Abstract
Background:
Individual measures of socioeconomic status (SES) have been associated with an increased risk of neural tube defects (NTDs); however, the association between neighborhood SES and NTD risk is unknown. Using data from the National Birth Defects Prevention Study (NBDPS) from 1997 to 2011, we investigated the association between measures of census tract SES and NTD risk.
Methods:
The study population included 10,028 controls and 1829 NTD cases. We linked maternal addresses to census tract SES measures and used these measures to calculate the neighborhood deprivation index. We used generalized estimating equations to calculate adjusted odds ratios (aORs) and 95% confidence intervals (CIs) estimating the impact of quartiles of census tract deprivation on NTDs adjusting for maternal race–ethnicity, maternal education, and maternal age at delivery.
Results:
Quartiles of higher neighborhood deprivation were associated with NTDs when compared with the least deprived quartile (Q2: aOR = 1.2; 95% CI = 1.0, 1.4; Q3: aOR = 1.3, 95% CI = 1.1, 1.5; Q4 (highest): aOR = 1.2; 95% CI = 1.0, 1.4). Results for spina bifida were similar; however, estimates for anencephaly and encephalocele were attenuated. Associations differed by maternal race–ethnicity.
Conclusions:
Our findings suggest that residing in a census tract with more socioeconomic deprivation is associated with an increased risk for NTDs, specifically spina bifida.
Keywords: Anencephaly, Encephalocele, Neighborhood deprivation, Neural tube defects, Socioeconomic status, Spina bifida
Neural tube defects (NTDs) are congenital defects that occur when the neural tube fails to close properly. NTDs are the second most common congenital malformation worldwide and are estimated to impact 7 per 10,000 live births in the United States.1,2 Specific types of NTDs include spina bifida, anencephaly, and encephalocele. NTDs are associated with substantial mortality, morbidity, disability, and economic costs.2,3 Due to the severity of many forms of NTDs, prenatal diagnosis may lead to termination of the pregnancy.3 In the United States, the prevalence of NTDs varies by race–ethnicity with Hispanic women having a higher prevalence of spina bifida and non-Hispanic Black women having a higher prevalence of encephalocele when compared with non-Hispanic White women.4
NTDs have a complex etiology that includes both genetic and environmental causes.5 Known risk factors for NTDs include folate deficiency, maternal pregestational diabetes, maternal obesity, certain medications (e.g., valproic acid), and insufficient folate intake.6,7 Women who have had an NTD-affected pregnancy have a higher risk of having a future affected pregnancy.8 The US Preventive Services Task Force recommends that all women who are planning or capable of pregnancy take a daily supplement containing 0.4–0.8 mg of folic acid.9 The United States began fortifying cereal grains with folic acid in 1998 to prevent NTDs in pregnancy.10 The mandatory fortification resulted in an initial decrease in NTD prevalence, but the prevalence of NTDs in the United States has remained stable since 1999.2,11
In several studies, individual measures of socioeconomic status (SES), such as maternal education and household income, have been associated with an elevated risk of NTDs.11-14 However, measures of neighborhood SES, which capture both the physical environment (e.g., resources, services, and housing) and social environment (e.g., safety and social connections), may influence health through their contributions to individual behaviors and stress and other pathways.15 Measures of neighborhood SES are recognized as different entities than measures of individual SES which may contribute differently to outcomes. Neighborhood context could impact NTD risk through exposures to environmental pollutants, lower folic acid intake due to lack of access to healthcare or nutritious foods, and increased maternal stress.
Results from previous studies have been inconclusive for the impact of neighborhood SES on NTD risk.11,12,16,17 Many of these studies have been limited by either the number of years of data, small sample sizes, or by only investigating individual census variables. Prior studies in the United States have been limited to California births.12,16 It has been suggested that due to high correlations between census variables and the multidimensionality of neighborhood deprivation it is more effective to use a calculated deprivation index.18 The neighborhood deprivation index (NDI) has been associated with adverse birth outcomes previously,18-21 but its association with NTDs is unknown.
To extend our understanding of this complex relationship, we investigated the association of measures of neighborhood SES on the risk of NTDs using data from 1997 to 2011 from the National Birth Defects Prevention Study (NBDPS), one of the largest population-based case–control studies of birth defects conducted in the United States. To the author’s knowledge, this is the first study to investigate the association between NTDs and the NDI.
METHODS
Study Design
The NBDPS is a population-based multistate case–control study of birth defects in the United States. The methods are described in more detail elsewhere.22 Briefly, data collection occurred for pregnancies that had dates of delivery on or after 1st October 1997, and estimated dates of delivery on or before December 31st, 2011. Cases were identified from birth defect surveillance programs and included live-born infants, stillbirths, and induced terminations. Controls were live-born infants without a birth defect diagnosis born in the same geographical area and birth years as cases and were randomly selected from hospital records or birth certificates. Women were contacted for a computer-assisted telephone interview between 6 weeks and 24 months after the estimated date of delivery. Interviews were conducted in English or Spanish. Case and control women were ascertained statewide from Arkansas, Iowa, and Utah (beginning in 2003) and from selected counties in California, Georgia, Massachusetts, New York (no data contributed from 2002 to 2004), North Carolina (beginning in 2003), and Texas. For this analysis, participants from New Jersey were not included as geocoded residential addresses were unavailable. The NBDPS study was approved by the institutional review board at CDC and at each center.
The following NTDs were eligible for inclusion in the NBDPS: anencephaly (including craniorachischisis), encephalocele (including cranial meningocele and encephalomyelocele), and spina bifida.23 Clinical geneticists reviewed abstracted medical record data for each case to ensure inclusion criteria were met. Cases with chromosome abnormalities or single-gene conditions were excluded. Case ascertainment, specifically the inclusion of terminations and stillbirths, changed over time for some centers.22 Georgia and Massachusetts expanded the existing inclusion of live births and stillbirths to include induced abortions in 1999 and 2011, respectively. New York began to include stillbirths and induced abortions in 2000.
The NBDPS interview collected information on demographics, pregnancy history, medications, medical conditions, and other exposures from the 3 months before pregnancy to the end of pregnancy. During the telephone interview, women were asked to report any addresses where they resided for more than 1 month from the 3 months before conception to the date of delivery. Reported addresses were geocoded and were linked to 2000 and 2010 census tracts using ArcGIS. Since the embryologically relevant period for NTD development is 17–28 days postfertilization,1 the address where the woman resided from the month before pregnancy to the end of the 1st month of pregnancy was selected as the exposed periconceptional residence. If overlapping addresses were reported during this time period, the address lived at the longest before the estimated date of conception was selected.
Exclusions
Women who did not report a residential address during the periconceptional period or whose reported address was unable to link to a census tract were excluded. Overlapping addresses with the same duration of stay before conception were excluded as it was not possible to determine the accurate address to attribute to the periconceptional period. Because of their strong associations with NTDs, women with pregestational diabetes or unknown diabetes status and women with unknown or reported use of folate antagonist medications (aminopterin sodium, carbamazepine, cholestyramine resin, methotrexate, oxcarbazepine, pyrimethamine, sulfasalazine, triamterene, trimethoprim, phenytoin, primidone, phenobarbital, or valproate sodium) during the month before pregnancy to end of the first month of pregnancy were also excluded.
Neighborhood SES Measures
We obtained census tract level socioeconomic measures from the 2000 decennial census, the 2005–2009 5-year American Community Survey (ACS), and the 2010–2014 5-year ACS. The census includes complete population counts for select questions and additional estimates that were obtained from a subset of the population (via the census “long form”). The ACS is an ongoing yearly survey that asks questions of a sample of the United States population. The ACS 5-year data include census tract level estimates.
