Summary
Background
Animal models of serious infection suggest that 24 h of induced hypothermia improves circulatory and respiratory function and reduces mortality. We tested the hypothesis that a reduction of core temperature to 32–34°C attenuates organ dysfunction and reduces mortality in ventilator-dependent patients with septic shock.
Methods
In this randomised, controlled, open-label trial, we recruited patients from ten intensive care units (ICUs) in three countries in Europe and North America. Inclusion criteria for patients with severe sepsis or septic shock were a mean arterial pressure of less than 70 mm Hg, mechanical ventilation in an ICU, age at least 50 years, predicted length of stay in the ICU at least 24 h, and recruitment into the study within 6 h of fulfilling inclusion criteria. Exclusion criteria were uncontrolled bleeding, clinically important bleeding disorder, recent open surgery, pregnancy or breastfeeding, or involuntary psychiatric admission. We randomly allocated patients 1:1 (with variable block sizes ranging from four to eight; stratified by predictors of mortality, age, Acute Physiology and Chronic Health Evaluation II score, and study site) to routine thermal management or 24 h of induced hypothermia (target 32–34°C) followed by 48 h of normothermia (36–38°C). The primary endpoint was 30 day all-cause mortality in the modified intention-to-treat population (all randomly allocated patients except those for whom consent was withdrawn or who were discovered to meet an exclusion criterion after randomisation but before receiving the trial intervention). Patients and health-care professionals giving the intervention were not masked to treatment allocation, but assessors of the primary outcome were. This trial is registered with ClinicalTrials.gov, number NCT01455116.
Findings
Between Nov 1, 2011, and Nov 4, 2016, we screened 5695 patients. After recruitment of 436 of the planned 560 participants, the trial was terminated for futility (220 [50%] randomly allocated to hypothermia and 216 [50%] to routine thermal management). In the hypothermia group, 96 (44·2%) of 217 died within 30 days versus 77 (35·8%) of 215 in the routine thermal management group (difference 8·4% [95% CI –0·8 to 17·6]; relative risk 1·2 [1·0–1·6]; p=0·07]).
Interpretation
Among patients with septic shock and ventilator-dependent respiratory failure, induced hypothermia does not reduce mortality. Induced hypothermia should not be used in patients with septic shock.
Funding
Trygfonden, Lundbeckfonden, and the Danish National Research Foundation.
Introduction
Septic shock is an acute life-threatening condition caused by a deleterious, non-resolving host response to pathogenic microorganisms that leads to organ dysfunction.1 Key pathophysiological aspects include endothelial dysfunction, vasodilation, coagulopathy, mitochondrial breakdown, and consequent organ failure.2 Respiratory failure requiring mechanical ventilation is a feared complication of septic shock and leads to high mortality.3 Sepsis remains a leading cause of death in hospitals,4 and multiple attempts to improve prognosis have been unsuccessful in recent decades.5–8
In rodents, induced hypothermia for sepsis (in the range of 31–34°C maintained for 24–72 h) has been associated with a substantial mortality reduction.9–11 The benefit of induced hypothermia appears to be a result of reduced sepsis-related damage to the lungs,12 heart,12,13 and liver.14 At the cellular level, improved intracellular metabolism has been observed in a pneumococcal challenge model, along with reduced dissemination of the infection to other organs in cooled animals.15 In rabbits challenged with bacteraemia, pyrexia has been associated with improved survival.16 Para doxically, physical cooling to reduce fever in a similar experiment improved survival.17 However, in human beings, spontaneous hypothermia in sepsis is associated with persistent lymphopenia and a worse prognosis.18
In human beings, fever prevention with antipyretic drugs does not improve organ function or survival in critically ill patients with severe infections.19 However, in a trial of 200 febrile patients in septic shock,20 external cooling to normothermia reduced the need for vasoactive therapy. Data from a small uncontrolled study21 of induced hypothermia in patients with sepsis and respiratory failure also suggested improved cardiac physiology and survival. On the basis of animal evidence and few human studies, induced hypothermia has been used as a treatment of serious infections for decades,22,23 although no convincing evidence exists that induced hypothermia improves survival in human septic shock.
In this trial, we decided to only recruit patients aged 50 years or older because of power concerns because we noted a low mortality rate among young patients with septic shock in a previous trial.24 When the intervention was designed, several members of the steering committee with experience in this field mentioned the challenge of rebound fever after therapeutic hypothermia. This phenomenon was estimated to be rather frequent and far from negligible, and the potential harm from severe hyperthermia was considered as a possible limitation of the intervention: if some patients would benefit from the intervention and the same or other patients would be harmed from rebound fever, interpretation of the trial results might eventually be compromised. The steering committee decided on a two-phased intervention to avoid rebound fever: 24 h of induced hypothermia followed by 48 h of fever control or normothermia. While our trial was underway, other studies25,26 found that rebound fever was frequent in patients with cardiac arrest, occurring in approximately 30–40% of patients. We defined normothermia in this study as a temperature in the range of 36–38°C as defined by others.27
The question of whether to aim for a hypothermia or fever control (normothermia) intervention was discussed within the steering committee: all members agreed that the rationale for induced hypothermia in this patient group was strong, as summarised by others,28 and since this intervention had never been tested in a trial setting, all members of the steering committee wanted to test this hypothesis. However, the steering committee also agreed that the rationale existed for testing fever control. Some members postulated that the effect on intracellular functions seemed to be more pronounced than fever control in hypothermia in animal studies. The possibility of a three-armed trial was discussed (no fever intervention, fever control, and induced hypothermia). However, this design would increase the required sample size substantially and would not be feasible in the planned setting since the recruitment period would be substantially extended. We were aware that a trial of fever control was already ongoing,20 so we decided to test the induced hypothermia intervention—ie, that a reduction of core temperature to 32–34°C for 24 h followed by slow rewarming and normothermia for 48 h (fever control) attenuates organ dysfunction and reduces mortality in patients with septic shock and accompanying acute respiratory failure.
Methods
Study design and participants
The Cooling And Surviving Septic Shock Study (CASS) was a randomised, controlled, open-label trial recruiting patients from ten intensive care units (ICUs) in two countries in Europe (Denmark and the Netherlands) and the USA. Patients with severe sepsis or septic shock were considered for enrolment if they had a mean arterial pressure of less than 70 mm Hg, were on mechanical ventilation in an ICU, were aged at least 50 years, were expected to stay in the ICU for more than 24 h, and could be recruited within 6 h after fulfilling all inclusion criteria. Exclusion criteria were uncontrolled bleeding, clinically important bleeding disorder (acute or chronic), recent open surgery, pregnancy or breast-feeding, or involuntary psychiatric admission.
We obtained written informed consent from patients or next of kin when possible or from two independent medical legal representatives (appendix), except in the Netherlands and the USA where the ethics board required informed consent from patients or next-of-kin in all cases. Data management and analysis were done by the Centre of Excellence for Health, Immunity and Infections, Rigshospitalet, Denmark, and University College London, London, UK. The original and final study protocol versions with a complete list of changes (inclusion of additional sites) is available in the appendix. The protocol was approved by the ethics committees at each institution.
Randomisation and masking
Enrolment, randomisation, and data entry were done via a locally developed online system. We randomly allocated patients 1:1 to induced hypothermia for 24 h and subsequent normothermia or to routine thermal management (control group). We based randomisation on computer-generated variable block sizes ranging from four to eight, stratified by age (≥65 years vs <65 years), Acute Physiology and Chronic Health Evaluation (APACHE) II score (≥25 vs <25), and study site. We chose stratification limits for APACHE II score and age according to the expected medians on the basis of a previous trial that we did.24 The randomisation sequence was prepared by the study statistician who did not take part in randomisation. Treatment allocation was concealed by our web-based system until qualifying patients consented and were ready for thermal management.
Health-care professionals taking part in the intervention were aware of treatment assignment because they were responsible for implementing the designated thermal management. However, assessors of the primary endpoint were fully masked to treatment. Investigators and steering committee members were unaware of all data until the trial concluded. Since safety of the patients was our primary concern, the data and safety monitoring board (DSMB), which was independent of the steering committee, was unmasked throughout the trial.
