Abstract
In food safety implementation, bacterial inactivation is an imperative aspect of hygiene and sanitation. Studies on lithium magnesium silicate (LMS) hydrosol incorporated with slightly acidic electrolyzed water (SAEW) for decontamination of pathogenic bacteria are limited. This present study aimed to investigate the bactericidal efficacy of LMS hydrosol incorporated with SAEW against Escherichia coli. Optimum combination conditions of SAEW, hydrosol concentration, and available chlorine concentration (ACC) were optimized by response surface methodology under the central composite design against the growth of E. coli. The optimum combination conditions of exposure time, hydrosol concentration, and ACC were 9.5 minutes, 1.7%, and 20.5 ppm, respectively. The results showed that the increase in ACC led to inactivation in the survival of E. coli compared with the control (p<0.05). It can be concluded that the best combination percentage between SAEW and hydrosol ranged from 1.5-1.7%, in which E. coli was reduced by 4.50 log10 CFU/mL at an ACC of 9.94 ppm. When increasing the ACC to 14.84 ppm, E. coli was reduced by 4.51 log10 CFU/mL compared with the initial number of bacteria (8.20 log10 CFU/mL) in the control group. The number of bacteria was undetected after increasing ACC to 19.93, 25.15, and 29.88 ppm at 10 min. This study suggests that LMS hydrosol incorporated with SAEW could potentially be used as an effective sanitizer.
Key words: bactericidal, lithium magnesium silicate, hydrosol, slightly acidic electrolyzed water, available chlorine concentration
Introduction
The most prominent food safety challenges are the consumer’s preferences and requirements for high-quality food, which are mainly associated with health aspects such as freshness, flavor, color, and good appearance (Andoni et al., 2021; Sri Prabakusuma et al., 2022; Su et al., 2023). Different molds, yeasts, and bacteria can grow in human foods, which then leads to various degrees of decomposition and deterioration, destroying their nutrients and posing adverse effects on human health (Naka et al., 2020). Bacteria have low requirements for nutrient use and are adaptable in many environmental conditions, making them easy to grow everywhere with enough water availability and as a source of biological contamination in slightly cooked or raw food materials and tap water. This bacterial contamination and subsequent infections pose a dangerous threat to human health, particularly to people who have immunodeficiency, open wounds, children, pregnant and lactating women, and the elderly as well. For many years, a variety of decontamination techniques, such as thermal and non-thermal sterilization technologies, chemical preservatives, and sanitizing techniques, have been used to reduce the number of pathogenic microorganisms and inactivate proteolytic enzymes to increase the safety and extend the shelf life of food products (Režek Jambrak et al., 2018; Ding et al., 2019).
In agriculture and in the food industry, many sanitizers, for instance, sodium hypochlorite, hydrogen peroxide, chlorine dioxide, quaternary ammonium compounds (quats), ozone, and organic acids, have been widely applied for decontamination purposes. Among those, the application of most traditional sanitizers has various limitations since they cause adverse effects on both the environment and human health. Besides, they are also corrosive, toxic, volatile, not easy to handle, and often very ineffective. Accordingly, slightly acidic electrolyzed water (SAEW) has been developed as an eco-friendly, easy-returning water after use and an effective antibacterial agent for the substitution of chlorine-based sanitizers with minimum operating costs. Previous studies reported SAEW as an alternative and novel method to prevent, control, and disinfect microorganisms in many fields, from chemical pesticide removal on food, environmental disinfection, sterilization of livestock, obliterating moth infestation throughout the storage of fruits and vegetables, to contributing to prolonging the shelf life of seafood products compared to common conventional sanitizers (Ding et al., 2019; Zhang et al., 2021). SAEW was used for the first time in Japan as a food preservation agent by the Japanese Ministry of Agriculture and the Ministry of the Environment and Forestry (Naka et al., 2020). After that, it was widely applied as a disinfectant in markets, fisheries, care homes, kindergartens, hospitals, restaurants, households, and many other areas where personal hygiene implementation is required (Koide et al., 2011).
