Abstract
The indoor air quality of a residential home during winter in Fairbanks, Alaska, was investigated and contrasted with outdoor levels. Twenty-four-hour average indoor and outdoor filter samples were collected from January 17 to February 25, 2022, in a residential area with high outdoor PM2.5 concentrations. The oxidative potential of PM2.5 was determined using the dithiothreitol-depletion assay (OPDTT). For the unoccupied house, the background indoor-to-outdoor (I/O) ratio of mass-normalized OP (OPmDTT), a measure of the intrinsic health-relevant properties of the aerosol, was less than 1 (0.53 ± 0.37), implying a loss of aerosol toxicity as air was transported indoors. This may result from transport and volatility losses driven by the large gradients in temperature (average outdoor temperature of −19°C/average indoor temperature of 21 °C) or relative humidity (average outdoor RH of 78%/average indoor RH of 11%), or both. Various indoor activities, including pellet stove use, simple cooking experiments, incense burning, and mixtures of these activities, were conducted. The experiments produced PM2.5 with a highly variable OPmDTT. PM2.5 from cooking emissions had the lowest OP values, while pellet stove PM2.5 had the highest. Correlations between volume-normalized OPDTT (OPvDTT), relevant to exposure, and indoor PM2.5 mass concentration during experiments were much lower compared to those in outdoor environments. This suggests that mass concentration alone can be a poor indicator of possible adverse effects of various indoor emissions. These findings highlight the importance of considering both the quantity of particles and sources (chemical composition), as health metrics for indoor air quality.
Keywords: indoor air quality, subarctic region, residential heating, biomass burning, fine particulate matter (PM2.5), oxidative potential
Short abstract
This study reveals that PM infiltrating from outdoor to indoor environments exhibited substantial changes in oxidative potential and that various indoor activities generated particles with a wide range of toxicity showing the importance of both the quantity and sources (chemical composition) of particles as health metrics for indoor air quality.
1. Introduction
The air quality in Fairbanks, a high latitude (64.84°N) city in Alaska’s interior, often exceeds fine particle (PM2.5) standards during the wintertime. This is mainly due to severe meteorological conditions that limit dispersion of emissions largely from residential heating with wood.1−6 With average outdoor low and high temperatures of nominally −24 °C and −15 °C in the wintertime, residents spend most time indoors. To some extent, this may isolate residents from the poor outdoor air quality but also intensify exposures to indoor emissions due to minimized indoor/outdoor air exchange.
Indoor air pollution has emerged as a significant global health concern, contributing to a substantial burden of disease worldwide. Household air pollution was responsible for an estimated 3.2 million deaths per year in 2020, including over 237 000 deaths of children under the age of 5.7 It can produce immediate health risks, such as headache and dizziness, and long-term health consequences, including respiratory infection, pulmonary diseases, cardiovascular disorders, and cancer.8−11 Indoor PM generated from different sources has been linked to premature death in people with heart or lung disease, nonfatal heart attacks, irregular heartbeat, aggravated asthma, decreased lung function, and increased adverse respiratory symptoms.8 Exposure to solid fuel smoke, for example, has been linked to chronic obstructive pulmonary disease in women, acute respiratory infection in children, lung cancer in women, and 3.5 million premature deaths.12,13 Obesity may increase susceptibility to the effects of indoor fine and coarse PM exposure.14 Across the globe, much of the exposure to indoor smoke is from low-efficiency traditional cooking methods with biomass-fuel, but in cold climates in many nations, indoor wood burning can be common as a source of heat or for recreation.
Indoor air quality is influenced by a combination of factors, including both outdoor air infiltration and emissions from indoor sources. Continuous exchange between outdoor and indoor air impacts indoor air quality, especially when outdoor air quality is poor, highlighting the significance of the outdoor air concentration and composition. This may contribute to the observed associations between outdoor air quality and health issues in epidemiological studies, even though people spend the most time indoors. During winter in Fairbanks, residential heating with fuel oil and wood has been identified as a significant contributor to outdoor PM2.5 mass concentration, followed by sulfate and vehicular emissions.4 The infiltration of ambient PM2.5 into indoor environments involves the movement of outdoor air into the house through openings, such as through gaps around windows and doors as well as through walls, floors, ceilings, and vents. Species that infiltrate are affected by concentration gradients between the indoor and outdoor environments, as well as differences in temperature and relative humidity (RH). Higher indoor temperature and lower indoor RH both can contribute to specific chemical species losing mass when moving from outdoors to indoors.15,16 In extremely cold regions like wintertime Fairbanks, where there are significant indoor-to-outdoor temperature and RH differences, this effect could be substantial.17,18
Indoor emissions of PM2.5 can be episodic and include PM resuspension,19 cooking,20,21 and combustion emissions, such as from stoves, fireplaces, or other forms of space heating or recreational burning.22 Secondary PM can also be generated from the reactions between volatile organic compounds (VOCs) and oxidants.23 VOCs can be emitted from indoor sources and from evaporation of infiltrating particles in a heated house during cold periods.19,24,25 Oxidants, such as ozone from outdoors and hydroxyl radical (OH·) and nitrate radical (NO3·) produced indoors,25 can react with VOCs, generating secondary species, but these are often a minor contributor to indoor PM2.5 mass concentration.26
Indoor emissions from burning wood are especially important in Fairbanks since wood is widely utilized for heating3 and can be a source of oxidative products.27 For example, pellet stoves can emit large amounts of fine particles28 and high levels of CO and polycyclic aromatic hydrocarbons (PAHs),29 especially in the ignition phase when combustion efficiency is low.30,31 Cooking is another major source of indoor aerosols, which can emit fatty acids and dicarboxylic acids,32,33 as well as combustion products if burning occurs.
These emissions can produce a substantial PM2.5 mass concentration, especially in a relatively small, tightly sealed house. Outdoor PM2.5 mass concentrations have been linked to adverse health effects. By also considering particle chemical composition, specific components of emissions, and any changes they may undergo through various processes that lead to more hazardous aerosols, can provide more insights than mass concentration alone.34 This may be especially important when considering indoor air quality since not all chemical components that substantially contribute to mass concentration are equally toxic. An alternative approach is to assess the ability of a particle to induce oxidative stress when inhaled. This results from the accumulation of reactive oxygen or nitrogen species (ROS/RNS) in vivo that cause cellular, tissue, and DNA damage,35 leading to a wide range of adverse effects.36 Various chemical assays have been developed to quantify an aerosol particle’s ability to generate ROS through the measurement of oxidative potential (OP). The dithiothreitol-depletion (DTT) assay is one of the most commonly used assays and has been linked to adverse effects, including both acute and chronic diseases.37−41 Other OP assays have also been associated with adverse health effects.41−45 OPDTT is most sensitive to certain metals and organic species, such as Cu, Mn, and various aromatic species.46−48 It can be used to provide insight into potential dose (OP normalized by the volume of air, OPv) and the particle’s intrinsic health-relevant properties (OP normalized by particle mass concentration, OPm). A more comprehensive assessment of air quality is likely achieved by utilizing several complementary assays that are sensitive to different sources and aerosol species.49−51
Most studies on aerosol particle OP have focused on outdoor air, and we have reported on Fairbanks wintertime outdoor OPDTT.51 Fewer studies have explored OP of indoor particulate matter from indoor-outdoor air exchange52,53 and from various indoor sources.54 Here we report on the effects of infiltration of outdoor air and the intrinsic health-relevant properties of different indoor activities using the OPDTT assay.
2. Methods
2.1. Sampling Location and House Description
This research was part of the Alaskan Layered Pollution And Chemical Analysis (ALPACA) field campaign. Indoor and outdoor daily PM2.5 samples were collected from 17 January 17, 2022 to 25 February 25, 2022, at the ALPACA House field site (64.850°N, 147.676°W) located in a Fairbanks residential area (Shannon Park Neighborhood). Residential areas of Fairbanks generally have higher concentrations of PM2.5 species emitted from residential heating, such as organic species from wood burning.55 The house was in a residential area and similar to other types of housing in Fairbanks.56 It was a single-level (ranch style) structure covering approximately 1549 square feet (excluding the garage). Prior to the intensive study, a house pressurization test and energy audit were conducted. The house was depressurized by 50 Pa, and leakage into the house was measured. A door blower test estimated an air exchange rate of 0.12/h under natural conditions. Additionally, the house ranked at the bottom of a 4-star rating (on a scale ranging from 1 to 6 stars), implying it was moderately above the typical Fairbanks residential housing standards in terms of air tightness, thermal resistance, and indoor ventilation patterns.
The main heating system was oil-burning with forced air distribution. The furnace blower fan was run continuously throughout the study to ensure adequate mixing of indoor air. The thermostat was set to 20 °C (68 °F). Online instruments (AMS and AE33) were sampled via small tubing from a hallway off the kitchen, whereas filters were collected in two rooms away from the kitchen and one room (through a doorway) from the pellet stove (see Figure S1), with the doorway being consistently open. Because this study reports nominally 24 h averaged data, we do not investigate the time evolution of indoor emissions or other perturbations.
