Abstract
Background
New strategies in immunotherapy with specific antigens that trigger an anti‐tumour immune response in people with lung cancer open the possibility of developing therapeutic vaccines aimed at boosting the adaptive immune response against cancer cells.
Objectives
To evaluate the effectiveness and safety of different types of therapeutic vaccines for people with advanced non‐small cell lung cancer.
Search methods
We searched CENTRAL, MEDLINE, Embase, Wanfang Data, and China Journal Net (CNKI) up to 22 August 2023.
Selection criteria
We included parallel‐group, randomised controlled trials evaluating a therapeutic cancer vaccine, alone or in combination with other treatments, in adults (> 18 years) with advanced non‐small cell lung cancer (NSCLC), whatever the line of treatment.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Our primary outcomes were overall survival, progression‐free survival, and serious adverse events; secondary outcomes were three‐ and five‐year survival rates and health‐related quality of life.
Main results
We included 10 studies with 2177 participants. The outcome analyses included only 2045 participants (1401 men and 644 women). The certainty of the evidence varied by vaccine and outcome, and ranged from moderate to very low. We report only the results for primary outcomes here.
TG4010
The addition of the vector‐based vaccine, TG4010, to chemotherapy, compared with chemotherapy alone in first‐line treatment, may result in little to no difference in overall survival (hazard ratio (HR) 0.83, 95% confidence interval (CI) 0.65 to 1.05; 2 studies, 370 participants; low‐certainty evidence). It may increase progression‐free survival slightly (HR 0.74, 95% CI 0.55 to 0.99; 1 study, 222 participants; low‐certainty evidence). It may result in little to no difference in the proportion of participants with at least one serious treatment‐related adverse event, but the evidence is very uncertain (risk ratio (RR) 0.70, 95% CI 0.23 to 2.19; 2 studies, 362 participants; very low‐certainty evidence).
Epidermal growth factor vaccine
Epidermal growth factor vaccine, compared to best supportive care as switch maintenance treatment after first‐line chemotherapy, may result in little to no difference in overall survival (HR 0.82, 95% CI 0.66 to 1.02; 1 study, 378 participants; low‐certainty evidence), and in the proportion of participants with at least one serious treatment‐related adverse event (RR 1.32, 95% CI 0.88 to 1.98; 2 studies, 458 participants; low‐certainty evidence).
hTERT (vx‐001)
The hTERT (vx‐001) vaccine compared to placebo as maintenance treatment after first‐line chemotherapy may result in little to no difference in overall survival (HR 0.97, 95% CI 0.70 to 1.34; 1 study, 190 participants).
Racotumomab
Racotumomab compared to placebo as a switch maintenance treatment post‐chemotherapy was assessed in one study with 176 participants. It may increase overall survival (HR 0.63, 95% CI 0.46 to 0.87). It may make little to no difference in progression‐free survival (HR 0.73, 95% CI 0.53 to 1.00) and in the proportion of people with at least one serious treatment‐related adverse event (RR 1.03, 95% CI 0.15 to 7.18).
Racotumomab versus docetaxel as switch maintenance therapy post‐chemotherapy was assessed in one study with 145 participants. The study did not report hazard rates on overall survival or progression‐free survival time, but the difference in median survival times was very small – less than one month. Racotumomab may result in little to no difference in the proportion of people with at least one serious treatment‐related adverse event compared with docetaxel (RR 0.89, 95% CI 0.44 to 1.83).
Personalised peptide vaccine
Personalised peptide vaccine plus docetaxel compared to docetaxel plus placebo post‐chemotherapy treatment may result in little to no difference in overall survival (HR 0.80, 95% CI 0.42 to 1.52) and progression‐free survival (HR 0.78, 95% CI 0.43 to 1.42).
OSE2101
The OSE2101 vaccine compared with chemotherapy, after chemotherapy or immunotherapy, was assessed in one study with 219 participants. It may result in little to no difference in overall survival (HR 0.86, 95% CI 0.62 to 1.19). It may result in a small difference in the proportion of people with at least one serious treatment‐related adverse event (RR 0.95, 95% CI 0.91 to 0.99).
SRL172
The SRL172 vaccine of killed Mycobacterium vaccae, added to chemotherapy, compared to chemotherapy alone, may result in no difference in overall survival, and may increase the proportion of people with at least one serious treatment‐related adverse event (RR 2.07, 95% CI 1.76 to 2.43; 351 participants).
Authors' conclusions
Adding a vaccine resulted in no differences in overall survival, except for racotumomab, which showed some improvement compared to placebo, but the difference in median survival time was very small (1.4 months) and the study only included 176 participants.
Regarding progression‐free survival, we observed no differences between the compared treatments, except for TG4010, which may increase progression‐free survival slightly. There were no differences between the compared treatments in serious treatment‐related adverse events, except for SRL172 (killed Mycobacterium vaccae) added to chemotherapy, which was associated with an increase in the proportion of participants with at least one serious treatment‐related adverse event, and OSE2101, which may decrease slightly the proportion of people having at least one serious treatment‐related adverse event.
These conclusions should be interpreted cautiously, as the very low‐ to moderate‐certainty evidence prevents drawing solid conclusions: many vaccines were evaluated in a single study with small numbers of participants and events.
Keywords: Adult; Female; Humans; Male; Carcinoma, Non-Small-Cell Lung; Carcinoma, Non-Small-Cell Lung/therapy; Docetaxel; EGF Family of Proteins; Lung Neoplasms; Lung Neoplasms/therapy; Mycobacteriaceae; Quality of Life; Randomized Controlled Trials as Topic; Vaccines
Plain language summary
Do cancer vaccines help people with advanced non‐small cell lung cancer?
Key messages
‐ The vaccines evaluated in this review do not improve peoples' survival, or progression‐free survival, or do so to a negligible extent.
‐ Unwanted effects of the vaccines are not frequent.
What is lung cancer?
Lung cancer is one of the most common cancers worldwide. Non‐small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for around 87% of lung cancers. Non‐small cell lung cancer is often diagnosed when it is at an advanced stage, which is associated with high death rates and a short life expectancy.
How is non‐small cell lung cancer treated?
Most of these cancers are treated first with chemotherapy – that is, medicine consisting of powerful chemicals to kill fast‐growing cancer cells. New therapies to improve survival rates for people with NSCLC are focused on treatment with immunotherapy after chemotherapy. Cancer vaccines are a type of immunotherapy. Unlike vaccines to protect us from disease, cancer vaccines are for people who already have cancer. Therapeutic cancer vaccines aim to stimulate the immune system to recognise and destroy cancer cells.
What did we want to find out?
We wanted to find out whether vaccines lengthen people's survival time and time without disease progression, and whether they are associated with any unwanted effects.
What did we do?
We searched for studies that looked at therapeutic cancer vaccines alone or in combination with chemotherapy compared with supportive care, no treatment, or placebo (inactive or 'dummy' medicine) in people with advanced NSCLC.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 10 studies that involved 2177 participants with advanced NSCLC. The biggest study involved 419 people and the smallest study 50. Seven different types of vaccines were evaluated. Three vaccines were evaluated in 2 studies each: TG4010 vector‐based vaccine; epidermal growth factor vaccine; and racotumomab. The remaining 4 vaccines were each evaluated in a single study.
Main results
‐ None of the vaccines increased participants' survival time, except racotumomab, which may improve it slightly compared to placebo. The median survival time for those in the racotumomab vaccine group was 8.2 months, compared to 6.8 months in the group that did not receive the vaccine. (The median is the middle value of a set of numbers.)
‐ None of the vaccines improved progression‐free survival time, except TG4010, which may increase it slightly. The median progression‐free survival time for people in the TG4010 vaccine group was 5.9 months, compared to 5.1 months in the non‐vaccine group.
‐ The 7 different vaccines tested largely appear to be safe: there were no differences between the people given vaccines and those not given vaccines in terms of serious adverse (unwanted) events. However, 1 vaccine (SLR172) added to chemotherapy increased the proportion of people having at least 1 serious adverse event. A different vaccine (OSE2101) may result in a slight decrease in the proportion of people having at least 1 serious adverse event.
What are the limitations of the evidence?
Our confidence in the evidence varied from moderate to very low for the different vaccines and outcomes assessed, mainly because the studies were small and there were not enough studies to be sure of the results.
How up to date is this evidence?
The evidence is current to August 2023.
Summary of findings
Summary of findings 1. TG4010 added to chemotherapy compared to chemotherapy alone in first‐line treatment.
| TG4010 added to chemotherapy compared to chemotherapy alone in first‐line treatment | ||||||
| Patient or population: adults with advanced non‐small cell lung cancer (NSCLC) Setting: outpatients Intervention: TG4010 added to chemotherapy Comparison: chemotherapy alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with chemotherapy alone | Risk with TG4010 added to chemotherapy | |||||
| Overall survival | HR 0.83 (0.65 to 1.05) | 370 (2 RCTs) | ⊕⊕⊝⊝ Lowa | TG4010 vaccine may result in little to no difference in overall survival. | ||
| Progression‐free survival | HR 0.74 (0.55 to 0.99) | 222 (1 RCT) | ⊕⊕⊝⊝ Lowa | TG4010 vaccine may slightly increase progression‐free survival. | ||
| Participants with at least one serious adverse event | 251 per 1000 | 176 per 1000 (58 to 551) | RR 0.70 (0.23 to 2.19) | 362 (2 RCTs) | ⊕⊝⊝⊝ Very lowb | TG4010 vaccine may result in little to no difference in the number of participants that have at least one serious adverse event but the evidence is very uncertain. |
| Survival rates at 3 years | 68 per 1000 | 68 per 1000 (20 to 224) | RR 1.00 (0.30 to 3.31) | 148 (1 RCT) | ⊕⊕⊝⊝ Lowa | TG4010 vaccine may result in little to no difference in survival rates at 3 years. |
| Survival rates at 5 years | Neither study assessed this outcome (and none of the participants were alive at five years). | |||||
| Health‐related quality of life | Neither study assessed this outcome. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised clinical trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a For the outcomes of overall survival, progression‐free survival, and survival rates at 3 years, we downgraded the certainty of the evidence by two levels for imprecision (the confidence interval includes the threshold of clinical relevance and no clear conclusions can be drawn). b For serious adverse events, we downgraded the certainty of the evidence by two levels for imprecision, and one level for inconsistency (high I2 statistic).
Summary of findings 2. Epidermal growth factor versus best supportive care for switch maintenance after first‐line treatment.
| Epidermal growth factor versus best supportive care for switch maintenance after first‐line treatment | ||||||
| Patient or population: adults with advanced non‐small cell lung cancer (NSCLC) Setting: outpatients Intervention: epidermal growth factor vaccine Comparison: best supportive care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with chemotherapy alone | Risk with epidermal growth factor vaccine added to chemotherapy | |||||
| Overall survival | HR 0.82 (0.66 to 1.02) | 378 (1 RCT) | ⊕⊕⊝⊝ Lowa | Epidermal growth factor vaccine may result in little to no difference in overall survival. | ||
| Progression‐free survival | Neither study assessed this outcome. | |||||
| Participants with at least one serious adverse event | 151 per 1000 | 200 per 1000 (133 to 299) | RR 1.32 (0.88 to 1.98) | 458 (2 RCTs) | ⊕⊕⊝⊝ Lowa | Epidermal growth factor vaccine may result in little to no difference in the rate of participants that have at least one serious adverse event. |
| Survival rates at 3 years | 87 per 1000 | 126 per 1000 (72 to 222) | RR 1.45 (0.82 to 2.54) | 458 (2 RCTs) | ⊕⊕⊝⊝ Lowa | Epidermal growth factor vaccine may result in little to no difference in survival rates at 3 years. |
| Survival rates at 5 years | 23 per 1000 | 77 per 1000 (23 to 256) | RR 3.40 (1.02 to 11.27) | 378 (1 RCT) | ⊕⊕⊝⊝ Lowa | Epidermal growth factor vaccine may result in a small difference in survival rates at 5 years. |
| Health‐related quality of life Assessed with EORTC QLQ‐C30 global health status score at 6 months | The mean health‐related quality of life score at 6 months was 54.9, as assessed with EORTC QLQ‐C30 global health status | MD 7.9 higher (0.49 lower to 16.29 higher) | 86 (1 RCT) | ⊕⊝⊝⊝ Very lowb | Epidermal growth factor vaccine may result in little to no difference in health‐related quality of life but the evidence is very uncertain. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EORTC QLQ‐C30: European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire; HR: hazard ratio; MD: mean difference; RCT: randomised clinical trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a For the outcomes of overall survival, participants with at least one serious adverse event, and survival rates at three and five years, we downgraded the certainty of the evidence by two levels for imprecision (the confidence interval contains the threshold of clinical relevance and no clear conclusions can be drawn). bWe downgraded the certainty of the evidence by two levels for risk of bias (lack of blinding, incomplete outcome data) and by one level for imprecision.
Background
Description of the condition
Lung cancer is the most common cancer worldwide. In records from the GLOBOCAN database of the International Agency for Research on Cancer in 2022, lung cancer ranked as the most commonly diagnosed cancer, with an incidence of 2,480,675 new cases (1,572,045 in men and 908,630 in women), representing 12.4% of the total of all new cancers (GLOBOCAN 2024). It was also the main cause of death from cancer, accounting for 1,817,469 deaths (1,233,241 in men and 584,228 in women), representing 18.7% of total cancer deaths (GLOBOCAN 2024).
Non‐small cell lung cancer (NSCLC) is the most common type of lung cancer, making up 87% of all lung cancers, while small cell lung cancer (SCLC) represents about 13% of all lung cancer cases (Goldstraw 2016).
Most people with NSCLC are diagnosed at an advanced stage (stage IIIB or IV), according to the TNM stage classification for lung cancer (Appendix 1). Of people diagnosed with NSCLC, about 17.6% of non‐small cell lung cancers are stage IIIB when diagnosed, and 40% are stage IV (Lemjabbar‐alaoui H 2015).
New strategies in immunotherapy target immune‐modulating mechanisms that help tumour cells defend themselves against the immune system (Remon 2017). This approach targets immune checkpoint pathways, which include blockade of the inhibitory receptors, cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA‐4), programmed cell death‐1 (PD‐1), and its ligand, PD‐L1. Immune checkpoint inhibitors are now an important part of the therapeutic armamentarium for NSCLC, both in locally advanced and metastatic stages.
For people with unresectable locally advanced stages, the usual recommendations are curative radiotherapy combined with chemotherapy and anti‐PD‐L1 treatment as consolidation treatment (Antonia 2017; Antonia 2018).
The treatment of advanced lung cancer consists of platinum‐based doublet chemotherapy with anti‐PD‐1 treatment (immunotherapy), independent of the PD‐L1 status (Paz‐Ares 2018; Gandhi 2018). In the case of a PD‐L1 tumour proportion score of 50% or higher, an anti‐PD‐1 monoclonal antibody as monotherapy can be proposed (Reck 2016). In cases of epidermal growth factor receptor (EGFR) activating mutations or ALK (anaplastic lymphoma kinase) translocation, patients receive an EGFR or ALK tyrosine kinase inhibitor. New data are likely to expand the role of immunotherapy in combination with other therapies in the coming years (Ramamurthy 2017).
With the development of immune checkpoint inhibitors, research has led to a better understanding of the interactions between the immune system and cancer cells and the mechanisms by which cancer evades the immune response. Immunotherapy represents a broad class of treatments designed to elicit immune‐mediated destruction of tumour cells (Domingues 2014). It has been shown that malignant cells can express mutated proteins that can be recognised as foreign antigens, over‐expressed normal proteins, or expressed foetal antigens, which are normally absent in healthy adults. If these tumour‐associated antigens are recognised as foreign by the immune system, they can activate them by stimulating antigens‐presenting cells (APC), and eliciting a targeted adaptive immune response (Rosenberg 1999). The discovery of specific malignant antigens that trigger an anti‐tumour immune response in people with lung cancer opens the possibility of developing therapeutic vaccines aimed at boosting the adaptive immune response against cancer cells that express those antigens.
Therapeutic vaccines can be given at different moments, from the initial diagnosis of advanced lung cancer or as the disease progresses:
as part of the initial treatment (first‐line treatment);
as second‐line treatment after failure or non‐tolerated side effects of first‐line treatment;
