Abstract
We have previously shown that phosphatidylinositol 3-kinase α (PI 3-Kα) (p85α-p110α) is required for DNA synthesis induced by various growth factors (S. Roche, M. Koegl, and S. A. Courtneidge, Proc. Natl. Acad. Sci. USA 91:9185–9189, 1994) in fibroblasts. In the present study, we have investigated the function of PI 3-Kβ (p85α-p110β) during mitogenesis. By using antibodies specific to p110β we showed that PI 3-Kβ is expressed in NIH 3T3 cells. PI 3-Kβ and PI 3-Kα have common features: PI 3-Kβ is tightly associated with a protein serine kinase that phosphorylates p85α, it interacts with the Src-middle T antigen complex and the activated platelet-derived growth factor (PDGF) receptor in fibroblasts in vivo, and it becomes tyrosine phosphorylated after PDGF stimulation. PI 3-Kβ was also activated in Swiss 3T3 and Cos7 cells stimulated with lysophosphatidic acid (LPA), a mitogen that interacts with a heterotrimeric G protein-coupled receptor. In contrast PI 3-Kα was activated to a lesser extent in these cells. Microinjection of neutralizing antibodies specific for p110β into quiescent fibroblasts inhibited DNA synthesis induced by both insulin and LPA but poorly affected PDGF receptor signaling. Therefore, PI 3-Kβ plays an important role in transmitting the mitogenic response induced by some, but not all, growth factors. Finally, we show that while oncogenic V12Ras interacts with type I PI 3-Ks, it could induce DNA synthesis in the absence of active PI 3-Kα and PI 3-Kβ, suggesting that Ras uses other effectors for DNA synthesis.
Phosphatidylinositol 3-kinases (PI 3-Ks) belong to a family of enzymes that phosphorylate phoshoinositides at the 3′ position of the inositol ring, leading to the formation of phosphatidylinositol 3-phosphate [PI(3)P], phosphatidylinositol 3,4-bisphosphate [PI(3,4)P2], and phosphatidylinositol 3,4,5-trisphosphate (PIP3). PI 3-K activity was first identified by its association with tyrosine kinases in mammalian cells. PI 3-Ks have now been identified in various organisms, including plants, yeasts, flies, and mammals (for reviews, see references 40 and 46). They are involved in the regulation of multiple biological responses, including mitogenesis, apoptosis, vesicular trafficking, and cytoskeleton rearrangement. In agreement with such important functions, PI 3-K activities are highly regulated in vivo. Also, their products PI(3,4)P2 and PIP3 are present in very low levels in quiescent cells but are rapidly produced during cell stimulation. These lipids are thought to have a secondary messenger function. Several targets for these lipids have been identified. These are serine/threonine kinases of the protein kinase C (PKC) family, the product of the proto-oncogene Akt (also called PKB), a protein kinase involved in prevention of apoptosis; p70S6k, a kinase important for mitogenesis; and GRP1 (18) and cytohesin, two proteins involved in cell adhesion and membrane trafficking. Additionally, PI 3-K may also affect the activity of small GTP-binding proteins, such as Rac, leading eventually to cytoskeleton rearrangement during membrane ruffling. The mechanism by which these novel lipids initiate their signaling pathway has been recently unravelled: PI(3,4)P2 and PIP3 have been shown to have binding affinity for conserved peptidic sequences, including the pleckstrin homology domain (PH) and the Src homology region 2 domain (SH2) (29). One consequence of such associations may be localization of a PH-containing signaling molecule to the membrane or regulation of complex dissociation in the case of SH2-containing proteins. PIP3 also activates intrinsic enzymatic activities, as has been shown for several serine/threonine kinases, including members of the PKC family, PDK, and Akt (1, 19, 38).
The first PI 3-K identified was a heterodimer composed of the regulatory subunit p85 and the catalytic subunit p110. Later, other enzymes with PI 3-K activity were identified. They are now grouped into three classes depending on their substrate specificity. Enzymes of class I phosphorylate PI, PI(4)P, and PI(4,5)P2, whereas PI 3-Ks of class II prefer PI and PI(4)P as substrates. Class III members include enzymes with PI as the sole substrate, the first member being the S. cerevisiae PI 3-K Vps34p, identified by its involvement in intracellular protein trafficking (for a review, see reference 46).
Type I enzymes include heterodimers composed of p110α (the first p110 originally identified) (11), p110β (12), and p110δ (3, 47), all of which are tightly associated with regulatory subunits (p85α, p85β, PIK55, and p50). While p110s encode PI 3-K activity, p85 contains multiple domains that regulate interaction of PI 3-K with signaling proteins, e.g., two SH2 domains, one SH3 domain, two proline-rich regions, and a Bcr homology domain which is involved in rho-like binding regions. These enzymes interact with and are regulated by tyrosine kinases. p110γ also belongs to this family, but it does not associate with p85 (39); rather, it associates with a recently cloned p101 regulatory subunit which has no homology with any known protein (37). Recent studies have shown that this member is involved in G-protein-coupled receptor signaling (25). In addition, all these enzymes contain a region in the catalytic subunit involved in the interaction with the small GTP-binding protein Ras (35, 46, 47), and it has been shown that PI 3-Kα is a Ras effector (17, 33, 34). Therefore, type I PI 3-Ks may also be part of the Ras-dependent signaling pathway.
While p110δ is almost exclusively present in leukocytes (3, 47), PI 3-Kα (p85α-p110α) and PI 3-Kβ (p85α-p110β) are broadly expressed (11, 12). α and β catalytic subunits show 42% identity (12). Although the regulation and function of PI 3-Kα have been extensively studied, much less is known about PI 3-Kβ. In a recent study, we used a microinjection approach in order to assess the function of PI 3-Kα during mitogenesis: neutralizing antibodies specific to p110α were microinjected into quiescent fibroblasts, and the capacity of injected cells to respond to growth factors was subsequently analyzed. This study led us to conclude that the α form is required for DNA synthesis induced by various growth factors (30). In the present study we investigated the role of PI 3-K β in fibroblasts during mitogenesis by using antibodies specific for p110β.
MATERIALS AND METHODS
Antibodies and protein purification.
p110α.1 and p110α.2 were described in reference 31 and were generated in rabbits as antibodies to the 16 carboxy-terminal amino acids and and amino acids 776 to 791 of the bovine brain p110α subunit; p110β.2 and p110β.3 sera were generated as antibodies to amino acids 738 to 752 and 420 to 435 of human p110β; and polyclonal antibody (PAb) αp110γ was generated as antibody to amino acids 742 to 756 of human p110γ (39). Monoclonal p110γ (generous gift of B. Stoyanov) and PAb 762 (7) (generous gift of S. Dilworth) were used for Western blotting analyses and recognize p110γ and mouse middle T (mT) antigen, respectively. Platelet-derived growth factor PDGF receptor antibodies (αPR4), mT antibodies (αmT), and p85α antibodies (αp85α) have been described elsewhere (31). PP2A-C and PP2A-R antibodies have been described elsewhere (43). Antiphosphotyrosine monoclonal antibody 4G10 was from Upstate Biochemical Inc., and anti-hemagglutinin (HA) monoclonal antibody 12CA5 was from Babco. Antibodies were affinity purified as described previously (30). Briefly, ammonium sulfate-precipitated sera were first loaded onto a Sepharose column to which peptides were coupled and then were washed with 10 mM sodium phosphate–500 mM NaCl buffer (pH 7). Antibody was eluted with 1 mM propionic acid, and fractions were collected into 1 M ice-cold sodium phosphate buffer (pH 7.0) and stored at −70°C. Nonimmune and preimmune rabbit immunoglobulin G (IgG) was purified on a protein A-Sepharose (Pharmacia) column and eluted as described previously (30). p85α, PI 3-Kα (p85α-p110α) expressed in Sf9 insect cells, and histidine-tagged V12Ras expressed in Escherichia coli were purified as described in references 20, 30, and 33, respectively. PI 3-Kβ (p85α–histidine-tagged p110β) expressed in insect Sf9 cells was purified by using His-bind resin (Novagen) according to the manufacturer’s instructions. All proteins were concentrated to >1 mg/ml with a Minicon microconcentrator (Amicon) for in vivo studies.
Cell culture and transfection.