2.2. PM2.5 Sampling
Separate systems were used for outdoor and indoor filter sampling. For the outdoor filters, a total of 49 PM2.5 filter samples (including 7 blanks) were collected using a Tisch PM2.5 high-volume (Hi-Vol) sampler (flow rate normally 1.13 m3/min). Each filter was collected over approximately 23.5 h (10 am to 9:30 am next day) using quartz filters (20.32 × 25.40 cm; Whatman® QM-A quartz filter). Inside the house, 35 PM2.5 filter samples were collected during the same period and synchronized with the outdoor sampling times using a PM2.5 Partisol-Plus sampler (Model 2025 sequential air sampler, Rupprecht & Patashnick Co., Inc, flow rate normally 16.7 L/min) with Teflon filters (46.2 mm in diameter with PP ring supported, pore size of 2 μm, Whatman® PTFE membrane filter). Neither set of filters was denuded of gases. A previous study found no statistically significant difference when comparing PM2.5 OPDTT collected on quartz filters to that collected on Teflon filters,57 although another study reported a 21% reduction in OPvDTT associated with quartz filters relative to Teflon filters.58 The collected samples were stored at −20 °C until analysis, which occurred approximately 100 days following the study.
2.3. Acellular Oxidative Potential Measurements
Both indoor and outdoor filter samples were analyzed for total OP by the DTT depletion assay (OPDTT) for PM2.5 particles. (This is often referred to as OPtotal DTT. The OP measurement method used in this study was designed to include both insoluble and soluble particle components, in contrast to the often-measured water-soluble fraction.) A fraction from each filter was placed in a sterile polypropylene centrifuge vial (VWR International LLC, Suwanee, GA, USA) for extraction and analysis. Due to the possible nonlinear response of the DTT assay to extract mass,46 the punched filter fraction and the volume of water used for extraction were adjusted, based on the PM2.5 mass loading on each filter, to achieve a relatively constant extract particle concentration of 10 μg/mL for both indoor and outdoor filters. Extraction of filters was performed using deionized Milli-Q water (DI, Nanopure InfinityTM ultrapure water system; resistivity > 18 MΩ/cm) via sonication (Ultrasonic Cleanser, VWR International LLC, West Chester, PA, USA) for 60 min. The filter punch remained in the extracts throughout the OP analysis to allow insoluble species to interact with the reagents. Details of the established protocol can be found in Gao et al. (2017).57 Both volume-normalized (OPvDTT) and mass-normalized (OPmDTT) results are discussed. Details of the outdoor OPDTT can be found in Yang et al. (2024).51
2.4. Aerosol Mass and Composition Measurements
The online instrumentation was in an attached garage but isolated from the house (Figure S1). They were equipped with continuous flow switching inlets to consecutively sample indoor/outdoor air. PM1 composition measurements were made with a High-Resolution Time-of-Flight Aerosol Mass Spectrometer (HR-ToF-AMS, Aerodyne Research, Inc., USA)15 with a 10 min indoor/10 min outdoor sampling cycle. Following a Nafion dryer, the HR-ToF-AMS measured non-refractory PM1 species, including NH4+, NO3–, SO42–, Cl–, and organic aerosol (OA). Indoor and outdoor light-absorbing particles were measured by an aethalometer (AE-33, Magee Scientific, Berkeley, CA) at 1 Hz by employing the same I/O switching inlet. To align with the start/stop times of the AMS, the AE-33 time series data were converted to 1 min averages for subsequent analysis. These datasets were then merged to the filter sampling times and separated into indoor and outdoor sampling periods. Light absorption measured at the 880 nm wavelength was used to determine the mass equivalent black carbon (BC) concentration based on the manufacturer’s stated mass absorption cross-section.
Neither indoor nor outdoor PM2.5 mass concentration was directly measured. Indoor PM2.5 mass concentration was determined by summing the AMS-measured non-refractory PM1 species and AE33-measured BC and merging them to the filter sampling time (24 h). This estimate was in reasonable agreement with the PM measurements using a medium-cost MODULAIR-PM sensor which combines a nephelometer and optical particle counter to measure PM1, PM2.5, and PM10 after correcting for humidity59 (Figure S2, slope 0.91, intercept 1.36 μg/m3, r2 = 0.92). For outdoor PM2.5 mass estimation, the AMS-measured species were combined with elemental carbon and metals analyzed from the filters, as described in Yang et al. (2024).51 This estimation showed good agreement with the PM2.5 mass concentrations measured by the Alaska Department of Environmental Conservation for the U.S. Environmental Protection Agency using a Beta Attenuation Monitor (BAM) at the National Core (NCore) monitoring site (roughly 2.6 km from the house) (slope 1.04, intercept 2.07 μg/m3, r2 = 0.70). While a similar assessment of the indoor PM2.5 mass concentration was not possible, missing species in the indoor measurement, such as metals and other refractory species, contributed only a small fraction (about 1%) to outdoor PM2.5 mass concentration. Therefore, these components were also expected to have a limited impact on the mass of indoor aerosol particles.
2.5. Indoor Experiments
The study involved various indoor activities, such as heating with a pellet stove, simple stovetop (electrical heating) cooking activities, and burning incense. The pellet stove was an open-hearth fireplace insert and was installed just prior to the study. It experienced issues throughout the study with exhaust leakage at the point where the pellet stove exhaust pipe connected to the existing chimney flue. This resulted in indoor smoke levels that were higher than is typically expected for this stove. The simple cooking experiments mainly involved the generation of aerosols from fats and oils from stove-top heating of vegetable oil with or without added food (e.g., chicken), as well as heating pasta and noodles. Specific details are given in Table S1. The particles and gases produced by these various indoor activities could interact with both the infiltrating air and other indoor components and surfaces. Mixed experiments, involving simultaneous cooking and pellet stove activities, with both contributing to indoor PM emissions, were also conducted (see Table S1). The house was unoccupied when there were no experiments or necessary instrument-related activities. This allowed for a focused analysis during periods when infiltration was the primary source of indoor particulate matter.
3. Results
3.1. Indoor PM Oxidative Potential
Throughout the study period, for a house with minimal indoor activities, the average indoor volume-normalized oxidative potential (OPvDTT) was 0.12 ± 0.10 nmol/min/m3 (mean ± standard deviation). This amount was approximately one-fourth of the corresponding average of the outdoor value (0.418 ± 0.215 nmol/min/m3), indicating significantly reduced exposures to OP of PM indoors. However, the mean intrinsic OP values (toxicity) of particles during the entire study period were comparable for indoor and outdoor settings, with OPm,inDTT = 31 ± 19 pmol/min/μg and OPm,outDTT = 35 ± 17 pmol/min/μg, meaning that, on average, the toxicity (measured by this assay) of the aerosol was similar between indoors and outdoors.
3.2. Effects of Outdoor Air Infiltration on OPDTT: Insights from Comparison with Sulfate
To determine the effect of infiltration of outdoor air on indoor particle health-related properties, we compared the indoor-to-outdoor (I/O) ratio of PM1 sulfate and PM2.5 OPDTT based on 24 h average data. Assuming sulfate was nonvolatile, lost only by mass-transport processes (e.g., impaction, interception, diffusion), and had no indoor sources, differences in I/O ratios of other PM species compared to sulfate would indicate changes due to gas/particle partitioning, or contributions from indoor sources, or both.15
Comparisons are made for two periods, both when there was no activity in the house: (1) Background periods, the time at the beginning of the study before any perturbation experiments; (2) No-experiment periods, the time between perturbation experiments throughout the study. See Figure S3 for a time series of the study, with periods of indoor perturbation experiments identified.
The average I/O ratio of sulfate during the combined Background and No-experiment periods was 18% (Figure S4, regression results of I/O ratio of sulfate; slope = 0.183, intercept = 0.049, r2 = 0.91), demonstrating that during the infiltration of outdoor air into the house, most of the sulfate-contained particles were removed, effectively lowering indoor exposures by about 80%. Similarly, BC is nonvolatile and may be externally mixed with some fraction of the sulfate, meaning it would potentially have a similar I/O ratio.15,16 Unfortunately, even after an inline nafion drier upstream of the aerosol instruments, the T and RH variation between indoor and outdoor sampling significantly reduced the accuracy of determining BC from the AE-33 measurement, making the calculations of the I/O ratio of BC less accurate. Hence, we assumed a similar I/O ratio as sulfate for BC and other nonvolatile PM components. Particle losses due to deposition during transport are size-dependent,60 potentially leading to varying removal efficiencies for different PM components compared to sulfate during the infiltration process, which has been observed in previous indoor/outdoor studies.15,16 This effect is likely small since by mass most particle chemical components of PM2.5 (e.g., sulfate, water-soluble metals, and organic aerosol) were in the size range of 0.1 to 1 μm. For semivolatile aerosol species, substantially lower I/O ratios would be expected due to the evaporation of these semi-volatile components, driven by the large I/O temperature and RH gradients. For the study period, the outdoor temperature ranged from roughly −35 °C to 5 °C, and outdoor RH ranged from 47% to 97% (1-h average). The indoor temperature ranged from 19.4 °C to 24.4 °C, and indoor RH ranged from 8.6% to 15.6% (1-h average).
