Abstract
The dose-dependent pharmacological response to dapagliflozin in patients with type 2 diabetes mellitus (T2DM) with regard to weight loss remain unknown. The aim of the present study was to investigate the effects of dapagliflozin on weight loss in patients with T2DM. A total of 8,545 patients with T2DM from 24 randomized controlled trials reported in the literature were selected for inclusion in the study. Data from these trials were analyzed using maximal effect (Emax) models with nonlinear mixed effects modeling; the evaluation index was the body weight change rate from baseline values. Patients treated with 2.5 mg/day dapagliflozin exhibited an Emax of -3.04%, and the time taken for therapy to reach half of the Emax (ET50) was estimated to be 30.8 weeks for patients treated with this dose. Patients treated with 5, 10 and 20 mg/day dapagliflozin exhibited Emax values of -6.57, -4.12 and -3.23%, respectively, and their ET50 values were estimated to be 27.3, 20.4 and 4.23 weeks, respectively. The data indicated ideal linear relationships between individual predictions and observations, suggesting the optimal fitting of the final models. The present study is the first systematic analysis of the effect of dapagliflozin on weight loss in patients with T2DM. The application of dapagliflozin at 5 mg/day exhibited a greater weight loss effect compared with the other doses used, and the weight loss onset time shortened as the dose of dapagliflozin increased.
Keywords: effect, dapagliflozin, weight loss, type 2 diabetes mellitus, evidence-based practice
Introduction
It is estimated that the global prevalence of diabetes is currently 463 million worldwide and will increase to 700 million by 2045(1). Type 2 diabetes mellitus (T2DM), a condition in which patients experience hyperglycemia due to impaired insulin action and insufficient insulin secretion, is the most common type of diabetes worldwide (1). In addition, patients with T2DM often present with hypertension, dyslipidemia, atherosclerotic disease and obesity (2,3). It has been reported that >50% of patients with T2DM are obese (3,4). Patients with T2DM who are overweight or obese have a higher risk of cardiovascular disease and higher mortality rate, which are vital determinants of T2DM prognosis (4,5). Therefore, it is crucial to improve the management of T2DM in patients who are overweight or obese (6).
Dapagliflozin is a sodium glucose cotransporter 2 (SGLT2) inhibitor and was the first drug with this mechanism to be approved for the treatment of T2DM. It is considered to be an important treatment option as an adjunct to diet and exercise for the improvement of glycemic control in adult patients with T2DM (7). In addition, dapagliflozin can cause a modest reduction in weight (7). The weight loss achieved with dapagliflozin is clinically meaningful in terms of improving overall health outcomes and reducing the risk of complications associated with T2DM (7).
However, the extent to which dapagliflozin causes weight reduction and the dose-dependent pharmacological response to dapagliflozin in patients with T2DM with regard to weight loss remain unknown. Therefore, the present study aimed to explore the dose-dependent weight loss response to dapagliflozin in patients with T2DM.
Materials and methods
Included data
The data of patients with T2DM treated with dapagliflozin were extracted from published articles, and the details of patients assigned to the treatment or control groups were obtained from the selected literature (8-31). These studies had all been approved by the ethics committee of each participating center (8-31). The inclusion criteria for the present study were as follows: i) Patients with T2DM, ii) dapagliflozin treatment, iii) randomized controlled trial (RCT), iv) availability of body weight information and v) availability of the exact dosage and duration of therapy with dapagliflozin. No additional specific criteria were required to be met. The source, grouping, dapagliflozin dosage, duration of treatment, sample size and patient age were extracted from these published articles.
In order to eliminate the potential baseline effect, the present study calculated the body weight change rate from baseline for use as an evaluation index. The equation (I) used was as follows.
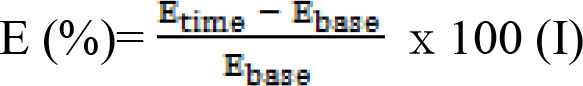 |
Etime is weight at a specific time and Ebase is weight at baseline.
Model establishment
The effects of dapagliflozin on weight loss in patients with T2DM varied with time and eventually reached a plateau. Maximal effect (Emax) models were used to assess these effects. In addition, the actual effects of dapagliflozin on weight loss in patients with T2DM were assessed by subtracting the control effect from the sum effect using equations (II) and (III) as follows:
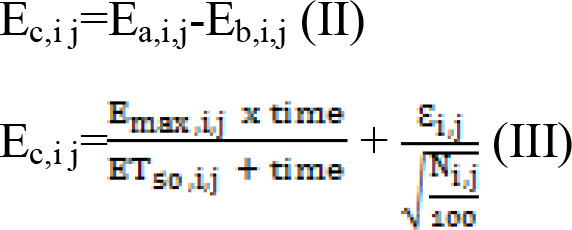 |
Ea,i,j represents the sum effect of dapagliflozin on weight loss in patients with T2DM; Eb,i j represents the weight loss in the control group of patients with T2DM; Ec,i,j represents the actual effect of dapagliflozin on weight loss in patients with T2DM; i represents a specific study; j represents the time point of the study; ET50 is the duration of treatment required to reach half the Emax; Ɛi,j represents the residual error of study i with time j; and Ni,j represents the sample size in study i at time point j. Ɛi,j was weighted by sample size and assumed to be normally distributed, with a mean of 0 and variance of σ2/(Ni,j/100).
