Abstract
Introduction:
Microplastics may be present in food and drinks from various sources, exposing pregnant women to these particles. Consumption of contaminated food can lead to the ingestion of microplastics by pregnant women, potentially causing adverse health effects on the fetus. This study aims to investigate the presence of microplastics in the stools of pregnant women.
Methods:
The research was conducted in the Makassar City region of South Sulawesi, Indonesia. Thirty healthy pregnant women from 2 community health centers, Pattingalloang and Jumpandang Baru, participated in the study. Their stools were analyzed using Fourier Transform Infrared (FTIR) microspectroscopy to detect the presence of microplastics.
Result:
The analysis revealed the presence of a total of 359 microplastics in the participants’ stools, with particle counts ranging from 4 to 21 and sizes ranging from 0.2 to 4.9 mm per 25 g of stool. The polymers identified included Polyethylene Terephthalate (PET), Polyamide/Nylon, Polyethylene Chlorinated, HDPE, and Ethylene Propylene. The amount of microplastics varied significantly among groups with different levels of seafood consumption.
Conclusion:
Indonesian pregnant women have been exposed to some microplastic polymers.
Keywords: Microparticles, plastics, feces, pregnancy
Introduction
Microplastics (MPs) are present in all marine environments, from the coastline and ocean surface to the depths, and are easily absorbed by small marine organisms such as fish and shellfish, potentially causing harm. 1 MPs are also widespread in both freshwater and terrestrial ecosystems.2,3 Large (macro) objects, including bottles, wrappers, plastic straws, cartons, and cosmetic products highly resistant to degradation, all contribute to their ubiquitous presence. 4 Over 690 marine species have been affected by microplastic contamination, which has been detected in their digestive tracts and may also pose a risk to humans.5,6
Numerous studies have demonstrated that microplastics enter the human body by crossing the food chain via food, beverages, 7 and air 8 through inhalation and ingestion. 9 Microplastics have been monitored in various commodities, such as drinking water,10,11 salt,12 -16 and rice. 17 Adverse effects can occur on human health after consuming microplastic-contaminated foods or beverages. There are 2 degraded plastic materials, microplastic <5 mm and nanoplastic <0.1 μm. Micrometer-sized plastic is more easily digested, while nanometer-sized particles can pass through cell membranes. 8 Once microplastics are ingested, they can be transported throughout various organ tissues and expelled through pseudo-feces or collected in certain bodily tissues. Accumulation of microplastics in body tissues can lead to many detrimental effects on an organism’s health, such as infertility, stunted growth, internal or external damage, and blockage of body tracts. 1
Pregnant women are a susceptible group to pollutants. Most of what is recognized about the effects of MPs on the health of pregnant women and fetal development is based on animal studies. Various evidence of toxicity in animal models has been observed. It was discovered that MPs interact with the production of energy and lipid metabolism, thereby increasing oxidative stress and neurotoxic reactions. 18 Their presence in cell cultures has been linked to toxic effects, including apoptosis, inflammation, mitochondrial and lysosomal dysfunction, and genotoxicity. 19 Their interference with the immune system has seemed detrimental, with genetic modifications to the expression of immune response-related genes. As evidenced by brain abnormalities in the offspring of mice whose mothers were fed plastic microparticles, 20 MPs can alter the phenotype and the expression of genes and epigenetics in mice. The exposure to MPs during the newborn period is associated with the onset of several diseases in adulthood. Despite the limited amount of studies on children’s exposure to MPs, it is evident that exposure to MPs and other plastic additives during crucial developmental periods leads to significant alterations in the digestive, reproductive, central nervous, immune, and circulatory systems of a kid. 21
Jeong et al 20 demonstrated that maternal exposure to polystyrene nano plastics (ie, plastic fragments with a size of 100 nm) during pregnancy and lactation altered the neural cell compositions and brain histology of offspring. Polystyrene (PS) nanoparticles also activated molecular and functional problems in neural cells cultured in vitro. Mice with abnormal brain development induced by exposure to elevated levels of polystyrene nanoplastics display gender-specific neurophysiological and cognitive deficits. Adult offspring of female mice exposed to polybrominated diphenyl ethers exhibit short- and long-term deficits in social recognition, decreased sociability, and elevated repetitive behavior. 22 Exposure to 2 different microplastic sizes in pregnant women increases the risk of metabolic disorders for the fetus.
In recent years, several studies have been conducted in various countries to investigate the extent of microplastic contamination in humans. These studies have provided valuable insights into the prevalence of microplastics in different populations and the potential risks to human health associated with exposure to these pollutants. Some warranted the presence of microplastics in the human body through stool samples. 23 However, related studies from Indonesia are still limited24-27 and all were conducted in the general population.
Susceptible groups, like pregnant women and children, are an important target. Thus, this study aims to identify the existence of microplastics in the feces of pregnant women in Indonesia. This study is the first study in Indonesia exploring MPs in pregnant women; hence, it will provide new information about chemical exposure to pregnant women.
Methods
Sample collection
For this study, 30 pregnant women from 2 community health centers in Makassar—the Pattingalloang and Jumpandang Baru Health Centers—were included voluntarily. They were included with some inclusion criteria: pregnant mothers who had visited the health centers and checked up there since the beginning of their pregnancy and lived in Makassar city. The women who had a medical indication (diarrhea) and felt disgusting to get their stool were excluded from the study.