Mouse mT-transformed NIH 3T3 cells, NIH 3T3 cells, NIH 3T3 cells stably overexpressing insulin receptor (NIH 3T3 HIR), Swiss 3T3 cells, and Cos7 cells were maintained in Dulbecco’s modified Eagle medium (DMEM) (GIBCO) or RPMI 1640 (Eurobio) (in the case of BI-141 lymphoid T cells) containing 10% fetal calf serum (FCS). For growth factor stimulation experiments, fibroblasts were growth arrested at confluence, then incubated for 40 to 48 h in DMEM supplemented with 0.5% FCS, and then stimulated or not with PDGF BB (25 ng/ml; Upstate Biochemical Inc.), 5% FCS insulin (100 μg/ml; Sigma), or LPA (10 μM; Sigma). Cos7 cells were incubated overnight in the absence of serum before lysophosphatidic acid (LPA) stimulation. For inhibitor treatment, cells were preincubated for 30 min with wortmannin, LY294002, or 0.1% dimethyl sulfoxide (as a control) before growth factor stimulation or protein microinjection. For transient-expression experiments, Cos7 cells were transfected for 2 days with 5 μg of pRK5 vector encoding human p110β tagged at the C terminus with influenza virus HA epitope as previously described (12) or pSG5 vector encoding human p110γ (generous gift of B. Stoyanov) by using Lipofectamine (Life Technology) according to the manufacturer’s instructions.
Biochemistry.
Methods for immunoprecipitation of proteins, kinase assay, reimmunoprecipitation, and sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis have been described before (30). Briefly, cells were rinsed twice in cold TBS (20 mM Tris [pH7.5], 150 mM NaCl, 0.1 mM sodium orthovanadate) and then lysed into LB (20 mM Tris [pH 8], 150 mM NaCl, 1% Nonidet P-40, 1% aprotinin, 20 μM leupeptin, 0.1 mM sodium orthovanadate, 10 mM NaF) at 4°C. For some experiments cells were lysed in 0.5% SDS, boiled for 3 min at 95°C, and then diluted in fivefold radioimmunoprecipitation assay (RIPA) buffer (see above) lacking SDS. Cleared lysate was incubated with the indicated serum for 1 h, followed by a further incubation of 30 min with protein A-Sepharose. Immunoprecipitates were washed three times with LB or RIPA buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1% aprotinin, 20 μM leupeptin, 0.1 mM sodium orthovanadate) as appropriate and once with TBS. Kinase assays were performed in 20 μl of 20 mM HEPES (pH 7.5)–10 mM MnCl2–2 mM dithiothreitol containing 2 to 10 μCi of [γ-32P]ATP (3,000 to 5,000 Ci/mmol; Amersham) for 10 min at 30°C. For reimmunoprecipitation experiments immunoprecipitates were boiled at 95°C for 3 min in the presence of 0.5% SDS in order to dissociate the complex, diluted in 5 volumes of RIPA buffer without SDS, and then reimmunoprecipitated with the indicated antibody (31). The samples were then resolved on SDS-polyacrylamide gels and analyzed by autoradiography. Serine protein kinase activity of p110β was tested by performing the kinase assay in the presence of 1 μg of purified p85α (20) that was expressed in insect Sf9 cells (20) containing 2 to 10 μCi of [γ-32P]ATP (3,000 to 5000 Ci/mmol; Amersham) and 10 μM ATP (Boehringer Mannheim). For Western blotting experiments, the transfer of proteins to nitrocellulose (BA85; Schleicher and Schuell) was performed with a semidry apparatus according to the manufacturer’s instructions (Millipore), blocked in 3% bovine serum albumin, and blotted with the indicated antibodies. Bound antibodies were detected with horseradish-conjugated anti-protein A or horseradish-conjugated anti-mouse IgG (for the 4G10, αp110γ, PAb 762, and 12CA antibodies) and followed by enhanced chemiluminescence detection (Amersham). Phosphoamino acid analysis was described previously (41). An immunodepletion experiment was performed as described elsewhere (30). Briefly, p110α and p110β were immunoprecipitated overnight with 20 μl of αp110α.1 and αp110β.3 or nonimmune serum as control, followed by another round of immunoprecipitation. Immunodepleted cell lysate was then used for further biochemical analysis as indicated.
In vitro binding assay.
For V12Ras in vitro experiments, purified protein coupled to Affigel 10 was first incubated with 10 mM GTPγS or GDPβS (Sigma) and then incubated with purified PI 3-Kα for 1 h at 4°C. The beads were then washed extensively with 20 mM HEPES [pH 7.5]–150 mM NaCl–0.1 mM sodium orthovanadate–2 mM dithiothreitol and then further incubated for 15 min with 5 μg of affinity-purified anti-PI 3-K antibody or nonimmune IgG before PI 3-K activity was determined.
Measurement of PI-3K activity.
The in vitro PI kinase assay was performed as described previously (30, 31). PI(4,5)P2 or a mixture of PI–PI(4)P–PI(4,5)P2 (1:1:1; Sigma) was used as the substrate as indicated. Products were separated by chromatography on silica gel 60 plates impregnated with 1% potassium oxalate–1 mM EDTA in chloroform-methanol-acetone-glacial acetic acid-water (60:20:23:18:11) for 2 h. Phosphorylated products were detected by autoradiography and quantified by using a PhosphorImager. PI 3-K activity in vivo was analyzed by measurement of the levels of PI(4,5)P2, PI(3,4)P2, and PIP3 as described in reference 13.
Microinjection of cells.
Cells were grown on coverslips to 70% confluence and then made quiescent by incubating for 24 to 30 h (48 h in the case of NIH 3T3 HIR cells and Swiss 3T3 cells) in DMEM containing 0.5% FCS, as well as in insulin (5 μg/ml) and transferrin (5 μg/ml) in the case of NIH 3T3 cells. Purified antibody or purified V12Ras premixed with antibodies or purified p85α (1 mg/ml) as indicated was then injected into the cytoplasm by using either an automatic (AIS; Zeiss) or a semiautomatic (Eppendorf) microinjection system as described earlier (30, 32) and then stimulated with growth factor in the presence or the absence of wortmannin or LY294002 when indicated. Typically antibodies were injected into 100 to 150 cells per coverslip. The needles used for microinjection were pulled with a Fleming-Brown micropipette puller. DNA synthesis was monitored by adding bromodeoxyuridine (BrdU) (Sigma). The cells were further incubated for 18 h (22 h in the case of Swiss 3T3 cells) and then fixed for immunostaining. Coverslips were washed once with PBS and fixed for 5 min with cold methanol. Cells with injected antibodies were detected by incubating the coverslips with fluorescein-conjugated goat anti-rabbit antibody (Dianova) diluted in PBS (1:100) for 30 min and then washing them three times with PBS. To analyze DNA synthesis, cells were incubated for 10 min with 1.5 M HCl, washed three times with PBS, stained with monoclonal anti-BrdU antibody (Boehringer Mannheim), and then stained with Texas red-conjugated anti-mouse antibody (Dianova). All coverslips were finally washed in PBS containing Hoechst 33258 (Sigma), rinsed in water, and mounted in Moviol (Hoechst) on glass slides. The slides were viewed with an Axiophot fluorescence microscope. Percent DNA synthesis was calculated by using the formula (number of injected cells that were BrdU positive)/(total number of injected cells) × 100. Results from several independent experiments (n > 3) have been averaged; the mean and standard deviation are shown.
RESULTS
Characterization of PI 3Kβ antibodies.
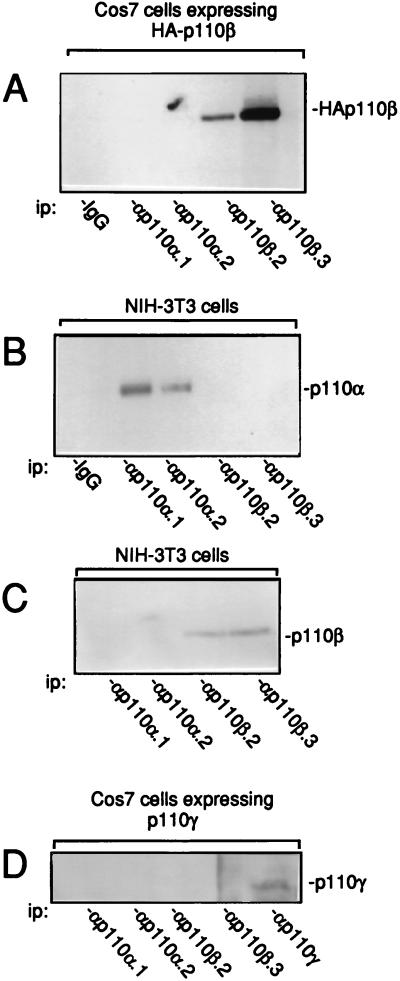
Antibodies to p110β were first generated. They were raised against two distinct peptides corresponding to specific regions of the human p110β. These peptides had no homology with any cloned type I PI 3-K sequence and therefore were expected to be specific to PI 3-Kβ. p110β was immunoprecipitated with various PI 3-K antibodies from a lysate of Cos7 cells that were transiently transfected with an expression vector encoding HA-tagged human p110β. The presence of p110β was detected by immunoblotting with antitag antibody. As shown in Fig. 1A, both p110β.2 and p110β.3 antibodies recognized the expressed p110β. Antibody specificity was next investigated. While anti-p110β antibodies recognize endogenous p110β in NIH 3T3 cells (Fig. 1C), they did not immunoprecipitate p110α. As a control, p110α was detected in both p110α.1 and p110α.2 immunoprecipitates (Fig. 1B). Conversely no p110β was detected in anti-p110α immunoprecipitates when lysates of Cos7 cells expressing HA-p110β or NIH 3T3 cells were used (Fig. 1A and C). Finally, none of these antibodies cross-reacted with p110γ that was transiently expressed in Cos7 cells (Fig. 1D). Therefore, both sets of antibodies are specific to each subunit.
