Abstract
Two cell lines originating from a common ancestral tumor, CSML0 and CSML100, were used as a model to study AP-1 transcription factors at different steps of tumor progression. CSML0 cells have an epithelial morphology; they express epithelial but not mesenchymal markers and are invasive neither in vitro nor in vivo. CSML100 possesses all characteristics of a highly progressive carcinoma. These cells do not form tight contacts, are highly invasive in vitro, and are metastatic in vivo. AP-1 activity was considerably higher in CSML100 cells than in CSML0 cells. There was a common predominant Jun component, namely, JunD, detected in both cell lines. We found that the enhanced level of AP-1 in CSML100 cells was due to high expression of Fra-1 and Fra-2 proteins, which were undetectable in CSML0 nuclear extracts. Analysis of the transcription of different AP-1 members in various cell lines derived from tumors of epithelial origin revealed a correlation of fra-1 expression with mesenchymal characteristics of carcinoma cells. Moreover, we show here for the first time that the expression of exogenous Fra-1 in epithelioid cells results in morphological changes that resemble fibroblastoid conversion. Cells acquire an elongated shape and become more motile and invasive in vitro. Morphological alterations were accompanied by transcriptional activation of certain genes whose expression is often induced at late stages of tumor progression. These data suggest a critical role of the Fra-1 protein in the development of epithelial tumors.
Progression of breast cancer is often accompanied by changes in the pattern of gene expression in cells of growing carcinomas, resulting in highly tumorigenic and invasive cell types (23). Activation of a number of mesenchymal genes has been implicated in the development of a more malignant phenotype. In addition, loss of epithelial markers such as the cellular adhesion protein E-cadherin and epithelial cytokeratins often occurs at certain stages of tumor progression (reviewed in reference 12). These changes are reminiscent of an epithelial-mesenchymal transition, a process that is distinctive for several critical stages in development, such as gastrulation, organogenesis, and neural crest cell emigration (reviewed in reference 73). Promoters and enhancers of many genes whose expression is affected in a developing carcinoma bear functional elements capable of binding the Fos and Jun transcription factors (so-called 12-O-tetradecanoylphorbol-13-acetate (TPA) response elements [TREs]). Moreover, inducible c-FosER and c-JunER fusion proteins may trigger an epithelial-mesenchymal conversion of nontumorigenic immortalized mammary epithelial Ep-1 cells (22, 67). Therefore, AP-1 seems to belong to a group of factors defining tumor progression.
AP-1 (activator protein-1) is thought to play a central role in reprogramming of the gene expression pattern in response to external stimuli. Being a downstream event of various signal transduction cascades, activation of AP-1 has been implicated in fundamental processes occurring in mammalian cells: differentiation (8, 28, 55), cell proliferation (39, 46, 47), oncogenic transformation (reviewed in reference 4), and apoptosis (14, 65). AP-1 consists of bZIP transcription factors belonging to two protein families: Jun and Fos. In mammalian cells, three members of the Jun family (c-Jun, JunB, and JunD) and four members of the Fos family (c-Fos, FosB, Fra-1, and Fra-2) have been identified to date. In addition, as a result of alternative splicing, a dominant negative mutant of FosB, FosB2, may naturally occur (57, 58). These proteins form Jun-Jun homodimers and more stable Fos-Jun heterodimers and activate transcription from the TRE-containing enhancers. Moreover, Fos and Jun may efficiently dimerize with other bZIP transcription factors, such as ATF/CREB (30) or Maf/Nrl family members (42, 44), as well as with the bHLHZip proteins MyoD (10), FIP (13), and USF (64).
The Jun and Fos proteins act cooperatively in DNA binding and, therefore, in the control of transcription. There are no direct data showing preferential binding of certain AP-1 dimers to specific TREs in vivo. However, in vitro, the adjacent sequences may differently influence the stability of the AP-1 complex (70). When the Fos proteins are bound to DNA as heterodimers, the contributions of individual family members to transcriptional activation are different. This difference is due to the lack of the C-terminal transactivation domain in the Fra-1, Fra-2, and FosB2 proteins, while c-Fos and FosB harbor the regions which are sufficient to activate transcription (87). Cellular transformation by the c-Fos protein depends on the presence of the transactivation domain (25, 40, 87). Consistent with this concept, no transforming potential could be assigned to the Fra-1 and Fra-2 proteins upon their overexpression in 208F fibroblasts (87). Similarly, the Fra-1 protein was unable to induce morphological transformation of Rat-1 cells. However, Fra-1 protein became a predominant Fos component upon Ras-induced transformation of NIH 3T3 cells (56), and Rat-1 fibroblasts transfected with a fra-1 expression construct were capable of tumor formation in nude mice and anchorage-independent growth in vitro (11). The view that different Fos proteins have distinct functions in many cellular processes is further supported by the study of Fos activation in response to stimulation of serum-depleted fibroblasts by serum. At early times after serum stimulation, the most abundant Fos proteins are c-Fos and FosB, whereas at later times Fra-1 and Fra-2 become more abundant (29, 47). Since Fra-1 and Fra-2 have been shown to inhibit c-Fos- and c-Jun-dependent transactivation in a transient-transfection assay (79), these proteins may act as negative regulators which limit the duration of the AP-1 response (74, 87).
In the present work, we analyze the expression and functional activity of AP-1 in two mouse adenocarcinoma cell lines that originate from the same tumor but differ in morphology and metastatic potential. We show a correlation of Fra-1 expression with the mesenchymal characteristics of epithelial tumors. In contrast to data obtained by study of fibroblastoid cell lines 208F and Rat-1 (11, 87), the overexpression of Fra-1 in epithelioid carcinoma cells greatly influences cell morphology, motility, and invasiveness and activates the transcription of a number of genes. We suggest that Fra-1 plays a pivotal role in the progression of mouse mammary tumors.
MATERIALS AND METHODS
Plasmids.
The coding parts of fra-1, fra-2, and c-fos mRNAs were amplified by reverse transcription-PCR and cloned in the PCR3-Uni (Invitrogen) (fra-1 and fra-2) or in pSVK3 (Pharmacia) (c-fos) vector. These vectors contain cytomegalovirus and simian virus 40 promoters, respectively. RNA for amplification of fra-1 and fra-2 sequences was isolated from CSML100 cells, whereas to obtain the coding part of c-fos, we used CSML0 mRNA. The sequences of the primers used are as follows: fra-1, TCCAGCCCAGGGCATGTA (forward, coordinates 184 to 201) and GTGGCTGGGTGCCTCACAAAG (reverse, coordinates 1011 to 1031); fra-2, CGGATCATGTACCAGGATTTATC (forward, coordinates 872 to 894) and TTACAGGGCTAGAAGTGTGGG (reverse, coordinates 1837 to 1857); and c-fos, TCTACCCCTGGACCCCTTGC (forward coordinates 106 to 125) and TCTGGATGCCGGCTGCCTTG (reverse, coordinates 1168 to 1187). The inserts of the obtained constructs pCMVFra-1, pCMVFra-2 and pSVc-Fos were verified by the dideoxynucleotide sequencing procedure. A JunD expression vector, pSVJunD, was kindly provided by Peter Herlich. For conditional expression, the insert of the pCMVFra-1 plasmid was excised and cloned in pUHD 10-3 (26), generating pUHDFra-1. To obtain a clone of CSML0 cells producing reverse tetracycline-controlled transactivator (rtTA), the pUHD172-1neo construct (27) was used. Plasmid pBabeHyg, a gift from J. Lukas, contains a hygromycin resistance gene cloned in the pBabe retroviral vector. The pfLUC reporter construct contains the Photinius pyralis luciferase gene under transcriptional control of the minimal c-fos promoter (71). pcfLUC is based on pfLUC but contains the TRE from human collagenase (collTRE) (36). To construct pfLUC5×TRE, five copies of the collTRE-containing oligonucleotide were cloned in pfLUC upstream of the c-fos minimal promoter in a head-to-tail orientation. The β-galactosidase expression plasmid pCMVβ-gal and the pEGFP-N1 construct, which expresses enhanced green fluorescent protein (EGFP), were purchased from Clontech.
Nuclear extract preparation and EMSA.
Nuclear extracts were prepared as previously described (3). Besides phenylmethylsulfonyl fluoride (1 mM), the protease inhibitors benzamidin (0.5 mM) and pepstatin A, aprotinin, and bestatin (10 μg/ml each) were added to both lysis and extraction buffers. All inhibitors were purchased from Sigma. Electrophoretic mobility shift assay (EMSA) was performed as described previously (82). The AP-1 complex was detected with a double-stranded end-labeled oligonucleotide that contained the consensus TRE derived from the collagenase gene promoter (CGCTTGATGAGTCAGCCGGAA) (53). To detect Oct-1, we used an oligonucleotide that contained the Oct-1 binding site (TGCGAATGCAAATCACTAGAA) (51). To perform gel supershift analysis, anti-Jun and anti-Fos antibodies (all purchased from Santa Cruz Biotechnology Inc.) were added to the EMSA reaction mixtures. The incubation was carried out for 1 h at 4°C after the binding reactions were completed.
RNA analysis.
The acid guanidine thiocyanate method (17) was used to isolate total RNA. RNA blotting and hybridization were performed as described previously (72). Radioactive DNA probes were synthesized with a random-primed labeling kit (Amersham). Plasmids that contain cDNAs of different jun and fos family members were used for preparation of radioactive probes and are described in “Plasmids” above. The coding part of the high-mobility group protein I (HMGI) gene was amplified by reverse transcription-PCR in the presence of the primers AGGAGAATGAGCGAGTCGGG (forward, nucleotides 196 to 215) and CTGCGAGTGGTGATCACTGC (reverse, nucleotides 486 to 505) and cloned in the pSVK3 vector (Pharmacia). The sequence of the insert was found to be identical to the previously published HMGI sequence (38). Other probes used were mouse mts1 cDNA (21), rat extracellular matrix (ECM)-degrading metalloproteinase-3 (MMP-3) cDNA (15), human MMP-9 cDNA (86), E-cadherin cDNA (68), mouse urokinase-type plasminogen activator (uPA) cDNA (9), rat uPA inhibitor (PAI-1) cDNA (88), and mouse tissue inhibitor of metalloproteinase (TIMP-1) cDNA (61).