A glass bottle was distributed to each of them, and they were asked to put around 25 mg of their morning stool in it. The enumerators explain the approximate limit for the number of stools required in the bottle to the respondents. Those who had constipation might take their stool anytime. Enumerators collected the stool-filled bottle within 2 to5 days. The pregnant women also were interviewed using a questionnaire to get information on their characteristics. A Food Frequency Questionnaire (FFQ) regarding seafood consumption within the last year was applied to obtain their consumption behavior.
Microplastic analysis
Feces extraction
Extraction was applied to the feces before observing the microplastic. As much as 12.5 ml 1% Phenol solution (Merck, Germany) and 62.5 ml distilled water (Merck, Germany) were mixed with the feces and stirred with a stir bar and vortex. Phenol Solution was half the stool weight and acted as an antibacterial. The mixtures were poured into a petri dish covered with aluminum foil and incubated at 60°C for 48 hours. After drying, the feces were crushed and put into a 200 ml jar glass bottle.
Microplastic characterization using microscopy
The dry feces were then dissolved with Kalium Hydroxide (KOH) (Merck, Germany) 10% with a ratio of 1:3 and kept for 2 weeks until they fused and changed from solid to colloidal. The sample was observed under a stereomicroscope (Euromex SB-1902, Arnhem, The Netherlands) with a magnification of 25x for bigger particles and 45x for smaller particles. The microplastics were separated using a pin and were classified according to their size, shape, and color according to the GESAMP guidelines.28,29 Microplastics discovered were photographed, counted, and measured using Image J.
Microplastic identification using FTIR
Some MPs were selected based on the variations of color and shape and further subjected to Fourier Transformed Infra-Red Spectroscope (FT-IR Shimadzu Prestice-21, Japan) for plastics polymer identification. The selected MPs were kept between the cover glass and mixed with kalium bromide UV-sol (KBr) with a ratio of 1:10, then crushed and compressed in stainless steel dish (1 cm2) to produce a pellet. The pellet should be transparent. Pellets were then subjected to FT-IR, which emitted infrared radiation that turned out as spectrum values or wavelengths number. Using the method described by Primpke et al, 30 the spectra were recorded in absorbance mode in the range of 4000 to 400 cm−1. Percentage values of polymer types were obtained by grouping similar spectrums of all MPs samples processed. The Attenuated total reflectance (ATR) spectra were processed online with Open Specy, an open-access software produced by OFSHOME. 31
Quality assurance and control
Several procedures were implemented to ensure the absence of contamination in the samples. Stool samples were taken using the aluminum spoon and glass bottles with aluminum cover. In the laboratory, all materials were covered with aluminum foil until used. Likewise, samples were covered with aluminum foil before and after use. Each equipment component was washed with distilled water prior to utilization. Additionally, dust was eliminated from the visual observation workstation of the MPs before the identification process commenced. Blanks as control that the environment in the laboratory was maintained and free from microplastic were made. The blank uses a petri dish containing distilled water and was placed in the laboratory during the analysis process. Blanks were placed next to the work area during sample processing to measure possible contamination from the surrounding environment.
No traces of microplastic were found in the sample blanks of the airborne controls. Therefore, it is assumed that contamination does not affect the observation of microplastics in the stool samples.
Statistical analysis
The mean difference of MPs’ amounts was analyzed according to seafood consumption frequency, amount, and type. We categorize seafood consumption into 2 groups: frequently (more than 3x/week) and rarely (less than 3x/week). Then, the seafood consumption amount is categorized into 3 groups based on the Food and Drug Administration (FDA) recommendation on fish consumption for the pregnant group. 32 including high >12 once/week, middle 8 to 12 once/week, and low <8 once/week. The mean difference was analyzed using a t-test for the variable within 2 groups, one-way ANOVA for variables within 3 groups, and P-value < .05 is used for the significant difference.
Ethical clearance
All respondents were involved voluntarily after signing an informed consent form. The ethical clearance was obtained from the Faculty of Public Health Ethics Commission, Hasanuddin University, with Document Number 9068/UN4.14.1/TP.01.02/2022.
Results
Characteristics of respondents
Table 1 shows the characteristics of respondents and pregnancy. The majority of the respondents experienced elementary school and having no job but as a housewife. Their income is mostly under the Regional Minimum Wage (RMW). Most pregnant women were in the second trimester of pregnancy, having been pregnant more than once, and had normal Body Mass Index (BMI). None of them was smoking. Half of them consumed fast food once to twice a week.
Table 1.
Characteristics and pregnancy condition of pregnant women (N = 30).
| Variables | Range | Mean (SD) | n (%) |
|---|---|---|---|
| Age | 20-42 | 28.1 (6.8) | |
| Education | |||
| No formal education | 1 (3.0) | ||
| Elementary school | 13 (43.0) | ||
| High school | 11 (37.0) | ||
| Higher level | 5 (17.0) | ||
| Work | |||
| Not working | 27 (90.0) | ||
| Working | 3 (10.0) | ||
| Income | |||
| Less than RMW * | 26 (87) | ||
| More than RMW | 4 (13) | ||
| Age of pregnancy | |||
| First trimester | 11 (36.7) | ||
| Second trimester | 17 (56.7) | ||
| Third trimester | 2 (6.6) | ||
| Gravidity | |||
| Primigravida | 7 (23.0) | ||
| Multigravida | 23 (77.0) | ||
| BMI (N = 30) | |||
| Underweight | 8 (26.7) | ||
| Normal | 13 (43.3) | ||
| Overweight | 9 (30.0) | ||
| Smoking | |||
| Smoking | 0 (0) | ||
| Never | 30 (100) | ||
| Fast food consumption | |||
| <once a week | 8 (26.7) | ||
| 1 to 2x a week | 15 (50.0) | ||
| 3 to 4x a week | 4 (13.3) | ||
| 5 to 6x a week | 1 (3.3) | ||
| Every day | 2 (6.7) | ||
Regional minimum wave for Makassar City 2022.