FIG. 1.
Characterization of p110β antibodies. (A) Ectopic HA-p110β was immunoprecipitated from lysates of Cos7 cells that had been transfected for 2 days with the expression vector encoding HA-tagged human p110β with nonimmune IgG or antibodies specific to PI 3-Kα or PI 3-Kβ as indicated. p110α (B) and p110β (C) were immunoprecipitated from an NIH 3T3 cell lysate with the indicated antibodies. (D) p110γ was immunoprecipitated with the indicated antibodies from a lysate of Cos7 cells that had been transfected with the expression vector encoding human p110. The presence of p110α, p110β, HA-p110β, and p110γ was detected by immunoblotting using αp110α.2, αp110β.3, 12CA5 anti-tag, and monoclonal anti-p110γ antibodies, respectively. ip, immunoprecipitate.
Expression of PI 3-Kβ in fibroblasts and lymphoid T cells.
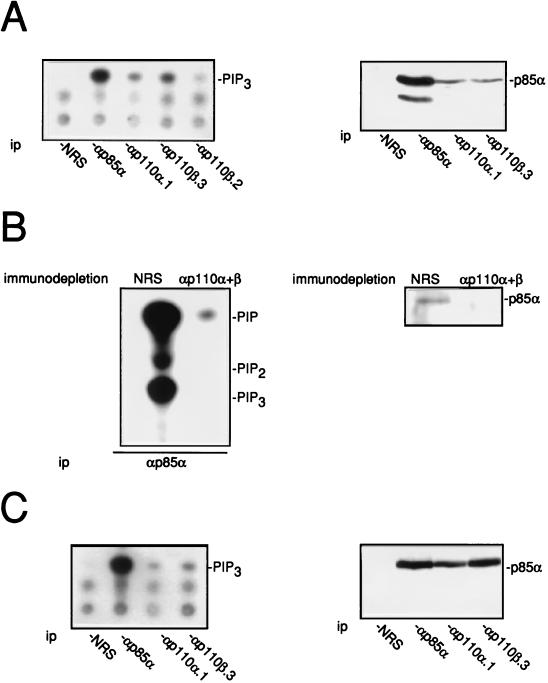
PI 3-Kβ expression was next investigated in mammalian cells. Two approaches were used: the detection of PI 3-K activity present in the immunocomplex and the level of coimmunoprecipitated p85α. Figure 2A shows that a PI(4,5)P2 3-kinase activity was detected in both p110β immunoprecipitates from NIH 3T3 cell lysates. More activity was found associated with the p110β.3 antibody than with anti-p110β.2 and anti-p110α.1. This suggests that NIH 3T3 cells do express PI 3-Kβ. Similar results were obtained with Swiss 3T3 fibroblasts (30a). When looking at the level of p85α coimmunoprecipitated from the cell lysate, a similar amount of p85α was found associated with p110α and p110β immunocomplexes. Whether p85α associates with proteins other than p110α or p110β was also investigated. As shown in Fig. 2B, immunodepletion of PI 3-Kα and PI 3-Kβ with p110 antibodies strongly reduced both the p85α level present in the cell lysate and the PI 3-K activity present in the p85α immunoprecipitate. This suggests that p85α is mainly, if not exclusively, associated with p110α and p110β in fibroblasts. PI 3-Kβ was also found expressed in other cell types, as shown by the example of the lymphoid T-cell line BI-141 (Fig. 2C).
FIG. 2.
Expression of PI 3-Kα and PI 3-Kβ in NIH 3T3 cells (A) and in BI-141 lymphoid T cells (C). PI 3-Kα and PI 3-Kβ were immunoprecipitated from cell lysates by using nonimmune rabbit serum (NRS) or anti-PI 3-K sera as indicated followed by an in vitro PI 3-K assay using PI(4,5)P2 as a substrate. Products were resolved on thin-layer chromatography plates. The presence of p85α in the immunocomplex is also shown and was determined by Western blotting analysis using anti-p85a antibody. (B) Association of p85α subunit with p110α and p110β in NIH 3T3 cells. Fibroblast lysate was immunodepleted of p110α and p110β molecules by using a mixture of αp110α.1 and αp110β.3 or nonimmune serum as indicated. The presence of p85α was detected as described for panel A. p85α was also immunoprecipitated from depleted cell lysates followed by an in vitro PI 3-K assay using PI–PI(4)P–PI(4,5)P2 (1:1:1) as substrates. Shown are the labelled products that were resolved by thin-layer chromatography. The positions of phosphatydylinositol phosphate (PIP), PIP2, PIP3, and the origin (Ori) are shown. ip, immunoprecipitate.
PI 3-Kβ associates with a serine protein kinase that phosphorylates the p85 subunit.
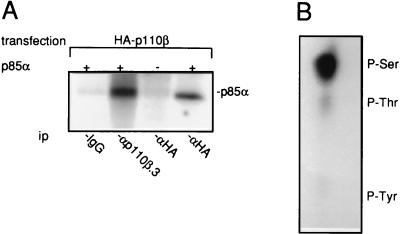
Biochemical characterization of PI 3-Kβ was next investigated. First p110β showed no PI 3-K activity in vitro when expressed alone in Cos7 cells (30a). This was reminiscent of what was observed with p110α (11) in these cells and suggested that PI 3-Kβ is regulated in the same way as PI 3-Kα. We and others have previously demonstrated that p110α has a serine kinase activity toward p85 that negatively regulates its lipid kinase activity (2, 5). We tested whether such activity is associated with PI 3-Kβ. For this purpose, a lysate of Cos7 cells expressing tagged p110β was used as a source of free p110β. Indeed most p110 expressed in these cells was not associated with p85α (30a), perhaps because of the very high level of ectopic protein expression. Tagged p110β was immunoprecipitated, and after extensive washing, an in vitro kinase assay was performed with purified p85α as a substrate. As shown in Fig. 3A, a kinase activity toward p85α was detected from p110β immunocomplexes. No specific phosphorylation was observed when p110β was immunopurified with a nonrelevant antibody. Also, similar kinase activity was detected in both anti-p110β.3 and anti-tag immunoprecipitates, showing that the kinase activity is not due to antibody cross-reactivity. Phosphoamino acid analysis showed that p85α was mainly phosphorylated on serine residues (Fig. 3B). Taken together, these data strongly suggest the existence of a serine kinase activity tightly associated with p110β that phosphorylates p85α.
FIG. 3.
PI 3-Kβ is tightly associated with a serine protein kinase that phosphorylates p85α. (A) In vitro phosphorylation of purified p85α by immunopurified p110β. HA-p110β was immunoprecipitated (ip) with nonimmune IgG or anti-HA-p110β antibodies from cell lysate of Cos7 cells transiently transfected with HA-p110β expression vector as shown. After extensive washing, immunoprecipitates were assayed for an in vitro kinase activity in the presence or the absence of 1 μg of purified p85α as indicated, and phosphoproteins were resolved on 9% acrylamide gel. The presence of phosphorylated p85α is shown. ip, immunoprecipitate. (B) Phosphoamino acid analysis of labelled p85α. The positions of phosphorylated Ser, Thr, and Tyr are shown.
PI 3-Kβ associates with tyrosine kinases in vivo and becomes tyrosine phosphorylated.
