Abstract
The yeast vacuolar H+-ATPase (V-ATPase) is a multisubunit complex responsible for organelle acidification. The enzyme is structurally organized into two major domains: a peripheral domain (V1), containing the ATP binding sites, and an integral membrane domain (V0), forming the proton pore. Dissociation of the V1 and V0 domains inhibits ATP-driven proton pumping, and extracellular glucose concentrations regulate V-ATPase activity in vivo by regulating the extent of association between the V1 and V0 domains. To examine the mechanism of this response, we quantitated the extent of V-ATPase assembly in a variety of mutants with known effects on other glucose-responsive processes. Glucose effects on V-ATPase assembly did not involve the Ras-cyclic AMP pathway, Snf1p, protein kinase C, or the general stress response protein Rts1p. Accumulation of glucose 6-phosphate was insufficient to maintain or induce assembly of the V-ATPase, suggesting that further glucose metabolism is required. A transient decrease in ATP concentration with glucose deprivation occurs quickly enough to help trigger disassembly of the V-ATPase, but increases in cellular ATP concentrations with glucose readdition cannot account for reassembly. Disassembly was inhibited in two mutant enzymes lacking ATPase and proton pumping activities or in the presence of the specific V-ATPase inhibitor, concanamycin A. We propose that glucose effects on V-ATPase assembly occur by a novel mechanism that requires glucose metabolism beyond formation of glucose 6-phosphate and generates a signal that can be sensed efficiently only by a catalytically competent V-ATPase.
Vacuolar H+-ATPases (V-ATPases) couple hydrolysis of cytoplasmic ATP to transport of protons from the cytosol into intracellular compartments in all eukaryotic cells. Organelle acidification by V-ATPases has been linked to normal cell growth, cellular ion homeostasis, zymogen activation, protein sorting in the biosynthetic and endocytic pathways, and other fundamental biological processes (for reviews, see references 22, 43, 44, and 58). The yeast V-ATPase is a multisubunit complex composed of at least 13 different subunits. The enzyme is structurally organized into two domains, V1 and V0. The V1 domain, consisting of subunits of 69 (Vma1p), 60 (Vma2p), 54 (Vma13p), 42 (Vma5p), 32 (Vma8p), 27 (Vma4p), 14 (Vma7p), and 13 (Vma10p) kDa, is peripherally attached to the cytoplasmic side of the membrane and contains both catalytic and noncatalytic ATP binding sites (reviewed in reference 58). The V0 domain forms a transmembranous proton channel to which V1 is attached and consists of a 100-kDa subunit (Vph1p), a 36-kDa subunit (Vma6p), and three isoforms of a proteolipid subunit (Vma3p, Vma11p, and Vma16p) (58).
ATP-driven proton transport by V-ATPases requires a functional association of V1 and V0 sectors. Unlike the F0 sector of the evolutionarily and structurally related F-ATPases (21), the V0 domain of the V1V0-ATPases is not an open proton pore when V1 is not attached to it (69, 76). The extent of ATPase activity in the soluble V1 domain is more uncertain. Even though it was widely accepted that the V1 sector does not retain Mg2+-dependent hydrolytic activity upon dissociation from the membrane (5, 25, 30, 46, 51, 74), it appears that a cryptic Mg2+-dependent ATPase activity can be uncovered in V1 sectors under certain conditions (25, 73). The inability of the separated V1 and V0 domains to translocate protons across the membrane suggests regulated association of V1 with V0 as a potential mechanism for regulating enzyme activity in vivo.
In fact, the assembly state of the yeast V-ATPase is posttranslationally regulated by glucose in vivo (29). Glucose-grown cells briefly deprived of any carbon source or shifted to a less optimal carbon source rapidly dissociate most of the assembled V1V0 complexes into cytoplasmic V1 sectors and membrane bound V0 sectors, and they also show lower levels of V-ATPase activity in isolated vacuoles. This effect is entirely reversible; readdition of glucose to glucose-deprived cells results in functional reassembly of the dissociated complexes. The assembly state of the yeast V-ATPase also shows a long-term regulation by carbon source. Cells grown overnight in raffinose medium contain a higher proportion of disassembled V1 and V0 sectors than glucose-grown cells, and the dissociated sectors remain competent for assembly upon addition of glucose. The V-ATPase of Manduca sexta shows a similar type of regulation. V1 sectors dissociate from the membrane of the larval midgut in Manduca at a specific developmental stage characterized by cessation of feeding, and dissociation can also be specifically induced by starvation (60). These results indicate that nutrient regulation of V-ATPases may be a general phenomenon. We have speculated that downregulation of V-ATPase activity by enzyme dissociation in response to glucose deprivation might conserve cellular reserves of ATP and that reassembly of the enzyme in response to glucose readdition might help the cell to handle the intracellular acidification generated by glucose metabolism. A similar physiological justification has been suggested for glucose regulation of the yeast plasma membrane proton pump (Pma1p) (55). Like the V-ATPase, the enzymatic activity of Pma1p is posttranslationally downregulated by glucose deprivation and upregulated by glucose readdition (18, 55). Evidence from a number of systems has suggested that under certain conditions, the V-ATPase and plasma membrane proton pump, or the functionally equivalent plasma membrane pumps of higher eukaryotes, may cooperate to regulate intracellular pH (7, 61).
There is no single glucose-signaling pathway in yeast that integrates all glucose-induced responses into a comprehensive scheme. Addition of glucose to glucose-starved yeast cells or to cells growing on a nonfermentable carbon source leads to a variety of changes aimed toward establishing efficient glucose metabolism and reducing or eliminating enzymatic activities not used during fermentation (reviewed in references 28, 50, and 62). Glucose-induced effects may be transcriptional or posttranslational. Glucose repression reduces the expression of proteins not required at high levels for growth on glucose. Glucose inactivation accelerates the turnover of enzymatic activity not required for fermentation by a variety of posttranslational mechanisms, including phosphorylation and targeted degradation. Activation of Pma1p in response to glucose appears to involve phosphorylation of the enzyme (11), but the exact mechanism of activation remains unclear (11, 18).
In this study we further examine the effects of changes in carbon source on V-ATPase assembly and begin to probe the molecular basis for these changes. We show that glucose-induced effects on assembly of the V-ATPase are independent of the most thoroughly characterized signal transduction pathways for glucose-induced responses, the Ras-cyclic AMP (cAMP) and main glucose repression/derepression pathways. Assembly changes in the V-ATPase in response to glucose are also independent of the Rts1p-containing protein phosphatase 2A, which has been shown to act in a number of stress-induced responses including glucose deprivation (19, 57), and protein kinase C, which has been implicated in regulation of Pma1p (39, 55). In contrast to most glucose-induced responses (62), accumulation of glucose 6-phosphate does not appear to be sufficient to allow the V-ATPase to sense glucose readdition to glucose-starved yeast cells. Rather, continuous glycolytic metabolism appears to be required to maintain the intracellular pool of assembled V1V0 complexes. We demonstrate that only catalytically active V-ATPase complexes efficiently disassemble in response to glucose deprivation and present evidence suggesting a rapid and transient decrease of the intracellular ATP pool immediately after glucose deprivation may be important for signaling disassembly of the enzyme. The implications of substrate availability and catalysis for disassembly and reassembly are discussed.
MATERIALS AND METHODS
Materials and strains.
Zymolyase 100 T and Tran35S-label were purchased from ICN. Dithiobis(succinimidylpropionate) was obtained from Pierce. 14C-labeled molecular mass markers (high range) were obtained from Life Technologies, Inc. Concanamycin A was obtained from Wako Biochemicals. ATP bioluminescence assay kit HS II was purchased from Boehringer Mannheim. Luciferin-luciferase was also purchased from Analytical Luminescence Laboratory. All other reagents were purchased from Sigma.
Saccharomyces cerevisiae strains used in this study and their genotypes are listed in Table 1. Cells were grown in YEPD (1% yeast extract, 2% peptone, 2% glucose) or fully supplemented synthetic medium (SD; 0.67% yeast nitrogen base, 2% dextrose) lacking individual amino acids (56). The pgi1 mutant strain was grown in YEP (1% yeast extract, 2% peptone) containing 2% fructose (YEPF). The pkc1Δ mutant strain was grown in YEPD containing 1 to 1.2 M sorbitol. Strain MM112 was transformed with pVIP1-78, a YCp50 plasmid containing the entire VPH1 open reading frame (42), to obtain an isogenic wild-type strain for the experiments with the vph1-E789Q mutant. The wild-type strain SF838-5Aα was treated with ethidium bromide to induce a [rho0] strain as described by Fox et al. (23). The [rho0] phenotype of the petite cells was confirmed by 4′,6-diamidino-2-phenylindole (DAPI) staining and their inability to grow in 1% yeast extract–2% peptone–3% glycerol plates.
TABLE 1.