Microplastics
The characteristics of the microplastics identified are presented in Table 2 and Figure 1. It was found 359 MP from all the stool samples, ranging in size from 200 to 4900 µm. Most of the microparticles were found in the stools of mothers in the pregnancy age of second trimester (10-21 pieces). The identified MPs in the stool samples were fragments, films, and fibers. Most of the shapes are film (41.23%). Most stool samples (76.7%) contained the 3 shapes of MPs, and the remains were 2. Fibers were discovered in all samples. Brown was the predominant color identified in the feces samples (36.77%), followed by yellow (28.77%) and transparent (27.02%).
Table 2.
Microplastic characteristics in the feces of pregnant women.
| Variables | Min | Max | Mean | SD | N (%) |
|---|---|---|---|---|---|
| Amount of MP per sample | 4 | 21 | 11.9 | 4.7 | |
| Size (mm) | 0.2 | 4.9 | |||
| Type of polymer (N = 10) | |||||
| PET | 2 (20.0) | ||||
| HDPE | 2 (20.0) | ||||
| Polyamide | 3 (30.0) | ||||
| Polyethylene chlorinated | 2 (20.0) | ||||
| Ethylene propylene | 1 (20.0) | ||||
Figure 1.
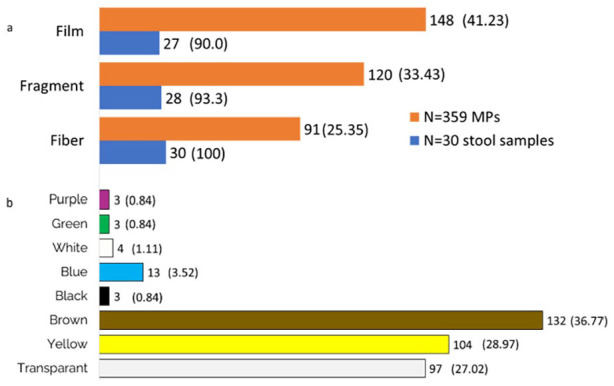
(a) The shape of MPs found in the pregnant women stools and (b) the color of MPs in the pregnant women stools.
The samples of MPs in various colors and shapes are shown in Figure 2, and the wavelengths are presented in Figure 3.
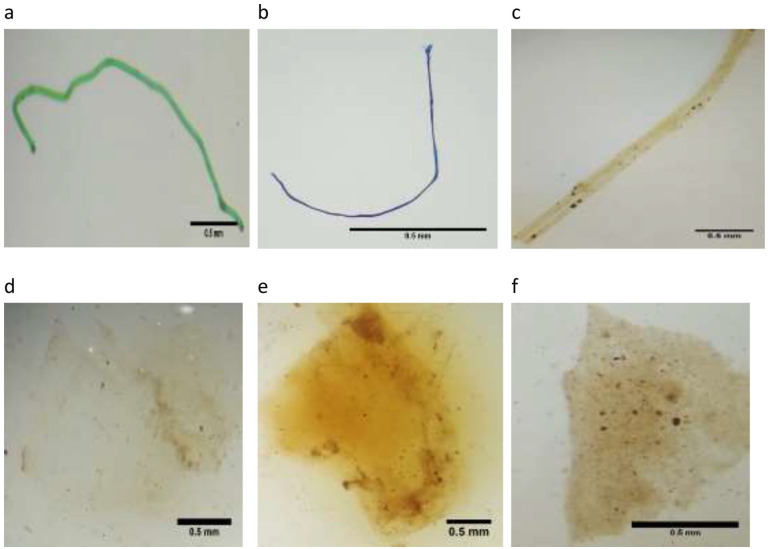
Figure 2.
The sample shape of microplastics in the stool of the pregnant women ((a–c) Fiber, (d)Film, and (e and f) Fragment).
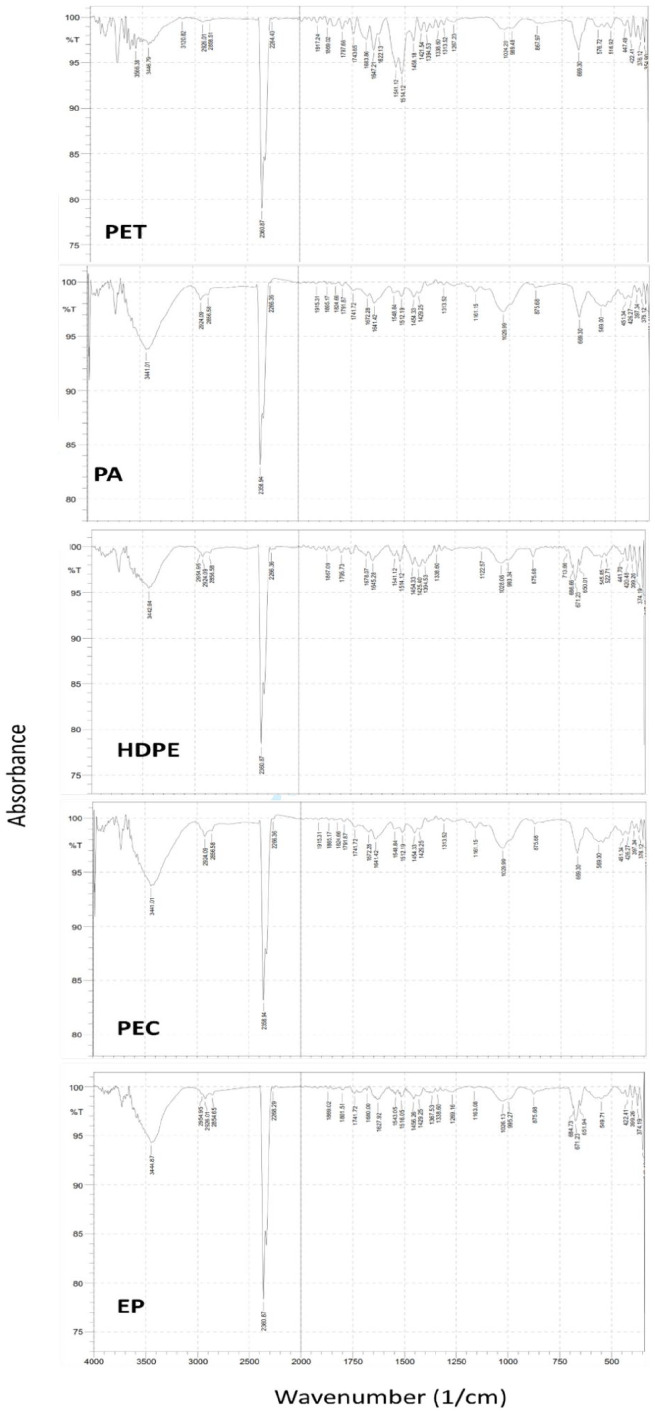
Figure 3.
