Abstract
Whereas emotion theorists often keep their distance from the embodied approach, theorists of embodiment tend to treat emotion as a mainly physiologic process. However, intimate links between emotions and the body suggest that emotions are privileged phenomena to attempt to reintegrate mind and body and that the body helps the mind in shaping emotional responses. To date, research has favored the cerebrum over other parts of the brain as a substrate of embodied emotions. However, given the widely demonstrated contribution of the cerebellum to emotional processing, research in affective neuroscience should consider embodiment theory as a useful approach for evaluating the cerebellar role in emotion and affect. The aim of this review is to insert the cerebellum among the structures needed to embody emotions, providing illustrative examples of cerebellar involvement in embodied emotions (as occurring in empathic abilities) and in impaired identification and expression of embodied emotions (as occurring in alexithymia).
Keywords: embodiment, emotions, empathy, alexithymia, internal models, affective neuroscience
The Embodied Foundations of Emotions
Many disciplines deal with emotions (psychology, philosophy, anthropology, linguistics, literary studies, history, cognitive science, neuroscience, medicine, etc.), although none considers emotions its central core. This cross-disciplinarity makes the situation somewhat slippery, given that each scientific area adopts a self-contained approach and addresses the aspects of emotion that are most related to its main concerns. A central theme of scientific thinking on emotion is whether the body and the mind, as distinct entities, can influence each other during the generation of an emotional response. Some psychological theories suggest that changes in the body cause changes in the mind; others suggest the opposite; and others suggest that the body and mind interact to produce an emotional response. Therefore, the history of embodied emotions has been a long and controversial one (Semin and Smith 2008).
Short Historical Notes on Embodied Emotions
An appropriate point of departure may be Aristotle (384–322 bc). Although there had already been discussions on the relationship between mind (soul) and body in the period of Plato, Parmenides, and Democritus, Aristotle was the first to systematically propose that emotions (pathê) are passive states, located within a ground that contrasts active and passive, form and matter, and actuality and potentiality. At the end of On the Soul (De Anima), Aristotle argues that the pathê are enmattered forms (logoi enuloi), intelligible structures inseparable from the matter of which they are forms. Therefore, the soul is inseparable from the body, and emotions are psychic states grounded in bodily feelings (Colombetti and Thompson 2008; Huang 2021). The pathē are the first responses of the embodied individual to the input from the outside world. They are actualized by external causes in the experience of an incipient emotion, and it is suggested that the capacity to even experience pathē requires a determinate form: a soul. For these reasons, emotions can be attributed to the soul insofar as the soul informs the body. Aristotle’s innovation was to insist that the soul “neither acts nor is affected without the body.” Thus, his interest in unifying the body and soul and giving the body an essential, although subordinate, role in the emotional experience may have anticipated of some of the latest research in cognitive science.
Supporters of embodied emotions include Spinoza (1632–1677), who claimed that although mind and body do not interact, every bodily state implies the existence of a corresponding and isomorphic mental state, and mind and body are parallel manifestations of the same underlying substance; Hobbes (1588–1679), who treated passions as motions within the body; and Hume (1711–1776), who defined emotions as “sensations arising in the soul from the body” (Colombetti 2021).
A somewhat prejudicially opposed scenario was proposed by René Descartes (1596–1650), who in addition to developing a revolutionary philosophical method in traditional areas such as physics, mathematics, and physiology, developed a pioneering approach to the study of emotions, considering them from an essentially scientific point of view (en physicien). According to Descartes, reason and emotion are independent substances acting as antagonistic forces (Strejcek and Zhong 2014), and the existence of mind is ascertained before, and independently of, that of the body.
The influential position of Descartes on mind-body dualism permeated and still permeates thinking on cognition and emotion and favored the notion of the disembodied mind. Emotion theories of the 1960s and 1970s, the golden years of cognitivism, rest on the hypothesis that emotions are essentially intentional events and cognitive antecedents. Emotions are necessarily “about something” in the sense that they are evaluative judgments or “appraisals” that refer to our well-being and assess the significance of the situations in which we find ourselves. The cognitivists’ disembodied point of view considers bodily events as nonspecific and concomitant by-products of cognition, which do not contribute to the motley emotional experience. Cognitivist theories rejected Darwin’s interest in the bodily expression of emotions and James’s idea that emotions are bodily processes. Namely, Darwin (1872) maintained that the perception of behavioral and bodily manifestations of emotion constitutes feelings and that facial expressions are universal, biologically innate, and adaptive; for example, people aware of a danger easily communicate to others through facial expression of fear. James (1884) posited that emotion is the mind’s perception of physiologic conditions that result from some stimulus, and he failed to assign any cognitive role to the emotions. Notably, long before neuroscientists came to demonstrate how our emotions affect our bodies, in the essay titled “What Is an Emotion?” (1884), James asserted “the bodily changes follow directly the perception of the exciting fact, and our feeling of the same changes as they occur is the emotion.” Subsequently, in the volume The Principles of Psychology (James and others 1890), he claimed “the world experienced comes at all times with our body as its center, center of vision, center of action, center of interest.” Contesting common presuppositions about the ordering of an emotional episode, James argued that it is not an emotion causing bodily changes; rather, corporeal reverberations are actually the raw material of the emotion itself: “the perception of bodily changes, as they occur, is the emotion.”
Even without adopting this “extreme” position, emotions may be considered the best field to attempt to rejoin the mind and the body. In fact, theories on disembodied cognition and affect have been challenged by the rise of embodied approaches. These approaches consider emotions to involve cognitive processes (perception, attention, and evaluation) and bodily events (arousal, behavior, facial, and vocal expressions), and they emphasize the coupled interactions of the brain, body, and environment. In particular, embodiment theory posits that cognitive and emotional processes are shaped and rooted in our biological constitution (Critchley and Garfinkel 2017). The general idea that the mind is grounded in the whole body and not only in the brain and that emotional states arise from physiologic changes from within the whole body is well suited to bridge the Cartesian dichotomies between mind and body, cognition and emotion, nature and nurture, rationality and irrationality, and conscious and unconscious (Damasio and Carvalho 2013). According to embodiment theory, the processing of information about concrete facts or abstract concepts is triggered, influenced, updated by, associated with, and even dependent on perceptual, somatosensory, motor, neuroendocrine, visceral, and autonomic nervous system activities, such as smiling, weeping, frowning, sweating, cringing, or getting a feeling in the pit of the stomach (Niedenthal and others 2005).
Interoception and Embodied Emotions
We experience the world factually, but it is the visceral reaction that validates the experience as “real” (Duncan and Barrett 2007). According to emotional constructivism (Barrett 2017), emotions are the brain’s creation of what bodily sensations mean in relation to what is going on around us in the world. Crucially, bodily sensations include not only exteroceptive sensations from the external environment but also interoceptive sensations from the internal milieu of the body that relay signals to the brain about the current status of the body. Interoception encompasses the following signals:
Proprioceptive: perception of joint position and movement, muscle force, stiffness, and viscosity
Visceral: perception of heartbeat, breath, or gastric/bladder contraction
Hormonal: messages released from endocrine glands, such as thyroid, pituitary, adrenal, and pancreatic
Immune: cascades of adaptors, kinases, and transcription factors, leading to the expression of antimicrobial genes and cytokines
Metabolic: metabolite sensors for sugars, lipids, amino acids, and metabolic intermediates
When mapped onto the brain, interoceptive information allows for a nuanced representation of the bodily physiologic state, which is important for maintaining homeostatic conditions (Craig 2014).
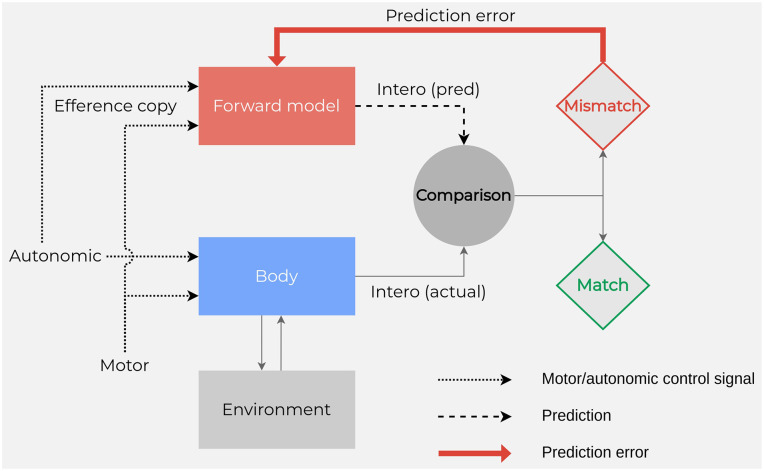
On such a basis, recent formulations on embodied emotions consider that speaking about an embodied process means speaking about a process related to interoceptive sensations. In this framework, the model of interoceptive predictive coding (Clark 2013; Seth and others 2012) proposed a data-processing strategy whereby signals are generated by predictive models (Figure 1).
Figure 1.
Interoception and predictive coding. Motor and autonomic signals evoke interoceptive responses, “intero (actual),” which are compared with predicted responses, “intero (pred).” These predictions are generated by hierarchically organized forward models informed by motor and autonomic efference copy signals. The comparison might take place in the inferior olive and generates a prediction error to be sent to the cerebellum. Adapted from Seth and others (2012).
