123 (72%) | <0.001 |
| BMI, kg/mm2 | 23.1 (3.7) | 24.5 (4.2) | 24.5 (4.1) | <0.001 |
| Hypertension, n (%) | 196 (70%) | 181 (78%) | 153 (89%) | <0.001 |
| Dyslipidemia, n (%) | 158 (56%) | 190 (82%) | 136 (80%) | <0.001 |
| Systolic BP, mmHg | 140 (20) | 141 (22) | 145 (22) | 0.097 |
| Heart rate, beats/min | 76 (14) | 76 (13) | 78 (13) | 0.246 |
| Current or past smoker, n (%) | 75 (27%) | 69 (30%) | 48 (28%) | 0.746 |
| Atrial fibrillation, n (%) | 21 (7.4%) | 14 (6.0%) | 17 (9.9%) | 0.350 |
| Laboratory parameters | ||||
| Triglyceride, mg/dL | 90 (70–111) | 176 (145–223) | 126 (81–167) | <0.001 |
| HDL-C, mg/dL | 70 (19) | 56 (16) | 57 (17) | <0.001 |
| LDL-C, mg/dL | 117 (34) | 127 (38) | 107 (34) | <0.001 |
| Fasting plasma glucose, g/dL | 104 (18) | 119 (31) | 159 (67) | <0.001 |
| HbA1c, % | 5.6 (0.3) | 5.8 (0.3) | 7.0 (1.4) | <0.001 |
| CRP, mg/L | 0.7 (0.3–1.7) | 1.2 (0.5–2.6) | 0.9 (0.4–2.7) | 0.013 |
| TyG index, median (IQR) | 8.4 (8.2–8.6) | 9.2 (9.0–9.5) | 9.1 (8.6–9.5) | <0.001 |
| eGFR, mL/min/1.73 mm2 | 67 (15) | 67 (16) | 64 (15) | 0.202 |
| Medication | ||||
| ACE inhibitor or ARB, n (%) | 63 (22%) | 65 (28%) | 68 (40%) | <0.001 |
| Calcium channel blocker, n (%) | 75 (27%) | 59 (25%) | 67 (39%) | 0.005 |
| β-blocker, n (%) | 25 (8.8%) | 19 (8.2%) | 23 (13%) | 0.172 |
| Statins, n (%) | 69 (25%) | 69 (30%) | 81 (47%) | <0.001 |
| Oral antidiabetic drugs, n (%) | 0 (0%) | 0 (0%) | 104 (61%) | – |
| Insulin, n (%) | 0 (0%) | 0 (0%) | 7 (4.1%) | – |
Values are presented as mean (standard deviation) or number (%).
ACE, angiotensin converting enzyme; ARB, angiotensin Ⅱ receptor blocker; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IQR, interquartile range; TyG, triglyceride glucose; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
3.2. CCTA analysis
Table 2 shows the baseline CCTA findings of the three groups. The prevalence of a CAC score >400 was the highest in Group C (24%) (p < 0.001). There were no significant differences in the frequency of obstructive CAD or nonobstructive CAD between the groups. Group C had a greater volume of noncalcified and calcified plaque volume than Group A. Group B had the highest abdominal adipose tissue area, while the greatest EAT volume was observed in Group C.
Table 2.
Coronary computed tomography angiography findings.
