Abstract
We report a baby with neonatal herpes simplex virus (HSV) encephalitis concurrent with Rrhesus (Rh) incompatibility. He was delivered by a Ggravida 2 mother with a history of miscarriage in her previous pregnancy at a gestation age of 4 months. She had Bblood group 0 and Rrhesus negative. The baby was noticed to have jaundice on day one1 of life accompanied by generalised petechiae on the face and upper chest. A full blood picture revealed severe anaemia and severe thrombocytopaenia and HSV 1/2 IgM was positive. MRI of the brain showed multiple extensive haemorrhagic lesions on the frontal-temporal regions.
Keywords: Neonatal intensive care, Infection (neurology), Neuroimaging, Haematology (incl blood transfusion)
Background
Herpes simplex virus (HSV) produces significant disease in the neonatal period and early infancy. It has a very high mortality rate and neurological effects.1 The appearance of HSV infection in newborns can be classified into three groups: skin, eye, and mouth; central nervous system (CNS) disease; and disseminated disease. Since 40%–60% of infants with CNS infections may present without skin lesions, hence the encephalitic type frequently goes undetected.1 Mortality has been reported to be between 50% and 75% percent, and those who survive have been noted to have significant rates of morbidity, including mental disabilities, epilepsy, impaired growth, retinopathy, and cystic encephalomalacia.2 The best shot to reduce neonatal herpes encephalitis morbidity and mortality is to have a high degree of suspicion, timely supportive medical and surgical care, and prompt commencement of acyclovir therapy.
Case presentation
A male baby born to a woman in her late 20s, at term with a birth weight of 2.5 kg and APGAR score of 6 and 8 at 1stst and 5thth min,utes respectively. He was delivered by emergency caesarian section due to non-reassuring fetal status. The mother had a rupture of membrane 2 days before delivery, and a history of miscarriage fourth-month gestation (Did n’ot receive Anti-D at that time). During this pregnancy, she attended six antenatal clinic visits where she received folic acid, iron supplements and 2two doses of tetanus toxoid. She received Aanti-D at gestation age of 28 weeks and had a negative serology result for Venereal Disease Research Laboratory (VDRL) and HIV. The pregnancy was uneventful with no history of any febrile illness, skin rashes, lower abdominal pain or abnormal vaginal discharge, and she was normotensive and euglycaemic throughout. A maternal full blood picture done during third trimester showed normal levels of Hhaemoglobin and platelets being 13.4 g/dl and 256×109/L, respectively. Maternal examination did not reveal any genital, skin, oral or breast lesion.
The Bbaby was noted to be jaundiced on first day of life and had prolonged bleeding following venipuncture. Mother did not report history of fever or convulsions. On examination, the baby had a slightly bulging and tense anterior fontanelle, petechiae on the face and upper trunk and palmar pallor. He had reduced primitive reflexes with inability to suck, and slightly increased truncal and appendicular tone. There were no skin blisters, vesicles or scars and no hepatosplenomegaly. He had normal occipital frontal circumference of 35.2 cm and length of 49 cm.
Investigations
Full blood picture on first day of life revealed normal WBC white cell count of 7.8×109/L, severe anaemia with Hhaemoglobin of 9.6 g/dL and severe thrombocytopaenia with platelet count of 30.5×109 /L.
The baby’s blood group is O and Rrhesus Ppositive.
Blood culture done twice at birth and on day 7 of life, both were negative.
There was a raised Lactate Dehrogenase (LDH) of 1995 U/L, Eelevated total serum bilirubin 408 mmol/L (23.7 mg/dL), conjugated bilirubin of 88 mmol/L (4.4 mg/dL) and Eelevated reticulocyte count of 8.2%.
Alanine Transferase (ALT) was 39 U/L and Aspartate Tranferase (AST) 106 U/L.
Direct Coomb’s test was positive.
Prolonged bleeding time of 9 min, with normal PT of 2.0, PTT of 23.7 and INR of 1.7.
Lumbar puncture was postponed due to thrombocytopaenia and history of prolonged bleeding after venipunctures.
To exclude Nneonatal Aauto-Iimmune Tthrombocytopaenia (NAIT), platelet crossmatch studies were done using mother’s and father’s blood and found to be compatible.
HSV serology revealed HSV 1/2 IgM positive on day 9 of life.
