Abstract
We report the use of simple 1,4-dihydropyridine anions as a general platform for promoting single-electron photoreductions. In the presence of a mild base, 1,4-dihydropyridines were shown to effectively promote the hydrodechlorination and borylation of aryl chlorides and the photodetosylation of N-tosyl aromatic amines under visible light irradiation. Our studies also demonstrate that the C4 substituent can influence the reactivity of these anions, reducing unwanted side reactions like hydrogen atom transfer and back-electron transfer.
1,4-Dihydropyridines (DHPs) have emerged as a valuable class of reagents in organic synthesis, often used as reductants in catalytic hydrogenation reactions and more recently as a class of sacrificial single-electron reductants or as the hydrogen atom source in photoredox catalyzed reactions.1 Further demonstrating the versatility of these reagents, 4-alkyl-1,4-DHPs have been extensively employed as precursors to alkyl radicals in C–C bond forming reactions.2 A common theme in these methodologies is the requirement for some chemical entity to oxidize the DHP to the radical cation to ultimately furnish the alkyl radical, which is normally achieved either by using a photocatalyst or through direct excitation of the DHP in the presence of an oxidant. In previous work from our lab, we demonstrated that, in the presence of a suitable base, 4-tert-alkyl-1,4-DHPs bearing cyano groups at C3 and C5 could be directly photolyzed under blue LED irradiation, which allowed for these DHPs to be used as tert-alkyl radical precursors in photochemical Giese reactions in the absence of a photocatalyst or external oxidant (Scheme 1A).3 Mechanistic studies suggested that the reaction proceeds via excitation of the DHP anion, which possesses a significantly red-shifted absorption in comparison to the neutral DHP. During the course of this work, we observed that 1,4-DHPs bearing secondary alkyl groups did not yield the corresponding Giese products, with hydrogenation of the Michael acceptor being predominantly observed in these cases.3 Inspired by this switch in mechanism afforded by a simple change in the substitution pattern at C4, we envisioned that these 1,4-DHPs, in the presence of a suitable mild base, could serve as a general platform for visible light mediated single-electron photoreduction reactions (Scheme 1B).4 Furthermore, as 1,4-DHPs are long established to be competent hydrogen atom donors,1a this strategy would eliminate the need for separate chemical entities to promote the desired single-electron reduction and the subsequent hydrogen atom transfer, addressing a common drawback of many modern photochemical approaches in this space.
Scheme 1. (A) Previous Work: 4-tert-Alkyl-1,4-DHP Anions as Radical Precursors. (B) This Work: 1,4-DHP Anions as Potent Single-Electron Photoreductants.
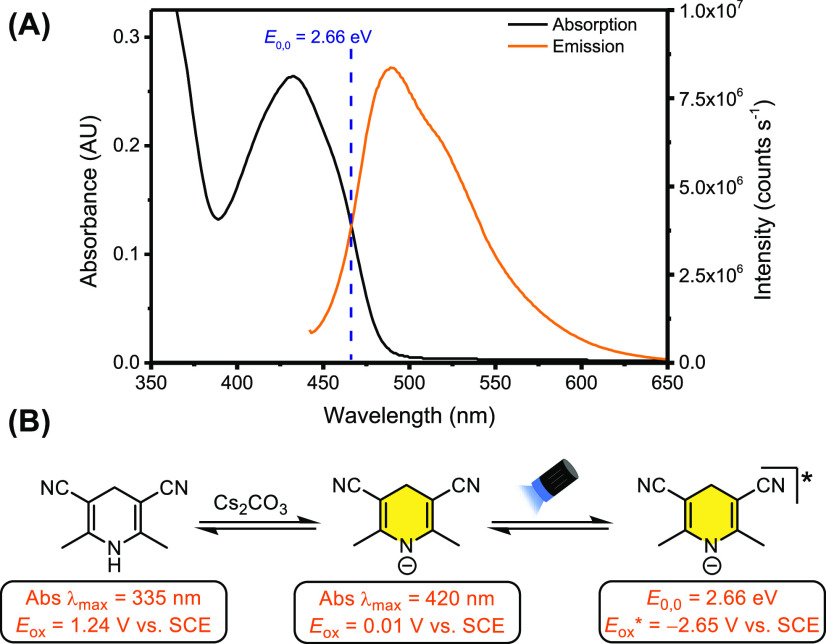