Yeast strains and genotypes
| Strain | Genotype | Reference(s) |
|---|---|---|
| SF838-1D | MATα ade6 leu2-3,112 ura3-52 pep4-3 gal2 | 59 |
| SF838-5A | MATα leu2-3,112 ura3-52 ade6 gal2 | 59 |
| W303-1A | MATa ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 | 32 |
| MT544, W303-1A pde2Δ | MATa ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 pde2Δ::TRP1 CLN1-HA | 64 |
| W303-1A snf1Δ | MATa ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 snf1Δ::URA3 | 33 |
| W303-1A rts1Δ | MATa ura3-1 leu2-3,112 his3-11,15 trp1-1 ade2-1 rts1Δ::HIS3 | 57 |
| DL100-1783 | MATa leu2-3,112 ura3-52 trp1-1 his4 can1r | 70 |
| DL376, DL100-1783 pkc1Δ | MATa leu2-3,112 ura3-52 trp1-1 his4 can1rpkc1Δ::LEU2 | 70 |
| 093CJM | MATα his6 pgi1 | 49 |
| RHA108 | MATα ade6 leu2-3,112 ura3-52 pep4-3 gal2 vma11Δ::LEU2 | 27 |
| RHA108/vma11-E145L | MATα ade6 leu2-3,112 ura3-52 pep4-3 gal2 vma11Δ::LEU2 [pRS316-vma11-E145L URA3 CEN6] | 27 |
| RHA108/VMA11 | MATα ade6 leu2-3,112 ura3-52 pep4-3 gal2 vma11Δ::LEU2 [pRS316-VMA11 URA3 CEN6] | 27 |
| MM112 | MATa his3-Δ200 leu2 lys2 ura3-52 vph1Δ::LEU2 stv1Δ::LYS2 | 41 |
| MM112/vph1-E789Q | MATa his3-Δ200 leu2 lys2 ura3-52 vph1Δ::LEU2 stv1Δ::LYS2 [pRS316-vph1-E789Q URA3 CEN6] | 34 |
| MM112/pVIP1-78 | MATa his3-Δ200 leu2 lys2 ura3-52 vph1Δ::LEU2 stv1Δ::LYS2 [pVIP1-78: entire VPH1 ORF in a YCp50 plasmid] | 41, 42 |
Immunoprecipitations.
For each immunoprecipitation of the V-ATPase performed in a mutant strain, its isogenic wild-type strain was immunoprecipitated in parallel, except for the pgi1 mutant strain, for which an isogenic strain was not available. Cells were grown overnight to a density of 0.4 to 0.7 optical density unit (OD)/ml in supplemented minimal medium lacking methionine containing 2% glucose (SD-Met), and immunoprecipitations were carried out under nondenaturing conditions as described previously (29), with the following exceptions: (i) the pkc1Δ mutant strain was maintained at 25°C in the presence of osmotic support (1 to 1.2 M sorbitol) throughout; (ii) the pgi1 mutant strain was grown, converted to spheroplasts, and radiolabeled in the presence of 2% fructose plus 0.02% glucose; and (iii) the vph1-E789Q mutant and its isogenic wild type were grown in supplemented minimal medium lacking methionine and uracil (SD-Met-Ura) and treated as described in reference 34. For each immunoprecipitation, 0.5 × 107 spheroplasts were labeled for 1 h with 50 μCi of Tran[35S]-label. At the end of the labeling period, unlabeled methionine and cysteine were added to a final concentration of 0.16 mg/ml and spheroplasts were chased as indicated. For immunoprecipitations with antibody 10D7, 250 μl of 10D7 cultured supernatant plus 250 μl of phosphate-buffered saline (PBS; 137 mM NaCl, 2.6 mM KCl, 12 mM sodium phosphate [pH 7.2]) containing 5 mg of bovine serum albumin (BSA) per ml were added to the samples. Immunoprecipitations with antibody 8B1 were carried out by adding 5 μl (12.5 μg) of purified 8B1 or 300 μl of the 8B1 cultured supernatant plus 495 μl or 200 μl of PBS containing 5 mg of BSA per ml. Antibody incubations were carried out overnight, followed by 1 h incubation with 50 μl of a 40% (vol/vol) suspension of protein A-Sepharose CL-4B. Immunoprecipitated protein was solubilized by adding 50 μl of cracking buffer (50 mM Tris-HCl [pH 6.8], 8 M urea, 5% sodium dodecyl sulfate, 1 mM EDTA, 5% β-mercaptoethanol) that had been preheated at 70°C, followed by incubation at 70°C for 10 min. Samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. Immunoprecipitation results were quantitated on a Molecular Dynamics PhosphorImager 425.
Estimation of intracellular ATP concentration.
To quantify intracellular ATP concentrations, yeast cells were converted to spheroplasts, washed, and incubated as for immunoprecipitations. After incubation, a 250-μl aliquot was pelleted by centrifugation, frozen at −80°C, and later used to estimate protein. In parallel, a 50-μl aliquot was diluted 20-fold in the same chase medium to a final cell density of 0.165 OD/ml. Each dilution was placed on ice and immediately used to determine ATP levels in duplicate. To determine ATP concentrations, 10-μl aliquots were mixed with 50 μl of lysis buffer and brought to a final volume of 100 μl/sample with solubilization buffer or 20 mM HEPES (pH 7.5). Samples were heated for 5 min at 100°C in a boiling water bath, mixed, and spun for 2 min in a microcentrifuge. A 50-μl aliquot of each sample was transferred to a luminometer tube and used to estimate the total intracellular ATP concentration by using a luciferin-luciferase system kit in an Autolunat LB953 luminometer. A calibration curve was prepared for each experiment. Duplicate points showed linearity for ATP values between 10−15 and 10−9 mol of ATP/reaction. The bioluminescence (in relative luminescence units) was assayed with 100 μl of twofold-diluted luciferase reagent, and for each reaction the light signal was integrated for 10 s after a delay of 1.2 s. The intracellular ATP levels were estimated by transforming the recorded values with the Sigma Plot curve-fitting application program.
Protein determination.
The total protein in spheroplasts was calculated by a modified Lowry assay (Bio-Rad DC Protein Assay Kit II). Frozen pellets from 250 μl of spheroplasts at a density of 3.3 OD/ml were resuspended in 100 μl of cracking buffer without β-mercaptoethanol prewarmed at 70°C. Samples were immediately heated at 100°C in a boiling water bath for 10 min. A calibration curve for 0- to 80-μg BSA samples in the same buffer showed linearity and was prepared in duplicate each time.
Indirect immunofluorescence microscopy.
SF838-5Aα cells were grown in YEPD to mid-log phase or stationary phase. Cells were prepared for microscopy as described in Roberts et al. (53). Monoclonal antibodies 13D11 (against the 60-kDa subunit) and 10D7 (against the 100-kDa subunit) were used as described in reference 29. Cells were observed with a 100× oil immersion objective, using a Zeiss Axioskop microscope with an MC 100 SPOT camera.
RESULTS
V1 and V0 naturally disassemble in cells at stationary phase.
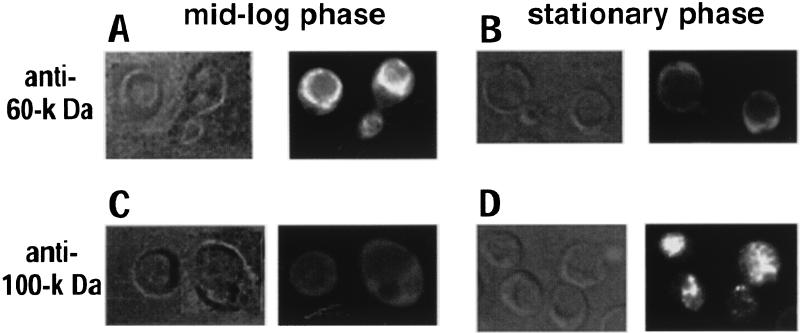
S. cerevisiae ferments almost all of the available glucose over time, even when grown aerobically (28). When the glucose available to yeast is depleted, the cells continue to grow by utilizing the ethanol formed during fermentation as a carbon source. During this phase, several physiological changes take place and almost all ATP must be produced by mitochondrial oxidative phosphorylation. It has been previously established that disassembly and reassembly of the yeast V-ATPase are efficiently induced by glucose removal and readdition (29), but such sudden and acute changes in carbon source may be rather artificial. If the presence of glucose in the medium is required to keep the enzyme assembled, however, we expect that the vacuolar H+-ATPase will disassemble during stationary phase because glucose is depleted. To determine whether dissociation of V1 from the vacuolar membrane naturally occurs when exponentially growing cells reach stationary phase, we performed indirect immunofluorescence microscopy to localize the V1 and V0 sectors of yeast cells during mid-log phase and stationary phase. Indirect immunofluorescence microscopy provides a qualitative picture of the assembly state of the V-ATPase (29). It is possible to quantitate the extent of disassembly by biosynthetically labeling yeast cells and immunoprecipitating proteins under nondenaturing conditions (see below), but it is difficult to make biosynthetically active spheroplasts from cells in stationary phase. As shown in Fig. 1, yeast vacuoles appear as depressions under Nomarski optics. In log-phase cells, antibody 13D11, which recognizes the 60-kDa peripheral subunit of the enzyme, brightly stained the vacuolar membrane (Fig. 1A), indicating the presence of fully assembled V1V0 complexes at the vacuole of cells in mid-log phase. When the same antibody was used to visualize the enzyme during stationary phase, it did not show vacuolar staining and instead appeared to show diffuse cytoplasmic staining (Fig. 1B), suggesting dissociation of V1 from the vacuolar membrane. Disassembly was confirmed by using antibody 10D7, which recognizes its epitope on the 100-kDa V0 subunit only when the peripheral V1 subunits are not associated to V0 at the membrane (31). This antibody gave very little staining of the vacuoles during mid-log phase (Fig. 1C) but brightly stained the vacuoles of cells at stationary phase (Fig. 1D). We concluded that dissociation of V1 from V0 naturally occurs as part of a long-term response to glucose depletion with growth.
FIG. 1.
Indirect immunofluorescence microscopy of the 60- and 100-kDa subunits in yeast cells at mid-log and stationary phases. Yeast cells grown to mid-log phase (A and C) or to stationary phase (B and D) were fixed and then stained with monoclonal antibodies 13D11 to visualize the V1 domain (A and B) and 10D7 to visualize the free V0 domain (C and D). The same fields were viewed under Nomarski (micrograph on left in each panel) or fluorescein fluorescence (micrograph on right in each panel) optics. Micrographs in panels A and C are composites of two fields.
Extracellular glucose concentrations modulate the level of V-ATPase assembly.