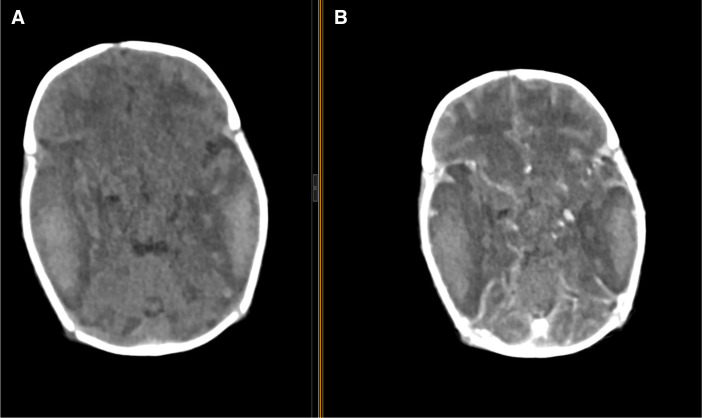
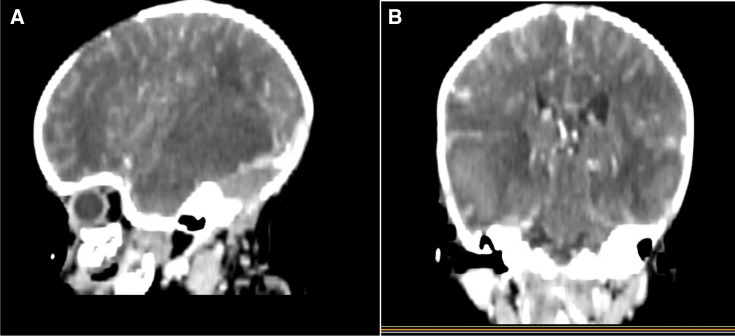
CT scan brain reported acute intracerebral haemorrhage at the right frontal-temporal and parietal regions and also left temporal-parietal regions. Axial non-enhanced CT and axial contrast -enhanced CT show multiple non-enhancing hyperdense (49.7 HU) areas with perilesional oedema in the right frontal-temporal parietal lobes (figure 1) and sagittal and coronal contrast- enhanced CT show bilateral multiple non-enhanced lesions with perilesional oedema at frontal temporal lobes and effacement of ipsilateral ventricles noted (figure 2) both indicating haemorrhage in the brain.
Figure 1.
Axial non-enhanced contrast CT (A) and axial contrast E-enhanced CT (B) show multiple non- enhancing hyper dense (49.7 HU) areas with perilesional oedema in the right frontal –-temporal and left temporal parietal lobes.
Figure 2.
Sagittal and Ccoronal. (A and, B) Contrast E-enhanced CT shows bilateral multiple non- enhanced lesions with perilesional oedema at frontal temporal lobes and effacement of ipsilateral lateral ventricles noted.
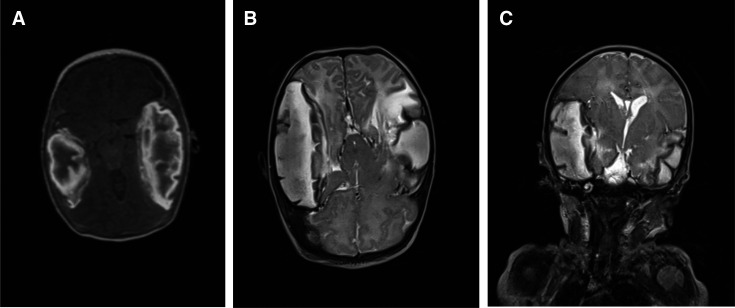
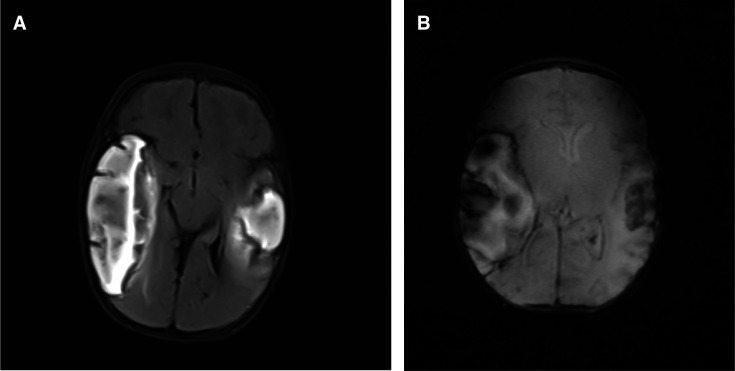
Brain MRI showed multiple extensive hyperintense lesions in the right frontal-temporal regions and left temporal region with effacement of right lateral ventricle. Axial non-contrasted T1W1 shows multiple extensive hyper-intense lesions in the right frontal-temporal regions and temporal region (figure 3). While the coronal T2W1 image shows significant effacement of the right lateral ventricles due to lesion mass- effect (figure 3C). There was loss of signal width noted in both transverse sinuses with axial FLAIRfluid-attenutaed inversion recovery showing bilateral multiple extensive hyperintense lesions at the frontal temporal regions (figure 4A) and axial fast field echo FFE shows bilateral frontal temporal lesion with blooming artefacts in keeping with areas of cerebral haemorrhages (figure 4B).