as third‐line treatment when both first‐ and second‐line treatment do not work, stop working, or are not well‐tolerated;
as an ongoing maintenance treatment given to help keep cancer from coming back after a good response to first‐ or second‐line treatment;
as switch maintenance, in patients in which the tumour did not progress with first induction chemotherapy, using a vaccine with a different mechanism of action.
Description of the intervention
Therapeutic vaccines stimulate the immune system to target cancer cells by boosting the innate and adaptive immune response (Zhou 2016). These vaccines may induce cellular and humoral immune responses against tumour‐specific or associated antigens. However, there are several obstacles, such as tolerance, poorly‐defined immunogenic tumour antigens, and several suppression mechanisms, which decrease the effectiveness of the immune system, mainly by protecting the tumour‐suppressive micro‐environment, especially in advanced‐stage NSCLC (Vesely 2011).
Several clinical trials that examine vaccination strategies have been developed for people with advanced NSCLC, looking primarily at allogeneic whole‐cell vaccines, protein‐based vaccines, peptide vaccines, anti‐idiotype vaccines, and viral‐based vaccines (Declerck 2014; Yang 2016; Zhou 2016; Zhu 2017).
How the intervention might work
There are data to support the hypothesis that cancer vaccines can induce a specific anti‐tumour immune response in people with cancer (Leone 2013). This is achieved through the administration of either immunogenic tumour‐associated antigens or cells in conjunction with immunoadjuvants that enhance the immune response (Mountzios 2016). The immune system plays a dual role in cancer development: it can promote tumour growth by mechanisms that interfere with immune surveillance, but it can also suppress tumour growth by activating innate and adaptive immune mechanisms (Schreiber 2011; Vesely 2013).
Therapeutic cancer vaccines try to elicit an effective immune response; in this setting, innate and adaptive immune cells recognise antigens in transformed cells and destroy them (Monteiro 2016; Vesely 2011). The tumour antigens are taken up, processed, and presented to T cells by specific antigen‐presenting cells, such as dendritic cells and macrophages. Peptides derived from these antigens are then presented in the context of class I major histocompatibility complex molecules to CD8+ T cells (anti‐tumour cytotoxic T cells) and in the context of class II major histocompatibility complex molecules to CD4+ T cells. The antigen‐presenting cells, mainly dendritic cells, express co‐stimulatory molecules, such as CD80 and CD86, which provide the signals required to activate tumour antigen‐specific CD8+ T cells, and tumour antigen‐specific CD4+ helper T cells, in a process called cross‐presentation. The helper T cells may secrete cytokines, such as tumour necrosis factor‐alpha (TNF‐α), interleukin (IL‐2), and interferon‐gamma (IFN‐γ) that can activate macrophages and natural killers to kill tumour cells. Helper T cells can also help in the activation and differentiation of B cells to plasma cells, promoting the production of specific anti‐tumour antigen antibodies. Antibodies may kill tumour cells by activating complementary, antibody‐dependent cell cytotoxicity, or other mechanisms (Mittal 2014; Pardoll 2015; Schreiber 2011; Vesely 2013).
TG4010
TG4010 is formed by an attenuated vaccinia Ankaravirus, genetically modified to express the coding sequences of the mucin‐1 (MUC1) antigen and interleukin‐2 (Suzuki 2014; Quoix 2017). IL‐2 plays a key role in activating the adaptive and innate immune response, especially T cells and natural killer cells in tumour‐associated environments. TG4010 has been shown to induce adaptive responses in participants with NSCLC in several studies (Hillman 2017; Schaedler 2017; Tosch 2017).
CIMAvax‐EGF
CIMAvax‐EGF vaccine is made from human recombinant epidermal growth factor (EGF) linked to P64, a carrier recombinant protein of the meningitis B bacteria, and an oily adjuvant (Ascarateil 2015). The mechanism of action of CIMAvax‐EGF is based on the formation of antibodies against the epidermal growth factor, a self‐protein overexpressed in NSCLC. Its overexpression has been associated with poor prognosis, lower survival, and resistance to treatment in cancer. The vaccine induces antibodies that remove EGF, thus blocking the EGF–EGFR interaction. The response against this self‐protein is due to a chemical bond between recombinant epidermal growth factor and the P64k protein derived from Neisseria meningitidis bacteria (Saavedra 2016; Saavedra 2017).
hTERT (vx‐001) vaccine
The hTERT (human telomerase reverse transcriptase) (vx‐001) vaccine is made with “optimised cryptic peptides”, a family of tumour antigens derived from universal tumour antigens. Vx‐001 comprises two 9‐amino acid peptides, the optimised Vx‐001/TERT572Y and the wild‐type (WT) Vx‐001/TERT5. Vx‐001 targets TERT (telomerase reverse transcriptase). Optimised cryptic peptides are recognised by the immune system as foreign and are strongly immunogenic (Gridelli 2020).
Racotumomab
Racotumomab (anti‐idiotype vaccine) is a murine monoclonal antibody IgG1 directed to membrane glycoconjugates expressed in aggressive solid tumours. It was developed as a mirror image of the idiotype of another antibody against N‐glycolyl‐containing molecules, such as the N‐glycolyl GM3 ganglioside (NeuGcGM3 ganglioside) (Hernandez 2021). These glycolipids are generally not expressed in healthy individuals. The vaccine develops antibodies against that ganglioside and induces complementary, independent, oncotic necrosis for tumour cells(Hernandez 2008; Hernandez 2011).
Personalised peptide vaccination (PPV)
The earlier generations of peptide vaccines were composed of one to several human leukocyte antigen (HLA)‐class I‐restricted peptides of a single HLA‐type. Personalised peptide vaccination (PPV) includes vaccine antigens selected and administered based on pre‐existing host immunity before vaccination: 12 peptides for HLA‐A2, 14 peptides for HLA‐A24, nine peptides for HLA‐A3 supertype, and four peptides for HLA‐A26 (Takayama 2016).
OSE2101
The vaccine includes modified HLA‐A2+‐restricted neoepitopes that target tumour‐associated antigens frequently overexpressed in NSCLC (human epidermal growth factor receptor 2 (HER2/neu), carcinoembryonic antigen (CEA), melanoma antigen genes 2 and 3 (MAGE 2 and 3), and p53) and generate a specific cytotoxic T cell response, stimulating killer T cells, allowing them to detect and kill cancer cells (Besse 2023).
SRL172 ‐ killed Mycobacterium vaccae
SRL172 is a suspension of killed Mycobacterium vaccae, a rapidly growing mycobacterium that normally grows as an environmental saprophyte (O'Brien 2004). It has several functions relevant to its activity in cancer, including activation of antigen‐presenting cells (APCs), Th1 adjuvant properties, suppression of pre‐existing Th2 responses (via activation of regulatory T cells), and activation of natural killer (NK) cells (O'Brien 2004).
Why it is important to do this review
The evaluation of potential therapies aimed at treating advanced non‐small cell lung cancer has a crucial role in guiding future medical treatment to improve the survival rates of this deadliest malignant disease. As previously described, immunotherapy has become a cutting‐edge clinical approach to treating cancer (Domingues 2014). Therapeutic vaccines represent a viable immunotherapy option that stimulates the immune system to fight against tumour antigens. Thus, a review of current clinical trials is important to evaluate the clinical outcomes of this treatment.
Existing systematic reviews in this area are scarce and have limitations. A recent Cochrane review assessed the effect of vaccines in people with non‐small cell lung cancer, but it did not include studies on people with advanced stages (Zhu 2021). Two reviews that did include studies in people with advanced lung cancer are now rather dated and do not include recently published studies (Dammeijer 2016; Wang 2015). Furthermore, the Dammeijer 2016 and Wang 2015 reviews did not address the health‐related quality of life of the participants, which is paramount for people with advanced lung cancer, and they also pooled studies examining different types of vaccines.
Thus, this review is important because it provides an up‐to‐date synthesis and analysis of the current evidence on the effects of therapeutic vaccines in people with advanced non‐small cell lung cancer, covers health‐related quality of life, and evaluates separately the specific effects of each type of vaccine.
The present review focuses only on therapeutic vaccines for advanced NSCLC, whatever the line of treatment or maintenance treatment.
Objectives
To evaluate the effectiveness and safety of different types of therapeutic vaccines for people with advanced non‐small cell lung cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included only parallel‐group, randomised controlled trials (RCTs) in which participants were assigned to interventions by chance. We did not include cluster‐randomised trials or quasi‐randomised trials.
Types of participants
We included participants older than 18 years with histologically‐confirmed, advanced‐stage NSCLC (stages IIIB or IV), whatever the line of treatment.
We excluded studies if, in addition to participants with advanced NSCLC, they also included participants with non‐advanced NSCLC (cancer stages lower than IIIB) and did not provide separate data for participants with advanced NSCLC.
Types of interventions
Therapeutic cancer vaccine interventions targeting tumour‐associated antigens. Therefore, we included the following interventions in this review.
Therapeutic cancer vaccines alone or in combination with chemotherapy versus supportive care, no treatment, or placebo
Therapeutic cancer vaccines alone or in combination with chemotherapy versus chemotherapy alone, as stated in current treatment guidelines.
Vaccines can be used as first‐ or second‐line treatments or as maintenance treatment post‐induction treatment.
We excluded studies that gave both groups vaccines and studies with multicomponent interventions which would preclude isolating for the effect of the vaccine.
We also excluded studies that gave vaccines withdrawn by their manufacturers for being ineffective in treating advanced NSCLC, as these are unavailable for use in clinical practice: tecemotide, belagenpumatucel‐L, and melanoma antigen gene (MAGE)‐A3 peptide vaccines.
Types of outcome measures
We considered the following outcomes in this review.
Primary outcomes
Overall survival: defined as the interval between the date of randomisation and the date of death from any cause.
Progression‐free survival: defined as the interval between the date of randomisation and the appearance of new lesions, or the progression of the primary tumour, preferably according to RECIST criteria for studies done after the year 2009 (Response Evaluation Criteria in Solid Tumour (RECIST 2009)), or death.
Serious treatment‐related adverse events, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (Freites‐Martinez 2021).
Secondary outcomes
Survival rates: proportion of participants in a study who were still alive at: (1) three years and (2) five years.
Health‐related quality of life (HRQoL), measured with standard and psychometrically validated instruments with application in cancer, such as the 30‐item European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ‐C30 (Aaronson 1993; Damm 2013; Smith 2014)). Improvement was defined as a 10‐point or greater increase in functional scores or a 10‐point or greater decrease in symptom scores of the EORTC QLQ‐C30 questionnaire (Maringwa 2011). For the Functional Assessment of Cancer Therapy‐Lung (FACT‐L) Questionnaire, a 2‐ to 3‐point change in the Lung Cancer Subscale (LCS) and a 5‐ to 6‐point change in the Trial Outcome Index (TOI) are considered minimally important differences (Cella 2002).
Search methods for identification of studies
The Cochrane Lung Cancer Information Specialist designed and ran our search strategies for the three main databases (Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase).
Electronic searches
We searched for eligible trials, without language restrictions, in:
The Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, from inception to 22 August 2023;
MEDLINE (via PubMed from 1966 to 22 August 2023);
Embase (from 1974 to 22 August 2023);
Wanfang Data (from 2017 to July 2022);
China Journal Net (often referred to as CNKI) (from 2017 to July 2022).
The search strategy combined terms from the Medical Subject Heading (MeSH), free‐text terms, and appropriate indexing terms relevant to other information sources. Our MEDLINE search string was developed according to the Cochrane Highly Sensitive Search Strategy, sensitivity‐maximizing version (2008 version) as referenced in Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2023). Our search strategies are presented in Appendix 2 (CENTRAL), Appendix 3 (MEDLINE), Appendix 4 (Embase), Appendix 5 (Wanfang Data), and Appendix 6 (China Journal Net).
Searching other resources
We reviewed the reference lists of the included studies to identify other primary clinical trials.
We searched the grey literature by conducting a manual search for potentially eligible trials in abstracts from the following conference proceedings for the years 2020 to 2023:
American Society of Clinical Oncology (ASCO; www.asco.org/), up to 22 September 2023;
European Society of Medical Oncology (ESMO; www.esmo.org), up to 22 September 2023;
American Association for Cancer Research (AACR; www.aacr.org/Meetings/Pages/MeetingDetail.aspx?EventItemID=54#.WsZl0C7waM9), up to 22 September 2023;
Tumor & Cancer Immunology and Immunotherapy (tumorimmunology.conferenceseries.com/), up to 22 September 2023.
We searched for errata or retractions from included trials, according to the guidance in Chapter 4 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2023).
Data collection and analysis
Four review authors (MC‐J, JR, MR‐E, EJ) performed the data collection and analysis, using standard Cochrane methodological procedures (Higgins 2023a). We assessed all potentially eligible studies for inclusion, regardless of the language of publication.
Selection of studies
Four review authors (MC‐J, JR, MR‐E, EJ) independently screened articles by titles and abstract; we retrieved full‐text documents that possibly met the review's inclusion criteria. Three review authors (MC‐J, JR, MR‐E) independently screened the full‐text articles for eligibility. We used Covidence to manage duplication detection and full‐text evaluation. We contacted study authors when necessary to help us make a decision about the inclusion of a study and to request additional data. A consensus was reached regarding the inclusion or exclusion of a trial. We reported the reasons for excluding a trial. We created a PRISMA flowchart to show the process graphically (Moher 2009).
Data extraction and management
Four review authors (MC‐J, JR, MR‐E, EJ) independently extracted clinical and methodological information. Two review authors (JR, MR‐E) independently extracted quantitative data for effect sizes, using a standard data collection form. We resolved any discrepancies regarding the extracted data by consensus or by consulting another review author.
We used a standardised form designed for this review to collect data for each included study, and we extracted the following information.
Methods: design of the study, setting and year, duration of follow‐up, publication status (published or unpublished).
Participants: main characteristics (sex, age), number randomised to trial arms, and baseline clinical characteristics (clinical stage or severity at inception, time since first‐line treatment).
Interventions: details of experimental intervention and comparison, dosage, and timing.
Outcomes: primary and secondary outcomes of the study as reported in publications, obtained from trial protocols, or both.
Other: trial registration code; funding; conflicts of interest reported.
If reports did not provide appropriate or sufficient information, we contacted study authors, requesting additional information.
Assessment of risk of bias in included studies
Three review authors (MC‐J, JR, MR‐E), working in two‐person subgroups, independently assessed the methodological quality of each study using the Cochrane risk of bias (RoB 1) tool, described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If we could not resolve disagreements through discussion, we consulted a third review author.
For each risk of bias domain, we assigned a rating of low, unclear, or high risk of bias, based on the following definitions.
Was the allocation sequence adequately generated? We considered randomisation adequate (low risk of bias) if the allocation sequence was generated from a table of random numbers or by computer. We judged a study to have an unclear risk of bias if the study report stated that the trial was randomised, but did not describe the method.
Was allocation adequately concealed? We deemed allocation concealment to be adequate (low risk of bias) if the report stated that it was undertaken using sequentially pre‐numbered, sealed opaque envelopes, or by a centralised system. We judged a study to have an unclear risk of bias if the study report stated that the allocation was concealed, but did not describe the method.
Was knowledge of the allocated intervention adequately prevented during the study? Effective blinding of participants can be difficult to apply to trials of anticancer treatment because of the known potential toxicity of chemotherapy. We evaluated the risk of bias separately for personnel, participants, and outcomes assessors, and for each outcome, when applicable. We considered that lack of blinding of participants and personnel could be a source of performance bias and detection bias for subjective outcomes (quality of life and progression‐free survival), but not for objective outcomes (overall survival, survival rates at three and five years, and severe adverse events).