Our design plan was further motivated by the recent reports of organic anions serving as potent single-electron photoreductants upon visible light irradiation.5 For example, Xia and co-workers, among others,6 have shown that phenolate anions possess highly reducing excited states upon photoexcitation with visible light (Eox* = −3.16 V vs SCE).7 Furthermore, Melchiorre and co-workers recently reported that thiolate anions generated from the deprotonation of cyclic thioamides possess highly reducing excited states (E*ox = −3.38 V vs SCE) capable of activating C–Cl, C–F, and C–O bonds as well as promoting the Birch reduction of unfunctionalized arenes.8 Given this precedent, we set out to determine the excited state oxidation potentials of simple 1,4-DHP anions to assess their potential as potent single-electron photoreductants. UV–vis studies demonstrated that a significant red shift in the absorption of 1,4-DHP I is observed upon treatment with Cs2CO3, supporting the formation of the anion (Scheme 2). Excitation of the anion resulted in an emission centered at ∼490 nm, supporting the formation of an excited state upon irradiation of the anion. From these data, the E0,0 of 2.66 eV could be inferred from the crossing point between the absorption and emission spectra (466 nm).9 Using the ground state oxidation potential of 1,4-DHP I anion (Ep/2 = 0.01 V vs SCE, see SI), an excited state oxidation potential [E(DHP•/[DHP–]*)] was estimated as −2.65 V vs SCE using the Rehm–Weller equation.10 Given the highly reducing nature of the excited state, we hypothesized that these 1,4-DHP anions could serve as a general platform for the single-electron photoreduction of C–Cl bonds of aryl halides11 as well as the photodetosylation of N-Ts aromatic amines.12
Scheme 2. (A) Absorption (black trace) and Emission (orange trace) Spectra of the 1,4-DHP I Anion. (B) Summary of Electrochemical and Photophysical Data for the 1,4-DHP I Anion.
We began our investigation of 1,4-DHP anions as potent photoreductants by studying the hydrodechlorination of methyl 4-chlorobenzoate (1) as a model system (Table 1). Gratifyingly, hydrodechlorinated product 2 was observed in 60% yield using only 1 equiv of 1,4-DHP I in the presence of 5 equiv of Cs2CO3 in MeCN under 456 nm LED irradiation (entry 1). Increasing the loading of 1,4-DHP I led to a further increase in yield (entries 2, 3). Finally, running the reaction under more dilute conditions was found to be beneficial, providing 2 in a 78% isolated yield (entry 4). Control experiments demonstrated that both Cs2CO3 and light were essential for promoting reactivity (entries 5, 6). Finally, we tested an alternative approach using Hantzsch ester (II) and tBuOK under 456 nm irradiation previously reported by Budén and co-workers,13 which afforded 2 in only 22% yield under comparable reaction conditions (entry 7).
Table 1. Hydrodechlorination Optimization and Control Reactions.
| entry | DHP | [MeCN] (mM) | base | yield of 2a (%) |
|---|---|---|---|---|
| 1 | I, 1.0 equiv | 50 | Cs2CO3 | 60 |
| 2 | I, 1.2 equiv | 50 | Cs2CO3 | 69 |
| 3 | I, 1.5 equiv | 50 | Cs2CO3 | 75 |
| 4 | I, 1.5 equiv | 62.5 | Cs2CO3 | 78b |
| 5 | I, 1.5 equiv | 62.5 | none | c |
| 6 | I, 1.5 equiv | 62.5 | Cs2CO3 | c,d |
| 7 | II, 1.5 equiv | 62.5 | tBuOKe | 22 |
Yield calculated by 1H NMR using 1,3,5-trimethoxybenzene as an external standard.
