Abstract
The present study aimed to compare the diagnostic performance of three imaging tests: X-ray, computed tomography (CT) and magnetic resonance imaging (MRI), for subtle Lisfranc injuries and three anatomical subtype injuries. The non-weight-bearing X-ray, CT and MRI imaging results of patients with subtle Lisfranc injuries from September 2013 to March 2022 were retrospectively reviewed. Subtle Lisfranc injuries and three anatomical subtypes (first, second and cuneiform rays) were diagnosed based on the surgical reports. The diagnostic performance of X-ray, CT and MRI was compared. The sensitivity (Sn), specificity (Sp), positive predictive value, negative predictive value, area under the receiver operating characteristic curve (AUC) and κ coefficient were reported. A total of 31 patients were included in the study. The correct diagnosis was made in 48.4% (15/31), 87.1% (27/31) and 96.8% (30/31) of patients by X-ray, CT and MRI, respectively. A total of 54 different anatomical injuries were found intraoperatively in all patients, with MRI and CT having high agreement (Sn, 72.2 and 87.0%; κ, 0.69 and 0.78, respectively) and X-ray having a low agreement (Sn, 29.6%; κ, 0.26) with the surgical findings. Regarding the first-ray injuries, CT had the highest Sn (76.9%), Sp (100%) and AUC (0.885) in diagnosing subtle Lisfranc injuries. MRI showed the best Sn (88.5 and 93.3%, respectively) and AUC (0.942 and 0.904, respectively) in both second and cuneiform rays. In conclusion, non-weight-bearing X-rays had poor diagnostic accuracy for subtle Lisfranc injuries and their subtypes. CT was superior to X-rays and MRI in diagnosing first-ray injuries. Although not significantly different from CT in terms of overall diagnosis, MRI was superior to X-ray and CT in diagnosing second and cuneiform-ray injuries.
Keywords: subtle Lisfranc injury, tarsometatarsal joint, X-ray, computed tomography, magnetic resonance imaging, imaging diagnosis
Introduction
The Lisfranc joint includes the tarsometatarsal, intermetatarsal and anterior intertarsal joints, which have an essential role in maintaining the longitudinal and transverse stability of the midfoot. Lisfranc injuries were reported to account for ~0.2% of all fractures (1). Based on the etiology, Lisfranc injuries may be categorized into high-energy or low-energy injuries (2). Low-energy injuries are common in midfoot sprains and sports activities and are difficult to diagnose due to atypical imaging features (3,4). Certain studies have defined the Lisfranc ligament injury, ligament avulsion fracture and joint instability caused by low-energy injury as subtle Lisfranc injury (5-9). Its delayed diagnosis and treatment may lead to chronic pain, arch collapse and dysfunction in the midfoot (3,10,11). Therefore, it is essential to diagnose these injuries early and accurately.
The common diagnostic tests for midfoot injuries are X-ray, computed tomography (CT) and magnetic resonance imaging (MRI) examinations. Each of these examinations has advantages and limitations (3). X-ray has the advantages of convenience, affordability and low radiation exposure. Weight-bearing X-ray may also detect dynamic joint instability. However, the complex midfoot anatomy often leads to overlapping bony structures, contributing to a high missed diagnosis rate of X-rays in subtle Lisfranc injuries. A CT scan may cause more radiation exposure but has the advantage of better visualization of the bony structures, fracture situations and joint spaces. MRI has a high sensitivity (Sn) in diagnosing Lisfranc injuries with ligament injuries, at the expense of high medical cost. In addition, certain patients have contraindications for the MRI examination. Compared with the weight-bearing X-ray, commonly used CT and MRI only provide static views to estimate joint stability. In addition, most of these previous studies compared the diagnostic efficacy of different imaging examinations with each other but not with the intraoperative findings as the ‘gold standard’. The diagnostic efficacy of these imaging examinations in different subtypes of subtle Lisfranc injuries was also rarely reported (12,13).
In the present study, the operative findings were used as the ‘gold standard’ to diagnose subtle Lisfranc injuries and anatomical subtypes and the diagnostic performance of X-ray, CT and MRI examinations in these injuries was investigated. Furthermore, the clinical applications of these examinations in patients with suspected subtle Lisfranc injuries were discussed.
Materials and methods
Study design and participant selection
A retrospective study was performed and the hospital's medical records of patients with surgically confirmed Lisfranc injuries at Shenzhen Hospital of Peking University (Shenzhen, China) from September 2013 to March 2022 were reviewed. The study protocol was approved by the ethics committee at Shenzhen Hospital of Peking University (approval no. 201400233).
The inclusion criteria were as follows: i) A history of unilateral foot low-energy injury with midfoot pain and discomfort during walking and exercise, midfoot joint tenderness and plantar pain on weight-bearing or stress tests; ii) available bilateral foot non-weight-bearing X-rays, CT and MRI of the involved side; and iii) surgery by foot open reduction and internal fixation, and confirmed subtle Lisfranc injury and its subtype. Patients with previous foot surgery or incomplete medical records were excluded from the study. In addition, those patients who opted for conservative treatments (lower limb cast immobilization for four weeks with no weight bearing) were excluded from the study, since they had no intraoperative results for comparison.