| Group A DM (−) elevated TyG index (−) n = 281 |
Group B DM (−) elevated TyG index (+) n = 232 |
Group C DM (+) n = 171 |
p-value | |
|---|---|---|---|---|
| CAC score parameters | ||||
| CAC score, median (IQR) | 18.9 (0–188.8) | 24.7 (0–192.8) | 155.5 (11.0–392.6) | <0.001 |
| CAC 0, n (%) | 119 (42%) | 82 (35%) | 35 (20%) | <0.001 |
| CAC 1–100, n (%) | 75 (27%) | 65 (28%) | 46 (27%) | 0.940 |
| CAC 101–400, n (%) | 49 (17%) | 56 (20%) | 49 (29%) | 0.017 |
| CAC >400, n (%) | 38 (14%) | 29 (13%) | 41 (24%) | 0.003 |
| Stenosis severity on CCTA | ||||
| Nonobstructive CAD, n (%) | 91 (32%) | 78 (34%) | 60 (35%) | 0.846 |
| Obstructive CAD, n (%) | 61 (22%) | 57 (25%) | 48 (28%) | 0.287 |
| Plaque burden | ||||
| Coronary plaque volume, mm3 | 1461 (1183–1785) | 1660 (1306–2006) | 1912 (1332–2391) | 0.001 |
| Calcified plaque volume, mm3 | 13 (4–58) | 18 (6–74) | 30 (4–136) | <0.001 |
| Noncalcified plaque volume, mm3 | 1400 (1134–1712) | 1578 (1248–1931) | 1817 (1264–2291) | <0.001 |
| Adipose tissue parameters | ||||
| Abdominal visceral adipose tissue area, cm2 | 90 (51) | 119 (57) | 116 (66) | <0.001 |
| Abdominal subcutaneous fat area, cm2 | 142 (80) | 161 (83) | 144 (82) | 0.019 |
| EAT volume, mL | 117 (48) | 133 (49) | 144 (53) | <0.001 |
Values are presented as mean (standard deviation) or number (%).
CAC, coronary artery calcium; CAD, coronary artery disease; EAT, epicardial adipose tissue; CCTA, coronary computed tomography angiography; IQR, interquartile range.
3.3. Primary endpoint
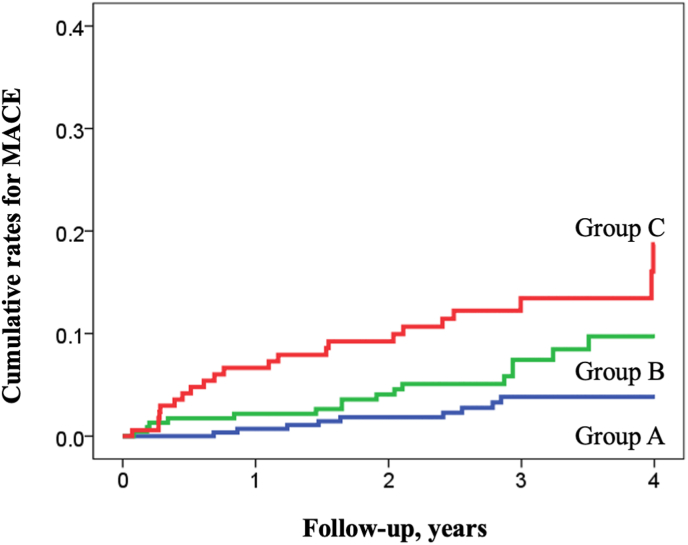
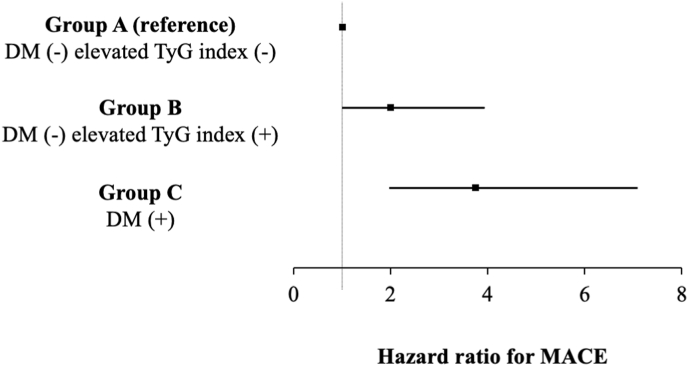
During a mean follow-up period of 3.0 ± 0.9 years, the composite endpoint defined as a of all-cause mortality and MACE was observed in 47 patients (6.9%, 9 in Group A, 16 in Group B, and 22 in Group C). The incidence rate of the composite endpoint was the highest in Group C, followed by Group B and Group A (p < 0.001, log-rank test; Fig. 2). Multivariable Cox proportional hazards models adjusted for age, sex, and total plaque volume demonstrated that DM (HR, 2.45; 95% confidence interval [CI], 1.35–4.41; p = 0.003) and an elevated TyG index (HR, 2.84; 95% CI, 1.46–5.53; p = 0.002) were independent predictors of the composite endpoint. Similarly, with an adjustment for CAC score >400 (model 2 in Table 3) or obstructive CAD (model 3 in Table 3), DM and elevated TyG index remained independent predictors of the composite endpoint. Fig. 2 illustrates the Kaplan–Meier curve analysis stratified by the presence or absence of DM and an elevated TyG index to predict the composite endpoint. Group C had the worst prognosis among the three groups (log-rank test, p < 0.001). The age- and sex-adjusted HRs for each group are shown in Fig. 3. Group C (HR, 3.74; 95% CI, 1.97–7.08, p < 0.001) and Group B (HR, 1.99; 95% CI, 1.01–3.91; p = 0.045) had an increased risk of the composite endpoint compared with Group A.