Predictive coding (Friston 2010) is implemented by functional architectures in which top-down predictions counterflow with bottom-up prediction errors (actual sensory signals; Clark 2013). The brain generates many predictions, each with its own prior probability based on past experiences. The predictions with major prior probabilities function as hypotheses about the world and are tested against prediction errors. Once the difference between predictions and unanticipated information from the world is minimized, predictions become the inferences about the causes of sensory events and plans for movement to deal with them. By applying this approach, the constructionist theory of emotion (Barrett 2017; Barrett and others 2016) posits that emotions are internal states constructed on the basis of previous experiences as predictive schemes to react to external stimuli. The brain predicts body responses by drawing on prior sensorimotor experiences in similar situations, and the interoceptive predictions produce basic affective feelings with specific properties of valence (pleasure or displeasure) and arousal (agitation or calmness; Barrett and Simmons 2015; Gu and others 2013; Seth 2013). Prediction signals are embodied representations continuously anticipating interoceptive and exteroceptive sensory events as well as the best action to deal with these incoming sensory events. According to the embodied predictive interception coding model (Barrett and Simmons 2015), the brain infers the likely causes of upcoming sensory events by modulating ongoing visceromotor actions (i.e., inner movements associated with the immune, endocrine, and autonomic nervous systems) and motor actions to deal with the sensory events. By integrating the predictive coding account with neuroanatomic data, it has been proposed that the predictions originating in the agranular limbic visceromotor cortices (i.e., the cingulate cortex, ventromedial prefrontal cortex, orbitofrontal cortex, and ventral anterior insula) are sent to the primary interoceptive cortex (i.e., the posterior insular cortex) and to the granular cortical regions (i.e., all the primary sensory cortices). These regions compute prediction errors and update the internal model, thereby correcting visceromotor and action plans and sensory representations (Barrett 2017). Notably, the amygdala, basal ganglia, and, importantly for the present review, cerebellum also compute prediction errors that are forwarded to the cortex to correct internal models—that is, the neural representations of context-specific dynamics to facilitate predictive control of the system (Figures 1 and 2; Box 1). Note that the ventromedial prefrontal cortex and cerebellum belong to the default mode network (“mentalizing network”) and can therefore transmit predictions to the rest of the network during cognitive and emotional phenomena, such as imagery, memory, and empathy. The cited cortical and subcortical regions create a multisensory representation of the body in the world so that our sensations are influenced by our interoceptive and exteroceptive predictions. The result is a multisensory representation of the world from the perspective of “someone with a body” (Barrett and Simmons 2015). The view that interoceptive and exteroceptive representations are central parts of every mental event is wholly consistent with embodied accounts of perception, cognition, and emotion (Barsalou 2008).
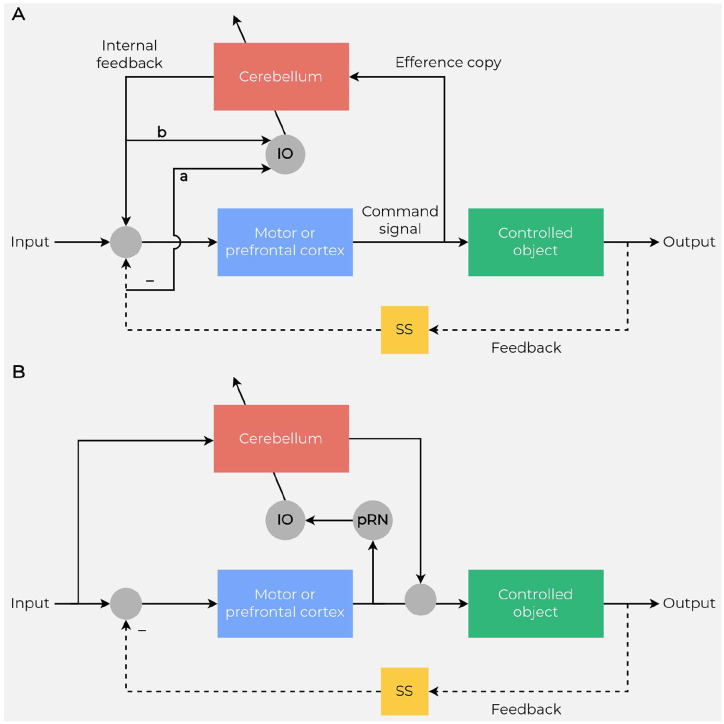
Figure 2.
Schematics of the internal model control. (A) A forward model is implemented in the cerebellum. It mimics the dynamic properties of the controlled object: a body part or a mental model. The sensory system (SS) mediates feedback (indicated by –). Circles indicate junctions at which signals converge or are relayed. In the inferior olive (IO), the outputs of (a) the controlled object (monitored by the SS) and (b) the cerebellum are compared to produce the error signals that are then sent to the cerebellum to eventually modify its internal models. (B) An inverse model is implemented in the cerebellum. It mimics the reciprocal of the dynamic properties of the controlled object. The oblique arrows in panels A and B represent the pathway signals that tune the dynamics of the forward model or the inverse dynamics of the inverse model. pRN = parvocellular red nucleus. Adapted from Ito (2008).
Box 1. Internal Models.
In neuroscience, an internal model is the neural network that simulates input-output relationships of a given process to continuously anticipate events in the environment. Internal models are classified as either forward (Figure 2A) or inverse (Figure 2B).
While a forward internal model calculates a sensory prediction for an ongoing motor command, an inverse internal model calculates a predicted motor command for the desired movement. The forward model highlights the comparison between the intentional content of the actions and their outcomes and relies on two main functions: prediction and detection/processing of prediction errors (Sokolov and others 2017; Tanaka and others 2019). The forward model of movement has, as inputs, the current state of the body part (position and velocity) and the efferent copies of motor commands representing the motor intention (corollary discharge or efference copy) and, as output, an estimate/prediction of the sensory consequences of the forthcoming action. The efferent copy is processed by a comparator that detects the presence of a mismatch between the predicted sensory outcome and the sensory feedback (prediction signal error; Figure 1). The signal error is used to retune the internal model so that in the future repetitions, through a learning process, the prediction is progressively better matched to the actual feedback. Thus, cortical areas may generate a suitable command for the next moment depending on the predicted consequence before a feedback signal is available (Ito 2008).
Initially proposed as a computational model of voluntary motor control, the forward model has subsequently been applied to a large repertoire of actions—for instance, the use of different tools (Imamizu and Kawato 2012) and cognitive functions (Honda and others 2018; Ito 2008; Kawato and Cortese 2021).
Internal models are conceivably located in many brain regions that have high synaptic plasticity and receive and send a large amount of information. In this framework, considering its uniform cytoarchitecture, its plastic properties (long-term depression and long-term potentiation), and its massive bidirectional connectivity with virtually all major brain subdivisions, the cerebellum is one of the most likely sites for containing forward internal models (Kawato and others 2003) and optimally acting as comparator. The extensive cortical and subcortical inputs reaching cerebellar granule cells from the pontine nuclei allow for the encoding of multimodal contexts (Sawtell 2010), including efference copies. The learning-induced changes in the activity of Purkinje cells, the output of the cerebellar cortex, can be viewed as a prediction of a future event (Rasmussen and others 2013). This prediction is compared with the actual feedback relayed to the cerebellar cortex by climbing fibers, originating in the inferior olivary nuclei, with mismatches about errors across a range of tasks resulting in the generation of complex spikes (Eccles and others 1967). Thus, the complex spike represents an error signal between the actual and predicted output that serves as a teaching signal that modifies Purkinje cell synapses and improves the internal model (Kawato and others 2021).
Because the uniformity of cellular organization across the cerebellar cortex strongly suggests identity in the computations, the same plastic events occurring in the motor domain might support the automation of cognitive processes (Argyropoulos 2016; Barrett and Simmons 2015; Ito 2008) such that the cerebellar forward models may even provide computational mechanisms for thought processes. Specifically, as the cerebellum models prediction errors from the periphery and relays them to the cortex to modify motor predictions, the same may be true for visceromotor and somatosensory predictions, given the connectivity of the cerebellum with the cingulate cortex, hypothalamus, and amygdala, as well as with frontal and parietal cortices via the thalamus (Buckner and others 2011; Schmahmann and Pandya 1997; Strick and others 2009). These computations would provide the cerebellum with a central role in emotions (Wager and others 2015).
Interestingly, it was recently argued that inner bodily states are those that more strongly correlate with core features of the self-concept (Monti and others 2021), and in the higher-order theory of emotional consciousness proposed by LeDoux and Brown (2017), the sense of self is core to emotional experiences. Crucially, embodiment involves the central processing of bottom-up signals afferent from the body with top-down regulatory directives in a bidirectional relationship. Ultimately, embodiment theories suggest that we understand others’ emotions because we are able to embody their emotions within ourselves (Colombetti and Thompson 2008; Damasio 1996; Niedenthal and others 2005).
The Cerebellum and Embodied Emotions
In regard to the brain as the structure involved in embodied emotions, it nearly always refers to the cerebrum and rarely to the cerebellum, which is considered ancillary to the telencephalic structures. However, in recent years, broad agreement has been reached around the cerebellar contribution to various aspects of emotional processing—from the perception and recognition of emotional cues to the evaluation of emotional contexts and from facial expressions to social behavior linked to emotions (Adamaszek and others 2017). Thus, research into the physiology of emotional states should consider the cerebellum more seriously than what is often the case in affective neuroscience, and embodiment theories may provide a useful approach for evaluating the cerebellum’s role in emotion.
During the last decades, rather than the preconception of cerebellar function being exclusively motor related, it has become clear that it is rare to find tasks that do not engage the cerebellum. Indeed, experimental and clinical strands of research have convincingly demonstrated cerebellar involvement in a large set of domains, such as the timing and monitoring of events, the integration of somatic and visceral information, attentional control, spatial and executive functions, working memory, linguistic processing, the modulation of intellect and mood, social cognition, personality, and the regulation of emotion and affect (Baumann and Mattingley 2012; Petrosini and others 2017; Sokolov and others 2017; Stoodley and Schmahmann 2010; Van Overwalle and others 2014; Zhu and others 2006). Structural and functional neuroimaging studies describing cerebellar functional topographic organization indicated that the anterior cerebellum (lobules I–V) and lobule VIII sustain motor and sensorimotor functions; the posterior cerebellum (lobules VI and VII, including Crus I and II and lobule VIIb) is the anatomic substrate of cognitive functions; and the posterior vermis, the so-called limbic cerebellum (lobules VI, VII, VIIb, and VIII, Crus I and II), is involved in emotional regulation (Stoodley and Schmahmann 2010; Figures 3 and 4).