Imaging methods and diagnostic criteria
Non-weight-bearing X-rays, CT and MRI were performed prior to the operation. Pre-operative non-weight-bearing X-rays were obtained using the Philips Digital TH DR machine for anteroposterior (AP) and oblique foot views. CT examination was performed using a Philips Brilliance 6-layer spiral CT machine with the following scanning parameters: Voltage, 120 kV; current, 35 mA; pitch, 1.0 mm; and slice thickness, 0.6 mm. MRI examination was completed in a Philips Ingenia 3.0T MRI machine (with MR Systems Ingenia Release 6.0 software installed). Two experienced orthopedic doctors (with >3 years of experience) who did not participate in the operation read the images diagnosed the subtle Lisfranc injuries and classified them into different anatomical subtypes according to the published diagnostic criteria described below. Images were interpreted separately, with inconsistent results discussed and a consistent conclusion reached.
Imaging diagnostic criteria
In the foot X-ray, compared with the other side, the presence of diastasis >2 mm between the first metatarsal (M1) and second metatarsal (M2) joint in the AP view, diastasis over 2 mm between the medial cuneiform (C1) and second metatarsal (M2) joint in the AP view, diastasis over 2 mm between C1 and the intermediate cuneiform (C2) in the AP or oblique view, discontinuity of the lateral cortical line of C1 and M1 in the AP view, discontinuity of the medial cortical border between the C2 and M2 bone in the AP view, and/or avulsion fracture between C1 and M2 (spot sign) were diagnosed as subtle Lisfranc injuries (13-16). In the CT scan, in addition to the above-mentioned diagnostic criteria, a distance of C1-M2 >2 mm compared with the healthy side measured in the coronal view, and subtle fractures between each metatarsophalangeal joint were also diagnosed as subtle Lisfranc injury (17,18). During the MRI examination, in addition to the above criteria, the presence of Lisfranc ligament and dorsal, interosseous and plantar ligament tears, laxity, edema and/or subtle fractures were the diagnostic criteria for subtle Lisfranc injury. MRI assesses the ligament injuries (Lisfranc's ligament, interosseous ligament, dorsal collateral ligament and plantar ligament) by the ligament visibility, appearance, configuration, signal intensity, length, width and thickness. The MRI images are primarily based on hydrogen atom protons (particularly in water in the human body). A high water content usually results in high signal intensity. Acute bone injuries and peripheral inflammatory reactions may cause bone marrow edema with local interstitial fluid accumulation. This may lead to high signal intensity in the local areas, including the bone marrow, in the T2 image. MRI diagnoses fractures and bone contusions by the high signal intensity due to bone marrow edema after the injury (19) (Figs. 1 and 2).
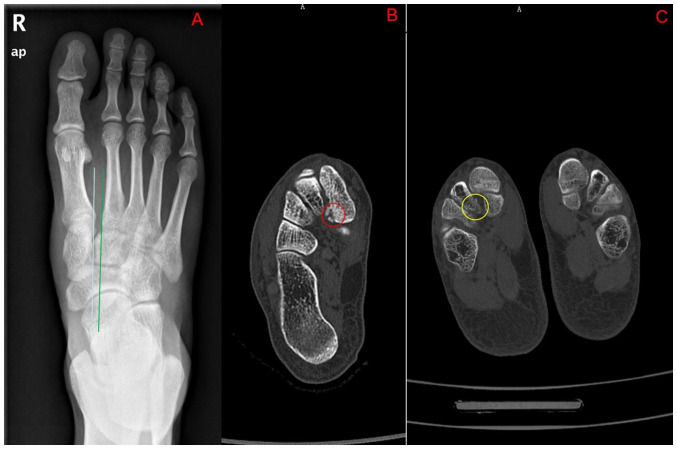
Figure 1.
(A) X-ray anterior-posterior view showing that the alignment of the second metatarsal bone and the middle cuneiform bone was lost. The basal space of the first and second metatarsals was significantly widened. (B) Small fracture of the medial cuneiform bone can be seen on the axial CT (red circle). (C) Small avulsion fracture of the middle cuneiform bone can be seen on the axial CT (yellow circle).
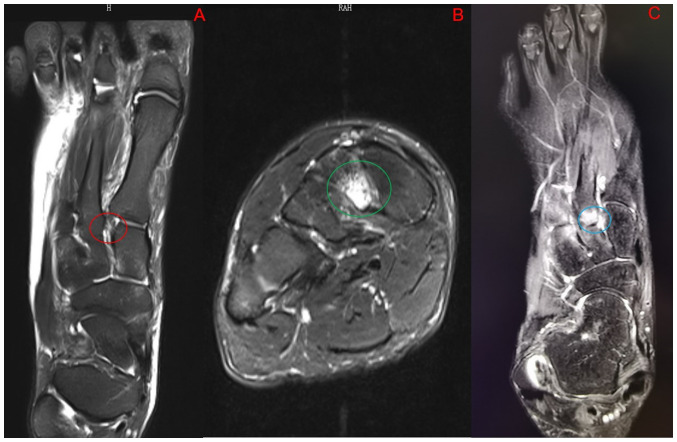
Figure 2.