Wavelength from FTIR.
We characterized 10 MP samples out of the 359 plastics found for the polymer. The 10 MPs were selected as representation of 10 variations of colors and shapes, such as film-brown, line blue, fragment brown, etc. Polyethylene Terephthalate (PET), Polyamide/Nylon, Polyethylene Chlorinated (PEC), High-Density Polyethylene (HDPE) and Ethylene propylene (EP) were present in those samples, and polyamide was the most polymer.
Some factors related to MPs exposure is presented in Table 3. Regular consumption of seafood was common among the women (>75%), and almost half of the women consumed a high amount of seafood. However, the majority of them usually consumed small fish. Another risk factor for MPs exposure is eating food from a plastic bowl poured with hot water. However, two-thirds of them were rarely to do this activity.
Table 3.
Microplastic amount according to seafood consumption of pregnant women.
| Variables | n (%) | Microplastic amount | P-value | |
|---|---|---|---|---|
| n | Mean (SD) | |||
| Seafood consumption frequency | ||||
| Frequently | 23 (76.7) | 5-21 | 12.7 (4.7) | .09 a |
| Rarely | 7 (23.3) | 6-15 | 9.3 (3.8) | |
| Seafood consumption amount | ||||
| High | 13 (43.3) | 10-21 | 16.0 (3.2) | .00 b |
| Middle | 7 (23.3) | 11.43 | 11.4 (2.2) | |
| Low | 10 (33.3) | 5-10 | 6.9 (1.3) | |
| Seafood type consumed | ||||
| Big fish | 5 (16.7) | 8-21 | 15.6 (5.3) | .14 b |
| Small fish | 18 (60.0) | 5-21 | 10.9 (4.5) | |
| Mollusk, shell | 7 (23.3) | 7-15 | 11.9 (3.7) | |
| Using plastic bowl for food with hot water | ||||
| Rarely | 20 (66.7) | 6-15 | 10.4 (3.2) | .46 b |
| Frequently | 7 (23.3) | 7-21 | 14.1 (5.4) | |
| Everyday | 3 (10.0) | 10-17 | 13.0 (3.6) | |
t-test.
One-way Anova. Significantly different when P < .05.
Discussion
MPs characteristic
Studies on MPs in stool are still rare. The previous study 23 has provided evidence that microplastics were present in all human stool samples, and the current study supports the result. While the study identified a median of 20 microplastic pieces per 10 g of fecal matter, the recent study detected a mean of 11.3 pieces per 25 g stool. Other studies in Indonesia 25 found the existence of MPs in seven of eleven stool samples with a concentration of 6.94 µg/g of feces (µg/g) to 16.6 µg/g. While another study discovered MPs in six of eleven feces samples ranging from 3.3 to 13.9 µg/g. 24
The size of plastic particles is crucial in producing toxic effects on various organisms. However, the magnitude of the destruction that these particles can produce is greatly influenced by the tissue structure and anatomy of each organism. 33 The current study found the size of MPs discovered from the stool ranged from 0.2 to 4.9 mm. A size of 0.2 is the minimum MPs size that can be detected from microscopy, while a size less than that can not be detected anymore, although they were there.