We linked periconceptional addresses of NBDPS case and control women to census tracts based on the case or control’s year of delivery. Addresses from mothers of NBDPS cases and controls delivered between 1997 and 2004 were linked to 2000 census tracts and the associated 2000 census measures. Those with cases or controls delivered between 2005 and 2009 were linked to the 2000 census tracts and the associated 2005–2009 ACS measures. Addresses from mothers of NBDPS cases and controls born in 2010 or 2011 were linked to the 2010 census tracts and the associated 2010–2014 ACS measures.
We extracted census and ACS measures relevant to the calculation of the NDI. The NDI has been shown to be associated with birth outcomes and has frequently been used to operationalize neighborhood SES.18-21,24,25 The NDI considers the multidimensional contributions of eight SES components to neighborhood deprivation: (1) percent of owner-occupied housing units with more than 1.01 occupants per room among total occupied housing units (“crowding”); (2) percent of female-headed households with dependents (“female-headed households”); (3) percent of males in management and professional occupations (“males in management”); (4) percent of the employed civilian labor force 16 years and over who are unemployed (“unemployment”); (5) percent of sample whose highest level of education at the age of 25 was less than a high school diploma (“low education”); (6) percent of those with an income to poverty ratio <1 among the total in the sample (“poverty”); (7) percent of households on public assistance (“public assistance”); and (8) percent of households earning <$30,000 per year (“low income”).
We used principal components analysis to calculate variable factor loadings for the eight selected variables. The NDI was the resulting summary score calculated by weighting each of the eight variables by their respective factor loading values (crowding = 0.73742, female-headed households = 0.48000, males in management = −0.66620, unemployment = 0.63126, low education = 0.89745, poverty = 0.88970, public assistance = 0.78283, and low income = 0.86382). The NDI was standardized to have a mean of 0 and a standard deviation of 1. Higher NDI values represent more deprivation.
Analysis
We compared maternal and infant characteristics among controls between quartiles of NDI. Maternal characteristics examined included maternal educational attainment at delivery (less than high school, high school graduate, some college or higher), maternal age at delivery in years (less than 20 years, 20–34 years, and 35 or more years), maternal race–ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other), maternal birthplace (United States, Mexico, other), prepregnancy body mass index (BMI) in kg/m2 (underweight [<18.5], normal weight [18.5–24.9], overweight [25.0–29.9], obese [>=30.0]), number of previous pregnancies (no prior pregnancies, 1–2 prior pregnancies, 3 or more prior pregnancies), and maternal cigarette smoking between the month before pregnancy and the third month of pregnancy (yes, no). In addition, maternal reported household income (less than $10,000, $10,000–$19,999, $20,000–$29,999, $30,000–$39,999, $40,000–$49,999, and $50,000 or more), first-degree family history of NTD (no, yes), and infant sex (male, female) were examined. We categorized each census tract SES measure based on quartiles of the distribution among controls. Birth and maternal characteristics were additionally compared between NTD cases and controls.
Unconditional logistic regression models were used to calculate the crude odds ratio (OR) and 95% confidence intervals (CI) between each census tract SES measure and the NDI for each NTD category. In addition, we analyzed the components of the NDI separately to understand the individual contributions of each element. We calculated adjusted odds ratios (aOR) and associated 95% CIs using a generalized estimating equation (GEE) model with logistic links. This model accounted for the correlation of observations within census tracts with an exchangeable correlation matrix. We adjusted models for individual-level measures of maternal race–ethnicity and maternal age and educational attainment at delivery. Subjects with missing values for the exposure or confounders were excluded in the models. We selected potential confounders using a directed acyclic graph (DAG) created via the program Daggity26 (eFigure 1; http://links.lww.com/EDE/C56). Unadjusted and adjusted models were additionally stratified by maternal race–ethnicity (non-Hispanic White, non-Hispanic Black, and Hispanic). As a sensitivity analysis to evaluate the impact of racial composition of the census tract, the race–ethnicity stratified models were further adjusted to include the percent African American race in the census tract and the percent non-White race in the census tract. Because the severity of NTDs may lead to terminations, we performed an additional sensitivity analysis restricting the dataset to study centers that ascertained terminations, with the assumption that this subgroup would be likely to show stronger associations. To align with the American Statistical Association guidelines,27 we considered ORs meaningful if they had an effect size 15% greater than the null.
RESULTS
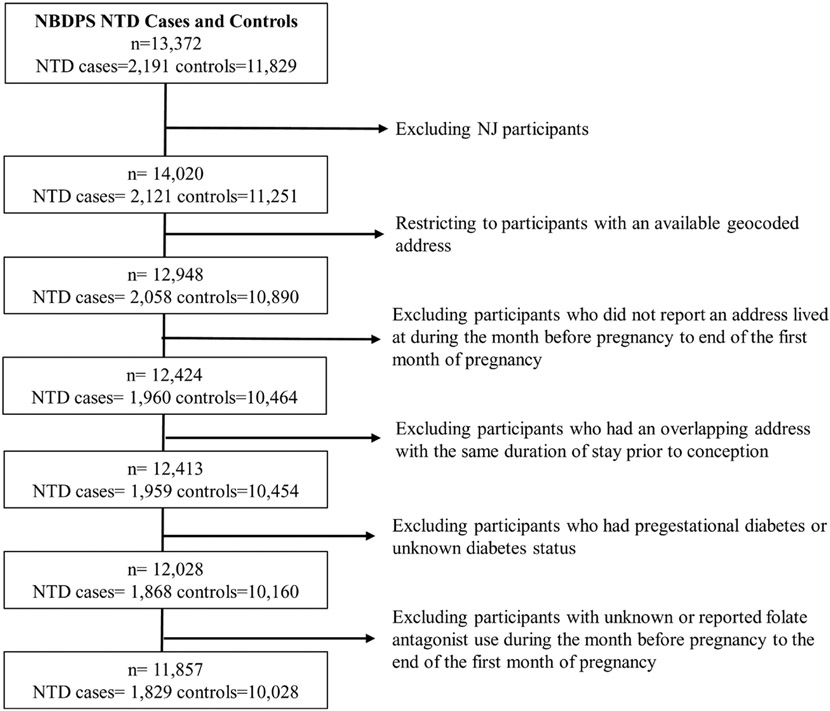
Overall, addresses from 2120 NTD cases and 11,241 control women in NBDPS underwent geocoding. Of those, 3% of both NTD case (n = 63) and control (n = 361) women had periconceptional addresses that were unlinkable to a census tract and were excluded from this analysis. Characteristics of NTD cases and controls with linkable addresses were similar to those with addresses unable to link to a census tract (eTable 1; http://links.lww.com/EDE/C56). After additional exclusions (n = 228 cases, n = 852 controls), the final analytic dataset included 1085 spina bifida cases, 555 anencephaly cases, 189 encephalocele cases, and 10,028 controls (Figure 1).
FIGURE 1.
Study population and exclusion criteria.
Birth and maternal characteristics of controls varied by quartile of NDI. Control women who had a periconceptional residence in the highest quartile of deprivation were more likely to have lower educational attainment and to be younger than control women who had a residence in the lowest quartile of deprivation (Table 1). In addition, control women who had a residence in the highest quartile of deprivation were more likely to be non-Hispanic Black or Hispanic, have a birthplace outside of the United States, have a lower household income, and to be obese. Control women with a residence in the highest quartile of deprivation were also more likely to have either no prior pregnancies or three or more prior pregnancies and to report smoking during pregnancy. Additionally, birth and maternal characteristics of NTD cases differed from the characteristics of controls (eTable 2; http://links.lww.com/EDE/C56).