(A) The Lisfranc ligament injury (red circle) can be seen on the MRI transverse section T2 image. (B) MRI coronal T2 image shows prominent bone marrow edema in the middle cuneiform bone (green circle). (C) The second metatarsal basal bone marrow edema can be seen on the MRI transverse section T2 image. The Lisfranc ligament is not visible (blue circle).
According to the classification proposed by Haraguchi et al (20), which is based on the Kaar typology, the injuries were categorized into three different anatomical subtypes, namely first-ray injuries (injuries between M1 and C1), second-ray injuries (injuries between M2 and C1 and C2) and cuneiform-ray injuries (injuries between the three cuneiform bones) in the present study.
The first-ray injuries were diagnosed when there was a discontinued lateral cortical line of C1 and M1 in the X-ray AP view, subtle fracture of the first metatarsophalangeal joint in the CT images, or plantar and dorsal ligament tears on MRI. According to the above criteria, M1-M2, C1-M2 separation displacement, C2-M2 medial cortical border discontinuity, C1-M2 avulsion fracture, subtle fracture at the second metatarsophalangeal joint on CT, and Lisfranc ligament and dorsal, interosseous and plantar ligament tears on MRI were diagnosed as second-ray injuries. Dislocation and microfracture between cuneiform bones on X-ray or CT images or injuries of interosseous, plantar and dorsalis pedis ligaments in each cuneiform bone area on MRI images were diagnosed as cuneiform-ray injuries.
Surgical diagnostic criteria
The stress test was performed during the operation and each joint was inspected under direct observation. Tarsometatarsal joint dislocation or instability, avulsion fracture, ligament relaxation and rupture, and joint capsule damage were considered subtle Lisfranc injuries (Fig. 3). These abnormalities were classified into different subtypes according to the anatomical location of the injury (13,20).
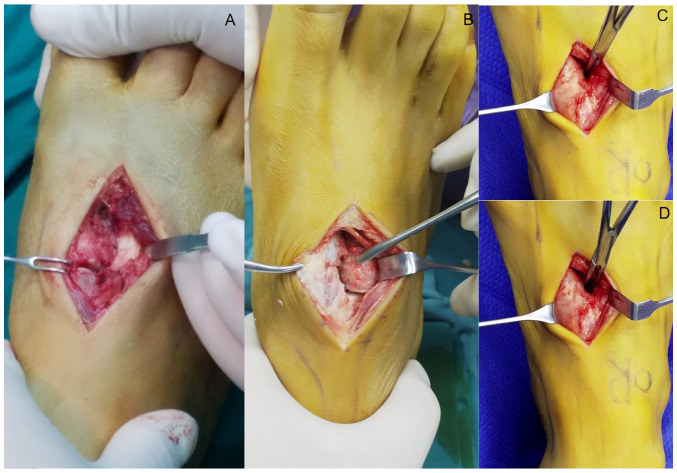
Figure 3.
Intraoperative findings of the subtle Lisfranc injury. (A) Rupture of the dorsal ligament and instability of the first tarsometatarsal joint was classified as a first-ray injury. (B) Rupture of the Lisfranc ligament and the dorsal ligament of the second tarsometatarsal joint was classified as a second-ray injury. (C) The first and (D) second tarsometatarsal joint gaps further widened during the intraoperative stress test.
Statistical analysis
Statistical analysis was performed in SPSS 25.0 (IBM Corp.). First, the overall subtle Lisfranc injury detection rate (%) was calculated for X-ray, CT and MRI compared with the final surgery report. Patients without a certain subtype injury were used as the negative controls in this subtype analysis when evaluating the subtype injuries. For example, a patient with first-ray injury was a positive case in the first-ray injury analysis but a negative case in the second-ray and cuneiform-ray injury analysis. In this way, the Sn, specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) of the X-ray, CT and MRI examinations in each anatomical subtype were calculated. The receiver operating characteristic curve (ROC) was plotted and the area under the ROC curve (AUC) values were reported. During the calculations, the intraoperative findings were used as the gold standard to compare with the X-ray findings in each ray. True-positive was defined as positive radiographic and intraoperative findings, while true-negative was defined as negative radiographic and intraoperative findings. False-positive was defined as positive radiographic findings but negative intraoperative findings, and false-negative was defined as negative radiographic findings but positive intraoperative findings. Sn (true-positive rate) was calculated by dividing the number of true-positive cases by the number of cases with positive intraoperative findings. Sp (true negative rate) was calculated by dividing the true-negative cases by the number of cases with negative intraoperative findings. The PPV was calculated by dividing the number of true-positive cases by the number of radiograph-positive cases. The NPV was calculated by dividing the number of true-negative cases by the number of radiograph-negative cases. The Cohen κ statistic was used to assess the agreement between the surgical diagnosis and each imaging examination. The results are presented according to the Landis and Koch criteria: 0.00-0.20, slight agreement; 0.21-0.40, fair; 0.41-0.60, moderate; 0.61-0.80, substantial; and 0.81-1.00, almost perfect (21). The categorical data were compared by the χ2 test among different examinations. P<0.05 was considered to indicate a statistically significant difference.