Fig. 2.
Kaplan–Meier analysis for prediction of major adverse cardiovascular events stratified by the presence of elevated triglyceride glucose index and diabetes mellitus The Kaplan–Meier curves demonstrate significant differences among the three groups in the cumulative event rates of MACE. The highest event rates are observed in Group C, (patients with DM; log-rank test, p < 0.001), followed by Group B (patients without DM with an elevated TyG index; log-rank test, p = 0.047) and Group A (patients without DM without an elevated TyG index). MACE, major adverse cardiovascular events; TyG, triglyceride glucose.
Table 3.
Cox hazard proportional analysis to predict major cardiovascular adverse events.
| Predictor | Hazard ratio | 95% confidence interval | p-value | |
|---|---|---|---|---|
| Model 1 | DM | 2.45 | 1.35–4.41 | 0.003 |
| TyG index >8.8 | 2.84 | 1.46–5.53 | 0.002 | |
| Model 2 | DM | 2.10 | 1.15–3.83 | 0.016 |
| TyG index >8.8 | 2.77 | 1.42–5.43 | 0.003 | |
| Model 3 | DM | 2.31 | 1.18–4.54 | 0.015 |
| TyG index >8.8 | 3.34 | 1.50–7.42 | 0.003 |
Models 1 is adjusted for age, sex, and plaque volume.
Model 2 is adjusted for age, sex, and CAC score >400.
Model 3 is adjusted for age, sex, and obstructive CAD.
CAD, coronary artery disease; CAC, coronary artery calcium; TyG, triglyceride glucose; DM, diabetes mellitus.
Fig. 3.
Subgroup analysis of the major adverse cardiovascular event The hazard ratio for the composite endpoint of all-cause mortality and MACE was calculated in the subgroups stratified by the presence of diabetes mellitus and an elevated TyG index. Hazard ratios adjusted for age and sex are reported as point estimates with 95% confidence intervals. MACE, major adverse cardiovascular event; TyG, triglyceride glucose.
4. Discussion
In this study, which included 684 patients with CCS who underwent CCTA, the presence of DM and an elevated TyG index were independently associated with increased risks of MACE, irrespective of age and coronary plaque burden. Among the three groups, patients with DM (Group C) exhibited the highest risk of MACE, followed by those with an elevated TyG index (Group B) and those without DM and an elevation of TyG index (Group A). Additionally, patients with an elevated TyG index had greater plaque and EAT volumes compared with other groups, indicating that this pre-DM group is a high-risk population for progression to adverse events. Taken together, our results indicate the possible contribution of IR to all-cause mortality and MACE, suggesting the importance of identifying patients with an elevated TyG index to prevent progression to DM and poorer outcomes.
4.1. IR and cardiovascular risks
IR is reportedly associated with MACE in both healthy individuals and patients with DM [[6], [7], [8]]. The TyG index is a useful marker of IR, serving as a marker for ASCVD risk [9]. The cutoff values for the TyG index to predict the incidence of cardiovascular events and mortality vary depending on the patient population [23,24]. In a meta-analysis comprising eight cohort studies, Ding et al. demonstrated that the highest TyG index category had an increased risk of ASCVD (HR, 1.61; p < 0.001), where the HR was found to be 1.61 per one unit increase in the TyG index [23]. In patients without DM with chronic kidney disease, Quiora et al. demonstrated that the TyG index >8.63, defined on the basis of the median value of the population, was an independent predictor of MACE [25]. Wang et al. demonstrated that the optimal cutoff value for the TyG index to predict MACE in patients with DM presenting with acute coronary syndrome was 9.323 [26]. Our results indicate that an elevated TyG index is a useful marker for identifying patients with pre-DM at high risk of MACE in patients with CCS.