In our earlier experiments, we did not determine whether the disassembly of the V-ATPase in response to glucose concentration was a graded response, in which intermediate glucose concentrations gave intermediate levels of assembly, or a threshold response, in which a whole population of V-ATPases disassembled when a threshold concentration of glucose was reached. To address this question, we quantitated the intracellular pool of assembled V-ATPases while adjusting the amount of glucose present in the medium. Yeast cells were converted to spheroplasts and radiolabeled with [35S]methionine as described in Materials and Methods. Immediately after labeling, an excess of unlabeled methionine and cysteine was added and spheroplasts were chased for 20 min in medium containing varied amounts of added glucose, from none (YEP) to 2% glucose (YEPD). The V-ATPase was immunoprecipitated under nondenaturing conditions using monoclonal antibodies 8B1 and 10D7, against the 69-kDa V1 subunit and the 100-kDa V0 subunit, respectively. Antibody 8B1 recognizes its epitope when the 69-kDa subunit is alone, assembled into V1 complexes, or assembled into V1V0 complexes (30); antibody 10D7 recognizes the 100-kDa subunit, either alone or assembled into V0 complexes free of V1 subunits, but does not recognize assembled V1V0 complexes (31). The use of both antibodies allows us to estimate the proportion of V0 domains assembled into V1V0 complexes, by comparing the quantity of three V0 subunits immunoprecipitated by antibody 8B1 to the total immunoprecipitated by both antibodies. Figure 2A shows an autoradiograph of the enzyme complexes immunoprecipitated by each antibody. In the absence of glucose (YEP medium), monoclonal antibody 8B1 coimmunoprecipitated several V1 peripheral subunits (69, 60, 32, and 27 kDa) with relatively little V0 complex. PhosphorImager quantitation indicated that in the absence of glucose, only 8 to 18% of the individual V0 subunits (100, 36, and 17 kDa) were assembled into V1V0 complexes (Fig. 2B). Addition of increasing amounts of glucose to the chase medium induced various degrees of association of V1 and V0 (lanes 2 to 6). In the presence of 0.1 and 0.3% added glucose, 25 and 50% of the total pool of V0 subcomplexes, respectively, were immunoprecipitated with antibody 8B1, indicating that they were assembled into V1V0 complexes. At both 1% (not shown) and 2% glucose (lane 6), 60% of the total pool of V0 domains formed V1V0 complexes. Very similar results were obtained with a 5-min chase time (data not shown), indicating that glucose depletion from the 0.1 and 0.3% glucose samples is not the major trigger for disassembly. From these results, we conclude that the presence of 2% glucose in the medium induced maximal assembly under the conditions used in our experiments; thus, further experiments designed to study disassembly and reassembly were carried out by adding either 0 or 2% glucose to YEP. Moreover, these results indicate that intermediate levels of assembly can be induced by addition of subsaturating levels of extracellular glucose because glucose-induced disassembly and reassembly of V1V0 complexes is not an all-or-none (threshold) response.
FIG. 2.
Effect of extracellular glucose concentration on the intracellular pool of assembled V1V0 complexes. The level of assembled V1V0 complexes is modulated by the amount of extracellular glucose added. Wild-type yeast cells were converted to spheroplasts and labeled with Tran[35S]-label for 1 h in SD-Met as described in Materials and Methods. An excess of unlabeled cysteine and methionine was added to the radiolabeled spheroplasts, which were immediately washed in the corresponding chase medium. Each pellet was resuspended in the same chase medium and chased for 20 min at 30°C. Chase medium was YEP (no added glucose) (lane 1) or YEP containing 0.01% (lane 2), 0.03% (lane 3), 0.1% (lane 4), 0.3% (lane 5), and 2% (YEPD) (lane 6) glucose, plus 1.2 M sorbitol in each case. Monoclonal antibodies 8B1, against the 69-kDa V1 peripheral subunit, and 10D7, against the 100-kDa V0 subunit, were used in the immunoprecipitations. (A) Immunoprecipitated proteins were visualized by autoradiography. Positions of 14C-labeled molecular mass markers are indicated on the right (from the top, 200, 97, 68, 43, 29, 18.4, and 14.3 kDa), and previously identified subunits of the yeast V-ATPase are indicated by arrows on the left. (B) The total amount of radioactivity in the 100-, 36-, and 17-kDa subunits immunoprecipitated by both monoclonal antibodies was quantitated on a PhosphorImager. The percentage of each subunit coimmunoprecipitated as part of fully assembled V1V0 was calculated as described in the text. The bars represent the average of two independent experiments, and the error bars indicate the range of the two experiments.
Signaling pathways involved in disassembly and reassembly of V1 and V0.
In an attempt to characterize the molecular triggers of dissociation and reassociation of V1 and V0, we investigated whether the signaling pathway that mediates disassembly and reassembly of the V-ATPase shares components with other signal transduction pathways implicated in glucose-induced responses. We used mutant strains deficient in specific signaling pathways to study the effects of glucose depletion and glucose readdition on the assembly state of the V-ATPase for each mutant and its isogenic wild-type strain.
One of the earliest events known to occur after glucose addition is a transient but pronounced increase of the intracellular cAMP concentration due to activation of the adenylate cyclase in response to a drop in cytosolic pH (52, 68). Activation of the Ras-cAMP pathway in response to glucose has been implicated in a number of glucose-induced responses (62). We investigated whether changes in the intracellular cAMP level mediate the glucose-induced disassembly and reassembly of the yeast V-ATPase by manipulating the intracellular levels of cAMP and examining the assembly state of the V-ATPase. Addition of exogenous cAMP (5 to 200 mM) to wild-type yeast cells had no effect on disassembly and reassembly (not shown), but the intracellular cAMP phosphodiesterases, the PDE1 and PDE2 gene products (8, 54, 72), may have prevented the cAMP levels from changing inside the cells. A pde2Δ mutant strain lacks the cAMP-phosphodiesterase responsible for conversion of the majority of cytosolic cAMP to AMP, and as a consequence, addition of exogenous cAMP to the mutant cells increases the cytosolic cAMP concentration (64, 71, 72). This strain has been used previously to look at cAMP signals (64). We found that pde2Δ cells disassembled and reassembled the complex normally in response to glucose, as shown in Table 2. The same results were obtained when an isogenic strain containing a wild-type copy of the PDE2 gene was used for our experiments (not shown). Addition of 5 mM cAMP to glucose-depleted pde2Δ cells did not replace glucose in triggering reassembly, indicating that an increase in intracellular cAMP is not enough to trigger this response (Table 2). Moreover, the presence of cAMP in the chase medium did not affect the extent of assembly of the enzyme. Addition of 5 mM cAMP to YEPD or YEP neither induced nor prevented disassembly of V1V0 complexes. Preincubation of the cells in YEPD containing 5 mM cAMP did not prevent the disassembly of the enzyme induced by glucose deprivation. Complementary results were obtained in experiments performed with a cdc35ts strain, in which the catalytic subunit of adenylate cyclase has been mutated, resulting in very low levels of cAMP at the nonpermissive temperature. At both the permissive and nonpermissive temperatures, the enzyme disassembled and reassembled normally (not shown). We conclude that the glucose-induced disassembly and reassembly of the V-ATPase is a cAMP-independent response.
TABLE 2.
Disassembly and reassembly of V-ATPase in mutant strainsa
| Strain | % of 100-kDa subunit assembled into V1V0 complexes
|
||
|---|---|---|---|
| In YEPD | After YEP treatment | After glucose readdition | |
| SF838-1D | 77.5 ± 11.5 | 18.6 ± 3.6 | 74.5 ± 14.4 |
| W303-1A snf1Δ | 52.9 | 12.3 | 62.6 |
| W303-1A rts1Δ | 42.3 | 13.4 | 62.6 |
| W303-1A | 49.2 ± 0.8 | 13.6 ± 6.4 | 40.7 ± 0.7 |
| DL100-1783 pkc1Δ | 77.4 ± 4.9 | 19.2 ± 0.2 | 70.5 ± 8.9 |
| DL100-1783 | 71.8 ± 9.1 | 20.2 ± 1.9 | 60.0 ± 5.0 |
| SF838-5A [rho0] | 63.6 | 19.5 | 60.7 |
| W303-1A pde2Δ | |||
| No added cAMP | 56.3 | 21.2 | 55.5 |
| +5 mM cAMP with YEPD | 53.4 | 13.7 | |
| +5 mM cAMP with YEP | 15.9 | ||
| +5 mM cAMP after 5 min with YEP | 21.5 | ||
Mutant strains and their isogenic wild-type strains were labeled with Tran35S-label and chased in YEPD, YEP, or YEP followed by addition of 2% glucose. V1V0 and free V0 complexes were immunoprecipitated by antibodies 8B1 and 10D7, respectively. The percentage of 100-kDa subunit assembled into V1V0 complexes was calculated by quantitating the amount immunoprecipitated by each antibody on a PhosphorImager and dividing the amount immunoprecipitated by antibody 8B1 by the total immunoprecipitated by both antibodies. SF838-1D is the wild-type strain used throughout the rest of this work, and it is included for comparison. W303-1A is the isogenic wild-type strain for the snf1Δ, rts1Δ, and pde2Δ mutants, and DL100-1783 is the isogenic wild-type strain for the pkc1Δ mutant. The genetic background of the [rho0] strain (SF838-5A) is very similar to that of SF838-1D and gave qualitatively similar results in disassembly and reassembly experiments. Results are expressed as mean ± standard deviation for SF838-1D (n = 5) and mean ± range of two experiments for W303-1A, DL100-1783, and DL100-1783 pkc1Δ. Quantitation for the other strains was from a single experiment.