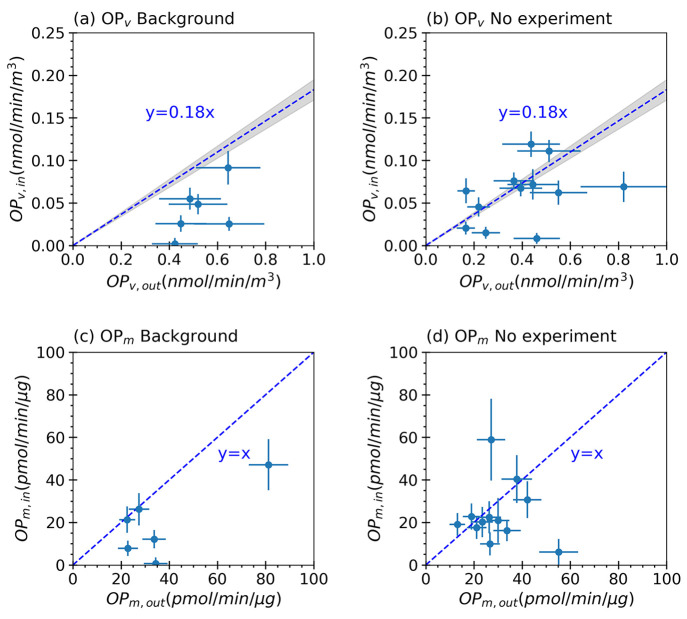
OPvDTT appeared to be influenced by the loss of semivolatile species that the DTT assay is sensitive to. For the Background period (prior to any indoor activities), the indoor OPvDTT relative to outdoor OPvDTT was consistently below the 18% observed for sulfate (Figure 1a). This was also observed when comparing the I/O ratio of mass-normalized OPDTT (OPmDTT), shown in Figure 1c, where data points were on or below the 1-to-1 line.
Figure 1.
Indoor versus outdoor levels of volume-normalized and mass-normalized OP determined with the DTT assay for both Background and No-experiment periods. Panels (a) and (b) depict volume-normalized OP, while panels (c) and (d) show mass-normalized OP. The shaded gray regions within (a) and (b) correspond to areas calculated based on the uncertainty arising from the regression slope of indoor versus outdoor PM1 sulfate concentrations (as shown in Figure S3). The error bars denote the measurement uncertainty for OP.
We have shown that outdoor OPDTT is sensitive to BC, biomass-burning organic aerosol (AMS-determined BBOA) species, and copper (Cu).51 It is likely that specific species associated with BBOA, such as oxygenated PAHs originating from biomass burning emissions,61−63 including quinones—to which the DTT assay is known to be very responsive64—may exhibit varying levels of volatility. Their particle-phase concentrations could be negatively correlated with temperature.65−67
However, no correlation was observed between the I/O ratio of OPvDTT and outdoor BBOA (r = 0) or outdoor temperature (r = 0.09). This lack of correlation is likely due to additional factors, such as variations in particle composition and changes in indoor/outdoor relative humidity (RH).
The infiltration process led to aerosol drying that would result in a reduction in water content due to the significant I/O RH gradient (mean of ΔRH = −65%). This could cause the evaporation of certain water-soluble DTT-responsive species, such as benzoquinone, since the outdoor OPvDTT was largely water-soluble (mean water-soluble to total was 77%).51 The I/O ratio of OPvDTT was negatively correlated with outdoor RH (r = −0.77); a larger I/O ratio of OPvDTT was observed when outdoor RH was lower and closer to the relatively constant indoor RH of about 10%. The I/O ratio of OPvDTT was positively correlated with outdoor EC and Cu (r = 0.78 for both), both of which are nonvolatile and expected to behave similarly to sulfate during air infiltration. These findings suggest that a significant decrease in RH and variability in particle composition affected the volatility losses, consequently impacting indoor OP levels. We used concentration of outdoor PM2.5 components here for the correlation analysis since some indoor chemical species, including EC and metals, were not measured due to insufficient PM mass collected on our filters to enable their analysis. A difference in the transmission efficiency of the gas-phase PAHs and related species relative to corresponding PM2.5 species may also alter the equilibrium when air moves between indoors and outdoors, but this is likely a minor effect relative to the extreme I/O differences in temperature and RH observed in this study.
The overall effect is that the health-related properties of the infiltrated outdoor PM2.5, as measured by the DTT assay, were substantially lower. This is despite the use of a filter sampling system to collect the particles for the OP measurement, which is in general known to miss more short-lived species, such as semivolatile organic compounds68,69 that may contribute to OPDTT,70 and that the filters had been archived for an extended period prior to analysis. It is likely that the I/O ΔT and ΔRH values were so high in this case that the effect was observed for a filter sampling system.
A similar analysis was performed for the No-experiment periods (Figure 1b,d). Indoor experiments were performed during the daytime, and measurements of different VOCs showed that VOC concentrations decay exponentially within 1 to 4 h after emissions ceased (e.g., when cooking ended or the pellet stove was shut off, etc.). There were 12 periods when the 24-h filter samples were not directly affected by the perturbation experiments from the prior days (see Figure S3). For these No-Experiment periods, most of the I/O ratio of OPvDTT data points were either close to or below the sulfate ratio line (Figure 1b), and for OPmDTT, they clustered near or below the unity slope line (Figure 1d). This pattern resembled the Background results, but there was greater dispersion in the No-experiments data. This dispersion could be due to more data points, or the residual presence of pollutants emitted in previous-day experiments. These pollutants may be adsorbed by indoor surfaces, subsequently repartitioning to particles, or lead to the production of secondary species that repartitioned to the particles, both cases raising the indoor OPDTT. The long sample time of these OPDTT data limits a more detailed investigation of the repartitioning of the DTT-active species. We speculate this partitioning would be mainly from pellet stove emissions adsorbing to indoor surfaces, providing a reservoir for subsequent re-emission.71 From these data, we cannot conclude that there is a definitive effect. In the further analysis below, given the substantial variability in OPDTT observed during the No-experiment period, the pollutant enhancements and PM2.5 OPDTT in perturbation experiments were exclusively compared to the Background levels.
3.3. PM2.5 Mass Concentration and OPDTT for Specific Indoor Perturbation Experiments
The perturbation experiments do not necessarily represent indoor concentrations for a typical household performing these various activities. Therefore, we compared the intrinsic health-relevant properties of these activities by focusing on OPmDTT and compared correlations between OPvDTT and PM2.5 mass concentrations. However, indoor OA concentrations can influence partitioning, potentially altering the relative concentration of components contributing to OP and, consequently, mass-normalized OP, which is not considered in this study.
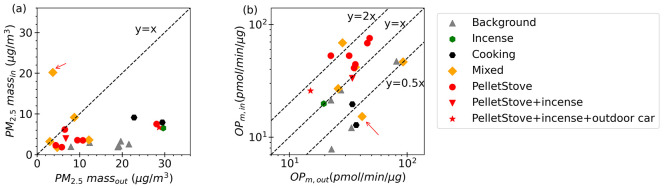
Some indoor perturbation experiments produced notably elevated PM2.5 mass concentrations (1 min average, as per AMS + BC data, peaked at 754 μg/m3 during a mixed experiment.). These peaks were of limited temporal extent, so when averaged over the 24 h filter sampling time, the concentrations were much lower. Thus, the majority of indoor PM mass had concentrations of less than 10 μg/m3, with values either less than or equal to the 24-h average outdoor PM mass (Figure 2a).
Figure 2.
Comparison of (a) PM2.5 mass concentration and (b) OPm (log scale) between indoor and outdoor environment based on 24-h averaged data. Note that one background data point is not plotted in (b) since lower than the range of the vertical axis plotted. The outlier of high PM2.5 indoor mass highlighted with an arrow in (a) is also identified in plot (b).
When compared to Background conditions within the house, the mean PM2.5 mass concentration was higher for all perturbation experiments (Table 1). Cooking and mixed experiments had the most pronounced elevation; the mean PM2.5 mass concentration exceeded the Background level by more than a factor of 3. Pellet stove experiments produced a smaller increase relative to that in the Background (Table 1). The highest 24 h average indoor PM mass concentration of 20.18 μg/m3 occurred during a mixed experiment when both cooking activities and pellet stove emissions contributed to the indoor PM levels (the outlier in Figure 2a identified with an arrow, where very high cooking emissions occurred when water dripped into a pan of hot cooking oil, but no cooking activities took place).
Table 1. Indoor PM2.5 Mass Concentration, Volume- and Mass-Normalized OPDTT and Indoor-to-Outdoor (I/O) Ratio of OPmDTT during Background and Indoor Perturbation Experiments Based on 24-h Average Filter Samplesa.
| N | PM2.5 mass (μg/m3) | OPvDTT (nmol/min/m3) | OPmDTT (pmol/min/μg) | I/O OPmDTT | |
|---|---|---|---|---|---|
| Background | 6 | 2.45 ± 0.58 | 0.041 ± 0.031 | 19.2 ± 16.5 | 0.53 ± 0.37 |
| No-experiment | 12 | 2.86 ± 1.76 | 0.061 ± 0.034 | 23.8 ± 14.2 | 0.90 ± 0.54 |
| Pellet Stove | 6 | 4.14 ± 2.23 | 0.232 ± 0.140 | 55.9 ± 13.5 | 1.57 ± 0.43 |
| Pellet Stove + incense | 1 | 3.96 | 0.132 | 33.4 | 0.98 |
| Pellet Stove + incense + outdoor car | 1 | 6.67 | 0.172 | 25.8 | 1.71 |
| Incense | 1 | 6.53 | 0.130 | 19.8 | 1.01 |
| Cooking | 2 | 8.50 ± 0.85 | 0.136 ± 0.027 | 16.2 ± 4.8 | 0.46 ± 0.16 |
| Mixed | 5 | 7.58 ± 7.59 | 0.195 ± 0.079 | 39.9 ± 20.3 | 1.10 ± 0.82 |
Means and standard deviations are shown.
