Abstract
The low-density lipoprotein receptor-related protein 6 (LRP6) is an essential Wnt co-receptor of the Wnt/β-catenin signaling pathway. Although studies have shown an increased expression of LRP6 in several types of cancer, its function in tumor development and progression remains to be elucidated. We herein demonstrated that LRP6 expression is up-regulated in human triple negative breast cancer (TNBC) patients and human TNBC cell lines, and that knockdown of LRP6 expression and treatment of recombinant Mesd protein (a specific inhibitor of LRP6) significantly decreased cell migration and invasion of TNBC MDA-MB-231 and BT549 cells. Interestingly, the effects of LRP6 knockdown and Mesd treatment on TNBC cell migration and invasion were more prominent than on TNBC cell proliferation/viability. Mechanistically, LRP6 knockdown and Mesd treatment inhibited Wnt/β-catenin signaling and decreased the expression of S100A4, a mediator of cancer metastasis and a specific target of Wnt/β-catenin signaling, in TNBC cells. Together, our data suggest that LRP6 promotes TNBC cell migration and invasion by regulating the expression and function of S100A4 via the Wnt/β-catenin signaling pathway.
Keywords: LRP6, Mesd, S100A4, migration, invasion, TNBC cells
The low-density lipoprotein receptor (LDLR)-related protein 6 (LRP6), a member of the LDLR family (Schneider and Nimpf, 2003), is an indispensable coreceptor of the canonical Wnt/β-catenin signaling pathway (He et al., 2004). Wnt proteins can activate the canonical Wnt/β-catenin signaling pathway only in the presence of LRP6 (He et al., 2004). Upon binding of Wnt to its cell-surface receptors Frizzled (FZD) and LRP6, β-catenin, an essential transcriptional co-activator of the Wnt/β-catenin signaling pathway, is stabilized and then translocates into the nucleus where it interacts with T-cell factor/lymphoid enhancing factor (TCF/LEF) to induce the expression of downstream target genes (He et al., 2004).
Deregulation of Wnt/β-catenin signaling is involved in the pathogenesis of many diseases including cancer (Clevers and Nusse, 2012; Madan and Virshup, 2015). LRP6 is up-regulated in a broad panel of cancers, including breast cancer (Lindvall et al., 2009; Liu et al., 2010; Yang et al., 2011), prostate cancer (Liu et al., 2012), hepatocellular carcinoma (Tung et al., 2012) and retinoblastoma (Wang et al., 2014). Activation of Wnt/β-catenin signaling is associated with a poorer prognosis in breast cancer patients (Lin et al., 2000), and is preferentially found in triple negative breast cancer (TNBC) which is distinguished by negative immunohistochemical assays for expression of the estrogen and progesterone receptors (ER/PR) and human epidermal growth factor receptor-2 (HER2) (Dey et al., 2013; Geyer et al., 2011; Khramtsov et al., 2010). Accordingly, it was found that LRP6 is significantly up-regulated in TNBC (Lindvall et al., 2009; Liu et al., 2010; Yang et al., 2011). In mice, mammary gland development and MMTV-Wnt1-induced mammary tumorigenesis are delayed in LRP6+/− mice (Lindvall et al., 2009), while MMTV-LRP6 transgenic mice develop hyperplasia in their mammary glands due to LRP6-mediated Wnt/β-catenin signaling activation (Zhang et al., 2010). Moreover, transcriptional knockdown of LRP6 and inhibition of LRP6 on the cell surface in TNBC cells significantly decreased Wnt/β-catenin signaling, cell proliferation and tumor growth in vivo (Lin et al., 2013; Liu et al., 2010). Together, these studies clearly indicate that LRP6 plays a critical role in breast cancer development and progression.
TNBC is clinically characterized as more aggressive and less responsive to standard treatment with a poorer overall patient prognosis (Carey et al., 2010). Women with TNBC have an increased likelihood and earlier appearance of distant recurrence, as compared with those with non-TNBC subtypes (Dent et al., 2009). The S100 calcium binding protein A4 (S100A4) is a direct transcriptional target of the Wnt/β-catenin signaling pathway (Stein et al., 2006), and has been established as a mediator of cancer metastasis (Dahlmann et al., 2016; Garrett et al., 2006; Mishra et al., 2012). S100A4 promotes breast cancer motility and invasion (Jenkinson et al., 2004; Wang et al., 2012), induces metastasis in rodent models for breast cancer (Ambartsumian et al., 1996; Davies et al., 1996; Lloyd et al., 1998), and is associated with poor patient survival in breast cancer patients (Platt-Higgins et al., 2000; Rudland et al., 2000). While LRP6/β-catenin signaling is highly activated in TNBC, the role of LRP6 in TNBC metastases remains to be elucidated. In the present study, we present evidence that LRP6 regulates TNBC cell migration and invasion by altering the expression of S100A4.
MATERIALS AND METHODS
MATERIALS
Preparation of recombinant mouse Mesd protein has been described before (Li et al., 2005; Lu et al., 2010). Plasmid pGST-E-cadherin was provided by Dr. Gail Johnson (University of Rochester). The Super8XTOPFlash luciferase construct was provided by Dr. Randall T. Moon (University of Washington, Seattle). A β-galactosidase-expressing vector was from Promega. Polyclonal anti-LRP6 was from Santa Cruz Biotechnology. Monoclonal anti-phospho-LRP6, anti-LRP5, anti-axin2 and anti-S100A4 were purchased from Cell Signaling Technology. Monoclonal anti-β-catenin was from BD Biosciences. Monoclonal anti-actin was from Sigma. Peroxidase labeled anti-mouse antibody and ECL system were purchased from Amersham Life Science. The luciferase and β-galactosidase assay systems were from Promega. Tissue culture media, fetal bovine serum (FBS), and plastic-ware were obtained from Life Technologies, Inc. Proteinase inhibitor cocktail Complete™ was obtained from Boehringer Mannheim.
ANALYSIS OF LRP6 EXPRESSION WITH BREAST CANCER DATASET OBTAINED FROM THE CANCER GENOME ATLAS (TCGA)
TCGA Data Portal (https://tcga-data.nci.nih.gov/tcga) was used to download expression data of breast invasive carcinoma samples (n=1,070). The RNAseqV2 level 3 data that includes fragments per kilobase of exon per million fragments mapped (FPKM)-normalized gene level data were used before statistics. In addition, idf file and sdrf file were also downloaded for sample mapping and annotation. The clinical outcome data was downloaded for correlation and model building. Group wise comparison as well as ANOVA was used to select genes of significance. False Discovery Rate (FDR)-corrected p values were used for multiple hypothesis testing purpose.
CELL CULTURE
All cell lines were obtained from American Type Culture Collection (Manassas, VA), where the cell lines were authenticated by STR profiling before distribution. All cell lines were expanded upon receipt to prepare frozen cell stocks that were cultured in vitro for no longer than 3 months after thawing. MDA-MB-231, MDA-MB-435s, HS578T, BT549, T-47D, ZR-75-1 and MCF-7 cells were cultured in DMEM medium (GIBCO-Invitrogen, Carlsbad, CA) containing 10% of FBS, 2 mM of L-glutamine, 100 units/ml of penicillin, and 100 μg/ml of streptomycin, and grown under standard cell culture conditions at 37°C in a humidified atmosphere with 5% CO2. The MCF-10A human breast epithelial cells were cultured in DMEM/Ham’s F-12 (GIBCO-Invitrogen, Carlsbad, CA) supplemented with 100 ng/ml cholera toxin, 20 ng/ml epidermal growth factor (EGF), 0.01 mg/ml insulin, 500 ng/ml hydrocortisone, and 5% chelex-treated horse serum.
SiRNA DEPLETION OF LRP6
For RNAi experiments, ON-TARGETplus double-stranded siRNA oligomers against human LRP6 and non-specific scrambled siRNA control (Stealth RNAi™ siRNA Negative Control, Med GC) were purchased from Thermo Scientific. siRNA LRP6-1, siRNA LRP6-2 and control siRNA were Thermo Scientific catalog numbers J-003845-09, J-003845-11 and 12935300, respectively. Cells were transfected with Lipofectamine RNAiMAX (Invitrogen) with a final siRNA concentration of 50 nM according to the manufacturer’s instructions.