Isolated yield.
No reaction.
No light.
2.2 equiv.
With the optimized conditions in hand, we examined the scope of compatible aryl chlorides (Scheme 3). To facilitate product isolation, we employed slightly modified conditions to afford the corresponding borylated products by adding bis(pinacolato)borane ((Bpin)2). For the borylation of aryl chlorides, it was also found that using 1,4-DHP III with a methyl group at C4 was beneficial, as it reduced the amount of hydrodechlorinated byproduct formed from an unwanted hydrogen atom transfer reaction (see SI for full reaction optimization). Aryl chlorides possessing electron-withdrawing groups (3–5) were efficiently photoreduced, giving the corresponding borylated products in moderate to good yields. Chlorobenzene (6) and 4-chlorobiphenyl (7) were also found to be compatible substrates, which was anticipated based on the measured reduction potential of 7 (Ep/2 = −2.34 V vs SCE, see SI). Aryl chlorides bearing electron-donating groups did not undergo photoreduction, which was expected as the reduction potentials required exceeded the excited state oxidation potential of the 1,4-DHP III anion (E(DHP•/[DHP–]*) = −2.59 V vs SCE, see SI).12a
Scheme 3. Reaction Scope for the Borylation of Aryl Chlorides.
Yields of isolated, purified products after 15 h of irradiation using the optimized conditions (see General Procedure C3 in the Supporting Information).
Next, we examined the potential of 1,4-DHP anions for the photodetosylation of N-Ts aromatic amines, given the similarities in the reported reduction potentials of these substrates in comparison to aryl chlorides.12a,14 As a model reaction, we examined the photodetosylation of N-Ts indole (10) using 1,4-DHP I as the excited state anionic photoreductant. In an initial attempt using only 1 equiv of DHP I in the presence of 5 equiv of Cs2CO3 in MeCN, detosylated indole 11 was observed in 72% yield after 18 h of 456 nm irradiation (Table 2, entry 1). Varying the loadings of DHP I and Cs2CO3 revealed that 1.2 equiv of DHP I and 4 equiv of Cs2CO3 were optimal, giving 11 in 99% yield (entries 2–4). Control reactions demonstrated that light and Cs2CO3 were essential for reactivity (entries 5 and 6). Finally, a time course revealed that the reaction was complete after 12 h, giving a 97% yield of 11 (entries 7 and 8).
Table 2. Photodetosylation Optimization and Control Reactionsa.
| entry | equiv of DHP I | equiv of Cs2CO3 | time (h) | yield of 11b (%) |
|---|---|---|---|---|
| 1 | 1.0 | 5.0 | 18 | 72 |
| 2 | 1.2 | 5.0 | 18 | 81 |
| 3 | 1.5 | 5.0 | 18 | 83 |
| 4 | 1.2 | 4.0 | 18 | 99 |
| 5 | 1.2 | 0 | 18 | c |
| 6 | 1.2 | 4.0 | 18 | c,d |
| 7 | 1.2 | 4.0 | 8 | 89 |
| 8 | 1.2 | 4.0 | 12 | 97 |
Ts: tosyl.
Yields calculated by 1H NMR using 1,3,5-trimethoxybenzene as an external standard.
No reaction.
No light.
With the optimized conditions in hand, we explored the scope of compatible aromatic amines and heterocycles for the photodetosylation reaction (Scheme 4). N-Ts heterocycles such as indoles (11, 12), benzimidazole (13), and carbazole (14) could all be photodetosylated in excellent yields using this method, and no loss in reactivity was observed when the detosylation was performed at 1 mmol scale. Secondary N-Ts anilines 15–17 were also effectively photodetosylated using this protocol. N-Ts melatonin (18) also underwent photodetosylation in 55% yield. Furthermore, 1,4-DHP I was able to mediate the selective deprotection of an N-Ts group in the presence of both Boc (19) and Cbz (20) protecting groups, highlighting the potential utility of this method for selective deprotections of aromatic amines in organic synthesis.