Like PM2.5 mass, there was a noticeable increase in indoor OP related to exposure (OPvDTT) throughout all of the indoor perturbation experiments compared to the Background level (Table 1), highlighting the substantial possible health impact of indoor activities. Notably, experiments involving the use of the pellet stove had the most significant increase in OPvDTT compared to that of the Background level, reaching an average of 0.232 nmol/min/m3. Conversely, particles generated from cooking and incense had comparatively lower levels of OPvDTT, despite cooking producing the highest PM2.5 mass concentration. Comparing differences in PM2.5 mass concentration between the various experiments to the Background levels and corresponding OPvDTT, shows no trend between PM2.5 mass concentration and OPvDTT (Table 1), pointing to highly variable OPmDTT between the different indoor emissions tested.
Another way to assess the relative OPDTT of the indoor experiments is to compare the OPmDTT (i.e., OPvDTT divided by the indoor PM2.5 mass concentration) for each indoor experiment to the outdoor OPmDTT. This comparison shows the intrinsic OP properties (like toxicity) of indoor air relative to that of outdoor air. Figure 2b shows large differences between indoor and outdoor OPmDTT values among perturbation experiments. Pellet stove-based experiments produced higher average OPmDTT relative to outdoor levels (data points above the 1:1 line in Figure 2b and I/O ratio of OPmDTT > 1 in Table 1). All cooking experiments resulted in OPmDTT lower than outdoor levels (I/O ratio of OPmDTT < 1). The OPmDTT levels in cooking emitted particles were comparable with a previous study on both primary and secondary emissions from heated cooking oils (5–20 pmol/min/μg).72 In addition, the low toxicity of cooking-emitted particles was consistent with OP measurements obtained through electron paramagnetic resonance (OPEPR) in another study.73 Toxicity of incense-burning particle emissions closely resembled that of outdoor air (I/O ratio of OPmDTT ≈ 1). OPmDTT of the incense-burning particles was notably lower than that in a previous study (65.3–68.3 pmol/min/μg),54 likely attributed to the extended sampling time (24-h) in this study, averaging the toxicity of incense burning over a longer period relative to the 50 min burning time. Mixed experiments were scattered between these ranges, dependent on the relative amounts of pellet stove and cooking emissions. For example, the mixed experiment with extremely high PM2.5 mass concentration due to large cooking oil emissions (data point noted by the arrow in Figure 2a) had a low OPmDTT (Figure 2b).
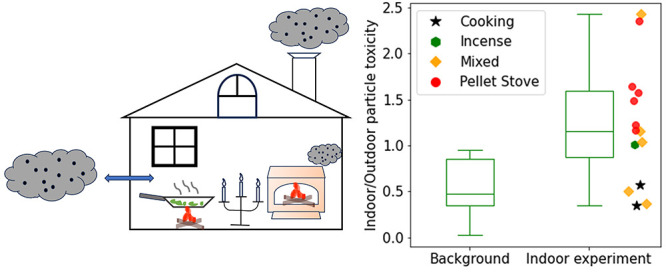
Differences in PM2.5 OPmDTT (toxicities) for the various indoor activities and relative to the background levels due to outdoor air infiltration are shown in Figure 3. The pellet stove emissions had the highest OPmDTT (average of 55.9 pmol/min/μg, Table 1), followed by mixed experiments involving pellet stove plus cooking and pellet stove plus incense emissions. Cooking emissions were associated with the lowest toxicity (mean OPmDTT of 16.2 pmol/min/μg), even below the Background OPmDTT levels when only infiltration of outdoor air contributed to indoor OPDTT. This is because the OPvDTT was low for cooking, although the mass concentration was high. These intrinsic OPDTT values were consistent with those reported by Bates et al. (2019), summarizing results from multiple studies.74 The OPmDTT associated with pellet stove emissions fell within the range of values associated with biomass burning (20–200 pmol/min/μg), while cooking emissions were comparable with the lower limit of biogenic secondary OA (1–50 pmol/min/μg). Mixed experiments were similar to the average outdoor OPmDTT of PM2.5 reported in a number of studies (nominally 30 pmol/min/ug).74
Figure 3.
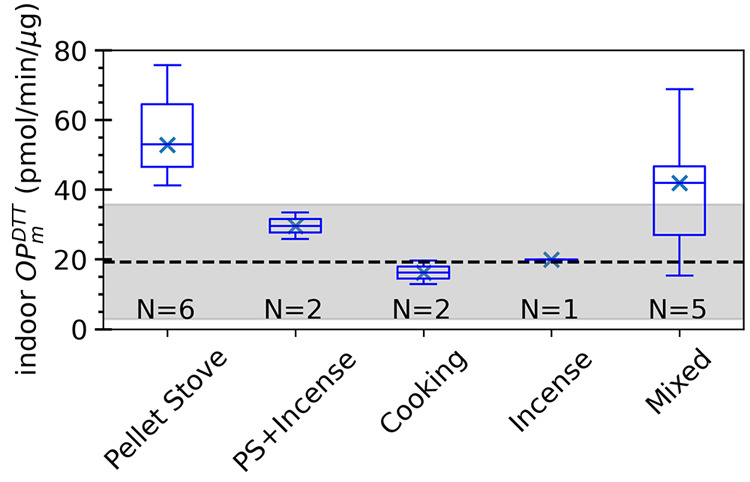
Boxplots of indoor OPmDTT during different perturbation experiments relative to indoor Background OPmDTT, where Background is from measurements in the empty house prior to any indoor experiments and only due to infiltration of outdoor air. The mean Background OPmDTT is indicated by the dashed line, and the shaded region represents its standard deviation. The two categories, pellet stove + incense and pellet stove + incense + outdoor car, with only one data point each, have comparable OPmDTT levels and have been merged (PS + Incense) in this plot. The marker and line in the box indicate the median value (Q2), the lower and upper box boundaries indicate the first quartile (Q1) and the third quartile (Q3), and the whiskers indicate the minimum and maximum values for each corresponding category.
Overall, these results highlight the significance of considering both the chemical composition and the quantity (mass concentration) of particles as health metrics for indoor air quality.
3.4. Relationship between PM2.5 Mass Concentration and OPDTT
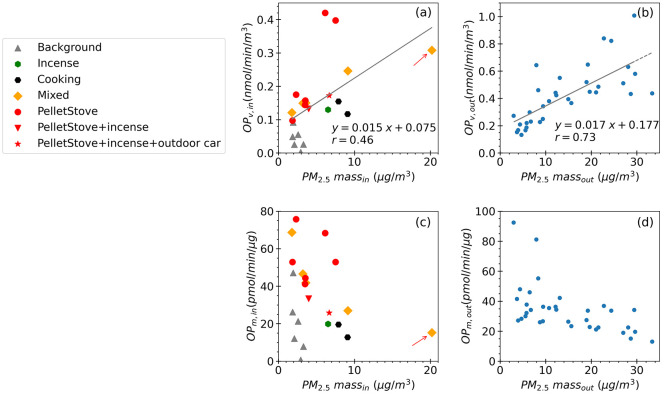
Given that effects of PM on health are often based on correlations between PM2.5 mass concentrations and adverse health end points, correlations between PM2.5 mass concentration and OPv are of interest. Comparing Figure 4a,b shows a much lower Pearson correlation between the OPvDTT and PM2.5 mass concentration for indoor experiments (r = 0.46) compared to outdoor air (r = 0.73). The scattering of indoor data is attributed to the differences in various types of indoor emissions. The two data points significantly deviating from the regression line with relatively high OPvDTT values in Figure 4a correspond to emissions from the pellet stove, indicating heightened toxicity. Moderate to high correlations (e.g., often in the range of 0.44 to 0.75) between OPvDTT and PM mass concentration have been reported in ambient (outdoor air) studies.48,75,76 These findings, along with the considerable variability in OPmDTT for the indoor experiments, suggest that the PM2.5 mass concentration may not adequately represent the relative adverse health responses from exposures to different indoor sources.
Figure 4.
Scatter plots between indoor PM2.5 mass concentration and (a) indoor OPv (c) indoor OPm during different perturbation experiments, and between outdoor PM2.5 mass concentration and (b) outdoor OPv, (d) outdoor OPm during the whole study period based on 24-h averaged data. Orthogonal regression was applied in (a) and (b). The outlier of high PM2.5 indoor mass highlighted with an arrow in (a) is also identified in plot (c).
Correlations between OPm and PM2.5 mass concentration can also show the extent of all PM2.5 species on particle toxicity. Both indoor and outdoor OPm were negatively correlated with PM2.5 mass (Figure 4c,d), indicating that many PM components that contributed to PM2.5 mass did not significantly contribute to the OP response. Conversely, there were other PM components that contributed to the OP but had minor contributions to the overall PM2.5 mass concentration. Specifically, in terms of sources, pellet stove emissions were mainly concentrated in the upper left of Figure 4c, indicating relatively low PM loadings and high intrinsic toxicity. In contrast, cooking emissions in the middle to lower part of Figure 4c produced large amounts of PM (about 10 μg/m3) with an OPm less than 20 pmol/min/μg. The mixed experiment involving concurrent emissions from both pellet stoves and cooking, showed the properties of both sources. The mixed experiment with very high cooking emissions (aerosolized hot oil in a pan, the outlier identified by the arrow in Figure 4) had a low OPmDTT.