Figure 3.
(A:) MRI image showing Aaxial non -contrasted T1WI show multiple extensive hyper intense lesions in the right frontal-temporal regions and left temporal region. (B:) MRI image showing Aaxial non -contrasted T2WI show multiple extensive hyper intense lesions in the right frontal-temporal regions and left temporal region. (C:) MRI images showing Ccoronal T2WI image shows significance effacement of the right lateral ventricles due to lesion mass effect. NB: T1W1 is a Magnetic resonance imaging term referring to the spin-lattice relaxation time and used to provide the consistency of a tissue
Figure 4.
(A) Axial Ffluid A-attenutaed Iinversion Rrecovery (FLAIR) shows bilateral multiple extensively hyperintense lesions at frontal temporal regions shown on MRI. (B:) Axial Ffast Ffield Eecho (FFE) shows bilateral frontal temporal lesion with blooming artefacts in keeping with areas of cerebral haemorrhages in MRI.
Diagnosis
Herpes Eencephalitis, Rrhesus Iincompatibility.
Differential diagnosis
Neonatal thrombocytopaenia has specific and non-specific causes. These include NAIT and NAIT. It has a protean presentation including petechia, purpura, ecchymosis, and bleeding into various organs including the brain. The presence of multiple haemorrhages in the brain in the neonate, however, is a hallmark of neonatal Hherpes Ssimplex infection.
Treatment
Baby was kept on intensive phototherapy for 4 days. He received injections of vitamin K, tranexamic acid, fresh frozen plasma, packed red blood cells, and 4four packs of platelets transfusions. Prophylactic antibiotics were given due to a history of prolonged rupture of membrane which werewas stopped when the C reactive protein was reported normal and blood culture was negative. IVIntravenous Aacyclovir was given at a dose of 20 mg/kg/dose for 3 weeks then changed to oral acyclovir that was prescribed to be used for 6 months. The baby was fed expressed breastmilk via nasogastric tube during the admission period.
Outcome and follow-up
The baby improved and was discharged home after 4 weeks of hospital admission.
At the 2-week follow-up visit, there was remarkable improvement. He was able to suck and was increasing his weight appropriately. The weight was 3.4 kg and an Ooccipital-Ffrontal Ccircumference 41 cm (50thth percentile). He had a haemoglobin of 11.8 g/dL and a platelet count of 168×109/L.
The Bbaby is to continue with acyclovir suppression therapy for 6 months and a monthly scheduled follow-up plan at the paediatric neurology clinic.
Discussion
HSV infection during pregnancy poses a significant risk to the developing fetus and newborn. Neonates can acquire HSV infection by intrauterine, perinatal, or postnatal transmission of the virus.1 The most frequent (70%) cause of neonatal Hherpes simplex encephalitis (HSE) is HSV-2 as compared with HSV-1 which is the primary cause of HSE in adults. The fetus normally contracts HSV-2 infection through the shedding of the virus during vaginal birth.3 To reduce the risk of virus transmission from pregnant women who are suspected of being carriers or infected with HSV, an elective C-section is typically performed. In our case scenario, a mother was asymptomatic and again she was delivered by emergency C-section but the newborn baby still got infected. With this, we think the baby acquired an ascending infection due to early and prolonged rupture of membrane before the C-section was performed.
With regards to clinical presentation, the majority of neonatal with HSV infection typically present with skin involvement commonly manifesting as classic eruption of grouped vesicular lesions; however, approximately one-third (1/3) of neonates with Hherpes encephalitis will not have skin lesions at the time of presentation, as it was seen in our case.3 Conversely, lack of multi-organ involvement and almost normal liver enzymes diagnosis of disseminated herpes is less likely for this baby.