SAEW is produced in a non-membrane electrolytic cell by electrolyzing 2-6% dilute hydrochloric acid with a nearly neutral pH value of 5.0 to 6.5, an available chlorine concentration (ACC) of usually around 30 mg/L, and an oxidation-reduction potential (ORP) of 800-1000 mV (Ding et al., 2019; Liu et al., 2022). At that pH range, the effective form of chlorine compound in SAEW is primarily 95% hypochlorous acid (HOCl), a weak acid (pKa=7.53, 25°C), which generates the antibacterial activity (Naka et al., 2020). Additionally, it was also reported that HOCl leads to damage to bacterial cell membranes that causes the death of cells and the inactivation of intracellular enzymes owing to its high antibacterial activity (Tango et al., 2017; Kim et al., 2019). After SAEW enters the cell, the generated active substance and HOCl trigger a series of complex modifications in intracellular metabolites (Liu et al., 2022). Other studies have reported that SAEW has a promising bactericidal effect on foodborne pathogens and bacterial spores, including Escherichia coli, Salmonella spp., Staphylococcus aureus, Vibrio parahaemolyticus, and Vibrio vulnificus, even at low concentrations, and has lower potential hazards to human health due to its pH range (Hussain et al., 2019; Zhang et al., 2019). Subsequently, lithium magnesium silicate (Li2Mg2O9Si3) (LMS) hydrosol, an inorganic silicate layered nano mineral material, which was selected in this work to present an antibacterial effect in SAEW solution, exhibits good cation exchangeability, strong chemical stability, suspensibility, absorbability, and cohesiveness (Zhang et al., 2019). LMS can be decomposed into Na+, Mg2+, H4SiO4, and Li+, where these products are not toxic and harmful and have good cytocompatibility and biosafety, which are determined by in vivo and in vitro studies (Mohanty and Joshi, 2016; Tomás et al., 2018). Many studies have found that LMS can be used as a food antibacterial chemical or preservation agent, as an additive ingredient, and as an adsorption toxin (Hassan et al., 2019; Zhang et al., 2019). The LMS hydrosol combined with SAEW has antibacterial characteristics with a strong oxidant profile that, in proper concentrated form, can react effectively to reduce unwanted pathogenic microorganisms and increase food safety. At present, there is still limited understanding and information on the efficiency of SAEW to reduce and inactivate the growth of microorganisms. Particularly, studies about the unraveling of the effectiveness of SAEW combined with other potential bactericidal agents are needed. Therefore, it is important to study the bactericidal activity of SAEW with silicate-layered nanomineral-based antibacterial agents at different exposure times, ACC, and LMS hydrosol concentrations. E. coli is commonly used as an indicator of bacterial contamination (Huebsch et al., 2005; Dewaele et al., 2011; Xu et al., 2023). This gram-negative bacterium is more resistant than gram-positive bacteria due to its distinctive structure. Thus, this study aimed to investigate the bactericidal efficacy of LMS hydrosol incorporated with SAEW against E. coli.
Materials and Methods
Materials and reparation of bacterial suspension
LMS (99%) was purchased from Shanghai Yuanye Biotechnology Co., Ltd. (China). All the other analytical-grade chemicals used in this study were purchased from standard Chinese companies. The E. coli CICC 10305 strain was purchased from the China Center for Industrial Culture Collection, which was then conserved and preserved in the laboratory. After the activation of the E. coli CICC 10305 strain, it was inoculated in Luria-Bertani (LB) broth containing 5.0 g of yeast extract, 10.0 g of NaCl, 10.0 g of peptone, 20.0 g of agar, and 1000 mL of distilled water with a pH of 7.0. The sterile filtrate of the E. coli was then prepared as follows: i) E. coli was inoculated in 100 mL of LB liquid broth; ii) it was incubated for 24 hours at 30°C in a thermostatic oscillator HZQ-X300C (Shanghai Yiheng Science Instrument Co. Ltd., China) with constant shaking at 150 rpm. The inoculation ring was used to dip the bacterial liquid into LB broth and inoculate with the streaking method. E. coli was incubated at 37°C for 12 hours. A single colony was selected with a pipette gun head and continuously activated in LB broth for 2 generations, incubated at 37°C for 18-24 hours with constant shaking at 190 rpm.
Preparation of slightly acidic electrolyzed water
The SAEW, with an ACC of 40±1.27 ppm, an ORP of 870-900 mV, and a pH of 6.29±1.33, was produced by electrolysis of a 6% (v/v) hydrochloric acid solution using a non-membrane electrolytic chamber generator (HD-240, Shanghai Want Want Holdings Ltd., China) at a voltage of 220 V. The pH and ORP values were determined using a dual-scale pH/ORP meter (CON60, Trans-Wiggens, Singapore). The effective chlorine concentration of SAEW was controlled by adjusting the current with a stable flow of 4 L/min, and the products of SAEW were collected after being generated for more than 20 minutes to make sure that the current became stable and reached the target ACC as shown in Figure 1.
The ACC was measured according to the method described by Zeng et al. (2010), with slight modifications. Briefly, 50 mL of each solution was mixed with 10 mL of potassium iodide solution (0.1 mol/L) and 10 mL of 10% acetic acid in a 250-mL conical beaker. The mixture was kept in the dark for 10 minutes, then a total of 20 mL of distilled water was added. The solution was titrated using sodium thiosulfate (Na2S2O3) solution (0.01 mol/L) until the treated sample turned pale yellow, and then the 5% starch solution (10 g/L) was added until the color turned blue. The titration with Na2S2O3 continued until the sample turned an achromatic color. The ACC (mg/L) was measured by Eq. 1:
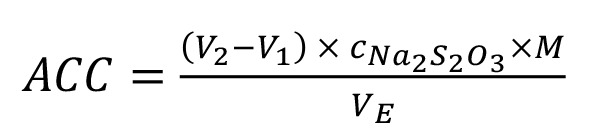 |
[Eq. 1] |
where cNa2S2O3 denotes the concentration of Na2S2O3 titrating solution (mol/L), V2 denotes the volume of Na2S2O3 titrating solution consumed in the treated sample (mL), V1 denotes the volume of Na2S2O3 titrating solution consumed in the blank sample (mL), VE denotes the volume of the treated sample (mL), and M denotes the mole mass of chlorine (35,453 mg/mol).