The variabilities observed between studies were described using additive error or exponential error models. The equations used (IV)-(VII) were as follows:
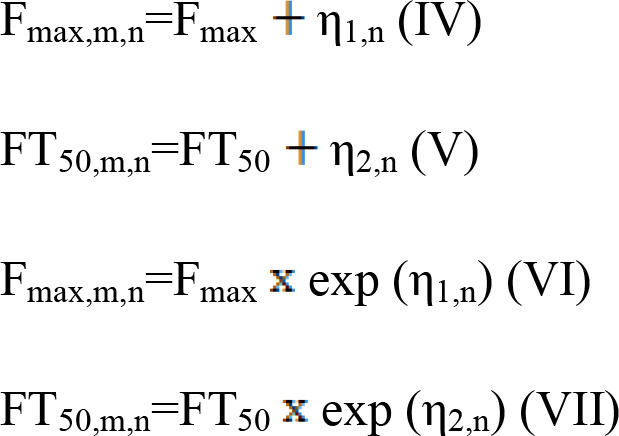 |
In these equations, Fmax represent Emax, FT50 represent ET50, m represents a specific study; n represents the time point of the study; η1,n and η2,n represent the inter-study variability, when available, which was assumed to be normally distributed, with a mean of 0 and variance of ω1,i2, ω2,i2, respectively.
Furthermore, categorical and continuous covariates (source, weight and age) were evaluated using equations (VIII)-(X):
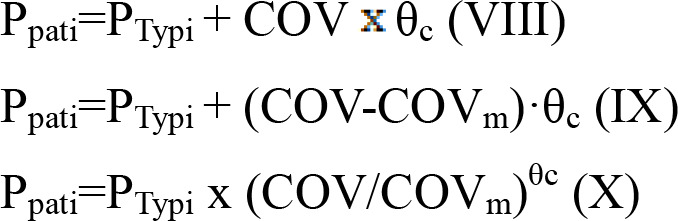 |
Ppati represents the value of an individual parameter; PTypi represents the value of a typical parameter; COV represents the covariate; COVm represents the median value of COV; and θc represents a correction coefficient.
Statistical analysis
Nonlinear mixed effects modeling software (NONMEM®; edition 7; ICON Development Solutions Ltd.) was used to establish the model and conduct statistical analysis. A change in the objective function value (OFV), which is a function that quantifies the difference between predicted values from the model and the actual observed data, was used as the criterion for covariate inclusion, which was the criterion to determine the fitting of the model. When the OFV was decreased [>3.84; χ2, α=0.05, degrees of freedom (d.f.)=1], the inclusion criterion was met. When the OFV was increased (>6.63; χ2, α=0.01, d.f.=1), significance was achieved in the final model. Our previous studies were mainly based on the methodology used in the present study, and indicated that the present method was reliable and acceptable (32-35).
Model validation
Individual predictions were compared with observations in individual plots and used to evaluate the final model. Prediction-corrected visual predictive check (VPC) plots were used to assess the predictive effectiveness of the final model.
Results
Included studies
A total of 24 RCTs, which included 8,545 patients with T2DM, were selected for analysis (8-31). These studies included 44 dapagliflozin treatment groups, which comprised 5 with a dose of 2.5 mg/day, 12 with a dose of 5 mg/day, 23 with a dose of 10 mg/day, and 4 with a dose of 20 mg/day. Drug safety at high doses was evaluated and no significant adverse reactions were found; in particular, no serious adverse events associated with the liver, kidney or pancreas were reported in these studies (8-31). In addition, in the included studies, the duration of dapagliflozin treatment was 12-104 weeks, and the mean age range of the patients with T2DM was 49.9-68.0 years (Table I).
Table I.
Studies identified for analysis.
| First author/s, year | Source | Groups | Dapagliflozin, mg/day | Duration of treatment, weeks | Body weight, kg mean median (SD); mean ± SD; (inter-quartile range); median [5-95th percentile] | Patient no. | Age, years | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Iacobellis and Gra-Menendez, 2020 | USA | Dapagliflozin | 10 | 24 | 104(28) | 50 | 52(9) | (13) |
| Control | - | 24 | 96.9(23) | 50 | 51(11) | |||
| Yamakage et al, 2020 | Japan | Dapagliflozin | 5 | 24 | 80.5±22.6 | 27 | 58.4±13.0 | (28) |
| Control | - | 24 | 79.0±16.3 | 27 | 60.7±11.9 | |||
| Aso et al, 2019 | Japan | Dapagliflozin | 5 | 24 | 73.6 (61.9, 80.8) | 33 | - | (8) |
| Control | - | 24 | 74.9 (65.6, 81.6) | 24 | - | |||
| Yang et al, 2018 | Asia | Dapagliflozin | 10 | 24 | 71.1±12.0 | 139 | 56.5±8.4 | (30) |
| Control | - | 24 | 72.4±13.1 | 133 | 58.6±8.9 | |||
| Yang et al, 2016 | Asia | Dapagliflozin | 5 | 24 | 70.8±12.2 | 147 | 53.1±9.1 | (29) |
| Dapagliflozin | 10 | 24 | 71.4±12.0 | 152 | 54.6±9.5 | |||
| Control | - | 24 | 70.9±11.4 | 145 | 53.5±9.2 | |||
| Matthaei et al, 2015 | - | Dapagliflozin | 10 | 52 | 88.6 (17.6) | 108 | - | (21) |
| Control | - | 52 | 90.1 (16.2) | 108 | - | |||
| Mathieu et al, 2015 | - | Dapagliflozin | 10 | 24 | 85.8±18.4 | 160 | 55.2±8.6 | (20) |
| Control | - | 24 | 88.2±18.1 | 160 | 55.0±9.6 | |||
| Cefalu et al, 2015 | Europe, Asia, USA, Canada, and Argentina | Dapagliflozin | 10 | 52 | 92.6 (20.5) | 455 | 62.8 (7.0) | (10) |
| Control | - | 52 | 93.6 (19.5) | 459 | 63.0 (7.7) | |||
| Rosenstock et al, 2015 | USA | Dapagliflozin | 10 | 24 | 87.1±18.0 | 179 | 53±10 | (22) |
| Control | - | 24 | 88.0±18.7 | 176 | 55±10 | |||
| Schumm-Draeger et al, 2015 | Europe and South Africa | Dapagliflozin | 2.5 | 16 | 92.49 (18.632) | 100 | 58.3 (9.0) | (24) |
| Dapagliflozin | 5 | 16 | 93.62 (16.641) | 99 | 55.3 (9.3) | |||