4.2. TyG index to predict mortality and cardiovascular events
The link between an elevated TyG index and MACE can be explained by the effects of elevated triglycerides and FPG on cardiovascular outcomes. In a meta-analysis, Sarwar et al. demonstrated that FPG concentration is modestly associated with the risk of vascular diseases in individuals without DM [3]. Triglycerides are associated with the residual risk of atherosclerotic cardiovascular disease. Triglycerides reflect the concentration of circulating atherogenic remnant cholesterol and atherogenic small, dense low-density lipoproteins as well as IR, which promote increased fatty acid secretion from the adipose tissue [27,28]. Pharmacological therapy focusing on lowering triglycerides in addition to low-density lipoprotein-cholesterol, which can reduce the risk of ASCVD, is increasingly being recognized [29].
Although we observed that the TyG index was comparable between Group B and Group C, patients with DM had higher FPG and lower triglyceride levels than those without DM. This indicates a different metabolically unhealthy status, leading to MACE. In addition to the independent association of DM and an elevated TyG index with cardiovascular outcomes, patients with DM exhibited the highest risk of mortality and cardiovascular events across the groups. This may be partly explained by the fact that patients with DM had the greatest plaque burden and CAC scores among the three groups. In a clinical CCTA study investigating CAC progression, an increased TyG index was associated with an increased risk of CAC progression and could serve as a marker for ASCVD risk [10]. Our findings are consistent with these observations, as an elevated TyG index was associated with an increased disease burden of coronary atherosclerosis. Considering that patients with DM have the worst prognosis, a strategy to prevent the progression of IR to DM is required.
4.3. IR and ectopic fat
In addition to metabolic status, ectopic adiposity has attracted attention in the pathogenesis of atherosclerosis [5,30]. Despite their distinct characteristics, obesity and atherosclerotic diseases share a common pathophysiology, such as inflammation, which is associated with IR and type 2 DM [31]. The EAT has been hypothesized to have a paracrine effect on coronary vessels and microcirculation, which accelerates atherogenesis and coronary calcification. Given that patients with DM and those with an increased TyG index have increased EAT volume and visceral adipose tissue area, increased ectopic fat and metabolic disorders may partially explain the worse outcomes in this population. Our results indicate that elevated TyG levels and increased plaque burden could help identify patients who are at a high risk and require intensive therapy to control dyslipidemia and IR.
4.4. Limitations
This study had several limitations. First, the indication of CCTA imaging was in line with clinical indication, where we did not include patients with severe renal insufficiency defined as estimated glomerular filtration ratio <30 ml/min/1.73 mm2 or severe medical illness who were life expectancy of less than 1 year. Second, we did not have data on HOMA-IR, which is a common marker of IR. Although the TyG index can serve as a marker of IR, discordance between the TyG index and HOMA-IR can exist in terms of the identification of patients with IR. Third, the antidiabetic drugs used to control hyperglycemia can influence the measurement of the TyG index in this mixed cohort of patients with and without DM. However, an association between an elevated TyG index and poor outcomes was observed in patients without DM with an elevated TyG index compared with those without an elevated TyG index (Fig. 3). Our findings indicate that IR, as assessed by TyG index, can help identifying high-risk patients with CCS. Further studies are required to investigate the effects of pharmacological intervention strategies on improving the metabolic status.
5. Conclusions
Patients with DM or an elevated TyG index had a significantly higher risk of incident MACE than those without DM or an elevated TyG index. The present study indicates the usefulness of the TyG index in identifying patients with pre-DM who are at a high risk of progression to MACE, irrespective of coronary plaque volume. Further studies are required to investigate the modification of metabolic status related to IR on clinical outcomes in patients with CCS.
Ethics approval and consent to participate
In this retrospective observational study, opt-out was performed for the use of all de-identified data, including clinical information, laboratory tests, and CCTA imaging. The Fujiikai Kashibaseiki Hospital Institutional Review Board approved the pooled data analysis.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
Funding
MSD Life Science Foundation, Public Interest Incorporated Foundation (K.O.)