Western blotting.
Nuclear extracts (20 or 50 μg) were denatured by being heated to 100°C prior to fractionation on 10 or 12% polyacrylamide gels. Fra-1, Fra-2, and c-Fos proteins were synthesized in vitro by using the TnT Coupled Reticulocyte Lysate System (Promega Corp.). pCMVFra-1, pCMVFra-2, or pSVc-Fos plasmid DNA was used as a template for in vitro transcription. Proteins were transferred to Immobilon-P membranes (Millipore) by standard procedures and incubated in blocking solution with primary antibodies at a dilution of 1:3,000. All antibodies used were purchased from Santa Cruz Biotechnology. Immunoreactive proteins were detected by using the enhanced chemiluminescence system (ECL; Amersham).
Cell lines and transfection.
The following 13 mouse adenocarcinoma cell lines were used in this study. The CSML (CSML0 and -100) (76) and VMR (VMR-0, -Li, and -Ly) (77) cell lines originated from two mammary adenocarcinomas in A/Sn mice. The RAC cell lines (10P, 311C, 34E, and 5E) originated from a spontaneous mammary adenocarcinoma in a BALB/c mouse (78). The MT1 cell lines (Tc1 and Tc3) were derived from a mammary adenocarcinoma in a CBA/Ca mouse (7). Line 1 is a metastatic cell line established from an alveolar adenocarcinoma in a BALB/c mouse (6), and LLMet originated from a 3LL Lewis lung tumor in a C57BL/6 mouse (63). Additionally, 10T1/2 fibroblasts were used. Cells were cultivated as described in the references given above.
CSML0 or 1f9 cells (2 × 106) in 100 μl of phosphate-buffered saline were transfected by electroporation with a single pulse of 250 V and 250 μFd by using the Bio-Rad electroporation system and seeded on 6-cm-diameter dishes. Alternatively, cells were transfected by using LipofectAMIN PLUS reagent (Gibco BRL) according to the manufacturer’s protocol. In transient-transfection experiments, the efficiency of each transfection was monitored by use of a cotransfected β-galactosidase expression vector, pCMVβ-gal. At 2 days posttransfection, cells were lysed and the luciferase activity was measured with a luminometer (Promega Corp.). The lysates obtained were also tested for β-galactosidase activity by using o-nitrophenyl-β-d-galactopyranoside (Sigma) as a chromogenic substrate. To obtain transfectants expressing Fra-1, CSML0 cells were transfected with the pCMVFra-1 expression vector with subsequent selection of neomycin-resistant clones in the presence of G418 at a concentration of 400 μg/ml. To generate cell lines which express Fra-1 in a doxycycline (DOX)-inducible manner, we first transfected CSML0 cells with the pUHD-172-1 plasmid, encoding rtTA, a DOX-inducible transactivator. The selected neomycin-resistant clone (CSML0-tet-on22) was then cotransfected with pUHDFra-1 and pBabeHyg, a hygromycin resistance plasmid. Cell clones were selected in the presence of hygromycin B (200 μg/ml) (Calbiochem). Drug-resistant colonies were picked and cultivated in the absence or presence of DOX (2 μg/ml) (Sigma).
Matrigel invasion assay.
The basement membrane Matrigel (Biomedical Technologies Inc.) was applied to 13-mm filters (8-μm pore size; Nucleopore) as described previously (1). The coated filters were placed in Boyden chambers. Cells (105) maintained in the presence or in the absence of DOX (2 μg/ml) were collected, resuspended in Dulbecco modified Eagle medium containing 5% fetal calf serum with or without DOX, and added to the upper compartment of a chamber. Assays were carried out at 37°C in 5% CO2 for 24 h. Cells on filters were then fixed in methanol and stained with eosin G-thiazine solution (Diff-Quik; Baxter). The cells on the upper part of the filters were completely removed by wiping with a swab. The remaining cells, i.e., all of the cells attached to the lower surface of the filters, were counted. Each assay was performed in duplicate and repeated five times.
Determination of random cell motility.
Subconfluent cells were dislodged with 0.5 mg of trypsin per ml–0.75 mM EDTA in a modified Puck’s saline (Gibco BRL) and seeded on six-well tissue culture plates (35-mm-diameter wells) at a density of 3 × 103 cells/cm2. The dishes were coated with Matrigel (10 μg/ml) (from a murine Engelbreth-Holm-Swarm tumor; a generous gift of Hynda K. Kleinman, National Institutes of Health, Bethesda Md.). The cells were grown for 48 h in Dulbecco modified Eagle medium containing 10% fetal calf serum in the presence or absence of DOX (2 μg/ml). Video recording and determination of random cell motility were performed as described previously (84). Briefly, the six-well tissue culture dishes (Nunc, Roskilde, Denmark) were placed on a thermostatically controlled stage (Lincam Scientific Instruments Ltd., Surrey, England) mounted on a Diaphot 300 inverted microscope equipped with phase-contrast optics and a modified Plexiglas incubator (Nikon, Tokyo, Japan). The temperature inside the incubator was maintained at 37°C by a thermostatically controlled heating fan (DFA, Copenhagen, Denmark). A motorized stage was mounted on the microscope, allowing simultaneous recording from many different microscopic fields in an individual experiment. Automated time-lapse 512 by 512 pixel image acquisition was performed with the software PRIMA (Protein Laboratory, Copenhagen, Denmark). The positions of individual cells were determined by marking the centers of the nuclei. Coordinates of nuclear centers from consecutive video frames were utilized to generate tracks of moving cells. Data were expressed as the mean square displacement of the cells, <d2>, at time t, the time of observation, and fitted to the following equation describing random cell motility (20): <d2> = 2S2P [t − P(1 − e−t/P)], where S (distance/time) is the root mean square speed and P (time) is the time of persistence in direction. The equation also allows for determination of the rate of diffusion, R (distance2/time): R = 2S2P. To test the effect of Fra-1 in transiently transfected cells, CSML0 cells were cotransfected with pCMVFra-1 along with the pEGFP-N1 construct at a molar ratio of 8:1. A control culture was cotransfected with the empty vector and pEGFP-N1. At 24 h posttransfection, the cells were plated on plastic at a density of 3.5 × 103 cells/cm2 and incubated for additional 24 h at 37°C in a humidified atmosphere of 5% CO2. Video recording was performed as described above, except the first recordings (at time zero) were made by using fluorescence light and excitation and fluorescence filters appropriate for fluorescein isothiocyanate in order to identify transfected cells expressing EGFP.
RESULTS
Senin et al. applied different passage conditions in syngenic animals to establish two tumor lines from a spontaneous mouse mammary adenocarcinoma (76). The CSML0 line was obtained by passing primary tumors with subsequent selection of low-metastatis variants. The resulting cells had no metastatic potential in either spontaneous or experimental metastatic assays, had an epithelial morphology, and formed tight contacts. The initial spontaneous tumor gave rise to lung metastasis with low incidence. The consecutive passing of the cells taken from metastatic nodules in the course of 30 passages led to the establishment of a highly metastatic line, CSML100, that manifested the features of carcinosarcoma (76). When subcutaneously injected, CSML100 cells formed multiple metastatic nodules in lungs of syngenic mice. CSML100 cells have a more elongated morphology, and they do not form tight cell contacts (24, 76).
Expression of invasion-associated genes in CSML0 and CSML100 cells.
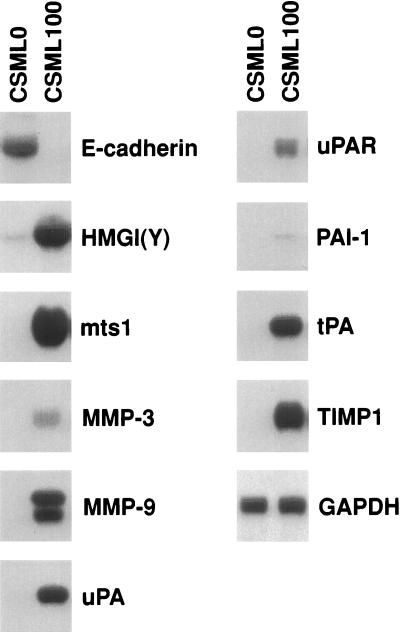
To explore the molecular basis of the differences in biological features of CSML0 and CSML100 cells, we analyzed the transcription of a number of genes whose protein products have been implicated in invasion and tumor progression. E-cadherin was highly expressed in epithelioid CSML0 cells but was entirely lost in CSML100 cells (Fig. 1), suggesting that the development of the CSML tumor comprised some elements of an epithelial-mesenchymal transition. We next analyzed the transcription of the gene coding for HMGI(Y), whose elevated expression was associated with progressive transformation of mammary and thyroid epithelia (16, 66). Transcription of this gene as well as that of another tumor progression marker that belongs to the S100 family, mts1 (21), is elevated in CSML100 cells (Fig. 1). Similarly, two ECM-degrading metalloproteinases shown to be involved in breast cancer development, MMP-3 and MMP-9, are up-regulated in CSML100 cells and practically not expressed in CSML0 cells (Fig. 1). In clear contrast to the case for the CSML0 cell line, genes encoding three components of the ECM-degrading uPA system, i.e., uPA, its receptor (uPAR), and PAI-1, were expressed in the CSML100 cells. Likewise, the tissue type serine proteinase (tPA) and TIMP-1 showed differential expression in CSML0 and CSML100 cells (Fig. 1).
FIG. 1.
Transcription of genes associated with tumor progression in CSML0 and CSML100 cells. Twenty micrograms of total RNA isolated from CSML0 and CSML100 cells was analyzed on Northern blots by using 32P-labeled specific probes, as indicated. Hybridization to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) confirms equal loading.