Preparation of hydrosol solutions
A certain amount of LMS powder was weighed, a small amount of SAEW was added several times, and the mixture was stirred until LMS was dissolved and evenly dispersed. In this study, LMS hydrosol was prepared with 1.5%, 2.5%, and 3.5% (w/v).
Sterilization effects of slightly acidic electrolyzed water lithium magnesium silicate hydrosol concentration
The sterilization test of this work was performed by spread plate count methods; 1 mL of bacterial suspension (approximately 8.0 log10 CFU/mL) was incubated with 9 mL of each group as follows: phosphate-buffered saline (PBS) (group 1, control); hydrosol concentration dissolved by sterile distilled water (group 2, control); SAEW solution (group 3); and hydrosol dissolved by SAEW solution (group 4). Those treatment groups were exposed for 0, 2.5, 5, 7.5, and 10 minutes with different ACC of 9.94, 14.84, 19.93, 25.15, and 29.88 ppm and different hydrosol concentrations of 1.5%, 2.5%, and 3.5%. The hydrosol groups were mixed with a glass rod using a small petri dish to ensure the spread of the bacteria, whereas other treatments were mixed in test tubes. Then, sterilization was conducted by transferring 0.172 mL of 0.5% Na2S2O3 solution into each treated sample as a neutralizer to stop the bactericidal reaction. After neutralization, the viable count of bacteria cells in each sample was serially diluted (1:10) in a sterile PBS solution (pH 7.2-7.4). A total of 1 mL of diluted cell mixture was spread on a selective growth medium of Violet Red Bile agar, and the plates were incubated at 37°C for 24 hours. According to the Box-Behnken combined test design principle, ACC, time, and hydrosol concentration were selected as varying factors, and the number of survival bacteria was designated as the response value to carry out the response surface analysis of 3 factors as shown in Table 1.
Figure 1.
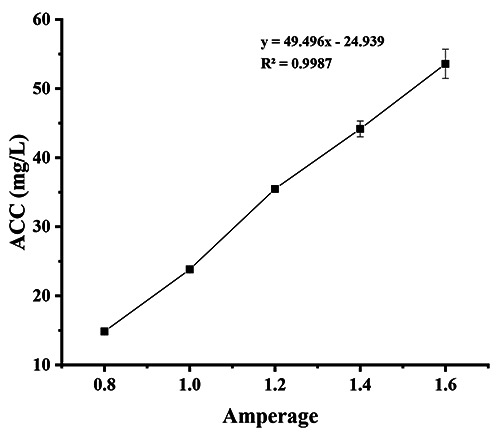
Relationship between current (Amperage) and available chlorine concentration (ACC).
Data analysis
In this work, OriginPro 2019b statistic software (OriginLab Corp., Northampton, MA, USA) was used for plotting and nonlinear fitting analysis; Excel 2019 for Windows software (Microsoft Corp., Redmond, WA, USA) was used for data processing; and IBM SPSS 23.0 statistic software (IBM Corp., Armonk, NY, USA) was used for significant difference analysis of data. To obtain the quadratic multiple regression equation of the total number of viable bacteria, the Design-Expert version 12 software (Stat-Ease, Inc., Minneapolis, MN, USA) was used.
Results and Discussion
Disinfection efficacy of slightly acidic electrolyzed water and combination of slightly acidic electrolyzed water and hydrosol concentration
The available chlorine in SAEW mainly exists in the form of HClO molecules, which are neutral molecules that can inactivate bacteria via reactions with nucleic acids and proteins in bacteria cells (Kim et al., 2019). However, SAEW has been perceived as a novel and effective technology for inactivating bacteria and preserving the freshness of foods. Until now, the effect of SAEW-containing hydrosol on bacteria growth and food preservation has been unclear. In our study, the effectiveness of SAEW with different levels of hydrosol on microorganisms’ growth with different ACC levels and exposure times was investigated. Compared with the control group, we found that SAEW-containing hydrosol was characterized by greater effectiveness in the inactivation of bacteria than the control. Furthermore, the survival of E. coli was greatly decreased along with the increasing ACC (p<0.05).