Were incomplete outcome data adequately addressed? We examined whether imbalance across intervention groups could be seen in the numbers or reasons for missing data, the type of measures undertaken to handle missing data, and whether the analysis was carried out on an intention‐to‐treat (ITT) basis.
Were reports of the study free of the suggestion of selective outcome reporting? We evaluated whether each predefined outcome was measured, analysed, and reported.
Were there any other potential sources of bias?
We completed a risk of bias table for each included study and summarised risks of bias across studies, as recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2023b).
Measures of treatment effect
For overall survival and progression‐free survival, we measured the effect of treatment on time‐to‐event outcomes using the hazard ratio (HR) with a 95% confidence interval (CI). We extracted HRs and standard errors from the reported data or estimated them from other data or graphs if possible (Tierney 2007). We measured the proportions of participants surviving at three and five years, and the percentages of participants with at least one serious adverse event using the relative risk or risk ratio (RR) with 95% CI. For continuous outcomes (HRQoL), we used mean differences (MDs) for measures using the same scale, and standardised mean differences (SMDs) for measures using different scales.
Unit of analysis issues
We included only parallel‐group randomised trials in this review, and thus, there was no unit of analysis issues related to the inclusion of cluster‐randomised trials or cross‐over trials.
If trials included several intervention comparisons, we followed standard methodological approaches, as recommended in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2023c).
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Also, for studies with full texts available, we attempted to obtain more information from study authors if details relevant to our analysis had not been reported. In future updates, if we cannot obtain additional data necessary for meta‐analysis, we will try to estimate values from reported data (for example, estimating the HR from published survival curves (Tierney 2007)). We conducted the main analyses as 'available‐data analysis', using ITT data from the included studies where they were available, and any reported data otherwise.
Assessment of heterogeneity
We used the I² statistic to assess heterogeneity amongst trials for each meta‐analysis. The I² statistic describes the percentage of variability in effect estimates that is due to heterogeneity rather than sampling error. We interpreted the I² value according to the following thresholds (Deeks 2023): 0% to 40% heterogeneity might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity. We investigated substantial heterogeneity (I² > 50%) by prespecified subgroup analysis.
Assessment of reporting biases
To address reporting bias and related small‐study effects, we planned to draw funnel plots for each meta‐analysis when advisable. If the required statistical conditions were met (i.e. inclusion of about 10 studies in a meta‐analysis), we planned to use asymmetry tests (Page 2023). In future updates of this review, if there are sufficient studies, for dichotomous outcomes we will test asymmetry with the Harbord test if Tau² is less than 0.1 (Harbord 2006), and with the Rücker test if Tau² is more than 0.1 (Rücker 2008); and for continuous outcomes, we will use the regression asymmetry test (Egger 1997).
Data synthesis
We used meta‐analysis to combine individual effect sizes when event percentages were available or could be calculated. We used Review Manager Web to analyse data for each comparison and outcome (RevMan Web 2022), using a random‐effects model. This model assumes between‐study variability in the observed effect beyond that due to random error. We presented all combined effect estimates with 95% CI.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses according to the following clinical characteristics:
clinical stage (III or IV);
sex of participants;
NSCLC histological type (squamous versus non‐squamous);
time interval between the previous treatment line and beginning therapy with vaccines (three, six, or 12 months);
Eastern Cooperative Oncology Group (ECOG) performance status (0 versus 1, or 0‐1 versus 2) (Oken 1982; Prasad 2018).
However, ultimately, we did not attempt subgroup analyses for two reasons: (1) four of the therapeutic vaccines included in this review were evaluated in a single RCT; three vaccines were evaluated in two studies each, and in those cases, we did not find heterogeneity amongst their results; and (2) the final numbers of participants in the subgroups were too small for all proposed subgroup analyses and thus did not guarantee enough statistical power.
Sensitivity analysis
If relevant, in future updates of the review, we will conduct sensitivity analyses to assess whether the results are robust to decisions made during the review process. We will perform sensitivity analyses to explore the influence on the effect size by: (1) excluding unpublished studies; and (2) excluding lower‐quality studies (i.e. those at high risk of bias).
Summary of findings and assessment of the certainty of the evidence
We present separate summary of findings tables for TG4010 and epidermal growth factor (Table 1; Table 2), as both of these vaccines were evaluated for efficacy and safety in two RCTs each, and these were sufficiently alike for pooling of results to make sense. We did not pool results from the two RCTs that examined racotumomab as one study compared racotumomab to placebo, whilst the other compared it to docetaxel. We created the summary of findings tables using the methods and recommendations described in the GRADE Handbookand using GRADEpro GDT software (GRADEpro GDT; Schünemann 2013). For the summary of findings tables, we used the five GRADE considerations (study limitations (i.e. risk of bias); consistency of the effect (heterogeneity if I2 was higher than 50%); imprecision (if the confidence interval contains the threshold of clinical relevance and no clear conclusions can be drawn); indirectness; and publication bias) to assess the certainty of the body of evidence.
We included the following outcomes: overall survival, progression‐free survival, serious treatment‐related adverse events, survival rates at three and five years, and health‐related quality of life.
When assessing the certainty of the evidence for imprecision, we considered a clinically relevant improvement for the EORTC QLQ‐C30 questionnaire to be a 10‐point or greater increase in functional scores or a 10‐point or greater decrease in symptom scores (Maringwa 2011). For the Functional Assessment of Cancer Therapy‐Lung (FACT‐L) Questionnaire, a 2‐ to 3‐point change in the Lung Cancer Subscale (LCS) and a 5‐ to 6‐point change in the Trial Outcome Index (TOI) are considered minimally important differences (Cella 2002).
When data aggregation was not possible, we presented the results of individual studies narratively and discussed them in the text.
Results
Description of studies
Details are available in Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We identified 18,411 records through electronic database searches. After the removal of duplicates, we screened 16,888 records by titles and abstracts and discarded 16,815 as irrelevant to the review. We retrieved the remaining 73 records in full text for further assessment. Of these, we included 10 studies (21 references) in the review, and we excluded 11 studies (12 references) with reasons. We discarded the remaining 40 references as irrelevant to the review (see Figure 1).
1.
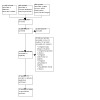
Study flow diagram
Included studies
Four studies were conducted in European countries (Gridelli 2020; O'Brien 2004; Quoix 2011; Quoix 2016); four studies were conducted in Cuba (Alfonso 2014; Hernandez 2021; Neninger 2008; Rodriguez 2016); one study was conducted in several different European countries, Israel, and the USA (Besse 2023); and the remaining study was conducted in Japan (Takayama 2016). Taken together, the 10 studies randomised a total of 2177 participants. Our outcome analyses included only 2045 participants (1401 men and 644 women).
Two studies assessed TG4010, a vector‐based vaccine (Quoix 2011; Quoix 2016); two studies assessed an epidermal growth factor vaccine (Neninger 2008; Rodriguez 2016); two studies assessed racotumomab‐alum (Alfonso 2014; Hernandez 2021); and one study each assessed hTERT (vx‐001) (Gridelli 2020), a personalised peptide vaccine (Takayama 2016), OSE2101 (Besse 2023), and SRL172 (killed Mycobacterium vaccae) (O'Brien 2004).
In two studies, vaccines were part of first‐line treatment (Quoix 2011; Quoix 2016); in four studies, participants received the vaccines as maintenance treatment after finishing first‐line chemotherapy (Alfonso 2014; Gridelli 2020; Hernandez 2021; Takayama 2016). In two studies, the vaccine was used for switch maintenance after first‐line treatment (Neninger 2008; Rodriguez 2016); in one study, participants had previously received one line of immune checkpoint blockers (Besse 2023); and one study included participants in first‐line or maintenance treatment (O'Brien 2004).
In the included studies, whatever the line of treatment (first‐ or second‐line), all participants received chemotherapy or a usual anticancer treatment and were then randomised to receive a therapeutic vaccine added to it.
Eight of the included studies were prospectively registered in publicly accessible clinical trial registers. We found no registry information for two studies (Neninger 2008; O'Brien 2004).
In terms of the review's prespecified outcomes, seven studies reported data on rates of overall survival (Alfonso 2014; Besse 2023; Gridelli 2020; Quoix 2011; Quoix 2016; Rodriguez 2016; Takayama 2016), and three on rates of progression‐free survival (Alfonso 2014; Quoix 2011; Takayama 2016). All included studies provided data on severe adverse events. Six studies provided data on survival percentages at three years and five years (Alfonso 2014; Besse 2023; Gridelli 2020; Neninger 2008; Quoix 2011; Rodriguez 2016), and only four reported on health‐related quality of life (Besse 2023; O'Brien 2004; Quoix 2011; Rodriguez 2016).
Two studies were terminated prematurely. The Rodriguez 2016 trial on epidermal growth factor vaccine CIMAvax‐EGF was stopped before reaching the intended sample size, at the second interim analysis, after the Cuban National Regulatory Agency approved the vaccine for marketing. The sponsor of the Besse 2023 study on OSE2101 stopped the trial prematurely in April 2020 at the recommendation of an independent data monitoring committee, due to the risk that the coronavirus disease 2019 (COVID‐19) pandemic posed to data integrity.
Excluded studies
We excluded 64 full‐text articles in total at the full‐text screening stage. Following the guidance in the Cochrane Handbook of Systematic Review of Interventions (Lefebvre 2023), we selected 11 excluded studies (12 articles in total) that readers might plausibly expect to see amongst the included studies, and we have listed these, together with reasons for exclusion, in the Characteristics of excluded studies table. We discarded the remaining 52 articles as irrelevant to the review. We excluded the 11 studies for the following reasons:
ineligible study design: single‐arm studies (two studies: Saavedra 2017; Sebastian 2014);
ineligible participants: planned to include people with stage IIIA cancer (as well as higher stages); study results not published (one study: Wu 2011).
ineligible intervention: (1) vaccine given as part of a multicomponent intervention, which precluded assessing the separate effect of the vaccine (two studies: Cohen 2014; Ramalingam 2014); (2) vaccine withdrawn by manufacturers (two studies: Butts 2014; Katakami 2017).
ineligible comparison: comparison of different schedules or maintenance schemes for the same vaccine (three studies: Gray 2018; Ramlau 2008; Saavedra 2021);
unpublished results (one study: Govindan 2014).
Risk of bias in included studies
In most cases, we assessed the risk of bias in the included studies using information published in full‐text papers, available in trial protocols publicly accessible from clinical trial registries, or both. We requested additional information from more than 30 authors; only three responded with partial information on their studies (Besse 2023; Hernandez 2021; Neninger 2008). For those studies whose authors did not respond to our requests, we deemed the risk of bias for some domains to be unclear.
In Figure 2, we present a graph displaying our global assessment of the risk of bias for each domain for all included trials, presented as percentages. In Figure 3, we present a summary of our judgements about each risk of bias domain for each included study. For detailed explanations of our judgements for each study, see the risk of bias tables in the Characteristics of included studies section.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Six included studies used adequate methods of sequence generation and allocation concealment, and therefore we considered them at low risk of selection bias (Alfonso 2014; Besse 2023; Gridelli 2020; Neninger 2008; Quoix 2011; Quoix 2016).
For the remaining four studies, we did not find relevant information on sequence generation procedures, allocation concealment, or both, and we classified them as being at unclear risk of selection bias. We sent emails requesting additional information (where we had contact information for corresponding authors or contact persons in clinical trials registers), but we received no replies.
Blinding
Blinding of participants and personnel (performance bias)
In three included studies, participants and personnel were blinded (Alfonso 2014; Gridelli 2020; Quoix 2016); we judged these to be at low risk of performance bias for subjective outcomes.
Six included studies had an open‐label design (Besse 2023; Hernandez 2021; Quoix 2011; O'Brien 2004; Rodriguez 2016; Takayama 2016). We considered these, by default, to be at high risk of performance bias for the review's subjective outcomes of progression‐free survival and HRQoL, if they measured these outcomes in their studies: Takayama 2016 assessed only progression‐free survival; Rodriguez 2016 and O'Brien 2004 assessed only HRQoL; and Besse 2023, Hernandez 2021, and Quoix 2011 evaluated both outcomes. However, we considered that the non‐blinding of participants and personnel was not a source of bias for the review's objective outcomes related to death (overall survival and survival rate at three and five years) and for severe adverse events.
One study did not report blinding (Neninger 2008).
Blinding of outcome assessors (detection bias)
We considered that the lack of blinding of outcome assessors could be a source of high risk of detection bias for the same cases described above for performance bias.
Incomplete outcome data
We did not detect a risk of attrition bias in any of the included studies. There were few losses to follow‐up in the studies.
Selective reporting
For the studies registered in publicly‐accessible clinical trials registers, we compared the outcomes described in the registries with those reported in study publications, and we judged them as having a low risk of reporting bias. For studies that were not prospectively registered, we checked whether their publications reported on all outcomes that could reasonably be expected to be assessed in these types of trials.
Most of the studies did not provide sufficient detail about their statistical methods, which impeded a proper evaluation of selective reporting bias.
Other potential sources of bias
For those studies that provided sufficient information, we checked for possible baseline differences between the intervention and comparator groups. We did not detect any potential bias related to baseline differences.
We did not conduct publication bias analysis using funnel plots since there were insufficient studies for each treatment comparison (Page 2023).
Effects of interventions
Comparison 1. TG4010 plus chemotherapy compared to chemotherapy alone in first‐line treatment
Two studies with 370 participants analysed the effects of a vector‐based vaccine, TG4010, as part of first‐line treatments (Quoix 2011; Quoix 2016). Participants had stage IIIB or IV non‐small cell lung cancer without a known activating EGFR mutation and with MUC1 expression in at least 50% of tumoural cells, previously untreated.
The Quoix 2011 study was conducted in 23 centres in France, Poland, Germany, and Hungary. It included 148 participants: 107 men and 41 women. Of these, 74 were randomised to vaccine plus chemotherapy and 74 to chemotherapy alone. Twelve participants had stage IIIB cancer and 136 had stage IV cancer. Their mean age was 58 years. They were followed up for 50 months.
The Quoix 2016 study was conducted in 45 centres in France, Belgium, the UK, Italy, Spain, Hungary, Poland, Israel, and the USA. It included 222 participants: 142 men and 80 women. Of these, 111 were randomised to vaccine plus chemotherapy and 111 to chemotherapy alone. All participants had stage IV cancer. Their mean age was 61 years. They were followed up for 36 months.
See Table 1.
Primary outcomes
Overall survival
Pooled results showed that adding TG4010 to first‐line chemotherapy may result in little to no difference in overall survival compared with chemotherapy alone (HR 0.83, 95% CI 0.65 to 1.05; I2 = 0%; 2 studies, 370 participants; low‐certainty evidence; Analysis 1.1). We downgraded the certainty of the evidence by two levels for imprecision. Median survival for the vaccine and no‐vaccine groups was 10.7 months versus 10.3 months, respectively, in Quoix 2011, and 12.7 months and 10.6 months in Quoix 2016.
1.1. Analysis.