WESTERN BLOTTING
Cells in 6-well plates were lysed in 0.5 ml of lysis buffer (phosphate-buffered saline containing 1% Triton X-100 and 1 mM PMSF) at 4°C for 10 min. Equal quantities of protein were subjected to SDS-PAGE under reducing conditions. Following transfer to immobilon-P transfer membrane, successive incubations with a primary antibody, and a horseradish peroxidase-conjugated secondary antibody were carried out for 60–120 min at room temperature. The immunoreactive proteins were then detected using the ECL system. Films showing immunoreactive bands were scanned by Hp Scanjet 5590.
CYTOSOLIC FREE β-CATENIN ANALYSIS WITH THE GST-E CADHERIN BINDING ASSAY
The GST-E-cadherin binding assay was conducted exactly as previously described (Lin et al., 2011). Uncomplexed cytosolic free β-catenin present in 100 μg of total cell lysate was subjected to SDS-PAGE and detected using the monoclonal antibody to β-catenin.
LUCIFERASE REPORTER ASSAY FOR WNT/β-CATENIN SIGNALING
Cancer cells were plated into 24-well plates. After overnight culture, the cells were transiently transfected with the Super8XTOPFlash luciferase construct and β-galactosidase-expressing vector. After 24 h incubation, cells were treated with Mesd at the indicated concentrations. Cells were then lysed 24 h later and both luciferase and β-galactosidase activities.
CELL MIGRATION AND INVASION ASSAYS
Breast cancer cell migration and invasion assays were performed as described previously, with minor modifications (Li et al., 1998; Song et al., 2009). The migration assay was carried out in 6.5-mm diameter transwell chambers with pore size of 8.0 μm from BD Biosciences. MDA-MB-231 and BT549 cells (1 × 105) in 200 μl of the serum-free DMEM medium with 0.1% BSA and 2 mM L-glutamine were placed in the upper compartment of the transwell chambers. The lower compartment was filled with 600 μl of complete medium (DMEM medium with 10% FBS and 2 mM L-glutamine). After incubation for 15 h at 37°C, cells on the lower surface of the filter were fixed in 4% paraformaldehyde and stained with crystal violet. The images were scanned and counted at 100 × magnification.
Invasion of cells through Matrigel was determined using 24-well BD invasion chambers (8.0-μm pore size with polycarbonate membrane; BD Biosciences) according to the manufacturer’s instructions. MDA-MB-231 and BT549 cells (5 × 104) in 100 μl of the serum-free DMEM medium with 0.1% BSA and 2 mM L-glutamine were placed in the upper compartment of the invasion chambers, and the lower compartment was filled with 600 μl of complete medium. After incubation for 22 h at 37°C, cells on the lower surface of the filter were fixed, stained and counted at 100 × magnification.
CELL PROLIFERATION/VIABILITY ASSAY
Cells were seeded at 2000 cells/well in opaque-walled 96 well plate 24 hours prior to Mesd and siRNAs exposure. At the indicated time, cell proliferation/viability was determined by CellTiter-Glo luminescent cell viability assay kit according to the manufactures protocol.
STATISTICAL ANALYSIS
The unpaired two-tailed student’s t-test was used to determine statistically significant differences between treatment effects and calculated using GraphPad Prism version 5 for Windows (GraphPad Software, La Jolla California USA). Significance was defined as P < 0.05.
RESULTS
LRP6 IS UP-REGULATED IN TNBC CELLS
It has been reported that LRP6 expression is up-regulated in TNBC (Lindvall et al., 2009; Liu et al., 2010; Yang et al., 2011). Through the TCGA Data Portal, we characterized LRP6 expression in breast cancer patients. We found that the level of LRP6 expression is higher in ER, PR and HER2 negative than in corresponding ER, PR, and HER2 positive breast tumors (Figure 1A), and confirmed that the level of LRP6 expression is higher in TNBC than in non-TNBC (Figure 1B).
Fig. 1.
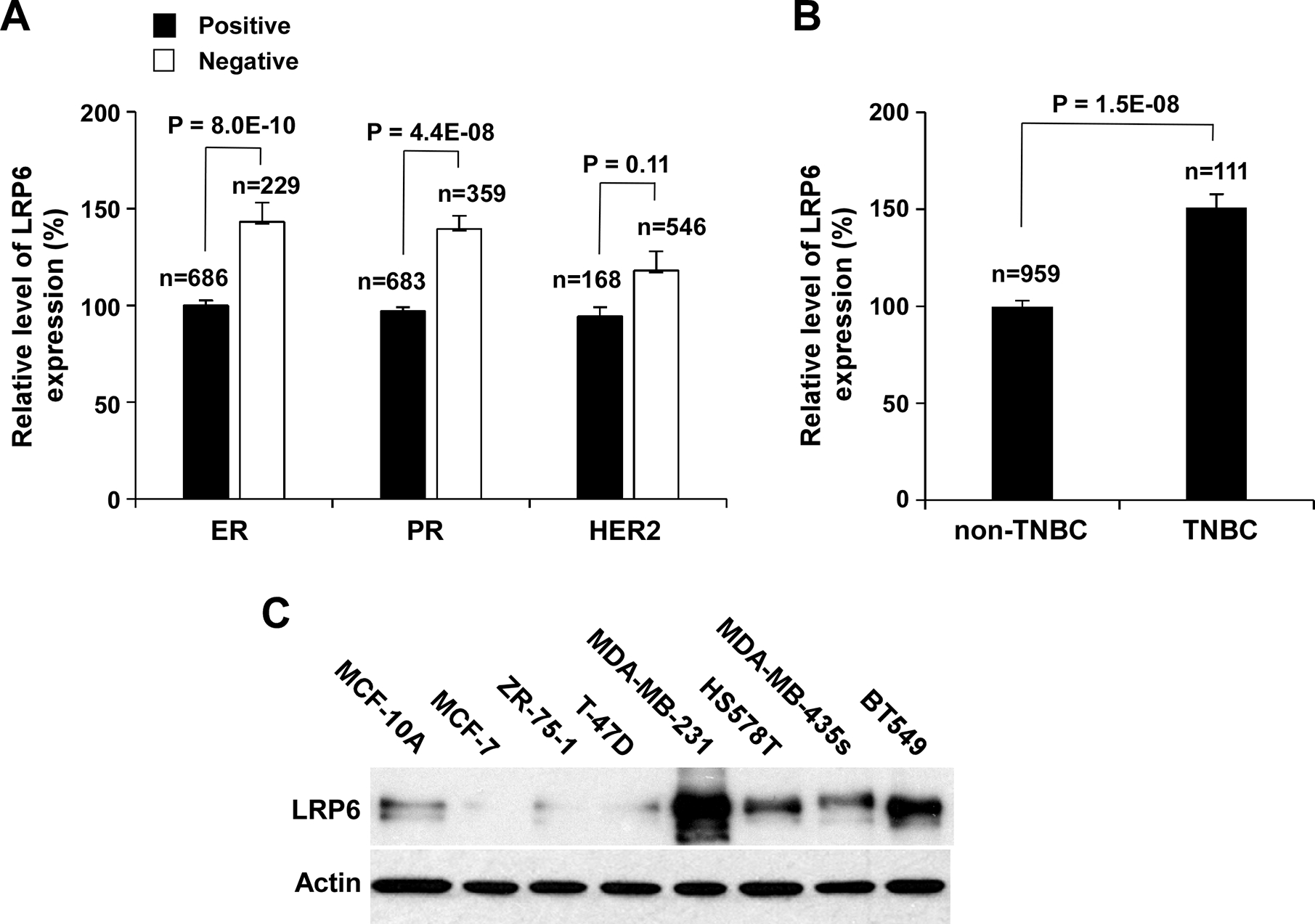
LRP6 is upregulated in TNBC cells. (A, B) LRP6 expression in breast cancer as related to TNBC markers. Data were collected from TCGA Data Portal and analyzed as described in Materials and Methods, and were presented as relative levels to the LRP6 expression level in ER positive breast cancer (A) or the LRP6 expression level in non-TNBC (B). All the values are the average with the s.d. indicated by error bars. (C) LRP6 expression in non-cancerous human breast epithelial cell line MCF-10A, three ER positive breast cell lines and four TNBC cell lines was examined by Western blotting. Samples were also probed with the anti-actin antibody to verify equal loading.