TABLE 1.
Birth and Maternal Characteristics of Neural Tube Defect (NTD) Controls by Neighborhood Deprivation Index (NDI) Quartile (Q), National Birth Defects Prevention Study, 1997–2011
| Characteristic | NDI Q1 | NDI Q2 | NDI Q3 | NDI Q4 |
|---|---|---|---|---|
| (Lowest Deprivation) | (Highest Deprivation) | |||
| n (%) | n (%) | n (%) | n (%) | |
| Maternal education | ||||
| <High school | 89 (4) | 219 (9) | 442 (18) | 851 (34) |
| High school graduate | 248 (10) | 541 (22) | 731 (29) | 803 (32) |
| Some college | 2161 (86) | 1732 (69) | 1306 (52) | 794 (32) |
| Missing | 8 (0) | 15 (1) | 27 (1) | 59 (2) |
| Maternal age at delivery (years) | ||||
| <20 | 70 (3) | 161 (6) | 256 (10) | 490 (20) |
| 25–34 | 1854 (74) | 1972 (79) | 2000 (80) | 1842 (74) |
| 35+ | 582 (23) | 374 (15) | 250 (10) | 175 (7) |
| Maternal race/ethnicity | ||||
| Non-Hispanic White | 2064 (82) | 1845 (74) | 1466 (59) | 560 (22) |
| Non-Hispanic Black | 101 (4) | 200 (8) | 336 (13) | 434 (17) |
| Hispanic | 143 (6) | 297 (12) | 556 (22) | 1383 (55) |
| Other | 197 (8) | 163 (7) | 146 (6) | 129 (5) |
| Missing | 1 (0) | 2 (0) | 2 (0) | 1 (0) |
| Maternal birthplace | ||||
| United States | 2184 (87) | 2159 (86) | 2025 (81) | 1627 (65) |
| Mexico | 50 (2) | 124 (5) | 248 (10) | 661 (26) |
| Other | 264 (11) | 211 (8) | 209 (8) | 163 (7) |
| Missing | 8 (0) | 13 (1) | 24 (1) | 56 (2) |
| Household income | ||||
| <$10,000 | 97 (4) | 242 (10) | 434 (17) | 892 (36) |
| $10,000–$19.999 | 89 (4) | 223 (9) | 407 (16) | 490 (20) |
| $20,000–$29,999 | 166 (7) | 313 (13) | 426 (17) | 361 (14) |
| $30,000–$39,999 | 161 (6) | 315 (13) | 274 (11) | 163 (7) |
| $40,000–$49,999 | 195 (8) | 241 (10) | 192 (7) | 109 (4) |
| $50,000+ | 1653 (66) | 990 (40) | 562 (22) | 183 (7) |
| Missing | 145 (6) | 183 (7) | 211 (8) | 309 (12) |
| First-degree family history of NTD | ||||
| No | 2497 (100) | 2500 (100) | 2500 (100) | 2506 (100) |
| Yes | 9 (0) | 7 (0) | 6 (0) | 1 (0) |
| Prepregnancy BMI (kg/m2) | ||||
| Underweight (<18.5) | 114 (5) | 130 (5) | 135 (5) | 130 (5) |
| Normal weight (18.5–24.9) | 1536 (61) | 1347 (54) | 1213 (48) | 1059 (42) |
| Overweight (25.0–29.9) | 522 (21) | 564 (23) | 554 (22) | 566 (23) |
| Obese (>=30.0) | 314 (13) | 423 (17) | 508 (20) | 536 (21) |
| Missing | 20 (1) | 43 (2) | 96 (4) | 216 (9) |
| Number of previous pregnancies | ||||
| No prior pregnancies | 718 (29) | 703 (28) | 782 (31) | 781 (31) |
| 1–2 prior pregnancies | 1275 (51) | 1276 (51) | 1181 (47) | 1121 (45) |
| 3+ prior pregnancies | 513 (21) | 527 (21) | 543 (22) | 604 (24) |
| Missing | 0 (0) | 1 (0) | 0 (0) | 1 (0) |
| Maternal cigarette smoking | ||||
| Yes | 302 (12) | 493 (20) | 554 (22) | 427 (17) |
| No | 2196 (88) | 2006 (80) | 1933 (77) | 2038 (81) |
| Missing | 8 (0) | 8 (0) | 19 (1) | 42 (2) |
| Infant sex | ||||
| Male | 1282 (51) | 1245 (50) | 1267 (51) | 1327 (53) |
| Female | 1222 (49) | 1262 (50) | 1238 (49) | 1174 (47) |
BMI indicates body mass index.
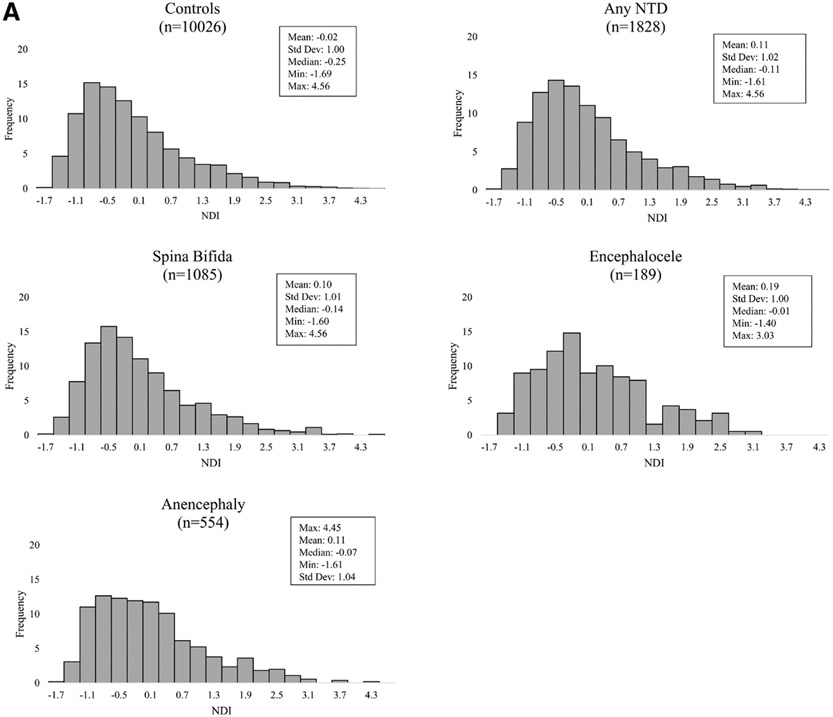
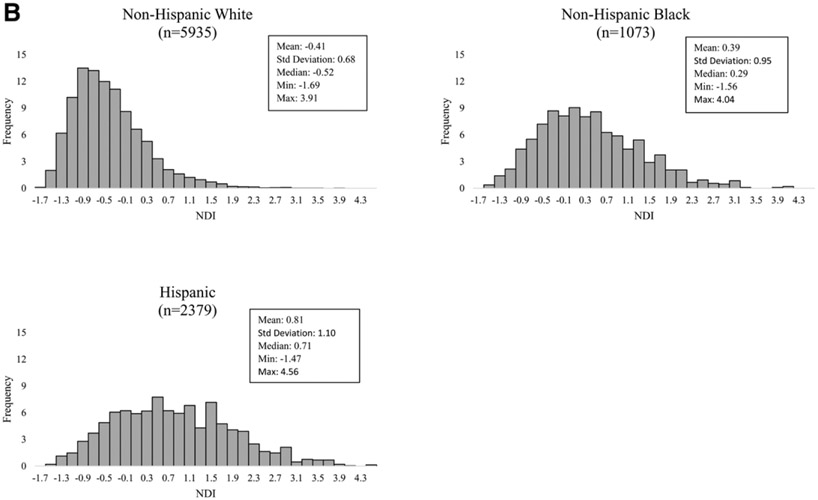
The mean NDI for pregnancies affected by a NTD was similar (mean 0.11; range: −1.61 to 4.56) to that of controls (mean: −0.02; range: −1.69 to 4.56) (Figure 2A). Among controls, non-Hispanic Black (mean: 0.39; range: −1.56 to 4.04) and Hispanic (mean: 0.81; range: −1.47 to 4.56) women had a residence in census tracts with more deprivation when compared with non-Hispanic White women (mean: −0.41; range: −1.69 to 3.91) (Figure 2B).