Procedures
In all aspects of treatment, except regarding temperature management, all patients in both groups of the trial were treated according to the most recent Surviving Sepsis Campaign29 guidelines at the time. In the routine thermal management group, no physical or pharmacological thermal interventions were permitted during the initial 24 h unless a specific indication for hypothermia treatment emerged, such as cardiac arrest. Thereafter, we allowed antipyretic drugs as part of standard treatment. In patients assigned to hypothermia, we started the thermal intervention immediately after randomisation. The target was to reduce core body temperature to 32–34°C within 2 h. We used two types of induced hypothermia intervention: external pad-based hypothermia, either with an Artic Sun device (Medivance, Louiseville, CO, USA) or Flex.Pads (Emcools, Traiskirchen, Austria), or with an intravenous catheter—ie, an Intravascular Temperature Management device (Zoll, Chelmsford, MA, USA). The intravenous catheter method was used as a backup method when other hypothermia devices were unavailable at two of the study sites. We maintained mild hypothermia for 24 h. Thereafter, we rewarmed patients to 37°C at a rate of 0·5°C per h. For the next 48 h, patients in the induced hypothermia group were kept normothermic (36–38°C), with additional cooling if necessary to prevent fever. We initiated antibiotic treatment within 1 h after severe sepsis or septic shock was diagnosed, with drug selection based on the relevant national guidelines, accounting for differences in distribution and susceptibility of the suspected causative microorganisms.
Outcomes
The primary outcome was 30 day all-cause mortality. Secondary outcomes were all-cause mortality at 180 days and length of ICU stay (total and separated between survivors and non-survivors). We considered patients discharged from the ICU not to need vasopressors, inotropics, or mechanical ventilation. A specific organ failure secondary outcome (acute respiratory failure [on or not on mechanical ventilation]) and a respiratory failure secondary outcome (the ratio of partial pressure of arterial oxygen [PaO2] to the fraction of inspired oxygen [FiO2]) were both assessed at 72 h, at the end of the two-phased intervention. Secondary outcomes relating to circulatory failure or shock were mean arterial pressure, whether or not the patient was on any vasoactive support, vasoactive-inotropic score (VIS), accumulated VIS, and whether or not the patient achieved a minimum of a 50% decrease in VIS. We estimated VIS according to Gaies and colleagues.30 Renal failure secondary outcomes were diuresis, creatinine concentration, any renal replacement therapy, and acute kidney injury according to Risk, Injury, Failure, Loss of Kidney Function, and End-Stage Kidney Disease criteria.31 Coagulation secondary outcomes were International Normalized Ratio, platelet count, a platelet count of fewer than 150 × 106 per L, and a platelet count decrease of more than 25% from baseline. Liver secondary outcomes were bilirubin concentration and a bilirubin concentration of more than 21 mmol/L. Progress of infection secondary outcomes were C-reactive protein (CRP) concentration and a CRP concentration decrease of more than 30% from baseline. Cerebral function and sedation secondary outcomes were whether or not the patient received sedatives, score on the Richmond Agitation-Sedation Scale, and whether or not the patient was diagnosed with delirium. We estimated Sequential Organ Failure Assessment score as defined,32 except that we collected data at 0600 h daily (and not as the worst value in the last 24 h). Post-hoc secondary outcomes were days alive and without mechanical ventilation within 30 days, days alive and without vasopressors or inotropics within 30 days, and days alive and without dialysis within 30 days (patients who died within 30 days were given a score of 031). The primary outcome was centrally assessed.
Statistical analysis
We estimated mortality in qualifying patients in the participating centres to be 40–56% on the basis of a data draw from participating sites and on the basis of the available literature.33,34 We did two sample size calculations to cover this range of presumed control group event proportion. First, an estimated 560 patients would provide 80% power at a two-sided α level of 0·05 to detect a relative 21% change in the primary endpoint, corresponding to a change in absolute risk from 56% to 44%. Second, this sample size, with equal power and α, also allowed us to detect a relative 28% change in the primary endpoint, corresponding to a change in absolute risk from 40% to 29%. We included no loss to follow-up in the sample-size estimate due to complete follow-up of the primary outcome in central registries.
Since no trials in human beings had previously explored hypothermia for severe sepsis or septic shock, safety was a particular concern, especially the risk of coagulopathy.35 We assessed ongoing safety at three levels. First, we planned ordinary, full-data interim analyses after recruitment of 140, 280, and 420 patients. These analyses consisted of data for baseline characteristics, the primary outcome, safety endpoints, the intervention (time to target temperature and temperature maintenance), and recruitment rates. Second, we assessed complications, with a focus on bleeding and coagulopathy, after the initial ten and 24 patients were recruited. Finally, we assessed seven organ-related outcomes on a patient-by-patient basis (cardiac or circulatory, respiratory, renal, cerebral, hepatic, coagulation, and infection). The DSMB requested an additional interim analysis at 337 patients, but this analysis was not disclosed to the Steering Committee until after study closure. For the interim analyses, we used a group-sequential design for normally distributed data, based on the approach of O’Brien and Fleming.36 For the ordinary, full-data interim analyses, we used the following terms regarding efficacy and harm: if the z value for mortality analysis was larger than the upper boundary value (efficacy) or smaller than the lower boundary (harm) at the specified interim analysis, the trial can be prematurely stopped. The z value used for stopping for efficacy at 140 patients was 3·359 versus −2·241 for harm, the values at 280 patients were 2·760 versus −2·125, and the values at 420 patients were 2·359 versus −2·019.
Regarding the planned futility analysis, at the third ordinary interim analysis, the DSMB did a formal futility analysis. Two distinct assumptions to show benefit were made about unobserved future data: first, that the under lying effect of the intervention was going to remain the same in the remaining 140 patients as the rate seen up to the third planned interim analysis and, second, that the underlying effect of induced hypothermia was as hypothesised when the trial was planned. At the actual analysis at 420 patients, the conditional power to show a benefit from induced hypothermia was effectively zero, whatever was assumed about the underlying effect of the intervention in the remaining 140 patients.
All analyses were done in the modified intention-to-treat population, defined as all randomly allocated patients except those in whom the patient or relatives withdrew consent and demanded their data be deleted and those for whom pre-existing fulfilment of exclusion criteria were dis covered after randomisation and who never received the trial intervention. Patients who had at least one major protocol violation were considered as not fulfilling the protocol and additional per-protocol analyses were done excluding these patients. A list of protocol violations is available in the appendix.
We analysed the primary endpoint with Kaplan-Meier survival curves and corresponding log-rank tests; Cox proportional hazards models, adjusted for pre stratification variables according to published principles;37 and subgroup analysis using Cox proportional hazards models with interactions tested across stratification layers. We analysed secondary endpoints of categorical variables using χ2 tests for equal proportions (or Fisher’s exact tests when events were seldom) and compared continuous outcome measures with Mann-Whitney U tests (non-normally distributed data) or Student’s t tests (normally distributed data). All analyses were done with R software version 3.02 and SAS version 9.4. Tests were two-sided and we considered p values of less than 0·05 significant. This trial is registered at ClinicalTrials.gov, number NCT01455116.
Role of the funding source
Initially, the sites financed the cooling equipment. During the trial, Bard, Emcools, and Zoll agreed to donate cooling equipment. The funders had no role in study design, data collection, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between Nov 1, 2011, and Nov 4, 2016, we screened 5695 patients (figure 1). At the third scheduled interim analysis, the independent DSMB recommended the trial to be closed for futility. At that point, 436 (78%) of a planned 560 patients had been enrolled and the conditional power for showing a positive effect of the intervention on the primary outcome was zero. 220 (50%) patients were allocated to receive mild induced hyperthermia and 216 (50%) were assigned routine thermal management. Next of kin withdrew consent for three (1%) patients (two [1%] in the induced hypothermia group and one [<1%] in the routine thermal management group). Another patient in the induced hypothermia group proved to have a severe bleeding disorder that was considered a contraindication to hypothermia. The intervention and control groups were fairly balanced at baseline, although acute renal failure seemed more frequent in the induced hypothermia group than in the routine thermal management group and the induced hypothermia group also had a lower median platelet count than did the routine thermal management group (table 1). Hypothermia was induced in 217 (99%) patients (figure 1). We used two types of induced hypothermia intervention: 202 (93%) patients received external pad-based induced hypothermia and 15 (7%) had hypothermia induced by intravenous catheter. The median time to target temperature was 3·2 h (IQR 2·2–4·8), and all but 23 (11%) patients reached the target temperature within 6 h. 26 (12%) patients did not complete 24 h of induced hypothermia and 48 h of normothermia (appendix). Figure 2 shows the temperature in the control and intervention groups during the initial 72 h after randomisation. Temperatures differed significantly between treatment groups at all times during the 24 h period of induced hypothermia, except at baseline (p<0·0001).