The main glucose repression/derepression pathway is independent of the Ras-cAMP pathway. This signaling pathway involves cytosolic and nuclear components that cooperate to control expression of glucose-repressed genes (reviewed in reference 28). In the cytosol, Snf1p is considered a master kinase in glucose repression because of its central role in numerous regulatory mechanisms (9). We used an snf1Δ mutant strain to investigate the role of Snf1p in the glucose-induced disassembly and reassembly of V1 and V0, and found that the ATPase disassembled and reassembled normally in this strain (Table 2). We conclude that glucose-induced assembly of the V-ATPase is probably not regulated by the Ras-cAMP pathway and the main glucose repression/derepression pathway.
Glucose addition to yeast cells triggers phosphorylation and activation of the plasma membrane H+-ATPase, possibly by a protein kinase C-dependent signaling pathway (39, 55). To address whether protein kinase C (Pkc1p) was essential for disassembly and reassembly of the V-ATPase, we used a pkc1Δ strain. Immunoprecipitation of the V-ATPase showed that the enzyme also disassembled and reassembled normally in a pkc1Δ strain (Table 2), indicating that signal transduction does not involve protein kinase C. In addition, we used an rts1Δ mutant strain, deficient in a key component of the general stress response. RTS1/SCS1 encodes the B regulatory subunit of one of the three cytoplasmic yeast protein phosphatases 2A and appears to be specifically involved in the stress response to osmotic stress, heat shock, nitrogen starvation, and glucose starvation (19, 57). We carried out the previously described immunoprecipitation experiments with an rts1Δ mutant strain. As shown in Table 2, the rts1Δ mutation does not affect disassembly or reassembly. Consequently, glucose-induced assembly of the yeast V-ATPase seems to be independent of key signal transduction components that control most other glucose-induced responses.
Immediately after transport into the cell, glucose is phosphorylated by one of several hexokinases (28, 62). For many glucose-induced regulatory effects, transport and phosphorylation of glucose, in the absence of further glucose metabolism, allow the cell to sense and respond to the presence of glucose (28, 62). Although most glucose 6-phosphate will be used for glycolysis in yeast, glucose 6-phosphate is an intermediate in the pentose phosphate shunt, gluconeogenesis, and glycogenolysis, and a potential second messenger for the Ras-cAMP and main glucose repression/derepression signal transduction pathways (28, 62). To determine whether glucose phosphorylation was sufficient to trigger reassembly of V1V0 complexes in glucose-deprived yeast cells, we first replaced glucose with the glucose analog 2-deoxyglucose. 2-Deoxyglucose is transported into the cells as efficiently as glucose and is efficiently phosphorylated into 2-deoxyglucose 6-phosphate (78). The phosphorylated form is not further metabolized and accumulates inside the cells, causing energy depletion. Even though 2-deoxyglucose is highly toxic, it has been commonly used to study early steps in glucose metabolism and the glucose repression/derepression pathways (10, 38, 78). Addition of 2% 2-deoxyglucose to glucose-depleted cells did not induce reassembly of V1 and V0 (not shown), suggesting that phosphorylation of glucose may not be enough to trigger reassembly, possibly because further glucose metabolism is required. The following experiments were designed to test this hypothesis.
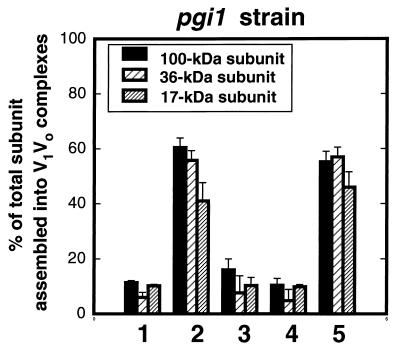
Fructose is efficiently transported into the cells and then phosphorylated to produce fructose 6-phosphate, bypassing the formation of glucose 6-phosphate. Its metabolism then follows the same steps as glucose in the glycolytic pathway. We used a glycolytic mutant strain deficient in phosphoglucose isomerase activity (pgi1), which cannot interconvert glucose 6-phosphate and fructose 6-phosphate and thus cannot perform glycolysis using glucose as a substrate. As a consequence, this strain cannot grow in glucose and grows only in fructose (24, 26, 49). Addition of glucose to yeast pgi1 mutants produces accumulation of glucose 6-phosphate and a decrease in the ATP content (12, 24, 26, 67, 40). We grew pgi1 mutant cells overnight on minimal medium containing 2% fructose as described in Materials and Methods. Spheroplasts were radiolabeled with [35S]methionine and chased as indicated in Fig. 3. In the presence of fructose (YEPF chase), about 60% of the total V0 subunits were immunoprecipitated as part of assembled V1V0 complexes. Fructose depletion of the cells, achieved by chasing in either YEP or YEPD, triggered disassembly of 70 to 80% of the V1V0 complexes. Readdition of 2% fructose, but not of 2% glucose, for 10 min to fructose-depleted pgi1 cells triggered full reassembly of V1 and V0 (Fig. 3). Our results clearly demonstrate that intracellular accumulation of glucose 6-phosphate is not enough to trigger reassembly and that further glucose metabolism through glycolysis may be required. Moreover, these results support prior data (Table 2) indicating that reassembly of the V-ATPase may be independent of the two main glucose-induced signaling pathways in yeast: the Ras-cAMP and the main glucose repression/derepression signal transduction pathways for which glucose 6-phosphate is an early intermediate (10, 28, 62).
FIG. 3.
Initiation of glycolysis triggers reassembly of V1 and V0. Yeast pgi1 mutant cells were converted to spheroplasts and labeled in medium containing 2% fructose plus 0.02% glucose as described in Materials and Methods. Labeled spheroplasts were chased for 15 min in YEPD (position 1), 15 min in YEPF (position 2), 5 min in YEP (position 3), and 5 min in YEP followed by an additional 10 min with 2% glucose (position 4) or 2% fructose (position 5) added. Immunoprecipitations were performed and analyzed as described for Fig. 2.
A higher percentage of disassembled V1 and V0 sectors is observed when yeast cells are grown overnight in raffinose or glycerol-ethanol medium (29), suggesting that these carbon sources cannot maintain assembly of V1 and V0 even after long-term incubations. A number of carbon sources (fructose, mannose, raffinose, glycerol, ethanol, galactose, lactate, and xylulose) were added to cells after brief glucose depletion in order to investigate whether they would trigger reassembly of the V-ATPase. Only the rapidly fermentable carbon sources fructose and mannose substituted for glucose in triggering reassembly of the V1 and V0 domains (not shown). Metabolism of these three sugars starts early in the glycolytic pathway; fructose and mannose are incorporated as fructose 6-phosphate, and then all three sugars follow the same steps of glycolysis. In combination with the experiments performed with the pgi mutant strain, these results suggest that glycolysis may participate in the signaling pathway that mediates reassembly. In agreement with this, we previously showed that the V-ATPase disassembled when cells reached a stationary phase (Fig. 1).
Although fermentation is the predominant metabolic pathway in glucose-grown yeast cells, it is not totally exclusive. A [rho0] mutant strain was constructed and used to investigate whether respiration is required for disassembly and reassembly of the V-ATPase. The [rho0] mutants are unable to derive energy from respiration because they lack mitochondrial DNA and therefore contain incomplete respiratory complexes (13, 23). Glucose depletion triggered disassembly of 70% of the immunoprecipitated V-ATPase complexes of the [rho0] strain into V1 and V0 domains, and addition of either 2% glucose or 2% fructose to glucose-depleted [rho0] cells efficiently triggered reassembly (Table 2 and data not shown). These results prove that glucose-induced disassembly/reassembly of the V-ATPase is independent of respiration and that fermentation of glucose is sufficient to maintain or restore ATPase assembly.
Glucose depletion alters the intracellular pool of ATP.
Our results implicate glycolysis as a metabolic pathway modulating disassembly and reassembly of V1 and V0. Slowing glycolysis by glucose depletion or changes in extracellular nutrients may affect the ATP concentration in the cells. We determined whether changes in the ATP pool of the cells could be correlated to disassembly and reassembly of the V-ATPase. First, we analyzed the effect of glucose depletion on intracellular ATP levels quantitated with the luciferin-luciferase system as described in Materials and Methods. The estimated intracellular ATP pool in yeast cells incubated in a glucose-rich medium was relatively constant for 1 to 20 min at about 8 nmol of ATP/mg of protein (Table 3). Withdrawal of glucose was accompanied by a transient decrease in the intracellular pool of ATP. Wild-type yeast cells showed a 50% drop in total intracellular ATP after a 1-min incubation in YEP. However, these cells started to restore their ATP pool within a few minutes in the same medium. Cells recovered 63% of the initial ATP level after 5 min, and 83% after 20 min, of incubation in YEP. Disassembly, which can be detected at 2 min of glucose depletion, and the initial drop in ATP levels occur over similar time courses; thus, a transient drop of ATP levels seems to be parallel to disassembly of the enzyme.
TABLE 3.
Intracellular ATP concentration upon glucose depletion and when glucose is added back to glucose-depleted cellsa
| Condition | ATP level (nmol/mg of protein)
|
||
|---|---|---|---|
| 1 min | 5 min | 20 min | |
| In YEPD | 7.3 ± 0.8 | ND | 8.5 ± 1.0 |
| After YEP treatment | 3.6 ± 0.6 | 4.6 ± 0.5 | 6.1 ± 0.5 |
| After glucose readdition | 4.0 ± 0.5 | 5.4 ± 0.6 | 6.1 ± 0.9 |
Wild-type yeast cells were converted to spheroplasts as described in Materials and Methods. After 15 min at 30°C, spheroplasts were pelleted, resuspended in YEPD or YEP medium at a final density of 3.3 OD/ml, and immediately incubated at 30°C. Samples were removed at 1 min, 5 min, and 20 min. To measure the intracellular ATP concentration after glucose readdition, YEP incubation was followed by an additional incubation with 2% glucose for 1, 5, and 20 min. ATP levels were determined in duplicate, using a luciferin-luciferase system as described in Materials and Methods. The average values ± standard errors from five independent experiments are shown. ND, not done.