4. Limitations and Implications
The indoor perturbation experiments of this study do not necessarily reflect a “typical” indoor home environment. All indoor experiments were conducted mainly in the afternoon and had relatively short durations. The cooking activities were highly simplified, and there were no other concurrent human activities. The pellet stove was not operated for extended periods and often produced high levels of smoke indoors despite being recently installed and serviced during the study. Pellet stove indoor emissions are expected to be much lower than other indoor wood-heating methods since the combustion chamber is isolated from the room, in contrast to open-hearth fireplaces and airtight stoves that require opening for loading wood (although ideally, a properly functioning airtight stove, once warmed, draws room air up the hot chimney, reducing emissions into the room during fueling).
The DTT assay utilized in this study, while sensitive to a wide range of chemical components, primarily focuses on the formation of superoxide (O2•–) and does not include the generation of hydroxyl radicals (·OH), which are crucial steps in the ROS cascade.77 Furthermore, DTT is a surrogate for reducing agents in cells, (e.g., NADH and NADPH)78 and may not perfectly replicate the biological processes of oxidative stress in vivo. Future research should incorporate multiple assays, such as assays capable of measuring PM’s ability to generate ·OH, or cellular OP assays that consider additional biological responses involving the generation of ROS within biological organisms, to better assess the health-relevant properties of indoor PM.
Although OPvDTT values reported here may not be broadly comparable to other houses or locations, the Background and No-experiment cases may be representative of what occurs in other houses in Fairbanks and in other cold urban environments. OPmDTT for the perturbation experiments provides a measure of the intrinsic health-relevant properties of these emissions (based on this assay) and can be compared to values reported in the literature for various other types of sources.79 The OPmDTT (toxicity) of the higher-than-normal emitting pellet stove may be similar to emissions from other forms of wood-burning, which are expected to be high.80 Based on the DTT assay, we find that incomplete combustion emissions are of greatest concern, for both outdoor and indoor environments, which is well-established for outdoor pollution.
These OP data also enable comparisons to the PM2.5 mass concentration as an air quality metric. Several studies for outdoor aerosols (ambient air) have shown that OP may be more strongly linked to specific health end points than PM2.5 mass concentration.49,79 The indoor experiments show that divergence between OP and mass may be even greater for emissions in an indoor environment due to strong influences from individual sources, many of which may be non-combustion related and can have lower OPmDTT. More studies on the I/O ratio of OP in various locations using multiple assays are needed. Online measurement approaches would allow the assessment of volatile OP species81−83 and provide larger datasets. Overall, this study demonstrated the complex interplay between indoor and outdoor aerosol sources on indoor air quality characterized by the PM2.5 oxidative potential.
Acknowledgments
We thank the entire ALPACA science team of researchers for designing the experiments, acquiring funding, making measurements, and ongoing analysis of the results. The ALPACA project was initiated as a part of PACES under IGAC and with the support of IASC. We thank the University of Alaska Fairbanks and the Geophysical Institute for logistical support, and Fairbanks for welcoming and engaging with this research. We thank the Alaska Department of Environmental Conservation (ADEC) for data collection at the NCORE site. K.C.E., S.K., T.F., and M.S. collected the Hi-Vol samples and graciously provided portions of the Hi-Vol filter samples for our analysis. Y.Y., M.A.B., and R.J.W. were supported by the National Science Foundation’s (NSF) Atmospheric Geoscience Program (grant no. AGS-2029730) and the NSF Navigating the New Arctic Program (grant no. NNA-1927778). Y.Y. was also supported in part by the Phillips 66 Company (grant no. AGR DTD 10/05/2020). M.A.B. was also supported by NASA (grant No. 80NSSC18K0557). E.S.R. and P.F.D. were supported by the NSF Navigating the New Arctic Program (grant nos. NNA-90086753 and NNA-1927750). K.C.E., S.K., T.F., and M.S. were supported by the National Science Foundation (grant no. AGS-1654104). M.C.-M. and W.R.S. were supported by the NSF Sustainably Navigating Arctic Pollution Through Engaging Communities (SNAP-TEC) Program (grant no. 1927750). J.R.C. and J.M. were supported by the NSF Atmospheric Geoscience Program (grant no. AGS-2029747) and the NSF Navigating the New Arctic Program (grant no. NNA-1927750). A.N. was supported by the European Research Council (ERC) project “PyroTRACH” (Grant agreement No. 726165).
Data Availability Statement
Data is available on arcticdata.io: https://arcticdata.io/catalog/view/doi%3A10.18739%2FA23R0PV7J.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsestair.3c00067.
Detailed description of the indoor experiments (Table S1); 3-D rendering of the one-story house’s plan (Figure S1); comparison between indoor PM2.5 mass concentration estimated by PM1 + BC and PM2.5 measurement using a low-cost optical PM sensor (Figure S2); time series of indoor–outdoor (I/O) PM2.5 mass, I/O OPmDTT, and the time slot of different experiments (Figure S3); daily average PM1 sulfate concentrations measured indoors and outdoors with an Aerosol Mass Spectrometer merged to the filter sampling time during periods of no indoor activities (Figure S4) (PDF)
Author Present Address
◆ U.S. Army DEVCOM CBC, Aberdeen Proving Ground, Maryland, 21010 USA
Author Present Address
□ Sustainable Energy and Environment Thrust, The Hong Kong University of Science and Technology (Guangzhou), Guangzhou, 511400 China
The authors declare no competing financial interest.
Supplementary Material
References
- Baklanov A.; Wmo U.; Bell D.; Descari S.; Ganzenfeld L.; Konstantinov P.; Raut J.-C.; Latmos F.; Thomas J.; Weber R.. Alaskan Layered Pollution And Chemical Analysis (ALPACA) White Paper, 2019.
- Cesler-Maloney M.; Simpson W. R.; Miles T.; Mao J.; Law K. S.; Roberts T. J. Differences in Ozone and Particulate Matter Between Ground Level and 20 m Aloft are Frequent During Wintertime Surface-Based Temperature Inversions in Fairbanks, Alaska. Journal of Geophysical Research: Atmospheres 2022, 127 (10), e2021JD036215 10.1029/2021JD036215. [DOI] [Google Scholar]
- Busby B. D.; Ward T. J.; Turner J. R.; Palmer C. P. Comparison and evaluation of methods to apportion ambient PM2.5 to residential wood heating in Fairbanks, AK. Aerosol and air quality research 2016, 16 (3), 492–503. 10.4209/aaqr.2015.04.0235. [DOI] [Google Scholar]
- Ward T.; Trost B.; Conner J.; Flanagan J.; Jayanty R. Source apportionment of PM2.5 in a subarctic airshed-fairbanks, Alaska. Aerosol and Air Quality Research 2012, 12 (4), 536–543. 10.4209/aaqr.2011.11.0208. [DOI] [Google Scholar]
- Kotchenruther R. A. Source apportionment of PM2.5 at multiple Northwest US sites: Assessing regional winter wood smoke impacts from residential wood combustion. Atmospheric Environment 2016, 142, 210–219. 10.1016/j.atmosenv.2016.07.048. [DOI] [Google Scholar]
- Ye L.; Wang Y. Long-term air quality study in Fairbanks, Alaska: Air pollutant temporal variations, correlations, and PM2.5 source apportionment. Atmosphere 2020, 11 (11), 1203. 10.3390/atmos11111203. [DOI] [Google Scholar]
- World_Health_Organization Household Air Pollution. https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health#:~:text=The%20combined%20effects%20of%20ambient,(COPD)%20and%20lung%20cancer (accessed 9 January 2024).