Comparison 1: TG4010 added to chemotherapy versus chemotherapy alone in first line treatment, Outcome 1: Overall survival
Progression‐free survival
Only Quoix 2016 evaluated this outcome and found that adding the TG4010 vaccine may slightly increase participants' progression‐free survival compared with chemotherapy alone (HR 0.74, 95% CI 0.55 to 0.99; 1 study, 222 participants; low‐certainty evidence; Analysis 1.2). We downgraded the certainty of the evidence by two levels for imprecision. Median progression‐free survival for the vaccine and non‐vaccine groups in the study was 5.9 versus 5.1 months, respectively.
1.2. Analysis.

Comparison 1: TG4010 added to chemotherapy versus chemotherapy alone in first line treatment, Outcome 2: Progression‐free survival
Serious treatment‐related adverse events (CTCAE grades 3 to 5)
Both studies evaluated the percentages of participants who had at least one serious adverse event. Pooled results showed that adding the TG4010 vaccine to first‐line chemotherapy may result in little to no difference in the proportion of participants with at least one serious adverse event compared with chemotherapy alone, but the evidence is very uncertain (RR 0.70, 95% CI 0.23 to 2.19; I2 = 75%; 2 studies, 362 participants; very low‐certainty evidence; Analysis 1.3). We downgraded the certainty of the evidence by two levels for imprecision and one level for heterogeneity.
1.3. Analysis.