| Dapagliflozin | 10 | 16 | 90.58 (15.929) | 99 | 58.5 (9.8) | |||
| Control | - | 16 | 88.82 (15.327) | 101 | 58.5 (9.4) | |||
| Kaku et al, 2014 | Japan | Dapagliflozin | 5 | 24 | 65.81 (14.37) | 86 | 58.6 (10.4) | (15) |
| Dapagliflozin | 10 | 24 | 69.70 (13.82) | 88 | 57.5 (9.3) | |||
| Control | - | 24 | 65.96 (12.91) | 87 | 60.4 (9.7) | |||
| Leiter et al, 2014 | USA, Canada, Australia, Chile, Argentina and five European countries | Dapagliflozin | 10 | 24 | 94.5±17.8 | 480 | 63.9±7.6 | (18) |
| Control | - | 24 | 93.2±16.8 | 482 | 63.6±7.0 | |||
| Grandy et al, 2014 | Bulgaria, Czech Republic, Hungary, Poland and Sweden | Dapagliflozin | 10 | 102 | 92.1 (14.1) | 89 | 60.6 (8.2) | (11) |
| Control | - | 102 | 90.9 (13.7) | 91 | 60.8 (6.8) | |||
| Ji et al, 2014 | China, Korea and India | Dapagliflozin | 5 | 24 | 68.89 (11.43) | 128 | 53.0 (11.07) | (14) |
| Dapagliflozin | 10 | 24 | 70.92 (11.64) | 133 | 51.2 (9.89) | |||
| Control | - | 24 | 72.18 (13.23) | 132 | 49.9 (10.87) | |||
| Kohan et al, 2014 | USA, Argentina, Canada, India, Mexico, Peru, Italy, Australia, France, Spain, Denmark, Puerto Rico and Singapore | Dapagliflozin | 5 | 104 | 95.2±20.9 | 83 | 66±8.9 | (16) |
| Dapagliflozin | 10 | 104 | 93.2±17.3 | 85 | 68±7.7 | |||
| Control | - | 10 | 89.6±20.0 | 84 | 67±8.6 | |||
| Wilding et al, 2014 | Worldwide | Dapagliflozin | 2.5 | 104 | 93.0 (16.7) | 202 | 59.8 (7.6) | (27) |
| Dapagliflozin | 10 | 104 | 94.5 (16.8) | 194 | 59.3 (8.8) | |||
| Control | - | 104 | 94.5 (19.8) | 193 | 58.8 (8.6) | |||
| Lambers et al, 2013 | Canada, Netherlands and USA | Dapagliflozin | 10 | 12 | 93.2 (18.0) | 24 | 53.7 (9.4) | (17) |
| Control | - | 12 | 96.2 (19.5) | 25 | 58.0 (9.5) | |||
| Bailey et al, 2013 | Argentina, Brazil, Canada, Mexico, and USA | Dapagliflozin | 2.5 | 102 | 84.90 (17.77) | 137 | - | (9) |
| Dapagliflozin | 5 | 102 | 84.73 (16.26) | 137 | - | |||
| Dapagliflozin | 10 | 102 | 86.28 (17.53) | 135 | - | |||
| Control | - | 102 | 87.74 (19.24) | 137 | - | |||
| Rosenstock et al, 2012 | Argentina, Canada, India, Mexico, Peru, China, Philippines and USA | Dapagliflozin | 5 | 48 | 87.8±20.7 | 141 | 53.2±10.9 | (23) |
| Dapagliflozin | 10 | 48 | 84.8±22.2 | 140 | 53.8±10.4 | |||
| Control | - | 48 | 86.4±21.3 | 139 | 53.5±11.4 | |||
| Henry et al, 2012 | North America, Latin America, Europe and Asia | Dapagliflozin | 5 | 24 | 84.1 (19.5) | 194 | 51.7 (9.3) | (12) |
| Dapagliflozin | 10 | 24 | 88.4 (19.7) | 211 | 51.0 (10.1) | |||
| Control | - | 24 | 87.2 (19.4) | 208 | 52.7 (10.4) | |||
| Strojek et al, 2011 | Czech Republic, Hungary, Republic of Korea, Philippines, Poland, Thailand and Ukraine | Dapagliflozin | 2.5 | 24 | 81.89 | 154 | 59.9±10.14 | (25) |
| Dapagliflozin | 5 | 24 | 81.00 | 142 | 60.2±9.73 | |||
| Dapagliflozin | 10 | 24 | 80.56 | 151 | 58.9±8.32 | |||
| Control | - | 24 | 80.94 | 145 | 60.3±10.16 | |||
| Zhang et al, 2010 | - | Dapagliflozin | 10 | 12 | 86.6 [60.6, 115] | 45 | 55.0[41.0,71.0] | (31) |
| Dapagliflozin | 20 | 12 | 86.6 [60.6, 115] | 57 | 55.0[41.0,71.0] | |||
| Control | - | 12 | 89.8 [59.2, 122] | 49 | 52.0[34.4,70.6] | |||
| Dapagliflozin | 10 | 12 | 104 [82.0, 120] | 19 | 57.0[38.0,71.6] | |||
| Dapagliflozin | 20 | 12 | 104 [82.0, 120] | 25 | 57.0[38.0,71.6] | |||
| Control | - | 12 | 95.7 [77.3, 113] | 14 | 60.0[49.6,69.0] | |||
| Wilding et al, 2009 | USA and Canada | Dapagliflozin | 10 | 12 | 103.4±10.2 | 24 | 55.7±9.2 | (26) |
| Dapagliflozin | 20 | 12 | 101.2±15.3 | 24 | 56.1±10.6 | |||
| Control | - | 12 | 101.8±16.5 | 23 | 58.4±6.5 | |||
| List et al, 2009 | USA, Canada, Mexico and Puerto Rico | Dapagliflozin | 2.5 | 12 | 90±20 | 59 | 55±11 | (19) |
| Dapagliflozin | 5 | 12 | 89±17 | 58 | 55±12 | |||
| Dapagliflozin | 10 | 12 | 86±17 | 47 | 54±9 | |||
| Dapagliflozin | 20 | 12 | 88±18 | 59 | 55±10 | |||
| Control | - | 12 | 89±18 | 54 | 54±9 |
Modeling and validation
The actual dapagliflozin effect on weight loss in patients with T2DM is shown in Table II. Four Emax models were established, one for each dose of dapagliflozin (2.5, 5, 10 and 20 mg/day) to investigate the effect of the treatment on weight loss in patients with T2DM. The calculated values of Emax and ET50 were as follows: 2.5 mg/day, -3.04% and 30.8 weeks, respectively; 5 mg/day dapagliflozin, -6.57% and 27.3 weeks, respectively; 10 mg/day dapagliflozin, -4.12% and 20.4 weeks, respectively; and 20 mg/day dapagliflozin, -3.23% and 4.23 weeks, respectively. Information was obtained for all 8,545 patients with T2DM and it was not found that the clinicopathological characteristics of the patients may have influenced their weight loss outcomes.