- Tran V. V.; Park D.; Lee Y.-C. Indoor air pollution, related human diseases, and recent trends in the control and improvement of indoor air quality. International journal of environmental research and public health 2020, 17 (8), 2927. 10.3390/ijerph17082927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Kesavachandran C. N.; Kamal R.; Bihari V.; Ansari A.; Azeez P. A.; Saxena P. N.; Ks A. K.; Khan A. H. Indoor air pollution and its association with poor lung function, microalbuminuria and variations in blood pressure among kitchen workers in India: a cross-sectional study. Environmental Health 2017, 16, 33. 10.1186/s12940-017-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkovich S. M.; Goodman D.; Roa C.; Crocker M. E.; Gianella G. E.; Kirenga B. J.; Wise R. A.; Checkley W. The health and social implications of household air pollution and respiratory diseases. NPJ. primary care respiratory medicine 2019, 29 (1), 12. 10.1038/s41533-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Wen Q.; Zhang R. Sources, health effects and control strategies of indoor fine particulate matter (PM2. 5): A review. Science of the Total Environment 2017, 586, 610–622. 10.1016/j.scitotenv.2017.02.029. [DOI] [PubMed] [Google Scholar]
- Pérez-Padilla R.; Schilmann A.; Riojas-Rodriguez H. Respiratory health effects of indoor air pollution. Int. J. Tuberc. Lung Dis. 2010, 14 (9), 1079–1086. [PubMed] [Google Scholar]
- Chafe Z. A.; Brauer M.; Klimont Z.; Van Dingenen R.; Mehta S.; Rao S.; Riahi K.; Dentener F.; Smith K. R. Household cooking with solid fuels contributes to ambient PM2. 5 air pollution and the burden of disease. Environ. Health Perspect. 2014, 122 (12), 1314–1320. 10.1289/ehp.1206340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack M. C.; Belli A. J.; Kaji D. A.; Matsui E. C.; Brigham E. P.; Peng R. D.; Sellers C.; Williams D. L.; Diette G. B.; Breysse P. N.; Hansel N. N. Obesity as a susceptibility factor to indoor particulate matter health effects in COPD. European Respiratory Journal 2015, 45 (5), 1248–1257. 10.1183/09031936.00081414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. M.; Waring M. S.; DeCarlo P. F. Real-time transformation of outdoor aerosol components upon transport indoors measured with aerosol mass spectrometry. Indoor Air 2017, 27 (1), 230–240. 10.1111/ina.12299. [DOI] [PubMed] [Google Scholar]
- Avery A. M.; Waring M. S.; DeCarlo P. F. Seasonal variation in aerosol composition and concentration upon transport from the outdoor to indoor environment. Environmental Science: Processes & Impacts 2019, 21 (3), 528–547. 10.1039/C8EM00471D. [DOI] [PubMed] [Google Scholar]
- Cummings B. E.; Shiraiwa M.; Waring M. S. Phase state of organic aerosols may limit temperature-driven thermodynamic repartitioning following outdoor-to-indoor transport. Environmental Science: Processes & Impacts 2022, 24 (10), 1678–1696. 10.1039/D2EM00093H. [DOI] [PubMed] [Google Scholar]
- Cummings B. E.; Li Y.; DeCarlo P. F.; Shiraiwa M.; Waring M. S. Indoor aerosol water content and phase state in US residences: impacts of relative humidity, aerosol mass and composition, and mechanical system operation. Environmental Science: Processes & Impacts 2020, 22 (10), 2031–2057. 10.1039/D0EM00122H. [DOI] [PubMed] [Google Scholar]
- Wang B.; Tang Z.; Li Y.; Cai N.; Hu X. Experiments and simulations of human walking-induced particulate matter resuspension in indoor environments. Journal of Cleaner Production 2021, 295, 126488. 10.1016/j.jclepro.2021.126488. [DOI] [Google Scholar]
- Men Y.; Li J.; Liu X.; Li Y.; Jiang K.; Luo Z.; Xiong R.; Cheng H.; Tao S.; Shen G. Contributions of internal emissions to peaks and incremental indoor PM2. 5 in rural coal use households. Environmental Pollution 2021, 288, 117753. 10.1016/j.envpol.2021.117753. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Son Y. J.; Li L.; Wood N.; Senerat A. M.; Pantelic J. Healthy home interventions: Distribution of PM2. 5 emitted during cooking in residential settings. Building and Environment 2022, 207, 108448. 10.1016/j.buildenv.2021.108448. [DOI] [Google Scholar]
- Zhang L.; Ou C.; Magana-Arachchi D.; Vithanage M.; Vanka K. S.; Palanisami T.; Masakorala K.; Wijesekara H.; Yan Y.; Bolan N.; Kirkham M. B. Indoor particulate matter in urban households: sources, pathways, characteristics, health effects, and exposure mitigation. International Journal of Environmental Research and Public Health 2021, 18 (21), 11055. 10.3390/ijerph182111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.; Zhang P.; Chu B.; Ma Q.; Ge Y.; Liu J.; He H. Secondary organic aerosol formation from mixed volatile organic compounds: Effect of RO2 chemistry and precursor concentration. npj Climate and Atmospheric Science 2022, 5 (1), 95. 10.1038/s41612-022-00321-y. [DOI] [Google Scholar]
- Vardoulakis S.; Giagloglou E.; Steinle S.; Davis A.; Sleeuwenhoek A.; Galea K. S.; Dixon K.; Crawford J. O. Indoor exposure to selected air pollutants in the home environment: A systematic review. International journal of environmental research and public health 2020, 17 (23), 8972. 10.3390/ijerph17238972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S.; Sexton K. G.; Turpin B. Oxygenated VOC s, aqueous chemistry, and potential impacts on residential indoor air composition. Indoor Air 2018, 28 (1), 198–212. 10.1111/ina.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W.; Zhao B. Contribution of outdoor-originating particles, indoor-emitted particles and indoor secondary organic aerosol (SOA) to residential indoor PM2. 5 concentration: A model-based estimation. Building and environment 2015, 90, 196–205. 10.1016/j.buildenv.2015.04.006. [DOI] [Google Scholar]
- Florou K.; Kodros J. K.; Paglione M.; Jorga S.; Squizzato S.; Masiol M.; Uruci P.; Nenes A.; Pandis S. N. Characterization and dark oxidation of the emissions of a pellet stove. Environmental Science: Atmospheres 2023, 3 (9), 1319–1334. 10.1039/D3EA00070B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaidullah M.; De Ruyck J.. Performance, gaseous and particle emissions from a residential pellet stove. In Renewable Energy-Technologies and Applications; IntechOpen: Rijeka, 2020; Chapter 20. [Google Scholar]
- Boman C.; Pettersson E.; Westerholm R.; Bostrom D.; Nordin A. Stove performance and emission characteristics in residential wood log and pellet combustion, part 1: pellet stoves. Energy Fuels 2011, 25 (1), 307–314. 10.1021/ef100774x. [DOI] [Google Scholar]
- Toscano G.; Duca D.; Amato A.; Pizzi A. Emission from realistic utilization of wood pellet stove. Energy 2014, 68, 644–650. 10.1016/j.energy.2014.01.108. [DOI] [Google Scholar]
- Heringa M. F.; DeCarlo P. F.; Chirico R.; Lauber A.; Doberer A.; Good J.; Nussbaumer T.; Keller A.; Burtscher H.; Richard A.; Miljevic B.; Prevot A. S. H.; Baltensperger U. Time-resolved characterization of primary emissions from residential wood combustion appliances. Environ. Sci. Technol. 2012, 46 (20), 11418–11425. 10.1021/es301654w. [DOI] [PubMed] [Google Scholar]
- Kenar J. A.; Compton D. L.; Peterson S. C.; Felker F. C. Characterization and properties of starch-dicarboxylic acid inclusion complexes prepared by excess steam jet cooking. Carbohydr. Polym. 2022, 296, 119955. 10.1016/j.carbpol.2022.119955. [DOI] [PubMed] [Google Scholar]
- Li Y.-J.; Wu A.-H.; Tong M.-X.; Luan S.-J.; Li Z.; Hu M. Emission characteristics of particulate organic matter from cooking. Huan Jing Ke Xue 2020, 41 (8), 3467–3474. 10.13227/j.hjkx.202001027. [DOI] [PubMed] [Google Scholar]
- Li B.; Ma Y.; Zhou Y.; Chai E. Research progress of different components of PM2.5 and ischemic stroke. Sci. Rep. 2023, 13 (1), 15965. 10.1038/s41598-023-43119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforge M.; Elbim C.; Frère C.; Hémadi M.; Massaad C.; Nuss P.; Benoliel J.-J.; Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nature Reviews Immunology 2020, 20 (9), 515–516. 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad M.; Anil Kumar N. V.; Zucca P.; Varoni E. M.; Dini L.; Panzarini E.; Rajkovic J.; Tsouh Fokou P. V.; Azzini E.; Peluso I.; Prakash Mishra A.; Nigam M.; El Rayess Y.; Beyrouthy M. E.; Polito L.; Iriti M.; Martins N.; Martorell M.; Docea A. O.; Setzer W. N.; Calina D.; Cho W. C.; Sharifi-Rad J. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Frontiers in physiology 2020, 11, 694. 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates J. T.; Weber R. J.; Abrams J.; Verma V.; Fang T.; Klein M.; Strickland M. J.; Sarnat S. E.; Chang H. H.; Mulholland J. A.; Tolbert P. E.; Russell A. G. Reactive oxygen species generation linked to sources of atmospheric particulate matter and cardiorespiratory effects. Environ. Sci. Technol. 2015, 49 (22), 13605–13612. 10.1021/acs.est.5b02967. [DOI] [PubMed] [Google Scholar]