The most abundant MPs colors discovered in the recent study are brown, followed by yellow and transparent. The MPs likely come from seafood consumed, contaminated by MPs from the sea. Pigments are a source of numerous chemicals, including certain metals. Discoloration (yellowing) and surface erosion imply an extended exposure to the environment. Yellowing is produced by quinone or semi-quinone compounds forming from phenolic additives, such as benzotriazoles, by photo-oxidative environmental degradation. 28 The accumulation of degradation products in the plastic matrix due to photo-oxidation typically results in yellow or amber hues (yellowing and tanning). Photo-oxidation typically causes changes in plastic color, like a gradual shift toward lighter hues (discoloration or bleaching) 34 Yellow and brown were also notably prevalent in investigating the colors of marine plastic litter. 35 Yellowing and tanning are often observed in the most prevalent plastic polymers, such as Polyethylene (PE), Polypropylene (PP), Polystyrene (PS), PET, and Polyvinyl Chloride (PVC).34,36 The extent of yellow or brown color is related to the number of photo-oxidation products and, thus, the extent of weathering. 37 The oxidative process can act on the polymer (eg, PVC, PC, PS) and the thermal stabilizers applied to the plastic resin. 34
The shape of plastic particles plays a crucial role in determining their toxic effects. Plastics can exist in fragments, fibers/filaments/lines, pellets, beads/spheres, and films/sheets. Plastic debris and microplastics have a similar appearance to their primary materials. Fiber is used to manufacture nylon rope and clothing. Some plastic objects contain considerable amounts of certain extra chemicals for their intended applications. Hence, the chemical concentration based on the plastics’ structure could indicate environmental exposure and chemical dispersion through specific plastic objects or pollution sources. Hundreds of different polymers and polymer mixes are made commercially; nevertheless, 6 polymers dominate the market. They account for around 80 % of total plastic manufacturing and are likely a substantial component of the majority of marine debris. The polymers are polyethylene (in HDPE and Low-density Polyethene/LDPE variants) and PET. The following materials are polyurethane (PUR), PP, PS, and PVC.
The current study noticed polyamide or nylon in most of the 10 analyzed samples. This polymer may come in the form of fiber, film and plastic. Polyamide is applied in textile industries, household appliances, kitchen appliances, and food packaging. 38 Polyamide fibers primarily result from sewage from washing clothes. Garments released >100 fibers and >180% in each liter of washing machine effluent. Currently, synthetic fibers are used mostly as a textile material, and the proportion of polyamide found in marine habitats and sewage is 9%. 38 When it comes to marine habitats, it pollutes the fish, go into the food chain, and reaches humans. This polymer also comes from plastic tea bags steeping at 95°C. 39 Plastic tea bag is made from nylon, degrading and fracturing when they get hold of high-temperature water. 40 Thus, drinking tea bags likely increases the risk of polyamide exposure.
Another plastic discovered in this study was PET. This plastic material is used in drinking packaging, such as water or milk bottles. Schymanski et al 41 observed PET and Polysulfone (PES) in the water of plastic bottles due to the application of these materials for the bottles and the cap. Beverage cartons also release MPs from the foils and the caps. 41 The current result suggested that the MP exposure of pregnant women was through ingestion from the source of packed food, teabag, packaged beverages, and seafood. All of the participants consume seafood.42,43 The previous study also found PET in all 8 stool samples 44 and 1 of 6 samples. 24 Supporting the current study, the prior study also discovered among 15 types of MPs identified in feces, PET (22.3%-34.0%) and polyamide (8.9%-12.4%) also being dominant, and sheets as well as fibers as the primary shapes. 45
HDPE is another polymer found. HDPE is a variation of polyethylene, the most polymer usually found in the environment. 46 HDPE has a high degree of crystallinity tenacity due to the chains being almost linear and having minimal branching. 47 HDPE and PE are commonly used to manufacture plastic pharmaceutical bottles. 48 Supporting the recent study, HDPE was also found in 6 of 11 feces samples from a fisherman community in Surabaya, Indonesia. 24 In that study, a brand of toothpaste they used was also contaminated with HDPE. Thus it became a possible source of HDPE.
Potential sources
Pregnant women might be exposed to MPs from many sources. The occurrence of MPs in some potential sources around the world has been reported in a review. 33 First is seafood consumption; as stated previously MPs exposure in humans possibly through the food chain, such as fish and other seafood.49 -52 The current study found that the MPs’ amount in the stools significantly differs according to the amount of seafood consumption (Table 3). However, there was no difference in MPs amount according to the seafood type and consumption frequency. The previous study in the Paotere fish market in Makassar, Indonesia, demonstrated microplastic-contained debris in the gastrointestinal tract of 28% of 76 fish. The fishes included silver-stripe round herring, mackerel, and shortfin scads. 53 Other studies also observed the MPs in blood clams (Anadara granosa) in the coastal areas around Makassar. 54 Thus, seafood consumption may contribute to the presence of MPs in pregnant women’s stool.
Moreover, MPs have been found at high concentrations in table salt from a variety of brands worldwide,55 -57 including Indonesia.13,58 They are also reported in high concentrations in drinking water that could be derived from the bottles.11,16,59,60An average of 118 to 325 particles per liter of bottled water,41,61 for a total of 90 000 microplastics annually contribute to the ingestion pathway with the assumption of all water intake comes entirely from bottled sources. 62 Using plastic food containers or packaging might also contribute to this pathway. However, a previous study in Indonesia did not observe a significant relationship between the frequency of ingesting products packed in plastic and seafood, food and water consumption and microplastics in the stool samples. 25
Health implication
MPs exposure likely results in health effects on pregnant women. The existence of MPs in the stool indicated that they had been exposed to plastics. Smaller particles (nanoparticles) might stay in the body and have negative effects. Mostly, the health effects of MPs are still based on animal studies. PET may affect the estrogen hormone as estrogenic contamination has been discovered in PET water bottles.42,43 MPs may induce different human-derived cells. Exposure to PE and PS may affect the nervous system by increasing Reactive Oxygen Species (ROS) in cerebral human cells (T98G).33,63 PS nanoparticles (NPs) affect the respiratory system by inducing cytotoxicity effects in a dose-dependent manner in 2 human cell lines (Calu-3 epithelial cells and THP-1 differentiated macrophages.33,64
Furthermore, the digestive system can also be affected through gene expression in gastric adenocarcinoma cells, which morphologically cause inflammatory responses and alterations.33,65 Study in mice showed that exposure to PE resulted in a decrease in the abundance of Firmicutes and an increase in the abundance of Bacteroides in the gut microbiota. 66 Mice exposed to both 36 and 116 μm PE microbeads orally experienced disruptions in their stomach and caused histomorphological changes throughout the entire digestive tract. 67 In addition, the average daily consumption of 166 mg of PET has been found to impact both the composition and variability of human gut microbial populations. 68
For pregnant women, plastic exposure will affect the fetus. MPs have accumulated in the syncytiotrophoblast of the placenta. Syncytiotrophoblast responsible for nutrient transport across the placental barrier. All polystyrene particles, despite their capacity to pass the placental barrier or the direction of perfusion, accumulated in the syncytiotrophoblast of the placental tissue. The syncytiotrophoblast regulates nanoparticle transfer throughout the human placenta. 69 Particles that are compatible with MPs may play a role in activating pathological traits like oxidative stress, apoptosis, and inflammation that are indicative of metabolic disorders that lead to potential diseases like obesity, diabetes, metabolic syndrome, and many others. 70
This study has explored whether MPs have exposed the pregnant mother through the existence of MPs in the stool. However, this study has some limitations. Only 10 MPs of 356 particles were observed to analyze the polymer type. Thus, it might not represent all MPs, although they were chosen based on their colors and shapes. The MPs observed using microscopic resulted in a flaw in observing the smaller particles.