Table II.
Parameter estimates of the final models.
| Model | Parameter | Estimate |
|---|---|---|
| A | Emax, % | -3.04 |
| ET50, week | 30.8 | |
| ωEmax | 1.360 | |
| ωET50 | 17.088 | |
| Ɛ | 0.062 | |
| B | Emax, % | -6.57 |
| ET50, week | 27.3 | |
| ωEmax | 2.773 | |
| ωET50 | 15.460 | |
| Ɛ | 0.100 | |
| C | Emax, % | -4.12 |
| ET50, week | 20.4 | |
| ωEmax | 0.585 | |
| ωET50 | 7.918 | |
| Ɛ | 0.327 | |
| D | Emax, % | -3.23 |
| ET50, week | 4.23 | |
| ωEmax | - | |
| ωET50 | 3.302 | |
| Ɛ | 0.010 |
Model A, patients treated with 2.5 mg/day dapagliflozin; model B, patients treated with 5 mg/day dapagliflozin; model C, patients treated with 10 mg/day dapagliflozin; D, patients treated with 20 mg/day dapagliflozin. Emax, maximal effect; ET50, treatment duration to reach half of the Emax; ωEmax, interstudy variability of Emax; ωET50, interstudy variability of ET50; Ɛ, residual error.
Models were constructed based on the Emax and ET50 values for the 2.5, 5, 10 and 20 mg/day doses of dapagliflozin. The effects of these doses on weight loss in patients with T2DM are described in equations (XI)-(XIV), respectively:
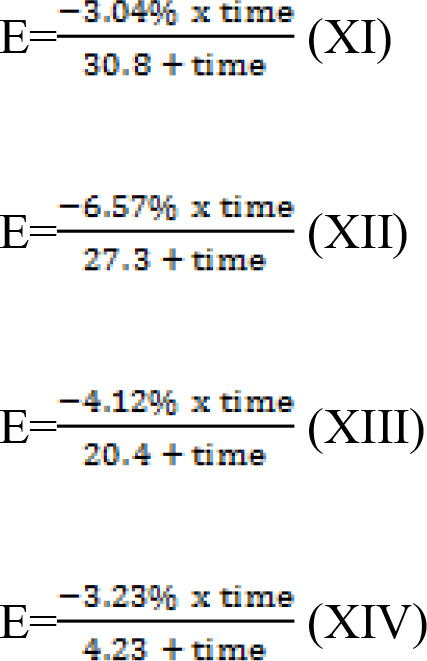 |
E represents the effect of dapagliflozin on the weight loss of patients with T2DM, and time is the duration of dapagliflozin treatment. Notably, these equations show that the only factor that ultimately affects body weight is the dose and duration of dapagliflozin.