Figure 1: Trial profile.
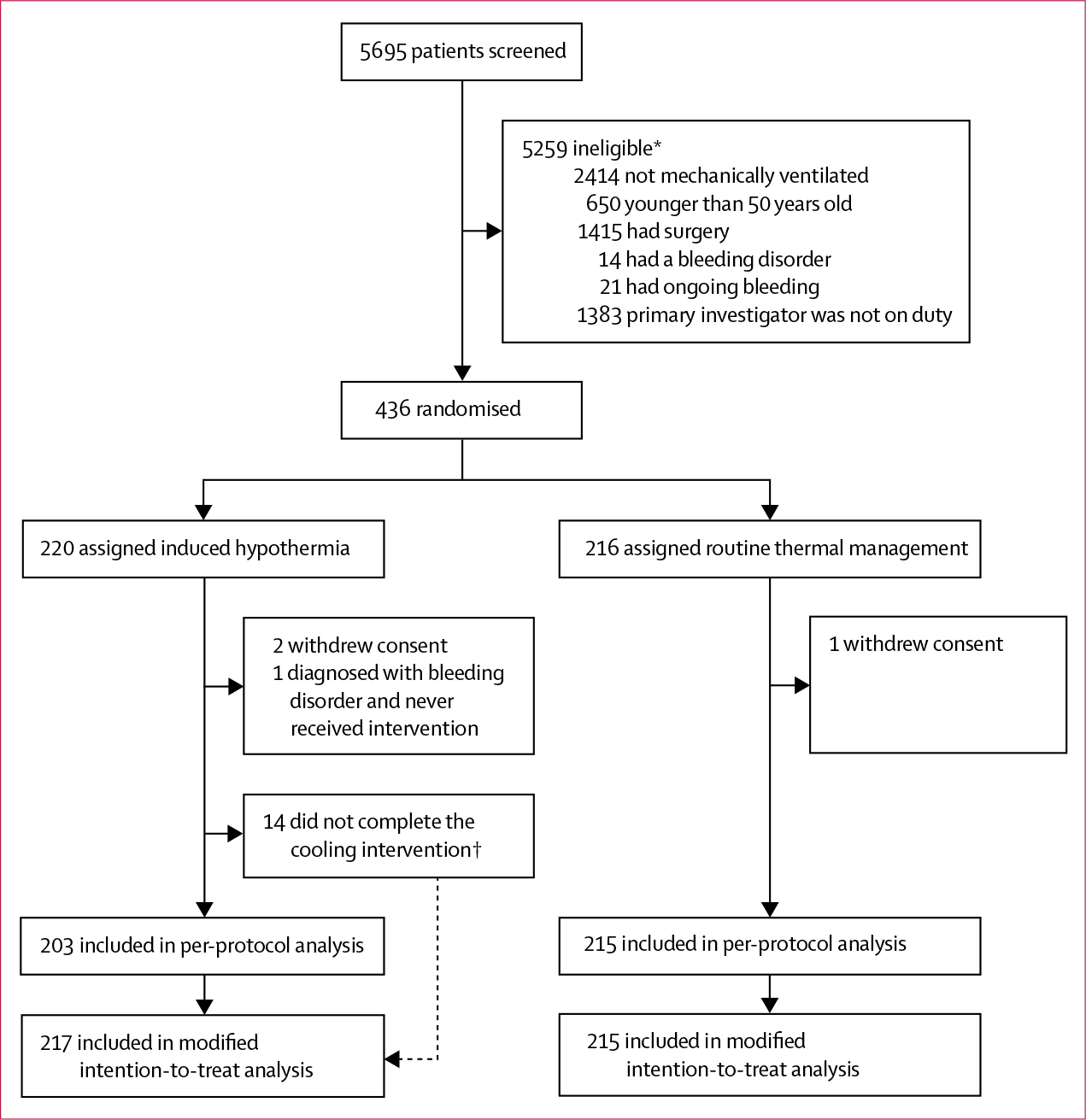
*Patients could have more than one exclusion criterion. †Causes listed in the appendix.
Table 1:
Baseline characteristics
| Routine thermal management (n=215) | Induced hypothermia (n=217) | |
|---|---|---|
|
| ||
| Age (years) | 71 (65–77) | 70 (64–77) |
| Female sex | 87 (40%) | 86 (40%) |
| Weight (kg) | 75 (65–85) | 80 (65–86) |
| Body-mass index | 24.7 (22.5-27.8) | 24.9 (22.2-28.1) |
| Pre-existing conditions | ||
| Chronic obstructive pulmonary disease | 56 (26%) | 55 (25%) |
| Ischaemic heart disease | 12 (6%) | 24 (11%) |
| Diabetes | 44 (20%) | 37 (17%) |
| Heart failure | 8 (4%) | 14 (6%) |
| Liver cirrhosis | 18 (8%) | 10 (5%) |
| Chronic kidney disease | 6 (3%) | 17 (8%) |
| APACHE II score | 23 (17–29) | 23 (16–29) |
| SOFA score | 12 (11–14) | 13 (11–15) |
| Acute organ failure | ||
| Cerebral failure* | 143 (67%) | 173 (80%) |
| Coagulopathy† | 91/201 (45%) | 103/205 (50%) |
| Hepatic failure‡ | 51/200 (26%) | 57/206 (28%) |
| Renal failure§ | 37/207 (18%) | 66/209 (32%) |
| Receiving supportive therapy | ||
| Sedative | 209 (97%) | 214 (99%) |
| Vasoactive medication | ||
| Dopamine | 3 (1%) | 5 (2%) |
| Adrenaline | 13 (6%) | 20 (9%) |
| Noradrenaline | 206 (96%) | 210 (97%) |
| Dobutamine | 7 (3%) | 25 (12%) |
| Mechanical ventilation | 215 (100%) | 217 (100%) |
| Renal replacement therapy | 26 (12%) | 31 (14%) |
| Physiological variables | ||
| Mean arterial pressure (mm Hg)¶ | 66 (60–75) | 65 (60–74) |
| Lactate concentration (mmol/L) | 1.8 (1.3-2.9) | 1.9 (1.3-3.4) |
| Lactate concentration of >2 mmol/L | 87/214 (41%) | 90/212 (42%) |
| pH | 7.31 (7.24-7.39) | 7.29 (7.21–7.37) |
| PaO2 to FiO2 ratio (kPa) | 19.5 (13.2-28.2) | 20 (13–27) |
| Fibrin D-dimer concentration (mg/L) | 3.3 (1.6-6.6) | 37 (1.4–6.7) |
| APTT (s) | 37 (32–46) | 37 (32–46) |
| INR | 1.3 (1.1-1.5) | 1.3 (1.1–1.6) |
| Albumin concentration (g/L) | 24 (21–29) | 25 (20–29) |
| Bilirubin concentration (μmol/L) | 11 (7–21) | 13 (7–22) |
| Alkaline phosphatase concentration (U/L) | 90 (64–150) | 88 (68–140) |
| Creatinine concentration (μmol/L) | 98 (66–162) | 132 (74–212) |
| Fluid administration (mL/kg per h) | 4.0 (2.8-6.6) | 3.9 (2.6-7.1) |
| Fluid output (mL/kg per h) | 1.4 (0.7-2.6) | 1.5 (0.7–2.7) |
| Sodium concentration (mmol/L) | 139 (134–143) | 138 (134–142) |
| Potassium concentration (mmol/L) | 4.0 (3.7–4.5) | 4.1 (3.7–4.7) |
| Ionized Ca2+ concentration (mmol/L) | 1.14 (1.07-1.22) | 1.13 (1.06–1.20) |
| Temperature (°C) | 37.3 (36.5-38.2) | 37.2 (36.2–38.0) |
| C-reactive protein concentration (mg/L) | 150 (67–266) | 168 (78–277) |
| Leucocyte count (× 109per L) | 15 (11–22) | 15 (9–21) |
| Platelet count (× 109 per L) | 240 (146–320) | 195 (136–284) |
| Haemoglobin concentration (mmol/L) | 6.6 (5.8-7.9) | 6.6 (5.8–7.5) |
| Cooling method | ||
| Internal | .. | 15 (7%) |
| External | .. | 202 (93%) |
Data are median (IQR), n (%), or n/N (%). APACHE=Acute Physiology and Chronic Health Evaluation. SOFA=Sequential Organ Failure Assessment. PaO2=partial pressure of arterial oxygen. FiO2=fraction of inspired oxygen. APTT=activated partial thromboplastin time. INR=International Normalized Ratio. Ca2+=calcium ion.