- Yang A.; Janssen N. A.; Brunekreef B.; Cassee F. R.; Hoek G.; Gehring U. Children’s respiratory health and oxidative potential of PM2. 5: the PIAMA birth cohort study. Occupational and Environmental Medicine 2016, 73 (3), 154–160. 10.1136/oemed-2015-103175. [DOI] [PubMed] [Google Scholar]
- Abrams J. Y.; Weber R. J.; Klein M.; Samat S. E.; Chang H. H.; Strickland M. J.; Verma V.; Fang T.; Bates J. T.; Mulholland J. A.; Russell A. G.; Tolbert P. E. Associations between ambient fine particulate oxidative potential and cardiorespiratory emergency department visits. Environ. Health Perspect. 2017, 125 (10), 107008. 10.1289/EHP1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strak M.; Janssen N.; Beelen R.; Schmitz O.; Vaartjes I.; Karssenberg D.; van den Brink C.; Bots M. L.; Dijst M.; Brunekreef B.; Hoek G. Long-term exposure to particulate matter, NO2 and the oxidative potential of particulates and diabetes prevalence in a large national health survey. Environ. Int. 2017, 108, 228–236. 10.1016/j.envint.2017.08.017. [DOI] [PubMed] [Google Scholar]
- Janssen N. A H; Strak M.; Yang A.; Hellack B.; Kelly F. J; Kuhlbusch T. A J; Harrison R. M; Brunekreef B.; Cassee F. R; Steenhof M.; Hoek G. Associations between three specific a-cellular measures of the oxidative potential of particulate matter and markers of acute airway and nasal inflammation in healthy volunteers. Occupational and environmental medicine 2015, 72 (1), 49–56. 10.1136/oemed-2014-102303. [DOI] [PubMed] [Google Scholar]
- Weichenthal S.; Lavigne E.; Traub A.; Umbrio D.; You H.; Pollitt K.; Shin T.; Kulka R.; Stieb D. M.; Korsiak J.; Jessiman B.; Brook J. R.; Hatzopoulou M.; Evans G.; Burnett R. T. Association of Sulfur, Transition Metals, and the Oxidative Potential of Outdoor PM 2.5 with Acute Cardiovascular Events: A Case-Crossover Study of Canadian Adults. Environ. Health Perspect. 2021, 129 (10), 107005. 10.1289/EHP9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maikawa C. L.; Weichenthal S.; Wheeler A. J.; Dobbin N. A.; Smargiassi A.; Evans G.; Liu L.; Goldberg M. S.; Pollitt K. J. G. Particulate oxidative burden as a predictor of exhaled nitric oxide in children with asthma. Environ. Health Perspect. 2016, 124 (10), 1616–1622. 10.1289/EHP175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyib O.; Lavigne E.; Traub A.; Umbrio D.; You H.; Ripley S.; Pollitt K.; Shin T.; Kulka R.; Jessiman B.; Tjepkema M.; Martin R.; Stieb D. M.; Hatzopoulou M.; Evans G.; Burnett R. T; Weichenthal S. Long-term exposure to oxidant gases and mortality: Effect modification by PM2.5 transition metals and oxidative potential. Epidemiology (Cambridge, Mass.) 2022, 33 (6), 767. 10.1097/EDE.0000000000001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E.; Burnett R. T.; Stieb D. M.; Evans G. J.; Godri Pollitt K. J.; Chen H.; van Rijswijk D.; Weichenthal S. Fine particulate air pollution and adverse birth outcomes: effect modification by regional nonvolatile oxidative potential. Environ. Health Perspect. 2018, 126 (7), 077012. 10.1289/EHP2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier J. G.; McFall A. S.; Vu K. K.; Baroi J.; Olea C.; Hasson A.; Anastasio C. A bias in the “mass-normalized” DTT response-An effect of non-linear concentration-response curves for copper and manganese. Atmospheric Environment 2016, 144, 325–334. 10.1016/j.atmosenv.2016.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier J. G.; Anastasio C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals. Atmospheric chemistry and physics (Print) 2012, 12 (5), 9321. 10.5194/acp-12-9321-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D.; Mulholland J. A.; Russell A. G.; Weber R. J. Characterization of water-insoluble oxidative potential of PM2.5 using the dithiothreitol assay. Atmospheric Environment 2020, 224, 117327. 10.1016/j.atmosenv.2020.117327. [DOI] [Google Scholar]
- Daellenbach K. R.; Uzu G.; Jiang J.; Cassagnes L.-E.; Leni Z.; Vlachou A.; Stefenelli G.; Canonaco F.; Weber S.; Segers A.; Kuenen J. J. P.; Schaap M.; Favez O.; Albinet A.; Aksoyoglu S.; Dommen J.; Baltensperger U.; Geiser M.; El Haddad I.; Jaffrezo J.-L.; Prevot A. S. H. Sources of particulate-matter air pollution and its oxidative potential in Europe. Nature 2020, 587 (7834), 414–419. 10.1038/s41586-020-2902-8. [DOI] [PubMed] [Google Scholar]
- Grange S. K.; Uzu G.; Weber S.; Jaffrezo J.-L.; Hueglin C. Linking Switzerland’s PM 10 and PM 2.5 oxidative potential (OP) with emission sources. Atmospheric Chemistry and Physics 2022, 22 (10), 7029–7050. 10.5194/acp-22-7029-2022. [DOI] [Google Scholar]
- Yang Y.; Battaglia M. A.; Moha M. K.; Robinson E. S.; DeCarlo P. F.; Edwards K. C.; Fang T.; Kapur S.; Shiraiwa M.; Cesler-Maloney M.; Simpson W. R.; Campbell J. R.; Nenes A.; Mao J.; Weber R. J.. Assessing the Oxidative Potential of PM2.5 in Wintertime Fairbanks, Alaska. ACS ES&T Air 2024 10.1021/acsestair.3c00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F.; Liu C.; Qian H. Comparison of indoor and outdoor oxidative potential of PM2. 5: pollution levels, temporal patterns, and key constituents. Environ. Int. 2021, 155, 106684. 10.1016/j.envint.2021.106684. [DOI] [PubMed] [Google Scholar]
- Szigeti T.; Dunster C.; Cattaneo A.; Cavallo D.; Spinazze A.; Saraga D. E.; Sakellaris I. A.; de Kluizenaar Y.; Cornelissen E. J.M.; Hanninen O.; Peltonen M.; Calzolai G.; Lucarelli F.; Mandin C.; Bartzis J. G.; Zaray G.; Kelly F. J. Oxidative potential and chemical composition of PM2. 5 in office buildings across Europe-The OFFICAIR study. Environ. Int. 2016, 92-93, 324–333. 10.1016/j.envint.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Hu H.; Ye J.; Liu C.; Yan L.; Yang F.; Qian H. Emission and oxidative potential of PM2. 5 generated by nine indoor sources. Building and Environment 2023, 230, 110021. 10.1016/j.buildenv.2023.110021. [DOI] [Google Scholar]
- Robinson E. S.; Cesler-Maloney M.; Tan X.; Mao J.; Simpson W.; DeCarlo P. F. Wintertime spatial patterns of particulate matter in Fairbanks, AK during ALPACA 2022. Environmental Science: Atmospheres 2023, 3 (3), 568–580. 10.1039/D2EA00140C. [DOI] [Google Scholar]
- Simpson W. R.; Mao J.; Fochesatto J.; Law K.; DeCarlo P. F.; Schmale J.; Pratt K. A.; Arnold S. R.; Stutz J.; Dibb J.; Creamean J.; Weber R. J.; Williams B.; Alexander B.; Hu L.; Yokelson R.; Shiraiwa M.; Decesari S.; Anastasio C.; D’Anna B.; Gilliam R.; Clair J. M. S.; Trost B.; Flynn J.; Savarino J.; Albertin S.; Baccarini A.; Barret B.; Bekki S.; Brado T.; Brett N.; Brus D.; Campbell J. R.; Cesler-Maloney M.; Cooperdock S.; Carvalho K. C. d.; DeMott P.; Dieudonné E.; Dingilian K.; Donateo A.; Edwards K. C.; Fahey K.; Fang T.; Guo F.; Heinlein L.; Holen A.; Huff D.; Ijaz A.; Kapur S.; Ketcherside D.; Levin E.; Lill E.; Moon A.; Onishi T.; Pappaccogli G.; Perkins R.; Pohorsky R.; Raut J.-C.; Ravetta F.; Roberts T.; Robinson E. S.; Scoto F.; Selimovic V.; Roussel B. T.; Tian X.; Wu J.; Yang Y., Overview of the Alaskan Layered Pollution And Chemical Analysis (ALPACA) field experiment. ACS ES&T Air 2024, 10.1021/acsestair.3c00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D.; Fang T.; Verma V.; Zeng L.; Weber R. J. A method for measuring total aerosol oxidative potential (OP) with the dithiothreitol (DTT) assay and comparisons between an urban and roadside site of water-soluble and total OP. Atmospheric Measurement Techniques 2017, 10 (8), 2821–2835. 10.5194/amt-10-2821-2017. [DOI] [Google Scholar]
- Yang A.; Jedynska A.; Hellack B.; Kooter I.; Hoek G.; Brunekreef B.; Kuhlbusch T. A.; Cassee F. R.; Janssen N. A. Measurement of the oxidative potential of PM2. 5 and its constituents: The effect of extraction solvent and filter type. Atmospheric Environment 2014, 83, 35–42. 10.1016/j.atmosenv.2013.10.049. [DOI] [Google Scholar]