Figure 3.
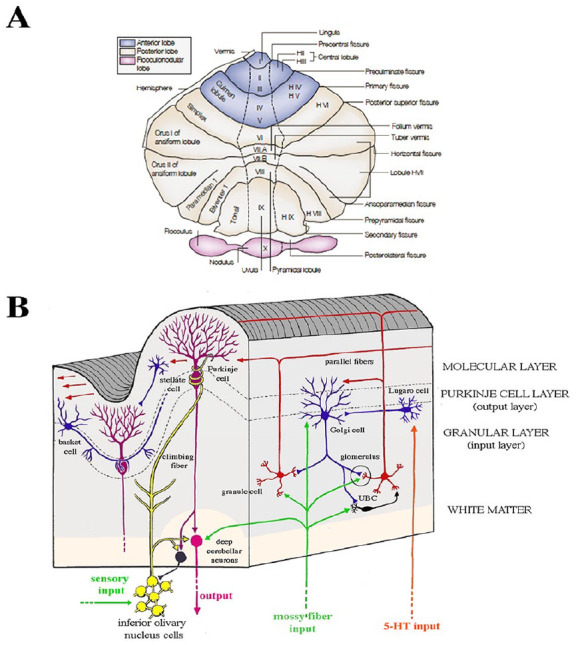
Macro- and microanatomy of the human cerebellum. (A) Unfolded view of the cerebellar cortex shows lobes, lobules by name and number, and main fissures. Hemispherical lobules are designed by the prefix “H” according to Larsell’s classification and are followed by Roman numerals indicating the corresponding vermian lobules. Adapted from Manni and Petrosini (2004). (B) Cellular and fiber elements of the cerebellar cortex. A vertical section of a single cerebellar folium, in longitudinal and transverse planes, illustrates the general organization of the cerebellar cortex. The cellular architecture of the cerebellar cortex is uniform throughout the folia. Purkinje cells, the sole output of the cerebellar cortex, mainly project to the deep cerebellar nuclei and receive excitatory input on their extensive arborization from a beam of parallel fibers arising from several granule cells and from a single climbing fiber arising from the inferior olive.
Figure 4.
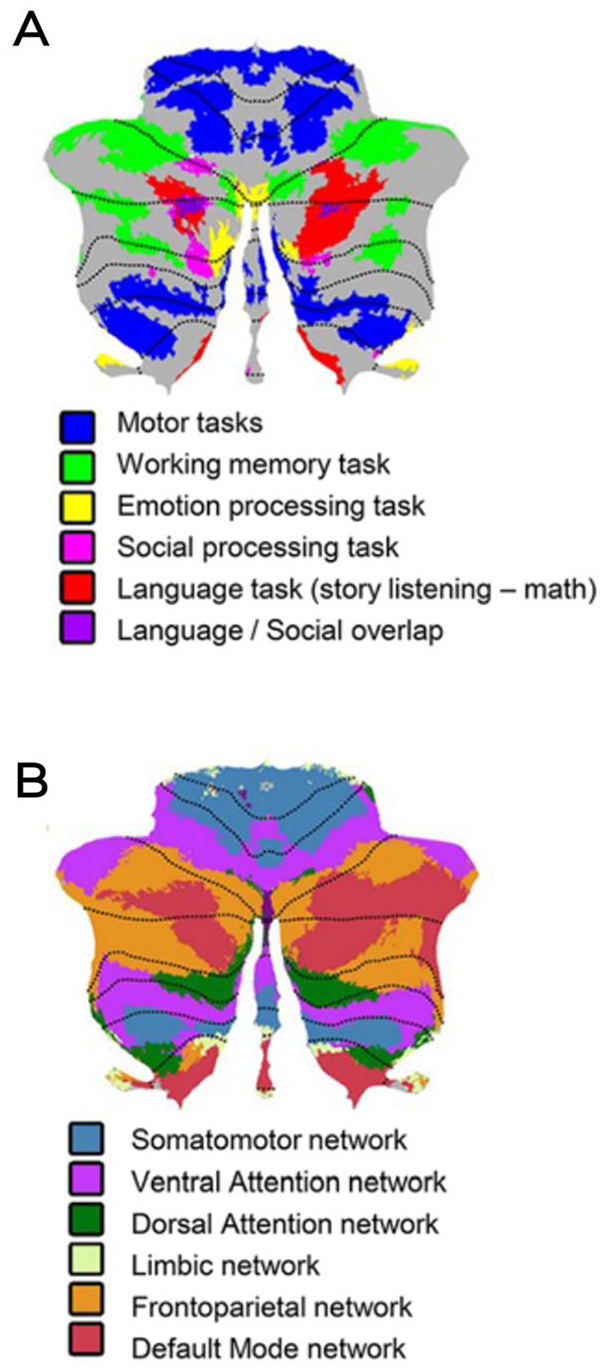
Functional specialization in the cerebellum. Cerebellar task activation maps (A) and resting-state networks (B). Adapted from Schmahmann (2021).
These cerebellar regions are reciprocally connected to areas implicated in the neural bases of reasoning, emotion, moral behavior, and social expression. Specifically, the medial part of the limbic cerebellum, especially lobules VI to VII, plays a key role in processing primary emotions; each emotion recruits specific cerebellar loci, with some spatial overlap (Schraa-Tam and others 2012). These areas communicate with those involved in the processing of emotional salience and control, such as the insula, frontal operculum, anterior cingulate cortex (ACC), medial prefrontal cortex, amygdala, and hippocampus (Bostan and others 2013; Habas 2018; Habas and others 2009). Additionally, the lateral part of the limbic cerebellum, encompassing the hemispheres (lobules VI–VIII and Crus I and II), plays a role in cognitive aspects of emotional processing, such as working memory, attention allocation, emotion evaluation, response selection, and affective prosody, and subserves cognitive and associative functions of the prefrontal cortex (Schmahmann 2021). An even more lateral part of the limbic cerebellum (Crus I and II) is functionally connected with the hypothalamus and ACC, while the anterior lobe and lobules VIIIB and VI are involved in automatic motor aspects of emotional processing, including facial expressions and the startle, withdrawal, avoidance reflexes, and pain processing (Moulton and others 2011).
In addition to brain imaging studies, neurophysiologic studies have offered a number of important insights into cerebellar involvement in emotional processing. Cerebellar participation in autonomic pathways and emotional regulation is indicated by impaired cardiovascular and autonomic responses during emotional processing in the presence of cerebellar lesions (Adamaszek and others 2017). In addition, event-related potential studies in patients with cerebellar lesions report alterations in early and late emotional processing stages, suggesting impairment in bottom-up and top-down control of emotional cue processing (Adamaszek and others 2017).
The introduction of noninvasive brain stimulation techniques, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), provided additional methods of investigating cerebellar contributions to emotional processing in healthy volunteers and clinical populations. Specifically, while low-frequency cerebellar repetitive TMS leads to impaired emotional regulation, high-frequency cerebellar repetitive TMS facilitates the processing of positive emotional stimuli and impairs the ability to discriminate emotional faces or emotional bodily postures (Adamaszek and others 2017). Facilitation of the recognition of negative emotional faces has also been reported by using cerebellar tDCS (Adamaszek and others 2017; Ruggiero and others 2022). Similarly, frontocerebellar tDCS improves mood measures in healthy participants (Adamaszek and others 2017).
In conclusion, one of the cerebellar functions seems to be the integration and forwarding of autonomic, emotional, and cognitive aspects of experience to the cerebral cortex (e.g., salience network and default mode network), resulting in adaptive behavioral responses. Analogous to motor symptomatology, cerebellar damage degrades the precision, efficiency, or coordination of cognitive and affective functions (Hoche and others 2016; Picazio and others 2020), and neuropsychiatric manifestations in cerebellar patients may be conceptualized as impairments in the implicit, automatic modulation of emotions (Schmahmann and others 2007). The original description of the Universal Cerebellar Transform hypothesizes that “singular anatomically determined universal transforms can manifest, through connectivity, as sensorimotor, cognitive, and affective functions resonates with the embodiment thesis that cognitive, affective, and sensorimotor systems are not independent” (Guell and others 2018). In the present review and in line with Guell and colleagues (2018), we address the issue that there is a mutual interaction between the fields of cerebellar neuroscience and embodied emotions, providing illustrative examples of cerebellar involvement in embodied emotions (as occurring in empathic abilities) and in impaired identification and expression of embodied emotions (as occurring in alexithymia).
Empathy as an Embodied Ability
After being a long-standing center of philosophical debate, the concept of empathy has crossed over from the philosophical domain and become the subject of study among social, developmental, clinical, and dynamic psychologists and subsequently even among neuroscientists. These multiple fields have incorporated the study of empathy, resulting in an overabundance of operational definitions. As Edmund Husserl, the founder of phenomenology, suggested in 1931, any intersubjective experience should be conceived as an empathic experience in which we consciously ascribe intentional acts and feelings to another subject. Such an experience is made possible because of physical, sensorial, and perceptual similarities with the “other”: seen as Leib, the component experientially based in our living body, which is distinguished from Körper, the physical structure. Subsequently, Heidegger advanced the same distinction in Being and Time (1962) and emphasized the world in which and with which the body as Leib is always engaged and how the body functions in this being-in-the-world (Heidegger 1996).