The majority of genes selectively up-regulated in CSML100 cells, i.e., the tPA, TIMP-1, mts1, MMP-9, PAI-1, MMP-3, uPAR, and uPA genes, have been reported to be AP-1 dependent under appropriate conditions (5, 18, 32, 33, 43, 45, 54, 59). Therefore, it was of interest to examine AP-1 in this cell system.
Quantitative and qualitative analyses of AP-1 complexes in CSML0 and CSML100 cells.
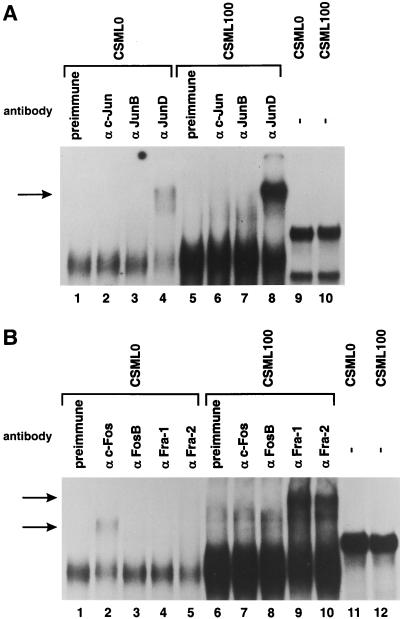
We analyzed AP-1 binding in CSML0 and CSML100 nuclear extracts by EMSA. An end-labeled oligonucleotide containing a consensus TRE sequence was mixed with 5 to 8 μg of CSML0 and CSML100 nuclear extracts, with the subsequent addition of specific antibodies against AP-1 proteins (Fig. 2) or control antibodies (Fig. 2A, lanes 1 and 5, and B, lanes 1 and 6). The binding activity in the two nuclear extracts was normalized by using an oligonucleotide containing an Oct-1 binding site. The Oct-1 factor has been shown to be equally expressed in normal and different types of malignant mammary epithelia (37). The in vitro binding of AP-1 to the consensus TRE was significantly increased in CSML100 versus CSML0 cells (Fig. 2). Antibodies recognizing the Jun family members revealed that the qualitative compositions of Jun components were identical in the two extracts; the predominant protein was JunD (Fig. 2A, lanes 4 and 8). Slight supershifts were seen with anti-c-Jun or anti-JunB antibodies in CSML100 extracts (Fig. 2A, lanes 6 and 7) and in CSML0 extracts when the film was exposed longer (not shown). The analysis of Fos family members demonstrated a qualitative difference in the composition of AP-1 complexes in asynchronously growing CSML0 and CSML100 cells. While anti-Fra-1 and anti-Fra-2 antibodies produced strong supershifts with CSML100 extracts, Fra-1 and Fra-2 proteins were entirely (Fra-1) or almost (Fra-2) not detected in CSML0 cells (Fig. 2B, lanes 4, 5, 9, and 10). Instead, anti-c-Fos antibodies produced a supershift when added to CSML0 but not CSML100 extracts (Fig. 2B, lanes 2 and 7). The FosB protein was not detected in either CSML0 or CSML100 extracts (Fig. 2B, lanes 3 and 8). The results of the EMSA indicated that in CSML0 cells, AP-1 consists of JunD and c-Fos proteins. In CSML100 cells, where this complex is more abundant, the predominant AP-1 components are JunD and the Fra-1 and Fra-2 proteins.
FIG. 2.
Levels and compositions of AP-1-containing complexes in CSML0 and CSML100 cells. 32P-labeled double-stranded oligonucleotides containing collTRE (lanes 1 to 8 in panel A and lanes 1 to 10 in panel B) or an Oct-1 binding site (lanes 9 and 10 in panel A and lanes 11 and 12 in panel B) were incubated with CSML0 or CSML100 nuclear extracts as shown and analyzed by EMSA. Anti-Jun (A) and anti-Fos (B) antibodies were added, as indicated. The antibody-supershifted complexes are marked by arrows.
c-Fos, but not JunD, is a functionally limiting component of the AP-1 complex in CSML0 cells.
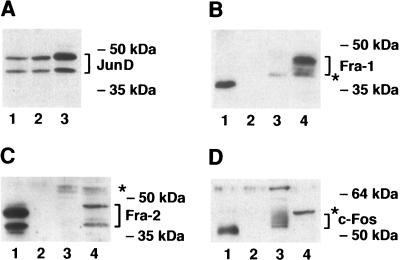
As complexes detected in EMSA are heterodimers, the relatively low level of AP-1 binding in CSML0 compared with CSML100 nuclear extracts might be due to a deficiency of only one component or both components. To determine which component limits the formation of the AP-1 complex in CSML0 nuclear extracts, we analyzed the protein levels of the individual AP-1 members observed in the supershift experiments. Immunoblot analysis of nuclear proteins isolated from CSML0 and CSML100 cells by using specific anti-JunD antibodies detected two bands of 41 and 46 kDa in both extracts (Fig. 3A, lanes 1 and 2). The 41-kDa band corresponds to the predicted size of JunD, whereas the origin of the 46-kDa immunoreactive band is unclear. However, we conclude that the 41- and 46-kDa polypeptides represent different forms of JunD protein. First, an increase in the intensities of both JunD immunoreactive bands was seen in nuclear extracts from CSML0 cells transfected with a JunD expression vector (Fig. 3A, lane 3). Second, both forms of the JunD protein of these molecular masses have previously been detected in nuclear extracts of primary keratinocytes (69). In general, the expression patterns of the JunD protein were found to be very similar in CSML0 and CSML100 cells. A different picture was observed when CSML0 and CSML100 cells were examined for expression of the Fra-1 and Fra-2 proteins. The use of Fra-1-specific antibodies revealed a major band of 45 kDa in CSML100 cells (Fig. 3B, lane 4), which is consistent with the mobility of fully phosphorylated Fra-1 protein detected in Swiss 3T3 cells as well as in primary keratinocytes (29, 69). Minor bands migrating slightly faster than the 45-kDa polypeptide indicated the existence of hypophosphorylated forms of Fra-1 in CSML100 extracts. No Fra-1 protein was detected in CSML0 cells (Fig. 3B, lane 3). Similarly, two Fra-2-immunoreactive bands of 38 and 48 kDa were observed in CSML100 but not CSML0 extracts (Fig. 3C, lanes 3 and 4). These forms of Fra-2 have been described previously (29, 69). We also examined the expression of c-Fos, whose presence in CSML0 cells was detected by the supershift analysis. Several c-Fos-immunoreactive bands with molecular masses ranging from 56 to 60 kDa, corresponding to the phosphorylated forms of p55 c-Fos, were detected exclusively in CSML0 extracts (Fig. 3D, lanes 3 and 4). The intensities of these bands increased when serum-depleted CSML0 cells were induced by serum (data not shown). We also performed similar experiments with CSML100 cells. Whereas the band in Fig. 3D, lane 4, was not affected upon serum stimulation of serum-depleted CSML100 cells, several novel c-Fos-immunoreactive bands appeared (data not shown). This allowed us to consider the band in lane 4 to be nonspecific. To confirm the specificity of antibodies to the Fos family members, we included in all Western blotting experiments the immunostaining of in vitro-synthesized proteins together with a negative control (rabbit reticulocyte lysate with no template added) (Fig. 3B, C, and D, lanes 1 and 2).
FIG. 3.
Expression of JunD, Fra-1, Fra-2, and c-Fos proteins in CSML0 and CSML100 cells. (A) Twenty micrograms of nuclear proteins extracted from CSML0 cells (lane 1), CSML100 cells (lane 2), or CSML0 cells transfected with a JunD expression construct (lane 3) were separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE) and immunostained with the anti-JunD antibody. (B to D) Immunoblotting analysis of the Fos proteins Fra-1 (B), Fra-2 (C), and c-Fos (D) in CSML0 (lanes 3) and CSML100 (lanes 4) cells. In vitro-synthesized Fra-1, Fra-2, and c-Fos proteins (lanes 1) were used as positive controls for the staining. As negative controls, mock-synthesized proteins (lanes 2) were used. Twenty (B and C) or fifty (D) micrograms of CSML0 and CSML100 nuclear extracts was separated by SDS–10% (B and C) or SDS–12% (D) PAGE. Nonspecific signals are shown by asterisks.
In summary, the results of the immunoblotting analysis confirmed the EMSA results and indicated that the predominant components of AP-1 in CSML0 cells are JunD and c-Fos, whereas those in CSML100 cells are the JunD, Fra-1, and Fra-2 proteins. Moreover, since JunD was nearly equally expressed in both the AP-1-abundant and AP-1-deficient cell lines, it seemed that the level of c-Fos limited the formation of the complex in CSML0 cells. However, as the activity of AP-1 members is regulated by protein phosphorylation, the amounts of the individual proteins might not reflect their functional activity. Therefore, it was important to confirm this conclusion by a functional study of AP-1.