To best prevent early and late complications following neonatal HSV encephalitis, diagnosis and treatment of HSV should have a low threshold of sensitivity. Recovery of the virus from scrapings of the skin lesions, oropharynx, urine, stool, or CSF provides the most definitive diagnosis of HSV infection. However, to do this, the sample must be frozen and packed in dry ice or transported using a special medium.4 In our setting, facilities for viral isolation are not frequently accessible. The fastest and most recommended alternative way to make the diagnosis of HSE is through the detection of specific IgM or IgG antibodies against HSV which may begin to show up as early as just one-to-21–2 weeks after the infection starts and lasts for six to twelve6–12 months.5 Our case had HSV IgM reactive in keeping with the literature.
Brain biopsy, which is among the most invasive diagnostic procedures, was not necessary in our case because it is only used in patients who do not respond to acyclovir therapy and if CT or MRI cannot confirm the aetiology. PCR assay of CSF is the gold -standard method for diagnosing HSE.6 However, in our case, the lumbar puncture was postponed due to thrombocytopaenia and a history of prolonged bleeding after venipunctures.
Brain imaging techniques are considered characteristic though they are not pathognomonic for neonatal HSE. From the literature search, the most frequent cause of sporadic encephalitis is HSE, which causes haemorrhagic encephalitis involving the frontal and medial temporal lobes as was the case in our patient.7 8
Patient’s perspective.
‘My first pregnancy ended up in a miscarriage. I was very excited to have this baby but was shocked to hear that he was bleeding in his brain. I was even more shocked when I was told my baby probably got the infection from me, when I did not have any symptoms during pregnancy. I thank the doctors and nurses for the care and support they have and continuing to offer.
I am happy that he is now growing well, and he can breastfeed well. I have been told that he will need to continue with regular follow up.’
Learning points.
Neonatal herpes simplex virus (HSV) infection can present with no skin eruptions.
Early diagnosis and administration of acyclovir require a high index of suspicion.
Prompt treatment prevents further complications of Hherpes Eencephalitis.
Actively identifying other comorbidities such as thrombocytopaenia and hyperbilirubinaemia is important to prevent complications.
Footnotes
Contributors: The following authors were responsible for drafting of the text, sourcing and editing of clinical images, investigation results, drawing original diagrams and algorithms, and critical revision for important intellectual content: PC, HM, MM and KPM. The following authors gave final approval of the manuscript: PC, HM, MM and KPM. All authors have reviewed and accepted the final submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
References
- 1. Samies NL, James SH, Kimberlin DW. Neonatal herpes simplex virus disease: updates and continued challenges. Clin Perinatol 2021;48:263–74. 10.1016/j.clp.2021.03.003 [DOI] [PubMed] [Google Scholar]
- 2. White JC, Magee SR. Neonatal herpes infection: case report and discussion. J Am Board Fam Med 2011;24:758–62. 10.3122/jabfm.2011.06.100257 [DOI] [PubMed] [Google Scholar]
- 3. Anderson E, Johns E, Conlon J, et al. Neonatal herpes simplex presenting as a zosteriform eruption. BMJ Case Rep 2023;16:1–5. 10.1136/bcr-2022-252627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J 2014;11:1–7.:83. 10.1186/1743-422X-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toth C, Harder S, Yager J. Neonatal herpes encephalitis: a case series and review of clinical presentation. Can J Neurol Sci 2003;30:36–40. 10.1017/s0317167100002419 [DOI] [PubMed] [Google Scholar]
- 6. Gelfand JM, Genrich G, Green AJ, et al. Encephalitis of unclear origin diagnosed by brain biopsy: a diagnostic challenge. JAMA Neurol 2015;72:66–72. 10.1001/jamaneurol.2014.2376 [DOI] [PubMed] [Google Scholar]
- 7. Erdem G, Vanderford PA, Bart RD. Intracranial hemorrhage in herpes simplex encephalitis: an unusual presentation. Pediatr Neurol 2002;27:221–3. 10.1016/s0887-8994(02)00428-9 [DOI] [PubMed] [Google Scholar]
- 8. Gupta SN, Gupta VS, Borad N. Late onset neonatal herpes encephalitis: a case-based CNS complication and neurological outcome. OALib 2015;02:1–7. 10.4236/oalib.1101280 [DOI] [Google Scholar]