The prerequisite of any empathic process needed to accurately comprehend others’ states is the self-awareness and sensitivity of our own emotional states (Decety and Moriguchi 2007; Moriguchi and others 2006). At a phenomenologic level, the psychological construct of empathy can be conceived as a primary interaction between individuals, with one experiencing and sharing the feelings of the other. In fact, empathic capacity allows exerting cognitive control and predicting and understanding others’ feelings, motivations, and actions without losing sight of whose feelings belong to whom and behaving accordingly (de Waal and Preston 2017). The empathic experience as a whole is produced by dynamic interactions of functional aspects, such as affective sharing between the self and the other, self-awareness, and cognitive flexibility (Decety and Moriguchi 2007). The crucial self/other distinction is one of the characteristics distinguishing empathy from other forms of “feeling with the other”: empathy presupposes alterity (Box 2). Empathic abilities promote prosocial and cooperative behaviors and enable people to navigate the social world they live in (de Waal and Preston 2017; Preston and de Waal 2002).
Box 2. Simulation versus Self-Other Differentiation.
Because successful social interactions, empathic understanding in particular, rely on evaluating similarities and differences between individuals, an essential aspect of empathy is to recognize the other person as like the self, while maintaining a clear separation between self and other, to keep track of the origin of the feeling. Thus, when speaking of empathy, it is necessary to face two contradicting elements working simultaneously during empathic experience: simulation and self-other differentiation.
Simulation based on the internal models is a “contagious” interaction style, where the self and other are overlapping and close to each other. The simulation theory of empathy holds that humans anticipate and make sense of the behavior of others by activating mental processes that produce similar behavior and emotions. It is considered a “low-level empathy,” putatively based on mirror neuron system (MNS) activation, which allows one to understand the emotions through a simulated shared body state (embodied simulation). The existence of simulative processes in emotion perception is supported by the finding that observing or imagining other’s emotions recruits brain regions (anterior insula and anterior cingulate cortex) involved in representing the emotional feeling and facial mimicry (the spontaneous tendency to mimic other’s facial expressions). Emotional simulation leads to emotional contagion and pain empathy based on the sensorimotor or affective autonomic matching system and on downstream effects in brain areas related to the production of affective experience.
In fact, MNS activation seems to be involved in providing a basic, automatic, and simulative understanding of the bodily expressions of the other person, and some kind of more explicit simulation routine apparently allows one to grasp the mental states that motivate the other’s actions.
The self-other differentiation is somewhat in conflict with simulation theory so that self and other keep their distance from each other. Any experience of true empathy, critically differentiated from emotional contagion, requires the crucial distinction that the primary source of one’s feeling is the perception of someone else’s experience. While failing to uphold a boundary between self and other when seeing another in pain can lead to feelings of personal distress, accurate processes of self-other differentiation underlie perspective taking and prevent our affective state egocentrically biasing how we empathize with others. Self-other differentiation represents a form of cognitive empathy based on perspective taking or meta-cognition, such as theory of mind or “theory theory.”
The co-occurrence in empathy of processes related to simulation and self-other differentiation is supported by developmental findings (Decety 2010). It has been demonstrated that each component of empathy—affective arousal, emotion understanding, and emotion regulation—has its own developmental trajectory. Humans are born with neural circuitries implementing core affect and binding interoceptive and sensory information. Bottom-up processing of affective arousal is present at an early age, is involuntary, and relies on mimicry and resonance between other and self. Such an automatic emotional resonance is based on a tight coupling between perceptual processing and emotion-related neural circuits, as the MNS. Emotion understanding develops during the second year of life. Toddlers respond to others’ distress by engaging in other-oriented empathic reactions and spontaneous helping behaviors. Such an empathic component requires the formation of an explicit representation of the other as a separate agent, and it needs additional computational mechanisms beyond the affect-sharing level. The cognitive components that result in empathic understanding develop later than the affective components, overlap with theory of mind–like processing, allow one to entertain perspective taking, and decouple between first- and second-person information. These processes help to transform the early developing affective empathic experience into other-focused experience, by more fully attaching one’s empathic feelings to a conceptualization of the other’s experience rather than one’s own. Emotional understanding mainly draws on prefrontal circuits.
Emotional regulation enables the control of emotions, develops throughout childhood and adolescence, and parallels the maturation of execution functions. Self-regulation and inhibitory processes recruited for emotional regulation are based on the activity of recursively connected neural regions (the prefrontal cortex, anterior cingulate cortex, amygdala, insula, superior temporal sulcus, and putatively the cerebellum) as well as interoceptive, autonomic, and neuroendocrine processes.
Taken as a whole, these findings suggest that simulation or matching systems that are lower-level autonomic processes are the basis for developing higher-level cognitive empathy, such as theory of mind or perspective taking. Note that neither component can account solely for the potential of empathy, being the intertwining of both that produces the subjective experience of empathy.
It is important to point out that empathy is categorized as affective and cognitive (Shamay-Tsoory and others 2009). Affective empathy refers to the ability to share the state of other persons through observation or imagination of their experience, and as a consequence of sharing the other’s state, there is usually an appropriate isomorphic emotional response. Cognitive empathy refers to the abilities of perspective taking and theory of mind (ToM) that allow predicting and understanding others’ mental states by using cognitive processes.
Empathic affective and cognitive components are mediated by specific and interacting systems, as indicated by the possibility of distinct impairments in the two forms of empathy in specific clinical disorders. For example, schizophrenia, depersonalization, and narcissistic personality disorders are characterized by deficits in affective empathy (Ritter and others 2011; Shamay-Tsoory and Aharon-Peretz 2007), while bipolar disorder and borderline personality traits are associated with impairments in cognitive empathy (Rijnders and others 2021). Even within nonclinical populations, the balance between the capacities of affective and cognitive empathy varies from one individual to another, uniquely defining the empathic experience for each person (Preston and de Waal 2002).
The richness of debates on the neurobiological correlates of empathic experience has been fed by multiple indications, obtained primarily through neuroimaging approaches but also through electrophysiologic and brain stimulation techniques in healthy and pathologic populations. Most research has assessed empathy as a state rather than a trait and has mainly focused on the neocortical activation associated with empathy-eliciting situations. Neuroimaging studies (Bilevicius and others 2018; Gu and others 2012) described consistent activation of neocortical structures specifically associated with each component of empathy. Namely, the ACC and anterior insula are mostly recruited in affective empathy, whereas the medial cingulate cortex and dorsomedial prefrontal cortex are mostly recruited in cognitive empathy.
The two empathic components are topographically distinct even in the cerebellum, with activation mainly in the posterior vermis associated with affective empathy and with activation in the lateral posterior cerebellum (particularly Crus I and Crus II) associated with cognitive empathy. Specifically, fMRI studies have reported that affective empathy for others’ pain was associated with activation of cerebellar lobule VI (Gu and others 2012; Moriguchi and others 2007; Singer and others 2004) and that patients with bilateral lesions of the cerebellar posterior vermis and hemispheres exhibited deficits in cognitive empathy and ToM abilities (Clausi and others 2019; Roldan Gerschcovich and others 2011; Sokolov 2018). Furthermore, cerebellar involvement in cognitive empathy fits with the repeatedly reported cerebellar (Crus I and II) involvement in social cognition (Van Overwalle and others 2014; Van Overwalle and others 2020), and activation of the right Crus I and II has been associated with ToM task performance (King and others 2018).
In a pediatric brain-injured sample, individual differences in cerebellar volumes predicted ToM outcomes, and volumetric reductions in the cerebrocerebellar mentalizing network predicted poor ToM performance (Ryan and others 2017). In children affected by autism spectrum disorders, voxel-based morphometry analyses revealed reduced volumes in the right Crus I and II, and the degree of cerebellar volumetric reductions correlated with the severity of symptoms in the domains of social interaction, communication, and repetitive behavior (D’Mello and others 2015). Furthermore, it has been reported that the size of empathic imbalance between cognitive and affective components positively correlated with autism traits in a neurotypical population (Shalev and Uzefovsky 2020). A recent study noted that patients with bipolar disorder have impaired cognitive empathy and display reduced correlations between empathy and resting-state functional connectivity of the cerebellum (culmen, lobules IV and V; Liang and others 2021). Finally, male perpetrators convicted for intimate partner crimes exhibited less affective empathy related to hyperconnectivity between Crus II and posterior areas of the default mode network (Amaoui and others 2022). Thus, cognitive empathy, in the case that it is predominant on affective empathy, is related to stronger connectivity among areas, including the cerebellum, implicated in interoception, autonomic monitoring, mentalizing, social-cognitive processing, and cognitive flexibility (Cox and others 2012).
Notably, a strong correlation has been described between cognitive flexibility in the social domain and activity in the right posterior cerebellum (lobule VI; Fujino and others 2017). Within a social cognition framework, perspective taking and cognitive flexibility appear to be as fundamental to empathic behaviors as emotional sensitivity and responsiveness. In fact, adopting someone else’s point of view is an effortful component of empathy that requires some form of active inhibitory mechanism (i.e., the deliberate suppression of a cognition or response to achieve an internally represented goal). By suppressing one’s own perspective, the inhibition allows one to evaluate the other’s point of view and engage in perspective taking. In other words, the ability to take another’s conceptual perspective requires cognitive flexibility to be able to generate and consider different possibilities of ideas and responses (Decety and Moriguchi 2007).
In contrast to the numerous functional findings assessing cerebellar involvement in state empathy, only recently was the involvement of cerebellar structures in trait empathy (affective and cognitive) in nonpathologic subjects addressed (Picerni and others 2021). Following the hypothesis that “larger is more powerful” (Box 3), it is reasonable to maintain the position that an increase in the volume of a cerebellar region may result in enhanced function. In a sample of 70 healthy subjects of both sexes, Picerni and others (2021) demonstrated a positive association between the volumes in the right Crus II (and in the pars triangularis of the inferior frontal gyrus) and the Fantasy subscale scores on the Interpersonal Reactivity Index, a well-validated self-report scale of empathy (Figure 5).
Box 3. Larger Is More Powerful?