The antibacterial activity of SAEW and the combination of SAEW and hydrosol at 9.94 ppm of ACC on E. coli is presented in Figure 2. The survival microorganisms of E. coli were greatly decreased along with the time until 10 minutes compared with the control group (p<0.05). The maximum reductions in the log number of E. coli were 4.50, 4.50, 1.73, and 1.30 log10 CFU/mL in SAEW, SAEW+hydrosol 1.5%, SAEW+hydrosol 2.5%, and SAEW+hydrosol 3.5%, respectively, at 10 minutes. On the one hand, the SAEW showed a great effect against the bacteria along with increasing the time, in which the reduction in the survival of bacteria was decreased to 4.0 log10 CFU/mL compared with 8.39 log10 CFU/mL in the control group (Figure 2). However, a single SAEW treatment against E. coli O78 on fresh-cut cilantro reduced it to 1.94 log10 CFU/g for 5 minutes (Hao et al., 2015). On the other hand, mixing hydrosol with SAEW showed fluctuating results on the number of survival bacteria at a concentration of 1.5%. In comparison, increasing the hydrosol concentration increased the number of survival bacteria at concentrations of 2.5% and 3.5% (Figure 2). Such a finding can be attributed to the alleviation of the strong effect of SAEW by the high amount of added hydrosol. Guo et al. (2021) found that the combination of SAEW with US exhibited a synergistic effect in the sterilization of E. coli and presented the highest reduction of E. coli with a value of 3.64 log10 CFU/mL. Notably, when the added concentration of hydrosol was 3.5%, the number of surviving bacteria was comparable to the control (Figure 2). Finally, it can be concluded that the best combination percentage between SAEW and hydrosol was 1.5%, which showed fewer survival bacteria than other treatments, and this effect increased with increased time.
Table 1.
Response surface test factor levels and coding.
| Code | ACC (mg/L) | Time (min) | Hydrosol concentration (%) |
|---|---|---|---|
| -1 | 20 | 5 | 1.5 |
| 0 | 25 | 7.5 | 2.3 |
| 1 | 30 | 10 | 3.5 |
ACC, available chlorine concentration.
Herein, the same abovementioned treatments were prepared in 15 ppm ACC, and the number of surviving E. coli was counted in every treatment and at different times. Figure 3 shows that increasing the concentration of ACC clearly reduced the growth of bacteria compared with 10 ppm (Figures 2 and 3). These results were consistent with those reported by Ratana-Arporn et al. (2014), who reported that the growth of microorganisms was negatively correlated with increasing ACC levels. When the ACC levels increased from 10 to 30, 50, 70, and 100 ppm, the survival of microorganisms such as E. coli and Salmonella enteritidis was gradually reduced by about 1 log10 at each intermission concentration for the 1-minute contact time. Kim et al. (2000) stated that electrolyzed oxidizing water treatment with 10 mg/L of ACC might reduce the population of microorganisms. In the same direction, the SAEW and SAEW+hydrosol 1.5% treatments showed great effectiveness against bacteria, with the number of survival bacteria reduced from 8.37 log10 CFU/mL in control to 4.51 log10 CFU/mL in both treatments. Besides, the SAEW+hydrosol 2.5% showed less effectiveness against the bacteria than the SAEW and SAEW+hydrosol 1.5%, as shown in Figure 3. At the same time, the SAEW+ hydrosol 3.5% treatment exhibited a more negligible effect compared to the other 3 groups and a slightly higher effect compared with the control. Interestingly, the reduction effect was gradually increased with increased time in all the treatments (Figure 3).
As was expected, increasing the concentration of ACC significantly reduced the number of surviving bacteria in all treatments compared with the control group. In Figure 4, it is clearly proven that the combined effect of ACC concentration and longer time reduced the number of bacteria to 0 in SAEW, SAEW+hydrosol 1.5%, and SAEW+hydrosol 2.5% at 10 minutes. These results were consistent with those found by Brychcy et al. (2015), who reported that the highest reduction of S. aureus and E. coli was obtained after treatment with carrageenan and gelatin hydrosols incorporated with 0.1% of electrolyzed sodium chloride solution exposed to electrolysis for 10 minutes. In addition, compared to the initial population of E. coli in the control group (8.39 log10 CFU/mL), SAEW, SAEW+hydrosol 1.5%, SAEW+hydrosol 2.5%, and SAEW+hydrosol 3.5% showed a log reduction of 2.49, 2.32, 1.59, and 1.34 CFU/mL after a treatment time of 2.5 minutes, respectively. Similarly, the survival of E. coli was reduced to 4.23, 3.15, 3.03, and 1.59 CFU/mL at 5 minutes and to 4.32, 3.94, 3.52, and 1.93 CFU/mL at 7.5 minutes in SAEW, SAEW+hydrosol 1.5%, SAEW+hydrosol 2.5%, and SAEW+hydrosol 3.5%, respectively (Figure 4). Another study proposed that the SAEW treatment’s bactericidal activity might be significantly correlated to the ACC (Cao et al., 2009), which is one of the main active factors that plays the main role in the bactericidal activity (Ren and Su, 2006).
Figure 2.