LRP5 is a closely related paralog of LRP6, but LRP5 is a much less potent transducer of Wnt/β-catenin signaling (Chin et al., 2015; MacDonald et al., 2011). We found that the level of LRP5 expression is not associated with ER and PR status (Figure S1A). Although the level of LRP5 expression is higher in TNBC than in non-TNBC, the change of LRP5 expression is much smaller than that of LRP6 (Figure S1B). Together, these data suggest that LRP6 is more important than LRP5 in activation of Wnt/β-catenin signaling in TNBC.
To further characterize LRP6 expression in breast cancer, we performed Western blotting to examine LRP6 expression in non-cancerous human breast epithelial cells line MCF-10A, three ER positive breast cell lines and four TNBC cell lines. As expected, LRP6 was highly expressed in 4 TNBC cell lines MDA-MB-231, HS578T, MDA-MB-435s and BT549, while low LRP6 expression was found in MCF-10A and three ER positive breast cell lines (MCF-7, ZR-75–1 and T-47D) (Figure 1C). As LRP6 is most abundant in MDA-MB-231 and BT549 cells, the rest of our experiments were focused on these two TNBC cell lines.
KNOCKDOWN OF LRP6 INHIBITS WNT/β-CATENIN SIGNALING IN TNBC CELLS AND SUPPRESSES CELL MIGRATION AND INVASION
We next examined the effects of modulating LRP6 expression on TNBC cell migration and invasion. Using two independent siRNAs targeting distinct regions of LRP6, we knocked down LRP6 expression in TNBC MDA-MB-231 and BT549 cells (Figure 2A). While the levels of LRP5 expression were not changed, the levels of LRP6 phosphorylation and cytosolic free β-catenin were greatly reduced in LRP6 siRNA-transfected TNBC cells (Figure 2A). When MDA-MB-231 and BT549 cells were transiently transfected with the Wnt/β-catenin signaling reporter Super8XTOPFlash, knockdown of LRP6 resulted in significant inhibition of the Super8XTOPFlash activity in TNBC cells (Figure 2B). In addition, levels of axin2, a specific target of Wnt/β-catenin signaling, were also significantly decreased in LRP6 siRNA-transfected TNBC cells (Figure 2A), indicating that LRP6 knockdown induces inhibition of Wnt/β-catenin signaling in TNBC cells. Importantly, knockdown of LRP6 significantly suppresses TNBC cell migration and invasion. Knockdown of LRP6 led to 61–70% inhibition of TNBC cell migration when compared to corresponding control cells (Figure 2C & 2D). Similar results were obtained in invasion assays. Knockdown of LRP6 induced 51 – 62% inhibition of TNBC cell invasion when compared to corresponding control cells (Figure 2E & 2F).
Fig. 2.
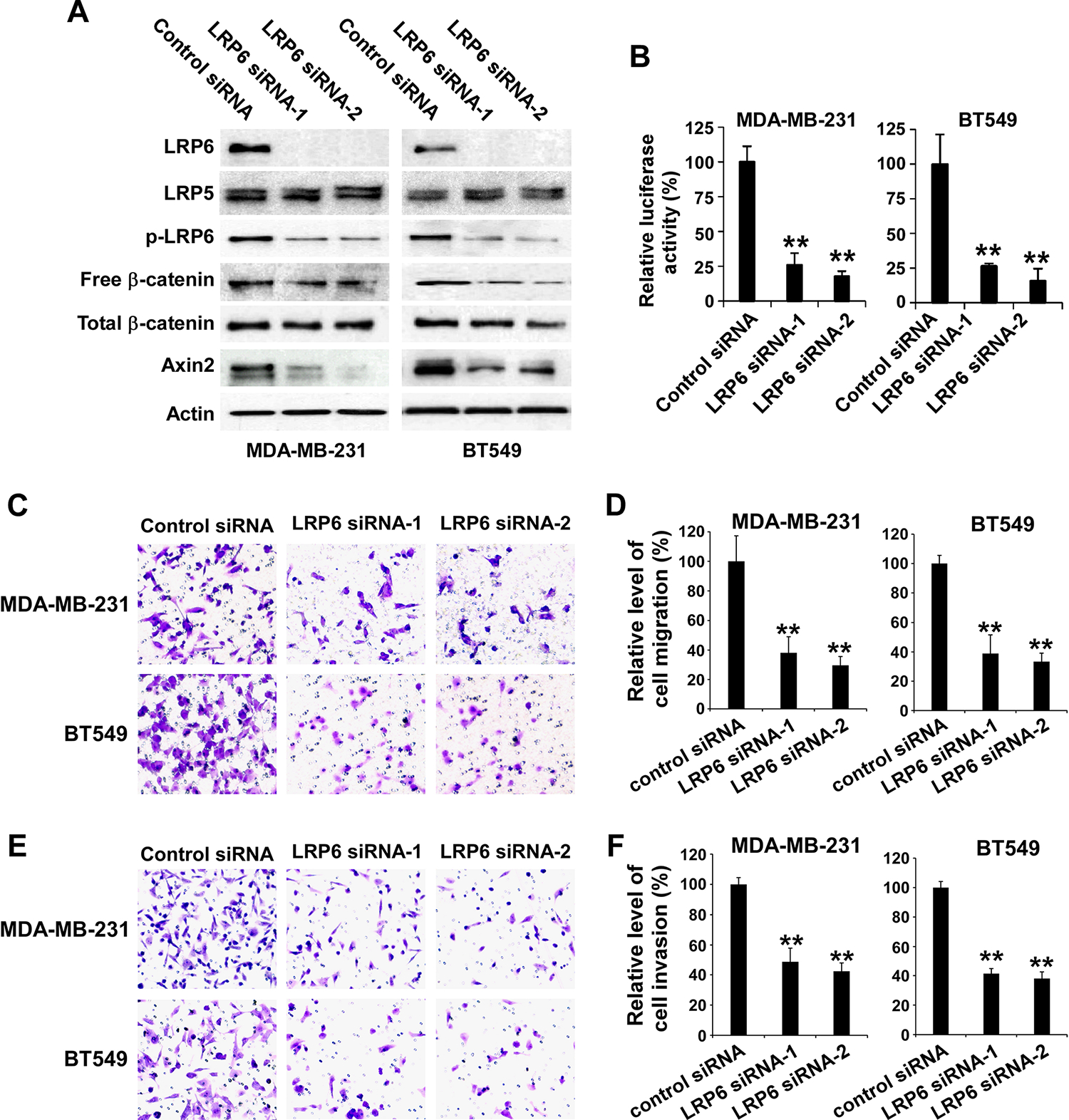
Depletion of LRP6 results in inhibition of Wnt/β-catenin signaling in TNBC cells and suppression of TNBC cell migration and invasion. (A) MDA-MB-231 and BT549 cells in 6-well plates were transiently transfected with 50 nM of LRP6 siRNA-1, LRP6 siRNA-2 or control siRNA. After incubation of 48 h, the levels of human LRP6, LRP5, phospho-LRP6 (p-LRP6), cytosolic free human β-catenin, total cellular human β-catenin, and axin2 were examined by Western blotting. All the samples were also probed with anti-human actin antibody to verify equal loading. (B) MDA-MB-231 and BT549 cells in 24-well plates were transiently transfected with 50 nM of LRP6 siRNA-1, LRP6 siRNA-2 or control siRNA. After incubation of 24 h, cells were transfected with the Super8XTOPFlash luciferase construct and β-galactosidaseexpressing vector in each well. The luciferase activity was then measured 24 h later with normalization to the activity of the β-galactosidase. Values are the average of triple determinations with the s.d. indicated by error bars. (C-F) MDA-MB-231 and BT549 cells were transiently transfected with 50 nM of LRP6 siRNA-1, LRP6 siRNA-2 or control siRNA. After incubation of 48 h, the transwell migration assay (C, D) and Matrigel invasion assay (E, F) were performed. (C) Representative images of cell migration. (D) Relative levels of cell migration are significantly decreased following LRP6 knockdown in MDA-MB-231 and BT549 cells. (E) Representative images of cell invasion. (F) Relative levels of cell invasion are significantly decreased following LRP6 knockdown in MDA-MB-231 and BT549 cells. Data shown are representative of three independent experiments. All the values are the average of quadruplicate determinations with the s.d. indicated by error bars. **P < 0.01 versus control cells.