Scheme 4. Reaction Scope for the Photodetosylation of N-Ts Aromatic Amines.
Yields of isolated, purified products after 12 h of irradiation using the optimized conditions (see General Procedure C4 in the Supporting Information).
While secondary N-Ts anilines underwent photodetosylation efficiently, photodetosylation of primary N-Ts aniline (21) was found to be significantly challenging under our standard conditions, despite the reaction being thermodynamically favorable based on the reported reduction potential of N-Ts aniline.12a We posited that the decreased reactivity was attributed to the poor stability of the resulting anilide anion leaving group. After further optimization (see SI), we found that a reasonable yield (53%) of 21 could be obtained using 1,4-DHP IV with a phenyl group at C4 and using water as a cosolvent to stabilize the leaving group (Table 3). We initially posited that adding the phenyl group to the C4 position of the 1,4-DHP helped facilitate the initial single-electron transfer by enabling the preassociation of IV with the Ts group in the ground state;15 however, a similar level of complexation was observed between N-Ts aniline and the corresponding anions of 1,4-DHPs I and IV (see SI). We next considered that the substitution at C4 may influence the rate of back-electron transfer (BET), given that BET following substrate reduction is a common challenge in many photoredox systems.16 Therefore, we decided to examine the driving force for BET (ΔGBET) for the reaction of N-Ts aniline with the corresponding anions from 1,4-DHPs I and IV, where ΔGBET is given by the negative of the energy stored in the radical (ion) pair, or Eox(donor) – Ered(acceptor).17 Using these data, we calculated that ΔGBET becomes more exothermic when employing the IV anion vs the parent I anion (−2.34 eV vs −2.02 eV, respectively). Given that the driving force in both these cases is so large, the BET reaction may occur in the Marcus inverted region, where the rate of BET decreases as ΔGBET decreases.18 This is also in good agreement with previous reports from Gould and Farid, who demonstrated that the rates of BET decreased as the ΔGBET decreased from −2 to −3 eV for different donor/acceptor systems.17,19 A similar effect was observed by Fukuzumi and co-workers for the photocatalytic oxidation of benzene to phenol.20 Given this precedent, we postulate that differences in BET rates, influenced by C4 substitution, may play a role in the increased reactivity for IV in the photodetosylation of N-Ts aniline. Finally, aliphatic N-Ts amines were found to be unreactive using our approach.
Table 3. Photodetosylation of N-Ts Aniline.
| entry | DHP | equiv of Cs2CO3 | ΔGBET (eV) | yield of 21a (%) |
|---|---|---|---|---|
| 1 | I | 5.0 | –2.02 | 22 |
| 2 | IV | 5.0 | –2.34 | 33 |
| 3 | IV | 2.0 | –2.34 | 53b |
Yields calculated by 1H NMR using 1,3,5-trimethoxybenzene as an external standard.
Isolated yield.
Given that this work was inspired by our previous observations that 1,4-DHP anions could mediate the hydrogenation of Michael acceptors under visible light irradiation,3 we briefly examined the ability of 1,4-DHP I anion to mediate these hydrogenations (Scheme 5). As anticipated, complete conversion of a range of Michael acceptors (22–25) was observed, giving the corresponding hydrogenated products in good yields. Given the propensity for Michael acceptors or other reducible functional groups such as aryl halides to undergo reduction in the presence of 1,4-DHPs under basic conditions, we believe the excited state reactivity of these anions should be carefully considered when developing and optimizing new transformations involving 1,4-DHPs.
Scheme 5. Reaction Scope for the Hydrogenation of Michael Acceptors.
Yields of isolated, purified products after 24 h of irradiation using the optimized conditions (see General Procedure C6 in the Supporting Information).