We have shown that the V-ATPase remained disassembled in cells chased in YEP for as long as 20 min (Fig. 2), even though under those conditions the cells have almost completely restored their initial ATP levels in YEP (Table 3). Therefore, even if a decrease in intracellular ATP is a signal that contributes to disassembly of the enzyme during glucose depletion, an increase in intracellular ATP is probably not the primary signal for reassembly. To monitor ATP concentration under reassembly conditions, we added a final concentration of 2% glucose to cells incubated in YEP for 5 min and monitored the intracellular ATP changes within 1 to 20 min of glucose readdition. A small decrease of the ATP levels was observed within the first minute of glucose readdition, followed by slow restoration of the intracellular ATP pool between 5 and 20 min (Table 3). A transient and rapid drop of ATP levels within 30 s of glucose addition to glucose-derepressed yeast cells has been previously detected by 31P nuclear magnetic resonance spectroscopy and attributed to formation of sugar phosphates (63). As shown in Table 3, following glucose addition, yeast cells showed kinetics of ATP recovery almost indistinguishable from kinetics for the recovery in YEP, although the mechanism of recovery is almost certainly different. Taken together, the results presented in Table 3 indicate that even if a drop in intracellular ATP or changes in the ATP/ADP concentration ratio helps to signal disassembly of V1 and V0, restoration of the ATP levels alone cannot trigger reassembly.
Does disassembly of the V-ATPase require enzymatic activity?
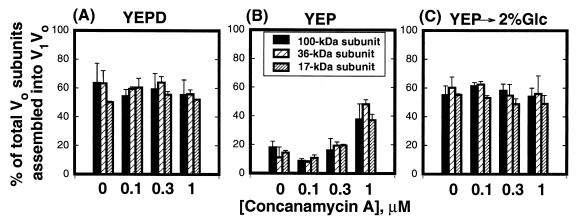
The results presented above suggest that the V-ATPase might sense a drop in extracellular glucose by sensing changes in cytosolic ATP concentration. It has been proposed that ATP hydrolysis generates a conformation of V-ATPases that is susceptible to dissociation. The enzyme can be disassembled into V1 and V0 domains in vitro by low concentrations of chaotropic agents in the presence of the enzyme substrate Mg-ATP (1, 2, 30, 48). Because the nonhydrolyzable ATP analog adenylyl-imidodiphosphate does not support in vitro disassembly (30), and mutations in the catalytic subunit that compromise catalysis also appear to compromise disassembly in vitro (36), it has been proposed that catalysis may induce a conformational change that makes the enzyme susceptible to disassembly in the presence of chaotropes (2, 30). To determine if catalysis is also required for disassembly of V-ATPase complexes in vivo, yeast cells labeled with [35S]methionine were chased in the presence of concanamycin A and varied concentrations of glucose. Concanamycin A is a potent inhibitor of V-ATPases (6, 17) that may interact with the V0 sector at the membrane to block catalysis (14, 77). Labeled spheroplasts were preincubated with three concentrations of the inhibitor (0.1, 0.3, and 1 μM) at 30°C. After 10 min, spheroplasts were pelleted and resuspended in chase medium (YEPD or YEP) containing equivalent concentrations of concanamycin A. Addition of concanamycin A partially prevented disassembly of V1 subunits from V0 when the cells were depleted of extracellular glucose (Fig. 4B). At 0.3 μM concanamycin A, disassembly of the complexes was only slightly reduced. However, at 1 μM concanamycin A, only 25% of the V1V0 complexes disassembled. The presence of the concanamycin A did not affect the pool of assembled V1V0 complexes when cells were chased either in YEPD (Fig. 4A) or in YEP after addition of 2% glucose (Fig. 4C), suggesting that its effect was specific to dissociation. To further address this specificity, we determined whether the addition of concanamycin A in the chase medium after disassembly could interfere with reassembly of V1 and V0. The presence of the inhibitor in the chase medium did not prevent reassociation of V1 and V0 (not shown). Although 100 nM concanamycin A is enough to inhibit the enzyme in isolated vacuolar membranes, higher concentrations are required to inhibit enzyme activity in vivo (16). Concanamycin A at 1 μM was shown to be sufficient to inhibit vacuolar acidification in vivo in Neurospora crassa (7), and up to 10 μM concanamycin A had no effect on plasma membrane or mitochondrial ATPase activities, suggesting good specificity (7, 17). Our results support a model in which dissociation of V1 and V0 occurs only if the enzyme is catalytically active.
FIG. 4.
Concanamycin A partially inhibits disassembly of the yeast V-ATPase. Wild-type yeast cells were converted to spheroplasts and labeled as described in Materials and Methods. Immediately after addition of unlabeled cysteine and methionine, spheroplasts were preincubated with concanamycin A (0.1, 0.3, and 1 μM) at 30°C in the same labeling medium. A control experiment was performed without concanamycin A. After 10 min of incubation, spheroplasts were pelleted and resuspended in YEPD or YEP containing the indicated concentrations of concanamycin A. (A) Spheroplasts were chased for 20 min in YEPD containing 0, 0.1, 0.3, and 1 μM concanamycin A. (B) Spheroplasts were chased for 10 min in YEP containing 0, 0.1, 0.3, and 1 μM concanamycin A. (C) Spheroplasts were chased for 10 min in YEP as described for panel B but followed by an additional 10 min with 2% glucose added. All samples contained 1% dimethyl sulfoxide, 0.2% (vol/vol) ethanol, and 1.2 M sorbitol. Immunoprecipitations were carried out as described in Materials and Methods, and the total amount of radioactivity in the 100-, 36-, and 17-kDa subunits was quantitated as for Fig. 2. The bars represent the average of two independent experiments, and the error bars indicate the range of the two experiments.
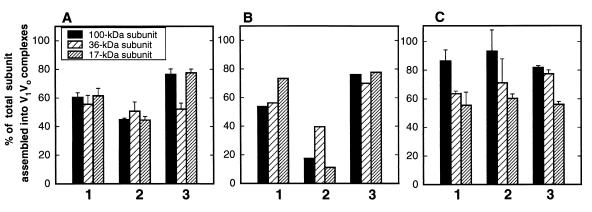
If catalysis is required to disassemble the enzyme, V1V0 complexes should not disassemble in vma mutant strains containing assembled but inactive V-ATPases. We first attempted to look at disassembly and reassembly in two peripheral subunit mutants that had been demonstrated to allow enzyme assembly, vma1-E740D (vma1-22 [36]) and vma2-Y352S (37). Although both mutations allow some assembly of V-ATPase complexes (36, 37), neither mutant contained wild-type levels of assembled enzyme when the immunoprecipitated complexes were quantitated, suggesting that the mutations have some effect on assembly or complex stability and thus are inappropriate for looking at glucose-specific effects. However, the vph1-E789Q strain, in which a point mutation in the 100-kDa V0 subunit completely abolishes both proton transport and ATPase activities in isolated vacuolar membranes (34), did show wild-type levels of assembled enzyme. In the vph1-E789Q strain, the mutated vph1 gene present in a low-copy-number plasmid is the only form of the 100-kDa subunit that is expressed because the chromosomal copies of both genes encoding the 100-kDa subunit, STV1 and VPH1, were deleted (Table 1) (34, 41, 42). To investigate whether disassembly of V1V0 complexes was prevented in the vph1-E789Q mutant strain, we carried out the previously described pulse-chase experiments followed by immunoprecipitation of the V-ATPase. Only 19.5% ± 3.5% of the immunoprecipitated V1V0 complexes disassembled when the vph1-E789Q mutant was chased in YEP medium for 5 min (not shown) or for as long as 20 min (Fig. 5A). Control experiments were performed with a stv1Δ vph1Δ double-mutant strain that carried the wild-type VPH1 gene in a low-copy-number plasmid (Fig. 5B); disassembly and reassembly occurred normally. These experiments support the results in Fig. 4 indicating that catalysis may play a role in disassembly of V1V0 upon glucose depletion. We performed the same experiment with a second vma mutant, vma11-E145L (27). This mutation, which occurs in one of the proteolipid subunits of the V0 sector, completely abolishes both ATP hydrolysis and proton pumping activity in the enzyme but allows very high levels of V1 V0 assembly in isolated vacuoles (27). Results for this mutant enzyme (Fig. 5C) confirm some hyperassembly of V1 and V0 relative to wild-type cells in the presence of YEPD but more importantly demonstrate an almost complete inhibition of disassembly upon glucose deprivation. Control experiments with an isogenic strain carrying the wild-type plasmid revealed normal levels of disassembly (51 to 70% for the three V0 subunits measured) and full reassembly (not shown). It would be interesting to distinguish between a requirement for ATP hydrolysis and a requirement for proton transport for disassembly, but at present, no yeast V-ATPase mutants that clearly exhibit uncoupled ATP hydrolysis and proton transport have been described.
FIG. 5.
Disassembly of the V-ATPase in mutant strains. (A and B) Yeast vph1Δ stv1Δ cells containing the vph1-E789Q allele (A) or a wild-type copy of the gene (B) in a low-copy-number plasmid were converted to spheroplasts and radiolabeled as described in Materials and Methods. Labeled spheroplasts were chased for 30 min in YEPD (position 1), 20 min in YEP (position 2), or 20 min in YEP, followed by an additional 10 min with 2% glucose added (position 3). (C) Yeast vma11Δ cells containing the vma11-E145L allele in a low-copy-number plasmid were converted to spheroplasts and chased as described for panels A and B. Immunoprecipitations were performed and analyzed as described for Fig. 2. The bars represent the average of two independent experiments in panels A and C and results from a single measurement in panel B. Error bars indicate the range of the two experiments. Disassembly and reassembly of the 100-, 36-, and 17-kDa subunits were measured in each experiment and are shown individually, as indicated.