We then examined the effects of LRP6 knockdown on TNBC cell proliferation/viability. Consistently with the study by Liu et al. (Liu et al., 2010), we found that the cell proliferation/viability was decreased by 14–47 % after transfection of LRP6 siRNA-1 or LRP6 siRNA-2 for 72–96 h in MDA-MB-231 cells and for 48–96 h in BT549 cells (Figure 3). LRP6 knockdown for 48 h in MDA-MB-231 had no significant effects on cell proliferation/viability (Figure 3A). The migration and invasion assays were conducted after LRP6 knockdown for 48 h in TNBC cells. Clearly, LRP6 knockdown in TNBC cells induced more profound effects on cell migration and invasion than on cell proliferation/viability.
Fig. 3.
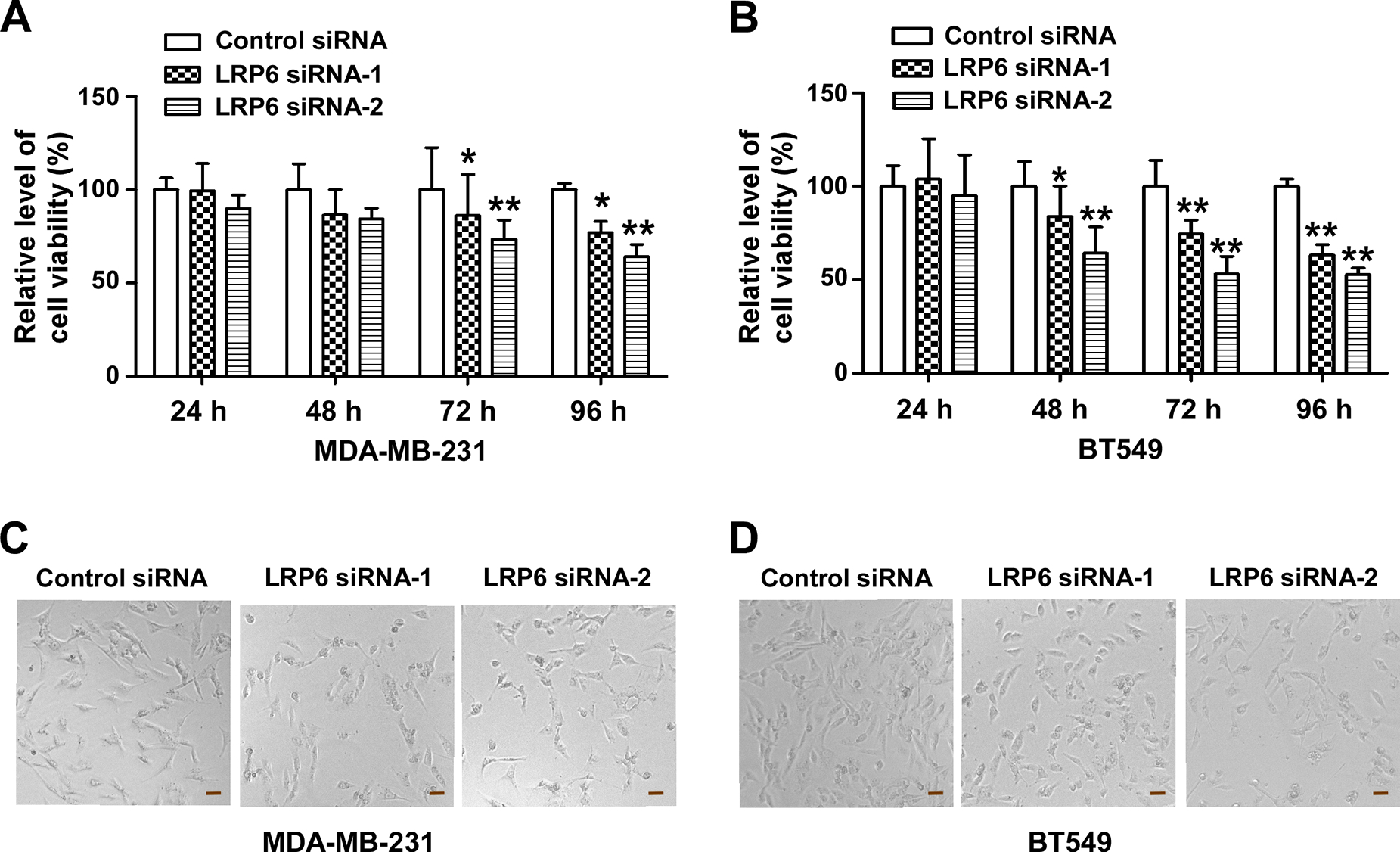
Depletion of LRP6 suppresses TNBC cell proliferation/viability. (A, B) MDA-MB-231 and BT549 cells in 96-well plates were transiently transfected with 50 nM of LRP6 siRNA-1, LRP6 siRNA-2 or control siRNA. After 24, 48, 72 or 96 h of transfection, cell viability was measured by the Cell Titer Glo Assay system. Data shown are representative of three independent experiments. All the values are the average of triplicate determinations with the s.d. indicated by error bars. *P < 0.05, **P < 0.01 versus control cells. (C, D) MDA-MB-231 and BT549 cells were transiently transfected with 50 nM of LRP6 siRNA-1, LRP6 siRNA-2 or control siRNA. After 96 h incubation, images were taken using phase contrast microscopy. Scale bar: 40 μm.
MESD PROTEIN INHIBITS LRP6 PHOSPHORYLATION AND WNT/β-CATENIN SIGNALING IN TNBC CELLS AND SUPPRESSES CELL MIGRATION AND INVASION
Mesd is a specialized chaperone for LRP6 (Culi and Mann, 2003; Hsieh et al., 2003). We have demonstrated that recombinant Mesd protein binds to LRP6 with a high affinity and is able to inhibit Wnt- and Rspondin-induced Wnt/β-catenin signaling in LRP6-expressing cells (Li et al., 2005; Lin et al., 2011; Lin et al., 2013; Lu et al., 2010). To confirm the role of LRP6 in TNBC cell migration and invasion, we examined the effects of recombinant Mesd protein on MDA-MB-231 and BT549 cell migration and invasion. It was found that the treatment of Mesd protein at 2 or 4 μM for 24 h significantly decreased the levels of LRP6 phosphorylation, cytosolic free β-catenin, axin2 expression, and activity of the Wnt/β-catenin signaling reporter Super8XTOPFlash in TNBC cells (Figure 4A & 4B), indicating that Mesd treatment induces inhibition of Wnt/β-catenin signaling in TNBC cells. Importantly, Mesd protein significantly inhibited TNBC cell motility and invasion in a dose-dependent manner. Mesd protein at 2 μM and 4 μM led to about 50% and 85% inhibition, respectively, of TNBC cell migration (Figure 4C & 4D). Similarly, Mesd protein at 2 μM and 4 μM led to about 25% and 60% inhibition, respectively, of TNBC cell invasion (Figure 4E & 4F).
Fig. 4.