Conclusion
It is concluded that 10 microplastic polymers were discovered in the stools of pregnant women. It might bring negative effect on the development of the fetus. It is suggested that further studies on the exposure source of the MPs and their effects on their pregnancy and the baby are necessary.
Acknowledgments
The authors acknowledge the Health Community Services that had supported this research by providing the facilities during data collection.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Indonesian Ministry of Education, Culture, Research and Technology [grant number 02381/UN4.22/PT.01.03/2023].
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: UH and ES havfe been involved in data collection. HA and MC have been involved in research concept and design. HA analyzed the data and wrote the manuscript. AZA reviewed and approved final manuscript.
References
- 1. Gola D, Kumar Tyagi P, Arya A, et al. The impact of microplastics on marine environment: a review. Environ Nanotechnol Monitor Manag. 2021;16:100552. [Google Scholar]
- 2. Wong JKH, Lee KK, Tang KHD, Yap PS. Microplastics in the freshwater and terrestrial environments: prevalence, fates, impacts and sustainable solutions. Sci Total Environ. 2020;719:137512. [DOI] [PubMed] [Google Scholar]
- 3. Du S, Zhu R, Cai Y, et al. Environmental fate and impacts of microplastics in aquatic ecosystems: a review. RSC Adv. 2021;11:15762-15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dowarah K, Devipriya SP. Microplastic prevalence in the beaches of Puducherry, India and its correlation with fishing and tourism/recreational activities. Mar Pollut Bull. 2019;148:123-133. [DOI] [PubMed] [Google Scholar]
- 5. Carbery M, O’Connor W, Palanisami T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ Int. 2018;115:400-409. [DOI] [PubMed] [Google Scholar]
- 6. Setälä O, Fleming-Lehtinen V, Lehtiniemi M. Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut. 2014;185:77-83. [DOI] [PubMed] [Google Scholar]
- 7. Osman AI, Hosny M, Eltaweil AS, et al. Microplastic sources, formation, toxicity and remediation: a review. Environ Chem Lett. 2023;21:1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vianello A, Jensen RL, Liu L, Vollertsen J. Simulating human exposure to indoor airborne microplastics using a breathing thermal manikin. Sci Rep. 2019;9:8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vethaak AD, Legler J. Microplastics and human health. Science. 2021;371:672-674. [DOI] [PubMed] [Google Scholar]
- 10. Zhou XJ, Wang J, Li HY, Zhang HM, Zhang DL. Microplastic pollution of bottled water in China. J Water Process Eng. 2021;40:101884. [Google Scholar]
- 11. WHO. Microplastics in drinking-water. Geneva: World Health Organization, 2019. [Google Scholar]
- 12. Tahir A, Taba P, Samawi M, et al. Microplastics in water, sediment and salts from traditional salt producing ponds. Glob J Environ Sci Manag. 2019;5:431-440. [Google Scholar]
- 13. Deswati D, Kurnia Hamzani B, Yusuf Y, Elsa Fitri W, Putra A. Detection of microplastic contamination in table salts in Padang City, Indonesia, and control strategies for choosing healthy salt. Int J Environ Anal Chem. 2023;104:1-16. [Google Scholar]
- 14. Yang D, Shi H, Li L, et al. Microplastic pollution in table salts from China. Environ Sci Technol. 2015;49:13622-13627. [DOI] [PubMed] [Google Scholar]
- 15. Iñiguez ME, Conesa JA, Fullana A. Microplastics in Spanish table salt. Sci Rep. 2017;7:8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Q, Xu EG, Li J, et al. A review of microplastics in table salt, drinking water, and air: direct human exposure. Environ Sci Technol. 2020;54:3740-3751. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Q, Zhao M, Meng F, et al. Effect of polystyrene microplastics on rice seed germination and antioxidant enzyme activity. Toxics. 2021;9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim X. Microplastics are everywhere — but are they harmful? Nature. 2021;593:22-25. [DOI] [PubMed] [Google Scholar]
- 19. Pironti C, Ricciardi M, Motta O, et al. Microplastics in the environment: intake through the food web, human exposure and toxicological effects. Toxics. 2021;9:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeong B, Baek JY, Koo J, et al. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny. J Hazard Mater. 2022;426:127815. [DOI] [PubMed] [Google Scholar]
- 21. Amran NH, Zaid SSM, Mokhtar MH, Manaf LA, Othman S. Exposure to microplastics during early developmental stage: review of current evidence. Toxics. 2022;10:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kozlova EV, Valdez MC, Denys ME, et al. Persistent autism-relevant behavioral phenotype and social neuropeptide alterations in female mice offspring induced by maternal transfer of PBDE congeners in the commercial mixture DE-71. Arch Toxicol. 2022;96:335-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwabl P, Köppel S, Königshofer P, et al. Detection of various microplastics in human stool: a prospective case series. Ann Intern Med. 2019;171:453-457. [DOI] [PubMed] [Google Scholar]