RichmondAgitation-Sedation Scale of less than or equal to −4.
INR of more than or equal to 1.5 or platelet count of fewer than 150 × 109 per L.
Bilirubin concentration of more than 21 μmol/L.
Estimated glomerular filtration rate of less than 30 mL/min per 1.73 m2 or renal replacement therapy.
Less than 70 mm Hg at screening. Patients were actively resuscitated simultaneously with inclusion in the trial; therefore, mean arterial pressure could be slightly higher after inclusion than at screening.
Figure 2: Patient temperature after randomization.
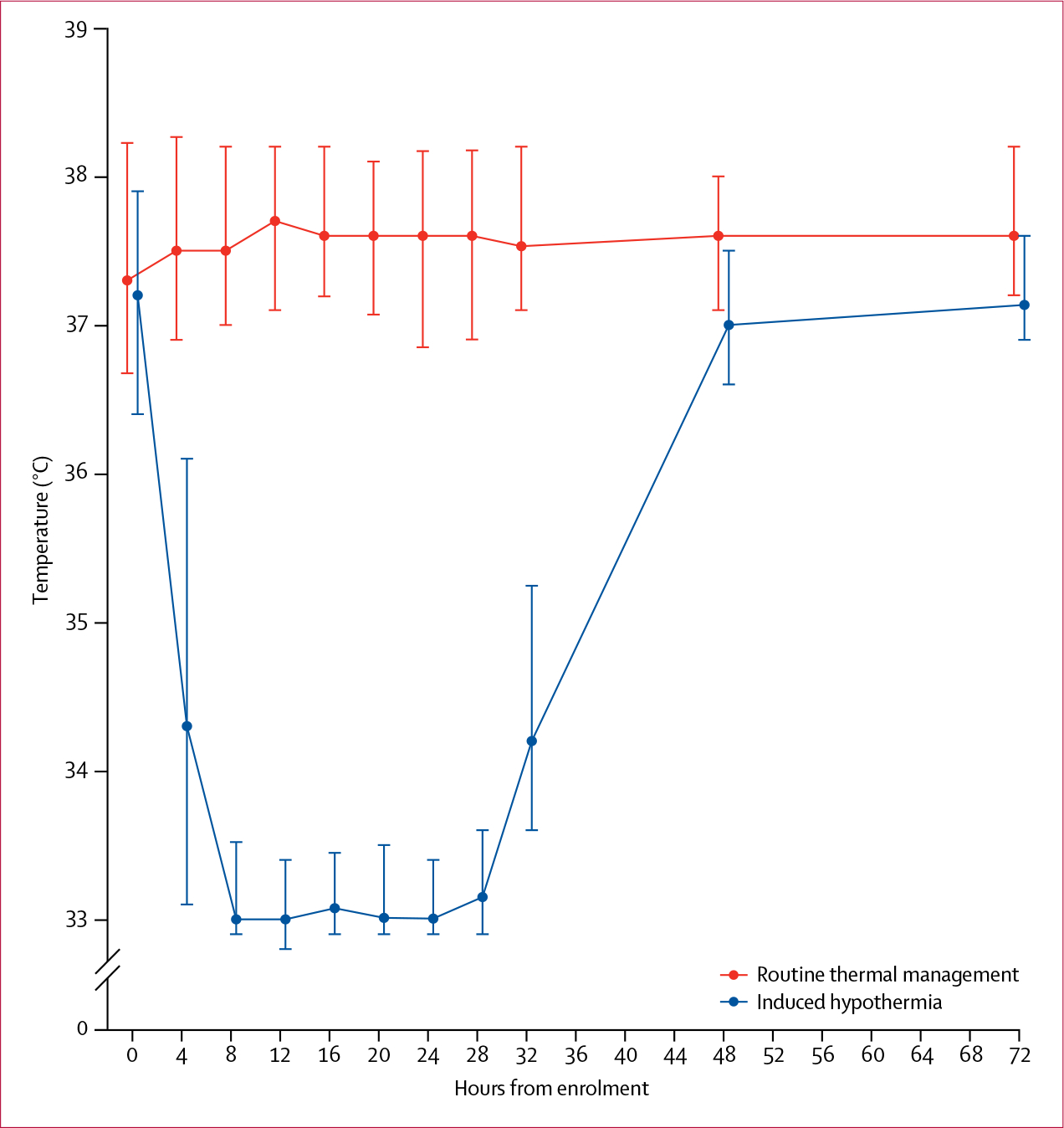
Values are medians and error bars are IQRs.
Follow-up for the primary outcome was complete in all patients. After 30 days, 96 (44·2%) of 217 patients had died in the induced hypothermia group compared with 77 (35·8%) of 215 in the routine thermal management group (difference 8·4% [95% CI −0·8 to 17·6]; relative risk 1·2 [1·0–1·6]; p=0·07; table 2, figure 3). Interim analysis data for the primary endpoint are available in the appendix. Within the first 30 days, patients in the induced hypothermia group had fewer days alive and without mechanical ventilation than did those in the routine thermal management group, fewer days alive without vasoactive treatment, and fewer days alive without renal replacement therapy (table 2). The duration of critical care was similar in each group.
Table 2:
Outcomes
| Routine thermal management (n=215) | Induced hypothermia (n=217) | Risk ratio (95% CI)* | Absolute difference (% [95% CI])* | p value | |
|---|---|---|---|---|---|
|
| |||||
| Primary outcome | |||||
| 30 day mortality | 77 (35.8%) | 96 (44.2%) | 1.2 (1.0 to 1.6)† | 8.4% (−0.8 to 17.6) | 0.07 |
| Secondary outcome ‡ | |||||
| 180 day mortality | 109 (50.7%) | 122 (56.2%) | 1.1 (0.9 to 1.3) | 5.5% (−3.9 to 14.9) | 0.25 |
| Days alive without respiratory support to day 30§ | 15 (0 to 26) | 3 (0 to 24) | .. | .. | 0.03 |
| Days alive without renal replacement therapy to day 30§ | 30 (0 to 30) | 20 (0 to 30) | .. | .. | 0.04 |
| Days alive without vasoactive medication to day 30§ | 23 (0 to 28) | 19 (0 to 27) | .. | .. | 0.006 |
| ICU length of stay (days)¶ | |||||
| All | 9 (3 to 17) | 8 (4 to 15) | .. | .. | 0.59 |
| Survivors | 9 (4 to 18) | 9 (6 to 19) | .. | .. | 0.12 |
| Non-survivors | 8 (2 to 15) | 7 (3 to 13) | .. | .. | 0.73 |
| Secondary outcomes and organ failure at 72 h‡ || | |||||
| SOFA score | 9 (6 to 12) | 11 (7 to 13) | .. | .. | 0.04 |
| Respiratory function | |||||
| On ventilator | 144/192 (75.0%) | 165/191 (86.4%) | 1.2 (1.0 to 1.3) | 11.4% (3.1 to 18.9) | 0.007 |
| PaO2 to FiO2 ratio | 25.4 (18.6 to 30.6) | 25.8 (18.7 to 34.3) | .. | .. | 0.52 |
| SOFA respiratory score | 2 (2 to 3) | 2 (2 to 3) | .. | .. | 0.74 |
| Circulatory function | |||||
| Mean arterial pressure (mm Hg) | 78 (70 to 86) | 75 (70 to 84) | .. | .. | 0.13 |
| Received vasoactive drugs | 102/192 (53.1%) | 132/191 (69.1%) | 1.3 (1.1 to 1.5) | 15.9% (6.8 to 26.4) | 0.002 |
| Vasoactive-inotropic score | 1 (0 to 14) | 10 (0 to 20) | .. | .. | <0.0001 |
| Accumulated vasoactive-inotropic score | 52 (27 to 104) | 74 (43 to 126) | .. | .. | <0.0001 |
| Achieved 50% reduction in vasoactive-inotropic score | 128/184 (69.6%) | 104/187 (55.6%) | 0.8 (0.7 to 0.9) | −14.0% (−23.7 to −4.2) | 0.006 |
| SOFA cardiovascular score | 3 (0 to 3) | 3 (0 to 4) | .. | .. | 0.002 |
| Renal function | |||||
| Diuresis (mL/kg per h) | 1.3 (0.6to 1.8) | 1.4 (0.6 to 2.0) | .. | .. | 0.25 |
| Creatinine concentration (μmol/L) | 83 (61 to 130) | 96 (64 to 146) | .. | .. | 0.08 |
| Renal replacement therapy | 36/192 (18.8%) | 43/191 (22.5%) | 1.2 (0.8 to 1.8) | 3.8% (−4.2 to 11.8) | 0.37 |
| Acute kidney injury (RIFLE)** | |||||
| Risk | 17/173 (9.8%) | 14/181 (7.7%) | 0.8 (0.4 to 1.6) | −2.1% (−8.0 to 3.8) | 0.49 |
| Injury | 4/173 (2.3%) | 2/181 (1.1%) | 0.5 (0.1 to 2.6) | −1.2% (−3.9 to 1.5) | 0.38 |