FIGURE 2.
A, Distribution of neighborhood deprivation scores of maternal periconceptional addresses among NTD cases, overall and by NTD type, and controls, National Birth Defects Prevention Study, 1997–2011. B, Distribution of neighborhood deprivation scores of maternal periconceptional addresses by maternal race/ethnicity among controls, National Birth Defects Prevention Study, 1997–2011. NTD indicates neural tube defect.
Maternal residence in quartiles two, three, and four of NDI were associated with delivering a fetus or infant with any NTD when compared with the least deprived (first) quartile; however, the estimates did not show a clear gradient across quartiles with the fourth quartile association having the smallest magnitude (Q2: aOR = 1.2; 95% CI = 1.0, 1.4; Q3: aOR = 1.3; 95% CI = 1.1, 1.5; Q4: aOR = 1.2; 95% CI = 1.0, 1.4) (Table 2). Any NTD was associated with residence in a census tract with a higher percentage of crowded households across all quartiles after adjustment. Meaningful unadjusted associations were also seen across all quartiles for census tracts with female-headed households with dependents, high census tract poverty, and lower census tract household income (eTable 3; http://links.lww.com/EDE/C56). After adjustment for covariates, several associations were attenuated. Residence in a census tract with the highest quartile of males in management and professional occupations showed reduced odds of any NTD (aOR = 0.8; 95% CI = 0.7, 0.9). Overall, results for spina bifida were similar to the results for any NTD. The results for anencephaly and encephalocele showed a similar pattern of elevated estimates, albeit less strong. The percent of crowded households in the census tract showed the most consistent association across NTD categories (aOR range: 1.1–1.6).
TABLE 2.
Adjusted Associations Between Maternal Periconceptional Census Tract Socioeconomic Measures and Neural Tube Defects (NTD), Overall and by NTD Type, National Birth Defects Prevention Study, 1997–2011
| Controls | Any NTD | Spina Bifida | Anencephaly | Encephalocele | |||||
|---|---|---|---|---|---|---|---|---|---|
| aOR | aOR | aOR | aOR | ||||||
| Census Tract Level Measurea | n | n | (95% CI)b | n | (95% CI)b | n | (95% CI)b | n | (95% CI)b |
| NDI total | |||||||||
| Q1 (lowest) | 2506 | 354 | Ref | 203 | Ref | 117 | Ref | 34 | Ref |
| Q2 | 2507 | 452 | 1.2 (1.0, 1.4) | 289 | 1.4 (1.2, 1.7) | 127 | 1.0 (0.8, 1.3) | 36 | 0.9 (0.6, 1.5) |
| Q3 | 2506 | 505 | 1.3 (1.1, 1.5) | 296 | 1.4 (1.2, 1.8) | 153 | 1.1 (0.8, 1.4) | 56 | 1.3 (0.8, 2.1) |
| Q4 (highest) | 2507 | 517 | 1.2 (1.0, 1.4) | 297 | 1.4 (1.1, 1.7) | 157 | 0.9 (0.7, 1.3) | 63 | 1.2 (0.7, 2.0) |
| Missing | 2 | 1 | 1 | ||||||
| NDI components | |||||||||
| Crowding | |||||||||
| Q1 (lowest) | 2506 | 355 | Ref | 217 | Ref | 105 | Ref | 33 | Ref |
| Q2 | 2506 | 427 | 1.2 (1.0, 1.4) | 253 | 1.2 (1.0, 1.4) | 134 | 1.2 (0.9, 1.6) | 40 | 1.2 (0.7, 1.8) |
| Q3 | 2508 | 521 | 1.4 (1.2, 1.6) | 315 | 1.4 (1.2, 1.7) | 143 | 1.2 (0.9, 1.6) | 63 | 1.6 (1.0, 2.4) |
| Q4 (highest) | 2507 | 525 | 1.2 (1.0, 1.4) | 300 | 1.2 (1.0, 1.5) | 172 | 1.2 (0.9, 1.6) | 53 | 1.1 (0.6, 1.7) |
| Missing | 1 | 1 | 1 | ||||||
| Female-headed households | |||||||||
| Q1 (lowest) | 2506 | 383 | Ref | 228 | Ref | 115 | Ref | 40 | Ref |
| Q2 | 2507 | 477 | 1.2 (1.0, 1.4) | 282 | 1.2 (1.0, 1.5) | 147 | 1.2 (0.9, 1.5) | 48 | 1.1 (0.7, 1.6) |
| Q3 | 2507 | 483 | 1.1 (1.0, 1.3) | 294 | 1.2 (1.0, 1.5) | 149 | 1.1 (0.8, 1.4) | 40 | 0.7 (0.5, 1.2) |
| Q4 (highest) | 2507 | 485 | 1.1 (1.0, 1.3) | 281 | 1.2 (1.0, 1.5) | 143 | 1.1 (0.8, 1.4) | 61 | 1.0 (0.6, 1.5) |
| Missing | 1 | 1 | 1 | ||||||
| Males in management | |||||||||
| Q1 (lowest) | 2506 | 534 | Ref | 306 | Ref | 169 | Ref | 59 | Ref |
| Q2 | 2506 | 488 | 1.0 (0.9, 1.2) | 286 | 1.0 (0.8, 1.2) | 142 | 1.0 (0.8, 1.2) | 60 | 1.2 (0.9, 1.8) |
| Q3 | 2507 | 442 | 0.9 (0.8, 1.1) | 280 | 1.0 (0.8, 1.2) | 124 | 0.9 (0.7, 1.2) | 38 | 0.9 (0.6, 1.3) |
| Q4 (highest) | 2508 | 365 | 0.8 (0.7, 0.9) | 213 | 0.7 (0.6, 0.9) | 120 | 0.9 (0.7, 1.2) | 32 | 0.8 (0.5, 1.2) |
| Missing | 1 | ||||||||
| Unemployment | |||||||||
| Q1 (lowest) | 2457 | 386 | Ref | 234 | Ref | 113 | Ref | 39 | Ref |
| Q2 | 2534 | 469 | 1.1 (1.0, 1.3) | 282 | 1.2 (1.0, 1.4) | 146 | 1.2 (0.9, 1.5) | 41 | 0.9 (0.6, 1.4) |
| Q3 | 2485 | 439 | 1.0 (0.9, 1.2) | 267 | 1.1 (0.9, 1.3) | 126 | 1.0 (0.7, 1.3) | 46 | 0.9 (0.6, 1.5) |
| Q4 (highest) | 2551 | 535 | 1.2 (1.0, 1.3) | 302 | 1.2 (0.9, 1.4) | 170 | 1.2 (0.9, 1.5) | 63 | 1.1 (0.7, 1.7) |
| Missing | 1 | ||||||||
| Low education | |||||||||
| Q1 (lowest) | 2487 | 381 | Ref | 232 | Ref | 121 | Ref | 28 | Ref |
| Q2 | 2527 | 415 | 1.0 (0.9, 1.2) | 239 | 1.0 (0.8, 1.2) | 124 | 0.9 (0.7, 1.2) | 52 | 1.6 (1.0, 2.6) |
| Q3 | 2503 | 504 | 1.2 (1.0, 1.4) | 317 | 1.3 (1.1, 1.6) | 138 | 1.0 (0.7, 1.3) | 49 | 1.3 (0.8, 2.2) |
| Q4 (highest) | 2511 | 529 | 1.1 (0.9, 1.3) | 297 | 1.1 (0.9, 1.4) | 172 | 1.0 (0.7, 1.3) | 60 | 1.3 (0.8, 2.3) |
| Poverty | |||||||||
| Q1 (lowest) | 2506 | 369 | Ref | 213 | Ref | 123 | Ref | 33 | Ref |
| Q2 | 2507 | 461 | 1.2 (1.0, 1.4) | 279 | 1.3 (1.1, 1.6) | 132 | 1.0 (0.8, 1.3) | 50 | 1.3 (0.8, 2.1) |
| Q3 | 2506 | 495 | 1.2 (1.0, 1.4) | 294 | 1.3 (1.1, 1.6) | 151 | 1.0 (0.8, 1.3) | 50 | 1.1 (0.7, 1.8) |
| Q4 (highest) | 2508 | 504 | 1.1 (0.9, 1.3) | 299 | 1.3 (1.0, 1.6) | 149 | 0.8 (0.6, 1.1) | 56 | 1.0 (0.6, 1.7) |