Results
Patients
A total of 31 patients with subtle Lisfranc injuries confirmed during the surgical operations were included in the present study. There were 21 males and 10 females, aged from 21 to 46 years, and the mean age was 32.26±5.76 years. The baseline demographics of these participants are shown in Table I.
Table I.
Clinicopathological characteristics of study participants.
| Demographics | First-ray injury (n=13) | Second-ray injury (n=26) | Cuneiform-ray injury (n=15) |
|---|---|---|---|
| Sex | |||
| Male | 9 (16.67) | 19 (35.19) | 11 (20.37) |
| Female | 4 (7.41) | 7 (12.96) | 4 (7.41) |
| Age, years | 33.92±6.49 | 31.54±5.32 | 34.53±6.08 |
Values are expressed as n (%) or the mean ± standard deviation.
Diagnostic efficacy of different imaging examinations in subtle Lisfranc injuries
Subtle Lisfranc injuries were correctly diagnosed in 48.4% (15/31), 87.1% (27/31) and 96.8% (30/31) of patients by X-ray, CT and MRI, respectively. The detection rate was statistically significantly lower for X-rays than for CT and MRI (both P<0.05). There was no significant difference in the detection rate between MRI and CT (P>0.05). A total of 54 different anatomical injuries were found intraoperatively in all patients, with X-ray being in slight agreement (Sn, 29.6%; κ, 0.26) and MRI and CT being in moderate agreement with the surgical diagnosis. The Sn of CT was 72.2%, with an AUC of 0.861 and κ=0.69. The Sn of MRI was 87.0%, with an AUC 0.897 and κ=0.78 (Table II; Fig. 4).
Table II.
Diagnostic efficacy of each imaging examination in each subtype injury and its consistency with surgical diagnosis.
| Examination | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC | 95% CI | κ |
|---|---|---|---|---|---|---|---|
| Total | |||||||
| X-ray | 29.6 | 100.0 | 100.0 | 50.6 | 0.648 | 0.538-0.758 | 0.26 |
| CT | 72.2 | 100.0 | 100.0 | 72.2 | 0.861 | 0.784-0.938 | 0.69 |
| MRI | 87.0 | 92.3 | 94.0 | 83.7 | 0.897 | 0.825-0.968 | 0.78 |
| First ray | |||||||
| X-ray | 30.8 | 100.0 | 100.0 | 66.7 | 0.654 | 0.448-0.860 | 0.34 |
| CT | 76.9 | 100.0 | 100.0 | 85.7 | 0.885 | 0.742-1.000 | 0.80 |
| MRI | 76.9 | 94.4 | 90.9 | 85.0 | 0.857 | 0.705-1.000 | 0.73 |
| Second ray | |||||||
| X-ray | 26.9 | 100.0 | 100.0 | 20.8 | 0.635 | 0.405-0.864 | 0.11 |
| CT | 80.8 | 100.0 | 100.0 | 50.0 | 0.904 | 0.797-1.000 | 0.58 |
| MRI | 88.5 | 100.0 | 100.0 | 62.5 | 0.942 | 0.861-1.000 | 0.71 |
| Cuneiform ray | |||||||
| X-ray | 33.3 | 100.0 | 100.0 | 61.5 | 0.667 | 0.470-0.863 | 0.34 |
| CT | 53.3 | 100.0 | 100.0 | 69.6 | 0.767 | 0.590-0.943 | 0.54 |
| MRI | 93.3 | 87.5 | 87.5 | 93.3 | 0.904 | 0.783-1.000 | 0.81 |
PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; CI, confidence interval.
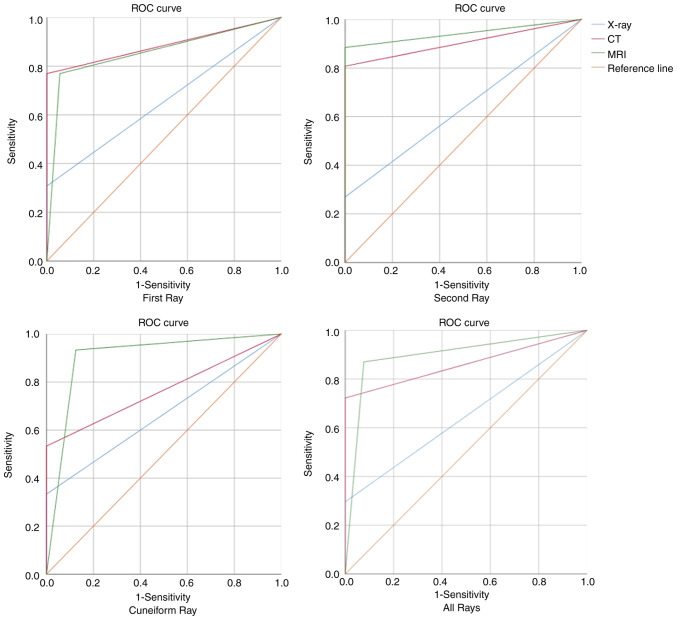
Figure 4.