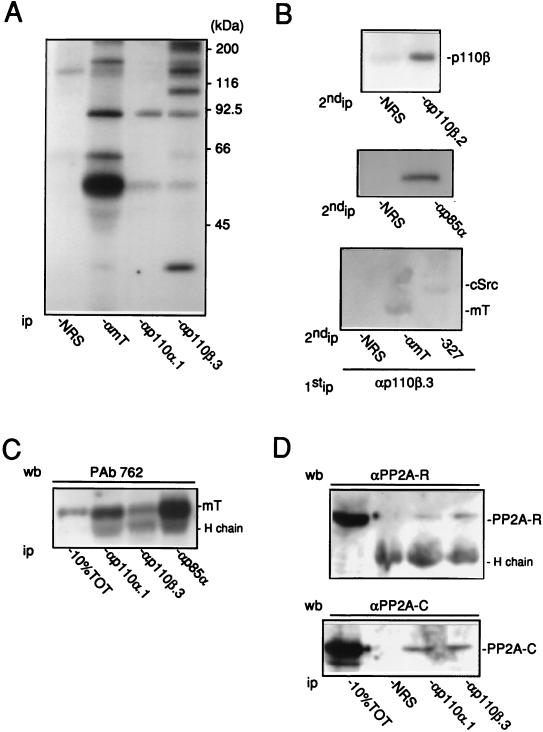
We next tested whether PI 3-Kβ interacts with tyrosine kinases in vivo. PI 3-K was first identified by its interaction with the Src-mT complex (51); therefore, association of PI 3-Kβ with Src-mT in mT-transformed NIH 3T3 cells was investigated. The enzyme was immunoprecipitated from cell lysates, and the presence of the Src-mT complex was detected by an in vitro kinase assay. We have previously shown by this same approach that PI 3-Kα associates with Src-mT in these cells (31). The protein kinase assay from the p110β immunocomplex revealed several phosphoproteins that comigrate with those observed in both mT and p110α immunocomplexes (Fig. 4A). Reimmunoprecipitation experiments identified mT, cSrc, p85α, and p110β as part of the labelled proteins (Fig. 4B). Therefore, like PI 3-Kα, PI 3-Kβ associates with the Src-mT complex in vivo. The mT antigen present in both PI 3-K immunoprecipitates was quantified by immunoblotting: mT was detected in both immunocomplexes but more viral antigen was found associated with PI 3-Kα than with PI 3-Kβ (Fig. 3C). Interestingly, additional phosphoproteins, including one of 35 kDa, were found associated with PI 3-Kβ but were absent in the PI 3-Kα immunocomplex. This suggested that PI 3-Kβ may be involved in specific complexes. The mT antigen has been shown to associate with the serine phosphatase PP2A (27), raising the possibility that the 35-kDa band is the catalytic subunit of the phosphatase (PP2A-C). However, we failed to identify PP2A-C or the regulatory subunit PP2A-R in a reimmunoprecipitation assay (30a). The presence of the phosphatase was then investigated by Western blotting, and as shown in Fig. 4D, both the 36- and 66-kDa subunits of PP2A were found associated with PI 3-Kβ. However, this was not specific to the enzyme, since PP2A proteins were also detected in the PI 3-Kα immunocomplex (Fig. 4D).
FIG. 4.
Association of PI 3-Kβ with the cSrc-mT complex in fibroblasts. (A) Association of PI 3-Kα and PI 3-Kβ with the Src-mT complex in mT-transformed cells. Immunoprecipitates with mT-transformed NIH 3T3 cells and nonimmune rabbit antibody (NRS) or antibody specific to mT, PI 3-Kα (αp110α.1), or PI 3-Kβ (αp110β.3) were assayed in vitro for associated kinase activity. The positions of the size markers are shown. (B) Reprecipitation analysis of the products detected by kinase assay of the p110β immunocomplex with nonimmune antibody and antibodies specific to mT, cSrc (327), p85α, and p110β (αp110β.2), as indicated. (C and D) Association of PI 3-Kα and PI 3-Kβ with mT and PP2A. After immunoprecipitation of PI 3-Kα or PI 3-Kβ as indicated, the presence of mT (C) and the catalytic (PP2A-C) and the regulatory (PP2A-R) subunits of PP2A (D) was detected by immunoblotting using PAb 762, αPP2A-C, and αPP2A-R antibodies, respectively. Cell lysate (10% of total [10%TOT]) used for immunoprecipitations was assayed by immunoblotting. The position of the antibody heavy chain (H chain) is also indicated. wb, Western blot; ip, immunoprecipitate.
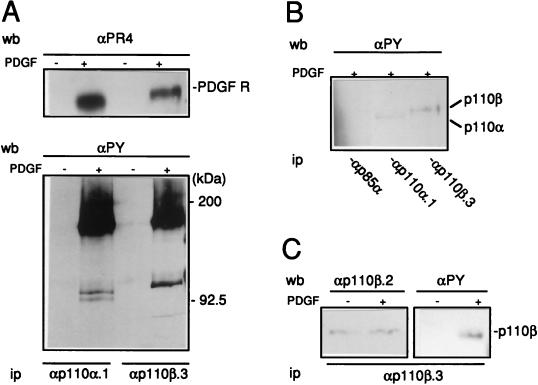
The in vivo association of PI 3-Kβ with activated PDGF receptor was next studied. p110α and p110β were immunoprecipitated from a lysate of quiescent NIH 3T3 cells either stimulated or not with PDGF. The presence of the PDGF receptor was subsequently detected by immunoblotting using specific antibodies to phosphotyrosine and to the receptor itself. As shown in Fig. 5A, PI 3-Kβ associated with the activated PDGF receptor in vivo as well as PI 3-Kα did. When interacting with the receptor, p110α becomes rapidly phosphorylated on tyrosine residues (31). We therefore tested whether a similar event occurred on the p110β subunit. Two major phosphotyrosine-containing proteins were detected in the p110β immunocomplex: a 180-kDa protein identified as the receptor and a protein of 120 kDa. The latter was identified as p110β by reprobing the membrane with affinity-purified p110β.2 antibody (Fig. 5B). Immunoprecipitation from SDS-denatured cell lysates confirmed the identity of the 110-kDa protein as the catalytic subunit of PI 3-Kβ. As a control, no tyrosine-phosphorylated p110 proteins were detected in the p85α immunocomplex, indicating that the p85-p110 heterodimer was dissociated under our conditions (Fig. 5C).
FIG. 5.
Association of PI 3-Kβ with activated PDGF receptor in fibroblasts. (A) Association of PI 3-Kα and PI 3-Kβ with the activated PDGF receptor (PDGF R) in NIH 3T3 in vivo. PI 3-Ks were immunoprecipitated from quiescent fibroblasts stimulated or not for 5 min with PDGF as shown, using αp110α.1 and αp110β.3 as indicated. The presence of the PDGF receptor and the phosphotyrosine proteins in immunocomplexes was detected by Western blotting using PR-4 antibody (αPR4) and 4G10 antiphosphotyrosine antibody (αPy), respectively. The presence of the PDGF receptor and the locations of the size markers are shown. (B and C) Tyrosine phosphorylation of p110α and p110β in NIH 3T3 cells stimulated by PDGF. (B) Antiphosphotyrosine blot of immunoprecipitates made using p85α, p110α.1, or p110β.3 antibodies as shown and cells stimulated with PDGF for 5 min and lysed under SDS denaturing conditions. (C) Anti-p110β and antiphosphotyrosine immunoblots of the p110β immunoprecipitate made of cells stimulated or not with PDGF for 5 min as indicated and αp110β.3. The presence of p110β is shown. wb, Western blot; ip, immunoprecipitate.
p110β antibodies reduce in vitro PI 3-Kβ activity.
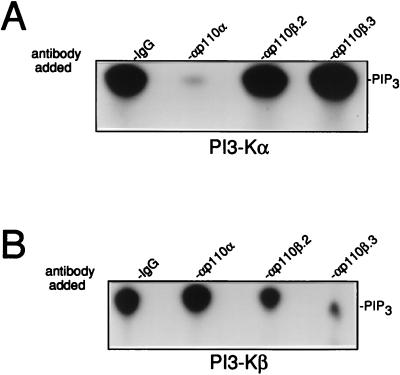
The function of PI 3-Kβ was next investigated in fibroblasts during mitogenesis. The p110β antibodies were first tested for their ability to affect in vitro lipid kinase activity. To address this question, PI 3-Kβ was affinity purified from Sf9 insect cells that were coinfected with baculoviruses expressing human p85α and histidine-tagged human p110β. The effect of antibodies on the activity of purified PI 3-Kβ was next determined. While anti-p110α antibodies did not affect PIP2 3-kinase activity, both p110β.2 and p110β.3 antibodies reduced the activity with a stronger effect for αp110β.3 (inhibition, 55 and 90%, respectively) (Fig. 6B). Conversely, p110β antibodies did not affect the activity of purified PI 3-Kα expressed in Sf9 cells, while αp110α strongly inhibited the activity as previously reported (30) (Fig. 6A). Collectively these data confirm the specificity of antibodies for their subunit and indicate that they reduce PI 3-K activities in vitro.