Comparison 1: TG4010 added to chemotherapy versus chemotherapy alone in first line treatment, Outcome 3: Adverse events grades 3 to 5 (Participants with at least one serious adverse event)
Quoix 2011 provided detailed data on the number of serious adverse events in each treatment group, and reported 88 serious adverse events in the 73 participants who received the vaccine and 99 in the 72 participants who did not receive the vaccine.
Secondary outcomes
Survival rates at three and five years
Only Quoix 2011 provided data on this outcome and found that adding the TG4010 vaccine to first‐line chemotherapy may result in little to no difference in the survival rate at three years (RR 1.0, 95% CI 0.30 to 3.31; 1 study, 148 participants; low‐certainty evidence; Analysis 1.4). We downgraded the certainty of the evidence by two levels for imprecision. None of the participants in the study were alive at five years.
1.4. Analysis.

Comparison 1: TG4010 added to chemotherapy versus chemotherapy alone in first line treatment, Outcome 4: Survival rates at 3 years
Health‐related quality of life (HRQoL)
None of the studies provided data to compare scores on a health‐related quality of life scale between groups before and after the treatments.
Quoix 2011 assessed HRQoL, using the Functional Assessment of Cancer Therapy‐Lung (FACT‐L) at baseline and every six weeks, measuring the "Time until definitive deterioration" (TUDD) of the four well‐being dimensions of the FACT‐L (physical (PWB), functional (FWB), emotional (EWB), and social well‐being (SWB)) and the Lung Cancer Subscale (LCS) domains for a 5‐point minimal clinically important difference. The study reported that "no difference of TUDD of HRQOL has been found between treatment arms".
Comparison 2. Epidermal growth factor vaccine versus best supportive care for switch maintenance after first‐line treatment
Two studies carried out in Cuba, which included 485 participants in total, compared adding an epidermal growth factor vaccine between one and two months after finishing chemotherapy to best supportive care (Neninger 2008; Rodriguez 2016).
The Neninger 2008 study included 80 participants: 59 men and 21 women. Forty participants were randomised to the vaccine and 40 to best supportive care. Fifty participants had stage IIIB cancer and 30 had stage IV. Their mean age was 56 years. They were followed up for 50 months.
The Rodriguez 2016 study included 405 participants: 264 men and 141 women. Of these, 270 were randomised to vaccine and 135 to control; 257 participants had stage IIIB and 134 had stage IV. Their mean age was not reported. They were followed up for 84 months. The Rodriguez 2016 trial on epidermal growth factor vaccine CIMAvax‐EGF was stopped before reaching the intended sample size, at the second interim analysis, after the Cuban National Regulatory Agency approved the vaccine for marketing.
See Table 2.
Primary outcomes
Overall survival
Only Rodriguez 2016 reported a hazard ratio for overall survival, showing that epidermal growth factor vaccine may result in little to no difference in overall survival compared with best supportive care (HR 0.82, 95% CI 0.66 to 1.02; 1 study, 378 participants; low‐certainty evidence; Analysis 2.1). We downgraded the certainty of the evidence by two levels for imprecision. Median survival times in the vaccine and control groups were 6.5 months versus 5.3 months, respectively (Neninger 2008), and 10.8 versus 8.9 months (Rodriguez 2016).
2.1. Analysis.

Comparison 2: Epidermal growth factor vaccines versus best supportive care for switch maintenance after first‐line treatment, Outcome 1: Overall survival
Progression‐free survival
Neither study assessed this outcome.
Serious treatment‐related adverse events (CTCAE grades 3 to 5)
Pooled results showed that adding the vaccine may result in little to no difference in the proportion of people with at least one serious adverse event compared with best supportive care (RR 1.32, 95% CI 0.88 to 1.98; 2 studies, 458 participants; low‐certainty evidence; Analysis 2.2). We downgraded the certainty of the evidence by two levels for imprecision.
2.2. Analysis.

Comparison 2: Epidermal growth factor vaccines versus best supportive care for switch maintenance after first‐line treatment, Outcome 2: Adverse events grades 3 to 5 (Participants with at least one serious adverse event)
Secondary outcomes
Survival rates at three and five years
Both studies provided data necessary to calculate survival rates at three years. Only Rodriguez 2016 followed participants for five years.
Survival rate at three years: pooled results showed that the vaccine may result in no difference in the survival rate at three years compared with best supportive care (RR 1.45, 95% CI 0.82 to 2.54; 2 studies, 458 participants; low‐certainty evidence; Analysis 2.3). We downgraded the certainty of the evidence by two levels for imprecision.
2.3. Analysis.

Comparison 2: Epidermal growth factor vaccines versus best supportive care for switch maintenance after first‐line treatment, Outcome 3: Survival rates at 3 years
Survival rate at five years: results showed that the vaccine may increase the survival rate slightly at five years compared with best supportive care (RR 3.40, 95% CI 1.02 to 11.27; 1 study, 378 participants; low‐certainty evidence; Analysis 2.4). We downgraded the certainty of the evidence by two levels for imprecision.
2.4. Analysis.

Comparison 2: Epidermal growth factor vaccines versus best supportive care for switch maintenance after first‐line treatment, Outcome 4: Survival rates at 5 years
Health‐related quality of life (HRQoL)
Rodriguez 2016 analysed the effect of the treatments on the HRQoL of the participants, evaluated with the EORTC QLQ‐C30 questionnaire at three to six months of treatment. Compared to best supportive care, the vaccine may result in little to no difference in global health status, but the evidence is very uncertain (MD 7.90, 95% CI ‐0.49 to 16.29; 1 study, 86 participants; very low‐certainty evidence; Analysis 2.5). We downgraded the certainty of the evidence two levels for risk of bias (lack of blinding; incomplete outcome data), and one level for imprecision.
2.5. Analysis.

Comparison 2: Epidermal growth factor vaccines versus best supportive care for switch maintenance after first‐line treatment, Outcome 5: Health‐related quality of life
Comparison 3. hTERT (vx‐001) vaccine versus placebo for maintenance treatment after first‐line chemotherapy
One study with participants from 70 places in Europe assessed this comparison (Gridelli 2020). The study included 221 participants, 109 were randomised to the vaccine and 112 to placebo (Gridelli 2020). Ultimately, 190 participants were included in the analyses: 132 men and 58 women.
All participants had metastatic NSCLC that did not progress after first‐line platinum‐based chemotherapy, and had human leukocyte antigen HLA‐A*0201 haplotype and tumoural expression of TElomerase Reverse Transcriptase (TERT).
Fifty‐three per cent of participants were older than 65 years and 46.5% were younger than 65 years. They were followed up for 50 months.
Primary outcomes
Overall survival
Data from the study showed that the vaccine may result in little to no difference in overall survival compared with placebo (HR 0.97, 95% CI 0.70 to 1.34; 1 study, 190 participants; Analysis 3.1). Median survival times in the vaccine and control groups were 14.3 versus 11.3 months, respectively.
3.1. Analysis.