How the volume of a brain area relates to function is a debated issue. In fact, the amount and synchronicity of neural activity strengthen the most relevant neuronal circuits, while weakening and allowing others to fade. The removal of weaker structures reallocates resources to the remaining structures, allowing them to become stronger and more stable, which is evidence that synaptic activity guides proper pruning. This process of eliminating and strengthening synapses and dendrites shapes the volume of the various brain areas. Based on the assumption that larger populations of neurons can produce larger outputs and therefore be more influential than smaller populations of neurons, a greater-than-average volume may signify greater-than-average power to carry out specific functions. However, a greater-than-average volume may signify a smaller-than-average power; for example, deficient dendritic pruning might render an area suboptimal in terms of a less finely tuned and functionally optimized structure. At the same time, a smaller-than-average volume may be related to increased and more tuned efficiency; for example, cortical thinning due to fewer inhibitory interneurons or enhanced pruning can result in enhanced processing. Furthermore, volumetric changes may stem from differences not only in the number, density, and morphology of various neuronal and glial populations but also in the degree of connectivity of circuits. Thus, the role of a specific region in a specific function also depends on the neuronal activity of many other regions at that same time, a concept named neural context, functional connectivity, or effective connectivity.
In summary, although some negative associations have been reported, the majority of investigations have provided data in favor of a positive association between brain size/volume and brain activity, supporting the “larger is more powerful” position. For example, training on particular tasks or experiencing complex environments increases the volume of functionally related brain structures (Boyke and others 2008; Di Paola and others 2013), providing data in favor of the position that volume tends to positively covary with function.
Figure 5.
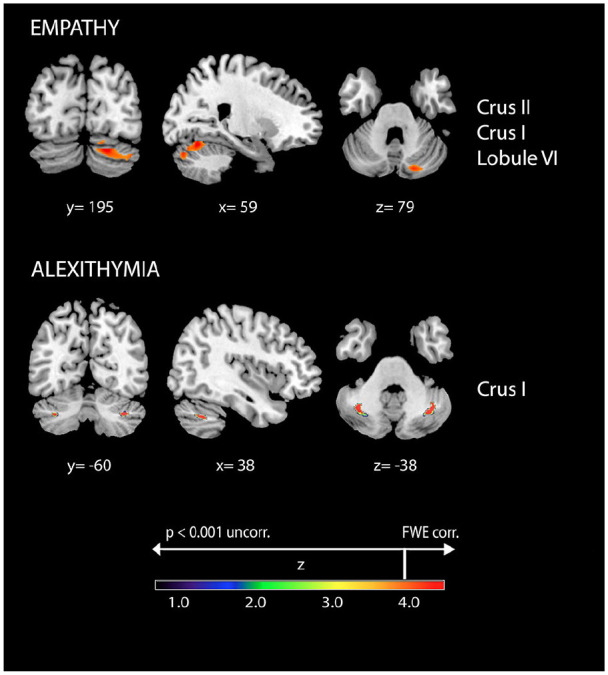
Positive associations between cerebellar gray matter volumes and empathy and alexithymia. Coordinates are in Montreal Neurological Institute space. Z below color bar indicates normalized t values. In figure left is left. FWE = familywise error rate.
Specifically, the Fantasy subscale examines the ability to imaginatively transpose the feelings and actions of fictitious characters in books, movies, and plays onto oneself. It is not surprising that there is empathy for characters, given that readers of narratives and spectators of films usually comprehend the depicted events by mentally representing the emotional states of the characters and assuming their perspectives. The crucial issue for the present discussion on embodied empathy is that the reader’s mental simulation elicits an emotional experience generally congruent with the character’s situation and equivalent to the emotional experiences encountered in the real world. Typically, the changes in the emotional state evoked by taking on emotions from others’ stories are associated with changes in the activity of the autonomous nervous system, providing an embodiment that facilitates an understanding of others’ emotional states (Niedenthal and others 2005; Nummenmaa and others 2014). Ultimately, the emotional narratives help the subjects to optimize decisions and actions, learn about existing or fictive worlds, and stimulate motivation and imagination, thus functioning as a sort of “emotional gym,” in which empathic capacities may be exerted. Thus, empathic abilities are deeply involved in emotional processing not only “online,” when we respond to real emotional objects, but also “offline,” when we represent emotional symbols (Niedenthal and others 2005).
The claim that empathic capacities are associated with interoceptive, autonomic, and somatosensory processes that tend to simulate those of another person can be seen as the basis for embodied empathy.
Cerebellar Internal Models in Empathy
Some characteristics of cerebellar internal models of actions (Box 1) can be extended to cognitive and emotional domains in general and to empathy in particular, and the principles of the predictive brain applied to social aspects of cognition (Brown and Brüne 2012; Imamizu and Kawato 2009) may be reasonably applied to the predictive cerebellum.
During emotion processing, the cerebellum checks whether the individual’s internal state deviates from the expected state, and if the prediction error exceeds a given threshold defined by the context, the cerebellum refines the cortical response and recalibrates the internal model. Thus, the cerebellar contribution to empathic abilities in interactions with real people, or even toward fictional characters, is characterized by forward modeling and error sensitivity that allow anticipating the other’s behavior or one’s own reactions (Sokolov 2018; Van Overwalle and others 2014). When the subject empathizes with other people, the cerebellar forward model potentially generates representations and predictions regarding others’ feelings. By using past interoceptive, motor, and socioemotional experiences of the empathizer, the internal models are developed and framed by the intentions, beliefs, and feelings of the other. The degree of matching between the subject’s and other’s state relies on such representations, but the subject can efficiently match the state of the other to the degree that she or he has preexisting representations for that state, emphasizing the experience-dependence of such an emotional process, which is analogous to what was previously described for the motor domain (Calvo-Merino and others 2006).
Models of mental state inference that incorporate predictive principles, such as Bayesian inferential statistics and generative forward models, can accurately simulate behavior through previous social experience. Specifically, the internal models of other people are decoded within our own motor system, thus forming the basis for ToM.
This proposal fits with recent studies on social interaction that suggest that the cerebellum modulates cortical activity by creating predictions based on similar previous experiences as well as the information received from the mentalizing regions (Sokolov 2018; Sokolov and others 2017; Van Overwalle and others 2020). A predictive model of ToM (Koster-Hale and Saxe 2013) has been proposed to model mental state inference, in which action understanding is acquired by integrating bottom-up information from observed actions and top-down constraints from prior experiences.
Because predictions are based on information sent from the cortex to the cerebellum (efferent copies) and error signals are sent from the cerebellum to the cortex, the coactivation of the cerebellum and neocortical areas is needed to successfully manage any mismatch (Figure 6).
Figure 6.
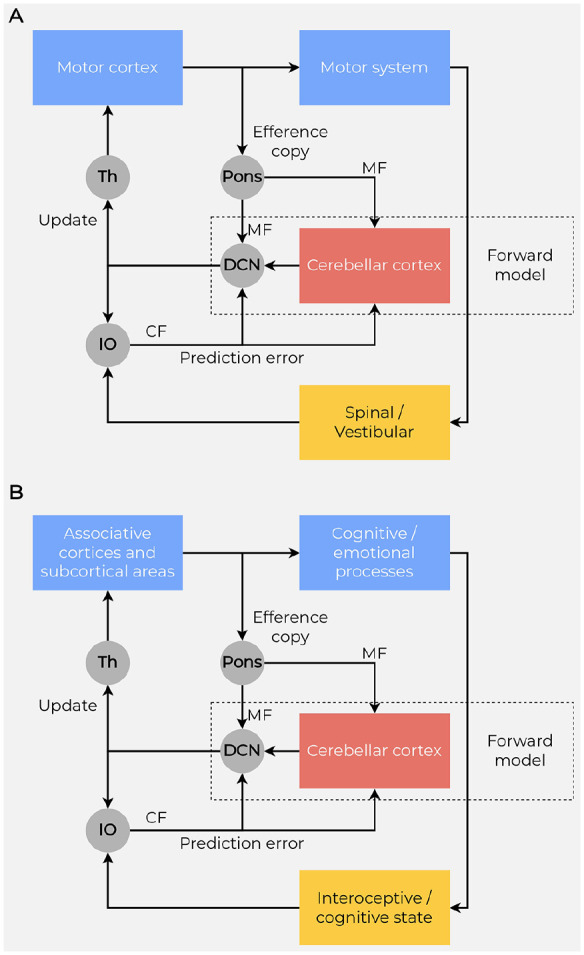
Forward models and prediction. (A) To predict the sensory consequences of actions, a forward model is implemented in the motor cerebellum by interacting with the motor cortex and using efference copies of motor commands, which reach the cerebellum through the mossy fibers (MF) originating in the pontine nuclei (Pons). The difference between the predicted and actual motor outcome (prediction errors) reaches the cerebellum through the climbing fibers (CF) originating in the inferior olive (IO). (B) Because the uniformity of cellular organization across the cerebellar cortex suggests identity in the computations, the cerebellar forward models may even provide computational mechanisms for cognitive/emotional processes. The cerebellum models interoceptive and cognitive prediction errors, given its connectivity with the cingulate cortex, hypothalamus, and amygdala, as well as with frontal and parietal cortices via the thalamus (Th). A copy of the output of the prefrontal and frontal cortex is sent via the pontine nuclei to the interconnected cerebellar lobules. The predictions generated from cerebellar lobules are transmitted from the Purkinje cells via the deep cerebellar nuclei (DCN) and the thalamus back to the same neocortical areas. Predicted and actual consequences of the process copied by these cerebellar lobules are compared in the inferior olive, and any mismatch between the two are fed via climbing fibers to the cerebellar cortex as an error signal. Long-term depression is triggered at the parallel fiber to Purkinje cell synapses, updating the internal model.
The cerebellum has widespread connections with the prefrontal area, which is a key node of the mirror neuron system (MNS; Rizzolatti and Craighero 2004). Given its observation-execution matching properties, the MNS provides the appropriate mechanism for empathy and imitation abilities (Iacoboni 2009) and allows the identification of goals and intentions of others by their resemblance to stored representations for the same states (experience-dependence). Thus, the MNS may facilitate the simulation of behavior, even emotional behavior, of the other (Kaplan and Iacoboni 2006).