FIG. 6.
p110β antibodies reduce PI 3-Kβ activity in vitro. Purified PI 3-Kα (A) and PI 3-Kβ (B) were incubated with nonimmune antibodies (IgG) or anti-p110 antibodies as indicated, followed by an in vitro lipid kinase assay using PIP2 as a substrate. Products were resolved on thin-layer chromatography plates and quantified by using a PhosphorImager. The position of PIP3 is shown.
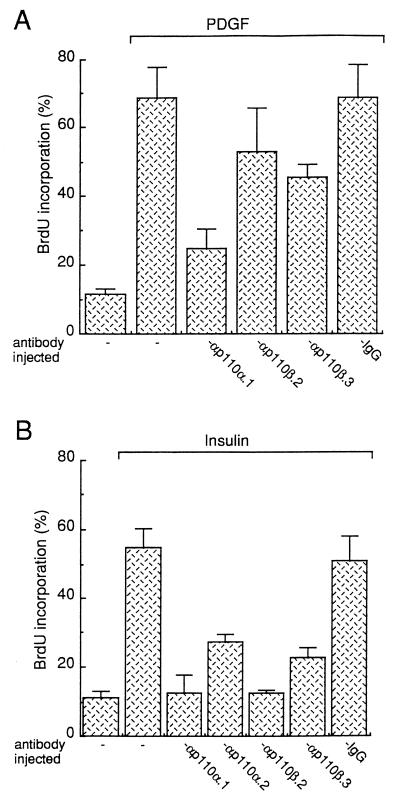
p110β antibodies inhibit the mitogenic response induced by insulin but have little effect on PDGF receptor signaling.
p110β antibodies were next microinjected into cells in order to specifically inhibit PI 3-Kβ in vivo. Quiescent NIH 3T3 cells were seeded onto coverslips, and purified p110β antibodies were injected into the cytoplasm. Cells were then stimulated with PDGF, and BrdU was added to the medium as a marker of S-phase entry. After 18 h of incubation at 37°C, cells were fixed and immunostaining was performed. The capacity of the injected cells to synthesize DNA was then compared to that of the surrounding noninjected cells. Statistical analysis of several independent experiments is summarized in Fig. 7A. p110β antibodies poorly affected the PDGF response: αp110β.3 gave about 35% inhibition, whereas αp110β.2 had no statistically significant effect. In contrast p110α.1 inhibited 80% of the PDGF response. Therefore, PI 3-Kβ may not be absolutely required for PDGF to induce DNA synthesis in these cells. The effect of PI 3-K-neutralizing antibodies on the mitogenic response induced by insulin was next analyzed. For this purpose fibroblasts that express a very high level of insulin receptor (NIH 3T3 HIR cells) were used. As shown in Fig. 7B, insulin induced a potent mitogenic response (50%) in these cells. PI 3-Kα-neutralizing antibodies inhibited this response (90% inhibition for p110α.1 and 60% for p110α.2), confirming a requirement of PI 3-Kα for insulin receptor signaling (26); p110β.2 and p110β.3 antibodies also strongly affected insulin-induced DNA synthesis (90 and 70%, respectively) (Fig. 7B). These inhibitory effects were specific, since control IgG had no effect on this biological response; also, p110β antibodies did not affect DNA synthesis induced by serum in these cells (30a). Therefore both PI 3-Kα and PI 3-Kβ are required for DNA synthesis induced by insulin in fibroblasts.
FIG. 7.
Inhibition of PDGF and insulin signaling by p110 antibodies in NIH 3T3 cells. Control IgG or affinity-purified anti-p110 antibodies were microinjected into quiescent fibroblasts (NIH 3T3 cells [A] and NIH 3T3 HIR cells [B]) as shown, and then the fibroblasts were stimulated with the growth factor indicated and processed for immunofluorescence as described in Materials and Methods. For each experiment, several coverslips were analyzed and the percentage of BrdU-positive cells in injected cells was calculated by using the formula (number of cells injected that were BrdU positive)/(total number of injected cells) × 100. The results from more than three experiments were averaged, and the means and standard deviations (error bars) are shown.
PI 3-Kβ is activated by LPA and is required for signaling.
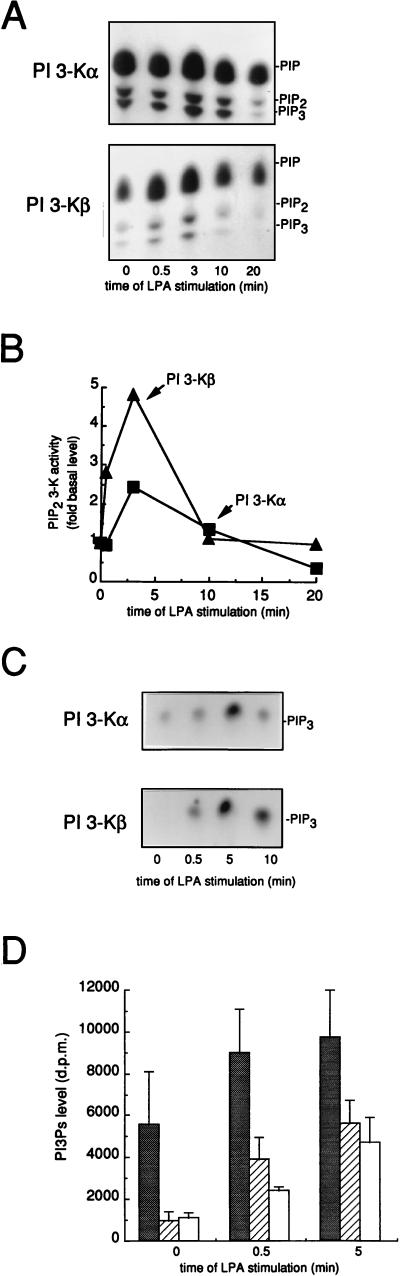
In addition to being induced by tyrosine kinase receptors, mitogenesis can be induced by ligands that interact with seven transmembrane receptors linked to heterotrimeric G protein. Therefore, we investigated the interaction of PI 3-Kβ with LPA, a lipid that interacts with a heterotrimeric protein G-coupled receptor and that induces a strong mitogenic response in fibroblasts (45). PI 3-Kα and PI 3-Kβ were immunoprecipitated from quiescent Swiss 3T3 cells stimulated for various times with LPA and assayed for in vitro lipid kinase activity. As shown in Fig. 8, LPA induced a rapid and strong activation of PI 3-Kβ (about fivefold) within the first 10 min of stimulation that was detected at 30 s and peaked at 3 min. PI 3-Kα was also found to be activated by LPA, but to a lesser extent and with different kinetics: this stimulation was detected at 3 min, suggesting a different mechanism for PI 3-Kα activation. PI 3-Kβ activation by LPA was also observed in Cos7 cells (Fig. 8C): LPA induced a rapid and strong increase in PI 3-Kβ activity accompanied by a delayed PI 3-Kα activation. In agreement with our in vitro data, in vivo stimulation of PI 3-K activity by LPA was also observed: a fivefold increase in PI(3,4)P2 and PIP3 levels was found in Cos cells stimulated with LPA (Fig. 8D).
FIG. 8.
PI 3-K stimulation by LPA. (A and B) Stimulation of PI 3-Kα and PI 3-Kβ by LPA in Swiss 3T3 cells. PI 3-Kα and PI 3-Kβ were immunoprecipitated from lysates of quiescent Swiss 3T3 cells stimulated with LPA at various times as indicated by using anti-p110α.1 and anti-p110β.3 antibodies, respectively, and in vitro activity measurement was performed with a mixture of PI–PI(4)P–PI(4,5)P2 (1:1:1) as substrates. (A) Shown are products resolved by thin-layer chromatography. The positions of PIP, PIP2, and PIP3 are indicated. (B) PIP2 3-kinase activities of PI 3-Kα (■) and PI 3-Kβ (▴) from panel A were quantified. (C) Stimulation of PI 3-Kα and PI 3-Kβ by LPA in Cos7 cells. Cos7 cells were incubated overnight in medium without serum and then stimulated with LPA at various times as indicated. PI 3-Kα and PI 3-Kβ were immunoprecipitated from cell lysates and assayed for an in vitro lipid kinase activity by using PI(4,5)P2 as a substrate. PIP3 was resolved by thin-layer chromatography. (D) Increase in PI(3)P (shaded bars), PI(3,4)P2 (hatched bars), and PIP3 (open bars) levels by LPA in Cos7 cells. [ortho-32P]-phosphate-labelled Cos cells were stimulated with 10 μM LPA at the indicated times, and the levels of various labelled phosphoinositides were determined. The results from several experiments were averaged, and the means and standard deviations (error bars) are shown. d.p.m., disintegrations per minute.