Comparison 3: hTERT (vx‐001) vaccine versus placebo as post‐chemotherapy maintenance treatment, Outcome 1: Overall survival
Progression‐free survival
The study did not assess this outcome.
Serious treatment‐related adverse events (CTCAE grades 3 to 5)
The authors did not provide detailed data on this outcome. They reported that no participant required treatment discontinuation because of severe grade 3 or 4 adverse events, and mentioned that a participant developed grade 3 fever, which completely resolved with paracetamol within two days.
Secondary outcomes
Survival rates at three and five years
Survival rate at three years: the study did not report survival data at three years. However, the authors reported on data at 40 months of follow‐up. Results showed that there might be no differences in survival at 40 months between the vaccine and best supportive care (RR 0.38, 95% CI 0.04 to 3.57; 1 study, 190 participants; Analysis 3.2).
3.2. Analysis.

Comparison 3: hTERT (vx‐001) vaccine versus placebo as post‐chemotherapy maintenance treatment, Outcome 2: Survival rates at 40 months
Survival rate at five years: no participants were alive at 50 months of follow‐up.
Health‐related quality of life (HRQoL)
The study did not assess this outcome.
Comparison 4. Racotumomab versus placebo for switch maintenance treatment after first‐line chemotherapy
One study conducted in Cuba assessed this comparison (Alfonso 2014). It included 176 participants: 118 men and 58 women; 87 were randomised to the vaccine and 89 to placebo. The participants had stage IIIB/IV NSCLC and had at least stable disease after first‐line chemotherapy. Ninety‐nine participants had stage IIIB cancer and 77 had stage IV cancer. Seventy‐nine participants were 60 years old or less and 97 were over 60 years. They were followed up for 84 months.
Primary outcomes
Overall survival
Data from the study showed that racotumomab may increase overall survival compared with placebo (HR 0.63, 95% CI 0.46 to 0.87; 1 study, 176 participants; Analysis 4.1). Median survival times in the vaccine and control groups were 8.2 versus 6.8 months, respectively.
4.1. Analysis.

Comparison 4: Racotumomab‐alum vaccine versus placebo for switch maintenance after first‐line treatment, Outcome 1: Overall survival
Progression‐free survival
Data from the study showed that racotumomab may have little or no effect on progression‐free survival compared with placebo (HR 0.73, 95% CI 0.53 to 1.00; 1 study, 176 participants; Analysis 4.2). Median progression‐free survival times in the vaccine and control groups were 5.3 versus 3.9 months, respectively.
4.2. Analysis.

Comparison 4: Racotumomab‐alum vaccine versus placebo for switch maintenance after first‐line treatment, Outcome 2: Progression‐free survival
Serious treatment‐related adverse events (CTCAE grades 3 to 5)
The study showed that the vaccine may result in little to no difference in the proportion of people with at least one serious adverse event compared with placebo (RR 1.03, 95% CI 0.15 to 7.18; 1 study, 175 participants; Analysis 4.3).
4.3. Analysis.

Comparison 4: Racotumomab‐alum vaccine versus placebo for switch maintenance after first‐line treatment, Outcome 3: Adverse events grade 3‐5 (Participants with at least one serious adverse event)
Secondary outcomes
Survival rates at three and five years
Survival rate at three years: the vaccine may increase the survival rate at three years compared with placebo (RR 4.09, 95% CI 1.20 to 14.00; 1 study, 176 participants; Analysis 1.4).
Survival rate at five years: the vaccine may result in little to no difference in the survival rate at five years compared with placebo (RR 2.05, 95% CI 0.38 to 10.88; 1 study, 176 participants; Analysis 4.5).
4.5. Analysis.

Comparison 4: Racotumomab‐alum vaccine versus placebo for switch maintenance after first‐line treatment, Outcome 5: Survival rates at 5 years
Health‐related quality of life (HRQoL)
The study did not assess this outcome.
Comparison 5. Racotumomab versus docetaxel for switch maintenance treatment after first‐line chemotherapy
One study conducted in Cuba assessed this comparison (Hernandez 2021). It included 145 participants: 88 men and 57 women; 93 were randomised to vaccine and 52 to docetaxel. Participants had stage IIIB or IV NSCLC, with an objective response or stable disease after first‐line chemotherapy. Forty‐six participants had stage IIIB cancer and 94 had stage IV cancer. The participants' mean age was 63 years. They were followed up for 45 months.
Primary outcomes
Overall survival
The study assessed survival but did not report the hazard rate for overall survival. Median survival times in the vaccine and control groups were 9.8 and 8.6 months, respectively.
Progression‐free survival
The study assessed survival but did not report the hazard rate for progression‐free survival. Median progression‐free survival times in the vaccine and control groups were 4.4 and 4.0 months, respectively.
Serious treatment‐related adverse events (CTCAE grades 3 to 5)
The study showed that racotumomab may result in little to no difference in the proportion of people with at least one serious adverse event compared with docetaxel (RR 0.89, 95% CI 0.44 to 1.83; 1 study, 145 participants; Analysis 5.1).
5.1. Analysis.

Comparison 5: Racotumomab‐alum vaccine versus docetaxel for switch maintenance treatment after first‐line treatment, Outcome 1: Adverse events grades 3 to 5 (Participants with at least one serious adverse event)
Secondary outcomes
Survival rates at three and five years
The study did not assess these outcomes.
Health‐related quality of life (HRQoL)
The study reported that quality of life assessment had similar results in both groups, but did not provide detailed data.
Comparison 6. Personalised peptide vaccine plus docetaxel versus docetaxel plus placebo after first‐line treatment
One study conducted in Japan assessed this comparison (Takayama 2016). It included 50 participants: 41 men and 9 women; 26 were randomised to vaccine plus docetaxel and 24 to docetaxel plus placebo. Participants had advanced NSCLC with epidermal growth factor receptor (EGFR) wild genotype previously treated by chemotherapy. Eleven participants had stage IIIB cancer, 34 had stage IV cancer, and five had recurrent cancers. The mean age was 65 years. They were followed up for 700 days (i.e. 23 months).
Primary outcomes
Overall survival
Data from the study showed that adding the personalised peptide vaccine to docetaxel may result in little to no difference in overall survival compared with docetaxel plus placebo (HR 0.80, 95% CI 0.42 to 1.52; 1 study, 50 participants; Analysis 6.1). Median survival times in the vaccine and control groups were 10.5 versus 7.7 months, respectively.
6.1. Analysis.

Comparison 6: Personalised peptide vaccine versus docetaxel plus placebo after first‐line treatment, Outcome 1: Overall survival
Progression‐free survival
Data from the study showed that adding the personalised peptide vaccine to docetaxel may result in little to no difference in progression‐free survival compared with docetaxel plus placebo (HR 0.78, 95% CI 0.43 to 1.42; 1 study, 50 participants; Analysis 6.2). The median progression‐free survival times for the vaccine and no‐vaccine groups in the study were 1.9 versus 1.7 months, respectively.
6.2. Analysis.

Comparison 6: Personalised peptide vaccine versus docetaxel plus placebo after first‐line treatment, Outcome 2: Progression‐free survival
Serious treatment‐related adverse events (CTCAE grades 3 to 5)
The study did not report the number of participants with at least one serious adverse event. Amongst the 26 participants treated with the vaccine, study authors reported 38 serious grade 3 to 4 adverse events, and 35 events amongst the 24 participants who did not receive the vaccine.
Secondary outcomes
Survival rates at three and five years
The study did not assess this outcome.
Health‐related quality of life (HRQoL)
The study did not assess this outcome.
Comparison 7. OSE2101 vaccine versus chemotherapy in HLA‐A2+ advanced NSCLC in second/third‐line treatment after failure with immune checkpoint inhibitors
One study carried out in the Czech Republic, France, Germany, Hungary, Italy, Israel, Poland, Spain, and the USA, assessed this comparison (Besse 2023). The study planned to recruit at least 363 participants, but recruitment was stopped prematurely in April 2020 at the recommendation of an independent data monitoring committee, due to the risk that the coronavirus disease 2019 (COVID‐19) pandemic posed to data integrity. Eligible participants had received one line of immune checkpoint blocker (ICB) therapy for locally advanced or metastatic epidermal growth factor receptor/anaplastic lymphoma kinase (EGFR/ALK)‐negative NSCLC, given sequentially (second‐line), or combined with platinum‐based chemotherapy (first‐line) with disease progression (measurable and non‐measurable disease), Eastern Cooperative Oncology Group (ECOG) performance status 0‐1, and central confirmation of HLA‐A2 positivity in total blood. Participants with baseline brain metastases were eligible if asymptomatic.
Ultimately, the study included 219 participants: 139 randomised to the vaccine and 80 to standard‐of‐care chemotherapy. There were 155 men and 64 women. The participants' mean age was 65 years. Two hundred and five participants had stage IV cancer, and 14 had stage III cancer. They were followed up for 24 months.
Primary outcomes
Overall survival
The study showed that the vaccine may result in little to no difference in overall survival compared with standard‐of‐care chemotherapy (HR 0.86, 95% CI 0.62 to 1.19; 1 study, 219 participants; Analysis 7.1). Median survival times in the vaccine and control groups were 8.8 versus 8.3 months, respectively.
7.1. Analysis.

Comparison 7: OSE2101 vaccine versus chemotherapy alone in HLA‐A2+ advanced NSCLC in second/third‐line treatment after failure with immune checkpoint inhibitors, Outcome 1: Overall survival
Progression‐free survival
Data on this outcome have not yet been published.
Serious treatment‐related adverse events (CTCAE grades 3 to 5)
Data showed that the vaccine may result in little difference in the proportion of people with at least one serious adverse event compared with chemotherapy (RR 0.95, 95% CI 0.91 to 0.99; 1 study, 219 participants; Analysis 7.2).
7.2. Analysis.

Comparison 7: OSE2101 vaccine versus chemotherapy alone in HLA‐A2+ advanced NSCLC in second/third‐line treatment after failure with immune checkpoint inhibitors, Outcome 2: Adverse events grades 3 to 5 (Participants with at least one serious adverse event)
Survival rates at three and five years
The study followed the participants for up to two years only.
Health‐related quality of life (HRQoL)
Data on this outcome have not yet been published.
Comparison 8. SRL172 (killed Mycobacterium vaccae) added to chemotherapy versus chemotherapy alone in first‐line treatment
One study conducted in centres in the UK, Austria, and Germany assessed this comparison (O'Brien 2004). It included 419 participants: 300 men and 119 women; 210 were randomised to vaccine plus chemotherapy and 209 to chemotherapy alone. Two hundred and fifty participants had stage IV cancer, 128 had stage IIIB, and 41 had stage IIIA. Fifty‐nine participants had previously been treated with surgery and 38 with radiotherapy. The participants' mean age was 61 years. They were followed up for 700 days (i.e. 23 months).
Overall survival
The study did not provide hazard ratios, but reported no difference between the treatment groups in overall survival, with a median survival time of 223 days in the chemotherapy plus SRL172 group, compared to 225 days in the chemotherapy‐alone group.
Progression‐free survival
The study did not evaluate this outcome.
Serious treatment‐related adverse events (CTCAE grades 3 to 5)
Data showed that adding the vaccine likely increases the proportion of participants having at least one serious adverse event compared with chemotherapy alone (RR 2.07, 95% CI 1.76 to 2.43; 1 study, 351 participants; Analysis 8.1).
8.1. Analysis.

Comparison 8: SRL172 (killed Mycobacterium vaccae) added to chemotherapy versus chemotherapy alone in first‐line or maintenance treatment, Outcome 1: Adverse events grade 3‐5 (Participants with at least one serious adverse event)
Secondary outcomes
Survival rates at three and five years
The study followed the participants for up to 23 months only.
Health‐related quality of life (HRQoL)
The study showed that adding the vaccine may result in a higher HRQoL for the participants, resulting in a smaller decrease in the "Global health status/QoL" score of the QLQ‐C30 (EORTC QLQ) questionnaire, compared with chemotherapy alone (MD 7.60, 95% CI 2.26 to 12.94; 1 study, 351 participants; Analysis 8.2), as measured at the end of the 15‐week treatment phase. The authors reported that by the end of the maintenance and survival phase, the differences observed at the end of the treatment phase in favour of the chemotherapy plus SRL172 group had diminished.
8.2. Analysis.