ROC curves for X-ray, CT and MRI examinations. ROC, receiver operating characteristic.
Diagnostic efficacy in different subtypes of injuries
There were 13, 26 and 15 patients with first-, second- and cuneiform-ray injuries, respectively. X-rays, CT and MRI imaging detected 4, 10 and 11 injuries in the first ray, 7, 21 and 23 injuries in the second ray, and 5, 8 and 16 injuries in the cuneiform ray. In these different anatomical subtypes, the X-rays showed poor consistency and low Sn compared with the surgical diagnosis in the first-, second- and cuneiform-ray injuries, with κ=0.34, 0.11 and 0.34, respectively, and an Sn of 26.9-33.3%. In the first ray, CT had the best agreement with the surgical diagnosis (Sn, 76.9%; AUC, 0.885; κ=0.80). MRI showed the best agreement with the surgical diagnosis in the second and cuneiform rays (Sn, 88.5 and 93.3%, respectively; AUC, 0.942 and 0.904, respectively; κ=0.71 and 0.81, respectively) (Table II; Figs. 4 and 5).
Figure 5.
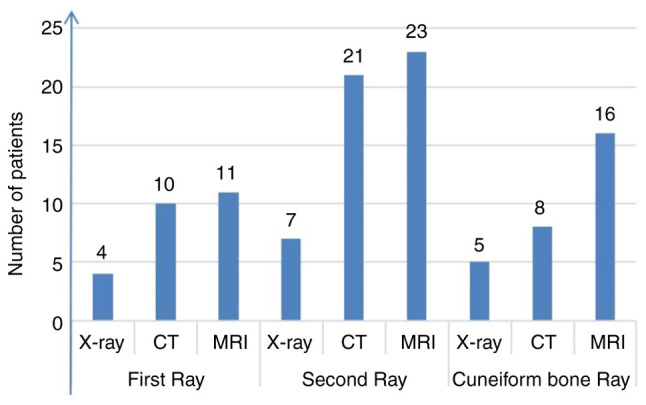
Number of cases correctly detected by X-ray, CT and MRI examinations in each subtype of subtle Lisfranc injury.
Discussion
Subtle Lisfranc injuries are usually caused by low-energy trauma, such as sprains and sports injuries. They typically occur when an axial load is sustained by the foot in the plantar flexed and slightly rotated position (16,22). Most imaging examinations of the injuries are atypical and may have certain subtle features. The incidence of low-energy Lisfranc injuries has increased in recent years. Renninger et al (2) found that in 60% of their surgically treated patients with Lisfranc injuries, they were caused by low-energy injuries. Ponkilainen et al (23) found that low-energy injuries caused most Lisfranc injuries and only 36.5% of injuries were caused by high-energy injuries. It was reported that the missed diagnosis rate of subtle Lisfranc injuries during the first clinic visit was as high as 58.8% (24), which may cause irreversible outcomes. Therefore, the early, accurate diagnosis of subtle Lisfranc injuries is crucial. X-rays have a high rate of missed diagnoses in subtle Lisfranc injuries, with Nunley and Vertullo (5) reporting a 50% missed diagnosis rate on X-rays. CT is useful in diagnosing subtle Lisfranc injuries, providing better visualization. When combined with 3D reconstruction, CT can identify subtle fractures and joint displacements (25). Preidler et al (26) found that CT showed 60% more metatarsal fractures and twice as many tarsal fractures and malalignments than X-rays. Haapamaki et al (25) found that 24% of subtle injuries not identified on the X-ray could be detected on CT. MRI is considered the best way to detect ligament injuries and has advantages in detecting subtle ones (3,10,18,27). MRI is often required to confirm the diagnosis of midfoot injuries that are difficult to diagnose with X-rays and CT. MRI diagnosis of subtle Lisfranc injuries benefits from its ability to directly visualize subtle ligament and soft tissue injuries, making it a susceptible test.
In the present study, the detection rates of X-ray, CT and MRI were 48.4% (15/31), 87.1% (27/31) and 96.8% (30/31), respectively, with a high rate of missed diagnoses for X-ray and no statistically significant difference between CT and MRI (P>0.05). The study on subtle Lisfranc injuries reported that 50% of patients had no abnormalities on initial non-weight-bearing X-rays (5,10), which was similar to the findings of the present study. In a study by Raikin et al (19), the MRI detection rate for subtle Lisfranc injury was 90.5%, similar to the result of the present study. Since X-ray has a high missed diagnosis rate, it was suggested that patients with negative X-ray but clinical suspicion of Lisfranc injuries should receive weight-bearing X-ray examination (28), which could improve the diagnostic efficacy when evaluating midfoot instability. Without the weight-bearing X-ray, 10-40% of Lisfranc injuries may be missed (29-31). Weight-bearing X-ray was able to better visualize the instability of the Lisfranc-injured joint dynamically (32).