- 24. Luqman A, Nugrahapraja H, Wahyuono RA, et al. Microplastic contamination in human stools, foods, and drinking water associated with indonesian coastal population. Environments. 2021;8:138. [Google Scholar]
- 25. Wibowo AT, Nugrahapraja H, Wahyuono RA, et al. Microplastic contamination in the human gastrointestinal tract and daily consumables associated with an Indonesian farming community. Sustainability. 2021;13:12840. [Google Scholar]
- 26. Wikurendra E, Aini S, Nagy I, et al. Source of microplastic pollution within human stool in the Surabaya River Basin Area. J Chem Heal Risks. 2022;12:581-586. [Google Scholar]
- 27. Daud A, Ishak H, Birawida AB, et al. Analysis of Micro Plastics on Feces of Community Consumed Shellfish at Coastal Area Takalar, South Sulawesi-Indonesia. Review of International Geographical Education Online. 2021;11(9). [Google Scholar]
- 28. GESAMP. Guidelines for the monitoring and assessment of plastic litter in the ocean. Nairobi: United Nations Environmental Programme (UNEP); 2019. Accessed January 9, 2022. http://www.gesamp.org/publications/guidelines-for-the-monitoring-and-assessment-of-plastic-litter-in-the-ocean [Google Scholar]
- 29. Kershaw PJ, Rochman CM. Sources, fate and effects of microplastics in the marine environment: part 2 of a global assessment. Reports and Studies-IMO/FAO/UNESCO-IOC/WMO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP) Eng No 93. [Google Scholar]
- 30. Primpke S, Wirth M, Lorenz C, Gerdts G. Reference database design for the automated analysis of microplastic samples based on Fourier transform infrared (FTIR) spectroscopy. Anal Bioanal Chem. 2018;410:5131-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cowger W, Steinmetz Z, Gray A, et al. Microplastic spectral classification needs an open source community: open specy to the rescue! Anal Chem. 2021;93:7543-7548. [DOI] [PubMed] [Google Scholar]
- 32. FDA. Advice about eating fish for those who might become or are pregnant or breastfeeding and children ages 1 - 11 years. US Food and Drug Administration; 2022. Accessed March 9, 2023. https://www.fda.gov/food/consumers/advice-about-eating-fish#pattern [Google Scholar]
- 33. Elizalde-Velázquez GA, Gómez-Oliván LM. Microplastics in aquatic environments: A review on occurrence, distribution, toxic effects, and implications for human health. Sci Total Environ. 2021;780:146551. [DOI] [PubMed] [Google Scholar]
- 34. Andrady AL, Searle ND, Crewdson LFE. Wavelength sensitivity of unstabilized and UV stabilized polycarbonate to solar simulated radiation. Polym Degrad Stab. 1992;35:235-247. [Google Scholar]
- 35. Martí E, Martin C, Galli M, et al. The colors of the ocean plastics. Environ Sci Technol. 2020;54:6594-6601. [DOI] [PubMed] [Google Scholar]
- 36. Brandon J, Goldstein M, Ohman MD. Long-term aging and degradation of microplastic particles: comparing in situ oceanic and experimental weathering patterns. Mar Pollut Bull. 2016;110:299-308. [DOI] [PubMed] [Google Scholar]
- 37. Ter Halle A, Ladirat L, Martignac M, et al. To what extent are microplastics from the open ocean weathered? Environ Pollut. 2017;227:167-174. [DOI] [PubMed] [Google Scholar]
- 38. Browne MA. Sources and pathways of microplastics to habitats. In: Bergmann M, Gutow L, Klages M, eds. Marine Anthropogenic Litter. SpringerOpen; 2015;229-244. [Google Scholar]
- 39. Hernandez LM, Xu EG, Larsson HCE, et al. Plastic teabags release billions of microparticles and nanoparticles into Tea. Environ Sci Technol. 2019;53:12300-12310. [DOI] [PubMed] [Google Scholar]
- 40. Tegge G. Handbook of Adhesives. Reinhold Publishing Corporation; 1964. [Google Scholar]
- 41. Schymanski D, Goldbeck C, Humpf H-U, Fürst P. Analysis of microplastics in water by micro-Raman spectroscopy: release of plastic particles from different packaging into mineral water. Water Res. 2018;129:154-162. [DOI] [PubMed] [Google Scholar]
- 42. Wagner M, Oehlmann J. Endocrine disruptors in bottled mineral water: total estrogenic burden and migration from plastic bottles. Environ Sci Pollut Res. 2009;16:278-286. [DOI] [PubMed] [Google Scholar]
- 43. Wagner M, Oehlmann J. Endocrine disruptors in bottled mineral water: estrogenic activity in the E-screen. J Steroid Biochem Mol Biol. 2011;127:128-135. [DOI] [PubMed] [Google Scholar]
- 44. Liebmann B, Köppel S, Königshofer P, et al. Assessment of microplastic concentrations in human stool: final results of a prospective study. In: Conference on nano and microplastics in technical and freshwater systems, 2018:28–31. [Google Scholar]
- 45. Yan Z, Liu Y, Zhang T, et al. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ Sci Technol. 2022;56:414-421. [DOI] [PubMed] [Google Scholar]