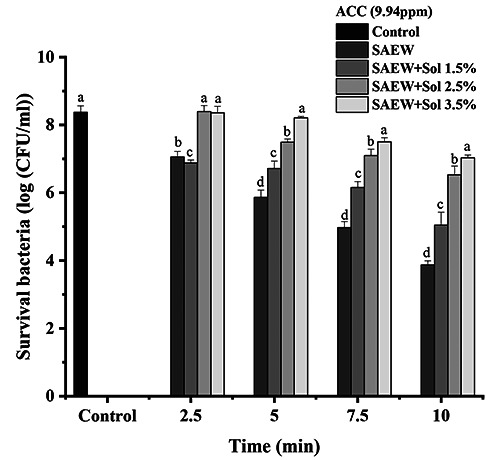
Effect of slightly acidic electrolyzed water (SAEW) and combination of SAEW and hydrosol at 9.94 ppm of available chlorine concentration on Escherichia coli. The different letters indicate a significant difference (p<0.05). ACC, available chlorine concentration; sol, hydrosol.
Figure 3.
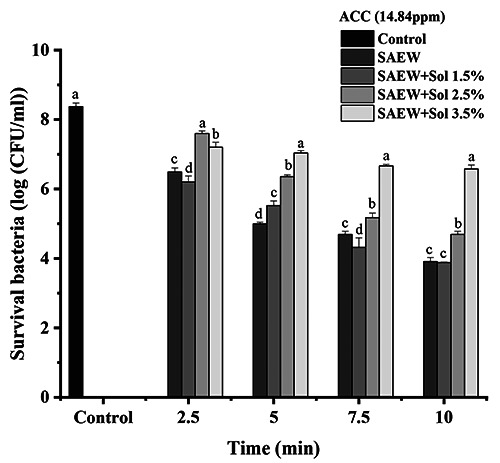
Effect of slightly acidic electrolyzed water (SAEW) and combination of SAEW and hydrosol at 14.84 ppm of available chlorine concentration on Escherichia coli. The different letters indicate a significant difference (p<0.05). ACC, available chlorine concentration; sol, hydrosol.
As shown in Figure 6, the log reduction of the survival of E. coli significantly increased as the time was increased from 2.5 to 10 minutes (p<0.05), especially in SAEW, SAEW+hydrosol 1.5%, SAEW+hydrosol 2.5%, and SAEW+hydrosol 3.5%. Compared to the initial population of E. coli of 8.39 log10 CFU/mL with the increase of ACC to 29.88 ppm, which is similar to the study conducted by Ding et al. (2019), the log reductions of treatments SAEW+hydrosol 1.5%, SAEW+hydrosol 2.5%, and SAEW+hydrosol 3.5% were reduced to 3.08, 3.38, 3.54, and 1.73 log10 CFU/mL at 2.5 minutes. At the same time, increasing the time to 5 minutes increased the reduction in E. coli survival to 4.12, 4.22, 4.46, and 2.46 log10 CFU/mL, respectively, compared to the control. Interestingly, increasing the time to 10 minutes completely affected the growth of bacteria, in which no survival bacteria were detected in SAEW, SAEW+hydrosol 1.5%, and SAEW+hydrosol 2.5% treatments. Similarly, with 7.5 minutes in SAEW and SAEW+hydrosol 2.5% (Figure 6), the combination of hydrogels and hydrosols with AEW, an exposure time of 10 minutes, and the highest concentration of ACC led to a reduction in the number of microorganisms (Brychcy et al., 2015).
Figure 4.
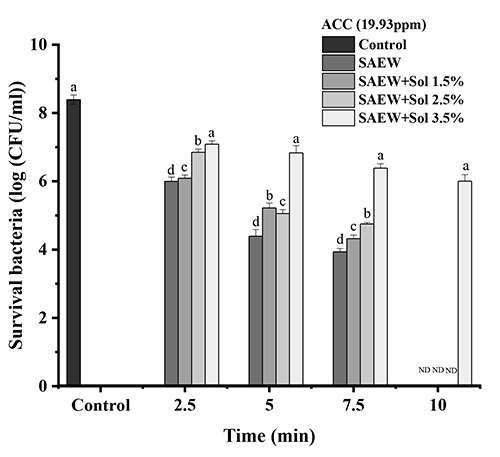
Effect of slightly acidic electrolyzed water (SAEW) and combination of SAEW and hydrosol at 19.93 ppm of available chlorine concentration on Escherichia coli. The different letters indicate a significant difference (p<0.05). ND, not detected; ACC, available chlorine concentration; sol, hydrosol.
Figure 5.