Most previous studies on diagnosing Lisfranc injury compared the diagnostic efficacy of imaging examinations. One of the novelties of the present study was the use of the surgical findings as the gold standard diagnosis for different subtle Lisfranc injuries. In addition, another novelty of the present study was that different diagnostic performances of different imaging examinations in different anatomical subtypes of subtle Lisfranc injuries were found. Compared with CT and MRI, X-ray had the lowest Sn and was consistent in all subtypes of subtle Lisfranc injuries. Both CT and MRI had comparable Sn to diagnose subtle Lisfranc injuries in the first ray (both sensitivities were 76.9%), which was similar to the Sn of 76.1% reported by Ponkilainen et al (33). However, in all 13 first-ray injuries, CT detected 10 injuries with no false-positive results (Sn, 76.9%; Sp, 100%) and MRI detected 11 injuries with one false-positive result (Sn, 76.9%; Sp, 94.4%), resulting in a lower Sp of MRI compared with CT in the first-ray injuries, which may lead to a specific misdiagnosis rate in clinical practice. The accuracy of CT diagnosis in this ray was better than that of MRI. Therefore, it may be recommended that CT is used as the first examination method for suspected subtle Lisfranc injuries in the first ray. The advantage of CT is in the diagnosis of microfractures and translocations. In the study by Wong et al (34), the most common types of injury in first-ray injuries were joint dislocations and microfractures, which further supported the diagnostic utility of CT in first-ray injuries. However, in the second and cuneiform rays, the CT diagnosis of subtle Lisfranc injuries was inferior to MRI. Particularly in the cuneiform ray, the Sn of CT was only 53.3%. A study by Kennelly et al (35) showed that 54% of patients with subtle Lisfranc injuries who had positive weight-bearing X-rays had undiagnostic or negative CT reports.
MRI images are primarily based on the hydrogen atom protons (particularly in water in the human body). Soft tissue injuries can cause local edema, which may be more easily detected by the MRI technique, whereas CT images are based on the radiologic density of body tissues, which cannot identify the soft tissue adequately, including ligament and tendon injuries. It has the same limitations as non-weight-bearing X-rays in identifying minor joint instability and ligament injuries. MRI has more advantages in the second and cuneiform rays than X-ray and CT, particularly in the cuneiform ray. It had almost perfect performance compared with the surgical diagnosis, with an Sn of 93.3%, which is superior to the other imaging examinations. MRI can directly image Lisfranc joint ligaments and capsule structures and is highly sensitive to minor ligament injuries (36). Low-energy subtle Lisfranc injuries commonly had minor joint displacements or ligament injuries (37). Therefore, MRI is advantageous in diagnosing subtle Lisfranc injuries in the second and cuneiform rays. However, MRI may have a false-positive rate in patients with significant soft tissue swelling in acute injuries due to its high Sn for soft tissue injuries. There is no previous literature report on the false-positive rate of MRI in Lisfranc injuries. In addition, MRI is not suitable for emergency and first-choice examinations due to its high cost and long appointment and examination times. The MRI can also not provide non-weight-bearing images.
The present study had certain limitations. First, it was a retrospective study with a small cohort of patients. Only those patients who had a surgical operation to confirm Lisfranc injuries were analyzed, while those patients who chose conservative treatments were not included in the present study. Negative controls of patients without subtle Lisfranc injuries were not included in the present study, either. All of the analyses, including the calculations of Sn, Sp, PPV and NPV, were performed in these patients with foot injuries. This may undoubtedly introduce bias in the results of the present study. The ideal negative control group for the study would be patients with clinically suspected subtle Lisfranc injuries who were found to have no injury during the surgical operation. However, these patients commonly received conservative non-surgical management without intraoperative reports. Second, the comparison did not include the results of weight-bearing X-ray, as this examination was not performed in every patient. Weight-bearing X-rays may improve X-ray performance in diagnosing subtle Lisfranc injuries.