It has been postulated that the prefrontal areas are activated when two or more emotional states, such as one’s own and that of the other (in real life or imaginary situations), are simultaneously processed and integrated to form a higher-order empathic state (Preston and de Waal 2002). Reading about or viewing a person who experiences a powerful emotion stimulates mirroring mechanisms and, through the implementation of the cerebellar internal models, forms embodied representations of that emotion grounded in visceral, autonomic, and sensorimotor loops. These embodiment circuitries act as a boost for subsequent socioemotional processes, allowing the remapping of other states into the corresponding subject’s visceral and sensorimotor brain areas, making the subject experience the same emotion of the other (Niedenthal and others 2005; Preston and de Waal 2002).
In summary, in addition to shared neural representations of others’ actions, sensory and emotional experiences can be shared when watching or imagining others. This then brings us closer to a conceptualization of empathy in which we experience not only the cognitive processes of others but also others’ emotional states. It is evident that anticipatory neural responses and predictive coding are crucial to empathetic representations of others’ experiences and consequently have a central role in emotional contagion (Decety and Ickes 2009) and affective and cognitive empathy (Gilbert and Wilson 2007).
Alexithymia as an Embodied Psychological Construct
In the mid-1960s, the psychiatrists J.C. Nemiah and P.E. Sifneos undertook systematic studies of the cognitive style of individuals who found it extremely difficult to describe their subjective feelings. This led Sifneos in 1972 to coin the term alexithymia, which is formed by the roots of several Greek words (a = lack, lexis = word, thymos = emotions) and literally means “lack of words for emotion.” Alexithymia is a construct of personality characterized by impairments in cognitive, emotional, and affective processing (Sifneos 1996; Taylor and Bagby 2004). It describes people with deficiencies in identifying or describing subjective feelings or emotional aspects of social interactions, difficulty in distinguishing between feelings and bodily sensations of emotional arousal, and limited affect-related fantasy and imagery. The marked dysfunction in emotional awareness is accompanied by impairments in social attachment and interpersonal relations (FeldmanHall and others 2013). Although it is not a psychological disorder in itself, alexithymia is associated with an enhanced risk of psychological impairment and is present in a broad spectrum of psychiatric and psychosomatic disorders, such as chronic pain, somatoform disorders, addictive behaviors, eating disorders, autism, anxiety, and depression (Taylor and Bagby 2004). People with high levels of alexithymia also have difficulty distinguishing and appreciating the emotions of others, which leads to unempathic and inadequate emotional responses (Moriguchi and others 2009).
Given that people with alexithymic traits have a tendency to focus on facts without affective involvement rather than inner experiences (Laricchiuta and others 2022; Taylor and Bagby 2004; ) and their attention is mainly focused externally, the symptoms of alexithymia have been attributed to alterations in interoception (Terasawa and others 2021), although research had proposed conflicting hypotheses on the direction of such alterations. One line of research has proposed that alexithymic individuals might be defective in noticing bodily signals and in interpreting interoceptive inputs (Rozenstein and others 2011), in support of the suggestion of a higher occurrence of alexithymic symptoms in clinical disorders associated with poor interoception, such as eating disorders. Conversely, another line of research proposed that alexithymic individuals might be characterized by heightened interoceptive abilities, leading them to “get trapped” at the level of bodily sensations without achieving symbolic representation of emotion, in support of the suggestion of a higher occurrence of alexithymic symptoms in clinical disorders based on misinterpretation of emotion-related visceral changes, such as somatization disorders (Sifneos 1996; Taylor and Bagby 2004). This approach fits the theoretical construct of emotion proposed by Lane and Schwartz (1987) in which emotional awareness can be graded in different “levels” derived from an integration of the cognitive development theory of Piaget (1981). In his model, awareness of physiologic cues and awareness of action tendencies are graded at a low level and are the basis for high cognitive levels of emotional awareness, for example allowing the differentiation of emotions. Accordingly, it has been reported that people with high levels of alexithymia have a low level of emotional awareness and even their high cognitive awareness is impaired, as if they “stagnate” in the development of empathy competencies, as remarkably formulated by Moriguchi and Komaki (2013). In fact, neuroimaging studies in subjects with high alexithymic traits have shown reduced activation in brain areas associated with emotional awareness, such as the ACC, fusiform gyrus, amygdala, parahippocampal gyrus, and insula (Nadeau 2021), and enhanced MNS activation (Moriguchi and others 2009). With regard to volumetric variations, negative correlations between alexithymia scores and volumes of the amygdala, insula, and ACC were described (Laricchiuta and others 2015), supporting the view that in alexithymia, the altered processing of emotional stimuli is accompanied by a reduction in activity and volume in limbic structures. More relevant to the focus of the present review, significant alterations in the activity (Moriguchi and others 2009) or volume (Laricchiuta and others 2015) of the cerebellum have been noted in individuals with alexithymia. Namely, alexithymia scores were positively associated with volumes in bilateral Crus I (Figure 5) and negatively associated with volumes in limbic and paralimbic areas (amygdala, insula, and parahippocampal gyrus; Laricchiuta and others 2015). These findings are consistent with the cerebellar activation (Crus I and lobules VI and VIIb) negatively correlated with the activation of limbic and paralimbic areas (parahippocampal gyrus, ACC, hypothalamus; Moulton and others 2011). Such an inverse link between the cerebellum and limbic system suggests that the increased volumes of Crus I could result in an enhanced inhibitory output of Purkinje cells on the deep cerebellar nuclei, thus reducing their excitatory output on extracerebellar targets including the limbic system (Bostan and others 2013). The limbic and paralimbic structures in turn could undergo a volumetric reduction because of the diminished activation level. Structural neuroimaging studies on patients affected by obsessive-compulsive disorder indicated reduced ACC volumes associated with enhanced cerebellar volumes (de Wit and others 2014), offering an additional insight into such a reciprocal structural relation between the cerebellum and the limbic and paralimbic areas. Notably, people with high alexithymic traits show reduced neural responses in the limbic system to external and internal emotional stimuli and, conversely, increased neural responses in somatosensory and sensorimotor areas to stimuli closely associated with physical information (Moriguchi and Komaki 2013), in line with their tendency to rely on or amplify physical symptoms. Notably, although the connectivity between the cerebellum and limbic system remains a disputed issue and further studies are needed to better define the putative limbic-related cerebellar regions (for details, see Jung and others 2022), the network comprising the cerebellum and limbic system is thought to be involved in monitoring physiologic bodily conditions (Critchley and Garfinkel 2017; Moulton and others 2011) and representing interoception within the context of ongoing activities.
Once more, the conceptualization of the cerebellum as an “embodying machine” emerges from the cerebellar property in forming internal models based on interoceptive and exteroceptive information.
Concerning alexithymia, the framework of the embodied predictive interoception coding model (Barrett and Simmons 2015) follows the top-down flow of information; as such, the limbic cortical regions, encompassing the cingulate cortex and insula, do not originate predictions. The prediction errors computed by the cerebellum are not adequately processed by the cortical areas; thus, it is not possible to modulate ongoing visceromotor actions and infer the likely causes of the upcoming sensory events. In other words, the cortical outputs do not exert relevant control over the cerebellar processing of interoceptive functions, producing a loss in flexibility of emotional responses and affective learning. Following the bottom-up flow of information, the interoceptive signal is not relocated from the cerebellar structures to the limbic areas, which manage the emotional responses, and neocortical regions, which manage the emotional experience. Interestingly, the enhanced volumes in Crus I described in subjects with high alexithymic traits may be related to the enduring work of the cerebellum that continues to provide prediction errors to noncollaborating cortical areas.
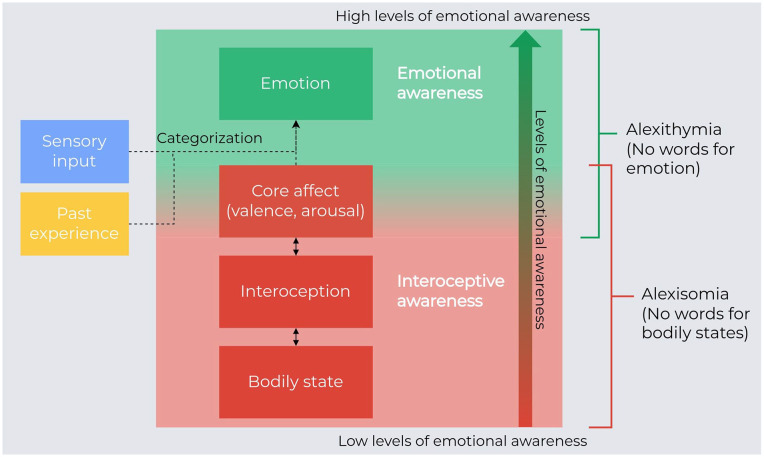
Finally, the conceptualization of alexithymia has to consider the intriguing phenomenon called alexisomia, describing the difficulty in the interoceptive awareness (Moriguchi and Komaki 2013). Considering the bottom-up component of emotional control, the altered awareness of bodily states featuring the alexisomia might be the low level of altered emotional awareness featuring alexithymia. If interoceptive awareness is the basis of emotional awareness, alexithymia (difficulty in emotional awareness) and alexisomia (difficulty in interoceptive awareness) are closely connected (Figure 7).
Figure 7.
Emotional awareness and putative mechanism of alexithymia and alexisomia. Proprioceptive, visceral, hormonal, immune, and metabolic signals on the bodily physiologic state constitute interoception, which is the basis of the core affect. The core affect represents the basic affective state with specific properties of valence (pleasure or displeasure) and arousal (agitation or calmness). Emotions are constructed and categorized through bodily internal information (core affect), information from past experiences, and external sensory information (visual, auditory, olfactive). Emotional awareness has different levels: at the lower level, there is the interoceptive awareness, which is strongly connected to bodily state and core affect. Difficulty in interoceptive awareness is called alexisomia. At higher levels of emotional awareness, there is the categorization process that integrates the three sources of information and constructs an emotional state. The difficulty in categorization results in a reduced cognitive awareness, which is called alexithymia.