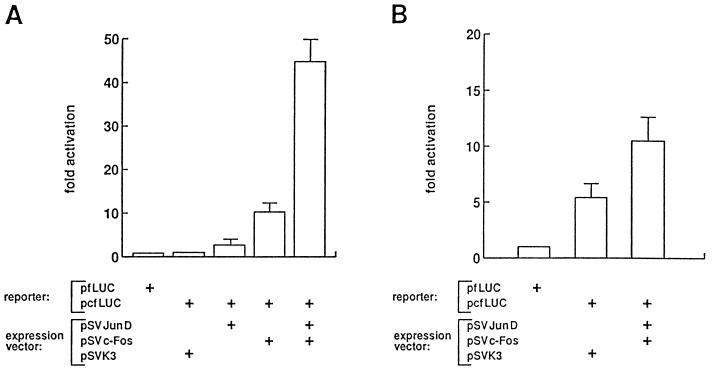
c-Fos needs the Jun counterpart to bind DNA; JunD was shown to be a weak activator in the absence of Fos (34). Therefore, we could compare in transient-transfection assay the influence of JunD and c-Fos overexpression on the activity of an AP-1-responsive reporter and hence determine which component is deficient. Two reporter constructs were used in these experiments: pfLUC, a reporter that contains the luciferase gene controlled by the minimal promoter (71), and pcfLUC, a derivative construct bearing a TRE from the human collagenase gene (36). When transfected in CSML0 cells, both constructs showed only a marginal level of luciferase expression (Fig. 4A), which was in good agreement with the low level of the AP-1 binding observed in CSML0 extracts (Fig. 2). We also performed cotransfection of CSML0 cells by pcfLUC together with JunD and c-Fos expression vectors. JunD stimulated the reporter activity by 2.7-fold, whereas stimulation by c-Fos gave a 10.3-fold induction. When simultaneously overproduced, JunD and c-Fos synergistically activated the reporter in CSML0 cells (about 45-fold activation) (Fig. 4A). These results closely resembled those obtained by Hirai et al., who studied the transactivation potential of JunD and c-Fos in serum-deprived NIH 3T3 fibroblasts (34). In resting fibroblasts, no Fos family members are expressed (19, 47, 60), and the Jun component is represented solely by JunD (34). Therefore, since our data are in a good accordance with the results obtained with serum-depleted NIH 3T3 cells, it is apparent that the component functionally limiting AP-1 activity in cycling CSML0 cells is c-Fos. In CSML100 cells, the pcfLUC construct was more active than the pfLUC reporter, reflecting the AP-1 abundance in these cells (Fig. 4B). We could detect only a minor activation of pcfLUC even by simultaneous overexpression of JunD and c-Fos proteins. The transactivation was likely suppressed by the abundant Fra-1 and Fra-2 proteins, whose complexes with JunD possess a higher stability but a lower transactivation potential than JunD–c-Fos heterodimers (70, 79).
FIG. 4.
Transactivation of the AP-1-responsive reporter pcfLUC by JunD and c-Fos. CSML0 (A) or CSML100 (B) cells were cotransfected with 4 μg of the reporter construct and 600 ng of JunD or c-Fos expression vector, or empty vector, as indicated. All transfections were normalized for β-galactosidase activity by cotransfection of 500 ng of a β-galactosidase expression vector, pCMVβ-gal. The results (means and standard deviations) of four independent experiments are expressed as fold activation relative to the basal activity of pcfLUC (A) or pfLUC (B).
In summary, these data show that the relatively high level of AP-1 activity in CSML100 cells can be attributed to an enhanced production of Fra-1 and Fra-2 proteins.
Transcription of fra-1 correlates with the mesenchymal characteristics of the carcinoma cells.
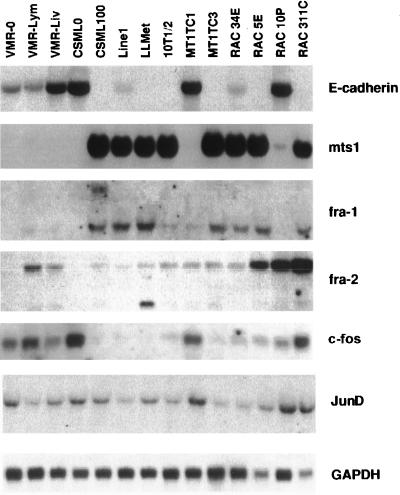
To examine whether the activation of fra-1 and fra-2 expression during progression of the CSML tumor is a unique feature of this cell system or an essential event in carcinoma development, we analyzed the transcription of AP-1 members in various carcinoma cell lines. Total RNA was isolated from 13 different tumor cell lines of epithelial origin and from 10T1/2 fibroblasts and subjected to Northern blotting analysis (Fig. 5). The two upper panels of Fig. 5 show the distribution of E-cadherin- and mts1-specific mRNAs in the cell lines analyzed. Transcription of the epithelial marker E-cadherin is characteristic of the initial steps of tumor development, while loss of its expression often occurs during transformation to malignancy (12). Transcription of the mts1 (S100A4) gene, belonging to the S100 family, is distinctive for highly metastatic carcinoma cells, which undergo mesenchymal transition (21, 62). In accordance with this view, there was a reverse correlation in the expression of the two progression-specific markers in all cell lines used for the analysis (Fig. 5). Hybridization with a fra-1-specific probe revealed an enhanced level of fra-1 mRNA not only in CSML100 cells but in all tumor cell lines which express mts1 but not E-cadherin. In contrast, expression of c-fos was predominantly detected in E-cadherin-expressing cells, although no absolute correlation was observed. There was no obvious correlation of fra-2 transcription with the E-cadherin/mts1 distribution (Fig. 5). junD was ubiquitously expressed in all cell lines analyzed. Therefore, activation of fra-1, but not fra-2 or junD, transcription might regularly occur during progression of an epithelial tumor towards a more malignant phenotype.
FIG. 5.
Northern blot analysis of E-cadherin, mts1, fra-1, fra-2, c-fos, and junD mRNAs in 13 mouse adenocarcinoma cell lines and 10T1/2 fibroblasts. Total RNA was extracted from cells growing at 50 to 70% confluence, and 20-μg samples were loaded as indicated. The filter was hybridized to the E-cadherin, mts1, fra-1, fra-2, c-fos, and junD cDNAs. Hybridization to the GAPDH probe was used as a control for RNA loading.
Activation of fra1 is sufficient to induce morphological alterations in CSML0 cells.
The pCMVFra-1 expression construct was tested for the ability to transactivate an AP-1-responsive promoter in transiently transfected CSML0 cells. We found that pCMVFra-1 transactivated the pcfLUC reporter four- to sixfold (data not shown), consistent with an excess of JunD protein in these cells. To obtain clones of CSML0 cells constantly expressing the Fra-1 protein, the parental cell line was transfected with the pCMVFra-1 construct, followed by selection of neomycin-resistant clones. While the phenotype of the majority of the clones obtained was indistinguishable from that of CSML0 cells, two clones exhibited a drastically altered morphology (data not shown). These cells lost their epithelioid appearance and acquired an elongated fibroblastoid shape. Exogenous fra-1 mRNA was detected only in the fibroblastoid clones and not in four randomly selected epithelium-like clones, suggesting that Fra-1 may function in morphological conversion of CSML0 cells.
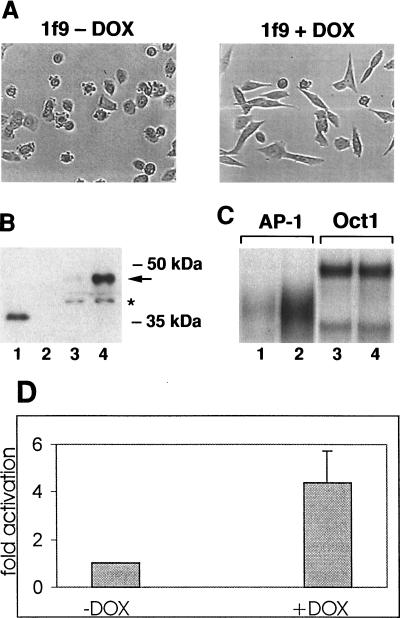
To confirm this assumption, we applied a tetracycline-regulated expression system, which enables the avoidance of artifacts resulting from subcloning of the parental cell line (27). Stable clones were generated by cotransfection of the CSML0-tet-on22 cell line, harboring rtTA, a DOX-inducible transactivator, with pUHDFra-1 and the pBabeHyg hygromycin resistance plasmid. The clones obtained were cultivated in the presence or absence of DOX, and the influence of this drug on cell morphology was used as a criterion for selection. Six clones were sensitive to DOX treatment. One of three clones whose phenotype in the absence of DOX was indistinguishable from that of the parental cell line (clone 1f9) was chosen for further studies. Morphological alterations became visible 24 h after addition of DOX, reached the maximum in 48 h, and were visible for 12 to 15 days (Fig. 6A). We analyzed the activation of fra-1 expression in response to treatment of lf9 cells with DOX through Western blotting. Whereas the protein was undetectable in cells maintained in the absence of DOX, addition of the drug led to the accumulation of the 45-kDa Fra-1-immunoreactive polypeptide (Fig. 6B, lanes 3 and 4), corresponding to the fully phosphorylated protein. The amount and phosphorylation level of the Fra-1 protein induced in lf9 cells in response to DOX treatment were similar to those detected in the CSML100 cell line (compare Fig. 3B and 6B). EMSA confirmed that Fra-1 produced in this system was functional for binding to the consensus TRE. Maintenance for 48 h in the presence of DOX led to a sevenfold increase of the AP-1 binding activity in lf9 cells (Fig. 6C, lanes 1 and 2). Binding activity in nuclear extracts prepared from DOX-treated and nontreated lf9 cells was normalized by using a probe for the Oct-1 transcription factor (Fig. 6C, lanes 3 and 4). Supershift analysis revealed that the predominant Fos component of the AP-1 complex detected in nuclear extracts of DOX-treated lf9 cells was Fra-1 (data not shown). We next examined how the DOX-mediated induction of Fra-1 affected the AP-1-dependent transcription. lf9 cells were transiently transfected with the pfLUC5×TRE reporter construct, which contained five copies of collTRE cloned in a direct orientation upstream of the minimal promoter. After transfection, cells were maintained in the absence or presence of DOX for 48 h. DOX stimulated the reporter activity by 4.5-fold (Fig. 6D). On the other hand, in similar experiments with an analogous construct bearing five copies of the mutated TRE, no effect of DOX treatment was observed (data not shown).
FIG. 6.