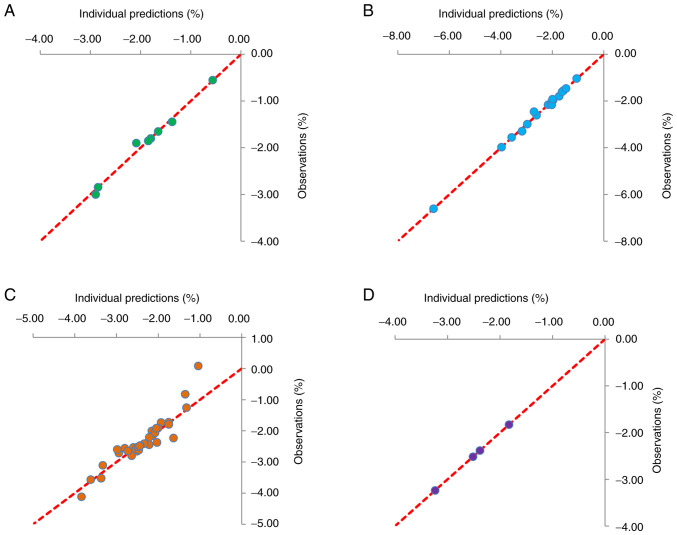
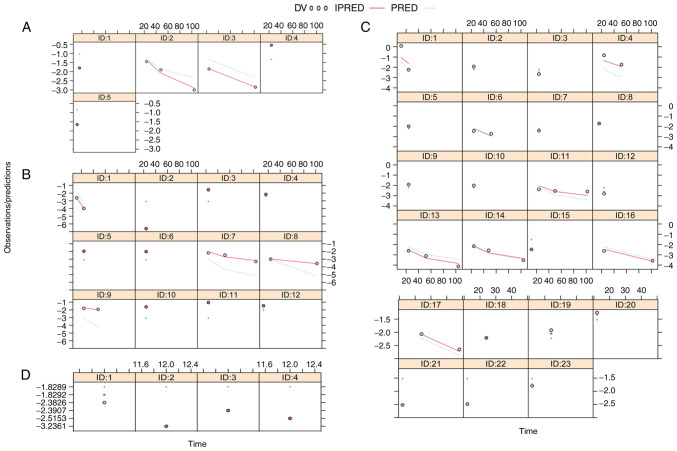
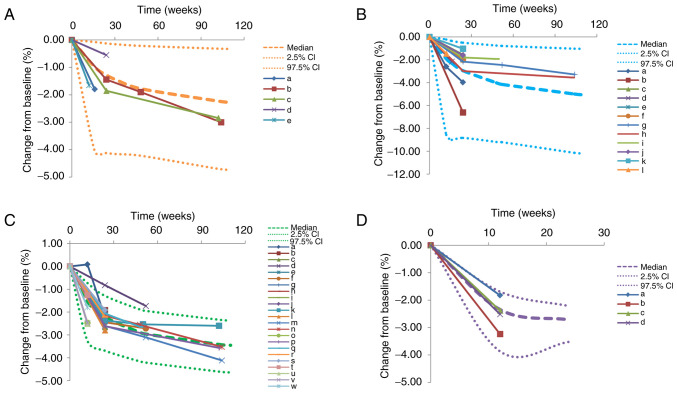
Fig. 1 presents plots of individual predictions compared with observations for patients treated with 2.5, 5, 10 and 20 mg/day dapagliflozin. The data indicate ideal linear relationships between individual predictions and observations, suggesting the optimal fitting of the final models. Plots for individuals treated with 2.5, 5, 10 and 20 mg/day dapagliflozin are shown in Fig. 2. These also demonstrate the optimal predictive ability of the models. VPC plots (Fig. 3) were established using data derived from patients treated with 2.5, 5, 10 and 20 mg/day dapagliflozin. The majority of the observed data fell within the 95% prediction intervals generated from the simulated data, which indicated the predictive power of the final models.
Figure 1.
Routine diagnostic plots of predictions and observations for different treatment groups. Plots for patients treated with (A) 2.5 mg/day, (B) 5 mg/day, (C) 10 mg/day and (D) 20 mg/day dapagliflozin are shown.
Figure 2.
Individual plots of predictions and observations for different treatment groups. Plots for patients treated with (A) 2.5 mg/day, (B) 5 mg/day, (C) 10 mg/day and (D) 20 mg/day dapagliflozin are shown. DV, observed value; IPRED, individual predicted value; PRED, population predicted value; ID, study identity.
Figure 3.
Prediction-corrected visual predictive check plots. Plots for patients treated with (A) 2.5 mg/day, (B) 5 mg/day, (C) 10 mg/day and (D) 20 mg/day dapagliflozin are shown. The median, 2.5 and 97.5% CI were simulated by the Monte Carlo method (n=1,000). CI, confidence interval; a-w, 44 dapagliflozin dose groups from 24 randomized controlled trials (8-31).
Dose-dependent pharmacological response to dapagliflozin
Fig. 4 indicates a dose-dependent pharmacological effect of dapagliflozin on weight loss in patients with T2DM. Fig. 4A indicates the relationship between Emax and dapagliflozin dosage, and Fig. 4B that between ET50 and dapagliflozin dosage. Based on these results, it can be deduced that among the four doses, 5 mg/day dapagliflozin exhibited the greatest weight loss effect, and the order of efficacy from high to low was as follows: 5 mg/day >10 mg/day >20 mg/day >2.5 mg/day. The onset time of weight loss reduced as the dose increased, and the order of onset from fast to slow was as follows: 20 mg/day >10 mg/day >5 mg/day >2.5 mg/day.
Figure 4.
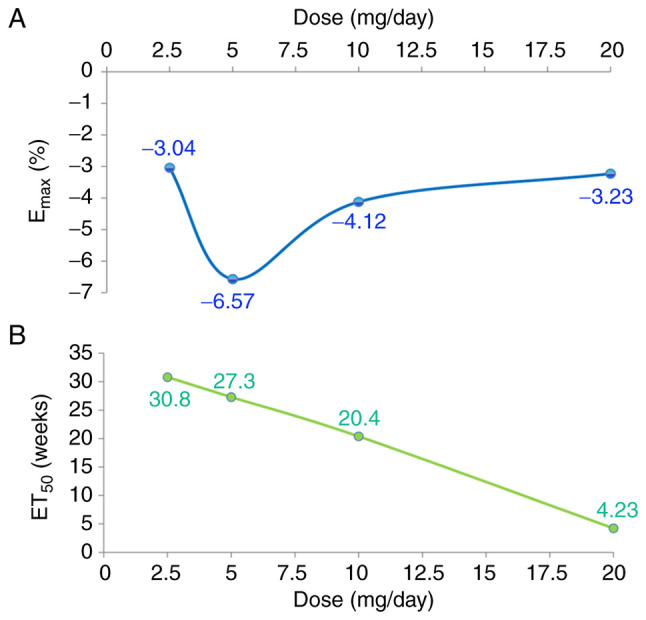
Pharmacological effect of dapagliflozin on weight loss. Relationships between (A) Emax and dapagliflozin dosage and (B) ET50 and dapagliflozin dosage. Emax, maximal effect; ET50, time taken to reach half the Emax.