In summary, simple 1,4-DHPs, which can be deprotonated using a mild base to form the corresponding anion, can serve as a general platform for visible light mediated single-electron reductions. Through a combination of electrochemical and photophysical studies, the excited state oxidation potentials of 1,4-DHP anions were estimated to be approximately −2.6 V vs SCE, which was shown to be sufficiently reducing to promote the borylation of aryl chlorides and the photodetosylation of N-Ts aromatic amines. Furthermore, 1,4-DHP anions can effectively play the role of both a single-electron donor and hydrogen atom donor, as exemplified in the hydrodechlorination reaction of an aryl chloride. Our studies have also demonstrated that the C4 substituent of the 1,4-DHP can be used to influence the reactivity of the resulting excited state anion, reducing unwanted side reactions such as hydrogen atom transfer and back-electron transfer. Given the highly reducing excited states that can be accessed under mild conditions and the wide array of substrates that can be reduced at these potentials, we anticipate that 1,4-DHPs could find widespread use as photoreductants in organic synthesis.
Acknowledgments
SPP would like to gratefully acknowledge Oklahoma State University for startup funds to support this work.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.4c00513.
Experimental procedures, reaction optimization, compound characterization, cyclic voltammetry studies, absorption and fluorescence studies and NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Zheng C.; You S.-L. Transfer Hydrogenation with Hantzsch Esters and Related Organic Hydride Donors. Chem. Soc. Rev. 2012, 41, 2498–2518. 10.1039/c1cs15268h. [DOI] [PubMed] [Google Scholar]; b Wang P.-Z.; Chen J.-R.; Xiao W.-J. Hantzsch Esters: An Emerging Versatile Class of Reagents in Photoredox Catalyzed Organic Synthesis. Org. Biomol. Chem. 2019, 17, 6936–6951. 10.1039/C9OB01289C. [DOI] [PubMed] [Google Scholar]; c Suresh Yedase G.; Venugopal S.; P A.; Reddy Yatham V. Catalyst-free Hantzsch Ester-mediated Organic Transformations Driven by Visible light. Asian J. Org. Chem. 2022, 11, e202200478 10.1002/ajoc.202200478. [DOI] [Google Scholar]; d You S.-L. Recent Developments in Asymmetric Transfer Hydrogenation with Hantzsch Esters: A Biomimetic Approach. Chem.—Asian J. 2007, 2, 820–827. 10.1002/asia.200700081. [DOI] [PubMed] [Google Scholar]; e Rueping M.; Dufour J.; Schoepke F. R. Advances in Catalytic Metal-free Reductions: From Bio-inspired Concepts to Applications in the Organocatalytic Synthesis of Pharmaceuticals and Natural Products. Green Chem. 2011, 13, 1084–1105. 10.1039/c1gc15027h. [DOI] [Google Scholar]; f Ouellet S. G.; Walji A. M.; Macmillan D. W. C. Enantioselective Organocatalytic Transfer Hydrogenation Reactions using Hantzsch Esters. Acc. Chem. Res. 2007, 40, 1327–1339. 10.1021/ar7001864. [DOI] [PubMed] [Google Scholar]