Characterization of the DOX-responsive lf9 clone. (A) Induction of Fra-1 expression alters cell morphology. lf9 cells were maintained in the presence or absence of DOX for 48 h. (B) Immunoblot analysis of Fra-1 expression. Nuclear extracts (20 μg) from lf9 cells maintained in the absence (lane 3) or presence (lane 4) of DOX were ressolved by 10% polyacrylamide gel electrophoresis and analyzed by immunoblotting with anti-Fra-1 antibody. In vitro-synthesized (lane 1) or mock-synthesized (lane 2) Fra-1 protein was used to control the specificity of the staining. (C) DOX treatment of lf9 cells induces binding to TRE in gel shift assay. Radiolabeled oligonucleotides that contained collTRE (lanes 1 and 2) or the Oct-1 binding site (lanes 3 and 4) were mixed with nuclear extracts prepared from lf9 cells maintained in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of DOX and analyzed by EMSA. (D) DOX activates the AP-1-responsive reporter pfLUCTRE×5. lf9 cells were cotransfected with 4 μg of the reporter construct and 500 ng of pCMVβ-gal control vector, used as a transfection standard. Transfection cells were grown for 48 h in the absence or presence of DOX. The results (means and standard deviations) of four independent experiments are expressed as fold activation relative to the activity of the reporter in cells maintained in the absence of DOX.
Fra-1 activates transcription of a number of tumor progression-associated genes.
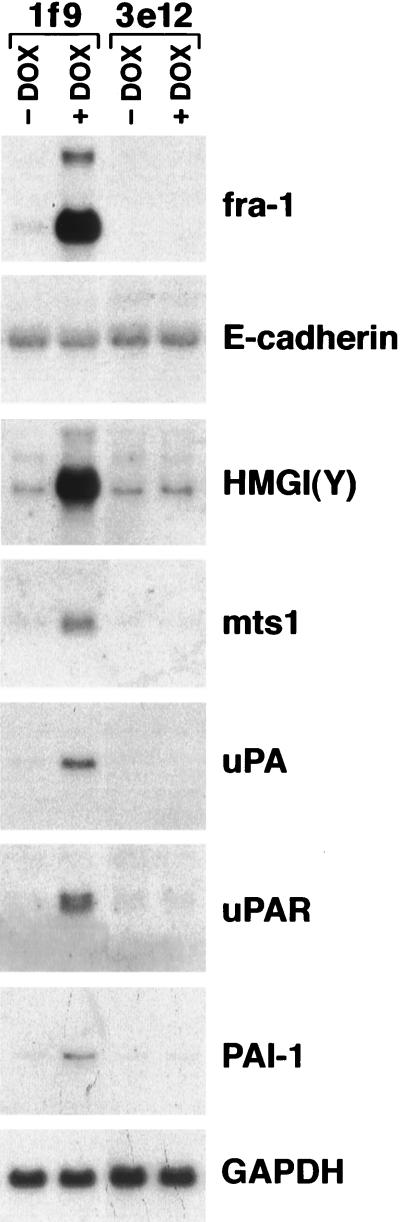
We next tested whether the Fra-1-induced morphological alterations in lf9 cells were accompanied by changes in the pattern of gene expression. The transcription of a number of genes differentially expressed in CSML0 and CSML100 cells (Fig. 1) was studied in the lf9 clone by Northern blot analysis. As a negative control, we used another hygromycin-resistant clone, the 3e12 clone, which did not express Fra-1 upon DOX treatment. A high level of the epithelial marker E-cadherin transcription was not affected by DOX treatment. On the other hand, we observed a DOX-dependent expression of two tumor progression markers, mts1 and HMGI(Y) (Fig. 7). Moreover, we analyzed the expression of four proteinases, MMP-3, MMP-9, uPA, and tPA, in DOX-stimulated and unstimulated cells. Although the transcription of all of these genes was reported to be AP-1 dependent, only uPA was activated by Fra-1 in lf9 cells (Fig. 7 and data not shown). Interestingly, in addition to that of uPA, transcription of two other components of the uPA system, uPAR and PAI-1, was markedly induced by Fra-1 (Fig. 7). On the other hand, TIMP-1 expression was not affected by DOX (data not shown).
FIG. 7.
Fra-1 stimulates transcription of a number of genes associated with tumor progression. RNA was isolated from lf9 or 3e12 cells after 48 h of stimulation by DOX or no stimulation. Twenty micrograms of total RNA was hybridized to cDNA probes, as indicated. Equal loading was controlled by hybridization to GAPDH.
Expression of Fra-1 enhances invasiveness and influences cell motility in in vitro assays.
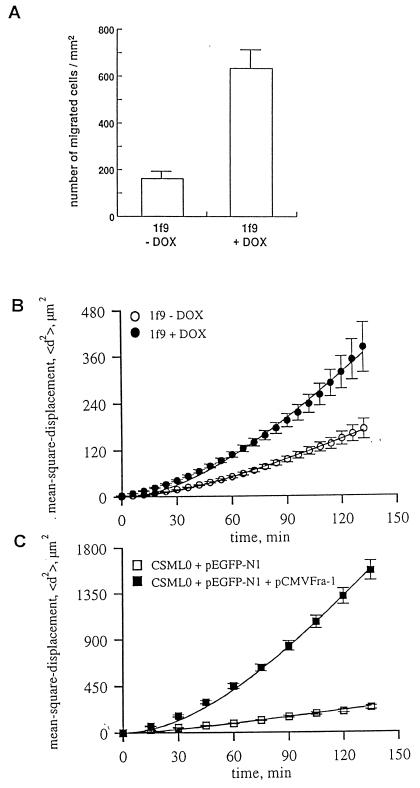
The uPA system plays a pivotal role in the process of cancer cell invasion into the surrounding tissue as a result of the proteolysis of the ECM (reviewed in reference 2). Since transcription of three key components of this system was induced by Fra-1 in lf9 cells, we investigated whether Fra-1 overexpression is sufficient to promote invasion through an artificial basement membrane composed of Matrigel. lf9 cells were grown for 48 h in the presence or in the absence of DOX, and then 105 cells were loaded onto the matrix-coated filters in Boyden chambers and cultured for 24 h. The number of cells reaching the underside of the filters increased approximately fourfold as a result of the induction of Fra-1 expression (Fig. 8A).
FIG. 8.
Effect of Fra-1 induction on cell invasion (A) and motility (B and C). (A) lf9 cells were maintained in the absence or presence of DOX for 48 h. The cultivation of 105 cells was prolonged for 24 h more in Boyden chambers, and the number of cells reaching the matrix-coated filters was calculated. Each experiment was performed in duplicate, and the results of five independent experiments are expressed as means and standard deviations. (B) Effect of Fra-1 induction on the motility of lf9 cells. Cells were grown for 48 h in the absence or presence of DOX. Video recordings and determinations of random cell motility were performed as described in Materials and Methods. (C) Effect of transient transfection with pCMVFra-1 on the motility of CSML0 cells. Cells were transfected with pCMVFra-1 along with the pEGFP-N1 construct. Selection of transfected cells and determination of random cell motility were carried out as described in Materials and Methods. The cell displacement was expressed as the mean square displacement over a period of 150 min.
Morphological transformation of lf9 cells in response to the induction of Fra-1 could reflect the deregulation of genes encoding cytoskeletal, cytoskeleton-associated, and/or cell adhesion proteins. These proteins, which determine cell morphology, also play a central role in cell motility (reviewed in references 31 and 49). In the present work, we did not focus on which components were affected by Fra-1 expression. Instead, we tested whether the expression of Fra-1 may in general contribute to tumor progression by modulation of cellular motility. We examined the motility of individual cells plated at a low density (3.5 × 103 cells/cm2) by using time-lapse video recording and computer-assisted image analysis. lf9 cells grown for 48 h in the presence and absence of DOX were transferred to a microscope, where images from a number of different areas were recorded. Data were expressed as the mean square displacement of the cells, <d2>, at time t (the time of observation) (Fig. 8B). According to the assumption that the motile behavior of cultured cells is a persistent random walk (20, 50), we determined the three major parameters characterizing single-cell motility, namely, the root mean square speed (S), the rate of diffusion (R), and the time of persistence in direction (P) (Table 1). When lf9 cells were maintained on Matrigel-coated plastic dishes for 48 h in the presence of DOX, the mean square displacement of the cells over the time of observation increased considerably compared to that of the control lf9 cells, where the expression of Fra-1 was not triggered (Fig. 8B). Moreover, the expression of Fra-1 resulted in increases of the root mean square speed and the rate of diffusion of 30 and 125%, respectively. The persistence time also increased by 30%. An even greater effect was observed when CSML0 cells were transiently cotransfected with pCMVFra-1 along with the pEGFP-N1 construct, coding for the green fluorescence protein. At 24 h after the transfection, cells were plated on plastic and incubated for additional 24 h before video recording was initiated. The expression of EGFP allowed the tracking of only transfected cells expressing Fra-1. The expression of Fra-1 in CSML0 cells caused a strong increase in mean square displacement (Fig. 8C). The root mean square speed, the rate of diffusion, and the persistence time increased by 65, 900, and 275%, respectively (Table 1).
TABLE 1.
Root mean square speed (S), rate of diffusion (R), and persistence time (P) for lf9 cells 48 h after addition of DOX and for CSML0 cells 48 h after transient transfection with pEGFP-N1 and pCMVFra-1a
| Cells and treatment | Substratum | No. of cells | S (μm/min) (mean ± SD) | R (μm2/min) (mean ± SD) | P (min) (mean ± SD) |
|---|---|---|---|---|---|
| lf9 | Matrigel | 152 | 0.15 ± 0.003 | 2.0 ± 0.06 | 44 ± 3 |
| lf9 + DOX | Matrigel | 130 | 0.20 ± 0.007 | 4.5 ± 0.40 | 57 ± 9 |
| CSML0 + pEGFP-N1 | Plastic | 68 | 0.23 ± 0.020 | 2.2 ± 0.06 | 20 ± 4 |
| CSML0 + pEGFP-N1 + pCMVFra-1 | Plastic | 39 | 0.38 ± 0.008 | 22 ± 0.8 | 75 ± 6 |
Data are derived from the results presented in Fig. 8.
In summary, Fra-1 expression led to an increase in random cell motility and in the time of cellular persistence in direction. The observed phenomenon may reflect a mesenchymal conversion induced by the ectopic expression of Fra-1.