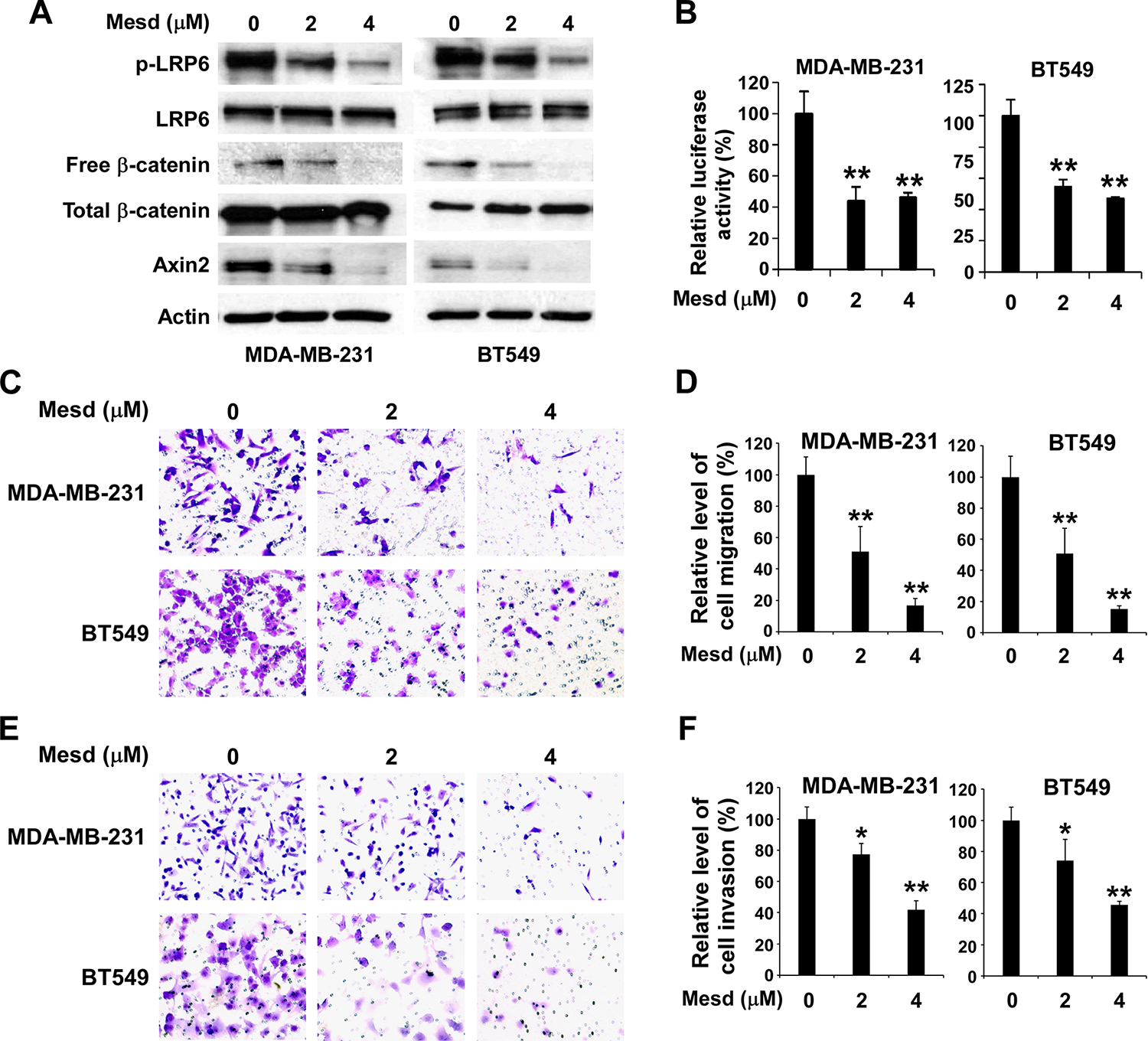
Mesd inhibits Wnt phosphorylation and Wnt/β-catenin signaling in TNBC cells and suppresses TNBC cell migration and invasion. (A) MDA-MB-231 and BT549 cells in 6-well plates were treated with Mesd at the indicated concentrations for 24 h. The levels of human LRP6, LRP5, phospho-LRP6 (p-LRP6), cytosolic free human β-catenin, total cellular human β-catenin, and axin2 were examined by Western blotting. All the samples were also probed with anti-human actin antibody to verify equal loading. (B) MDA-MB-231 and BT549 cells in 24-well plates were transiently transfected with the Super8XTOPFlash luciferase construct and β-galactosidase expressing vector in each well. After 24 h incubation, cells were treated with Mesd at the indicated concentrations. The luciferase activity was then measured 24 h later with normalization to the activity of the β-galactosidase. Values are the average of triple determinations with the s.d. indicated by error bars. (C-F) MDA-MB-231 and BT549 cells were treated with Mesd at the indicated concentrations. After incubation of 24 h, the transwell migration assay (C, D) and Matrigel invasion assay (E, F) were performed. (C) Representative images of cell migration. (D) Relative levels of cell migration are significantly decreased by Mesd treatment in MDA-MB-231 and BT549 cells. (E) Representative images of cell invasion. (F) Relative levels of cell invasion are significantly decreased by Mesd treatment in MDA-MB-231 and BT549 cells. Data shown are representative of three independent experiments. All the values are the average of quadruplicate determinations with the s.d. indicated by error bars. * P < 0.05, **P < 0.01 versus control cells.
Consistently with our previous study (Lin et al., 2013), we also found that Mesd protein significantly inhibited TNBC cell proliferation/viability after treatment at 2 and 4 μM for 96 h (Figure 5). However, Mesd protein had no significant effects on TNBC cell proliferation/viability after treatment at 2 and 4 μM for 24–72 h (Figure 5). In the migration and invasion experiments, TNBC cells were pretreated with Mesd protein for 24 h, and examined by the migration and invasion assays with the treatment of Mesd protein for 15 h and 22 h, respectively. These results indicate that treatment of Mesd protein in TNBC cells induced more profound effects on cell migration and invasion than on cell proliferation/viability.
Fig. 5.
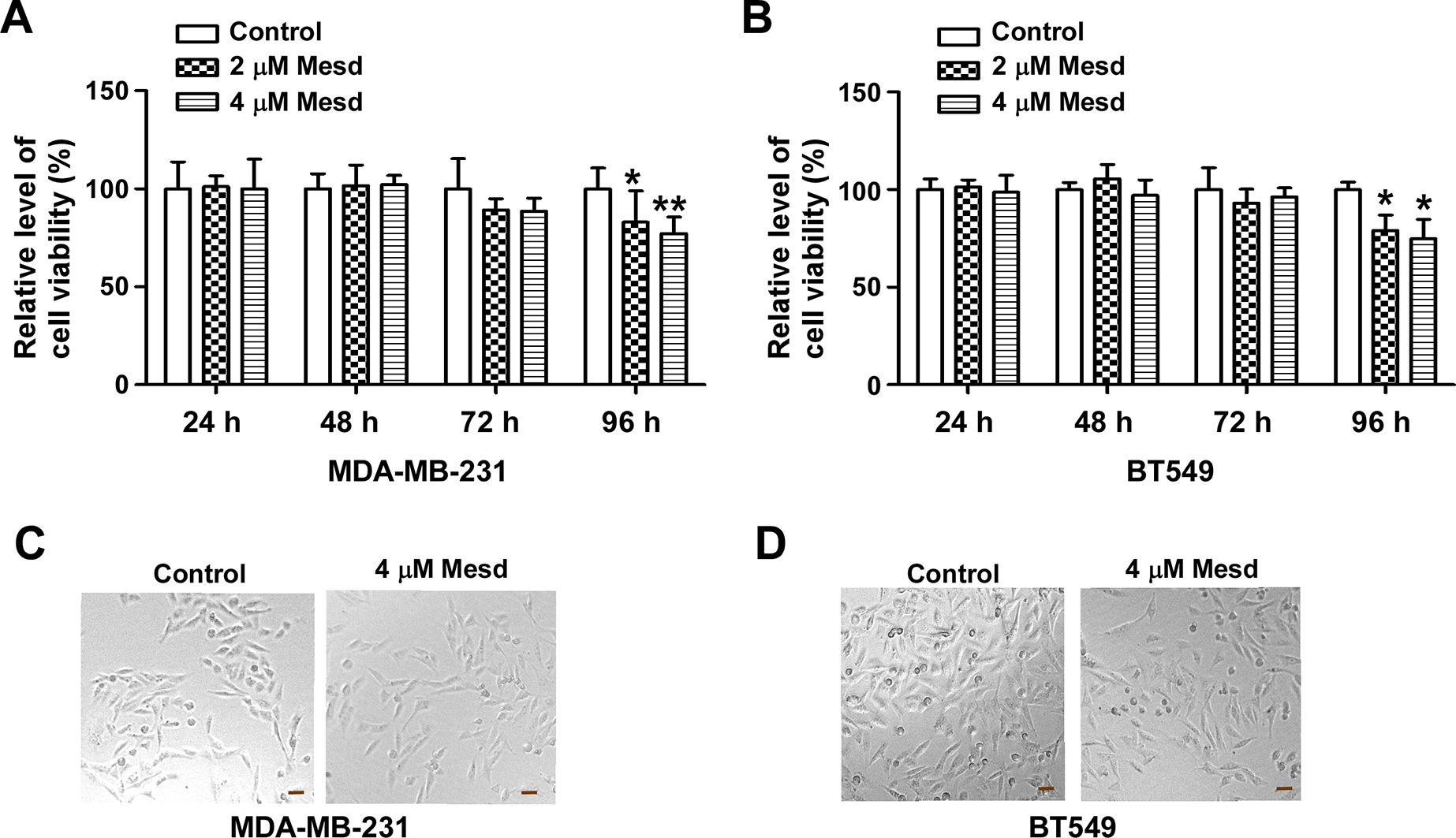
Mesd inhibits TNBC cell proliferation/viability. (A, B) MDA-MB-231 and BT549 cells in 96-well plates were treated with Mesd at the indicated concentrations. After incubation for 24, 48, 72 or 96 h, cell viability was measured by the Cell Titer Glo Assay system. Data shown are representative of three independent experiments. All the values are the average of triplicate determinations with the s.d. indicated by error bars. *P < 0.05, **P < 0.01 versus control cells. (C, D) MDA-MB-231 and BT549 cells were treated with Mesd (4 μM) for 96 h, and images were taken using phase contrast microscopy. Scale bar: 40 μm.