| Failure | 42/173 (24.3%) | 55/181 (30.4%) | 1.3 (0.9 to 1.8) | 6.1% (−3.2 to 15.4) | 0.19 |
| Any | 63/173 (36.4%) | 71/181 (39.2%) | 1.1 (0.8 to 1.4) | 2.8% (−7.3 to 12.9) | 0.59 |
| SOFA renal score | 0 (0 to 2) | 0 (0 to 2) | .. | .. | 0–08 |
| Coagulation and liver function | |||||
| INR | 1.3 (1.1 to 1.5) | 1.2 (1.1 to 1.5) | .. | .. | 0.80 |
| Bilirubin concentration (μmol/L) | 9 (6 to 16) | 11 (6 to 21) | .. | .. | 0.21 |
| Bilirubin concentration of >21 mmol/L | 33/172 (19.2%) | 45/188 (23.9%) | 1.3 (0.8 to 1.9) | 4.8% (−3.7 to 13.2) | 0.27 |
| ' SOFA liver score | 0 (0 to 0) | 0 (0 to 1) | .. | .. | 0.18 |
| Platelet count (× 109 per L) | 194 (115 to 282) | 156 (81 to 245) | .. | .. | 0.01 |
| Platelet count of <150 × 109 per L | 61/178 (34.3%) | 89/189 (47.1%) | 1.4 (1.1 to 1.8) | 12.8% (2.7 to 22.8) | 0.01 |
| Platelet count decrease of >25% from baseline | 60/168 (35.7%) | 79/177 (44.6%) | 1.3 (1.0 to 1.6) | 8.9% (−1.4 to 19.2) | 0.09 |
| SOFA coagulation score | 0 (0 to 1) | 0 (0 to 2) | .. | .. | 0.01 |
| C-reactive protein | |||||
| C-reactive protein concentration (mg/L) | 106 (59 to 191) | 153 (96 to 236) | .. | .. | 0.0001 |
| C-reactive protein concentration decrease of >30% from baseline | 88/175 (50.3%) | 60/180 (33.3%) | 0.7 (0.5 to 0.9) | −17.0% (−27.1 to −6.8) | 0.001 |
| Cerebral function | |||||
| Received sedatives | 130/192 (67.7%) | 150/191 (78.5%) | 1.2 (1.0 to 1.3) | 10.8% (2.0 to 19.6) | 0.02 |
| Richmond Agitation-Sedation Scale | −3 (−4 to 0) | −3 (−4 to −2) | .. | .. | 0.008 |
| Delirium†† | 16/84 (19.0%) | 19/71 (26.8%) | 1.4 (0.8 to 2.5) | 7.7% (−5.6 to 21.0) | 0.25 |
| SOFA CNS score‡‡ | 2 (0 to 4) | 3 (2 to 4) | .. | .. | 0.0004 |
Data are n (%), median (IQR), or n/N (%), unless otherwise indicated. Correction for multiple comparisons was not implemented according to the latest European Medicines Agency guidelines38 since the trial had one primary hypothesis and one primary outcome and all other endpoints were considered supportive. ICU=intensive care unit. SOFA=Sequential Organ Failure Assessment. PaO2=partial pressure of arterial oxygen. FiO2=fraction of inspired oxygen. RIFLE=Risk, Injury, Failure, Loss of Kidney Function, and End-stage Kidney Disease. INR=International Normalized Ratio.
We only report risk ratios and differences for binary outcomes.
The hazard ratio for the primary endpoint adjusted for stratifying variables (Cox regression with randomisation, site, age ≥65 years, and Acute Physiology and Chronic Health Evaluation II score of ≥25) was 1.31 (95% CI 0.97-1.77).
383 patients could be included in the analysis of secondary outcome at the end of the intervention (191 [50%] in the induced hypothermia group and 192 [50%] in the routine thermal management group). In one (<1%) patient the next of kin declined further data collection (in the induced hypothermia group) and 23 (11%) patients died in the routine thermal management group compared with 25 (12%) in the induced hypothermia group).
Days alive without organ failure to day 30 was for all assessments done according to Schoenfeld and colleagues39 and, accordingly, the score “0" was given to all patients who died before day 30.
Censored on day 30.
Secondary outcomes were evaluated 72 h after initiation of the intervention.
The maximum RIFLE class reached during 72 h was used. In RIFLE “F", patients in renal replacement therapy were included.
Delirium status could be established in 155 (84 [54%] in the routine thermal management group and 71 [46%] in the induced hypothermia group) patients for whom the Richmond Agitation-Sedation Scale was less than −4.
Calculated on the basis of the Richmond Agitation-Sedation Scale according to Vasilevskis and colleagues.40
Figure 3: Kaplan-Meier plot.
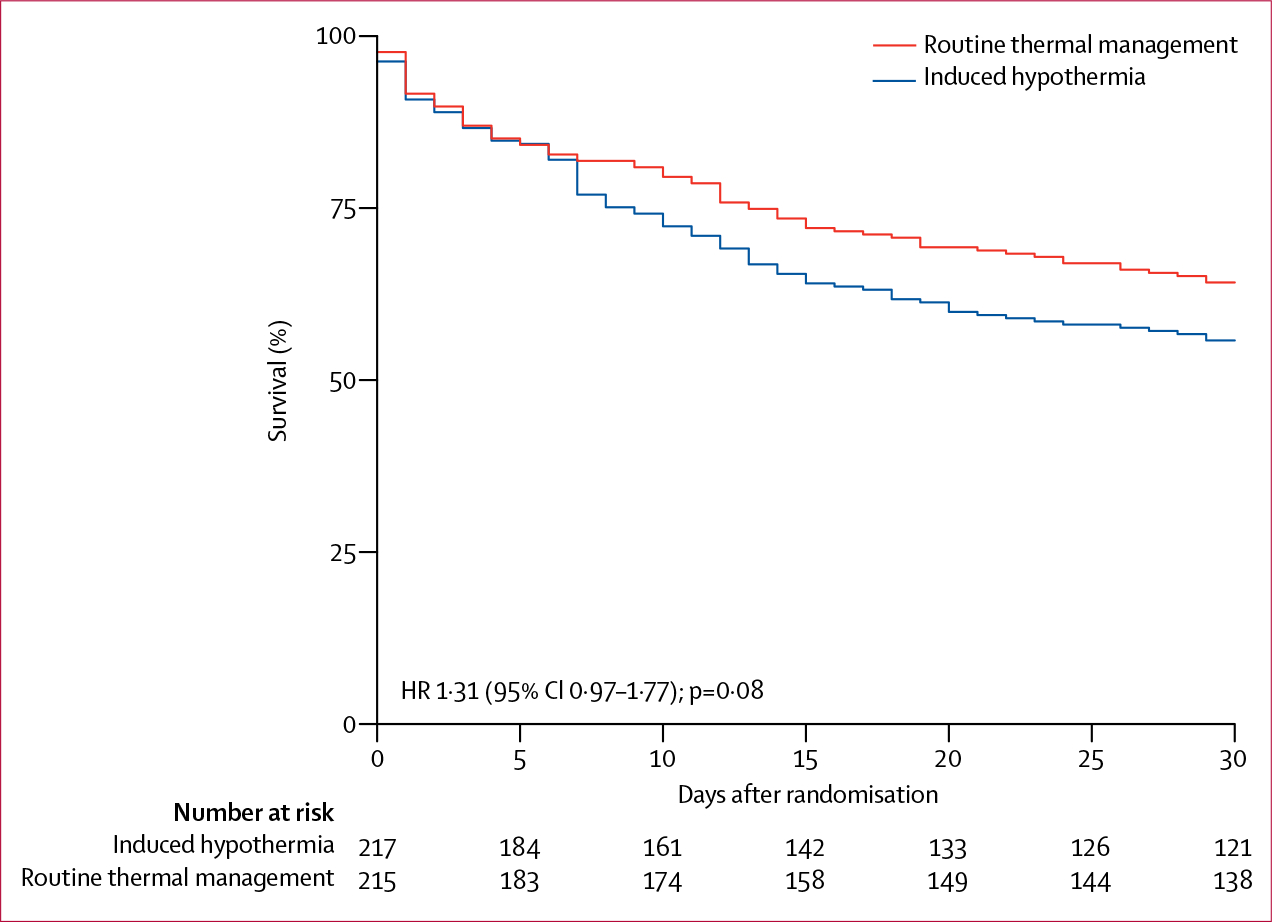
No patients were censored. HR=hazard ratio.