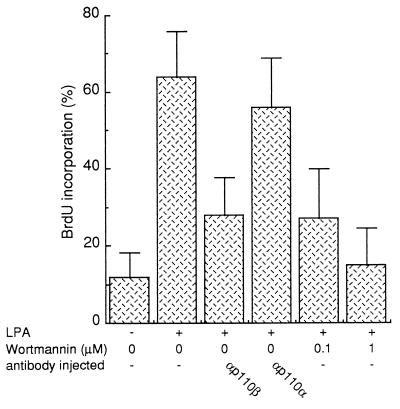
We next investigated the function of PI 3-Kβ in the mitogenic response induced by LPA. Our previous data suggested that PI 3-Kα was not involved in this response, and therefore we first analyzed whether LPA needed PI 3-K activity at all for signaling. Wortmannin, a drug that inhibits PI 3-K activities in vivo (42), was used for this purpose. As shown in Fig. 9, LPA induced a strong mitogenic response as 65% of cells entered the S phase. Addition of 0.1 μM wortmannin to the medium, a dose described to specifically inhibit PI 3-K activity, gave 70% inhibition. To confirm the wortmannin effect we next used the the microinjection approach described above: inhibition of PI 3-Kβ in vivo with p110β antibodies gave 70% reduction of the LPA response. This inhibition was specific to PI 3-Kβ, since anti-p110α.1 had no significant effect (Fig. 9). Together these data point to a crucial role of PI 3-Kβ in the mitogenic response induced by LPA.
FIG. 9.
Inhibition of the LPA-mitogenic response by wortmannin or by injection of p110β antibodies. Affinity-purified anti-p110 antibodies were microinjected into quiescent Swiss 3T3 fibroblasts as shown; the fibroblasts were then stimulated or not stimulated with LPA (10 μM), as indicated, and processed for immunofluorescence as described in the legend to Fig. 7. Alternatively, cells were treated with wortmannin during stimulation as indicated. For each experiment, several coverslips were analyzed and the percentage of BrdU-positive cells was calculated as described in the legend to Fig. 7. The results from more than three experiments were averaged, and the means and standard deviations (error bars) are shown.
PI 3-Kα and PI 3-Kβ are not needed for V12Ras-induced DNA synthesis.
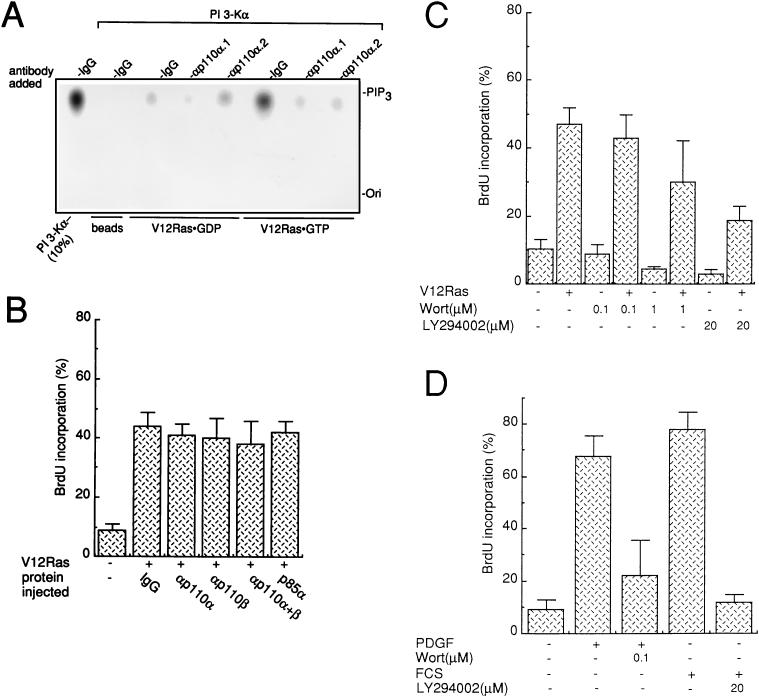
Finally we investigated the requirement of PI 3-Kα and PI 3-Kβ for oncogenic Ras to induce DNA synthesis in fibroblasts. When PI 3-Kα associates with V12Ras, its lipid kinase activity is increased (35). We therefore tested the effect of our neutralizing antibodies on PI 3-Kα activity associated with oncogenic Ras. For this purpose the V12Ras–PI 3-Kα complex was reconstituted in vitro: immobilized V12Ras was incubated with purified PI 3-Kα. After extensive washing, a PIP2 3-kinase assay was performed. A total of 3 to 5% of PI 3-K activity was found associated with Ras when the latter was in a GTP-bound form (Fig. 10A), which is in agreement with previous reports (33). Anti-p110α.1 and anti-p110α.2 strongly inhibited this lipid kinase activity (>80%), confirming that these antibodies affect PI 3-Kα activity regardless of whether the enzyme is associated with tyrosine kinases or activated Ras (Fig. 10A).
FIG. 10.
Inhibition of PI 3-Kα and PI 3-Kβ does not affect V12Ras-induced DNA synthesis in fibroblasts. (A) Anti-p110α antibodies reduce PI 3-Kα activity associated with oncogenic V12Ras. Purified PI 3-Kα was incubated with purified V12Ras that was coupled to Affigel 10 beads in a GDPβS (V12Ras · GDP) or GTPγS (V12Ras · GTP) prebound form or with uncoupled beads (beads) as a control. After extensive washings, in vitro PI 3-K activity was determined in the presence of 10 μg of purified control IgG or affinity-purified αp110α.1 or αp110α.2 as shown, and with PI(4,5)P2 as a substrate. PI 3-Kα (10%) used for the binding assay was assayed for PI 3-K activity as a control [PI 3-Kα–(10%)]. The positions of PIP3 and the origin (Ori) are shown. (B) Anti-PI 3-K antibodies inhibit type I PI 3-K activities present in NIH 3T3 cell lysate that were bound to activated Ras. The inhibition of PI 3-Kα and PI 3-Kβ did not affect V12Ras-induced DNA synthesis. Purified V12Ras together with control IgG, affinity-purified anti-p110 antibodies, or purified p85α was microinjected into quiescent fibroblasts as shown. (C and D) Quiescent fibroblasts were pretreated with 0.1% dimethyl sulfoxide (as a control), wortmannin (Wort), or LY294002 as indicated and then stimulated with PDGF (50 ng/ml) or 5% FCS (D) or microinjected with V12Ras (C) as indicated. In each case cells were incubated in the presence of BrdU for 15 to 18 h and then fixed and processed for immunofluorescence. Several coverslips were analyzed, and the percentage of BrdU-positive cells was calculated as described in the legend to Fig. 7. The results from more than three experiments were averaged, and the means and standard deviations (error bars) are shown.
The effect of these antibodies on the ability of activated Ras to induce DNA synthesis was next analyzed. A low dose of purified V12Ras was chosen to give a moderate cell response in order to avoid any maximal intracellular signaling. Purified V12Ras was premixed with neutralizing p110α antibodies or nonimmune IgG as a control and then microinjected into the cytoplasm of quiescent NIH 3T3 cells. BrdU was added to the medium, and after 15 h of incubation, cells were fixed and immunostained for the presence of injected antibody and for BrdU incorporation. The statistical analysis of these experiments is summarized in Fig. 10B. V12Ras drives 40% of cells into S phase; however, none of the PI 3-Kα- and PI 3-Kβ-neutralizing antibodies affected this response. While these data are in favor of an absence of PI 3-K requirement for signaling, other interpretations imply either a functional redundancy between these enzymes or the involvement of another PI 3-K. To test these hypotheses, a mixture of p110α and p110β antibodies was coinjected with Ras; however, none of these injected proteins affected the Ras cell response. Similarly, no effect was observed upon the coinjection of purified p85α, which was previously shown to inhibit V12Ras-induced cell transformation (34). Therefore, PI 3-Kα and PI 3-Kβ may not be required for DNA synthesis induced by V12Ras. Finally, we investigated whether Ras needed any PI 3-K activity for mitogenesis. PI 3-K activities were inhibited in vivo by the use of the PI 3-K inhibitors wortmannin and LY294002. When cells were treated with high concentrations of either compound, mitogenesis induced by PDGF and serum was inhibited (Fig. 10D). A stronger effect was observed with LY29400, probably because of a higher in vivo stability. Both drugs also affected mitogenesis induced by injected V12Ras, although not as efficiently (50% inhibition in the presence LY294002) (Fig. 10C), raising the possibility that a PI 3-K activity for Ras-induced DNA synthesis is involved.