- a Nakajima K.; Nojima S.; Sakata K.; Nishibayashi Y. Visible-Light-Mediated Aromatic Substitution Reactions of Cyanoarenes with 4-Alkyl-1,4-dihydropyridines through Double Carbon-Carbon Bond Cleavage. ChemCatChem. 2016, 8, 1028–1032. 10.1002/cctc.201600037. [DOI] [Google Scholar]; b Gutiérrez-Bonet Á.; Tellis J. C.; Matsui J. K.; Vara B. A.; Molander G. A. 1,4-Dihydropyridines as Alkyl Radical Precursors: Introducing the Aldehyde Feedstock to Nickel/Photoredox Dual Catalysis. ACS Catal. 2016, 6, 8004–8008. 10.1021/acscatal.6b02786. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Nakajima K.; Nojima S.; Nishibayashi Y. Nickel- and Photoredox-Catalyzed Cross-Coupling Reactions of Aryl Halides with 4-Alkyl-1,4-dihydropyridines as Formal Nucleophilic Alkylation Reagents. Angew. Chem., Int. Ed. 2016, 55, 14106–14110. 10.1002/anie.201606513. [DOI] [PubMed] [Google Scholar]; d Chen W.; Liu Z.; Tian J.; Li J.; Ma J.; Cheng X.; Li G. Building Congested Ketone: Substituted Hantzsch Ester and Nitrile as Alkylation Reagents in Photoredox Catalysis. J. Am. Chem. Soc. 2016, 138, 12312–12315. 10.1021/jacs.6b06379. [DOI] [PubMed] [Google Scholar]; e Gutiérrez-Bonet Á.; Remeur C.; Matsui J. K.; Molander G. A. Late-Stage C-H Alkylation of Heterocycles and 1,4-Quinones via Oxidative Homolysis of 1,4-Dihydropyridines. J. Am. Chem. Soc. 2017, 139, 12251–12258. 10.1021/jacs.7b05899. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Buzzetti L.; Prieto A.; Roy S. R.; Melchiorre P. Radical-Based C-C Bond-Forming Processes Enabled by the Photoexcitation of 4-Alkyl-1,4-dihydropyridines. Angew. Chem., Int. Ed. 2017, 56, 15039–15043. 10.1002/anie.201709571. [DOI] [PMC free article] [PubMed] [Google Scholar]; g van Leeuwen T.; Buzzetti L.; Perego L. A.; Melchiorre P. A Redox-Active Nickel Complex that Acts as an Electron Mediator in Photochemical Giese Reactions. Angew. Chem., Int. Ed. 2019, 58, 4953–4957. 10.1002/anie.201814497. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Phelan J. P.; Lang S. B.; Sim J.; Berritt S.; Peat A. J.; Billings K.; Fan L.; Molander G. A. Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis. J. Am. Chem. Soc. 2019, 141, 3723–3732. 10.1021/jacs.9b00669. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Xie S.; Li D.; Huang H.; Zhang F.; Chen Y. Intermolecular Radical Addition to Ketoacids Enabled by Boron Activation. J. Am. Chem. Soc. 2019, 141, 16237–16242. 10.1021/jacs.9b09099. [DOI] [PubMed] [Google Scholar]; j Guo Q.; Peng Q.; Chai H.; Huo Y.; Wang S.; Xu Z. Visible-light Promoted Regioselective Amination and Alkylation of remote C(sp3)-H bonds. Nat. Commun. 2020, 11, 1463. 10.1038/s41467-020-15167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Schwarz J. L.; Huang H.-M.; Paulisch T. O.; Glorius F. Dialkylation of 1,3-Dienes by Dual Photoredox and Chromium Catalysis. ACS Catal. 2020, 10, 1621–1627. 10.1021/acscatal.9b04222. [DOI] [Google Scholar]; l Bay A. V.; Fitzpatrick K. P.; Betori R. C.; Scheidt K. A. Combined Photoredox and Carbene Catalysis for the Synthesis of Ketones from Carboxylic Acids. Angew. Chem., Int. Ed. 2020, 59, 9143–9148. 10.1002/anie.202001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallage P. C.; Pitre S. P. Direct Photolysis of 4-tert-Alkyl-1,4-dihydropyridines under Blue-light Irradiation for the Generation of Tertiary Alkyl Radicals. Green Chem. 2022, 24, 6845–6848. 10.1039/D2GC02153F. [DOI] [Google Scholar]