72 h after randomisation, patients in the induced hypothermia group were more often given vasoactive medications than were those in the routine thermal management group (table 2). Fewer patients in the induced hypothermia group had at least 50% reductions in vasoactive medication than did those in the routine thermal management group and fewer had a more than 30% decrease in CRP concentration from baseline. More patients in the induced hypothermia group still required sedation than did those in the routine thermal management group and more were still mechanically ventilated. We noted no detectable differences in renal outcome variables. Platelet counts were lower in the induced hypothermia group than in the routine thermal management group, but were also lower at baseline (table 1). The need for blood transfusions and surgery was similar in each group (appendix).
The effects of hypothermia in preplanned subgroups are shown in figure 4; no subgroup seemed to benefit from the intervention, but some subgroups seemed to be harmed more than others (ie, in those comparisons where the p value for interaction was <0·05). On the basis of a request from the trial steering committee, we added additional exploratory subgroups: platelet count, PaO2 to FiO2 ratio, temperature, and lactate concentration. None of the exploratory subgroups showed a favourable effect of the intervention. Patients cooled with intravenous catheters had similar mortality to those cooled with external pads: seven (47%) of 15 versus 89 (44%) of 202 (p=0·84).
Figure 4: Effect of the intervention in subgroups.
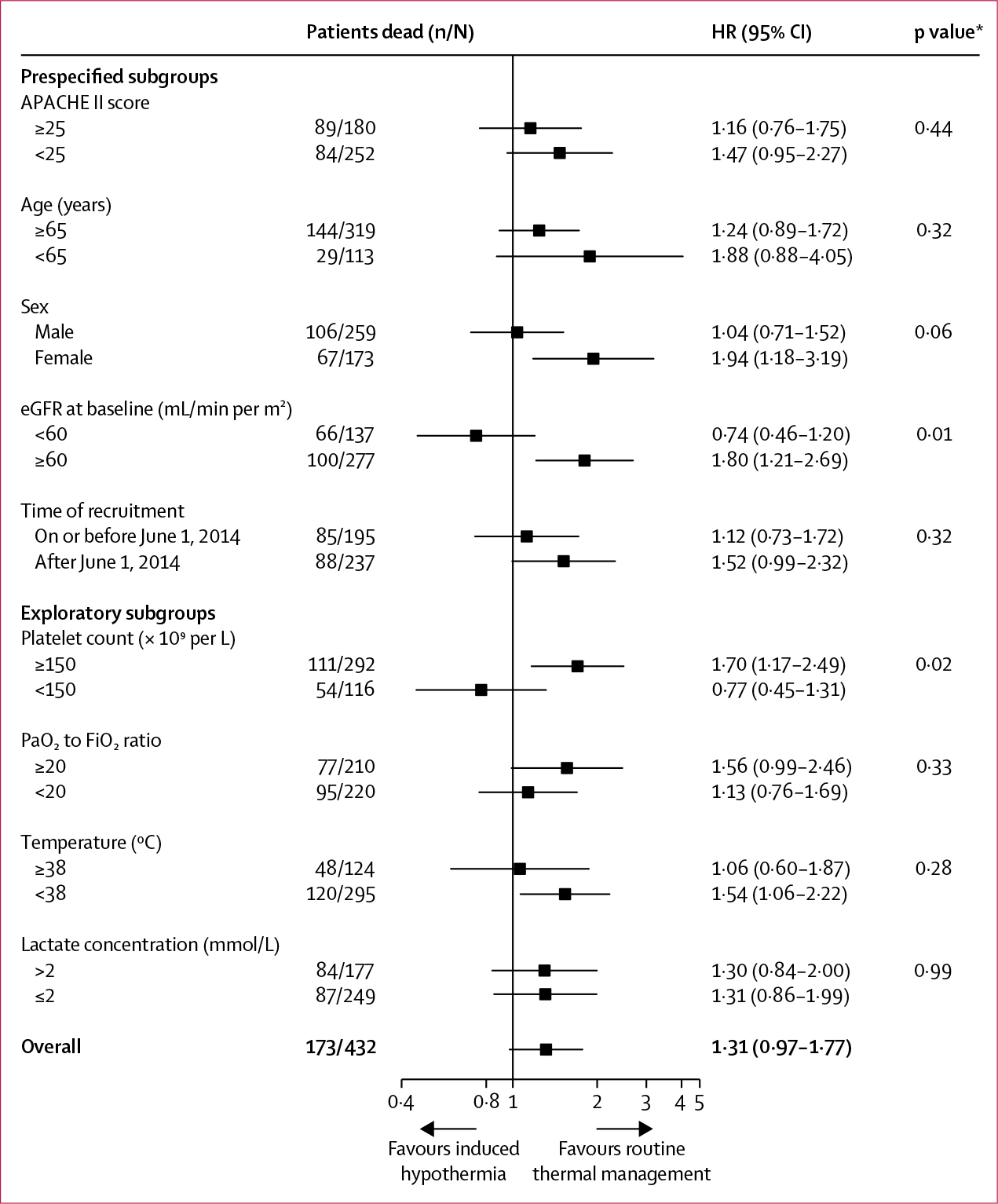
A test for interaction between site and intervention had a p value of 0·41. APACHE=Acute Physiology and Chronic Health Evaluation. eGFR=estimated glomerular filtration rate. HR=hazard ratio. PaO2=partial pressure of arterial oxygen. FiO2=fraction of inspired oxygen. *p values for the interaction between treatment effect and subgroup.
Discussion
This international randomised trial assessed patients with sepsis, circulatory failure, and ventilator-dependent respiratory failure who were at least 50 years old. Induced hypothermia to a target temperature of 32–34°C for 24 h, slow rewarming, and subsequent 48 h of fever suppression was not better than routine thermal management. Specifically, 30 day all-cause mortality (the primary outcome) was not improved by hypothermia, and was possibly worsened. Furthermore, hypothermia aggravated circulatory collapse, respiratory failure, and delayed the decrease in CRP protein concentration.
We selected a target temperature range of 32–34°C on the basis of experimental studies in animals showing pronounced immunomodulatory effects,41 reduced sepsis-related liver14,42 and lung12 damage, and improved survival in studies9–11 in which mammals were cooled to 32–34°C for 24–72 h. By contrast, short durations of hypothermia, especially combined with rapid rewarming, appear detrimental.43 During the course of this trial, additional studies were published showing hypothermia-induced reversion of sepsis-related mitochondrial dysfunction in rats15 and marked improvements in respiratory physiology in septic pigs.12
Perhaps the most striking aspect of our negative results is the extent to which they contrast with the positive findings in other mammals. Similar divergence in studies of therapeutic hypothermia has been shown in recent years for several clinical entities, including (but not restricted to) brain trauma44,45 and out-of-hospital cardiac arrest in adults.46 The most obvious explanation is that hypothermia usually takes several hours to induce in human beings by which time tissue damage might already have occurred. Tissue damage as a result of sepsis presumably develops over a far longer period than the roughly 3 h that our patients required to reach the hypothermic target, making sepsis an attractive target for therapeutic hypothermia. Since hypothermia was not beneficial, the effects of hypothermia on sepsis are likely to differ between elderly human beings on the one hand and young rodents and pigs on the other. Whether this difference is a true difference between these animals and human beings or rather simply an age difference remains to be established.
The most relevant previous human study20 reported that fever control in patients with septic shock reduced the need for vasoactive medications. Our study combined therapeutic hypothermia for 24 h with fever control from 24 h to 72 h without improving mortality and with worsening of other outcomes. Thus, although fever control appears beneficial in patients with sepsis, the combination of therapeutic hypothermia and subsequent fever control is not. A possible explanation for this pronounced difference is hypothermia-induced tryptophan catabolism and lymphocytopenia, leading to immune paresis.18,47
Predefined and post-hoc analyses did not identify any subgroups in which hypothermia was especially beneficial or harmful. Two subgroup analyses showed positive interactions; however, none had a significant benefit signal and thus we interpret the positive interactions as a signal that the harm effect was more pronounced among certain patient groups than among others. Since our trial was done at ten ICUs across Europe and North America, the results seem likely to apply broadly. Furthermore, the results were clear: mild hypothermia worsened organ function and tended to worsen mortality.