LRP6 MODULATES TNBC CELL MIGRATION AND INVASION BY REGULATING S100A4 EXPRESSION
S100A4 is a specific target of the Wnt/β-catenin signaling pathway (Stein et al., 2006), and has a well-established metastasis-promoting activity (Dahlmann et al., 2016; Garrett et al., 2006; Mishra et al., 2012). It has been demonstrated that S100A4 depletion effectively blocked MDA-MB-231 cell motility and invasion, whereas S100A4 overexpression induced opposite effects (Wang et al., 2012). Therefore, we investigated whether the depletion of LRP6 and treatment of Mesd protein inhibit S100A4 expression in TNBC cells. As shown in Figure 6A, LRP6 knockdown significantly reduced S100A4 expression in MDA-MB-231 and BT549 cells. Similarly, Mesd protein inhibited S100A4 expression in a dose-dependent manner in MDA-MB-231 and BT549 cells (Figure 6B). Taken together, these data suggest that LRP6-mediated cell migration and invasion are associated with the regulation of S100A4 expression in TNBC cells.
Fig. 6.
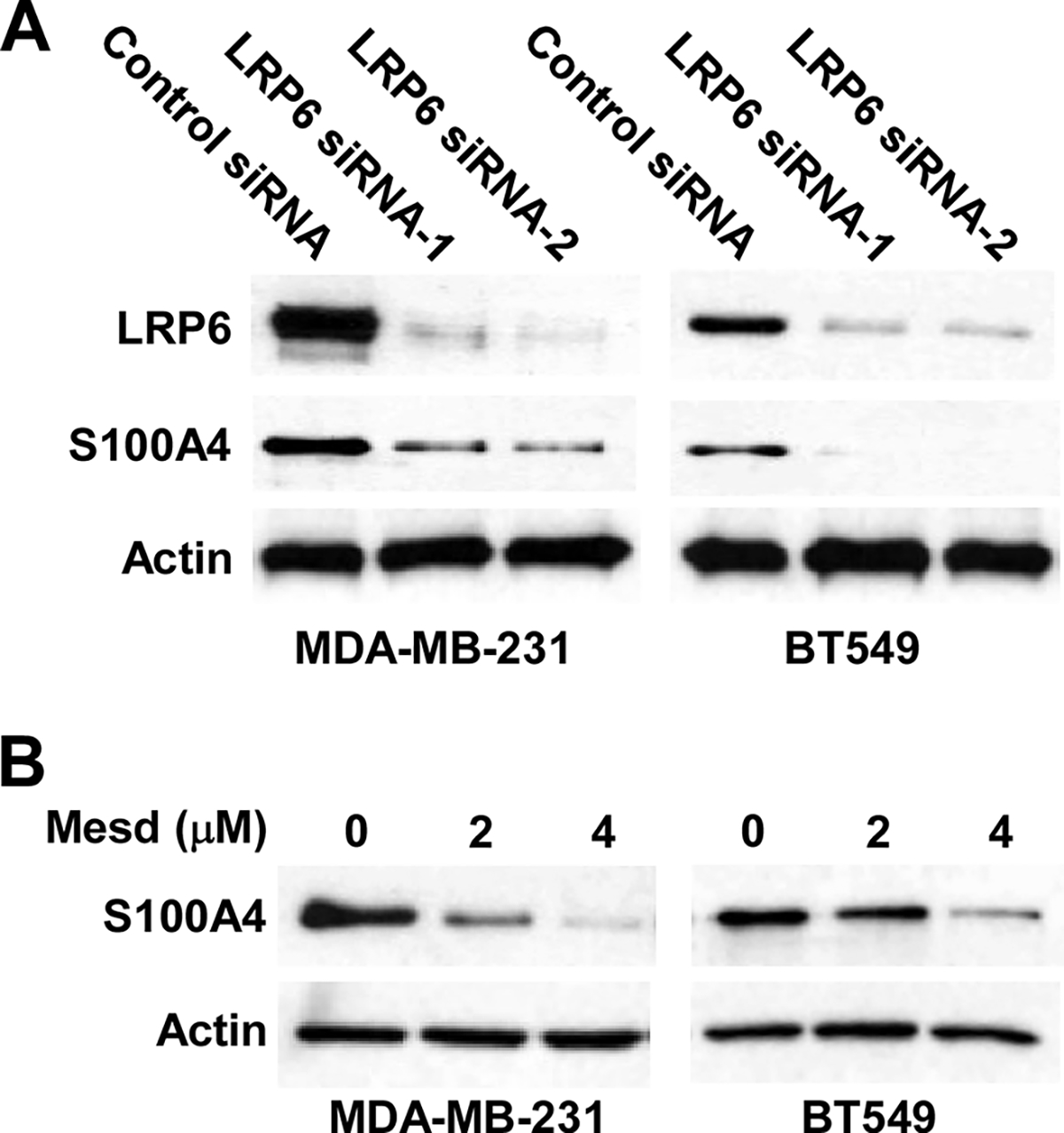
LRP6 depletion and Mesd treatment inhibit S100A4 expression in TNBC cells. (A) MDA-MB-231 and BT549 cells in 6-well plates were transiently transfected with 50 nM of LRP6 siRNA-1, LRP6 siRNA-2 or control siRNA. After incubation of 48 h, the levels of S100A4 expression were examined by Western blotting. (B) MDA-MB-231 and BT549 cells in 6-well plates were treated with Mesd at the indicated concentrations for 24 h. The levels of S100A4 were examined by Western blotting. Data shown are representative of three independent experiments. All the samples were also probed with anti-human actin antibody to verify equal loading.
DISCUSSION
As an essential Wnt co-receptor, LRP6 is up-regulated in many types of cancer (Lindvall et al., 2009; Liu et al., 2010; Liu et al., 2012; Tung et al., 2012; Wang et al., 2014; Yang et al., 2011), and overexpression of LRP6 promotes cancer cell proliferation in vitro and tumor growth in vivo (Li et al., 2004; Tung et al., 2012). Recent studies further suggest that LRP6 is associated with cancer cell motility, invasion and metastasis (Bernaudo et al., 2016; Deng et al., 2015; Marastoni et al., 2014; Nagaoka et al., 2013; Ren et al., 2015; Tung et al., 2012; Wang et al., 2014; Wen et al., 2015; Zhang et al., 2013). It has been demonstrated that the tumor metastasis suppressor gene-1 (NDRG1) interacts with LRP6, followed by blocking of LRP6 phosphorylation and Wnt/β signaling in breast and prostate cancer cells, and therefore, impairs the metastatic progression of tumor cells (Liu et al., 2012). Indeed, LRP6 expression is significantly up-regulated in prostate patients with metastatic disease compared to those without metastasis, and the elevated level of LRP6 is associated with a significantly increased risk of recurrent disease (Liu et al., 2012). Moreover, it has been reported that miR-183 suppresses retinoblastoma cell migration and invasion by targeting LRP6 (Wang et al., 2014), and that kallistatin, a plasma protein, inhibits cell motility via binding to LRP6 and suppressing LRP6 phosphorylation and Wnt/β-catenin signaling in TNBC cells (Zhang et al., 2013). In the present study, we confirm that LRP6 is up-regulated in TNBC patients and TNBC cell lines. More importantly, we demonstrated that knockdown of LRP6 expression and treatment of Mesd protein, a specific inhibitor of LRP6, significantly decreased cell migration and invasion of TNBC MDA-MB-231 and BT549 cells, providing direct evidence that LRP6 plays a critical role in TNBC cell motility and invasion.