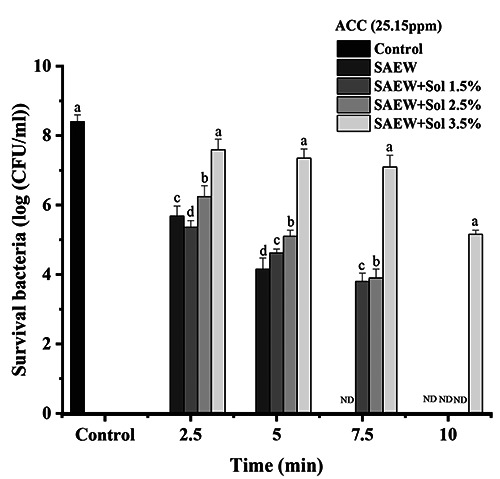
Effect of slightly acidic electrolyzed water (SAEW) and combination of SAEW and hydrosol at 25.15 ppm of available chlorine concentration on Escherichia coli. The different letters indicate a significant difference (p<0.05). ND, not detected; ACC, available chlorine concentration; sol, hydrosol.
Figure 6.
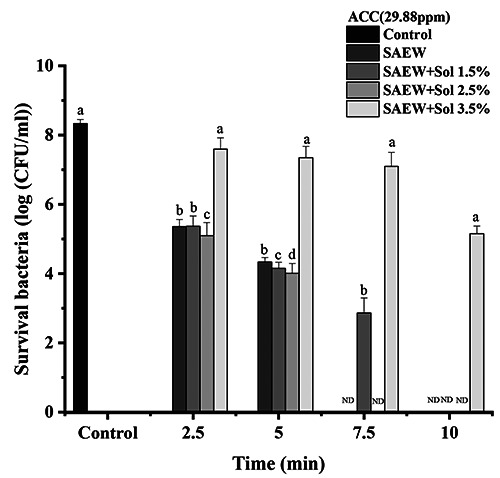
Effect of slightly acidic electrolyzed water (SAEW) and combination of SAEW and hydrosol at 29.88 ppm of available chlorine concentration on Escherichia coli. The different letters indicate a significant difference (p<0.05). ND, not detected; ACC, available chlorine concentration; sol, hydrosol.
Response surface analysis
The quadratic multiple regression equation of viable bacteria number (y) to hydrosol concentration, exposure time, and ACC through multiple regression fitting of test data established by Design-Expert is presented in Eq. 2:
| [Eq. 2] |
where, y represents the response value of the total number of survival bacteria (log10 CFU/mL); A, B, and C represent variables, including ACC, treatment time, and hydrosol concentration, respectively. The response surface (contour map and 3D chart) can be visually reflected in different factors in the test on the interaction between the 2 factors on the number of survival bacteria shown in Figure 7. The analysis of the interaction of hydrosol concentration (C) versusACC (A) in Figure 7A shows that as hydrosol concentration increased from 1.5% to 3.5%, the number of survival bacteria increased, whereas the ACC was in the range of 20 to 30 ppm.
Figure 7.
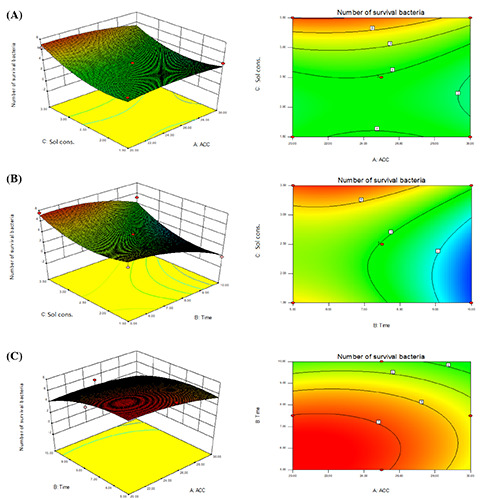
Response surface plot and contour plot of the optimal bactericidal effect of slightly acidic electrolyzed water hydrosol. Interactions between treatment parameters: A) hydrosol concentration and available chlorine concentration (ACC); B) hydrosol concentration and time; C) time and ACC. Sol cons, hydrosol concentration.
As a result of the response surface analysis results, the optimal storage conditions are shown in Figure 7A: exposure time of 9.5 minutes, hydrosol concentration of 1.7%, and ACC of 20.5 ppm. A similar study conducted by Song et al. (2021) found that the optimal concentration of free available chlorine of 22.17 ppm in SAEW showed maximum reduction values of Pectobacterium carotovorum subsp. carotovorum while the optimal exposure time was 180 seconds. Other studies have found that SAEW treatment can reach reduction values of >6.00 log10 CFU/mL against various pathogenic bacterial populations in suspension for 5 minutes (Deza et al., 2005; Ayebah et al., 2006). The contour diagram of the interaction of time (B) against hydrosol concentration (C) in Figure 7B clearly shows that the number of surviving E. coli increased as the hydrosol concentration escalated from 1.5% to 3.5%. In contrast, the number of survivors of E. coli was reduced with the increase in exposure time from 5 to 10 minutes. Figure 7C depicts the contour diagram of the interaction of time (B) and ACC (A). It is demonstrated that the increase in time from 5 to 8 minutes showed the growth of the number of survival bacteria. Similarly, the slight increase in ACC revealed the same inactivation in the number of surviving bacteria.