| Missing | 1 | ||||||||
| Public assistance | |||||||||
| Q1 (lowest) | 2506 | 426 | Ref | 245 | Ref | 146 | Ref | 35 | Ref |
| Q2 | 2505 | 440 | 1.0 (0.9, 1.2) | 269 | 1.1 (0.9, 1.3) | 125 | 0.8 (0.7, 1.1) | 46 | 1.3 (0.8, 2.0) |
| Q3 | 2509 | 441 | 1.0 (0.8, 1.1) | 259 | 1.0 (0.9, 1.2) | 130 | 0.8 (0.6, 1.0) | 52 | 1.3 (0.9, 2.1) |
| Q4 (highest) | 2507 | 521 | 1.0 (0.9, 1.2) | 312 | 1.2 (1.0, 1.4) | 153 | 0.8 (0.6, 1.1) | 56 | 1.2 (0.7, 1.8) |
| Missing | 1 | 1 | 1 | ||||||
| Low income | |||||||||
| Q1 (lowest) | 2506 | 369 | Ref | 211 | Ref | 125 | Ref | 33 | Ref |
| Q2 | 2506 | 472 | 1.2 (1.1, 1.4) | 295 | 1.4 (1.1, 1.7) | 133 | 1.0 (0.8, 1.3) | 44 | 1.2 (0.8, 1.9) |
| Q3 | 2508 | 487 | 1.2 (1.0, 1.4) | 276 | 1.3 (1.0, 1.5) | 155 | 1.0 (0.8, 1.3) | 56 | 1.4 (0.9, 2.1) |
| Q4 (highest) | 2507 | 500 | 1.2 (1.0, 1.4) | 303 | 1.4 (1.1, 1.7) | 141 | 0.8 (0.6, 1.1) | 56 | 1.2 (0.7, 1.9) |
| Missing | 1 | 1 | 1 | ||||||
Census tract measures were obtained from either the 2000 decennial census, the 2005–2009 5-year American Community Survey estimates, or the 2010–2014 5-year American Community Survey estimates. More information is detailed in the methods.
Adjusted on maternal race/ethnicity, maternal age at delivery, and maternal education; census tract was included as a correlation matrix.
aOR indicates adjusted odds ratio; CI, confidence interval; NDI, neighborhood deprivation index; NTD, neural tube defect.
The impact of neighborhood deprivation on any NTD varied by race–ethnicity (Table 3; eTable 4; http://links.lww.com/EDE/C56). Associations for non-Hispanic White women were similar to the overall results with less precision. The NDI was associated with any NTD for Q3 compared with Q1 of NDI (aOR = 1.3; 95% CI = 1.1, 1.6). The second quartile of NDI also had an elevated aOR. The associations between census tract level SES measures for non-Hispanic Black women and Hispanic women were less precise when compared with the overall results. Non-Hispanic Black women had elevated estimates for Q2 and Q4 of NDI (Q2: aOR = 1.6; 95% CI = 0.8, 3.2; Q4: aOR = 1.6; 95% CI = 0.8, 3.0), while Hispanic mothers had ORs around the null for all quartiles.
TABLE 3.
Adjusted Associations Between Maternal Periconceptional Census Tract Socioeconomic Measures and Any Neural Tube Defect (NtD), Stratified by Maternal Race/Ethnicity, National Birth Defects Prevention Study, 1997–2011
| Census Tract Level Measurea | Non-Hispanic White | Non-Hispanic Black | Hispanic | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls | Any NTD | Controls | Any NTD | Controls | Any NTD | ||||
| n | n | aOR (95% CI)b | n | n | aOR (95% CI)b | n | n | aOR (95% CI)b | |
| NDI total | |||||||||
| NDI | |||||||||
| Q1 (lowest) | 2064 | 283 | Ref | 101 | 14 | Ref | 143 | 35 | Ref |
| Q2 | 1845 | 309 | 1.2 (1.0, 1.4) | 200 | 39 | 1.6 (0.8, 3.2) | 297 | 77 | 1.0 (0.6, 1.6) |
| Q3 | 1466 | 280 | 1.3 (1.1, 1.6) | 336 | 40 | 1.0 (0.5, 2.0) | 556 | 147 | 1.0 (0.7, 1.6) |
| Q4 (highest) | 560 | 92 | 1.1 (0.9, 1.5) | 434 | 75 | 1.6 (0.8, 3.0) | 1383 | 319 | 0.9 (0.6, 1.3) |
| Missing | 1 | 2 | |||||||
| NDI components: | |||||||||
| Crowding | |||||||||
| Q1 (lowest) | 1974 | 273 | Ref | 199 | 29 | Ref | 153 | 29 | Ref |
| Q2 | 1882 | 293 | 1.1 (0.9, 1.3) | 234 | 40 | 1.2 (0.7, 2.0) | 236 | 62 | 1.3 (0.8, 2.2) |
| Q3 | 1458 | 283 | 1.4 (1.1, 1.6) | 357 | 56 | 1.2 (0.7, 1.9) | 529 | 147 | 1.5 (0.9, 2.3) |
| Q4 (highest) | 621 | 115 | 1.3 (1.0, 1.6) | 282 | 43 | 1.1 (0.7, 1.7) | 1461 | 340 | 1.2 (0.8, 1.8) |
| Missing | 1 | 1 | |||||||
| Female-headed households | |||||||||
| Q1 (lowest) | 2069 | 298 | Ref | 72 | 8 | Ref | 223 | 63 | Ref |
| Q2 | 1744 | 314 | 1.2 (1.0, 1.5) | 142 | 17 | 1.1 (0.5, 2.7) | 451 | 118 | 0.9 (0.7, 1.3) |
| Q3 | 1241 | 223 | 1.2 (1.0, 1.5) | 263 | 34 | 1.2 (0.5, 2.7) | 840 | 186 | 0.8 (0.6, 1.1) |
| Q4 (highest) | 881 | 129 | 1.0 (0.8, 1.2) | 595 | 109 | 1.8 (0.8, 3.8) | 865 | 211 | 0.9 (0.6, 1.2) |
| Missing | 1 | 1 | |||||||
| Males in management | |||||||||
| Q1 (lowest) | 840 | 163 | Ref | 401 | 61 | Ref | 1138 | 273 | Ref |
| Q2 | 1567 | 266 | 0.9 (0.7, 1.1) | 296 | 56 | 1.2 (0.8, 1.7) | 515 | 131 | 1.1 (0.8, 1.4) |
| Q3 | 1727 | 296 | 0.9 (0.7, 1.1) | 225 | 31 | 0.8 (0.5, 1.3) | 397 | 96 | 1.1 (0.8, 1.4) |
| Q4 (highest) | 1801 | 240 | 0.7 (0.6, 0.9) | 150 | 20 | 0.8 (0.5, 1.3) | 329 | 78 | 1.0 (0.7, 1.3) |
| Missing | 1 | ||||||||
| Unemployment | |||||||||
| Q1 (lowest) | 1974 | 302 | Ref | 110 | 16 | Ref | 224 | 48 | Ref |
| Q2 | 1739 | 295 | 1.1 (0.9, 1.3) | 232 | 38 | 1.2 (0.6, 2.2) | 404 | 110 | 1.2 (0.9, 1.8) |
| Q3 | 1385 | 224 | 1.0 (0.9, 1.3) | 277 | 38 | 0.9 (0.5, 1.8) | 662 | 142 | 1.0 (0.7, 1.4) |
| Q4 (highest) | 837 | 144 | 1.1 (0.9, 1.4) | 453 | 76 | 1.2 (0.7, 2.2) | 1089 | 278 | 1.1 (0.8, 1.6) |
| Missing | 1 | ||||||||
| Low education | |||||||||
| Q1 (lowest) | 2003 | 293 | Ref | 126 | 20 | Ref | 157 | 41 | Ref |
| Q2 | 1825 | 277 | 1.0 (0.8, 1.2) | 253 | 46 | 1.3 (0.7, 2.3) | 273 | 63 | 0.9 (0.5, 1.3) |
| Q3 | 1457 | 279 | 1.2 (1.0, 1.5) | 376 | 48 | 0.9 (0.5, 1.6) | 523 | 142 | 1.0 (0.7, 1.5) |
| Q4 (highest) | 650 | 116 | 1.1 (0.9, 1.4) | 318 | 54 | 1.3 (0.7, 2.3) | 1426 | 332 | 0.9 (0.6, 1.2) |
| Poverty | |||||||||
| Q1 (lowest) | 2059 | 293 | Ref | 117 | 17 | Ref | 155 | 39 | Ref |
| Q2 | 1798 | 299 | 1.1 (1.0, 1.4) | 214 | 42 | 1.3 (0.7, 2.5) | 317 | 94 | 1.1 (0.7, 1.8) |