Both LRP5 and LRP6 are essential Wnt co-receptors of the Wnt/β-catenin signaling pathway (He et al., 2004). While LRP5 has a Wnt-independent role in glucose uptake and growth for mammary epithelial cells (Chin et al., 2015), LRP6 is a more potent transducer of Wnt/β-catenin signaling (Chin et al., 2015; MacDonald et al., 2011). Furthermore, LRP5 knockdown induced caspase-dependent apoptosis in TNBC cells, whereas LRP6 knockdown had no such effect (Maubant et al., 2014). In the present study, we demonstrated that LRP6 knockdown or LRP6 inhibition by Mesd in TNBC cells induced more profound effects on cell migration and invasion than on cell proliferation/viability. Together, these findings suggest that LRP5 and LRP6 have different functions in TNBCs, with LRP6 playing an important role in cell motility.
Activation of Wnt/β-catenin signaling is required both FZD and LRP6 on the cell surface (He et al., 2004). It was reported that FZD7 was upregulated in TNBC and TNBC derived cell lines, and that FZD7 knockdown resulted in inactivation of Wnt/β-catenin signaling in TNBC cells, leading to inhibition of TNBC cell proliferation, migration and invasion in vitro and suppression of TNBC cell growth in vivo (Yang et al., 2011). It appears that aberrant Wnt/β-catenin signaling in TNBC cells is caused by overexpression of both LRP6 and FZD7 on the cell surface.
S100A4, a member of the S100 family of calcium-binding proteins, is able to activate and integrate pathways both intracellular and extracellular to generate a phenotypic response characteristic of cancer metastasis (Mishra et al., 2012). S100A4 is up-regulated in various types of cancers, and S100A4 expression levels in tumors are considered as a biomarker for the prognosis of both metachronous metastasis and survival of cancer patients (Dahlmann et al., 2016; Garrett et al., 2006; Mishra et al., 2012). Particularly, S100A4 is able to enhance TNBC cell motility and invasion in vitro and induces lung and brain metastasis in vivo (Sartorius et al., 2016; Wang et al., 2012), and the elevated levels of S100A4 expression are associated with the poor prognosis in human patients (Platt-Higgins et al., 2000; Rudland et al., 2000). Importantly, S100A4 is a direct transcriptional target of the Wnt/β-catenin signaling pathway, and a transcriptionally active β-catenin enhances the S100A4-induced migration and invasion of colorectal cancer cells (Stein et al., 2006). Wnt/β-catenin signaling is up-regulated in TNBC (Dey et al., 2013; Geyer et al., 2011; Khramtsov et al., 2010), and is positive associated with TNBC lung and brain metastasis (Dey et al., 2013). Moreover, the functional blockade of the Wnt/β-catenin pathway by either pharmacological Wnt inhibitors or β-catenin depletion results in inhibition of TNBC cell migration and invasion (De et al., 2016; Dey et al., 2013). In this study, we demonstrated that knockdown of LRP6 expression and treatment of Mesd protein inhibit Wnt/β-catenin signaling and S100A4 expression in TNBC cells, suggesting that S100A4 is associated with Wnt/β-catenin signaling-mediated TNBC cell migration, invasion and metastasis.
Mesd was original discovered as a specialized chaperone for LRP6 (Culi and Mann, 2003; Hsieh et al., 2003). We have demonstrated that recombinant Mesd protein is able to bind to mature LRP6 on the cell surface with a high affinity and acts as a specific inhibitor of LRP6 and Wnt/β-catenin signaling in cancer cells (Li et al., 2005; Lin et al., 2011; Lin et al., 2013; Lu et al., 2010). Mesd protein suppresses LRP6 phosphorylation and Wnt/β-catenin signaling in TNBC cells, inhibits TNBC cells proliferation in vitro and tumor growth in vivo (Lin et al., 2013; Liu et al., 2010). In this study, we demonstrated that Mesd protein blocks LRP6 phosphorylation and Wnt/β-catenin signaling in TNBC cells, and inhibits TNBC cells migration and invasion. Together, these results support the notion that, as a specific inhibitor of LRP6, recombinant Mesd protein has a therapeutic value in TNBC.
In summary, we have demonstrated that Wnt co-receptor LRP6 is up-regulated in human TNBC patients and cell lines, and that LRP6 knockdown via siRNA and LRP6 inhibition by recombinant Mesd protein not only inhibit TNBC cell proliferation/viability but also suppress TNBC cell migration and invasion. Furthermore, LRP6-mediated TNBC cell migration and invasion is associated with modulation of S100A4 expression in TNBC cells. These observations reveal a novel role of LRP6 in TNBC cell migration and invasion and further suggest LRP6 as a therapeutic target for TNBC.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health RO1CA124531 and R21CA182056 and Alabama Innovation Fund.
REFERENCES
- Ambartsumian NS, Grigorian MS, Larsen IF, Karlstrom O, Sidenius N, Rygaard J, Georgiev G, Lukanidin E. 1996. Metastasis of mammary carcinomas in GRS/A hybrid mice transgenic for the mts1 gene. Oncogene 13:1621–1630. [PubMed] [Google Scholar]
- Bernaudo S, Salem M, Qi X, Zhou W, Zhang C, Yang W, Rosman D, Deng Z, Ye G, Yang B, Vanderhyden B, Wu Z, Peng C. 2016. Cyclin G2 inhibits epithelial-to-mesenchymal transition by disrupting Wnt/beta-catenin signaling. Oncogene 35:4816–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey L, Winer E, Viale G, Cameron D, Gianni L. 2010. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol 7:683–692. [DOI] [PubMed] [Google Scholar]
- Chin EN, Martin JA, Kim S, Fakhraldeen SA, Alexander CM. 2015. Lrp5 Has a Wnt-Independent Role in Glucose Uptake and Growth for Mammary Epithelial Cells. Mol Cell Biol 36:871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. 2012. Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205. [DOI] [PubMed] [Google Scholar]
- Culi J, Mann RS. 2003. Boca, an endoplasmic reticulum protein required for wingless signaling and trafficking of LDL receptor family members in Drosophila. Cell 112:343–354. [DOI] [PubMed] [Google Scholar]
- Dahlmann M, Kobelt D, Walther W, Mudduluru G, Stein U. 2016. S100A4 in Cancer Metastasis: Wnt Signaling-Driven Interventions for Metastasis Restriction. Cancers 8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MP, Rudland PS, Robertson L, Parry EW, Jolicoeur P, Barraclough R. 1996. Expression of the calcium-binding protein S100A4 (p9Ka) in MMTV-neu transgenic mice induces metastasis of mammary tumours. Oncogene 13:1631–1637. [PubMed] [Google Scholar]
- De P, Carlson JH, Wu H, Marcus A, Leyland-Jones B, Dey N. 2016. Wnt-beta-catenin pathway signals metastasis-associated tumor cell phenotypes in triple negative breast cancers. Oncotarget 7:43124–43149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Wu B, Xiao K, Kang J, Xie J, Zhang X, Fan Y. 2015. MiR-146b-5p promotes metastasis and induces epithelial-mesenchymal transition in thyroid cancer by targeting ZNRF3. Cell Physiol Biochem 35:71–82. [DOI] [PubMed] [Google Scholar]
- Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. 2009. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 115:423–428. [DOI] [PubMed] [Google Scholar]
- Dey N, Barwick BG, Moreno CS, Ordanic-Kodani M, Chen Z, Oprea-Ilies G, Tang W, Catzavelos C, Kerstann KF, Sledge GW Jr, Abramovitz M, Bouzyk M, De P, Leyland-Jones BR. 2013. Wnt signaling in triple negative breast cancer is associated with metastasis. BMC Cancer 13:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett SC, Varney KM, Weber DJ, Bresnick AR. 2006. S100A4, a mediator of metastasis. J Biol Chem 281:677–680. [DOI] [PubMed] [Google Scholar]
- Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, Natrajan R, Reis-Filho JS. 2011. beta-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol 24:209–231. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. 2004. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 131:1663–1677. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Lee L, Zhang L, Wefer S, Brown K, DeRossi C, Wines ME, Rosenquist T, Holdener BC. 2003. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell 112:355–367. [DOI] [PubMed] [Google Scholar]
- Jenkinson SR, Barraclough R, West CR, Rudland PS. 2004. S100A4 regulates cell motility and invasion in an in vitro model for breast cancer metastasis. Bri J Cancer 90:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH. 2010. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol 176:2911–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen J, Lu W, McCormick LM, Wang J, Bu G. 2005. Mesd binds to mature LDL-receptor-related protein-6 and antagonizes ligand binding. J Cell Sci 118:5305–5314. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu W, He X, Schwartz AL, Bu G. 2004. LRP6 expression promotes cancer cell proliferation and tumorigenesis by altering beta-catenin subcellular distribution. Oncogene 23:9129–9135. [DOI] [PubMed] [Google Scholar]
- Li Y, Wood N, Grimsley P, Yellowlees D, Donnelly PK. 1998. In vitro invasiveness of human breast cancer cells is promoted by low density lipoprotein receptor-related protein. Invasion Metastasis 18:240–251. [DOI] [PubMed] [Google Scholar]
- Lin C, Lu W, Zhai L, Bethea T, Berry K, Qu Z, Waud WR, Li Y. (2011). Mesd is a general inhibitor of different Wnt ligands in Wnt/LRP signaling and inhibits PC-3 tumor growth in vivo. FEBS Lett 585:3120–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Lu W, Zhang W, Londono-Joshi AI, Buchsbaum DJ, Bu G, Li Y. 2013. The C-terminal region Mesd peptide mimics full-length Mesd and acts as an inhibitor of Wnt/beta-catenin signaling in cancer cells. PloS One 8:e58102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. 2000. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA 97:4262–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall C, Zylstra CR, Evans N, West RA, Dykema K, Furge KA, Williams BO. 2009. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PloS One 4:e5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Prior J, Piwnica-Worms D, Bu G. 2010. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci USA 107:5136–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Xing F, Iiizumi-Gairani M, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Kobayashi A, Mo YY, Fukuda K, Li Y, Watabe K. 2012. N-myc downstream regulated gene 1 modulates Wnt-beta-catenin signalling and pleiotropically suppresses metastasis. EMBO Mol Med 4:93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd BH, Platt-Higgins A, Rudland PS, Barraclough R. 1998. Human S100A4 (p9Ka) induces the metastatic phenotype upon benign tumour cells. Oncogene 17:465–473. [DOI] [PubMed] [Google Scholar]
- Lu W, Liu CC, Thottassery JV, Bu G, Li Y. 2010. Mesd is a universal inhibitor of Wnt coreceptors LRP5 and LRP6 and blocks Wnt/beta-catenin signaling in cancer cells. Biochemistry 49:4635–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Semenov MV, Huang H, He X. 2011. Dissecting molecular differences between Wnt coreceptors LRP5 and LRP6. PloS One 6:e23537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan B, Virshup DM. 2015. Targeting Wnts at the source--new mechanisms, new biomarkers, new drugs. Mol Cancer Ther 14:1087–1094. [DOI] [PubMed] [Google Scholar]
- Marastoni S, Andreuzzi E, Paulitti A, Colladel R, Pellicani R, Todaro F, Schiavinato A, Bonaldo P, Colombatti A, Mongiat M. 2014. EMILIN2 down-modulates the Wnt signalling pathway and suppresses breast cancer cell growth and migration. J Pathol 232:391–404. [DOI] [PubMed] [Google Scholar]
- Maubant S, Maire V, Tesson B, Némati F, Gentien D, Marty-Prouvost B, Depil S, Cruzalegui F, Tucker G, Roman-Roman S, Dubois T. 2014. The depletion of LRP5, unlike that of LRP6, promotes apoptosis in triple-negative breast cancer cells, making it an interesting therapeutic target. Cancer Res 74(19 Suppl): Abstract nr 2764. [Google Scholar]
- Mishra SK, Siddique HR, Saleem M. 2012. S100A4 calcium-binding protein is key player in tumor progression and metastasis: preclinical and clinical evidence. Cancer Metastasis Rev 31:163–172. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Karasawa H, Turbyville T, Rangel MC, Castro NP, Gonzales M, Baker A, Seno M, Lockett S, Greer YE, Rubin JS, Salomon DS, Bianco C. 2013. Cripto-1 enhances the canonical Wnt/beta-catenin signaling pathway by binding to LRP5 and LRP6 co-receptors. Cell Signal 25:178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt-Higgins AM, Renshaw CA, West CR, Winstanley JH, De Silva Rudland S, Barraclough R, Rudland PS. 2000. Comparison of the metastasis-inducing protein S100A4 (p9ka) with other prognostic markers in human breast cancer. Int J Cancer 89:198–208. [PubMed] [Google Scholar]
- Ren DN, Chen J, Li Z, Yan H, Yin Y, Wo D, Zhang J, Ao L, Chen B, Ito TK, Chen Y, Liu Z, Li Y, Yang J, Lu X, Peng Y, Pan L, Zhao Y, Liu S, Zhu W. 2015. LRP5/6 directly bind to Frizzled and prevent Frizzled-regulated tumour metastasis. Nature Commun 6:6906. [DOI] [PubMed] [Google Scholar]
- Rudland PS, Platt-Higgins A, Renshaw C, West CR, Winstanley JH, Robertson L, Barraclough R. 2000. Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res 60:1595–1603. [PubMed] [Google Scholar]
- Sartorius CA, Hanna CT, Gril B, Cruz H, Serkova NJ, Huber KM, Kabos P, Schedin TB, Borges VF, Steeg PS, Cittelly DM. 2016. Estrogen promotes the brain metastatic colonization of triple negative breast cancer cells via an astrocyte-mediated paracrine mechanism. Oncogene 35:2881–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider WJ, Nimpf J. 2003. LDL receptor relatives at the crossroad of endocytosis and signaling. Cell Mol Life Sci 60:892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Li Y, Lee J, Schwartz AL, Bu G. 2009. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res 69:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein U, Arlt F, Walther W, Smith J, Waldman T, Harris ED, Mertins SD, Heizmann CW, Allard D, Birchmeier W, Schlag PM, Shoemaker RH. 2006. The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology 131:1486–1500. [DOI] [PubMed] [Google Scholar]
- Tung EK, Wong BY, Yau TO, Ng IO. 2012. Upregulation of the Wnt co-receptor LRP6 promotes hepatocarcinogenesis and enhances cell invasion. PloS One 7:e36565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang X, Li Z, Liu H, Teng Y. 2014. MicroRNA-183 suppresses retinoblastoma cell growth, invasion and migration by targeting LRP6. FEBS J 281:1355–1365. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang X, Liang Y, Diao X, Chen Q. 2012. S100A4 promotes invasion and angiogenesis in breast cancer MDA-MB-231 cells by upregulating matrix metalloproteinase-13. Acta Biochim Pol 59:593–598. [PubMed] [Google Scholar]
- Wen Q, Zhao J, Bai L, Wang T, Zhang H, Ma Q. 2015. miR-126 inhibits papillary thyroid carcinoma growth by targeting LRP6. Oncol Rep 34:2202–2210. [DOI] [PubMed] [Google Scholar]
- Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, Chen L, Kim CC, Lau S, Somlo G, Yen Y. 2011. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene 30:4437–4446. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Liu Q, Lu W, Bu G. 2010. Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis. Oncogene 29:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang Z, Li P, Bledsoe G, Chao L, Chao J. 2013. Kallistatin antagonizes Wnt/beta-catenin signaling and cancer cell motility via binding to low-density lipoprotein receptor-related protein 6. Mol Cell Biochem 379:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