In conclusion, non-weight-bearing X-rays had poor diagnostic accuracy for subtle Lisfranc injuries and their subtypes. The diagnostic performance of CT and MRI in subtle Lisfranc injuries did not differ significantly. Regarding different anatomical subtypes, CT was better than MRI at diagnosing first-ray subtle Lisfranc injuries, whereas MRI was better than CT at diagnosing second- and cuneiform-ray subtle Lisfranc injuries.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
LB, WZ, LBT, CXW and CYX were involved in the conception and design of the study, data acquisition and follow-up, and editing of the manuscript. All authors have carefully reviewed the manuscript and have read and approved the final version. YXY and SMC confirm the authenticity of all raw data, and assisted in the collation of the raw data.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Shenzhen Hospital of Peking University (Shenzhen, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Smith N, Stone C, Furey A. Does open reduction and internal fixation versus primary arthrodesis improve patient outcomes for lisfranc trauma? A systematic review and meta-analysis. Clin Orthop Relat Res. 2016;474:1445–1452. doi: 10.1007/s11999-015-4366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renninger CH, Cochran G, Tompane T, Bellamy J, Kuhn K. Injury characteristics of low-energy lisfranc injuries compared with high-energy injuries. Foot Ankle Int. 2017;38:964–969. doi: 10.1177/1071100717709575. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui NA, Galizia MS, Almusa E, Omar IM. Evaluation of the tarsometatarsal joint using conventional radiography, CT, and MR imaging. Radiographics. 2014;34:514–531. doi: 10.1148/rg.342125215. [DOI] [PubMed] [Google Scholar]
- 4.Sripanich Y, Weinberg MW, Krähenbühl N, Rungprai C, Saltzman CL, Barg A. Reliability of measurements assessing the Lisfranc joint using weightbearing computed tomography imaging. Arch Orthop Trauma Surg. 2021;141:775–781. doi: 10.1007/s00402-020-03477-5. [DOI] [PubMed] [Google Scholar]
- 5.Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30:871–878. doi: 10.1177/03635465020300061901. [DOI] [PubMed] [Google Scholar]
- 6.Briceno J, Leucht A, Younger A, Veljkovic A. Subtle Lisfranc injuries: Fix it, fuse it, or bridge it? Foot Ankle Clin. 2020;25:711–726. doi: 10.1016/j.fcl.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum A, Dellenbaugh S, Dipreta J, Uhl R. Subtle injuries to the lisfranc joint. Orthopedics. 2011;34:882–887. doi: 10.3928/01477447-20110922-23. [DOI] [PubMed] [Google Scholar]
- 8.Faciszewski T, Burks RT, Manaster BJ. Subtle injuries of the Lisfranc joint. J Bone Joint Surg Am. 1990;72:1519–1522. [PubMed] [Google Scholar]
- 9.Norfray JF, Geline RA, Steinberg RI, Galinski AW, Gilula LA. Subtleties of Lisfranc fracture-dislocations. AJR Am J Roentgenol. 1981;137:1151–1156. doi: 10.2214/ajr.137.6.1151. [DOI] [PubMed] [Google Scholar]
- 10.Weatherford BM, Anderson JG, Bohay DR. Management of tarsometatarsal joint injuries. J Am Acad Orthop Surg. 2017;25:469–479. doi: 10.5435/JAAOS-D-15-00556. [DOI] [PubMed] [Google Scholar]
- 11.Curtis MJ, Myerson M, Szura B. Tarsometatarsal joint injuries in the athlete. Am J Sports Med. 1993;21:497–502. doi: 10.1177/036354659302100403. [DOI] [PubMed] [Google Scholar]
- 12.Kaar S, Femino J, Morag Y. Lisfranc joint displacement following sequential ligament sectioning. J Bone Joint Surg Am. 2007;89:2225–2232. doi: 10.2106/JBJS.F.00958. [DOI] [PubMed] [Google Scholar]
- 13.Seo DK, Lee HS, Lee KW, Lee SK, Kim SB. Nonweightbearing radiographs in patients with a subtle lisfranc injury. Foot Ankle Int. 2017;38:1120–1125. doi: 10.1177/1071100717717220. [DOI] [PubMed] [Google Scholar]
- 14.Kalia V, Fishman EK, Carrino JA, Fayad LM. Epidemiology, imaging, and treatment of Lisfranc fracture-dislocations revisited. Skeletal Radiol. 2012;41:129–136. doi: 10.1007/s00256-011-1131-5. [DOI] [PubMed] [Google Scholar]
- 15.Hatch RL, Alsobrook JA, Clugston JR. Diagnosis and management of metatarsal fractures. Am Fam Physician. 2007;76:817–826. [PubMed] [Google Scholar]
- 16.Desmond EA, Chou LB. Current concepts review: Lisfranc injuries. Foot Ankle Int. 2006;27:653–660. doi: 10.1177/107110070602700819. [DOI] [PubMed] [Google Scholar]
- 17.Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: Primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88:514–520. doi: 10.2106/JBJS.E.00228. [DOI] [PubMed] [Google Scholar]