DISCUSSION
Interaction of PI 3-Kβ with tyrosine kinases.
In this study we have investigated the function of PI 3-Kβ in fibroblasts. Using antibodies specific to p110β, we show that PI 3-Kβ and PI 3-Kα have common characteristics. First, PI 3-Kβ is tightly associated with a protein serine kinase that phosphorylates p85α. Second, PI 3-Kβ behaves like the α form in its interaction with tyrosine kinases; like PI 3-Kα, PI 3-Kβ was found associated with both the mT-Src complex and the activated PDGF receptor in vivo. This was expected, since p85α is responsible for the association (11, 31). In addition we found that p110α and p110β were both substrates of the activated PDGF receptor in vivo. The relevance of this phosphorylation is currently not known, but preliminary data suggests that it is not involved in enzymatic regulation (30a). Taken together, these observations suggest that tyrosine kinases may not discriminate between PI 3-Kα and PI 3-Kβ for protein interaction.
Interaction of PI 3-Kβ with Gi-coupled LPA receptor.
While PI 3-Ks are also activated by G-protein-coupled receptors, PI 3-Kα and PI 3-Kβ may interact in a distinct manner with these receptors. For example LPA, a mitogen that signals through a heterotrimeric Gi protein (45), induced a rapid and strong activation of PI 3-Kβ whereas PI 3-Kα was activated only after a longer period of stimulation. This suggests a different mechanism of α and β enzymatic activation by LPA. This idea is further supported by the work of Kurosu et al., who observed an in vitro stimulation of PI 3-Kβ by the βγ subunits of Gi not found with PI 3-Kα (22). This mechanism may account for the observed PI 3-Kβ activation by LPA. While not reported by Kurosu et al. (22), the interaction with the βγ heterodimer may occur through p110β. This suggests that PI 3-Kβ differs from PI 3-Kα at least by its ability to interact with specific receptors or signaling proteins, which may involve the catalytic subunit. Identification of specific interactors of p110β will be informative in this regard.
A link between type I PI 3-K and the G-protein-coupled receptors including the chemoattractant receptor in leukocytes has been previously established (28, 36), but other mechanisms for in vivo activation have been also proposed. First, an interaction between PI 3-Kγ and the βγ subunits of Gi was reported (25, 37). We, however, failed to detect a clear activation of this enzyme by the LPA receptor (30a). The reason for this observation is not known, but the existence of a p101 adapter that specifically interacts with p110γ and regulates PI 3-Kγ activity has been recently reported (37). Hence, failing to observe PI 3-Kγ activation may be due to an absence of p101 expression in fibroblasts. Second, the involvement of tyrosine kinases as mediators of PI 3-K activation as induced by G-protein-coupled receptors has been proposed. In particular, the association of a PI 3-K activity with the cytoplasmic tyrosine kinases of the Src family has been reported (28, 36). This raises the possibility that LPA activates PI 3-Kα through association with a member of the tyrosine kinase family, and this hypothesis is under current investigation.
Function of PI 3-Kβ during mitogenesis.
The role of PI 3-Kβ in DNA synthesis was next investigated. Most approaches described thus far involve mutagenesis of the tyrosine kinase receptor (9, 16, 44), overexpression of wild-type or mutated forms of p85α as a dominant negative form of PI 3-K (α and β forms) (14, 52), or drugs that inhibit PI 3-K activities (42, 48). None of these discriminate between PI 3-Kα and PI 3-Kβ. As an alternative we used an antibody microinjection approach in order to specifically inhibit PI 3-Kβ without affecting PI 3-Kα. This allowed us to show a requirement of PI 3-Kβ for the mitogenic response as induced by insulin and LPA. Indeed, a function of PI 3-K activity for insulin signaling has been previously reported (14, 26). Our data suggests in addition that both PI 3-Kα and PI 3-Kβ are involved for full mitogenic response. In the case of LPA, the antibody microinjection experiments confirm the importance of PI 3-Kβ for LPA receptor signaling and agree with several reports describing the involvement of a PI 3-K activity for LPA-induced mitogen-activated protein kinase activation (10, 21), an early event of G1 progression. However, the fact that PI 3-Kβ was not required for the mitogenic response induced by PDGF receptor is not clear and requires further investigation. Nevertheless, this data strongly suggests that PI 3-Kβ is required for DNA synthesis induced by some growth factors.
The role of the PI 3-Kβ form for signal transduction of extracellular stimulus is not known, but Logan et al. recently reported a function of PI 3-Kβ during epidermal growth factor induced Jun kinase activation (24), a key element of the cellular stress response (4, 23). From what is known about PI 3-K activity and mitogenesis induced by LPA and insulin, several roles can be attributed to PI 3-Kβ. First, PI 3-K inhibition blocked c-fos expression and mitogen-activated protein kinase activation induced by these mitogens (10, 14, 21, 49, 52), suggesting that PI 3-Kβ may regulate some elements of the Ras pathway. Second, PI 3-K was also shown to regulate the activation of the ribosomal protein S6 kinase induced by these stimuli, and PI 3-Kβ may also participate in this process. Finally, type I PI 3-Ks participate in the activation of PH-containing enzymes, including phospholipase Cγ (8) or cytoplasmic tyrosine kinases of the Btk family together with tyrosine kinases of the Src family (6, 10, 21, 28), and this may also involve the PI 3-Kβ.
Interaction of PI 3-Kα and PI 3-Kβ with Ras.
Since type I PI 3-Ks are also effectors of the small GTP-binding protein Ras (17, 33, 34), their function during G1-to-S phase entry induced by Ras was also investigated. We recently showed that PI 3-K is important for Ras-induced cell transformation and actin cytoskeleton rearrangement (34), which makes PI 3-K an important effector for Ras signaling. However our microinjection data strongly suggest that PI 3-Kα and PI 3-Kβ are not absolutely required for the Ras mitogenic response. While we cannot exclude the possibility that our antibody or purified p85α did not completely block PI 3-K activation in vivo, the partial effect of chemical inhibitors rather suggests the involvement of another PI 3-K less sensitive to these drugs. In agreement with this idea, a partial effect of LY294002 on PI 3-K activation induced by V12Ras in vivo was observed (34). Ras has been shown to interact with several effectors (15, 50), including the serine/threonine Raf, rasGAP, and Ral.GSD, and these effectors may be also important for the Ras mitogenic response.
ACKNOWLEDGMENTS
We thank P. Hu and J. Schlessinger for the construct encoding human p110β, B. Stoyanov and R. Wetzker for the construct encoding p110γ and the monoclonal anti-p110γ antibody, S. Christoforidis for the generous gift of baculovirus encoding human p110β, S. Dilworth for the monoclonal mT antibody, and P. Bello for critical reading of the manuscript.
S.R. was supported by the Institut National de la Santé et de la Recherche Médicale, the Fondation pour la Recherche Médicale, and the Association pour la Recherche sur le Cancer.