Comparison 8: SRL172 (killed Mycobacterium vaccae) added to chemotherapy versus chemotherapy alone in first‐line or maintenance treatment, Outcome 2: Quality of life: mean change from baseline in Global Health Status score
Discussion
In this review, we assessed the efficacy and safety of seven different types of vaccines in the treatment of people with advanced non‐small cell lung cancer (NSCLC). None of them resulted in large effects in the main outcomes of interest in this review: overall survival, progression‐free survival, serious treatment‐related adverse events, survival rates at three or five years, and participants' health‐related quality of life.
Summary of main results
We included a total of 10 studies in this review. We excluded studies on vaccines withdrawn by their manufacturers for being ineffective in treating advanced NSCLC, as these are unavailable for use in clinical practice: tecemotide, belagenpumatucel‐L, and MAGE A3 peptide vaccines.
TG4010, a vector‐based vaccine, added to chemotherapy as part of first‐line therapy, compared with chemotherapy alone, may increase slightly progression‐free survival. It may result in little to no difference in overall survival, the proportion of participants with at least one serious treatment‐related adverse event, and survival rates at three and five years.
Epidermal growth factor vaccine as switch maintenance treatment after first‐line chemotherapy, compared to best supportive care, may result in little to no difference in overall survival, in the proportion of participants who have at least one serious treatment‐related adverse event, and in the survival rate at three years. It may increase the survival rate slightly at five years.
The hTERT (vx‐001) vaccine compared to placebo as maintenance treatment after first‐line chemotherapy may result in little to no difference in overall survival and survival rates at five years.
Racotumomab as a switch maintenance treatment after first‐line chemotherapy, compared to placebo, may increase overall survival. It may make little to no difference in progression‐free survival, and in the proportion of participants with at least one serious treatment‐related adverse event. It may increase survival rates at three years, but not at five years.
Racotumomab as switch maintenance therapy post‐chemotherapy was compared to docetaxel, but researchers did not publish information on hazard rates for overall survival or progression‐free survival time. Anyway, differences in median survival times were very short, less than one month. Racotumomab may result in little to no difference in the proportion of people with at least one serious adverse event compared with docetaxel.
Personalised peptide vaccine plus docetaxel compared to docetaxel plus placebo after chemotherapy treatment may result in little to no difference in overall survival and progression‐free survival.
The OSE2101 vaccine compared with chemotherapy after chemotherapy or immunotherapy, in HLA‐A2+ advanced NSCLC in second/third‐line treatment after failure with immune checkpoint inhibitors, may result in little to no difference in overall survival. It may result in a slight decrease in the proportion of people having at least one serious treatment‐related adverse event.
The SRL172 vaccine of killed Mycobacterium vaccae, added to chemotherapy, compared to chemotherapy alone, may result in no difference in overall survival, and may increase the proportion of participants having at least one serious treatment‐related adverse event.
Overall completeness and applicability of evidence
Although we made every effort to conduct an exhaustive search for eligible studies, it is possible that we failed to identify relevant studies, particularly studies not registered in publicly accessible registers, or those published in journals not indexed in CENTRAL, MEDLINE, or Embase.
Of the 10 clinical trials included, only the most recent one – Besse 2023 – focused on what can be regarded as a modern clinical situation. It explored the efficacy and safety of the OSE2101 vaccine in people who had received one line of immune checkpoint blocker (ICB) therapy for locally advanced or metastatic epidermal growth factor receptor/anaplastic lymphoma kinase (EGFR/ALK)‐negative NSCLC, given sequentially (second‐line), or combined with platinum‐based chemotherapy (first‐line) for those with disease progression.
It should also be taken into account that the participants included in these clinical trials were selected because, for example, they had a better performance status than is typical for the population considered as a whole, and thus were probably not representative of all people with advanced NSCLC.
Few studies evaluated the impact of vaccines on the health‐related quality of life of their participants. This is a problem in itself and also a significant limitation, given that the effect of the assessed vaccines on prolonging the life of people with advanced NSCLC is, in general, small – in the best case, a few months. Therefore, it is essential to know whether an improvement in length of life is coupled with a better quality of life and acceptable side effects of the vaccines.
Today, none of these vaccines have been approved by authorities such as the American Food and Drug Administration or the European Medicines Agency. They are therefore not available on the market.
Certainty of the evidence
All the included studies were parallel‐group, randomised controlled trials.
We lacked information from some RCTs to properly assess some domains of risk of bias, even though we sent emails to authors requesting further information on their studies. We lacked detailed information on some studies' randomisation procedures or allocation concealment methods. Regarding blinding of participants, personnel, or evaluators, we believe that the lack of blinding of participants could be a source of performance bias and detection bias for subjective outcomes, such as progression‐free survival or quality of life. We did not find any cases of selective reporting of outcomes in the studies prospectively registered in clinical trials registers, or those for which the protocol of the study was available. However, most of the studies gave insufficient information about their statistical methods, which impeded our evaluation of selective reporting bias. As only one or two studies analysed each vaccine type, we could not investigate the risk of publication bias through funnel plot analysis.
For most comparisons, especially for those assessed by a single study, the total number of participants included was small and the analysis for some outcomes was based on a few events. In many cases, this situation resulted in wide confidence intervals of estimations of the efficacy and safety of the vaccines. We thus frequently downgraded the certainty of the evidence for imprecision, reducing the strength of the conclusions about the vaccines addressed in this review. This last problem is difficult to solve, given that the number of people with advanced NSCLC willing to participate in RCTs is limited.
Potential biases in the review process
Although we have done an extensive search of the literature, we cannot rule out the possibility that we have missed some trials, mainly because of the overlap between immunotherapy and vaccines, and the poor indexing of trials in this area. It is also possible that we did not identify trials that did not publish results, either because they did not manage to recruit the participants needed, or because they did not find favourable results for the vaccine they were evaluating.
In this review, we included 10 trials with a total of 2177 participants, which is insufficient to detect possible rare serious adverse effects of the vaccines evaluated.
There were also scant data on some vaccines' effects on participants' quality of life. And in some cases, where there were data, quality of life assessment was not blinded, because participants knew whether they had received the vaccine or not.
Agreements and disagreements with other studies or reviews
We have not found any other systematic review that specifically addressed the effects of vaccines in people with advanced NSCLC (that is, stages IIIB or higher) with separate analyses of the effects of each type of vaccine.
We found two reviews focused on people with advanced NSCLC (Wang 2015; Zhou 2016). Wang and colleagues pooled the results of 11 studies on different types of vaccines and found that vaccines improved overall survival, progression‐free survival, and resulted in fewer severe adverse effects in the vaccine groups, compared to the control groups (Wang 2015). Zhou and colleagues included studies on immunotherapies in general: studies on vaccines as well as studies on immune checkpoint inhibitors (Zhou 2016). They combined the results of the eight studies on vaccines in meta‐analysis, and found that therapeutic vaccines plus chemotherapy improved survival compared to chemotherapy plus placebo. They found no differences in the incidence of serious adverse events (≥ grade 3) between the two groups.
In addition, we found three reviews that included studies involving people with early‐stage NSCLC (Dammeijer 2016; Ding 2014; Zhu 2021), who have better prognoses than people with advanced NSCLC. Pooling the results of studies including participants with lower stages of cancer and a better prognosis would overestimate the real benefits of the vaccines in participants with advanced NSCLC.
All five reviews pooled in meta‐analyses the results of studies on different types of vaccines and with heterogeneous types of participants.
We decided against pooling the results of vaccines with very different mechanisms of action. We also decided against pooling results from studies with heterogeneous participant inclusion criteria. For example, some studies included only people receiving first‐line treatment, and others included participants in whom previous first‐line treatments had failed. For good reasons, patients and clinicians are more interested in the specific effect of a specific vaccine in a specific type of situation than in the average of the effects of different types of vaccines.
In our review, we decided not to include vaccines that had been withdrawn by their manufacturers for having poor clinical results, and are thus unavailable for medical use: tecemotide, belagenpumatucel‐L, and MAGE A3 peptide vaccines. Of the reviews mentioned above, four included studies on the effect of tecemotide in their meta‐analyses (Dammeijer 2016; Ding 2014; Wang 2015; Zhou 2016); two reviews included a study on belagenpumatucel‐L in their meta‐analyses (Dammeijer 2016; Zhou 2016); and one review also included a study on the MAGE‐A3 vaccine (Ding 2014).
None of the other reviews mentioned above included health‐related quality of life as a prespecified review outcome, as we did in this review.
Authors' conclusions
Implications for practice.
Therapeutic vaccines for advanced non‐small cell lung cancer may make little to no difference to survival, except for racotumomab. Racotumomab showed some improvement in survival time compared to placebo, but the difference in median survival was very short (1.4 months), and the study included only 176 participants.
Severe adverse events of vaccines were rare, but both the number of participants and the events in the studies were small.
Implications for research.
Future studies should implement blinded evaluation of the effect of the interventions on the health‐related quality of life of participants. They should also aim to include sufficient participants to reach enough statistical power for the outcomes that are most important to people with non‐small cell lung cancer.
History
Protocol first published: Issue 8, 2019
Acknowledgements
Thanks to Corynne Marchal, Managing Editor, Cochrane Lung Cancer Group, for providing administrative and logistical support for this review. We thank François Calais for helping us define the search strategies and conducting the searches for us. We thank Rolando Uranga, Ania Torres, Maria Del Carmen Arango, Iraida Caballero, Cecilia Pacheco, Rosa Maria Ortiz, Fernando Chuecas, and Pedro Mas Bermejo for their comments on the protocol. We thank Cochrane Iberoamérica, especially Marta Roque and Xavier Bonfill, for their support. We thank Javier Ballesteros for his support.
Thanks to Wai Tong Chien, YingYao Chen, and Yinghui Jin, from the Chinese Cochrane Center, for their contributions to the design of the literature searches in Chinese databases and the screening of the articles.
Thanks to Faith Armitage, Cochrane Central Production Service, for copy‐editing the review.
Thanks to Virginie Westeel, Cochrane Lung Cancer Group, who signed off on our review.
Appendices
Appendix 1. The eighth edition TNM stage classification for lung cancer
Definitions for TNM descriptors (Detterbeck 2018)
T (primary tumor)
T0 No primary tumor
Tis Carcinoma in situ (squamous or adenocarcinoma)
T1 Tumor ≤3 cm
T1mi Minimally invasive adenocarcinoma
T1a Superficial spreading tumor in central airways∗
T1a Tumor ≤1 cm
T1b Tumor >1 but ≤2 cm
T1c Tumor >2 but ≤3 cm
T2 Tumor >3 but ≤5 cm or tumor involving: visceral pleura, † main bronchus (not carina), atelectasis to hilum†
T2a Tumor >3 but ≤4 cm
T2b Tumor >4 but ≤5 cm
T3 Tumor >5 but ≤7 cm or invading chest wall, pericardium, phrenic nerve; or separate tumor nodule(s) in the same lobe
T4 Tumor >7 cm or tumor invading: mediastinum, diaphragm, heart, great vessels, recurrent laryngeal nerve, carina, trachea, esophagus, spine; or tumor nodule(s) in a different ipsilateral lobe
N (regional lymph nodes)
N0 No regional node metastasis
N1 Metastasis in ipsilateral pulmonary or hilar nodes
N2 Metastasis in ipsilateral mediastinal or subcarinal nodes
N3 Metastasis in contralateral mediastinal, hilar, or supraclavicular nodes
M (distant metastasis)
M0 No distant metastasis
M1a Malignant pleural or pericardial effusion‡ or pleural or pericardial nodules or separate tumor nodule(s) in a contralateral lobe
M1b Single extrathoracic metastasis
M1c Multiple extrathoracic metastases (1 or >1 organ)
∗ Superficial spreading tumor of any size but confined to the tracheal or bronchial wall.
† Atelectasis or obstructive pneumonitis extending to hilum; such tumors are classified as T2a if >3 and ≤4 cm, T2b if >4 and ≤5 cm.
‡ Pleural effusions are excluded that are cytologically negative, nonbloody, transudative, and clinically judged not to be due to cancer.
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Carcinoma, Non‐Small‐Cell Lung] explode all trees #2 nsclc #3 "lung cancer*" #4 "lung carcinom*" #5 "lung neoplasm*" #6 "lung tum*" #7 "non small cell*" #8 "nonsmall cell*" #9 (#3 or #4 or #5 or #6) and (#7 or #8) #10 #1 or #2 or #9 #11 MeSH descriptor: [Cancer Vaccines] explode all trees #12 vaccin* #13 MeSH descriptor: [Cell‐ and Tissue‐Based Therapy] explode all trees #14 "cell therap*" #15 "cellular therap*" 1 #16 MeSH descriptor: [Immunotherapy, Adoptive] explode all trees #17 "cellular immunotherap*" #18 "cell immunotherap*" #19 "adoptive immunotherap*" #20 MeSH descriptor: [Cytokine‐Induced Killer Cells] explode all trees #21 cytokine induced killer cell* #22 CIK cell* #23 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 #24 #10 AND #23
Appendix 3. MEDLINE search strategy
1 Carcinoma, Non‐Small‐Cell Lung[MeSH Terms] 2 nsclc 3 lung cancer* 4 lung carcinom* 5 lung neoplasm* 6 lung tumor* 7 lung tumour* 8 non small cell* 9 nonsmall cell* 10 (#3 OR #4 OR #5 OR #6 OR #7) AND (#8 OR #9) 11 #1 OR #2 OR #10 12 cancer vaccines[MeSH Terms] 13 vaccin* 14 Cell‐ and Tissue‐Based Therapy[MeSH Terms] 15 "cell therap*" 16 "cellular therap*" 17 cellular immunotherapy, adoptive[MeSH Terms] 18 "cellular immunotherap*" 19 "cell immunotherap*" 20 "adoptive immunotherap*" 21 Cytokine‐Induced Killer Cells[MeSH Terms] 22 "Cytokine‐Induced Killer Cell*" 23 CIK 24 "Lymphocyte Activated Killer Cell*" 25 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 26 #11 AND #25 27 ((((((randomized controlled trial[Publication Type]) OR randomized[Title/Abstract]) OR placebo[Title/Abstract]) OR drug therapy[MeSH Subheading]) OR randomly[Title/Abstract]) OR trial[Title/Abstract]) OR groups[Title/Abstract] 28 (animals[MeSH Terms]) NOT humans[MeSH Terms] 29 #27 NOT #28 30 #26 AND #29
Appendix 4. Embase search strategy
#1 'non small cell lung cancer'/exp #2 'nsclc':ti,ab #3 'lung cancer*':ti,ab #4 'lung carcinom*':ti,ab #5 'lung neoplasm*':ti,ab #6 'lung tumor*':ti,ab #7 'lung tumour*':ti,ab #8 'non small cell*':ti,ab #9 'nonsmall cell*':ti,ab #10 (#3 OR #4 OR #5 OR #6 OR #7) AND (#8 OR #9) #11 #1 OR #2 OR #10 #12 'cancer vaccine'/exp #13 'vaccine*':ti,ab #14 'vaccina*':ti,ab #15 'biological therapy'/exp #16 'cell therap*':ti,ab #17 'cellular therap*':ti,ab #18 'adoptive immunotherapy'/exp #19 'cellular immunotherap*':ti,ab #20 'cell immunotherap*':ti,ab #21 'adoptive immunotherap*':ti,ab #22 'cytokine induced killer cell'/exp #23 'cytokine induced killer cell*':ti,ab #24 'cik cell*':ti,ab #25 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 #26 #11 AND #25 #27 'crossover procedure'/exp OR 'double‐blind procedure'/exp OR 'randomized controlled trial'/exp OR 'single‐blind procedure'/exp OR random* OR factorial* OR crossover* OR (cross NEXT/1 over*) OR placebo* OR (doubl* NEAR/1 blind*) OR (singl* NEAR/1 blind*) OR assign* OR allocat* OR volunteer* #28 #26 AND #27
Appendix 5. Wanfang Data search strategy
#1 全部=细胞治疗 #2 全部=免疫治疗 #3 全部=疫苗 #4 #1 OR #2 OR #3 #5 全部=肺癌 #6 全部=肺*恶性肿瘤 #7 #5 OR #6 #8 全部=非小细胞 #9 全部=树*细胞 #10 全部=CIK #11 全部=killer cell #12 全部=DC #13 #8 OR #9 OR #10 OR #11 OR #12 #14 #4 AND #7 AND #13 #15 期刊论文 OR 学位论文 OR 会议论文 #16 #14 AND #15 #17 #16 AND Date 2017‐2022
Appendix 6. China Journal Net (often referred as CNKI) search strategy
#1 AB=细胞治疗 OR AB=免疫治疗 OR AB=疫苗
#2 AB=肺癌 OR AB=肺*肿瘤 OR AB=非小细胞
#3 AB=树*细胞 OR AB=CIK OR AB=杀伤细胞 OR AB=DC*细胞
#4 #1 AND #2 AND #3
#5 #4 AND 中英文拓展
#6 #5 in 期刊论文
#7 #5 in 学位论文
#8 #5 in 会议
#10 #6 OR #7 OR #8
#11 #10 in 中文
#12 #11 AND Date 2017‐2022
Data and analyses
Comparison 1. TG4010 added to chemotherapy versus chemotherapy alone in first line treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Overall survival | 2 | 370 | Hazard Ratio (IV, Random, 95% CI) | 0.83 [0.65, 1.05] |
| 1.2 Progression‐free survival | 1 | 222 | Hazard Ratio (IV, Random, 95% CI) | 0.74 [0.55, 0.99] |
| 1.3 Adverse events grades 3 to 5 (Participants with at least one serious adverse event) | 2 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.23, 2.19] |
| 1.4 Survival rates at 3 years | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.30, 3.31] |
Comparison 2. Epidermal growth factor vaccines versus best supportive care for switch maintenance after first‐line treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Overall survival | 1 | 378 | Hazard Ratio (IV, Random, 95% CI) | 0.82 [0.66, 1.02] |
| 2.2 Adverse events grades 3 to 5 (Participants with at least one serious adverse event) | 2 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.88, 1.98] |
| 2.3 Survival rates at 3 years | 2 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.82, 2.54] |
| 2.3.1 Epidermal growth factor | 2 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.82, 2.54] |
| 2.4 Survival rates at 5 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 Epidermal growth factor | 1 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 3.40 [1.02, 11.27] |
| 2.5 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 Global health status. EORTC QLQ‐C30. Score at six months. | 1 | 86 | Mean Difference (IV, Random, 95% CI) | 7.90 [‐0.49, 16.29] |
Comparison 3. hTERT (vx‐001) vaccine versus placebo as post‐chemotherapy maintenance treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Overall survival | 1 | 190 | Hazard Ratio (IV, Random, 95% CI) | 0.97 [0.70, 1.34] |
| 3.2 Survival rates at 40 months | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.04, 3.57] |
| 3.3 Survival rates at 5 years | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
3.3. Analysis.