- Hagan D. H.; Kroll J. H. Assessing the accuracy of low-cost optical particle sensors using a physics-based approach. Atmospheric measurement techniques 2020, 13 (11), 6343–6355. 10.5194/amt-13-6343-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Vet R. A review of current knowledge concerning size-dependent aerosol removal. China Particuology 2006, 4 (6), 272–282. 10.1016/S1672-2515(07)60276-0. [DOI] [Google Scholar]
- Zhang Y.; Shen Z.; Sun J.; Zhang L.; Zhang B.; Zou H.; Zhang T.; Hang Ho S. S.; Chang X.; Xu H.; Wang T.; Cao J. Parent, alkylated, oxygenated and nitrated polycyclic aromatic hydrocarbons in PM2. 5 emitted from residential biomass burning and coal combustion: A novel database of 14 heating scenarios. Environmental Pollution 2021, 268, 115881. 10.1016/j.envpol.2020.115881. [DOI] [PubMed] [Google Scholar]
- Vicente E. D.; Vicente A. M.; Musa Bandowe B. A.; Alves C. A. Particulate phase emission of parent polycyclic aromatic hydrocarbons (PAHs) and their derivatives (alkyl-PAHs, oxygenated-PAHs, azaarenes and nitrated PAHs) from manually and automatically fired combustion appliances. Air Quality, Atmosphere & Health 2016, 9, 653–668. 10.1007/s11869-015-0364-1. [DOI] [Google Scholar]
- Wang J.; Hang Ho S. S.; Huang R.; Gao M.; Liu S.; Zhao S.; Cao J.; Wang G.; Shen Z.; Han Y. Characterization of parent and oxygenated-polycyclic aromatic hydrocarbons (PAHs) in Xi’an, China during heating period: An investigation of spatial distribution and transformation. Chemosphere 2016, 159, 367–377. 10.1016/j.chemosphere.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Chan C. K. The oxidative potential of fresh and aged elemental carbon-containing airborne particles: a review. Environmental Science: Processes & Impacts 2022, 24 (4), 525–546. 10.1039/D1EM00497B. [DOI] [PubMed] [Google Scholar]
- Wei C.; Han Y.; Bandowe B. A. M.; Cao J.; Huang R.-J.; Ni H.; Tian J.; Wilcke W. Occurrence, gas/particle partitioning and carcinogenic risk of polycyclic aromatic hydrocarbons and their oxygen and nitrogen containing derivatives in Xi’an, central China. Science of the total environment 2015, 505, 814–822. 10.1016/j.scitotenv.2014.10.054. [DOI] [PubMed] [Google Scholar]
- Walgraeve C.; Demeestere K.; Dewulf J.; Zimmermann R.; Van Langenhove H. Oxygenated polycyclic aromatic hydrocarbons in atmospheric particulate matter: Molecular characterization and occurrence. Atmospheric environment 2010, 44 (15), 1831–1846. 10.1016/j.atmosenv.2009.12.004. [DOI] [Google Scholar]
- Huffman J. A.; Docherty K. S.; Aiken A. C.; Cubison M. J.; Ulbrich I. M.; DeCarlo P. F.; Sueper D.; Jayne J. T.; Worsnop D. R.; Ziemann P. J.; Jimenez J. L. Chemically-resolved aerosol volatility measurements from two megacity field studies. Atmospheric Chemistry and Physics 2009, 9 (18), 7161–7182. 10.5194/acp-9-7161-2009. [DOI] [Google Scholar]
- Biswas S.; Verma V.; Schauer J. J.; Cassee F. R.; Cho A. K.; Sioutas C. Oxidative potential of semi-volatile and non volatile particulate matter (PM) from heavy-duty vehicles retrofitted with emission control technologies. Environ. Sci. Technol. 2009, 43 (10), 3905–3912. 10.1021/es9000592. [DOI] [PubMed] [Google Scholar]
- Daher N.; Ning Z.; Cho A. K.; Shafer M.; Schauer J. J.; Sioutas C. Comparison of the chemical and oxidative characteristics of particulate matter (PM) collected by different methods: filters, impactors, and biosamplers. Aerosol Science and Technology 2011, 45 (11), 1294–1304. 10.1080/02786826.2011.590554. [DOI] [Google Scholar]
- Puthussery J. V.; Zhang C.; Verma V. Development and field testing of an online instrument for measuring the real-time oxidative potential of ambient particulate matter based on dithiothreitol assay. Atmospheric Measurement Techniques 2018, 11 (10), 5767–5780. 10.5194/amt-11-5767-2018. [DOI] [Google Scholar]
- Laguerre A.; Gall E. T. Polycyclic Aromatic Hydrocarbons (PAHs) in Wildfire Smoke Accumulate on Indoor Materials and Create Postsmoke Event Exposure Pathways. Environ. Sci. Technol. 2024, 58 (1), 639–648. 10.1021/acs.est.3c05547. [DOI] [PubMed] [Google Scholar]
- Wang S.; Takhar M.; Zhao Y.; Al Rashdi L. N. S.; Chan A. W. Dynamic oxidative potential of organic aerosol from heated cooking oil. ACS Earth and Space Chemistry 2021, 5 (5), 1150–1162. 10.1021/acsearthspacechem.1c00038. [DOI] [Google Scholar]
- Khurshid S. S.; Emmerich S.; Persily A. Oxidative potential of particles at a research house: Influencing factors and comparison with outdoor particles. Building and environment 2019, 163, 106275. 10.1016/j.buildenv.2019.106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates J. T.; Fang T.; Verma V.; Zeng L.; Weber R. J.; Tolbert P. E.; Abrams J. Y.; Sarnat S. E.; Klein M.; Mulholland J. A.; Russell A. G. Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects. Environ. Sci. Technol. 2019, 53 (8), 4003–4019. 10.1021/acs.est.8b03430. [DOI] [PubMed] [Google Scholar]
- Guascito M. R.; Lionetto M. G.; Mazzotta F.; Conte M.; Giordano M. E.; Caricato R.; De Bartolomeo A. R.; Dinoi A.; Cesari D.; Merico E.; Mazzotta L.; Contini D. Characterisation of the correlations between oxidative potential and in vitro biological effects of PM10 at three sites in the central Mediterranean. Journal of Hazardous Materials 2023, 448, 130872. 10.1016/j.jhazmat.2023.130872. [DOI] [PubMed] [Google Scholar]
- Dominutti P. A.; Borlaza L. J. S.; Sauvain J.-J.; Ngoc Thuy V. D.; Houdier S.; Suarez G.; Jaffrezo J.-L.; Tobin S.; Trebuchon C.; Socquet S.; Moussu E.; Mary G.; Uzu G. Source apportionment of oxidative potential depends on the choice of the assay: insights into 5 protocols comparison and implications for mitigation measures. Environmental Science: Atmospheres 2023, 3 (10), 1497–1512. 10.1039/D3EA00007A. [DOI] [Google Scholar]
- Xiong Q.; Yu H.; Wang R.; Wei J.; Verma V. Rethinking Dithiothreitol-Based Particulate Matter Oxidative Potential: Measuring Dithiothreitol Consumption versus Reactive Oxygen Species Generation. Environ. Sci. Technol. 2017, 51 (11), 6507–6514. 10.1021/acs.est.7b01272. [DOI] [PubMed] [Google Scholar]
- Cho A. K.; Sioutas C.; Miguel A. H.; Kumagai Y.; Schmitz D. A.; Singh M.; Eiguren-Fernandez A.; Froines J. R. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environmental research 2005, 99 (1), 40–47. 10.1016/j.envres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Bates J. T.; Fang T.; Verma V.; Zeng L.; Weber R. J.; Tolbert P. E.; Abrams J. Y.; Sarnat S. E.; Klein M.; Mulholland J. A.; Russell A. G. Review of acellular assays of ambient particulate matter oxidative potential: methods and relationships with composition, sources, and health effects. Environ. Sci. Technol. 2019, 53 (8), 4003–4019. 10.1021/acs.est.8b03430. [DOI] [PubMed] [Google Scholar]
- Miljevic B.; Heringa M. F.; Keller A.; Meyer N. K.; Good J.; Lauber A.; DeCarlo P. F.; Fairfull-Smith K. E.; Nussbaumer T.; Burtscher H.; Prevot A. S. H.; Baltensperger U.; Bottle S. E.; Ristovski Z. D. Oxidative potential of logwood and pellet burning particles assessed by a novel profluorescent nitroxide probe. Environ. Sci. Technol. 2010, 44 (17), 6601–6607. 10.1021/es100963y. [DOI] [PubMed] [Google Scholar]
- Wragg F. P.; Fuller S. J.; Freshwater R.; Green D. C.; Kelly F. J.; Kalberer M. An automated online instrument to quantify aerosol-bound reactive oxygen species (ROS) for ambient measurement and health-relevant aerosol studies. Atmospheric Measurement Techniques 2016, 9 (10), 4891–4900. 10.5194/amt-9-4891-2016. [DOI] [Google Scholar]
- Utinger B.; Campbell S. J.; Bukowiecki N.; Barth A.; Gfeller B.; Freshwater R.; Rüegg H.-R.; Kalberer M. An automated online field instrument to quantify the oxidative potential of aerosol particles via ascorbic acid oxidation. Atmospheric Measurement Techniques 2023, 16 (10), 2641–2654. 10.5194/amt-16-2641-2023. [DOI] [Google Scholar]
- Yu H.; Puthussery J. V.; Verma V. A semi-automated multi-endpoint reactive oxygen species activity analyzer (SAMERA) for measuring the oxidative potential of ambient PM2. 5 aqueous extracts. Aerosol Science and Technology 2020, 54 (3), 304–320. 10.1080/02786826.2019.1693492. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available on arcticdata.io: https://arcticdata.io/catalog/view/doi%3A10.18739%2FA23R0PV7J.