DISCUSSION
In the present work, we studied the transcription of a group of AP-1-dependent invasion-associated genes in two genetically related mammary adenocarcinoma cell lines, CSML0 and CSML100. Enhanced transcription of all of these genes was detected in the invasive CSML100 cells but not in the epithelioid CSML0 cells (Fig. 1). To elucidate the molecular basis of the differential expression of these genes in the CSML cell system, the AP-1 composition was studied. Fos was found to be a component that functionally limits the AP-1 activity in CSML0 cells, although EMSA and Western blotting analysis revealed the expression of the phosphorylated c-Fos protein in this cell line (Fig. 2 and 3). Sequence analysis of c-fos cDNA cloned from CSML0 cells revealed no mutations in the coding part of the gene. We are currently studying the mechanisms maintaining the inactive state of c-Fos in CSML0 cells, particularly the role of phosphorylation of the protein.
The study of c-fos transcription in a number of cell lines derived from various epithelial tumors revealed that the decreased c-fos mRNA level often (but not always) correlates with the malignant phenotype of adenocarcinoma cells (Fig. 5). This is consistent with the recently described suppression of c-fos transcription with the malignant transformation of human bronchial epithelial cells (52). In human bronchial epithelial cells, the suppression of c-fos transcription occurs through a CRE site located in a promoter of the gene. Presently it is not clear whether the suppression mechanism is cell type specific or can also be utilized in adenocarcinoma cells upon progression.
We found that the increased DNA binding and functional activity of AP-1 in CSML100 cells is due to a constitutive expression of Fra-1 and Fra-2 transcription factors (Fig. 2, 3, and 4), which could not be detected in CSML0 nuclear extracts. Furthermore, the activation of fra-1 might be a regular event in the progression of epithelial tumors (Fig. 5). Transcriptional activation of the fra-1 gene itself can be mediated by AP-1 via a TRE located in the first intron of the gene (11). The c-FosER fusion protein caused immediate induction of fra-1 transcription in NIH 3T3 fibroblasts (75) and in CSML0 cells as well (data not shown), likely through this site. On the other hand, the activation of AP-1 in CSML0 cells by overexpression of exogenous fra-1 did not lead to the activation of endogenous transcription (Fig. 7). Therefore, the accumulation of fra-1 mRNA in cells of malignant carcinomas does not reflect an enhanced level of AP-1, maintained by a positive autoregulatory loop, and the basis of the observed correlation seems to be more complex.
To study whether the Fra-1 protein is sufficient to affect features of epithelioid adenocarcinoma cells, we obtained CSML0 clones expressing exogenous Fra-1. Expression of the exogenous fra-1 led to a progressive elongation of cells, which adopted a more flattened, fibroblast-like morphology (Fig. 6). We examined whether the induction of Fra-1 synthesis may affect the transcription of nine genes previously implicated in mesenchymal transition and tumor progression: the mts1, HMGI(Y), MMP-3, MMP-9, uPA, uPAR, PAI-1, tPA, and TIMP genes. The most pronounced effect (65-fold activation) was observed in the case of HMGI(Y) expression (Fig. 7). The HMGI(Y) gene encodes a nonhistone chromatin protein which can influence the bending of DNA via an interaction with the minor groove. Moreover, HMGI(Y) physically interacts with a number of transcription factors and thereby modifies promoter activity (80, 81). To our knowledge, the up-regulation of HMGI(Y) by any of the AP-1 family members has not been described before. Interestingly, in thyroid tumor cell lines, fra-1 expression depends on the presence of the HMGI-C protein, which is encoded by a gene closely related to the HMGI(Y) gene (83). This interrelation between architectural components of the transcriptional apparatus and a transcription factor may contribute to the rearrangement of the genetic program occurring during tumor progression and may have important implications for epithelial tumorigenesis.
ECM-degrading proteinases, i.e., metalloproteinases and serine proteinases, have been implicated in tumor progression and cancer metastasis (reviewed in reference 85). Consistent with this view, two metalloproteinases, MMP-3 and MMP-9, and all tested components of the uPA and tPA systems are up-regulated at the RNA level in the metastatic CSML100 cell line. No transcription of these genes was seen in the nonmetastatic counterpart CSML0 (Fig. 1). Induction of Fra-1 synthesis in the lf9 clone led to a selective up-regulation of all components of the uPA system, while neither MMP-3, MMP-9, tPA, nor TIMP transcription was affected by DOX (Fig. 7). The selective activation of uPA, uPAR, and PAI-1 (but not MMP-3, MMP-9, tPA, and TIMP) was also seen in clones constantly expressing Fra-1 (data not shown). This example of the selective up-regulation of a group of functionally closely connected genes in CSML0 cells might be interpreted as an element of a certain differentiation pathway, utilized by cancer cells upon tumor progression. In CSML0 cells, the expression of all of the above-mentioned AP-1-dependent genes, including those for MMP-3, MMP-9, tPA, and TIMP-1, was highly activated upon c-FosER induction (data not shown). Therefore, the selectivity of the Fra-1-mediated activation can be attributed to the specific features of this protein rather than to the properties of the CSML0 cell line. These data are consistent with previous findings obtained by study of the c-fos-deficient 3T3-related fibroblasts. It was demonstrated that some AP-1-dependent genes, including that for MMP-3, require c-fos for full expression and inducibility by growth factors. In contrast, the metallothionein gene, also known to contain functional TREs, was expressed equally in c-Fos-deficient and control cells (35). Therefore, different AP-1 members may have distinctive and specific functions in transcriptional control depending on the context of a particular enhancer. This is supported by the observation that Fra-1 but not other Fos family members was capable of physical and functional interaction with the bHLHZip USF transcription factor (64). On the other hand, the transcription factor FIP specifically bound c-Fos and synergistically activated an AP-1-responsive reporter (13). These data are consistent with the idea that individual Fos family members may have specific functions in cancerogenesis.
Fra-1-mediated morphological alterations in CSML0 cells resembled an epithelial-mesenchymal transition, which often occurs during progression of epithelial tumors. However, in contrast to Ep-1 mammary epithelial cells, where E-cadherin was down-regulated upon c-FosER induction (67), neither Fra-1 (Fig. 7) nor c-FosER (data not shown) influenced the expression of this epithelial marker in CSML0 cells. Jooss and Müller (41) studied the changes in expression levels of 24 genes determining cell morphology occurring in the process of v-Fos-mediated transformation of 208F rat fibroblasts. A fourfold up-regulation in v-Fos transformed fibroblasts was seen with ezrin and tropomyosin-3, whereas tropomyosin-1 was down-regulated. Ezrin, a protein tethering the actin cytoskeleton to the plasma membrane, was shown to be essential for plasma membrane ruffling and for the extension of pseudopodia in v-Fos-transformed Rat-1 cells (48). Presently, it is not clear whether the ectopic expression of Fra-1 in CSML0 cells caused a reorganization of the cytoskeleton and affected cell adhesion similarly to that induced by v-Fos in rat fibroblasts. However, we found that these alterations are sufficient to affect cellular motility by increasing the root mean square speed as well as the rate of diffusion and persistence time (Fig. 8B and C; Table 1). The extended time of persistence in direction and the increased root mean square speed were seen in L fibroblasts (84). Fra-1-mediated induction of the fibroblastoid type of motility in epithelium-like carcinoma cells reflects certain elements of mesenchymal transition and may determine the fourfold increase of in vitro invasiveness through a basement membrane (Fig. 8A). Activation of the uPA system may also contribute to the formation of a more invasive phenotype.
Previous reports indicated that ectopic Fra-1 expression did not affect the morphology of immortalized fibroblasts (11, 87). The contrast between those data and the results reported here is probably due to the differences in genetic backgrounds of fibroblasts and epithelioid CSML0 cells; e.g., Fra-1-responsive genes may be up-regulated in fibroblasts and not expressed in the epithelium. Alternatively, the activity of Fra-1 may be cell type specific and depend on the expression of heterodimerization partners, or it might require specific posttranslational modifications.
The c-FosER chimera triggers irreversible fibroblastoid conversion of nontransformed immortalized (67) and transformed (data not shown) epithelia. However, as a rule, c-Fos protein is not detected in asynchronously growing cells. Fra-1 expression correlates with the mesenchymal characteristics of epithelial tumors (Fig. 5), and it can be up-regulated by c-FosER in CSML0 cells (data not shown). The role of the Fra-1 protein in the maintenance of the c-FosER-mediated fibroblastoid conversion will be a focus of our further investigations.
Recently, the essential role of the Fra-1 protein in oncogenic transformation of thyroid cells was demonstrated. The blocking of Fra-1 synthesis in neoplastic thyroid cell lines by using an antisense strategy caused a partial reversion of the transformed phenotype as evaluated by two criteria: morphological appearance and the ability to form colonies in soft agar. However, a construct expressing fra-1 in the sense orientation had no effect in normal thyroid epithelium (83). Here we show for the first time that the activation of fra-1 is sufficient to modulate the phenotype of epithelial tumor cells, induce the expression of some markers of malignant carcinoma, and activate motility and invasiveness. These data highlight the pivotal role of Fra-1 in the development of epithelial tumors.
ACKNOWLEDGMENTS
We thank M. Busslinger (Vienna, Austria) for the pMVc-fosER construct and GP+E packaging cell line, P. Herrlich (Karlsruhe, Germany) for JunD expression vector, and M. Jäättelä and J. Lukas (Copenhagen, Denmark) for pcfLUC and pBabeHyg plasmids. We are grateful to K. Danø (Copenhagen), D. Edwards (Calgary, Canada), R. Breatnach (Cambridge, United Kingdom), and S. Wilhelm (St. Louis, Mo.) for providing cDNA probes. We acknowledge N. Ambartsumian, M. Cohn, and M. Kriajevska for critical reading of the manuscript and T. Lukanidina and H. Nors for technical assistance.
This work was supported by grants from the Danish Cancer Society, Institute for Gene Biology (INTAS grant), Danish Medical Research Council, and Agnes and Poul Friis Foundation.