Conclusively, alexithymia might result from altered computation of the interoceptive information that remains embodied (trapped) and is not expressed or cognitively described.
Conclusions
Current psychological discourse has conceptualized emotions and psychological traits as embodied phenomena, suggesting that the body supports the mind in shaping emotional and cognitive responses. The main models of embodiment describe the self as an integration of the social or conceptual self with our physical self and suggest that emotional and psychological functions are not independent of sensorimotor functions. The idea of a strong mutual interaction between the fields of embodiment and cerebellar functionality may be fruitfully applied to explain the cerebellar contribution to emotions. Reminiscent of the seminal concept of the cerebellum as a neuronal machine (Eccles and others 1967), it is intriguing to propose the cerebellum as an embodying machine that provides internal models to integrate bodily information and emotional responses.
Footnotes
Author Contributions: All authors wrote and edited the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was partially funded by the Italian Ministry of Health, Ricerca Corrente, to L.P.
ORCID iDs: Laura Petrosini  https://orcid.org/0000-0001-7464-5168
https://orcid.org/0000-0001-7464-5168
Eleonora Picerni  https://orcid.org/0000-0002-7440-410X
https://orcid.org/0000-0002-7440-410X
Andrea Termine  https://orcid.org/0000-0003-4374-7430
https://orcid.org/0000-0003-4374-7430
References
- Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, and others. 2017. Consensus paper: cerebellum and emotion. Cerebellum 16:552–76. [DOI] [PubMed] [Google Scholar]
- Amaoui S, Marín-Morales A, Martín-Pérez C, Pérez-García M, Verdejo-Román J. 2022. Social mentalizing in male perpetrators of intimate partner violence against women is associated with resting-state functional connectivity of the Crus II. J Psychiatr Res 150:264–71. [DOI] [PubMed] [Google Scholar]
- Argyropoulos GPD. 2016. The cerebellum, internal models and prediction in “non-motor” aspects of language: a critical review. Brain Lang 161:4–17. [DOI] [PubMed] [Google Scholar]
- Barrett LF. 2017. The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci 12:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, Hamilton P. 2016. An active inference theory of allostasis and interoception in depression. Phil Trans R Soc B 371:20160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK. 2015. Interoceptive predictions in the brain. Nat Rev Neurosci 16:419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW. 2008. Grounded cognition. Annu Rev Psychol 59:617–45. [DOI] [PubMed] [Google Scholar]
- Baumann O, Mattingley JB. 2012. Functional topography of primary emotion processing in the human cerebellum. NeuroImage 61:805–11. [DOI] [PubMed] [Google Scholar]
- Bilevicius E, Kolesar T, Smith S, Trapnell P, Kornelsen J. 2018. Trait emotional empathy and resting state functional connectivity in default mode, salience, and central executive networks. Brain Sciences 8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. 2013. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci 17:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. 2008. Training-induced brain structure changes in the elderly. J Neurosci 28:7031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Brüne M. 2012. The role of prediction in social neuroscience. Front Hum Neurosci 6:147. doi: 10.3389/fnhum.2012.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106: 2322–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Merino B, Grèzes J, Glaser DE, Passingham RE, Haggard P. 2006. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr Biol 16:1905–10. [DOI] [PubMed] [Google Scholar]
- Clark A. 2013. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci 36:181–204. [DOI] [PubMed] [Google Scholar]
- Clausi S, Olivito G, Lupo M, Siciliano L, Bozzali M, Leggio M. 2019. The cerebellar predictions for social interactions: theory of mind abilities in patients with degenerative cerebellar atrophy. Front Cell Neurosci 12:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombetti G. 2021. The embodiment of emotion. In: A multidisciplinary approach to embodiment: understanding human being. Milton (UK): Routledge. Chapter 11. [Google Scholar]
- Colombetti G, Thompson E. 2008. The feeling body: towards an enactive approach to emotion. In: Developmental perspectives on embodiment and consciousness. New York (NY): Lawrence Erlbaum Associates. Chapter 3. [Google Scholar]
- Cox CL, Uddin LQ, Di Martino A, Castellanos FX, Milham MP, Kelly C. 2012. The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain’s intrinsic functional dynamics. Soc Cogn Affect Neurosci 7:727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. 2014. How do you feel? Princeton (NJ): Princeton University Press. [Google Scholar]
- Critchley HD, Garfinkel SN. 2017. Interoception and emotion. Curr Opin Psychol 17:7–14. [DOI] [PubMed] [Google Scholar]
- Damasio A, Carvalho GB. 2013. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci 14:143–52. [DOI] [PubMed] [Google Scholar]
- Damasio AR. 1996. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 351:1413–20. [DOI] [PubMed] [Google Scholar]
- Darwin C. 1872. The expression of the emotions in man and animals. London (UK): John Murray [Google Scholar]
- Decety J. 2010. The neurodevelopment of empathy in humans. Dev Neurosci 32:257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Ickes W, editors. 2009. The social neuroscience of empathy. Cambridge (MA): MIT Press. [Google Scholar]
- Decety J, Moriguchi Y. 2007. The empathic brain and its dysfunction in psychiatric populations: implications for intervention across different clinical conditions. Biopsychosocial Med 1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FBM, Preston SD. 2017. Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci 18:498–509. [DOI] [PubMed] [Google Scholar]
- de Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchón JM, and others. 2014. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. AJP 171:340–9. [DOI] [PubMed] [Google Scholar]
- Di Paola M, Caltagirone C, Petrosini L. 2013. Prolonged rock climbing activity induces structural changes in cerebellum and parietal lobe: cerebellum modifications in expert rock climbers. Hum Brain Mapp 34:2707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello AM, Crocetti D, Mostofsky SH, Stoodley CJ. 2015. Cerebellar gray matter and lobular volumes correlate with core autism symptoms. Neuroimage Clin 7:631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S, Barrett LF. 2007. Affect is a form of cognition: a neurobiological analysis. Cogn Emot 21:1184–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentágothai J. 1967. The cerebellum as a neuronal machine. Berlin (Germany): Springer Berlin Heidelberg. [Google Scholar]
- FeldmanHall O, Dalgleish T, Mobbs D. 2013. Alexithymia decreases altruism in real social decisions. Cortex 49: 899–904. [DOI] [PubMed] [Google Scholar]
- Friston K. 2010. The free-energy principle: a unified brain theory? Nat Rev Neurosci 11:127–38. [DOI] [PubMed] [Google Scholar]
- Fujino J, Tei S, Jankowski KF, Kawada R, Murai T, Takahashi H. 2017. Role of spontaneous brain activity in explicit and implicit aspects of cognitive flexibility under socially conflicting situations: a resting-state fMRI study using fractional amplitude of low-frequency fluctuations. Neuroscience 367:60–71. [DOI] [PubMed] [Google Scholar]
- Gilbert DT, Wilson TD. 2007. Prospection: experiencing the future. Science 317:1351–4. [DOI] [PubMed] [Google Scholar]
- Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, and others. 2012. Anterior insular cortex is necessary for empathetic pain perception. Brain 135:2726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, Fan J. 2013. Anterior insular cortex and emotional awareness: anterior insular cortex and emotional awareness. J Comp Neurol 521:3371–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell X, Gabrieli JD, Schmahmann JD. 2018. Embodied cognition and the cerebellum: perspectives from the dysmetria of thought and the universal cerebellar transform theories. Cortex 100:140–8. [DOI] [PubMed] [Google Scholar]
- Habas C. 2018. Research note: a resting-state, cerebello-amygdaloid intrinsically connected network. Cerebellum Ataxias 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, and others. 2009. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidegger M. 1996. Being and time. Stambaugh J, editor. Albany (NY): State University of New York Press [Google Scholar]
- Hoche F, Guell X, Sherman JC, Vangel MG, Schmahmann JD. 2016. Cerebellar contribution to social cognition. Cerebellum 15:732–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Nagao S, Hashimoto Y, Ishikawa K, Yokota T, Mizusawa H, and others. 2018. Tandem internal models execute motor learning in the cerebellum. Proc Natl Acad Sci U S A 115:7428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z. 2021. Aristotle’s “De Anima”—theoretical significance in contemporary cognitive psychology. Qingdao, China. In: Proceedings of the 2021 2nd International Conference on Mental Health and Humanities Education. doi: 10.2991/assehr.k.210617.027 [DOI] [Google Scholar]
- Iacoboni M. 2009. Imitation, empathy, and mirror neurons. Annu Rev Psychol 60:653–70. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Kawato M. 2009. Brain mechanisms for predictive control by switching internal models: implications for higher-order cognitive functions. Psychol Res 73:527–44. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Kawato M. 2012. Cerebellar internal models: implications for the dexterous use of tools. Cerebellum 11:325–35. [DOI] [PubMed] [Google Scholar]
- Ito M. 2008. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci 9:304–13. [DOI] [PubMed] [Google Scholar]
- James W. 1884. What is an emotion? Mind. Vol. os-IX. [Google Scholar]
- James W, Burkhardt F, Bowers F, Skrupskelis IK. 1890. The principles of psychology. London (UK): Macmillan. [Google Scholar]
- Jung SJ, Vlasov K, D’Ambra AF, Parigi A, Baya M, Frez EP, and others. 2022. Novel cerebello-amygdala connections provide missing link between cerebellum and limbic system. Front Syst Neurosci 16:879634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JT, Iacoboni M. 2006. Getting a grip on other minds: mirror neurons, intention understanding, and cognitive empathy. Soc Neurosci 1:175–83. [DOI] [PubMed] [Google Scholar]
- Kawato M, Cortese A. 2021. From internal models toward metacognitive AI. Biol Cybern 115:415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato M, Kuroda T, Imamizu H, Nakano E, Miyauchi S, Yoshioka T. 2003. Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog Brain Res 142:171–88. [DOI] [PubMed] [Google Scholar]