Discussion
Dapagliflozin is a SGLT2 inhibitor, which is used as a therapeutic strategy for the treatment of diabetes (7). The SGLT2 protein is specifically expressed in the renal tubular proximal S1 segment, where it mediates glucose reabsorption in the early proximal tubule; it is responsible for ~90% of glucose reabsorption in the kidney (7). SGLT2 inhibitors specifically inhibit the activity of SGLT2 and lower renal glucose reabsorption in the proximal convoluted tubule leading to increased urinary glucose excretion (7,36-38). The recommended initial dosage of dapagliflozin in the United States and China is 5 mg, which is rapidly absorbed following oral administration and enables the maximal plasma concentrations to be achieved in 2 h (7). In addition, the oral bioavailability following the administration of 10 mg dapagliflozin is 78%, and the mean half-life is 12.9 h (7). Dapagliflozin has been accepted as a monotherapy or adjuvant therapeutic strategy for T2DM in the European Union, United States and China (7).
Various clinical trials have verified that dapagliflozin is effective in reducing glycated hemoglobin, fasting plasma glucose and body weight with a low incidence of hypoglycemic events (7,39,40). In addition, dapagliflozin monotherapy (5-10 mg/day) is effective in achieving glucose control, and patients exhibit optimal adherence to the treatment due to it being easy to use (7,41-43). However, patients with T2DM often develop obesity (2,3); obese patients have an elevated risk of cardiovascular disease and mortality. Therefore, it is crucial to improve the management of overweight or obese patients with T2DM (6). Fortunately, in addition to improving the control of blood glucose, dapagliflozin is also able to reduce the weight of patients with T2DM, thus providing benefits to patients with T2DM from multiple perspectives.
However, the dose-dependent pharmacological response to dapagliflozin with regard to weight loss in patients with T2DM is unknown; specific clinical guidance for dapagliflozin in the promotion of weight loss in patients with T2DM is lacking. Therefore, the purpose of the present study was to probe the effects of dapagliflozin on weight loss in patients with T2DM. A total of 24 RCT studies containing 8,545 patients with T2DM were included for analysis in the present study. These included 44 dapagliflozin dose groups, of which 5 received a dose of 2.5 mg/day, 12 a dose of 5 mg/day, 23 a dose of 10 mg/day, and 4 a dose of 20 mg/day.
The Emax model was used to evaluate the dose-dependent pharmacological response of weight loss to dapagliflozin in patients with T2DM. In addition, in order to determine the actual weight loss effect of dapagliflozin in T2DM, the control effect was subtracted from the sum effect. Moreover, since RCTs were included, the experimental and control groups from the same source were essentially identical in terms of patient demographics, comorbidities and other factors that may influence weight loss in patients with T2DM. The literature data were processed by subtracting the possible effect on weight in the control group from that in the experimental group in order to obtain the actual weight loss effect of dapagliflozin in T2DM.
Finally, four Emax models were established, one for each dose of dapagliflozin (2.5, 5, 10 and 20 mg/day). The models represent the effect of dapagliflozin on weight loss in patients with T2DM. Patients treated with 2.5, 5, 10 and 10 mg/day dapagliflozin demonstrated Emax values of -3.04, -6.57, -4.12 and 3.23%, respectively and ET50 values of 30.8, 27.3, 20.4 and 4.23 weeks, respectively. The efficacy of dapagliflozin in the induction of weight loss in patients with T2DM was highest with a 5 mg/day dose, followed by 10, 20 and 2.5 mg/day, respectively. We hypothesize that the reason for the least favorable effect being achieved with 2.5 mg is that this dose is insufficient, while the optimal efficacy was obtained at 5 mg. However, the underlying mechanism of the effects of dapagliflozin on body weight require further study in the future. The onset time of weight loss was fastest with a dosage of 20 mg/day and slowed gradually as the dosage decreased from 10 to 2.5 mg/day. In addition, information was obtained from all 8,545 patients with T2DM and it was not found that different methodology, data collection methods, sample sizes, generalizability or other factors had any influence on weight loss outcomes.
The present study has certain objective limitations. Firstly, the included studies were all published, and included those with negative results or studies that did not show a significant effect of dapagliflozin on weight loss; However, unpublished literature data were not included, which may result in potential bias. Secondly, the deviations from the mean were not analyzed. Thirdly, the safety profile of the drug, particularly that associated with the liver, kidney and pancreas was not included or thoroughly considered. However, the doses explored were all administered in clinical trials or as recommended in the instructions of use, and the general safety was optimal and acceptable. It is important to note that the long-term effects require further assessment. Nevertheless, the security of long-term dapagliflozin use appears to be acceptable. Fioretto et al (44) reported that dapagliflozin treatment for ≤104 weeks was well tolerated in older patients. Although older patients treated with dapagliflozin, experienced more renal adverse events than placebo-treated patients, the majority of these events were non-serious small transient changes in serum creatinine. Durán-Martínez et al (45) reported that dapagliflozin had an appropriate safety profile in patients with T1DM following the careful selection of participants and implementation of strategies to reduce the risk of diabetic ketoacidosis, and the treatment also led to clinical improvements in this population.
In conclusion, to the best of our knowledge, the present study is the first to analyze the dose-dependent pharmacological effect of dapagliflozin on weight loss in patients with T2DM. Of the four doses used, 5 mg/day dapagliflozin exhibited the greatest weight loss effect, and the onset time of weight loss accelerated with increasing dose. The detailed mechanism underlying the effects of dapagliflozin on body weight will be investigated in future studies. In particular, proteomic investigations may be carried out using animal experiments to determine the specific signaling pathway of dapagliflozin.