- 18.Sripanich Y, Weinberg MW, Krähenbühl N, Rungprai C, Mills MK, Saltzman CL, Barg A. Imaging in Lisfranc injury: A systematic literature review. Skeletal Radiol. 2020;49:31–53. doi: 10.1007/s00256-019-03282-1. [DOI] [PubMed] [Google Scholar]
- 19.Raikin SM, Elias I, Dheer S, Besser MP, Morrison WB, Zoga AC. Prediction of midfoot instability in the subtle Lisfranc injury. Comparison of magnetic resonance imaging with intraoperative findings. J Bone Joint Surg Am. 2009;91:892–899. doi: 10.2106/JBJS.H.01075. [DOI] [PubMed] [Google Scholar]
- 20.Haraguchi N, Ota K, Ozeki T, Nishizaka S. Anatomical Pathology of subtle lisfranc injury. Sci Rep. 2019;9(14831) doi: 10.1038/s41598-019-51358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, Pang QJ, Yang CC. Functional outcome of tarsometatarsal joint fracture dislocation managed according to Myerson classification. Pak J Med Sci. 2014;30:773–777. [PMC free article] [PubMed] [Google Scholar]
- 22.Yeoh J, Muir KR, Dissanayake AM, Tzu-Chieh WY. Lisfranc fracture-dislocation precipitating acute Charcot arthopathy in a neuropathic diabetic foot: A case report. Cases J. 2008;1(290) doi: 10.1186/1757-1626-1-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponkilainen VT, Laine HJ, Mäenpää HM, Mattila VM, Haapasalo HH. Incidence and characteristics of midfoot injuries. Foot Ankle Int. 2019;40:105–112. doi: 10.1177/1071100718799741. [DOI] [PubMed] [Google Scholar]
- 24.Singh A, Lokikere N, Saraogi A, Unnikrishnan PN, Davenport J. Missed Lisfranc injuries-surgical vs conservative treatment. Ir J Med Sci. 2021;190:653–656. doi: 10.1007/s11845-020-02364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haapamaki V, Kiuru M, Koskinen S. Lisfranc fracture-dislocation in patients with multiple trauma: Diagnosis with multidetector computed tomography. Foot Ankle Int. 2004;25:614–619. doi: 10.1177/107110070402500903. [DOI] [PubMed] [Google Scholar]
- 26.Preidler KW, Peicha G, Lajtai G, Seibert FJ, Fock C, Szolar DM, Raith H. Conventional radiography, CT, and MR imaging in patients with hyperflexion injuries of the foot: Diagnostic accuracy in the detection of bony and ligamentous changes. AJR Am J Roentgenol. 1999;173:1673–1677. doi: 10.2214/ajr.173.6.10584818. [DOI] [PubMed] [Google Scholar]
- 27.Castro M, Melão L, Canella C, Weber M, Negrão P, Trudell D, Resnick D. Lisfranc joint ligamentous complex: MRI with anatomic correlation in cadavers. AJR Am J Roentgenol. 2010;195:W447–W455. doi: 10.2214/AJR.10.4674. [DOI] [PubMed] [Google Scholar]
- 28.Sands A, Grose A. Lisfranc injuries. Injury. 2004;35 (Suppl 2):SB71–SB76. doi: 10.1016/j.injury.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Arntz CT, Veith RG, Hansen ST Jr. Fractures and fracture-dislocations of the tarsometatarsal joint. J Bone Joint Surg Am. 1988;70:173–181. [PubMed] [Google Scholar]
- 30.Sherief TI, Mucci B, Greiss M. Lisfranc injury: How frequently does it get missed? And how can we improve? Injury. 2007;38:856–860. doi: 10.1016/j.injury.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Stein RE. Radiological aspects of the tarsometatarsal joints. Foot Ankle. 1983;3:286–289. doi: 10.1177/107110078300300508. [DOI] [PubMed] [Google Scholar]
- 32.Llopis E, Carrascoso J, Iriarte I, Serrano Mde P, Cerezal L. Lisfranc injury imaging and surgical management. Semin Musculoskelet Radiol. 2016;20:139–153. doi: 10.1055/s-0036-1581119. [DOI] [PubMed] [Google Scholar]
- 33.Ponkilainen VT, Partio N, Salonen EE, Riuttanen A, Luoma EL, Kask G, Laine HJ, Mäenpää H, Päiväniemi O, Mattila VM, Haapasalo HH. Inter- and intraobserver reliability of non-weight-bearing foot radiographs compared with CT in Lisfranc injuries. Arch Orthop Trauma Surg. 2020;140:1423–1429. doi: 10.1007/s00402-020-03391-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong LH, Chrea B, Atwater LC, Meeker JE. The first tarsometatarsal joint in Lisfranc injuries. Foot Ankle Int. 2022;43:1308–1316. doi: 10.1177/10711007221112090. [DOI] [PubMed] [Google Scholar]
- 35.Kennelly H, Klaassen K, Heitman D, Youngberg R, Platt SR. Utility of weight-bearing radiographs compared to computed tomography scan for the diagnosis of subtle Lisfranc injuries in the emergency setting. Emerg Med Australas. 2019;31:741–744. doi: 10.1111/1742-6723.13237. [DOI] [PubMed] [Google Scholar]
- 36.Hatem SF. Imaging of lisfranc injury and midfoot sprain. Radiol Clin North Am. 2008;46:1045–1060. doi: 10.1016/j.rcl.2008.09.003. vi. [DOI] [PubMed] [Google Scholar]
- 37.Lewis JS Jr, Anderson RB. Lisfranc injuries in the athlete. Foot Ankle Int. 2016;37:1374–1380. doi: 10.1177/1071100716675293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.