DISCUSSION
Reversible association between the peripheral and membrane sectors of V-ATPases is a newly described process that may regulate the amount of functional enzyme in a given membrane as well as prevent unnecessary ATP hydrolysis and proton pumping. In yeast (29) and in the tobacco hornworm M. sexta (25), this response appears to be triggered by extracellular nutrients. In both organisms, the pool of cytosolic V1 complexes increases during starvation due to dissociation from the membrane V0 sector. Although S. cerevisiae is the only organism in which the reversible nature of the process has been shown in vivo (29), indirect evidence obtained with M. sexta suggests that starvation-induced disassembly of the V-ATPase can be reversed by feeding. Therefore, regulation of enzyme activity by disassembly and reassembly may be a common feature of V-type ATPases.
Interestingly, the glucose-induced disassembly and reassembly of V1 and V0 appear to involve none of the previously characterized signaling pathways implicated in glucose-induced responses. We present evidence that signal transduction does not involve either protein phosphorylation by protein kinase C (Pkc1p) or protein dephosphorylation by the protein phosphatase 2A, which participates in the general stress signaling pathway. Moreover, components of the Ras-cAMP and glucose derepression signaling pathways do not appear to mediate the disassembly and reassembly of the yeast V-ATPase, based on results with pde2Δ, cdc35ts, and snf1Δ mutants. Supporting results with these mutant strains, we also showed that reassociation of V1 and V0 cannot be triggered by intracellular accumulation of glucose 6-phosphate, a common intermediate in the Ras-cAMP and main glucose repression/derepression pathways upstream of cAMP and Snf1p.
Associations between V1 and V0 are regulated by glucose metabolism via glycolysis, presumably by glycolytic products or cytosolic components of the glycolytic pathway. In support of this, disassembly of the yeast V-ATPase naturally occurs when exponentially growing cells reach stationary phase. A drop in the total number of particles, probably V-ATPases (5), per surface area of vacuolar membrane was previously observed by freeze-fracture microscopy when yeast cells reached stationary phase (45). During the diauxic shift, as the available glucose is depleted, mitochondrial enzymes are derepressed (50), and any further carbohydrate metabolism involves the tricarboxylic acid cycle and oxidative phosphorylation in mitochondria (28). Our results indicate that neither the tricarboxylic acid cycle nor oxidative phosphorylation supports full assembly of the V-ATPase; ethanol, lactate, and glycerol are also unable to trigger reassociation of V1 and V0 in short-term experiments. Galactose and raffinose were equally ineffective in triggering reassociation after glucose deprivation; in addition, raffinose was shown to give lower levels of assembled enzyme than glucose after long-term incubation (29). Although both galactose and raffinose undergo glycolysis, neither is used as efficiently as glucose, fructose, or mannose and therefore would give a reduced glycolytic rate.
In agreement with a model in which continuous glucose metabolism is required to keep the enzyme assembled, disassembly/reassembly was not an all-or-none response. The size of the intracellular pool of assembled V1V0 complexes was proportional to the amount of glucose in the medium over a range of 0.1 to 1% added glucose. The pool of immunoprecipitated V0 domains assembled into V1V0 complexes showed saturation at 1 to 2% glucose, but under these conditions 30 to 40% of the total V0 sectors remained free of peripheral subunits. An excess of V0 membrane domains over V1V0 complexes has been previously reported for yeast vacuoles and clathrin-coated vesicles (3, 76), and both yeast and bovine cells contain a significant pool of V1 domains free in the cytoplasm (15, 47, 65). Curiously, the vma11-E145L mutation appears to shift the balance between V1V0 and V0 complexes in cells, so that a higher percentage of the V0 subunits are assembled into V1V0 complexes (Fig. 5C), consistent with previous results for isolated vacuoles (27). It has not been established whether glucose promotes reassembly of only a fraction of the total pool of soluble V1 in wild-type cells. In that case, recruitment of V1 domains to membranes may even be triggered by a number of extracellular and intracellular signals. The results reported here, as well as previous work (29), also show that yeast cells contain a pool of fully assembled V1V0 complexes, representing 8 to 18% of the total V0 sectors, that never disassemble. We do not know if these complexes are inactive for some reason, and thus incompetent for disassembly, or if they are somehow conserved by the cell to maintain a baseline level of V-ATPase activity.
The effect of glycolysis on the V-ATPase is likely to be indirect. Cellular changes in ATP concentration resulting from impairment in glycolysis could be directly sensed by the V-ATPase. The transient decrease in the intracellular ATP concentration immediately after glucose deprivation of the cells occurred rapidly enough to account for the rapid disassembly of the enzyme. The ATP pool fell to 50% of the initial ATP levels in YEPD after 1 min of glucose depletion, and disassembly of the V-ATPase could well be an energy-saving mechanism to prevent ATP hydrolysis. Yeast cells efficiently buffered their intracellular ATP concentration, recovering up to 83% of their initial ATP in YEPD within 20 min of glucose depletion. In the presence of glucose, the preferred carbon source, yeast cells may obtain ATP entirely from glycolysis (28). However, cells incubated in YEP may metabolize storage carbohydrates (glycogen and trehalose) which appear to be utilized under conditions that demand an internal energy source, including starvation and adaptation to respiratory growth (28, 35). The ATP concentrations determined in our experiments corresponded to the total pool of ATP inside the cells, but the cytosol is the major reservoir of ATP in yeast. Therefore, our results likely reflect changes in the cytosolic pool of ATP, and the V-ATPase may respond to these changes. These results suggest that a decrease in ATP concentration, or, more likely, changes of the cytosolic ATP/ADP concentration ratio, could be a physiological trigger for disassembly of the vacuolar H+-ATPase.
By analogy with its closely related F1F0-ATPases, the V-ATPase may adopt different catalytic and structural properties in response to changes in substrate availability or ATP/ADP ratio. The enzyme possesses six potential nucleotide binding sites for ATP and ADP. Only three are catalytic sites located on the 69-kDa subunits (20, 66, 75). The remaining three are noncatalytic binding sites, presumed to be regulatory sites, that are located on the 60-kDa subunits (37, 75). Under conditions of limited ATP concentrations, or when the ATP/ADP concentration ratio is low, ADP binding or ATP release from nucleotide binding sites may destabilize interactions between V1 and V0. The full signal for disassembly may not be contained within the assembled V-ATPase itself or even in the vacuole; dissociation may involve other cellular components and additional cellular signals. Consistent with this, addition of ATP and Mg2+ to isolated vacuolar membranes is necessary but not sufficient to disassemble the enzyme in vitro (30, 48).
At low concentrations of ATP, catalysis may induce a structural conformation that favors dissociation of V1 from V0. A role for catalysis during disassembly is indicated by the partial inhibition of disassembly by concanamycin A or mutations that abolish the ATPase and proton pumping activities of the V-ATPase. We do not know the exact level of inhibition achieved by 1 μM concanamycin A in vivo and thus we cannot say how closely the level of disassembly paralleled the level of inhibition under these conditions. The vph1-E789Q mutant cells, which would be predicted to contain no active V-ATPases based on in vitro data, showed a substantial reduction of the amount of V1V0 complexes that disassembled on glucose depletion. The few complexes (16 to 23%) that disassembled may have been improperly assembled enzymes in the mutant strain. It is also possible that even a catalytically inactive enzyme, such as the vph1-E789Q mutant enzyme, can bind nucleotide and assume a conformation susceptible to disassembly. However, the results with the vma11-E145L mutant enzyme provide further evidence that catalysis, rather than nucleotide binding alone, is required for disassembly. This mutant enzyme undergoes Mg-ATP-dependent dissociation in vitro upon addition of low concentrations of chaotropic anion, suggesting that it can bind nucleotide and assume a conformation susceptible to dissociation (27). Nevertheless, it exhibits even less disassembly in response to glucose deprivation than the vph1-E789Q mutant enzyme (Fig. 5C). Disassembly of only catalytically active complexes offers the cell a means of ensuring that only potentially active V1 and V0 domains are available for reassembly. In this way the cells might prevent the formation of nonfunctional V1V0 complexes and guarantee restoration of full ATPase and proton pumping activities with reassembly.
In addition to glucose 6-phosphate and ATP production, initiation of glycolysis also results in transient cytosolic acidification. A sharp drop of cytosolic pH takes place shortly after addition of glucose to starving yeast cells (4, 52). This pH drop could provide both a signal for glucose-induced reassembly and a physiological reason for this response. Vacuolar and plasma membrane H+-ATPases play a major role in cellular homeostasis (55), and the two enzymes may work together to regulate cytosolic pH. Coordination between these two proton pumps was recently shown by Bowman et al. (7), who found that mutations in the pma-1 gene of N. crassa conferred resistance to concanamycin A but did not act by preventing its uptake. A reassembled V-ATPase may assist Pma1p in raising the cytosolic pH immediately after glucose addition to glucose-depleted cells. We showed that Pkc1p does not regulate the V-ATPase response, indicating that different intracellular triggers may induce Pma1p activation and the V1 and V0 reassembly. The signal for reassembly might be contained within the V-ATPase itself, which could sense, and reassemble in response to, a drop of the cytoplasmic pH. In vitro, functional reassembly of yeast V1 and V0 domains is strongly pH dependent and optimal at pH 5.5 (48), suggesting that one or more pH-sensitive groups may be involved in the interaction between V1 and V0. It is relatively difficult to accurately measure or manipulate the cytoplasmic pH of yeast cells in vivo (4), but it will be very important to address the link between cytoplasmic pH and V-ATPase assembly in the future.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant R01-GM50322 to P.M.K. P.M.K. is an American Heart Association Established Investigator.