Acknowledgements
Not applicable.
Funding Statement
Funding: The study was supported by The Innovative Practice Training Program for Students of Jiangsu Higher Education Institutions (grant no. 202210313053Z), The National Innovative Practice Training Program for Students of Higher Education Institutions (grant no. 202210313053), The Xuzhou Special Fund for Promoting Scientific and Technological Innovation (grant no. KC21257), The Initializing Fund of Xuzhou Medical University (grant nos. RC20552111 and RC20552222), The Fusion Innovation Project of Xuzhou Medical University (grant nos. XYRHCX2021011 and XYRHCX2022005), Jiangsu Province Education Science Planning Project (grant no. C/2022/01/36), Xuzhou Medical University Labor Education Special Support Project (grant no. X1d202209), Jiangsu Province Higher Education Informatization Research Topic (grant no. 2023JSETKT136) and Xuzhou Medical University Research Topic of Higher Education Teaching Reform (grant no. Xjyzrd202304).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
The study was conceived and designed by SMH and DDW. Collection of data was performed by YH, YFL, CWY, YYG, XC, QG, QQX and XMW. Data analysis and interpretation were performed by DDW, YH, YFL, CWY and YYG. The manuscript was written by YH, YFL, CWY and YYG. All authors read and approved the final version of the manuscript: YH and DDW confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nicholson MK, Ghazal Asswad R, Wilding JP. Dapagliflozin for the treatment of type 2 diabetes mellitus-an update. Expert Opin Pharmacother. 2021;22:2303–2310. doi: 10.1080/14656566.2021.1953471. [DOI] [PubMed] [Google Scholar]
- 2.González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, Martinez JA. Obesity. Nat Rev Dis Primers. 2017;3(17034) doi: 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- 3.Iglay K, Hannachi H, Joseph Howie P, Xu J, Li X, Engel SS, Moore LM, Rajpathak S. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32:1243–1252. doi: 10.1185/03007995.2016.1168291. [DOI] [PubMed] [Google Scholar]
- 4.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17(83) doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.8. Obesity management for the treatment of type 2 diabetes: Standards of medical care in diabetes-2020. Diabetes Care. 2020;43 (Suppl 1):S89–S97. doi: 10.2337/dc20-S008. American Diabetes Association. [DOI] [PubMed] [Google Scholar]
- 6.Uneda K, Kawai Y, Yamada T, Kinguchi S, Azushima K, Kanaoka T, Toya Y, Wakui H, Tamura K. Systematic review and meta-analysis for prevention of cardiovascular complications using GLP-1 receptor agonists and SGLT-2 inhibitors in obese diabetic patients. Sci Rep. 2021;11(10166) doi: 10.1038/s41598-021-89620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng M, Lv H, Xu X, Wang J, Lyu W, Fu S. Efficacy and safety of dapagliflozin as monotherapy in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2019;98(e16575) doi: 10.1097/MD.0000000000016575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aso Y, Kato K, Sakurai S, Kishi H, Shimizu M, Jojima T, Iijima T, Maejima Y, Shimomura K, Usui I. Impact of dapagliflozin, an SGLT2 inhibitor, on serum levels of soluble dipeptidyl peptidase-4 in patients with type 2 diabetes and non-alcoholic fatty liver disease. Int J Clin Pract. 2019;73(e13335) doi: 10.1111/ijcp.13335. [DOI] [PubMed] [Google Scholar]
- 9.Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: A randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013;11(43) doi: 10.1186/1741-7015-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin's effects on glycemia and cardiovascular risk factors in high-risk patients with type 2 diabetes: A 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. Diabetes Care. 2015;38:1218–1227. doi: 10.2337/dc14-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandy S, Hashemi M, Langkilde AM, Parikh S, Sjöström CD. Changes in weight loss-related quality of life among type 2 diabetes mellitus patients treated with dapagliflozin. Diabetes Obes Metab. 2014;16:645–650. doi: 10.1111/dom.12263. [DOI] [PubMed] [Google Scholar]
- 12.Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: Initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012;66:446–456. doi: 10.1111/j.1742-1241.2012.02911.x. [DOI] [PubMed] [Google Scholar]
- 13.Iacobellis G, Gra-Menendez S. Effects of dapagliflozin on epicardial fat thickness in patients with type 2 diabetes and obesity. Obesity (Silver Spring) 2020;28:1068–1074. doi: 10.1002/oby.22798. [DOI] [PubMed] [Google Scholar]
- 14.Ji L, Ma J, Li H, Mansfield TA, T'joen CL, Iqbal N, Ptaszynska A, List JF. Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: A randomized, blinded, prospective phase III study. Clin Ther. 2014;36:84–100.e9. doi: 10.1016/j.clinthera.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Kaku K, Kiyosue A, Inoue S, Ueda N, Tokudome T, Yang J, Langkilde AM. Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Metab. 2014;16:1102–1110. doi: 10.1111/dom.12325. [DOI] [PubMed] [Google Scholar]
- 16.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leiter LA, Cefalu WT, de Bruin TWA, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: A 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc. 2014;62:1252–1262. doi: 10.1111/jgs.12881. [DOI] [PubMed] [Google Scholar]
- 19.List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–657. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathieu C, Ranetti AE, Li D, Ekholm E, Cook W, Hirshberg B, Chen H, Hansen L, Iqbal N. Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009–2017. doi: 10.2337/dc15-0779. [DOI] [PubMed] [Google Scholar]
- 21.Matthaei S, Bowering K, Rohwedder K, Sugg J, Parikh S, Johnsson E. Durability and tolerability of dapagliflozin over 52 weeks as add-on to metformin and sulphonylurea in type 2 diabetes. Diabetes Obes Metab. 2015;17:1075–1084. doi: 10.1111/dom.12543. Study 05 Group. [DOI] [PubMed] [Google Scholar]