A limitation of our trial is that investigators and other health-care professionals treating participants were aware of study group allocation; this challenge is inherent to temperature and other physical interventions. However, bias was reduced by use of a robust primary endpoint assessed by masked investigators. At one site, a surgical ICU, most patients who met other criteria were excluded (before randomisation) because of recent major surgery; the steering committee nonetheless included this site to enhance accrual and increase generalisability. Additionally, we observed a higher use of sedation in the hypothermia group than in the routine thermal management group in the intervention period, and since substantial harm has been shown from sedation in patients like these,48 this sedation might, independently of the physiological effects of induced hypothermia, have resulted in some harm. Finally, we tested a specific target temperature range and duration of hypo thermia; results might have differed with other degrees and lengths of hypothermia.
We did not find a benefit of induction of hypothermia to 32–34°C followed by slow rewarming and 48 h of fever prevention in patients with sepsis who required vasopressors and had ventilator-dependent acute lung injury. In fact, hypothermia delayed recovery of several key organ functions. Our findings do not support use of induced hypothermia in patients with septic shock.
Supplementary Material
Research in context.
Evidence before this study
The influence of fever on the human host response to infection has been debated throughout the history of medicine. Septic shock complicated by respiratory failure is a major cause of mortality globally. Despite intensive research and multiple trials, no interventions have reduced the number of patients who die from septic shock. Induced hypothermia has been proposed as a potential intervention in systemic infections for decades. We searched PubMed on Dec 1, 2010, using the search terms “hypothermia” and “sepsis” without any limitations on language or date of publication and found that animal studies strongly suggest a beneficial survival effect of induced hypothermia in severe infections. Additionally, in animals, organ function preservation is enhanced in lungs, kidneys, and the liver. In rats, induced hypothermia has been shown to restore mitochondrial function in pneumococcal infection. Small studies of induced hypothermia in humans have substantiated the physiological benefits and improved survival; however, these studies were not powered for mortality analysis. Of note, spontaneous hypothermia is a well known complication of severe sepsis and septic shock and is known to pose an increased risk of death. Spontaneous hypothermia is a consequence of severe immunological derangement and signals severe disease and should not be extrapolated to induced hypothermia.
Added value of this study
This trial is the first to study, in a randomised manner, the effect of induced hypothermia followed by normothermia in patients with septic shock and acute ventilator-dependent respiratory failure. The sample size was sufficient to, within a reasonable clinical effect, make firm conclusions and to substantiate or refute the hypothesis. The trial was done across three countries in Europe and North America and the intervention was administered shortly after the patient developed septic shock. Induced hypothermia (target temperature 32–34°C) proved harmful with regards to respiratory function and it also prolonged septic shock. Mortality was not significantly different between the induced hypothermia group and the routine thermal management group. No subgroups of patients seemed to benefit from the treatment.
Implications of all the available evidence
Induced hypothermia should be discouraged as a treatment for septic shock. Furthermore, the pronounced discrepancy between our results and the results of preclinical and small clinical studies stresses the potential for errors when conclusions are extrapolated from insufficient evidence and, thus, especially among high-risk populations like patients with septic shock, the need for randomised trials powered for mortality.
Acknowledgments
This trial was funded by TrygFonden (application number 7-10-1301), Lundbeckfonden (application number R54-A5342), and the Danish National Research Foundation (grant number DNRF126).
Footnotes
Declaration of interests
J-UJ reports grants from TrygFonden, Lundbeckfonden, and the Danish National Research Foundation and non-financial support from Emcools, Medivance, and Zoll during the conduct of the study and grants from Boehringer Ingelheim and non-financial support from Roche and Boehringer Ingelheim outside of the submitted work. MB reports grants from the Nordsjællands Hospital Research Fund during the conduct of the study and personal fees from Bard outside of the submitted work. All other authors declare no competing interests.
Contributor Information
Theis Skovsgaard Itenov, Centre of Excellence in Immunity and Infection/Centre of Excellence for Personalised Medicine of Infectious Complications in Immune Deficiency, Department of Infectious Diseases, Rigshospitalet and University of Copenhagen, Copenhagen, Denmark; Department of Anesthesia and Intensive Care, Nordsjællands Hospital, Hillerød, Denmark.
Maria Egede Johansen, Centre of Excellence in Immunity and Infection/Centre of Excellence for Personalised Medicine of Infectious Complications in Immune Deficiency, Department of Infectious Diseases, Rigshospitalet and University of Copenhagen, Copenhagen, Denmark.
Morten Bestle, Department of Anesthesia and Intensive Care, Nordsjællands Hospital, Hillerød, Denmark.
Katrin Thormar, Department of Anesthesia and Intensive Care, Bispebjerg Hospital, Copenhagen, Denmark.
Lars Hein, Department of Anesthesia and Intensive Care, Nordsjællands Hospital, Hillerød, Denmark.
Louise Gyldensted, Department of Anesthesia and Intensive Care, Herlev and Gentofte Hospital, Hellerup, Denmark.
Anne Lindhardt, Department of Anesthesia and Intensive Care, Bispebjerg Hospital, Copenhagen, Denmark.
Henrik Christensen, Department of Anesthesia and Intensive Care, Herlev and Gentofte Hospital, Herlev, Denmark.
Stine Estrup, Department of Anesthesia and Intensive Care, Zealand University Hospital, Køge, Denmark.
Henrik Planck Pedersen, Department of Anesthesia and Intensive Care, Roskilde Hospital, Roskilde, Denmark.
Matthew Harmon, Department of Intensive Care, Academic Medical Center, Amsterdam, Netherlands.
Uday Kant Soni, Department of Anesthesia and Intensive Care, Horsens Hospital, Horsens, Denmark.
Silvia Perez-Protto, Center for Critical Care, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH, USA; Department of Outcomes Research, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH, USA.
Nicolai Wesche, Department of Anesthesia and Intensive Care, Nordsjællands Hospital, Hillerød, Denmark.
Ulrik Skram, Department of Anesthesia and Intensive Care, Nordsjællands Hospital, Hillerød, Denmark.
John Asger Petersen, Department of Anesthesia and Intensive Care, Bispebjerg Hospital, Copenhagen, Denmark.
Thomas Mohr, Department of Anesthesia and Intensive Care, Herlev and Gentofte Hospital, Hellerup, Denmark.
Tina Waldau, Department of Anesthesia and Intensive Care, Herlev and Gentofte Hospital, Herlev, Denmark.
Lone Musaeus Poulsen, Department of Anesthesia and Intensive Care, Zealand University Hospital, Køge, Denmark.
Ditte Strange, Department of Anesthesia and Intensive Care, Bispebjerg Hospital, Copenhagen, Denmark.
Nicole P Juffermans, Department of Intensive Care, Academic Medical Center, Amsterdam, Netherlands.
Daniel I Sessler, Department of Outcomes Research, Anesthesiology Institute, Cleveland Clinic, Cleveland, OH, USA.
Else Tønnesen, Department of Anesthesia and Intensive Care, Aarhus University Hospital, Aarhus, Denmark.
Kirsten Møller, Department of Neuroanesthesiology, Rigshospitalet and University of Copenhagen, Copenhagen, Denmark.
Dennis Karsten Kristensen, Centre of Excellence in Immunity and Infection/Centre of Excellence for Personalised Medicine of Infectious Complications in Immune Deficiency, Department of Infectious Diseases, Rigshospitalet and University of Copenhagen, Copenhagen, Denmark.
Alessandro Cozzi-Lepri, Centre for Clinical Research, Epidemiology, Modelling and Evaluation, Institute for Global Health, University College London, London, UK.
Jens D Lundgren, Centre of Excellence in Immunity and Infection/Centre of Excellence for Personalised Medicine of Infectious Complications in Immune Deficiency, Department of Infectious Diseases, Rigshospitalet and University of Copenhagen, Copenhagen, Denmark.