We thank Tom Stevens, Mike Tyers, Saul Honigberg, Richard Hallberg, David Levin, Carlos Gancedo, Morris Manolson, Michael Forgac, Mary Crivellone, and Kelly Tatchell for providing strains used in this work. We also thank David Amberg for the use of his microscope and Richard Cross and Mark Schmitt for helpful discussions.
REFERENCES
- 1.Adachi I, Puopolo K, Marquez-Sterling N, Arai H, Forgac M. Dissociation, cross-linking, and glycosylation of the coated vesicle proton pump. J Biol Chem. 1990;265:967–973. [PubMed] [Google Scholar]
- 2.Arai H, Pink S, Forgac M. Interaction of anions and ATP with the coated vesicle proton pump. Biochemistry. 1989;28:3075–3082. doi: 10.1021/bi00433a051. [DOI] [PubMed] [Google Scholar]
- 3.Bauerle C, Ho M N, Lindorfer M A, Stevens T H. The S. cerevisiae vma6 gene encodes the 36-kDa subunit of the vacuolar H+-ATPase membrane sector. J Biol Chem. 1993;268:12749–12757. [PubMed] [Google Scholar]
- 4.Beauvoit B, Rigoulet M, Raffard G, Canioni P, Guerin B. Differential sensitivity of the cellular compartments of Saccharomyces cerevisiae to protonophoric uncoupler under fermentative and respiratory energy supply. Biochemistry. 1991;30:11212–11220. doi: 10.1021/bi00111a004. [DOI] [PubMed] [Google Scholar]
- 5.Bowman B J, Dschida W J, Harris T, Bowman E J. The vacuolar ATPase of Neurospora crassa contains an F1-like structure. J Biol Chem. 1989;264:15606–15612. [PubMed] [Google Scholar]
- 6.Bowman E J, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman E J, O’Neill F J, Bowman B J. Mutations of pma-1, the gene encoding the plasma membrane H+-ATPase of Neurospora crassa, suppress inhibition of growth by concanamycin A, a specific inhibitor of vacuolar ATPases. J Biol Chem. 1997;272:14776–14786. doi: 10.1074/jbc.272.23.14776. [DOI] [PubMed] [Google Scholar]
- 8.Cannon J F, Tatchell K. Characterization of Saccharomyces cerevisiae genes encoding subunits of cyclic AMP-dependent protein kinase. Mol Cell Biol. 1987;7:2653–2663. doi: 10.1128/mcb.7.8.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celenza J L, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233:1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- 10.Cereghino G P, Scheffler I E. Genetic analysis of glucose regulation in Saccharomyces cerevisiae: control of transcription versus mRNA turnover. EMBO J. 1996;15:363–374. [PMC free article] [PubMed] [Google Scholar]
- 11.Chang A, Slyman C W. Maturation of the yeast plasma membrane [H+]-ATPase involves phosphorylation during intracellular transport. J Cell Biol. 1991;115:289–295. doi: 10.1083/jcb.115.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciriacy M, Breitenbach I. Physiological effects of seven different blocks in glycolysis in Saccharomyces cerevisiae. J Bacteriol. 1979;139:152–160. doi: 10.1128/jb.139.1.152-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox G B, Devenish R J, Gibson F, Howitt S M, Nagley P. The structure and assembly of ATP synthase. In: Ernster L, editor. Molecular mechanisms in bioenergetics. Amsterdam, The Netherlands: Elsevier; 1992. p. 283. [Google Scholar]
- 14.Crider B P, Xie X-S, Stone D K. Bafilomycin inhibits proton flow through the H+ channel of vacuolar proton pumps. J Biol Chem. 1994;269:17379–17381. [PubMed] [Google Scholar]
- 15.Doherty R D, Kane P M. Partial assembly of the yeast vacuolar H+-ATPase in mutants lacking one subunit of the enzyme. J Biol Chem. 1993;268:16845–16851. [PubMed] [Google Scholar]
- 16.Drose S, Altendorf K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J Exp Biol. 1997;200:1–8. doi: 10.1242/jeb.200.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Drose S, Bindseil K U, Bowman E J, Siebers A, Zeeck A, Altendorf K. Inhibitory effect of modified bafilomycins and concanamycins on P and V-type ATPases. Biochemistry. 1993;32:3902–3906. doi: 10.1021/bi00066a008. [DOI] [PubMed] [Google Scholar]
- 18.Estrada E, Agostinis P, Vandenheede J R, Goris J, Merlevede W, Francois J, Goffeau A, Ghislain M. Phosphorylation of yeast plasma membrane H+-ATPase by casein kinase I. J Biol Chem. 1996;271:32064–32072. doi: 10.1074/jbc.271.50.32064. [DOI] [PubMed] [Google Scholar]
- 19.Evangelista C C, Rodriguez Torres A M, Paulin Limbach M, Zitomer R S. Rox3 and Rts1 function in the global stress response pathway in baker’s yeast. Genetics. 1996;142:1083–1093. doi: 10.1093/genetics/142.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y, Forgac M. Cysteine 254 of the 73-kDa A subunit is responsible for inhibition of the coated vesicle (H+)-ATPase upon modification by sulfhydryl reagents. J Biol Chem. 1992;267:5817–5822. [PubMed] [Google Scholar]
- 21.Fillingame R H, Mosher M E, Negrin R S, Peters L K. H+-ATPase of Escherichia coli uncB402 mutation leads to loss of chi subunit of subunit of F0 sector. J Biol Chem. 1983;258:604–609. [PubMed] [Google Scholar]
- 22.Forgac M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol Rev. 1989;69:765–796. doi: 10.1152/physrev.1989.69.3.765. [DOI] [PubMed] [Google Scholar]
- 23.Fox D T, Folley L S, Mulero J J, McMullin T W, Thorsness P E, Hedin L O, Costanzo M C. Analysis and manipulation of yeast mitochondrial genes. Methods Enzymol. 1991;194:149–165. doi: 10.1016/0076-6879(91)94013-3. [DOI] [PubMed] [Google Scholar]
- 24.Gamo F J, Portillo F, Gancedo C. Characterization of mutations that overcome the toxic effect of glucose on phosphoglucose isomerase less strains of Saccharomyces cerevisiae. FEMS Microbiol Lett. 1993;106:233–238. doi: 10.1111/j.1574-6968.1993.tb05969.x. [DOI] [PubMed] [Google Scholar]
- 25.Graf R, Harvey W R, Wieczorek H. Purification and properties of a cytosolic V1-ATPase. J Biol Chem. 1996;271:20908–20913. doi: 10.1074/jbc.271.34.20908. [DOI] [PubMed] [Google Scholar]
- 26.Herrera L S, Pascual C. Genetical and biochemical studies of the glucosephosphate isomerase deficient mutants in Saccharomyces cerevisiae. J Gen Microbiol. 1978;108:305–310. [Google Scholar]
- 27.Hirata R, Graham L A, Takatsuki A, Stevens T H, Anraku Y. VMA11 and VMA16 encode the second and third proteolipid subunits of the Saccharomyces cerevisiae vacuolar membrane H+-ATPase. J Biol Chem. 1997;272:4795–4803. doi: 10.1074/jbc.272.8.4795. [DOI] [PubMed] [Google Scholar]
- 28.Johnston M, Carlson M. Regulation of carbon and phosphate utilization. In: Jones E W, Pringle J R, Broach J R, editors. The molecular and cell biology of the yeast Saccharomyces. Vol. 2. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1991. pp. 193–281. [Google Scholar]
- 29.Kane P M. Disassembly and reassembly of the yeast vacuolar H+-ATPase in vivo. J Biol Chem. 1995;270:17025–17032. [PubMed] [Google Scholar]
- 30.Kane P M, Yamashiro C T, Stevens T H. Biochemical characterization of the yeast vacuolar H+-ATPase. J Biol Chem. 1989;264:19236–19244. [PubMed] [Google Scholar]
- 31.Kane P M, Kuehn M C, Howald-Stevenson I, Stevens T H. Assembly and targeting of peripheral and integral membrane subunits of the yeast vacuolar H+-ATPase. J Biol Chem. 1992;267:447–454. [PubMed] [Google Scholar]
- 32.Koerner T J, Myers A M, Lee S, Tzagoloff A. Isolation and characterization of the yeast gene coding for the α subunit of mitochondrial phenylalanyl-tRNA synthetase. J Biol Chem. 1987;262:3690–3696. [PubMed] [Google Scholar]
- 33.Lee R H, Honigberg S M. Nutritional regulation of late meiotic events in Saccharomyces cerevisiae through a pathway distinct from initiation. Mol Cell Biol. 1996;16:3222–3232. doi: 10.1128/mcb.16.6.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leng X-H, Manolson M F, Liu Q, Forgac M. Site-directed mutagenesis of the 100-kDa subunit Vph1p of the yeast vacuolar H+-ATPase. J Biol Chem. 1996;271:22487–22493. doi: 10.1074/jbc.271.37.22487. [DOI] [PubMed] [Google Scholar]
- 35.Lillie S H, Pringle J R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980;143:1384–1494. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Kane P M. Mutational analysis of the catalytic subunit of the yeast vacuolar proton-translocating ATPase. Biochemistry. 1996;35:10938–10948. doi: 10.1021/bi9608065. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q, Kane P M, Newman P R, Forgac M. Site-directed mutagenesis of the yeast V-ATPase B subunit (Vma2p) J Biol Chem. 1996;271:2018–2022. doi: 10.1074/jbc.271.4.2018. [DOI] [PubMed] [Google Scholar]
- 38.Lobo Z, Maitra P K. Resistance to 2-deoxyglucose in yeast: a direct selection of mutants lacking glucose-phosphorylating enzymes. Mol Gen Genet. 1977;157:297–300. doi: 10.1007/BF00268666. [DOI] [PubMed] [Google Scholar]