CRediT authorship contribution statement
Kenichiro Otsuka: Conceptualization, Investigation, Methodology, Writing – original draft. Hiroki Yamaura: Investigation, Writing – original draft. Kenei Shimada: Conceptualization, Methodology, Writing – original draft. Takatoshi Sugiyama: Investigation, Methodology, Writing – review & editing. Kana Hojo: Investigation, Methodology, Writing – review & editing. Hirotoshi Ishikawa: Investigation, Methodology, Writing – review & editing. Yasushi Kono: Investigation, Methodology, Writing – review & editing. Noriaki Kasayuki: Investigation, Methodology, Supervision, Writing – review & editing. Daiju Fukuda: Investigation, Methodology, Supervision, Writing – review & editing.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Handling Editor: Dr D Levy
List of Abbreviations
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcium
- CAD
coronary artery disease
- CCS
chronic coronary syndrome
- CCTA
coronary computed tomography angiography
- CI
confidence interval
- CT
computed tomography
- DM
diabetes mellitus
- EAT
epicardial adipose tissue
- HR
hazard ratio
- HOMA-IR
homeostatic model assessment for insulin resistance
- IR
insulin resistance
- MACE
major adverse cardiovascular events
- TyG
triglyceride glucose
References
- 1.Neumann F.J., Sechtem U., Banning A.P., Bonaros N., Bueno H., Bugiardini R., Chieffo A., Crea F., Czerny M., Delgado V., Dendale P., Knuuti J., Wijns W., Flachskampf F.A., Gohlke H., Grove E.L., James S., Katritsis D., Landmesser U., Lettino M., Matter C.M., Nathoe H., Niessner A., Patrono C., Petronio A.S., Pettersen S.E., Piccolo R., Piepoli M.F., Popescu B.A., Räber L., Richter D.J., Roffi M., Roithinger F.X., Shlyakhto E., Sibbing D., Silber S., Simpson I.A., Sousa-Uva M., Vardas P., Witkowski A., Zamorano J.L., Achenbach S., Agewall S., Barbato E., Bax J.J., Capodanno D., Cuisset T., Deaton C., Dickstein K., Edvardsen T., Escaned J., Funck-Brentano C., Gersh B.J., Gilard M., Hasdai D., Hatala R., Mahfoud F., Masip J., Muneretto C., Prescott E., Saraste A., Storey R.F., Svitil P., Valgimigli M., Aboyans V., Baigent C., Collet J.P., Dean V., Fitzsimons D., Gale C.P., Grobbee D.E., Halvorsen S., Hindricks G., Iung B., Jüni P., Katus H.A., Leclercq C., Lewis B.S., Merkely B., Mueller C., Petersen S., Touyz R.M., Benkhedda S., Metzler B., Sujayeva V., Cosyns B., Kusljugic Z., Velchev V., Panayi G., Kala P., Haahr-Pedersen S.A., Kabil H., Ainla T., Kaukonen T., Cayla G., Pagava Z., Woehrle J., Kanakakis J., Toth K., Gudnason T., Peace A., Aronson D., Riccio C., Elezi S., Mirrakhimov E., Hansone S., Sarkis A., Babarskiene R., Beissel J., Cassar Maempel A.J., Revenco V., de Grooth G.J., Pejkov H., Juliebø V., Lipiec P., Santos J., Chioncel O., Duplyakov D., Bertelli L., Dikic A.D., Studencan M., Bunc M., Alfonso F., Back M., Zellweger M., Addad F., Yildirir A., Sirenko Y., Clapp B. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 3.Sarwar N., Gao P., Seshasai S.R.K., Gobin R., Kaptoge S., Di Angelantonio E., Ingelsson E., Lawlor D.A., Selvin E., Stampfer M., Stehouwer C.D.A., Lewington S., Pennells L., Thompson A., Sattar N., White I.R., Ray K.K., Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet (London, England) 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., Federici M., Filippatos G., Grobbee D.E., Hansen T.B., V Huikuri H., Johansson I., Jüni P., Lettino M., Marx N., Mellbin L.G., Östgren C.J., Rocca B., Roffi M., Sattar N., Seferović P.M., Sousa-Uva M., Valensi P., Wheeler D.C. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 5.Nishimoto S., Fukuda D., Higashikuni Y., Tanaka K., Hirata Y., Murata C., Kim-Kaneyama J.-R., Sato F., Bando M., Yagi S., Soeki T., Hayashi T., Imoto I., Sakaue H., Shimabukuro M., Sata M. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1501332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saely C.H., Aczel S., Marte T., Langer P., Hoefle G., Drexel H. The metabolic syndrome, insulin resistance, and cardiovascular risk in diabetic and nondiabetic patients. J. Clin. Endocrinol. Metab. 2005;90:5698–5703. doi: 10.1210/jc.2005-0799. [DOI] [PubMed] [Google Scholar]