REFERENCES
- 1.Albini A, Iwamoto Y, Kleinman H K, Martin G R, Aaronson S A, Kozlowsky J M, McEvan R N. A rapid in vivo assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- 2.Andreasen P A, Kjøller L, Christensen L, Duffy M J. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Andrews N C, Faller D. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 5.Arts J, Herr I, Lansink M, Angel P, Kooistra T. Cell type specific DNA-protein interactions at the tissue-type plasminogen activator promoter in human endothelial and HeLa cells in vivo and in vitro. Nucleic Acids Res. 1997;25:311–317. doi: 10.1093/nar/25.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahler D W, Lord E M, Kennel S J, Horan P K. Heterogeneity and clonal variation related to cell surface expression of a mouse lung tumor-associated antigen quantified using flow cytometry. Cancer Res. 1984;144:3317–3323. [PubMed] [Google Scholar]
- 7.Barnett S C, Eccles S A. Studies of mammary carcinoma metastasis in a mouse model system. I. Derivation and characterization of cells with different metastatic properties during tumor progression in vivo. Clin Exp Metast. 1984;2:15–36. doi: 10.1007/BF00132304. [DOI] [PubMed] [Google Scholar]
- 8.Basset-Séguin N, Demoly P, Moles J P, Tesniéres A, Gauthier-Rouviére C, Richard S, Blanchard J M, Guilhou J J. Comparative analysis of cellular and tissular expression of c-fos in human keratinocytes: evidence of its role in cell differentiation. Oncogene. 1994;9:765–771. [PubMed] [Google Scholar]
- 9.Behlin D, Vassalli J-D, Combepine C, Godeou F, Nagamine J, Reich E, Kocher H P, Duvoisin R M. Cloning, nucleotide sequencing and expression of cDNAs encoding mouse urokinase-type plasminogen activator. Eur J Biochem. 1985;148:225–232. doi: 10.1111/j.1432-1033.1985.tb08829.x. [DOI] [PubMed] [Google Scholar]
- 10.Bengal E, Ransone L, Scharfmann R, Dwark V T, Tapscott S J, Weintraub H, Verma I M. Functional antagonism between c-Jun and MyoD proteins: a direct physical association. Cell. 1992;68:507–519. doi: 10.1016/0092-8674(92)90187-h. [DOI] [PubMed] [Google Scholar]
- 11.Bergers G, Graninger P, Braselmann S, Wrighton C, Busslinger M. Transcriptional activation of the fra-1 gene by AP-1 is mediated by regulatory sequences in the first intron. Mol Cell Biol. 1995;15:3748–3758. doi: 10.1128/mcb.15.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 13.Blanar M A, Rutter W J. Interaction cloning: identification of a helix-loop helix zipper protein that interacts with c-Fos. Science. 1992;256:1014–1017. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- 14.Bossy-Wetzel E, Bakiri L, Yaniv M. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 1997;16:1695–1709. doi: 10.1093/emboj/16.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breatnach R, Matrisian L M, Gesnel M C, Staub A, Leroy P. Sequences coding for part of oncogene-induced transin are highly conserved in a related rat gene. Nucleic Acids Res. 1987;15:1139–1151. doi: 10.1093/nar/15.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiapetta G, Bandiera A, Berlingieri M T, Visconti R, Manfioletti G, Battista S, Martinez-Tello F J, Santoro M, Giancotti V, Fusco A. The expression of the high mobility group HMG I(Y) proteins correlates with the malignant phenotype of human thyroid neoplasias. Oncogene. 1995;10:1307–1314. [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidiniun thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Clark I M, Rowan A D, Edwards D R, Bech-Hansen T, Mann D A, Bahr M J, Cawston T E. Transcriptional activity of the human tissue inhibitor of metalloproteinases 1 (TIMP-1) gene in fibroblasts involves elements in the promoter, exon 1 and intron 1. Biochem J. 1997;324:611–617. doi: 10.1042/bj3240611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen D R, Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a Fos-related antigen. Mol Cell Biol. 1988;8:2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn G A. Characterizing a kinesis response: time averaged measures of cell speed and directional persistence. In: Keller H, Till G O, Abor A, editors. Leucocyte locomotion and chemotaxis. Basel, Switzerland: Birkhauser Verlag; 1983. pp. 14–33. [DOI] [PubMed] [Google Scholar]
- 21.Ebralidze A, Tulchinsky E, Grigorian M, Afanasjeva A, Senin V, Revazova E, Lukanidin E. Isolation and characterization of a gene specifically expressed in different metastatic cells and whose deduced gene product has a high degree of homology to a Ca-binding protein family. Genes Dev. 1989;3:1086–1093. doi: 10.1101/gad.3.7.1086. [DOI] [PubMed] [Google Scholar]
- 22.Fialka I, Schwarz H, Reichmann E, Oft M, Busslinger M, Beug H. The estrogen-dependent c-JunER protein causes a reversible loss of mammary epithelial cell polarity involving a destabilization of adherens junctions. J Cell Biol. 1996;132:1115–1132. doi: 10.1083/jcb.132.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fish E M, Molitoris B A. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med. 1994;330:1580–1588. doi: 10.1056/NEJM199406023302207. [DOI] [PubMed] [Google Scholar]
- 24.Ford H L, Salim M M, Chakravarty R, Aluiddin V, Zain S B. Expression of Mts1, a metastasis-associated gene, increases motility but not invasion of a nonmetastatic mouse mammary adenocarcinoma cell line. Oncogene. 1995;11:2067–2075. [PubMed] [Google Scholar]
- 25.Funk M, Poensgen B, Graulich W, Jérome V, Müller R. A novel transformation-relevant activation domain in Fos proteins. Mol Cell Biol. 1997;17:537–544. doi: 10.1128/mcb.17.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 28.Grigoriadis A E, Wang Z-Q, Cecchini M G, Hoffstetter W, Felix R, Fleisch H A, Wagner E F. cFos: a key regulator of osteoclast-macrophage lineage determination and bone remodelling. Science. 1994;266:443–447. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 29.Gruda M C, Kovary K, Metz R, Bravo R. Regulation of Fra-1 and Fra-2 phosphorylation differs during the cell cycle of fibroblasts and phosphorylation in vitro by MAP kinase affects DNA binding activity. Oncogene. 1994;9:2537–2547. [PubMed] [Google Scholar]
- 30.Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendrix M J C, Seftor E A, Chu Y-W, Trevor K T, Seftor R E B. Role of intermediate filaments in migration, invasion and metastasis. Cancer Metastasis Rev. 1996;15:507–525. doi: 10.1007/BF00054016. [DOI] [PubMed] [Google Scholar]
- 32.Hennigan R F, Hawker K L, Ozanne B W. Fos-transformation activates genes associated with invasion. Oncogene. 1994;9:3591–3600. [PubMed] [Google Scholar]
- 33.Himelstein B P, Lee E J, Sáto H, Seiki M, Muschel R J. Transcriptional activation of the matrix metalloproteinase-9 gene in an H-ras and v-myc transformed rat embryo cell line. Oncogene. 1997;14:1995–1998. doi: 10.1038/sj.onc.1201012. [DOI] [PubMed] [Google Scholar]
- 34.Hirai S-I, Ryseck R-P, Mechta F, Bravo R, Yaniv M. Characterization of junD: a new member of the jun proto-oncogene family. EMBO J. 1989;8:1433–1439. doi: 10.1002/j.1460-2075.1989.tb03525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu E, Mueller E, Oliviero S, Papaioannou V E, Johnson R, Spiegelman B M. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994;13:3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jäättelä M, Mouritzen H, Elling F, Bastholm L. A20 zinc finger protein inhibits TNF and IL-1 signaling. J Immunol. 1996;156:1166–1173. [PubMed] [Google Scholar]
- 37.Jehn B, Costello E, Marti A, Keon N, Deane R, Li F, Friis R R, Burry P H, Martin F, Jaggi R. Overexpression of Mos, Ras, Src, and Fos inhibits mouse mammary epithelial cell differentiation. Mol Cell Biol. 1992;12:3890–3902. doi: 10.1128/mcb.12.9.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson K R, Lehn D A, Elton T S, Barr P J, Reeves R. Complete murine cDNA sequence, genomic structure and tissue expression of the high mobility group protein HMG I(Y) J Biol Chem. 1988;263:18338–18342. [PubMed] [Google Scholar]
- 39.Johnson S J, van Lingen B, Papaioannou V E, Spiegelman B M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- 40.Jooss K U, Funk M, Müller R. An autonomous N-terminal transactivation domain in Fos protein plays a crucial role in transformation. EMBO J. 1994;13:1467–1475. doi: 10.1002/j.1460-2075.1994.tb06401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jooss K U, Müller R. Deregulation of genes encoding microfilament-associated proteins during Fos-induced morphological transformation. Oncogene. 1995;10:603–608. [PubMed] [Google Scholar]
- 42.Kataoka K, Fujiwara K T, Noda M, Nishizawa M. MafB, a new Maf family transcription activator that can associate with Maf and Fos but not with Jun. Mol Cell Biol. 1994;14:7581–7591. doi: 10.1128/mcb.14.11.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keeton M R, Curriden S A, van Zonneveld A-J, Loskutoff D J. Identification of regulatory sequences in the type 1 plasminogen activator inhibitor gene responsive to transforming growth factor β. J Biol Chem. 1991;266:23048–23052. [PubMed] [Google Scholar]
- 44.Kerppola T K, Curran T. Maf and Nrl can bind to AP-1 sites and form heterodimers with Fos and Jun. Oncogene. 1996;9:675–684. [PubMed] [Google Scholar]