Conclusions
SAEW alone and in combination with hydrosol demonstrated excellent bactericidal efficacy against E. coli as a pure culture. The effects of the increasing level of ACC and exposure time on the survival of E. coli greatly decreased the number of E. coli survivors compared with the control group (p<0.05). The best treatment of hydrosol incorporated with SAEW was at 1.5%, which greatly reduced E. coli at ACC to 9.94 ppm, which was better than SAEW treatment alone. However, all treatments were always better than the control group. The use of hydrosol incorporated with SAEW as a new active packaging material with antibacterial activity is a promising technique for food preservation. However, more studies regarding the role of hydrosol incorporated with SAEW in food preservation, especially its ability to inhibit or prevent microbiological spoilage during food storage, are needed.
Acknowledgments
The authors would like to express their heartfelt appreciation to the anonymous reviewers and colleagues for their constructive remarks and advice that helped enhance the manuscript’s content quality.
Funding Statement
Funding: financial support for the research was provided by the National Science Foundation of China (project numbers: 32060573; 31860474) and Yunnan Major Science and Technology Projects (project number: 202202AE090019).
References
- Andoni E, Ozuni E, Bijo B, Shehu F, Branciari R, Miraglia D, Ranucci D, 2021. Efficacy of non-thermal processing methods to prevent fish spoilage. J Aquat Food Prod Technol 30:228-45. [Google Scholar]
- Ayebah B, Hung YC, Kim C, Frank JF, 2006. Efficacy of electrolyzed water in the inactivation of planktonic and biofilm Listeria monocytogenes in the presence of organic matter. J Food Prot 69: 2143-50. [DOI] [PubMed] [Google Scholar]
- Brychcy E, Malik M, Drożdżewski P, Król Ż, Jarmoluk A, 2015. Physicochemical and antibacterial properties of carrageenan and gelatine hydrosols and hydrogels incorporated with acidic electrolyzed water. Polymers 7:2638-49. [Google Scholar]
- Cao W, Zhu ZW, Shi ZX, Wang CY, Li BM, 2009. Efficiency of slightly acidic electrolyzed water for inactivation of Salmonella enteritidis and its contaminated shell eggs. Int J Food Microbiol 130:88-93. [DOI] [PubMed] [Google Scholar]
- Dewaele I, Ducatelle R, Herman L, Heyndrickx M, De Reu K, 2011. Sensitivity to disinfection of bacterial indicator organisms for monitoring the Salmonella Enteritidis status of layer farms after cleaning and disinfection. Poul Sci 90:1185-90. [DOI] [PubMed] [Google Scholar]
- Deza MA, Araujo M, Garrido MJ, 2005. Inactivation of Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa, and Staphylococcus aureus on stainless steel and glass surfaces by neutral electrolysed water. Lett Appl Microbiol 40:341-6. [DOI] [PubMed] [Google Scholar]
- Ding T, Oh DH, Liu D, 2019. Electrolyzed water in food: fundamentals and applications. Springer, Singapore. [Google Scholar]
- Guo L, Zhang X, Xu L, Li Y, Pang B, Sun J, Wang B, Huang M, Xu X, Ho H, 2021. Efficacy and mechanism of ultrasound combined with slightly acidic electrolyzed water for inactivating Escherichia coli. J Food Qual 2021:6689751. [Google Scholar]
- Hao J, Li H, Wan Y, Liu H, 2015. Combined effect of acidic electrolyzed water (AcEW) and alkaline electrolyzed water (AlEW) on the microbial reduction of fresh-cut cilantro. Food Control 50:699-704. [Google Scholar]
- Hassan AA, Hafsa SHA, Elghandour MMMY, Reddy PRK, Monroy JC, Salem AZM, 2019. Dietary supplementation with sodium bentonite and coumarin alleviates the toxicity of aflatoxin B1 in rabbits. Toxicon 171:35-42. [DOI] [PubMed] [Google Scholar]
- Huebsch N, Gilbert M, Healy KE, 2005. Analysis of sterilization protocols for peptide-modified hydrogels. J Biomed Mater Res B Appl Biomater 74:440-7. [DOI] [PubMed] [Google Scholar]
- Hussain MS, Tango CN, Oh DH, 2019. Inactivation kinetics of slightly acidic electrolyzed water combined with benzalkonium chloride and mild heat treatment on vegetative cells, spores, and biofilms of Bacillus cereus. Food Res Int 116:157-67. [DOI] [PubMed] [Google Scholar]
- Kim C, Brackett RE, 2001. Roles of oxidation-reduction potential (ORP) in electrolyzed oxidizing (EO) and chemically modified water for the inactivation of food-related pathogens. J Food Prot 63:19-24. [DOI] [PubMed] [Google Scholar]