- 46. Dioses-Salinas DC, Pizarro-Ortega CI, De-la-Torre GE. A methodological approach of the current literature on microplastic contamination in terrestrial environments: current knowledge and baseline considerations. Sci Total Environ. 2020;730:139164. [DOI] [PubMed] [Google Scholar]
- 47. Zhang XM, Elkoun S, Ajji A, Huneault MA. Oriented structure and anisotropy properties of polymer blown films: HDPE, LLDPE and LDPE. Polymer. 2004;45:217-229. [Google Scholar]
- 48. FOREHAO. The difference between plastic PP, PET and PE. 2020. Accessed December 15, 2022. https://www.foerhao-pharmpack.com/news/the-difference-between-plastic-pp-pet-and-pe.html
- 49. Li B, Liang W, Liu Q-X, et al. Fish ingest microplastics unintentionally. Environ Sci Technol. 2021;55:10471-10479. [DOI] [PubMed] [Google Scholar]
- 50. Mistri M, Sfriso AA, Casoni E, et al. Microplastic accumulation in commercial fish from the Adriatic Sea. Mar Pollut Bull. 2022;174:113279. [DOI] [PubMed] [Google Scholar]
- 51. Abbasi S, Soltani N, Keshavarzi B, et al. Microplastics in different tissues of fish and prawn from the Musa estuary, Persian Gulf. Chemosphere. 2018;205:80-87. [DOI] [PubMed] [Google Scholar]
- 52. EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016;14:e04501. [Google Scholar]
- 53. Rochman CM, Tahir A, Williams SL, et al. Anthropogenic debris in seafood: plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci Rep. 2015;5:14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Namira N, Daud A, Mallongi A, et al. Risk analysis of microplastic exposure through consumption of Anadara granosa at Coastal Area. Pharmacogn J. 2023;15:558-562. [Google Scholar]
- 55. Ravikumar S, Jeyameenakshi A, Syed Ali M, Ebenezer KS. Assessment of microplastics in edible salts from solar saltpans and commercial salts. Total Environ Res Themes. 2023;6:100032. [Google Scholar]
- 56. Kapukotuwa RWMGK, Jayasena N, Weerakoon KC, Abayasekara CL, Rajakaruna RS. High levels of microplastics in commercial salt and industrial salterns in Sri Lanka. Mar Pollut Bull. 2022;174:113239. [DOI] [PubMed] [Google Scholar]
- 57. Kim J-S, Lee H-J, Kim S-K, Kim HJ. Global pattern of microplastics (MPs) in commercial food-grade salts: sea salt as an indicator of seawater MP pollution. Environ Sci Technol. 2018;52:12819-12828. [DOI] [PubMed] [Google Scholar]
- 58. Dwiyitno D, Sturm MT, Januar HI, Schuhen K. Influence of various production methods on the microplastic contamination of sea salt produced in Java, Indonesia. Environ Sci Pollut Res. 2021;28:30409-30413. [DOI] [PubMed] [Google Scholar]
- 59. Ali MGA. Presence And Characterization of Microplastics in Drinking (Tap/Bottled) Water and Soft Drinks. Master’s thesis. The University of North Dakota, 2019. [Google Scholar]
- 60. Pivokonsky M, Cermakova L, Novotna K, et al. Occurrence of microplastics in raw and treated drinking water. Sci Total Environ. 2018;643:1644-1651. [DOI] [PubMed] [Google Scholar]
- 61. Mason SA, Welch VG, Neratko J. Synthetic polymer contamination in bottled water. Front Chem. 2018;6:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cox KD, Covernton GA, Davies HL, et al. Human consumption of microplastics. Environ Sci Technol. 2019;53:7068-7074. [DOI] [PubMed] [Google Scholar]
- 63. Schirinzi GF, Pérez-Pomeda I, Sanchís J, et al. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ Res. 2017;159:579-587. [DOI] [PubMed] [Google Scholar]
- 64. Paget V, Dekali S, Kortulewski T, et al. Specific uptake and genotoxicity induced by polystyrene nanobeads with distinct surface chemistry on human lung epithelial cells and macrophages. PLoS One. 2015;10:e0123297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Forte M, Iachetta G, Tussellino M, et al. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol In Vitro. 2016;31:126-136. [DOI] [PubMed] [Google Scholar]
- 66. Sun H, Chen N, Yang X, Xia Y, Wu D. Effects induced by polyethylene microplastics oral exposure on colon mucin release, inflammation, gut microflora composition and metabolism in mice. Ecotoxicol Environ Saf. 2021;220:112340. [DOI] [PubMed] [Google Scholar]
- 67. Djouina M, Vignal C, Dehaut A, et al. Oral exposure to polyethylene microplastics alters gut morphology, immune response, and microbiota composition in mice. Environ Res. 2022;212:113230. [DOI] [PubMed] [Google Scholar]
- 68. Tamargo A, Molinero N, Reinosa JJ, et al. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci Rep. 2022;12:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Grafmueller S, Manser P, Diener L, et al. Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Environ Health Perspect. 2015;123:1280-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ragusa A, Matta M, Cristiano L, et al. Deeply in plasticenta: presence of microplastics in the intracellular compartment of human placentas. Int J Environ Res Public Health. 2022;19:11593. [DOI] [PMC free article] [PubMed] [Google Scholar]