| Q3 | 1414 | 260 | 1.2 (1.0, 1.5) | 329 | 43 | 1.0 (0.5, 1.8) | 616 | 155 | 0.9 (0.6, 1.4) |
| Q4 (highest) | 664 | 113 | 1.1 (0.9, 1.4) | 412 | 66 | 1.2 (0.7, 2.3) | 1291 | 290 | 0.9 (0.6, 1.3) |
| Missing | 1 | ||||||||
| Public assistance | |||||||||
| Q1 (lowest) | 1759 | 281 | Ref | 209 | 38 | Ref | 325 | 77 | Ref |
| Q2 | 1736 | 288 | 1.0 (0.9, 1.2) | 216 | 23 | 0.6 (0.3, 1.0) | 407 | 104 | 1.1 (0.8, 1.5) |
| Q3 | 1574 | 247 | 0.9 (0.8, 1.1) | 256 | 48 | 1.1 (0.7, 1.8) | 555 | 119 | 0.9 (0.7, 1.2) |
| Q4 (highest) | 866 | 148 | 1.0 (0.8, 1.2) | 391 | 59 | 0.9 (0.6, 1.4) | 1092 | 278 | 1.1 (0.8, 1.4) |
| Missing | 1 | 1 | |||||||
| Low income | |||||||||
| Q1 (lowest) | 1919 | 263 | Ref | 147 | 24 | Ref | 225 | 56 | Ref |
| Q2 | 1724 | 286 | 1.2 (1.0, 1.4) | 236 | 39 | 1.0 (0.6, 1.7) | 372 | 113 | 1.2 (0.8, 1.7) |
| Q3 | 1425 | 255 | 1.3 (1.0, 1.5) | 279 | 48 | 1.1 (0.6, 1.8) | 660 | 154 | 0.9 (0.7, 1.3) |
| Q4 (highest) | 867 | 160 | 1.3 (1.0, 1.6) | 410 | 57 | 1.0 (0.6, 1.6) | 1122 | 255 | 0.9 (0.6, 1.3) |
| Missing | 1 | 1 | |||||||
Census tract measures were obtained from either the 2000 decennial census, the 2005–2009 5-year American Community Survey estimates, or the 2010–2014 5-year American Community Survey estimates. More information is detailed in the methods.
Adjusted for maternal age at delivery and maternal education; census tract was included as a correlation matrix.
aOR indicates adjusted odds ratio; CI, confidence interval; NDI, neighborhood deprivation index; NTD, neural tube defect; OR, odds ratio.
As a sensitivity analysis, we further adjusted the race–ethnicity stratified NDI models for the racial composition of the census tract via two separate models (percent non-White living in the census tract and percent African American living in the census tract). These additional covariates did not affect the results for non-Hispanic White women and Hispanic women but resulted in attenuated estimates for non-Hispanic Black women (eTable 5; http://links.lww.com/EDE/C56). Restricting to centers that ascertained terminations did not result in meaningfully different estimates (data not shown).
DISCUSSION
This study investigated the relationship between residing in a census tract with higher deprivation during early pregnancy and the risk of NTDs. Neighborhood deprivation was measured by the creation of the NDI. This index is frequently used to operationalize neighborhood SES and contains the contributions of eight SES components. If a woman’s residence during early pregnancy was in a census tract with higher deprivation scores, there were higher odds of an NTD in the offspring, spina bifida in particular. We observed elevated ORs for anencephaly and encephalocele; however, these estimates had CIs that included the null after adjustments. For any NTD and spina bifida, specifically, we saw elevated ORs for the following census tract SES measures: crowded households, female-headed households with dependents, unemployment, low education, poverty, and low income. The percentage of males in management and professional occupations tended toward a negative association with NTDs across all NTD categories.
Two studies from California that investigated census block group measures found contradictory results. Wasserman et al.16 conducted a case–control study of a subset of California births between 1989 and 1991 and reported that lower neighborhood SES status in early pregnancy was associated with NTDs. These groups found calculated SES index to be associated with NTDs with a clear risk gradient. Our study is not directly comparable because only some SES measures overlapped (i.e., low education, unemployment, poverty, and crowding), differences in project methods (e.g., in-person interviews), and the NDI was not yet available for the Wasserman et al. analysis. We also observed an increased risk of NTDs for women living in more deprived areas, but our risk estimates for the NDI did not increase on a gradient. Grewal et al.12 more recently updated the Wasserman et al. analysis using California births from 1999 to 2003. They did not observe a relationship between neighborhood SES and NTDs. However, mandatory folic acid fortification began in the United States in 1998, after the Wasserman study, and the overall impact of mandatory fortification on the prevalence of NTDs in the United States might explain some of the differences in these studies.28,29
Two additional studies outside of the United States also investigated the impact of neighborhood SES and NTDs. A cohort study from Ontario investigated hospital births during 1994–2009.11 These studies found lower census tract income (relative risk [RR] = 1.29; CI = 1.15, 1.34) and educational attainment (RR = 1.25; CI = 1.14, 1.37) to be associated with an increased risk of having an infant born with an NTD. This is similar to the results that we observed for low education and low income. Finally, a case–control study from the United Kingdom (UK) investigated census enumeration district deprivation and congenital malformations among births during 1986–1993.17 When comparing the most deprived versus the most affluent areas as measured by the UK Carstairs index, the investigators observed no associations for NTDs. They were limited, however, by their sample size (n = 107 NTD cases).