- 7.Gast K.B., Tjeerdema N., Stijnen T., Smit J.W.A., Dekkers O.M. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X., Li J., Zheng S., Luo Q., Zhou C., Wang C. Fasting insulin, insulin resistance, and risk of cardiovascular or all-cause mortality in non-diabetic adults: a meta-analysis. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan S.H., Sobia F., Niazi N.K., Manzoor S.M., Fazal N., Ahmad F. Metabolic clustering of risk factors: evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol. Metab. Syndrome. 2018;10:74. doi: 10.1186/s13098-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park K., Ahn C.W., Lee S.B., Kang S., Nam J.S., Lee B.K., Kim J.H., Park J.S. Elevated TYG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42:1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 11.Park G.M., Cho Y.R., Won K.B., Yang Y.J., Park S., Ann S.H., Kim Y.G., Park E.J., Kim S.J., Lee S.G., Yang D.H., Kang J.W., Lim T.H., Kim H.K., Choe J., Lee S.W., Kim Y.H. Triglyceride glucose index is a useful marker for predicting subclinical coronary artery disease in the absence of traditional risk factors. Lipids Health Dis. 2020;19:1–7. doi: 10.1186/s12944-020-1187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J., Fan H., Wang T., Yu B., Mao S., Wang X., Zhang W., Wang L., Zhang Y., Ren Z., Liang B. TyG index is positively associated with risk of CHD and coronary atherosclerosis severity among NAFLD patients. Cardiovasc. Diabetol. 2022:1–11. doi: 10.1186/s12933-022-01548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdoğan A., İnan D., Genç Ö., Yıldız U., Demirtola A.İ., Çetin İ., Güler Y., Tekin A.F., Barutçu S., Güler A., Karagöz A. The triglyceride–glucose index might Be a better indicator for predicting poor cardiovascular outcomes in chronic coronary syndrome. J. Clin. Med. 2023;12 doi: 10.3390/jcm12196201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao S., Yu L., Li J., Xie Z., Huang L., Yang D., Tan Y., Zhang W., Huang X., Xue T. Prognostic value of triglyceride-glucose index in patients with chronic coronary syndrome undergoing percutaneous coronary intervention, Cardiovasc. Diabetol. 2023;22:1–14. doi: 10.1186/s12933-023-02060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iguchi T., Hasegawa T., Otsuka K., Matsumoto K., Yamazaki T., Nishimura S., Nakata S., Ehara S., Kataoka T., Shimada K., Yoshiyama M. Insulin resistance is associated with coronary plaque vulnerability: insight from optical coherence tomography analysis. Eur. Hear. Journal. Cardiovasc. Imaging. 2014;15:284–291. doi: 10.1093/ehjci/jet158. [DOI] [PubMed] [Google Scholar]
- 16.Yonetsu T., Kato K., Uemura S., Kim B.K., Jang Y., Kang S.J., Park S.J., Lee S., Kim S.J., Jia H., Vergallo R., Abtahian F., Tian J., Hu S., Yeh R.W., Sakhuja R., McNulty I., Lee H., Zhang S., Yu B., Kakuta T., Jang I.K. Features of coronary plaque in patients with metabolic syndrome and diabetes mellitus assessed by 3-vessel optical coherence tomography. Circ Cardiovasc Imaging. 2013;6:665–673. doi: 10.1161/CIRCIMAGING.113.000345. [DOI] [PubMed] [Google Scholar]