Jens-Ulrik Jensen, Centre of Excellence in Immunity and Infection/Centre of Excellence for Personalised Medicine of Infectious Complications in Immune Deficiency, Department of Infectious Diseases, Rigshospitalet and University of Copenhagen, Copenhagen, Denmark; Respiratory Medicine Division, Department of Internal Medicine, Herlev and Gentofte Hospital, Hellerup, Denmark.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ 2016; 353: i1585. [DOI] [PubMed] [Google Scholar]
- 3.Donnino MW, Andersen LW, Chase M, et al. Randomized, double-blind, placebo-controlled trial of thiamine as a metabolic resuscitator in septic shock: a pilot study. Crit Care Med 2016; 44: 360–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014; 312: 90–92. [DOI] [PubMed] [Google Scholar]
- 5.McCloskey RV, Straube RC, Sanders C, Smith SM, Smith CR. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS Trial Study Group. Ann Intern Med 1994; 121: 1–5. [DOI] [PubMed] [Google Scholar]
- 6.Abraham E, Anzueto A, Gutierrez G, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet 1998; 351: 929–33. [PubMed] [Google Scholar]
- 7.Lopez A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med 2004; 32: 21–30. [DOI] [PubMed] [Google Scholar]
- 8.Annane D, Vignon P, Renault A, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 2007; 370: 676–84. [DOI] [PubMed] [Google Scholar]
- 9.Huet O, Kinirons B, Dupic L, et al. Induced mild hypothermia reduces mortality during acute inflammation in rats. Acta Anaesthesiol Scand 2007; 51: 1211–16. [DOI] [PubMed] [Google Scholar]
- 10.L’Her E, Amerand A, Vettier A, Sebert P. Effects of mild induced hypothermia during experimental sepsis. Crit Care Med 2006; 34: 2621–23. [DOI] [PubMed] [Google Scholar]
- 11.Leon K, Pichavant-Rafini K, Ollivier H, Monbet V, L’Her E. Does induction time of mild hypothermia influence the survival duration of septic rats? Ther Hypothermia Temp Manag 2015; 5: 85–88. [DOI] [PubMed] [Google Scholar]
- 12.Schwarzl M, Seiler S, Wallner M, et al. Mild hypothermia attenuates circulatory and pulmonary dysfunction during experimental endotoxemia. Crit Care Med 2013; 41: e401–10. [DOI] [PubMed] [Google Scholar]
- 13.Scumpia PO, Sarcia PJ, Kelly KM, DeMarco VG, Skimming JW. Hypothermia induces anti-inflammatory cytokines and inhibits nitric oxide and myeloperoxidase-mediated damage in the hearts of endotoxemic rats. Chest 2004; 125: 1483–91. [DOI] [PubMed] [Google Scholar]
- 14.Kuboki S, Okaya T, Schuster R, et al. Hepatocyte NF-kappaB activation is hepatoprotective during ischemia-reperfusion injury and is augmented by ischemic hypothermia. Am J Physiol Gastrointest Liver Physiol 2007; 292: G201–07. [DOI] [PubMed] [Google Scholar]
- 15.Beurskens CJ, Aslami H, Kuipers MT, et al. Induced hypothermia is protective in a rat model of pneumococcal pneumonia associated with increased adenosine triphosphate availability and turnover. Crit Care Med 2012; 40: 919–26. [DOI] [PubMed] [Google Scholar]
- 16.Kluger MJ, Vaughn LK. Fever and survival in rabbits infected with Pasteurella multocida. J Physiol 1978; 282: 243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaughn LK, Veale WL, Cooper KE. The effect of venous blood stream cooling on survival of bacterially infected rabbits. Pflugers Arch 1987; 409: 635–37. [DOI] [PubMed] [Google Scholar]
- 18.Drewry AM, Fuller BM, Skrupky LP, Hotchkiss RS. The presence of hypothermia within 24 hours of sepsis diagnosis predicts persistent lymphopenia. Crit Care Med 2015; 43: 1165–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young P, Saxena M, Bellomo R, et al. Acetaminophen for fever in critically ill patients with suspected infection. N Engl J Med 2015; 373: 2215–24. [DOI] [PubMed] [Google Scholar]
- 20.Schortgen F, Clabault K, Katsahian S, et al. Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med 2012; 185: 1088–95. [DOI] [PubMed] [Google Scholar]
- 21.Villar J, Slutsky AS. Effects of induced hypothermia in patients with septic adult respiratory distress syndrome. Resuscitation 1993; 26: 183–92. [DOI] [PubMed] [Google Scholar]
- 22.Blair E, Buxton RW, Cowley RA, Mansberger AR Jr. The use of hypothermia in septic shock. JAMA 1961; 178: 916–19. [DOI] [PubMed] [Google Scholar]
- 23.Miorner G, Haeger K, Ryd H. Artificial hypothermia in poliomyelitis with hyperpyrexia. Lancet 1955; 269: 593–95. [DOI] [PubMed] [Google Scholar]
- 24.Jensen JU, Hein L, Lundgren B, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 2011; 39: 2048–58. [DOI] [PubMed] [Google Scholar]
- 25.Winters SA, Wolf KH, Kettinger SA, Seif EK, Jones JS, Bacon-Baguley T. Assessment of risk factors for post-rewarming “rebound hyperthermia” in cardiac arrest patients undergoing therapeutic hypothermia. Resuscitation 2013; 84: 1245–49. [DOI] [PubMed] [Google Scholar]
- 26.Leary M, Grossestreuer AV, Iannacone S, et al. Pyrexia and neurologic outcomes after therapeutic hypothermia for cardiac arrest. Resuscitation 2013; 84: 1056–61. [DOI] [PubMed] [Google Scholar]
- 27.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med 2009; 37: 1101–20. [DOI] [PubMed] [Google Scholar]
- 28.de Pont AC. Does cold-bloodedness protect against sepsis? Crit Care Med 2006; 34: 2692–93. [DOI] [PubMed] [Google Scholar]
- 29.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med 2008; 34: 17–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010; 11: 234–38. [DOI] [PubMed] [Google Scholar]
- 31.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 2006; 10: R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 1998; 26: 1793–800. [DOI] [PubMed] [Google Scholar]
- 33.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362: 779–89. [DOI] [PubMed] [Google Scholar]
- 34.COIITSS Study Investigators, Annane D, Cariou A, Maxime V, et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA 2010; 303: 341–48. [DOI] [PubMed] [Google Scholar]
- 35.Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008; 108: 71–77. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979; 35: 549–56. [PubMed] [Google Scholar]
- 37.Kahan BC, Morris TP. Improper analysis of trials randomised using stratified blocks or minimisation. Stat Med 2012; 31: 328–40. [DOI] [PubMed] [Google Scholar]
- 38.Hofstetter C, Boost KA, Flondor M, et al. Anti-inflammatory effects of sevoflurane and mild hypothermia in endotoxemic rats. Acta Anaesthesiol Scand 2007; 51: 893–99. [DOI] [PubMed] [Google Scholar]
- 39.Schoenfeld DA, Bernard GR, for the ARDS Network. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002; 30: 1772–77. [DOI] [PubMed] [Google Scholar]
- 40.Vasilevskis EE, Pandharipande PP, Graves AJ. Validity of a modified sequential organ failure assessment score using the Richmond Agitation-Sedation Scale. Crit Care Med 2016; 44: 138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.European Medicines Agency. Dec 15, 2016. Guideline on multiplicity issues in clinical trials. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/03/WC500224998.pdf (accessed Nov 16, 2017).
- 42.Lee JH, Kim K, Jo YH, et al. Therapeutic hypothermia attenuates liver injury in polymicrobial sepsis model of rats via Akt survival pathway. J Surg Res 2013; 181: 114–20. [DOI] [PubMed] [Google Scholar]
- 43.Torossian A, Ruehlmann S, Middeke M, et al. Deleterious effects of mild hypothermia in septic rats are ameliorated by granulocyte colony-stimulating factor. Anesthesiology 2003; 99: 1087–92. [DOI] [PubMed] [Google Scholar]
- 44.Andrews PJ, Sinclair HL, Rodriguez A, et al. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med 2015; 373: 2403–12. [DOI] [PubMed] [Google Scholar]
- 45.Adelson PD, Wisniewski SR, Beca J, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol 2013; 12: 546–53. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013; 369: 2197–206. [DOI] [PubMed] [Google Scholar]
- 47.Schefold JC, Fritschi N, Fusch G, et al. Influence of core body temperature on tryptophan metabolism, kynurenines, and estimated IDO activity in critically ill patients receiving target temperature management following cardiac arrest. Resuscitation 2016; 107: 107–14. [DOI] [PubMed] [Google Scholar]
- 48.Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet 2010; 375: 475–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