Neighborhood SES has been theorized to influence health through both the physical and social environments.15 The physical environment could impact NTD risk due to environmental exposures, such as increased pollution, and lack of access to quality nutrition and healthcare services. Exposure to certain environmental pollutants in early gestation has been shown to contribute to the development of NTDs30 and this association may be mediated by neighborhood socioeconomic factors.31,32 Lack of access to quality nutrition could impact dietary folic acid consumption—a known factor associated with lower NTD risk. In addition, women from low SES groups are less likely to use folic acid supplements11,33 perhaps due to inadequate access to quality health care services before pregnancy, lower educational resources, or ability to purchase. The social environment could impact NTD risk as social norms may be different in more deprived areas leading to more acceptance of negative health behaviors. Both alcohol intake and exposure to tobacco smoke have been associated with NTDs.34,35 Both the social environment and physical environment could impact maternal stress via concerns around safety/violence, lack of social support, and housing quality. Self-reported maternal stressful events during the periconceptional period have been associated with NTDs.36 Women who live in more deprived neighborhoods during pregnancy may have experienced a greater amount of economic deprivation in their lifetime. This accumulation of stress and other factors may also lead to an increased NTD risk that is not directly measured in our study.
In our study, the specific census tract level SES measures, and the cumulative NDI, were shown to have differing magnitudes of risk of any NTD by race–ethnicity. In this analysis, race–ethnicity is serving as a proxy for other social, environmental, and structural factors that we cannot measure in our data rather than an indicator of biological differences.37 Estimates among non-Hispanic White women of any NTD and the SES measures were similar—with some loss of precision—to the overall results. Estimates of the association between NDI and any NTD were attenuated among non-Hispanic Black and Hispanic women when compared with the overall results. This may be due to the sample size and composition of the study population, as 58% were non-Hispanic White, 10% were non-Hispanic Black, and 25% were Hispanic. Living in a racially incongruous neighborhood has been shown to amplify the impact of low SES and birth outcomes38; however, we did not find meaningful differences when adjusting for the racial composition of the census tract. Previous studies of neighborhood deprivation and NTD either did not find differences by race–ethnicity16 or did not report estimates stratified by race–ethnicity.11,12,17 In addition, the risk of NTDs among non-Hispanic Black and Hispanic populations may be driven by factors that were not measured by the NDI. For example, there have been reported differences in folic acid awareness and supplement use by maternal race and ethnicity.39-41
Other research has demonstrated the importance of considering the impact of SES measures by race–ethnicity. The Minorities Diminished Returns framework, which was created using three national longitudinal cohort studies with results generalizable to the United States population, suggests that indicators of high SES show weaker protective associations with health outcomes for Black families compared with White families.42 This may be due to smaller health gain from economic resources, such as education and employment, and psychological assets. The disparity in health outcomes in the United States persists even when there is equal access to resources and assets. When investigating birth outcomes, it has been shown that low neighborhood SES is only associated with preterm birth among White women43 or that the impact is attenuated for Black women.18,25 There is evidence that Hispanic populations may have better health outcomes, despite any lower SES measures.44 This may be due to cultural values that lead to greater social support and lower acceptance of negative health behaviors during pregnancy. Our study might provide support to this theory as we did not see an association between residence in a neighborhood with more deprivation and NTDs among Hispanic women.
Our study was subject to a number of limitations. While our overall study population was large, the stratified analyses were limited by the small number of cases in the non-Hispanic Black and Hispanic strata. We also did not have the statistical power necessary to investigate NTD categories by maternal race–ethnicity. Overall participation in the NBDPS was 67% for cases and 65% for controls.22 It is possible that participants and nonparticipants could have derived from neighborhoods with different levels of deprivation and this could be differential by case status. Neighborhood SES measures were at the census tract level, which includes between 2500 and 8000 people. There is the possibility of heterogeneity of deprivation within the census tract. Actual neighborhoods may also have different boundaries that cross census tract lines. Additionally, the level of deprivation in the census tracts may have changed over time. The United States Census did not obtain census tract level estimates of socioeconomic measures between the 2000 decennial census conclusion and the beginning of the ACS in 2005.45 The level of deprivation in the census tracts may have changed over the unmeasured time period.
This study had a number of strengths. NBDPS is a large, multisite, population-based study with rigorous methodology for case classification. The study population includes stillbirths and induced terminations, which are vital for studying more severe defects including certain NTDs.1 Other studies have been limited to using addresses listed at birth, which may not reflect the residential neighborhood during the critical period of fetal development.11,17 This analysis was strengthened by the ability to link census tract to the residence during the periconceptional period; this linkage may reduce nondifferential exposure misclassification due to moving during pregnancy.46 This study was strengthened by the high percentage of participants for whom the address could be linked to a census tract. Only 3% of case and control mothers could not be linked to a census tract and this did not meaningfully vary by maternal demographics.
In conclusion, this study of NBDPS participants showed that the association between individual census tract level SES measures and NTDs varied; however, the NDI was associated with any NTD, and spina bifida in particular, across all quartiles of neighborhood deprivation. When we stratified by maternal race–ethnicity, consistent associations across all quartiles remained among non-Hispanic White mothers, lending credence to the Minorities Diminished Returns framework. This study is an important contribution to the literature about the association between neighborhood SES and risk of NTDs. There have been few published studies in the United States investigating this relationship and there is more work to be done in this space to better understand the role of neighborhood deprivation. These results highlight the importance of considering maternal race–ethnicity when examining the impact of neighborhood deprivation during pregnancy.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a cooperative agreement from the Centers for Disease Control and Prevention.
This project was supported through Centers for Disease Control and Prevention (CDC) cooperative agreements under PA #96043, PA #02081, FOA #DD09-001, FOA #DD13-003, and NOFO #DD18-001 to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study (NBDPS) and/or the Birth Defects Study To Evaluate Pregnancy exposureS (BD-STEPS).
Footnotes
A supplemental video abstract is available online (http://links.lww.com/EDE/C71).
The authors report no conflicts of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
The process for accessing the data used in this analysis is described here: https://www.cdc.gov/ncbddd/birthdefects/nbdps-public-access-procedures.html. Computing code may be requested from the corresponding author. This analysis has been replicated by coauthor E.P.