- 39.Lopes Brandao R, Magalhaes-Rocha N M, Alijo R, Ramos J, Thevelein J M. Possible involvement of a phosphatidylinositol-type signaling pathway in glucose-induced activation of plasma membrane H+-ATPase and cellular proton extrusion in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1994;1223:117–124. doi: 10.1016/0167-4889(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 40.Maitra P K. Glucose and fructose metabolism in a phosphoglucoisomeraseless mutant of Saccharomyces cerevisiae. J Bacteriol. 1971;107:759–769. doi: 10.1128/jb.107.3.759-769.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manolson M F, Wu B, Proteau D, Taillon B E, Tibor Roberts B, Andrew Hoyt M, Jones E W. STV1 gene encodes functional homologue of 95-kDa yeast vacuolar H+-ATPase subunit Vph1p. J Biol Chem. 1994;269:14064–14074. [PubMed] [Google Scholar]
- 42.Manolson M F, Proteau D, Preston R A, Stenbit A, Roberts B T, Hoyt M A, Preuss D, Mulholland J, Botstein D, Jones E W. The VPH1 gene encodes a 95-kDa integral membrane polypeptide required for in vivo assembly and activity of the yeast vacuolar H+-ATPase. J Biol Chem. 1992;267:14294–14303. [PubMed] [Google Scholar]
- 43.Mellman I. The importance of being acid: the role of acidification in intracellular membrane traffic. J Exp Biol. 1992;172:39–45. doi: 10.1242/jeb.172.1.39. [DOI] [PubMed] [Google Scholar]
- 44.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 45.Moeller C H, Thomson W W. An ultrastructural study of the yeast tonoplast during the shift from exponential to stationary phase. J Ultrastruct Res. 1979;68:28–37. doi: 10.1016/s0022-5320(79)90139-4. [DOI] [PubMed] [Google Scholar]
- 46.Moriyama Y, Nelson N. Cold inactivation of vacuolar proton-ATPases. J Biol Chem. 1989;264:3577–3582. [PubMed] [Google Scholar]
- 47.Myers M, Forgac M. Assembly of the peripheral domain of the bovine vacuolar H+-ATPase. J Cell Physiol. 1993;156:35–42. doi: 10.1002/jcp.1041560106. [DOI] [PubMed] [Google Scholar]
- 48.Parra K J, Kane P M. Wild-type and mutant vacuolar membranes support pH-dependent reassembly of the yeast vacuolar H+-ATPase in vitro. Biol Chem. 1996;271:19592–19598. doi: 10.1074/jbc.271.32.19592. [DOI] [PubMed] [Google Scholar]
- 49.Pascual C, Alonso A, Perez C, Herrera L S. Glucose and fructose consumption in a phosphoglucose isomeraseless mutant in Saccharomyces cerevisiae. Arch Microbiol. 1979;121:17–21. [Google Scholar]
- 50.Perlman P S, Mahler H R. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974;162:248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- 51.Puopolo K, Forgac M. Functional reassembly of the coated vesicle proton pump. J Biol Chem. 1990;265:14836–14841. [PubMed] [Google Scholar]
- 52.Purwin C, Nicolay K, Scheffers W A, Holzer H. Mechanism of control of adenylate cyclase activity in yeast by fermentable sugars and carbonyl cyanide m-chlorophenylhydrazone. J Biol Chem. 1986;261:8744–8749. [PubMed] [Google Scholar]
- 53.Roberts C J, Raymond C K, Yamashiro C T, Stevens T H. Methods for studying the yeast vacuole. Methods Enzymol. 1991;194:644–661. doi: 10.1016/0076-6879(91)94047-g. [DOI] [PubMed] [Google Scholar]
- 54.Sass P, Field J, Nikawa J, Toda T, Wigler M. Cloning and characterization of the high-affinity cAMP phosphodiesterase of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1986;83:9303–9307. doi: 10.1073/pnas.83.24.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serrano R. Transport across yeast vacuolar and plasma membranes. In: Jones E, Pringle J R, Broach J R, editors. The molecular and cellular biology of the yeast Saccharomyces. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1991. pp. 523–585. [Google Scholar]
- 56.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. pp. 177–186. [Google Scholar]
- 57.Shu Y, Hallberg R L. SCS1, a multicopy suppressor of hsp60-ts mutant alleles, does not encode a mitochondrially targeted protein. Mol Cell Biol. 1995;15:5618–5626. doi: 10.1128/mcb.15.10.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens T H, Forgac M. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu Rev Cell Dev Biol. 1997;13:779–808. doi: 10.1146/annurev.cellbio.13.1.779. [DOI] [PubMed] [Google Scholar]
- 59.Stevens T M, Rothman J H, Payne G S, Schekman R. Gene dosage-dependent secretion of yeast vacuolar carboxypeptidase Y. J Cell Biol. 1986;102:1551–1557. doi: 10.1083/jcb.102.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sumner J-P, Dow J A T, Earley F G P, Klein U, Jager D, Wieczorek H. Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J Biol Chem. 1995;270:5649–5653. doi: 10.1074/jbc.270.10.5649. [DOI] [PubMed] [Google Scholar]
- 61.Swallow C J, Grinstein S, Sudsbury R A, Rotstein O D. Relative roles of Na+/H+ exchange and vacuolar-type H+ ATPases in regulating cytoplasmic pH and function in murine peritoneal macrophages. J Cell Physiol. 1993;157:453–460. doi: 10.1002/jcp.1041570304. [DOI] [PubMed] [Google Scholar]
- 62.Thevelein J M, Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem Sci. 1995;20:3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- 63.Thevelein J M, Beullens M, Honshoven F, Hoebeeck G, Detremerie K, Griewel B, Den Hollander J A, Jans A W H. Regulation of the cAMP level in the yeast Saccharomyces cerevisiae: the glucose-induced cAMP signal is not mediated by a transient drop in the intracellular pH. J Gen Microbiol. 1987;133:2197–2205. doi: 10.1099/00221287-133-8-2197. [DOI] [PubMed] [Google Scholar]
- 64.Tokiwa G, Tyers M, Volpe T, Futcher B. Inhibition of G1 cyclin activity by the Ras/cAMP pathway in yeast. Nature. 1994;371:342–345. doi: 10.1038/371342a0. [DOI] [PubMed] [Google Scholar]
- 65.Tomashek J J, Garrison B S, Klionsky D J. Reconstitution in vitro of the V1 complex from the yeast vacuolar proton-translocating ATPase. J Biol Chem. 1997;272:16618–16623. doi: 10.1074/jbc.272.26.16618. [DOI] [PubMed] [Google Scholar]
- 66.Uchida E, Ohsumi Y, Anraku Y. Characterization and function of catalytic subunit a of H+-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem. 1988;263:45–51. [PubMed] [Google Scholar]
- 67.Ugarova N N, Romay C, Garcia I, Pascual C. Intracellular ATP in glucosephosphate isomerase mutant of Saccharomyces cerevisiae. Folia Microbiol. 1986;31:113–119. doi: 10.1007/BF02926828. [DOI] [PubMed] [Google Scholar]
- 68.Van der Plaat J B. Cyclic 3′,5′-adenosine monophosphate stimulates trehalose degradation in baker’s yeast. Biochem Biophys Res Commun. 1974;56:580–587. doi: 10.1016/0006-291x(74)90643-3. [DOI] [PubMed] [Google Scholar]
- 69.Ward J M, Sze H. Subunit composition and organization of the vacuolar H+-ATPase of plants roots. Plant Physiol. 1991;99:170–179. doi: 10.1104/pp.99.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watanabe M, Chen C-Y, Levin D E. Saccharomyces cerevisiae PKC1 encodes a protein kinase C (PKC) homolog with a substrate specificity similar to that of mammalian PKC. J Biol Chem. 1994;269:16829–16836. [PubMed] [Google Scholar]
- 71.Wilson R B, Tatchell K. SRA5 encodes the low-Km cyclic AMP phosphodiesterase of Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:505–510. doi: 10.1128/mcb.8.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson R B, Renault G, Jacquet M, Tatchell K. The pde2 gene of Saccharomyces cerevisiae is allelic to rca1 and encodes a phosphodiesterase which protects the cell from extracellular cAMP. FEBS Lett. 1993;325:191–195. doi: 10.1016/0014-5793(93)81071-7. [DOI] [PubMed] [Google Scholar]
- 73.Xie X-S. Reconstitution of ATPase activity from individual subunits of the clathrin-coated vesicle proton pump. J Biol Chem. 1996;271:30980–30985. [PubMed] [Google Scholar]
- 74.Xie X-S, Stone D K. Partial reconstitution of the subunits of the clathrin-coated vesicle proton ATPase responsible for Ca2+-activated ATP hydrolysis. J Biol Chem. 1988;263:9859–9867. [PubMed] [Google Scholar]
- 75.Zhang J, Vasilyeva E, Feng Y, Forgac M. Inhibition and labeling of the coated vesicle V-ATPase by 2-azido-[32P]ATP. J Biol Chem. 1995;270:15494–15500. doi: 10.1074/jbc.270.26.15494. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Myers M, Forgac M. Characterization of the V0 domain of the coated vesicle (H+)-ATPase. J Biol Chem. 1992;267:9773–9778. [PubMed] [Google Scholar]
- 77.Zhang J, Feng Y, Forgac M. Proton conduction and bafilomycin binding by the V0 domain of the coated vesicle V-ATPase. J Biol Chem. 1994;269:23518–23523. [PubMed] [Google Scholar]
- 78.Zimmermann F K, Scheel I. Mutants of Saccharomyces cerevisiae resistant to carbon catabolite repression. Mol Gen Genet. 1977;154:75–82. doi: 10.1007/BF00265579. [DOI] [PubMed] [Google Scholar]