- During the submission of this manuscript, the following report outlining the excited state reactivity of the Hantzsch ester anion was disclosed:; Xu J.; Lan Y.; Liu B. Activation of Aryl and Alkyl Halides Enabled by Strong Photoreduction Potentials of a Hantzsch Ester/Cs2CO3 System. J. Org. Chem. 2024, 89, 599–604. 10.1021/acs.joc.3c02320. [DOI] [PubMed] [Google Scholar]
- Schmalzbauer M.; Marcon M.; König B. Excited State Anions in Organic Transformations. Angew. Chem., Int. Ed. 2021, 60, 6270–6292. 10.1002/anie.202009288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei B.; Gentile G.; Rosso C.; Filippini G.; Prato M. Turning the Light on Phenols: New Opportunities in Organic Synthesis. Chem.—Eur. J. 2021, 27, 16062–16070. 10.1002/chem.202102276. [DOI] [PubMed] [Google Scholar]
- a Liang K.; Liu Q.; Shen L.; Li X.; Wei D.; Zheng L.; Xia C. Intermolecular oxyarylation of olefins with aryl halides and TEMPOH catalyzed by the phenolate anion under visible light. Chem. Sci. 2020, 11, 6996–7002. 10.1039/D0SC02160A. [DOI] [Google Scholar]; b Wei D.; Li X.; Shen L.; Ding Y.; Liang K.; Xia C. Phenolate Anion-catalyzed Direct Activation of Inert Alkyl Chlorides Driven by Visible Light. Org. Chem. Front. 2021, 8, 6364–6370. 10.1039/D1QO01128F. [DOI] [Google Scholar]
- Wu S.; Schiel F.; Melchiorre P. A General Light-Driven Organocatalytic Platform for the Activation of Inert Substrates. Angew. Chem., Int. Ed. 2023, 62, e202306364 10.1002/anie.202306364. [DOI] [PubMed] [Google Scholar]
- Turro N. J.; Ramamurthy V.; Scaiano J. C.. Principles of Molecular Photochemistry; University Science Books, 2009. [Google Scholar]
- Rehm D.; Weller A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. 10.1002/ijch.197000029. [DOI] [Google Scholar]
- a Ghosh I.; Ghosh T.; Bardagi J. I.; König B. Reduction of Aryl Halides by Consecutive Visible Light-Induced Electron Transfer Processes. Science 2014, 346, 725–728. 10.1126/science.1258232. [DOI] [PubMed] [Google Scholar]; b Chmiel A. F.; Williams O. P.; Chernowsky C. P.; Yeung C. S.; Wickens Z. K. Non-innocent Radical Ion Intermediates in Photoredox Catalysis: Parallel Reduction Modes Enable Coupling of Diverse Aryl Chlorides. J. Am. Chem. Soc. 2021, 143, 10882–10889. 10.1021/jacs.1c05988. [DOI] [PubMed] [Google Scholar]; c Neumeier M.; Sampedro D.; Májek M.; de la Peña O’Shea V. A.; Jacobi von Wangelin A.; Pérez-Ruiz R. Dichromatic Photocatalytic Substitutions of Aryl Halides with a Small Organic Dye. Chem.—Eur. J. 2018, 24, 105–108. 10.1002/chem.201705326. [DOI] [PubMed] [Google Scholar]; d Widness J. K.; Enny D. G.; McFarlane-Connelly K. S.; Miedenbauer M. T.; Krauss T. D.; Weix D. J. CdS Quantum Dots as Potent Photoreductants for Organic Chemistry Enabled by Auger Processes. J. Am. Chem. Soc. 2022, 144, 12229–12246. 10.1021/jacs.2c03235. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Shen N.; Li R.; Liu C.; Shen X.; Guan W.; Shang R. Photocatalytic Cross-Couplings of Aryl Halides Enabled by o-Phosphinophenolate and o-Phosphinothiophenolate. ACS Catal. 2022, 12, 2788–2795. 10.1021/acscatal.1c05941. [DOI] [Google Scholar]; f Shen N.; Liu C.; Zhang X.; Shang R. o-Phosphinodiarylamides as Reductive Photocatalysts for Dehalogenative and Deaminative Cross-Couplings. ACS Catal. 2023, 13, 11753–11761. 10.1021/acscatal.3c03569. [DOI] [Google Scholar]