- 22.Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, Iqbal N. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: A randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376–383. doi: 10.2337/dc14-1142. [DOI] [PubMed] [Google Scholar]
- 23.Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473–1478. doi: 10.2337/dc11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schumm-Draeger PM, Burgess L, Korányi L, Hruba V, Hamer-Maansson JE, de Bruin TW. Twice-daily dapagliflozin co-administered with metformin in type 2 diabetes: A 16-week randomized, placebo-controlled clinical trial. Diabetes Obes Metab. 2015;17:42–51. doi: 10.1111/dom.12387. [DOI] [PubMed] [Google Scholar]
- 25.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: A randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928–938. doi: 10.1111/j.1463-1326.2011.01434.x. [DOI] [PubMed] [Google Scholar]
- 26.Wilding JPH, Norwood P, T'Joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: Applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–1662. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilding JPH, Woo V, Rohwedder K, Sugg J, Parikh S. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: Efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124–136. doi: 10.1111/dom.12187. Dapagliflozin 006 Study Group. [DOI] [PubMed] [Google Scholar]
- 28.Yamakage H, Tanaka M, Inoue T, Odori S, Kusakabe T, Satoh-Asahara N. Effects of dapagliflozin on the serum levels of fibroblast growth factor 21 and myokines and muscle mass in Japanese patients with type 2 diabetes: A randomized, controlled trial. J Diabetes Investig. 2020;11:653–661. doi: 10.1111/jdi.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang W, Han P, Min KW, Wang B, Mansfield T, T'Joen C, Iqbal N, Johnsson E, Ptaszynska A. Efficacy and safety of dapagliflozin in Asian patients with type 2 diabetes after metformin failure: A randomized controlled trial. J Diabetes. 2016;8:796–808. doi: 10.1111/1753-0407.12357. [DOI] [PubMed] [Google Scholar]
- 30.Yang W, Ma J, Li Y, Li Y, Zhou Z, Kim JH, Zhao J, Ptaszynska A. Dapagliflozin as add-on therapy in Asian patients with type 2 diabetes inadequately controlled on insulin with or without oral antihyperglycemic drugs: A randomized controlled trial. J Diabetes. 2018;10:589–599. doi: 10.1111/1753-0407.12634. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Feng Y, List J, Kasichayanula S, Pfister M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: Effects on glycaemic control and body weight. Diabetes Obes Metab. 2010;12:510–516. doi: 10.1111/j.1463-1326.2010.01216.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang DD, Mao YZ, He SM, Chen X. Analysis of time course and dose effect from metformin on body mass index in children and adolescents. Front Pharmacol. 2021;12(611480) doi: 10.3389/fphar.2021.611480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Wang DD, Li ZP. Time course and dose effect of metformin on weight in patients with different disease states. Expert Rev Clin Pharmacol. 2020;13:1169–1177. doi: 10.1080/17512433.2020.1822164. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Wang DD, Li ZP. Analysis of time course and dose effect of tacrolimus on proteinuria in lupus nephritis patients. J Clin Pharm Ther. 2021;46:106–113. doi: 10.1111/jcpt.13260. [DOI] [PubMed] [Google Scholar]
- 35.Wang DD, Li YF, Mao YZ, He SM, Zhu P, Wei QL. A machine-learning approach for predicting the effect of carnitine supplementation on body weight in patients with polycystic ovary syndrome. Front Nutr. 2022;9(851275) doi: 10.3389/fnut.2022.851275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeFronzo RA, Hompesch M, Kasichayanula S, Liu X, Hong Y, Pfister M, Morrow LA, Leslie BR, Boulton DW, Ching A, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care. 2013;36:3169–3176. doi: 10.2337/dc13-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27:136–142. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22:104–112. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaku K, Maegawa H, Tanizawa Y, Kiyosue A, Ide Y, Tokudome T, Hoshino Y, Yang J, Langkilde AM. Dapagliflozin as monotherapy or combination therapy in Japanese patients with type 2 diabetes: An open-label study. Diabetes Ther. 2014;5:415–433. doi: 10.1007/s13300-014-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M, Zhang L, Wu B, Song H, An Z, Li S. Dapagliflozin treatment for type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2014;30:204–221. doi: 10.1002/dmrr.2479. [DOI] [PubMed] [Google Scholar]
- 41.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60:215–225. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson C. Diabetes: Dapagliflozin: An insulin-independent, therapeutic option for type 2 diabetes mellitus. Nat Rev Endocrinol. 2010;6(531) doi: 10.1038/nrendo.2010.134. [DOI] [PubMed] [Google Scholar]
- 43.Gilor C, Niessen SJM, Furrow E, DiBartola SP. What's in a name? Classification of diabetes mellitus in veterinary medicine and why it matters. J Vet Intern Med. 2016;30:927–940. doi: 10.1111/jvim.14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fioretto P, Mansfield TA, Ptaszynska A, Yavin Y, Johnsson E, Parikh S. Long-term safety of dapagliflozin in older patients with type 2 diabetes mellitus: A pooled analysis of phase IIb/III studies. Drugs Aging. 2016;33:511–522. doi: 10.1007/s40266-016-0382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durán-Martínez M, Azriel S, Doulatram-Gamgaram VK, Moreno-Pérez Ó, Pinés-Corrales PJ, Tejera-Pérez C, Merino-Torres JF, Brito-Sanfiel M, Chico A, Marco A, et al. Real-world safety and effectiveness of dapagliflozin in people living with type 1 diabetes in Spain: The Dapa-ON multicenter retrospective study. Diabetes Metab. 2024;50(101501) doi: 10.1016/j.diabet.2023.101501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.