- Kim C, Hung YC, Brackett RE, 2000. Efficacy of electrolyzed oxidizing (EO) and chemically modified water on different types of foodborne pathogens. Int J Food Microbiol 61:199-207. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Tango CN, Chelliah R, Oh DH. 2019. Sanitization efficacy of slightly acidic electrolyzed water against pure cultures of Escherichia coli, Salmonella enterica, Typhimurium, Staphylococcus aureus and Bacillus cereus spores, in comparison with different water hardness. Sci Rep 9:4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide S, Shitanda D, Note M, Cao W, 2011. Effects of mildly heated, slightly acidic electrolyzed water on the disinfection and physicochemical properties of sliced carrot. Food Control 22:452-6. [Google Scholar]
- Liu L, Lan W, Wang Y, Xie J, 2022. Antibacterial activity and mechanism of slightly acidic electrolyzed water against Shewanella putrefaciens and Staphylococcus saprophytic. Biochem Biophys Res Commun 592:44-50. [DOI] [PubMed] [Google Scholar]
- Mohanty RP, Joshi YM, 2016. Chemical stability phase diagram of aqueous Laponite dispersions. Appl Clay Sci 119:243-8. [Google Scholar]
- Naka A, Yakubo M, Nakamura K, Kurahashi M, 2020. Effectiveness of slightly acidic electrolyzed water on bacteria reduction: in vitro and spray evaluation. PeerJ 8:e8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratana-Arporn P, Jommark N, 2014. Efficacy of neutral electrolyzed water for reducing pathogenic bacteria contaminating shrimp. J Food Prot 77:2176-80. [DOI] [PubMed] [Google Scholar]
- Ren T, Su YC, 2006. Effects of electrolyzed oxidizing water treatment on reducing Vibrio parahaemolyticus and Vibrio vulnificus in raw oysters. J Food Prot 69:1829-34. [DOI] [PubMed] [Google Scholar]
- Režek Jambrak A, Vukušić T, Donsi F, Paniwnyk L, Djekic I, 2018. Three pillars of novel nonthermal food technologies: food safety, quality, and environment. J Food Qual 2018: 8619707. [Google Scholar]
- Song H, Lee JY, Lee HW, Ha JH, 2021. Inactivation of bacteria causing soft rot disease in fresh cut cabbage using slightly acidic electrolyzed water. Food Control 128:108217. [Google Scholar]
- Sri Prabakusuma A, Zhu J, Shi Y, Ma Q, Zhao Q, Yang Z, Xu Y, Huang A, 2022. Prevalence and antimicrobial resistance profiling of Staphylococcus aureus isolated from traditional cheese in Yunnan, China. 3 Biotech 12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R, Wen Y, Sri Prabakusuma A, Tang X, Huang A, Li L, 2023. Prevalence, antibiotic resistance and virulence feature of Listeria monocytogenes isolated from bovine milk in Yunnan, Southwest China. Int Dairy J 144:105703. [Google Scholar]
- Tango CN, Khan I, Kounkeu PFN, Momna R, Hussain MS, Oh DH, 2017. Slightly acidic electrolyzed water combined with chemical and physical treatments to decontaminate bacteria on fresh fruits. Food Microbiol 67:97-105. [DOI] [PubMed] [Google Scholar]
- Tomás H, Alves CS, Rodrigues J, 2018. Laponite®: a key nanoplatform for biomedical applications? Nanomedicine 14:2407-20. [DOI] [PubMed] [Google Scholar]
- Vorobjeva NV, Vorobjeva LI, Khodjaev EY, 2004. The bactericidal effects of electrolyzed oxidizing water on bacterial strains involved in hospital infections. Artif Organs 28:590-2. [DOI] [PubMed] [Google Scholar]
- Xu M, Luo H, Rong H, Wu S, Zheng Z, Chen B, 2023. Calcium alginate gels-functionalized polyurethane foam decorated with silver nanoparticles as an antibacterial agent for point-of-use water disinfection. Int J Biol Macromol 231:123289. [DOI] [PubMed] [Google Scholar]
- Zeng X, Tang W, Ye G, Ouyang T, Tian L, Ni Y, Li P, 2010. Studies on disinfection mechanism of electrolyzed oxidizing water on E. coli and Staphylococcus aureus. J Food Sci 75:M253-60. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li B, Jadeja R, Hung Y, 2016. Effects of electrolyzed oxidizing water on inactivation of Bacillus subtilis and Bacillus cereus spores in suspension and on carriers. J Food Sci 81:M144-9. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhang Y, Zhao Z, Liu W, Chen Y, Yang G, Xia X, Cao Y, 2019. The application of slightly acidic electrolyzed water in pea sprout production to ensure food safety, biological and nutritional quality of the sprout. Food Control 104:83-90. [Google Scholar]
- Zhang Z, Zheng Z, Chen A, Wang S, Xu Y, 2021. The recognition of Gastrodia elata variants based on machine vision. 2021 ASABE Annual International Virtual Meeting 2100345. [Google Scholar]