- a MacKenzie I. A.; Wang L.; Onuska N. P. R.; Williams O. F.; Begam K.; Moran A. M.; Dunietz B. D.; Nicewicz D. A. Discovery and Characterization of an Acridine Radical Photoreductant. Nature 2020, 580, 76–80. 10.1038/s41586-020-2131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lenz P.; Oshimizu R.; Klabunde S.; Daniliuc C. G.; Mück-Lichtenfeld C.; Tendyck J. C.; Mori T.; Uhl W.; Hansen M. R.; Eckert H.; Yamaguchi S.; Studer A. Oxy-Borylenes as Photoreductants: Synthesis and Application in Dehalogenation and Detosylation Reactions. Angew. Chem., Int. Ed. 2022, 61, e202209391 10.1002/anie.202209391. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Art J. F.; Kestemont J. P.; Soumillion J. P. Photodetosylation of Sulfonamides Initiated by Electron Transfer from an Anionic Sensitizer. Tetrahedron Lett. 1991, 32, 1425–1428. 10.1016/0040-4039(91)80348-A. [DOI] [Google Scholar]
- Heredia M. D.; Guerra W. D.; Barolo S. M.; Fornasier S. J.; Rossi R. A.; Budén M. E. Transition-Metal-Free and Visible-Light-Mediated Desulfonylation and Dehalogenation Reactions: Hantzsch Ester Anion as Electron and Hydrogen Atom Donor. J. Org. Chem. 2020, 85, 13481–13494. 10.1021/acs.joc.0c01523. [DOI] [PubMed] [Google Scholar]
- Roth H. G.; Romero N. A.; Nicewicz D. A. Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett 2016, 27, 714–723. 10.1055/s-0035-1561297. [DOI] [Google Scholar]
- Hamada T.; Nishida A.; Matsumoto Y.; Yonemitsu O. Photohydrolysis of Sulfonamides via Donor-Acceptor Ion Pairs with Electron-donating Aromatics and its Application to the Selective Detosylation of Lysine Peptides. J. Am. Chem. Soc. 1980, 102, 3978–3980. 10.1021/ja00531a064. [DOI] [Google Scholar]
- a Ruccolo S.; Qin Y.; Schnedermann C.; Nocera D. G. General Strategy for Improving the Quantum Efficiency of Photoredox Hydroamidation Catalysis. J. Am. Chem. Soc. 2018, 140, 14926–14937. 10.1021/jacs.8b09109. [DOI] [PubMed] [Google Scholar]; b Williams O. P.; Chmiel A. F.; Mikhael M.; Bates D. M.; Yeung C. S.; Wickens Z. K. Practical and General Alcohol Deoxygenation Protocol. Angew. Chem., Int. Ed. 2023, 62, e202300178 10.1002/anie.202300178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould I. R.; Moser J. E.; Armitage B.; Farid S.; Goodman J. L.; Herman M. S. Electron-transfer Reactions in the Marcus Inverted Region. Charge Recombination versus Charge Shift Reactions. J. Am. Chem. Soc. 1989, 111, 1917–1919. 10.1021/ja00187a077. [DOI] [Google Scholar]
- Marcus R. A. On the Theory of Oxidation-Reduction Reactions Involving Electron Transfer. I. J. Chem. Phys. 1956, 24, 966–978. 10.1063/1.1742723. [DOI] [Google Scholar]
- Gould I. R.; Farid S. Dynamics of Bimolecular Photoinduced Electron-Transfer Reactions. Acc. Chem. Res. 1996, 29, 522–528. 10.1021/ar950053z. [DOI] [Google Scholar]
- Ohkubo K.; Fujimoto A.; Fukuzumi S. Visible-Light-Induced Oxygenation of Benzene by the Triplet Excited State of 2,3-Dichloro-5,6-dicyano-p-benzoquinone. J. Am. Chem. Soc. 2013, 135, 5368–5371. 10.1021/ja402303k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.