REFERENCES
- 1.Avagliano L, Massa V, George TM, Qureshy S, Bulfamante GP, Finnell RH. Overview on neural tube defects: from development to physical characteristics. Birth Defects Res. 2019;111:1455–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams J, Mai CT, Mulinare J, et al. ; Centers for Disease Control and Prevention. Updated estimates of neural tube defects prevented by mandatory folic acid fortification - United States, 1995-2011. MMWR Morb Mortal Wkly Rep. 2015;64:1–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Blencowe H, Kancherla V, Moorthie S, Darlison MW, Modell B. Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis. Ann N Y Acad Sci. 2018;1414:31–46. [DOI] [PubMed] [Google Scholar]
- 4.Canfield MA, Mai CT, Wang Y, et al. ; National Birth Defects Prevention Network. The association between race/ethnicity and major birth defects in the United States, 1999-2007. Am J Public Health. 2014;104:e14–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frey L, Hauser WA. Epidemiology of neural tube defects. Epilepsia. 2003;44:4–13. [DOI] [PubMed] [Google Scholar]
- 6.Copp AJ, Stanier P, Greene ND. Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol. 2013;12:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endalifer ML, Diress G. Epidemiology and determinant factors of neural tube defect: narrative review. Surg Neurol Int. 2020;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Langlois PH, Mitchell LE, Agopian AJ. Maternal occupation and the risk of neural tube defects in offspring. Arch Environ Occup Health. 2018;73:304–312. [DOI] [PubMed] [Google Scholar]
- 9.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force. Folic acid supplementation for the prevention of neural tube defects: US preventive services task force recommendation statement. JAMA. 2017;317:183–189. [DOI] [PubMed] [Google Scholar]
- 10.Murphy ME, Westmark CJ. Folic acid fortification and neural tube defect risk: analysis of the food fortification initiative dataset. Nutrients. 2020;12:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agha MM, Glazier RH, Moineddin R, Moore AM, Guttmann A. Food fortification and decline in the prevalence of neural tube defects: does public intervention reduce the socioeconomic gap in prevalence?. Int J Environ Res Public Health. 2013;10:1312–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grewal J, Carmichael SL, Song J, Shaw GM. Neural tube defects: an analysis of neighbourhood- and individual-level socio-economic characteristics. Paediatr Perinat Epidemiol. 2009;23:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olesen C, Thrane N, Ronholt AM, Olsen J, Henriksen TB. Association between social position and congenital anomalies: a population-based study among 19,874 Danish women. Scand J Public Health. 2009;37:246–251. [DOI] [PubMed] [Google Scholar]
- 14.Rosano A, Del Bufalo E, Burgio A. [Socioeconomic status and risk of congenital malformations]. Epidemiol Prev. 2008;32:21–26. [PubMed] [Google Scholar]
- 15.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 16.Wasserman CR, Shaw GM, Selvin S, Gould JB, Syme SL. Socioeconomic status, neighborhood social conditions, and neural tube defects. Am J Public Health. 1998;88:1674–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vrijheid M, Dolk H, Stone D, Abramsky L, Alberman E, Scott JE. Socioeconomic inequalities in risk of congenital anomaly. Arch Dis Child. 2000;82:349–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83:1041–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekeke P, Mendez DD, Yanowitz TD, Catov JM. Racial differences in the biochemical effects of stress in pregnancy. Int J Environ Res Public Health. 2020;17:6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elo IT, Culhane JF, Kohler IV, et al. Neighbourhood deprivation and small-for-gestational-age term births in the United States. Paediatr Perinat Epidemiol. 2009;23:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janevic T, Stein CR, Savitz DA, Kaufman JS, Mason SM, Herring AH. Neighborhood deprivation and adverse birth outcomes among diverse ethnic groups. Ann Epidemiol. 2010;20:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reefhuis J, Gilboa SM, Anderka M, et al. ; National Birth Defects Prevention Study. The national birth defects prevention study: a review of the methods. Birth Defects Res A Clin Mol Teratol. 2015;103:656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA; National Birth Defects Prevention Study. Guidelines for case classification for the national birth defects prevention study. Birth Defects Res A Clin Mol Teratol. 2003;67:193–201. [DOI] [PubMed] [Google Scholar]
- 24.McGuinn LA, Windham GC, Messer LC, et al. Air pollution, neighborhood deprivation, and autism spectrum disorder in the study to explore early development. Environ Epidemiol. 2019;3:e067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Campo P, Burke JG, Culhane J, et al. Neighborhood deprivation and preterm birth among non-Hispanic black and white women in eight geographic areas in the United States. Am J Epidemiol. 2007;167:155–163. [DOI] [PubMed] [Google Scholar]
- 26.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package “dagitty.”. Int J Epidemiol. 2016;45:1887–1894. [DOI] [PubMed] [Google Scholar]
- 27.Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. The Am Stat. 2016;70:129–133. [Google Scholar]
- 28.Williams LJ, Mai CT, Edmonds LD, et al. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology. 2002;66:33–39. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Spina bifida and anencephaly before and after folic acid mandate--United States, 1995-1996 and 1999-2000. MMWR Morb Mortal Wkly Rep. 2004;53:362–365. [PubMed] [Google Scholar]
- 30.Ren A. Chapter 61 - Environmental pollutants and neural tube defects. In: Gupta RC, ed. Reproductive and Developmental Toxicology (Third Edition). Academic Press; 2022:1221–1243. [Google Scholar]
- 31.Padula AM, Yang W, Carmichael SL, et al. Air pollution, neighborhood acculturation factors, and neural tube defects among Hispanic women in California. Birth Defects Res. 2017;109:403–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Padula AM, Yang W, Carmichael SL, et al. Air Pollution, neighbourhood socioeconomic factors, and neural tube defects in the San Joaquin valley of California. Paediatr Perinat Epidemiol. 2015;29:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Pal-de Bruin KM, de Walle HE, de Rover CM, et al. Influence of educational level on determinants of folic acid use. Paediatr Perinat Epidemiol. 2003;17:256–263. [DOI] [PubMed] [Google Scholar]
- 34.Wang M, Wang ZP, Gong R, Zhao ZT. Maternal smoking during pregnancy and neural tube defects in offspring: a meta-analysis. Childs Nerv Syst. 2014;30:83–89. [DOI] [PubMed] [Google Scholar]
- 35.Grewal J, Carmichael SL, Ma C, Lammer EJ, Shaw GM. Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies. Birth Defects Res A Clin Mol Teratol. 2008;82:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmichael SL, Ma C, Tinker S, Rasmussen SA, Shaw GM; National Birth Defects Prevention Study. Maternal stressors and social support as risks for delivering babies with structural birth defects. Paediatr Perinat Epidemiol. 2014;28:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adkins-Jackson PB, Chantarat T, Bailey ZD, Ponce NA. Measuring structural racism: a guide for epidemiologists and other health researchers. Am J Epidemiol. 2022;191:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kothari CL, Paul R, Dormitorio B, et al. The interplay of race, socioeconomic status and neighborhood residence upon birth outcomes in a high black infant mortality community. SSM Popul Health. 2016;2:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukhtar A, Kramer MR, Oakley GP Jr., Kancherla V. Race and ethnicity and preconception folic acid supplement use among pregnant women in Georgia, PRAMS 2009 to 2011. Birth Defects Res. 2017;109:38–48. [DOI] [PubMed] [Google Scholar]
- 40.Marchetta CM, Hamner HC. Blood folate concentrations among women of childbearing age by race/ethnicity and acculturation, NHANES 2001-2010. Matern Child Nutr. 2016;12:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chacko MR, Anding R, Kozinetz CA, Grover JL, Smith PB. Neural tube defects: knowledge and preconceptional prevention practices in minority young women. Pediatrics. 2003;112:536–542. [DOI] [PubMed] [Google Scholar]
- 42.Assari S. Unequal gain of equal resources across racial groups. Int J Health Policy Manag. 2018;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunlop AL, Essalmi AG, Alvalos L, et al. ; Program Collaborators for Environmental Influences on Child Health Outcomes. Racial and geographic variation in effects of maternal education and neighborhood-level measures of socioeconomic status on gestational age at birth: findings from the ECHO cohorts. PLoS One. 2021;16:e0245064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Markides KS, Eschbach K. Aging, migration, and mortality: current status of research on the Hispanic paradox. J Gerontol B Psychol Sci Soc Sci. 2005;60 Spec No 2:68–75. [DOI] [PubMed] [Google Scholar]
- 45.American Community Survey Information Guide 2017; Census.gov.
- 46.Canfield MA, Ramadhani TA, Langlois PH, Waller DK. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. J Expo Sci Environ Epidemiol. 2006;16:538–543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.