REFERENCES
- 1.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R J, Reese R B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter C L, Auger K R, Duckworth B C, Hou W-M, Schaffhausen B, Cantley L C. A tightly associated serine/threonine protein kinase regulates phosphoinositide 3-kinase activity. Mol Cell Biol. 1993;13:1657–1665. doi: 10.1128/mcb.13.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chantry D, Vojtek A, Kashishian A, Holtzman D A, Wood C, Gray P W, Cooper J A, Hoekstra M F. p110δ, a novel PI 3-K catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J Biol Chem. 1997;272:19236–19241. doi: 10.1074/jbc.272.31.19236. [DOI] [PubMed] [Google Scholar]
- 4.Dérijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 5.Dhand R, Hiles I, Panayotou G, Roche S, Fry M J, Gout I, Totty N F, Truong O, Vicendo P, Yonezawa K, Kasuga M, Courtneidge S A, Waterfield M D. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 1994;13:522–533. doi: 10.1002/j.1460-2075.1994.tb06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 7.Dilworth S M, Horner V P. Novel monoclonal antibodies that differentiate between the binding of pp60c-src or protein phosphatase 2A by polyomavirus middle T antigen. J Virol. 1993;67:2235–2244. doi: 10.1128/jvi.67.4.2235-2244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falasca M, Logan S K, Lehto V P, Baccante G, Lemmon M A, Schlessinger J. Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fantl W, Escobedo J A, Martin G A, Turck C W, del Rosario M, McCormick F, Williams L T. Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell. 1992;69:413–424. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- 10.Hawes B E, Luttrell L M, van Biesen T, Lefkowitz R J. Phosphatidylinositol 3-kinase is an early intermediate in the Gβγ-mediated mitogen-activated protein kinase signaling pathway. J Biol Chem. 1996;271:12133–12136. doi: 10.1074/jbc.271.21.12133. [DOI] [PubMed] [Google Scholar]
- 11.Hiles I D, Otsu M, Volinia S, Fry M J, Gout I, Dhand R, Panayotou G, Ruiz-Larrea F, Thompson A, Totty N F, Hsuan J J, Courtneidge S A, Parker P J, Waterfield M D. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992;70:419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- 12.Hu P, Mondino A, Skolnik E Y, Schlessinger J. Cloning of a novel, ubiquitously expressed human phosphatidylinositol 3-kinase and identification of its binding site on p85. Mol Cell Biol. 1993;13:7677–7688. doi: 10.1128/mcb.13.12.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 14.Jhun B H, Rose D W, Seely B L, Rameh L, Cantley L, Saltiel A R, Olefsky J M. Microinjection of the SH2 domain of the 85-kilodalton subunit of phosphatodylinositol 3-kinase inhibits insulin-induced DNA synthesis and c-fos expression. Mol Cell Biol. 1994;14:7466–7475. doi: 10.1128/mcb.14.11.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joneson T, White M A, Wigler M H, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- 16.Kazlauskas A, Kashishian A, Cooper J A, Valius M. GTPase-activating protein and phosphatidylinositol 3-kinase bind to distinct regions of the platelet-derived growth factor receptor β subunit. Mol Cell Biol. 1992;12:2534–2544. doi: 10.1128/mcb.12.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khwaja A, Rodriguez-Viciana P, Wennström S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klarlund J K, Guilherme A, Holik J J, Virbasius J V, Chawla A, Czech M P. Signaling by phosphoinositide-3,4,5-trisphosphate through proteins containing pleckstrin and Sec7 homology domains. Science. 1997;275:1927–1930. doi: 10.1126/science.275.5308.1927. [DOI] [PubMed] [Google Scholar]
- 19.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koegl M, Kypta R M, Bergman M, Alitalo K, Courtneidge S A. Rapid and efficient purification of Src homology 2 domain-containing proteins: Fyn, Csk, and phosphatidylinositol 3-kinase p85. Biochem J. 1994;302:737–744. doi: 10.1042/bj3020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kranenburg O, Verlaan I, Hordijk P L, Moolenaar W H. Gi-mediated activation of the Ras/MAP kinase pathway involves a 100KDa tyrosine-phosphorylated Grb2 SH3 binding protein, but not Src nor Shc. EMBO J. 1997;16:3097–3105. doi: 10.1093/emboj/16.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, Ui M, Hazeki O, Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110β is synergistically activated by the βγ subunits of G proteins and phosphotyrosyl peptide. J Biol Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- 23.Kyriakis J, Banerjee P, Nikolakaki E, Dai T, Rubie E, Ahmad M, Avruch J, Woodgett J. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 24.Logan S K, Falasca M, Hu P, Schlessinger J. Phosphatidylinositol 3-kinase mediates epidermal growth factor-induced activation of the c-Jun N-terminal kinase signaling pathway. Mol Cell Biol. 1997;17:5784–5790. doi: 10.1128/mcb.17.10.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Ilasaca M, Crespo P, Pellici P G, Gutkind J S, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase γ. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 26.McIlroy J, Chen D, Wjasow C, Michaeli T, Backer J M. Specific activation of p85-p110 phosphatidylinositol 3′-kinase stimulates synthesis by Ras- and p70S6 kinase-dependent pathways. Mol Cell Biol. 1997;17:248–255. doi: 10.1128/mcb.17.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallas D C, Sharrick L K, Martin B L, Jasper S, Miller T B, Brautigan D L, Roberts T M. Polyoma small and middle T antigen and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 28.Ptasznik A, Traynor-Kaplan A, Bokoch G M. G protein-coupled chemoattractant receptors regulate Lyn tyrosine kinase Shc adapter protein signaling complexes. J Biol Chem. 1995;270:19969–19973. doi: 10.1074/jbc.270.34.19969. [DOI] [PubMed] [Google Scholar]
- 29.Rameh L E, Chen C S, Cantley L C. Phosphatidylinositol (3,4,5)P3 interacts with SH2 domains and modulates PI 3-kinase association with tyrosine phosphorylated proteins. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 30.Roche S, Koegl M, Courtneidge S A. The phosphatidylinositol 3-kinase α is required for DNA synthesis induced by some, but not all, growth factors. Proc Natl Acad Sci USA. 1994;91:9185–9189. doi: 10.1073/pnas.91.19.9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Roche, S. Unpublished observations.
- 31.Roche S, Dhan R, Waterfield M D, Courtneidge S A. The catalytic subunit of phosphatidylinositol 3-kinase is a substrate for the platelet-derived growth factor receptor, but not for middle-T antigen-pp60c-src complexes. Biochem J. 1994;301:703–711. doi: 10.1042/bj3010703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roche S, Koegl M, Barone V M, Roussel M, Courtneidge S A. DNA synthesis induced by some, but not all, growth factors requires Src family protein tyrosine kinases. Mol Cell Biol. 1995;15:1102–1109. doi: 10.1128/mcb.15.2.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Vicinia P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Vicinia P, Warne P H, Vanhaesebroeck B, Waterfield M D, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens L, Eguinoa A, Corey S, Jackson T, Hawkins P T. Receptor stimulated accumulation of phosphatodylinositol (3,4,5)-trisphosphate by G-protein mediated pathways in human myeliod derived cells. EMBO J. 1993;12:2265–2273. doi: 10.1002/j.1460-2075.1993.tb05880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stephens L R, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka A S, Thelen M, Cadwallader K, Tempst P, Hawkins P T. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 38.Stokoe D, Stephens L R, Copeland T, Gaffney P R J, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 39.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck D R, Dhand B, Nürnberg R, Gierschik P, Seedorf K, Hsuan J J, Waterfield M D, Wetzker R. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 1995;269:690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 40.Toker A, Cantley L C. Signalling through the lipid products of phosphoinositide 3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 41.Twamley G, Hall B, Kypta R, Courtneidge S A. Association of Fyn with the activated PDGF receptor: requirements for binding and phosphorylation. Oncogene. 1992;7:1893–1901. [PubMed] [Google Scholar]
- 42.Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- 43.Ulug E T, Cartwright A J, Courtneidge S A. Characterization of the interaction of polyoma middle T antigen with type 2A protein phosphatase. J Virol. 1992;66:1458–1467. doi: 10.1128/jvi.66.3.1458-1467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valius M, Kazlauskas A. Phospholipase C-γ1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor’s mitogenic signal. Cell. 1993;73:321–334. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- 45.van Corven E J, Groenink A, Jalink K, Eichholtz T, Moolenaar W H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by Gi protein. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- 46.Vanhaesebroeck B, Leevers S, Panayotou G, Waterfield M D. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 47.Vanhaesebroeck B, Welham M J, Kotani K, Stein R, Warne P H, Zvelebil M J, Higashi K, Volinia S, Downward J, Waterfield M D. p110δ, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci USA. 1997;94:4330–4335. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 49.Welsh G I, Foulstone E J, Yong S W, Tavare J M, Proud C G. Wortmannin inhibits the effects of insulin and serum on the activities of glycogen synthase kinase-3 and mitogen-activated protein kinase. Biochem J. 1994;303:15–20. doi: 10.1042/bj3030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White M A, Nicolette C, Minden A, Polverino A, Vanaelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 51.Whitman M, Kaplan D R, Schaffhausen B, Cantley L, Roberts T M. Association of phosphatidylinositol kinase activity with polyoma middle T competent for transformation. Nature. 1985;315:239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- 52.Yamaushi K, Holt K, Pessin J E. Phosphatidylinositol 3-kinase functions upstream of Ras and Raf in mediating insulin stimulation of c-fos transcription. J Biol Chem. 1993;268:14597–14600. [PubMed] [Google Scholar]