- Kawato M, Ohmae S, Hoang H, Sanger T. 2021. 50 years since the Marr, Ito, and Albus models of the cerebellum. Neuroscience 462:151–74. [DOI] [PubMed] [Google Scholar]
- King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J. 2018. A multi-domain task battery reveals functional boundaries in the human cerebellum. Neuroscience. doi: 10.1101/423509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster-Hale J, Saxe R. 2013. Theory of mind: a neural prediction problem. Neuron 79:836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Schwartz GE. 1987. Levels of emotional awareness: a cognitive-developmental theory and its application to psychopathology. Am J Psychiatry 144(2):133–43. [DOI] [PubMed] [Google Scholar]
- Laricchiuta D, Petrosini L, Picerni E, Cutuli D, Iorio M, Chiapponi C, and others. 2015. The embodied emotion in cerebellum: a neuroimaging study of alexithymia. Brain Struct Funct 220:2275–87. [DOI] [PubMed] [Google Scholar]
- Laricchiuta D, Termine A, Fabrizio C, Passarello N, Greco F, Piras F, and others. 2022. Only Words Count; the Rest Is Mere Chattering: A Cross-Disciplinary Approach to the Verbal Expression of Emotional Experience. Behav Sci 12:292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Brown R. 2017. A higher-order theory of emotional consciousness. Proc Natl Acad Sci U S A 114:E2016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Zhou S, Zhang Y, Cai X, Wang Y, Cheung EFC, and others. 2021. Altered empathy-related resting-state functional connectivity in patients with bipolar disorder. Eur Arch Psychiatry Clin Neurosci. [DOI] [PubMed] [Google Scholar]
- Manni E, Petrosini L. 2004. A century of cerebellar somatotopy: a debated representation. Nat Rev Neurosci 5:241–9. [DOI] [PubMed] [Google Scholar]
- Monti A, Porciello G, Panasiti MS, Aglioti SM. 2021. The inside of me: interoceptive constraints on the concept of self in neuroscience and clinical psychology. Psycholog Res. doi: 10.1007/s00426-021-01477-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, and others. 2007. Empathy and judging other’s pain: an fMRI study of alexithymia. Cerebral Cortex 17: 2223–34. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Komaki G. 2013. Neuroimaging studies of alexithymia: physical, affective, and social perspectives. Biopsychosocial Med 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Decety J, Hirakata M, Maeda M, Matsuda H, and others. 2009. The human mirror neuron system in a population with deficient self-awareness: an fMRI study in alexithymia. Hum Brain Mapp 30:2063–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Lane RD, Maeda M, Mori T, Nemoto K, and others. 2006. Impaired self-awareness and theory of mind: an fMRI study of mentalizing in alexithymia. Neuroimage 32:1472–82. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. 2011. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci 31:3795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau SE. 2021. Neural mechanisms of emotions, alexithymia, and depression. Handbook Clin Neurol 183:299–313. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM, Barsalou LW, Winkielman P, Krauth-Gruber S, Ric F. 2005. Embodiment in attitudes, social perception, and emotion. Pers Soc Psychol Rev 9:184–211. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Saarimäki H, Glerean E, Gotsopoulos A, Jääskeläinen IP, Hari R, and others. 2014. Emotional speech synchronizes brains across listeners and engages large-scale dynamic brain networks. NeuroImage 102: 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosini L, Cutuli D, Picerni E, Laricchiuta D. 2017. Viewing the personality traits through a cerebellar lens: a focus on the constructs of novelty seeking, harm avoidance, and alexithymia. Cerebellum 16:178–90. [DOI] [PubMed] [Google Scholar]
- Piaget J. 1981. Intelligence and affectivity: their relationship during child development. Brown TA, Kaegi CE, Rosenzweig MR, editors. Palo Alto (CA): Annual Reviews Inc. [Google Scholar]
- Picazio S, Foti F, Oliveri M, Koch G, Petrosini L, Ferlazzo F, and others. 2020. Out with the old and in with the new: the contribution of prefrontal and cerebellar areas to backward inhibition. Cerebellum 19:426–36. [DOI] [PubMed] [Google Scholar]
- Picerni E, Laricchiuta D, Piras F, Vecchio D, Petrosini L, Cutuli D, and others. 2021. Macro- and micro-structural cerebellar and cortical characteristics of cognitive empathy towards fictional characters in healthy individuals. Sci Rep 11:8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FBM. 2002. Empathy: its ultimate and proximate bases. Behav Brain Sci 25:1–20. [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Jirenhed D-A, Zucca R, Johansson F, Svensson P, Hesslow G. 2013. Number of spikes in climbing fibers determines the direction of cerebellar learning. J Neurosci 33:13436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnders RJP, Terburg D, Bos PA, Kempes MM, van Honk J. 2021. Unzipping empathy in psychopathy: empathy and facial affect processing in psychopaths. Neurosci Biobehav Rev 131:1116–26. [DOI] [PubMed] [Google Scholar]
- Ritter K, Dziobek I, Preißler S, Rüter A, Vater A, Fydrich T, and others. 2011. Lack of empathy in patients with narcissistic personality disorder. Psychiatry Res 187:241–7. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. 2004. The mirror-neuron system. Annu Rev Neurosci 27:169–92. [DOI] [PubMed] [Google Scholar]
- Roldan Gerschcovich E, Cerquetti D, Tenca E, Leiguarda R. 2011. The impact of bilateral cerebellar damage on theory of mind, empathy and decision making. Neurocase 17:270–5. [DOI] [PubMed] [Google Scholar]
- Rozenstein MH, Latzer Y, Stein D, Eviatar Z. 2011. Perception of emotion and bilateral advantage in women with eating disorders, their healthy sisters, and nonrelated healthy controls. J Affect Disord 134:386–95. [DOI] [PubMed] [Google Scholar]
- Ruggiero F, Dini M, Cortese F, Vergari M, Nigro M, Poletti B, and others. 2022. Anodal transcranial direct current stimulation over the cerebellum enhances sadness recognition in Parkinson’s disease patients: a pilot study. Cerebellum 21:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NP, Catroppa C, Beare R, Silk TJ, Hearps SJ, Beauchamp MH, and others. 2017. Uncovering the neuroanatomical correlates of cognitive, affective and conative theory of mind in paediatric traumatic brain injury: a neural systems perspective. Soc Cogn Affect Neurosci 12:1414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NB. 2010. Multimodal integration in granule cells as a basis for associative plasticity and sensory prediction in a cerebellum-like circuit. Neuron 66:573–84. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. 2021. Emotional disorders and the cerebellum: neurobiological substrates, neuropsychiatry, and therapeutic implications. In: Handbook of Clinical Neurology. Vol. 183. Amsterdam (Netherlands): Elsevier. p. 109–54. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. 1997. The cerebrocerebellar system. Int Rev Neurobiol 41:31–60. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg JB, Sherman JC. 2007. The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum 6:254–67. [DOI] [PubMed] [Google Scholar]
- Schraa-Tam CKL, Rietdijk WJR, Verbeke WJMI, Dietvorst RC, van den Berg WE, Bagozzi RP, and others. 2012. fMRI activities in the emotional cerebellum: a preference for negative stimuli and goal-directed behavior. Cerebellum 11:233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semin GR, Smith ER. eds. 2008. Embodied grounding: social, cognitive, affective, and neuroscientific approaches. Cambridge (UK): Cambridge University Press. [Google Scholar]
- Seth AK. 2013. Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci 17:565–73. [DOI] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. 2012. An interoceptive predictive coding model of conscious presence. Front Psychology 2:395. doi: 10.3389/fpsyg.2011.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Uzefovsky F. 2020. Empathic disequilibrium in two different measures of empathy predicts autism traits in neurotypical population. Mol Autism 11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J. 2007. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia 45:3054–67. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. 2009. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132:617–27. [DOI] [PubMed] [Google Scholar]
- Sifneos PE. 1996. Alexithymia: past and present. Am J Psychiatry 153:137–42. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. 2004. Empathy for pain involves the affective but not sensory components of pain. Science 303:1157–62. [DOI] [PubMed] [Google Scholar]
- Sokolov AA. 2018. The cerebellum in social cognition. Front Cell Neurosci 12:145. [Google Scholar]
- Sokolov AA, Miall RC, Ivry RB. 2017. The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci 21:313–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. 2010. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46:831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strejcek B, Zhong C-B. 2014. Morality in the body. In: The Routledge handbook of embodied cognition. New York (NY): Routledge/Taylor & Francis Group. p. 220–30. [Google Scholar]
- Strick PL, Dum RP, Fiez JA. 2009. Cerebellum and nonmotor function. Annu Rev Neurosci 32:413–34. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Ishikawa T, Kakei S. 2019. Neural evidence of the cerebellum as a state predictor. Cerebellum 18:349–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM. 2004. New trends in alexithymia research. Psychother Psychosom 73:68–77. [DOI] [PubMed] [Google Scholar]
- Terasawa Y, Oba K, Motomura Y, Katsunuma R, Murakami H, Moriguchi Y. 2021. Paradoxical somatic information processing for interoception and anxiety in alexithymia. Euro J Neurosci 54:8052–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K, Mariën P, Vandekerckhove M. 2014. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. NeuroImage 86:554–72. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Manto M, Cattaneo Z, Clausi S, Ferrari C, Gabrieli JD, and others. 2020. Consensus paper: cerebellum and social cognition. Cerebellum 19:833–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Kang J, Johnson TD, Nichols TE, Satpute AB, Barrett LF. 2015. A Bayesian model of category-specific emotional brain responses. PLoS Comput Biol 11:e1004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-N, Yung W-H, Kwok-Chong Chow B, Chan Y-S, Wang J-J. 2006. The cerebellar-hypothalamic circuits: potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res Rev 52:93–106. [DOI] [PubMed] [Google Scholar]