- 45.Kerr L D, Holt J T, Matrisian L M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988;242:1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- 46.Kovary K, Bravo R. The Jun and Fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991;11:4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovary K, Bravo R. Existence of different Fos/Jun complexes during the G0-to-G1 transition and during exponential growth in mouse fibroblasts: differential role of Fos proteins. Mol Cell Biol. 1992;12:5015–5023. doi: 10.1128/mcb.12.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamb R F, Ozanne B W, Roy C, McGarry L, Stipp C, Mangeat P, Jay D G. Essential functions of ezrin in maintenance of cell shape and lamellopodial extension in normal and transformed fibroblasts. Curr Biol. 1997;7:682–688. doi: 10.1016/s0960-9822(06)00295-8. [DOI] [PubMed] [Google Scholar]
- 49.Lauffenburger D A, Horwitz A F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 50.Lauffenburger D A, Linderman J J. Receptors. Models for binding, trafficking and signalling. Oxford, United Kingdom: Oxford University Press; 1993. [Google Scholar]
- 51.LeBowitz J H, Kobayashi T, Staudt L, Baltimore D, Sharp P A. Octamer-binding proteins from B or HeLa cells stimulate transcription of the immunoglobulin heavy chain promoter in vitro. Genes Dev. 1988;2:1227–1237. doi: 10.1101/gad.2.10.1227. [DOI] [PubMed] [Google Scholar]
- 52.Lee H-Y, Chaudhary J, Walsh G L, Hong W K, Kurie J M. Suppression of c-Fos gene transcription with malignant transformation of human bronchial epithelial cells. Oncogene. 1998;16:3039–3046. doi: 10.1038/sj.onc.1201843. [DOI] [PubMed] [Google Scholar]
- 53.Lee W, Mitchell P, Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987;49:741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- 54.Lengyel E, Wang H, Stepp E, Juarez J, Wang Y, Doe W, Pfarr C M, Boyd D. Requirement of an upstream AP-1 motif for the constitutive and phorbol ester-inducible expression of the urokinase-type plasminogen activator receptor gene. J Biol Chem. 1996;271:23176–23184. doi: 10.1074/jbc.271.38.23176. [DOI] [PubMed] [Google Scholar]
- 55.Lord K A, Abdollahi A, Hoffman-Liebermann B, Liebermann D A. Proto-oncogenes of the Fos/Jun family of transcription factors are positive regulators of myeloid differentiation. Mol Cell Biol. 1993;13:841–851. doi: 10.1128/mcb.13.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mechta F, Lallemand D, Pfarr C M, Yaniv M. Transformation by ras modifies AP-1 composition and activity. Oncogene. 1997;14:837–847. doi: 10.1038/sj.onc.1200900. [DOI] [PubMed] [Google Scholar]
- 57.Mumberg D, Lucibello F C, Schuermann M, Müller R. Alternative splicing of fosB transcripts results in differentially expressed mRNAs encoding functionally antagonistic proteins. Genes Dev. 1991;5:1212–1223. doi: 10.1101/gad.5.7.1212. [DOI] [PubMed] [Google Scholar]
- 58.Nakabeppu Y, Nathans D. A naturally occuring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64:751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- 59.Nerlov C, Rorth P, Blasi F, Johnsen M. Essential AP-1 and PEA3 binding elements in the human urokinase enhancer display cell type-specific activity. Oncogene. 1991;6:1583–1592. [PubMed] [Google Scholar]
- 60.Nishina H, Sato H, Suzuki T, Sato M, Iba H. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc Natl Acad Sci USA. 1990;87:3619–3623. doi: 10.1073/pnas.87.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nomura S, Hogan B L, Wills A J, Heath J K, Edwards D R. Developmental expression of tissue inhibitor of metalloproteinase (TIMP) RNA. Development. 1989;105:575–583. doi: 10.1242/dev.105.3.575. [DOI] [PubMed] [Google Scholar]
- 62.Okada H, Danoff T M, Kallury R, Neilson E G. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273:563–574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- 63.Olson L, Forchhammer J. Induction of the metastatic phenotype in a mouse tumor model by 5-azacytidine, and characterization of an antigen associated with metastatic activity. Proc Natl Acad Sci USA. 1984;81:3389–3393. doi: 10.1073/pnas.81.11.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pognonec P, Boulukos K E, Aperlo C, Fujimoto M, Ariga H, Nomoto A, Kato H. Cross family interaction between the bHLHZip USF and bZIP Fra1 proteins results in down-regulation of AP-1 activity. Oncogene. 1997;14:2091–2098. doi: 10.1038/sj.onc.1201046. [DOI] [PubMed] [Google Scholar]
- 65.Preston G A, Lyon T T, Yin Y, Lang J E, Solomon G, Annab L, Srinivasan D G, Alcorta D A, Barret J C. Induction of apoptosis by c-Fos protein. Mol Cell Biol. 1996;16:211–218. doi: 10.1128/mcb.16.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ram G T, Reeves R, Hosick H L. Elevated high motility group-I(Y) gene expression is associated with progressive transformation of mouse mammary epithelial cells. Cancer Res. 1993;53:2655–2660. [PubMed] [Google Scholar]
- 67.Reichmann E, Schwarz H, Deiner E M, Leitner I, Eilers M, Berger J, Busslinger M, Beug H. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell. 1992;71:1103–1116. doi: 10.1016/s0092-8674(05)80060-1. [DOI] [PubMed] [Google Scholar]
- 68.Ringwald M, Schuh R, Vestweber D, Eistetter H, Lottspeich F, Engel J, Doelz R, Jahnig F, Epplen J, Mayer S, Müller C, Kemler R. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca-dependent cell adhesion. EMBO J. 1987;6:3647–3653. doi: 10.1002/j.1460-2075.1987.tb02697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rutberg S E, Saez E, Glick A, Dlugosz A A, Spiegelman B M, Yuspa S H. Differentiation of mouse keratinocytes is accompanied by PKC-dependent changes in AP-1 proteins. Oncogene. 1996;13:167–176. [PubMed] [Google Scholar]
- 70.Ryseck R-P, Bravo R. c-Jun, JunB and JunD differ in their binding affinities to AP-1 and CRE consensus sequences: effect of Fos proteins. Oncogene. 1991;6:533–542. [PubMed] [Google Scholar]
- 71.Saksela K, Baltimore D. Negative regulation of immunoglobulin kappa light-chain gene transcription by a short sequence homologous to the murine B1 repetitive element. Mol Cell Biol. 1993;13:3698–3705. doi: 10.1128/mcb.13.6.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 73.Savagner P, Boyer B, Vallés A M, Jouanneau J, Yamada K M, Thiery J-P. Modulations of the epithelial phenotype during embryogenesis and cancer progression. In: Dickson R, Lippman M, editors. Mammary tomorogenesis and malignant progression. Norwell, Mass: Kluwer Academic; 1994. pp. 229–249. [DOI] [PubMed] [Google Scholar]
- 74.Schreiber M, Poirier C, Franchi A, Kurzbauer R, Guenet J-L, Carle G F, Wagner E F. Structure and chromosomal assignment of the mouse fra-1 gene, and its exclusion as a candidate gene for oc (osteosclerosis) Oncogene. 1997;15:1171–1178. doi: 10.1038/sj.onc.1201460. [DOI] [PubMed] [Google Scholar]
- 75.Schuermann M, Hennig G, Müller R. Transcriptional activation and transformation by chimaeric Fos-estrogen receptor proteins: altered properties as a consequence of gene fusion. Oncogene. 1993;8:2781–2790. [PubMed] [Google Scholar]
- 76.Senin V M, Buntsevich A M, Afanasyeva A V, Kiseleva N S. A new line of murine carcinosarcoma. Exp Oncol USSR. 1983;5:35–39. . (In Russian.) [Google Scholar]
- 77.Senin V M, Ivanov A M, Afanasjeva A V, Buntsevich A M. New organospecific metastatic transplanted tumors of mice and their use for studying laser effect on dissemination. Vestnik USSR Acad Med Sci. 1984;5:85–91. . (In Russian.) [PubMed] [Google Scholar]
- 78.Sonnenberg A, Daams H, Calafat J, Hilgers J. In vitro differentiation and progression of mouse mammary tumor cells. Cancer Res. 1984;46:5913–5922. [PubMed] [Google Scholar]
- 79.Suzuki T, Okuno H, Yoshido T, Endo T, Nishina H, Iba H. Difference in transcriptional regulatory function between c-Fos and Fra-2. Nucleic Acids Res. 1991;19:5537–5542. doi: 10.1093/nar/19.20.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thanos D, Maniatis T. The high mobility group protein HMG I(Y) is required for NF-κB-dependent induction of the human IFNβ gene. Cell. 1992;71:777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- 81.Thanos D, Maniatis T. Virus induction of human IFNb gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 82.Tulchinsky E M, Georgiev G P, Lukanidin E M. Novel AP-1 binding site created by DNA-methylation. Oncogene. 1996;12:1737–1745. [PubMed] [Google Scholar]
- 83.Vallone D, Battista S, Pierantoni G M, Fedele M, Casalino L, Santoro M, Viglietto G, Fusco A, Verde P. Neoplastic transformation of rat thyroid cells requires the junB and fra-1 gene induction which is dependent on the HMGI-C gene product. EMBO J. 1997;16:5310–5321. doi: 10.1093/emboj/16.17.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walmod, P. S., A. Foley, A. Berezin, U. Ellerbeck, H. Nau, E. Bock, and V. Berezin. Cell motility is inhibited by the antiepileptic compound valproic acid and its teratogenic analogues. Submitted for publication. [DOI] [PubMed]
- 85.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 86.Wilhelm S M, Collier I E, Marmer B L, Eizen A Z, Grant G A, Goldberg G I. SV-40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989;264:17213–17221. [PubMed] [Google Scholar]
- 87.Wisdom R, Verma I M. Transformation by Fos proteins requires a C-terminal transactivation domain. Mol Cell Biol. 1993;13:7429–7438. doi: 10.1128/mcb.13.12.7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeheb R, Gelehrter T D. Cloning and sequencing of cDNA for the rat plasminogen activator inhibitor-1. Gene. 1988;73:459–468. doi: 10.1016/0378-1119(88)90510-0. [DOI] [PubMed] [Google Scholar]