- 17.Otsuka K., Fukuda S., Tanaka A., Nakanishi K., Taguchi H., Yoshikawa J., Shimada K., Yoshiyama M. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC. Cardiovasc. Imaging. 2013;6:448–457. doi: 10.1016/j.jcmg.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Yamaura H., Otsuka K., Ishikawa H., Shirasawa K., Fukuda D., Kasayuki N. Determinants of non-calcified low-attenuation coronary plaque burden in patients without known coronary artery disease: a coronary CT angiography study. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.824470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sagris M., Antonopoulos A.S., Simantiris S., Oikonomou E., Siasos G., Tsioufis K., Tousoulis D. Pericoronary fat attenuation index—a new imaging biomarker and its diagnostic and prognostic utility: a systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging. 2022;23:E526–E536. doi: 10.1093/ehjci/jeac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araki E., Goto A., Kondo T., Noda M., Noto H., Origasa H., Osawa H., Taguchi A., Tanizawa Y., Tobe K., Yoshioka N. Japanese clinical Practice guideline for diabetes 2019. Diabetol. Int. 2020;11:165–223. doi: 10.1007/s13340-020-00439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikawa H., Otsuka K., Kono Y., Hojo K., Yamaura H., Hirata K., Kasayuki N., Izumiya Y., Fukuda D. Extent of coronary atherosclerosis is associated with deterioration of left ventricular global longitudinal strain in patients with preserved ejection fraction undergoing coronary computed tomography angiography. Int. J. Cardiol. Hear. Vasc. 2023;44 doi: 10.1016/j.ijcha.2023.101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Garcia H.M., McFadden E.P., Farb A., Mehran R., Stone G.W., Spertus J., Onuma Y., Morel M.-A., van Es G.-A., Zuckerman B., Fearon W.F., Taggart D., Kappetein A.-P., Krucoff M.W., Vranckx P., Windecker S., Cutlip D., Serruys P.W. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Eur. Heart J. 2018;39:2192–2207. doi: 10.1093/eurheartj/ehy223. [DOI] [PubMed] [Google Scholar]
- 23.Ding X., Wang X., Wu J., Zhang M., Cui M. Triglyceride–glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc. Diabetol. 2021;20:1–13. doi: 10.1186/s12933-021-01268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q., Zhang T.Y., Cheng Y.J., Ma Y., Xu Y.K., Yang J.Q., Zhou Y.J. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc. Diabetol. 2020;19:1–19. doi: 10.1186/s12933-020-01086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quiroga B., Muñoz Ramos P., Sánchez Horrillo A., Ortiz A., Valdivieso J.M., Carrero J.J. Triglycerides-glucose index and the risk of cardiovascular events in persons with non-diabetic chronic kidney disease. Clin. Kidney J. 2022;15:1705–1712. doi: 10.1093/ckj/sfac073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L., Cong H.-L., Zhang J.-X., Hu Y.-C., Wei A., Zhang Y.-Y., Yang H., Ren L.-B., Qi W., Li W.-Y., Zhang R., Xu J.-H. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc. Diabetol. 2020;19:80. doi: 10.1186/s12933-020-01054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Packard C.J. Triglyceride lowering 2.0: back to the future? Eur. Heart J. 2020;41:95–98. doi: 10.1093/eurheartj/ehz810. [DOI] [PubMed] [Google Scholar]
- 28.Low S., Khoo K.C.J., Irwan B., Sum C.F., Subramaniam T., Lim S.C., Wong T.K.M. The role of triglyceride glucose index in development of Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2018;143:43–49. doi: 10.1016/j.diabres.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Pradhan A.D. A new beginning for triglyceride-lowering therapies. Circulation. 2019;140:167–169. doi: 10.1161/CIRCULATIONAHA.119.038770. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka K., Fukuda D., Sata M. Roles of epicardial adipose tissue in the pathogenesis of coronary atherosclerosis - an update on recent findings. Circ. J. 2020;85:2–8. doi: 10.1253/circj.CJ-20-0935. [DOI] [PubMed] [Google Scholar]
- 31.Soehnlein O., Libby P. Targeting inflammation in atherosclerosis — from experimental insights to the clinic. Nat. Rev. Drug Discov. 2021;20:589–610. doi: 10.1038/s41573-021-00198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.