Comparison 3: hTERT (vx‐001) vaccine versus placebo as post‐chemotherapy maintenance treatment, Outcome 3: Survival rates at 5 years
Comparison 4. Racotumomab‐alum vaccine versus placebo for switch maintenance after first‐line treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Overall survival | 1 | 176 | Hazard Ratio (IV, Random, 95% CI) | 0.63 [0.46, 0.87] |
| 4.2 Progression‐free survival | 1 | 176 | Hazard Ratio (IV, Random, 95% CI) | 0.73 [0.53, 1.00] |
| 4.3 Adverse events grade 3‐5 (Participants with at least one serious adverse event) | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.15, 7.18] |
| 4.4 Survival rates at 3 years | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 4.09 [1.20, 14.00] |
| 4.5 Survival rates at 5 years | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [0.38, 10.88] |
4.4. Analysis.

Comparison 4: Racotumomab‐alum vaccine versus placebo for switch maintenance after first‐line treatment, Outcome 4: Survival rates at 3 years
Comparison 5. Racotumomab‐alum vaccine versus docetaxel for switch maintenance treatment after first‐line treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Adverse events grades 3 to 5 (Participants with at least one serious adverse event) | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.44, 1.83] |
Comparison 6. Personalised peptide vaccine versus docetaxel plus placebo after first‐line treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Overall survival | 1 | 50 | Hazard Ratio (IV, Random, 95% CI) | 0.80 [0.42, 1.52] |
| 6.2 Progression‐free survival | 1 | 50 | Hazard Ratio (IV, Random, 95% CI) | 0.78 [0.43, 1.42] |
Comparison 7. OSE2101 vaccine versus chemotherapy alone in HLA‐A2+ advanced NSCLC in second/third‐line treatment after failure with immune checkpoint inhibitors.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Overall survival | 1 | 219 | Hazard Ratio (IV, Random, 95% CI) | 0.86 [0.62, 1.19] |
| 7.2 Adverse events grades 3 to 5 (Participants with at least one serious adverse event) | 1 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.91, 0.99] |
Comparison 8. SRL172 (killed Mycobacterium vaccae) added to chemotherapy versus chemotherapy alone in first‐line or maintenance treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Adverse events grade 3‐5 (Participants with at least one serious adverse event) | 1 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [1.76, 2.43] |
| 8.2 Quality of life: mean change from baseline in Global Health Status score | 1 | 351 | Mean Difference (IV, Random, 95% CI) | 7.60 [2.26, 12.94] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alfonso 2014.
| Study characteristics | ||
| Methods |
Study design: parallel, RCT, double‐blind (participants and professionals), placebo‐controlled phase II/III clinical trial. Recruitment period: from September 2006 to June 2010. Duration of study: from 2006 to 2013. Objective: to assess the efficacy and tolerability of switch maintenance treatment with racotumomab‐alum in patients with advanced NSCLC. |
|
| Participants |
Country: Cuba. Setting: hospital. Inclusion criteria: advanced NSCLC with favourable response [complete response (CR), partial response (PR), or stable disease (SD)] after the standard first‐line therapy. All participants had measurable disease, an Eastern Cooperative Oncology Group performance status (ECOG PS) 2, and adequate renal, hepatic, and haematologic functions. Exclusion criteria: participants who had received immunotherapy or other investigational drugs; patients with known hypersensitivity to any component of the formulation; participants who were pregnant or lactating; participants with uncontrolled chronic diseases, history of severe allergic reactions; participants with brain metastases or other primary neoplastic lesions; patients with active infections, symptomatic congestive heart failure, unstable angina, cardiac arrhythmia or psychiatric disorders; and participants receiving systemic corticosteroids at the time of inclusion and participants with positive serology for hepatitis B and C or HIV. Age: 18 years or older: 97 participants 60 years or older, 79 participants under 60. Sex: 118 male and 58 female. Disease stages: 99 stage IIIB and 77 stage IV. N randomised: 176 participants: 87 vaccine group; 86 placebo. N analysed: 176. |
|
| Interventions |
Intervention group: racotumomab‐alum. Comparison: placebo. Participants were injected intradermally and received 15 doses of 1 mg of racotumomab‐alum or placebo. The induction phase consisted of 5 doses administered every 2 weeks. After induction, participants were vaccinated every 4 weeks, for 1 year (10 doses). After treatment was completed (15 doses of vaccine or placebo), the blinding was opened, and only participants in the racotumomab arm continued vaccination every 4 weeks, even beyond progression. Criteria for stopping vaccination included voluntary withdrawal, unacceptable toxicity, or severe worsening of the participants. Participants who discontinued study treatment were followed until death or study termination. Concurrent antitumour therapy was not permitted. |
|
| Outcomes | 1. Overall survival. 2. Progression‐free survival. 3. Adverse events. 4. Tumour response. 5. Antibody response, and antibody‐binding assay by flow cytometry. 6. Induction of cell death, determined by flow cytometry. |
|
| Notes |
Funding for trial: financial support for the study was supplied by the Center of Molecular Immunology and Recom‐Bio S.L. Conflicts of interest: no potential conflicts of interest were disclosed. Registered: RPCEC00000009, in the Cuban Registry of Clinical Trials. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “An independent CRO (CENCEC) was responsible for conducting the study. Treatment allocation was done using the automatic MINIM system." Quote: “Randomization was not stratified but it was balanced according to gender, clinical stage, performance status, race, and response to first‐line treatment. The investigator provided this information before randomization." |
| Allocation concealment (selection bias) | Low risk | Randomisation was done centrally. Quote: “Active drug was composed of the anti‐idiotype antibody racotumomab, alum hydroxyl gel, mono‐ and dibasic sodium phosphatase salt, sodium chloride, and water for injection. The placebo had the same composition excluding racotumomab. The drug and placebo had the same organoleptic characteristics and were presented in the same primary and secondary containers.” |
| Blinding of participants and personnel (performance bias) Survival; Serious adverse effects | Low risk | Double‐blinded. Quote: “A sealed letter enclosing the information of the treatment group was sent together with the investigational product. Information on treatment was disclosed only after completing 15 doses or in case of severe or serious adverse events." |
| Blinding of participants and personnel (performance bias) Progression‐free survival; Health‐related quality of life | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) Survival; Serious adverse effects | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) Progression‐free survival; Health‐related quality of life | Low risk | Double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Causes for withdrawal from the study are well reported. Quote: "One patient with histologically unconfirmed NSCLC randomized to racotumomab‐alum group did not receive treatment and was excluded from the safety analysis." Quote: “Ten patients from the racotumomab arm and 2 patients from the placebo cohort were alive at the moment of the Efficacy analysis and were consequently censored." |
| Selective reporting (reporting bias) | Low risk | Authors present results on all outcome measures that were pre‐specified as relevant. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Besse 2023.
| Study characteristics | ||
| Methods |
Study design: parallel, unblinded, phase 3 RCT study. Recruitment period: from February 2016 to 2021. Duration of study: from February 2016 to January 2021. Objective: to compare the efficacy of the OSE2101 vaccine with standard treatment in participants "HLA‐A2+ NSCLC" with resistance to immunotherapy, in 2nd or 3rd line treatment. |
|
| Participants |
Countries: USA, Spain, France, Italy, Poland, Germany and Israel. Setting: hospital. Inclusion criteria: participants had received one line of immune checkpoint blockers (ICB) treatment for locally advanced or metastatic "EGFR/ALK‐negative NSCLC", given sequentially (second‐line) or combined with platinum‐based chemotherapy (first‐line) with disease progression (measurable and non‐measurable disease), Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0‐1 and central confirmation of "HLA‐A2 positivity" in total blood. Participants with baseline brain metastases were eligible if asymptomatic. Exclusion criteria: small‐cell lung cancer or mixed NSCLC histology, spinal cord compression, leptomeningeal carcinomatosis, interstitial lung disease, autoimmune disease, immunodeficiency, previous cancer within 5 years, severe acute or chronic medical or psychiatric conditions or active type B or C hepatitis. Participants were not eligible if immune checkpoint blockers (ICB) treatment was stopped due to toxicity. Age: 18 years and older. Mean age: 65 years. Sex: 155 men and 64 women. Disease stages: 14 stage IIIB; 205 stage IV. N randomised: 219: 139 vaccine group; 80 standard of care. N analysed: 197. |
|
| Interventions |
Intervention group: OSE2101. OSE2101 was administered at 5 mg, 1 mL subcutaneously on day 1 every 3 weeks for six cycles, then every 8 weeks until 1 year of treatment and thereafter every 12 weeks. Comparison: investigator’s choice of standard treatment chemotherapy with pemetrexed or docetaxel. ‐ pemetrexed, was administered at 500 mg/m2 intravenously over 10 minutes. ‐ docetaxel at 75 mg/m2 intravenously over 1 hour, both every 3 weeks with premedication, according to international guidelines. |
|
| Outcomes | 1. Overall survival. 2. Progression‐free survival. 3. Adverse events. 4. Post‐progression survival. 5. Quality of life, QLQ‐C30 global health status. |
|
| Notes |
Funding for trial: supported by OSE Immunotherapeutics (no grant number). Conflicts of interest: potential conflicts of interest of several authors were disclosed in the publication. Registered: NCT02654587ClinicalTrials.gov. EudraCT: 2015‐003183‐36. At the time of the planned interim analysis (cut‐off of February 2020) when the first 103 participants reached 12 months of follow‐up, the sponsor prematurely stopped the accrual due to the coronavirus disease 2019 (COVID‐19) pandemic which was rapidly expanding with a strong concern about its impact on patient safety and data integrity. Data on progression‐free survival, the mean number of adverse events, and health‐related quality of life in the ITT population not presented in the publication, were requested to the authors, who sent us the information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation by an IWRS (Interactive Web Response System). Randomised block design of size 6 (4 A + 2 B). |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation. |
| Blinding of participants and personnel (performance bias) Survival; Serious adverse effects | Low risk | Unblinded study, but unlikely to have biased the analysis of the impact of treatments on outcomes such as mortality or severe adverse events. |
| Blinding of participants and personnel (performance bias) Progression‐free survival; Health‐related quality of life | High risk | Open‐label, unblinded study. |
| Blinding of outcome assessment (detection bias) Survival; Serious adverse effects | Low risk | Open‐label, unblinded study, but important outcomes for this review are objective outcomes: overall survival, death rates at 3 or 5 years, and serious adverse events. |
| Blinding of outcome assessment (detection bias) Progression‐free survival; Health‐related quality of life | High risk | Open‐label, unblinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses in follow‐up and causes reported. No imbalances among compared treatments. |
| Selective reporting (reporting bias) | Low risk | Authors present results on all outcome measures that were pre‐specified as relevant in www.ClinicalTrials.gov registry. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Gridelli 2020.
| Study characteristics | ||
| Methods |
Study design: parallel, double‐blind (participants and professionals) phase 2b RCT. Recruitment period: from August 2012 to March 2016. Duration of study: from August 2012 to January 2017. Objective: to examine the role of the Vx‐001 vaccine as maintenance immunotherapy in NSCLC participants who experienced disease control after first‐line chemotherapy. |
|
| Participants |
Country: 70 sites in Europe. Setting: hospital. Inclusion criteria: histologically confirmed, metastatic NSCLC, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1 who experienced disease control [complete response (CR), partial response (PR), or stable disease (SD)] after four cycles of platinum‐based front‐line chemotherapy were eligible for this study. Additional key eligibility criteria included an "HLA‐A*0201 haplotype and tumoural expression of TERT [telomerase reverse transcriptase]" as assessed by in situ hybridisation in a central laboratory (Vaxon Biotech, Genopole, Evry, France), adequate marrow, renal and liver function tests. Exclusion criteria: participantswere excluded if they had brain metastases, autoimmune or immunodeficiency diseases. Age: > 18 years old. 99 older than 65 years and 91 younger. Sex: 132 men, 58 women. N randomised: 221 participants: 109 vaccine and 112 placebo. N analysed: 190, 89 vaccine and 101 placebo. |
|
| Interventions |
Intervention group: Vx‐001. Comparison: placebo. |
|
| Outcomes | 1. Overall survival. 2. Survival rate at 12 months. 3. Adverse events. 4. Time to treatment failure. |
|
| Notes |
Funding for trial: was sponsored and the investigational drug was provided free of charge by Vaxon‐Biotech. Conflicts of interest: potential conflicts of interest of some authors were disclosed in the publication. Registered: NCT01935154, in ClinicalTrials.gov. All data are available via the corresponding author and the NCT trial centre. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It was performed via an Interactive Web Response System (IWRS). |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed via an Interactive Web Response System (IWRS). |
| Blinding of participants and personnel (performance bias) Survival; Serious adverse effects | Low risk | Blinded study: participant, care provider, investigator, outcomes assessor. |
| Blinding of participants and personnel (performance bias) Progression‐free survival; Health‐related quality of life | Low risk | Blinded study: participant, care provider, investigator, outcomes assessor. |
| Blinding of outcome assessment (detection bias) Survival; Serious adverse effects | Low risk | Blinded study: participant, care provider, investigator, outcomes assessor. |
| Blinding of outcome assessment (detection bias) Progression‐free survival; Health‐related quality of life | Low risk | Blinded study: participant, care provider, investigator, outcomes assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In Supplement Figure 1, only two losses to follow‐up appear. Eleven patients in the placebo group and 20 in the vaccine group were excluded from the analysis due to violation of the main inclusion criteria (metastatic NSCLC, disease control before randomisation). |
| Selective reporting (reporting bias) | Low risk | Authors present results on all outcome measures that were pre‐specified in the ClinicalTrials.gov registry. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Hernandez 2021.
| Study characteristics | ||
| Methods |
Study design: parallel, open‐label, phase III RCT. Recruitment period: from February 2013 toJanuary 2016. Duration of study: from February 2013 to December 2016. Objective: to evaluate safety and efficacy of racotumomab‐alum or nimotuzumab versus docetaxel as switch maintenance therapy for advanced NSCLC. |
|
| Participants |
Country: Cuba. Setting: hospitals and primary care. Quote: "Both experimental treatments were safely administered at primary level of health assistance." Inclusion criteria: participants with cytological or histological confirmation of stage IIIB, IV NSCLC, with objective response or stable disease after first‐line chemotherapy. Eastern Cooperative Oncology Group performance status (ECOG PS) 0 to 2, age over 18 years old, written informed consent, life expectancy of 6 months or more, adequate bone marrow, renal, and hepatic function, a time from end of first‐line treatment to randomisation up to 2 months. Exclusion criteria: brain metastasis, acute infectious diseases, chronic or inflammatory uncontrolled diseases, severe allergic reaction history, pregnant or breastfeeding women; unfit participants for chemotherapy or previously treated with investigational drugs. Age: over 18 years old. 85 younger than 60 years and 153 older. Sex: 92 male and 53 female. N randomised: 238 participants: 93 vaccine group; 145 control groups. N analysed: 238. |
|
| Interventions |
Intervention group: racotumomab‐alum (n = 93). Comparisons: ‐ docetaxel (n = 52). ‐ nimotuzumab (n = 93). Participants allocated to racotumomab‐alum group received five bi‐weekly intradermal immunisations (1 mg) and re‐immunisations every 4 weeks. Docetaxel was used at 75 mg/m2 of body surface for six cycles if no progressive disease was documented after 3rd cycle. Both drugs were administered until severe worsening of performance status, unacceptable toxicity, or participants requested discontinuation. Treatment was not discontinued at disease progression, even when other treatment line was administered concomitantly. Nimotuzumab arm received 200 mg as a 1‐hour intravenous infusion weekly for 6 weeks, followed by bi‐weekly maintenance doses. The comparison between vaccine and nimotuzumab has not been assessed for this review. |
|
| Outcomes | 1. Overall survival. 2. Progression‐free survival. 3. Adverse events. |
|
| Notes |
Funding for trial: not specified. Conflicts of interest: nothing was reported in the publication. Registered: RPCEC00000179, in the Cuban Registry of Clinical Trials. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomization was performed using dynamic allocation of minimization system and balanced according to age, gender, ECOG performance status, response to prior therapy, disease stage, histology, and previous treatment." |
| Allocation concealment (selection bias) | Low risk | Central randomisation. |
| Blinding of participants and personnel (performance bias) Survival; Serious adverse effects | Low risk | Open‐label study. But it is unlikely that it would result in performance bias risk affecting the assessment of effects for objective outcomes such as survival and serious adverse effects. |
| Blinding of participants and personnel (performance bias) Progression‐free survival; Health‐related quality of life | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) Survival; Serious adverse effects | Low risk | Open‐label study. But it is unlikely that lack of blinding could bias the evaluation of the impact of treatments on outcomes such as mortality or severe adverse events. |
| Blinding of outcome assessment (detection bias) Progression‐free survival; Health‐related quality of life | High risk | No information is available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in intention‐to‐treat analysis. Five were lost to follow‐up: three in one group and two in the other group. |
| Selective reporting (reporting bias) | Low risk | The authors present results on all outcome measures that were pre‐specified as relevant in the Cuban registry of clinical trials. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Neninger 2008.
| Study characteristics | ||
| Methods |
Study design: parallel, phase II RCT. Recruitment period: from April 2002 to June 2004. Duration of study: from April 2002 to June 2006. Objective: to evaluate the effect of EGF vaccination on immunogenicity, safety, and effect on survival in advanced non‐small cell lung cancer (NSCLC) participants. |
|
| Participants |
Country: Cuba. Setting: hospital. Inclusion criteria: stage IIIB/IV NSCLC after finishing first‐line chemotherapy, required to have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of at least 2, adequate bone marrow reserve, white blood cellcount of at least 3000/L, platelet count of at least 100,000/L, haemoglobin of at least 10 g/dL, life expectancy longer than 3 months, and normal creatinine, bilirubin, and transaminase values according to each institutional standards. Exclusion criteria: pregnancy or lactation, secondary malignancies, or history of hypersensitivity to foreign proteins rendered participants ineligible. Disease progression to first‐line chemotherapy was not an exclusion criterion. Age: > 18 years of age. Mean age: 56 years. 53 were 60 years or less, 27 older than 60. Sex: 59 men and 21 women. N randomised: 80 participants: 40 vaccine; 40 control group. N analysed: 80. |
|
| Interventions |
Intervention group: epidermal growth factor (EGF) vaccine and best supportive care if required. Comparison: best supportive care. Participants in the vaccine group received a low‐dose of cyclophosphamide (200 mg/m2) 3 days before the first vaccine dose. The EGF vaccine was administered on days 1, 7, 14, and 28, and monthly afterwards. Participants in the control group received supportive care alone. |
|
| Outcomes | 1. Overall survival. 2. Safety. 3. Immunogenicity: anti‐EGF antibody. |
|
| Notes |
Funding for trial: not specified. Conflicts of interest: the authors indicated no potential conflicts of interest. Registered: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to enrollment in the vaccine or in the control group. Random assignment was performed centrally through a validated simple randomization system (ASAL) version 1.2." |
| Allocation concealment (selection bias) | Low risk | Random assignment was performed centrally. |
| Blinding of participants and personnel (performance bias) Survival; Serious adverse effects | Low risk | No mention of blinding. But it is unlikely that it could bias the analysis of the impact of treatments on outcomes such as mortality or serious adverse events. |
| Blinding of participants and personnel (performance bias) Progression‐free survival; Health‐related quality of life | Unclear risk | No mention of blinding. |
| Blinding of outcome assessment (detection bias) Survival; Serious adverse effects | Low risk | No mention of blinding. But important outcomes for this review are objective outcomes: overall survival, death rates at 3 or 5 years, and serious adverse events. |
| Blinding of outcome assessment (detection bias) Progression‐free survival; Health‐related quality of life | Unclear risk | No mention of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses in follow‐up were reported. Three participants in each group were not included in the overall survival analysis. |
| Selective reporting (reporting bias) | Low risk | Study protocol or registry information was not provided, but the authors present results on all outcome measures that were pre‐specified as relevant. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
O'Brien 2004.
| Study characteristics | ||
| Methods |
Study design: parallel, open‐label, phase III RCT. Recruitment period: not detailed. Duration of study: not detailed. Objective: to investigate the clinical activity and safety of SRL172 (killed Mycobacterium vaccae suspension) with chemotherapy in the treatment of non‐small‐cell lung cancer (NSCLC). |
|
| Participants |
Countries: United Kingdom, Germany and Poland. Setting: hospital. Age: median 61 years. Sex: 300 men and 119 women. N randomised: 419 participants: 210 in the vaccine plus chemotherapy group and 209 in the placebo group. N analysed: 416, 218 in the vaccine plus chemotherapy group and 208 in the placebo group. Inclusion criteria: at least 18 years, with histologically and/or cytologically confirmed unresectable NSCLC (stages IIIA, IIIB, and IV) were eligible for the study. Participants were to have measurable lesions in at least one site, which had not been previously irradiated, a European Organisation for Research and Treatment of Cancer–World Health Organisation (EORTC–WHO) performance status of < 2 and life expectancy of > 3 months in the opinion of the investigator. Exclusion criteria: if participants had severe, active, uncontrolled infection requiring systemic antibiotics, antivirals or antifungals; if they had received previous chemotherapy for NSCLC; if they had evidence of central nervous system (CNS) metastases or if they had any previous or concurrent malignancy (except adequately treated carcinoma in situ of the cervix, basal cell carcinoma of the skin and/or non‐melanoma skin cancer or if previous malignancy was more than 5 years prior and there were no signs of recurrence). They must not have received depot corticosteroids in the 6 weeks prior to day 0 or chronic systemic corticosteroids in the 2 weeks prior to administration of the study drug. Patients who had previously received SRL172 were also excluded. |
|
| Interventions |
Intervention group: SRL172 vaccine added to chemotherapy. SRL17 (a suspension of heat‐killed "M. vaccae NCTC 11659") was supplied in borate‐buffered saline at pH 8, 0.1 mL of which contained 1 mg wet weight corresponding to 109 bacilli. A dose of 0.1 mL was given intradermally over the deltoid muscle on day 0 and weeks 4, 8, 12 and 16 during the treatment phase. The vials of SRL172 were supplied and kept in refrigerators at 4°C. If there was no disease progression after the initial treatment phase, participants randomised to receive SRL172 entered a maintenance and follow‐up phase, during which they could continue to receive SRL172 and were clinically assessed every 8 weeks, until there was clear evidence of disease progression. Comparison: chemotherapy alone. |
|
| Outcomes | 1. Overall survival. 2. Progression‐free survival. 3. Survival rate at one year. 4. Safety: adverse events. 5. Health‐related quality of life. 6. Tumour response. |
|
| Notes |
Funding for trial: not detailed. In Acknowledgements: "We are grateful to SR Pharma plc for their support." Conflicts of interest: nothing was reported in the publication. Registered: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedures not detailed. |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was blinded, thereby avoiding bias in the allocation of individual patients to treatment." |
| Blinding of participants and personnel (performance bias) Survival; Serious adverse effects | Low risk | Objective outcomes. |
| Blinding of participants and personnel (performance bias) Progression‐free survival; Health‐related quality of life | High risk | Unblinded study. |
| Blinding of outcome assessment (detection bias) Survival; Serious adverse effects | Low risk | Objective outcomes. |
| Blinding of outcome assessment (detection bias) Progression‐free survival; Health‐related quality of life | High risk | Unblinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | Authors did not mention whether the study was recorded in a clinical trials registry. In the published article, they mentioned that progression‐free survival was a secondary outcome to be assessed in the study, but they did not report on it. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Quoix 2011.
| Study characteristics | ||
| Methods |
Study design: parallel, open‐label, phase IIB RCT. Recruitment period: from December 2005 to July 2007. Duration of study: from December 2005 to March 2010. Objective: to assess whether TG4010 improves the outcome of NSCLC patients receiving first‐line chemotherapy. |
|
| Participants |
Countries: France, Poland, Germany and Hungary. Setting: hospital. Inclusion criteria: male or female participants aged 18 years or older with histologically confirmed stage IIIB wet (pleural or pericardic effusion) or stage IV NSCLC, chemotherapy‐naive for this stage of the disease, at least one lesion measurable by CT according to WHO criteria, Eastern Collaborative Oncology Group (ECOG) performance status 0 or 1, life expectancy of at least 4 months, and tumour positive for "MUC1" [mucin‐1 antigen] as defined by expression in at least 25% of tumour cells analysed by immunohistochemistry. Exclusion criteria: participants were excluded if they had received prior systemic therapy for advanced stage NSCLC, or if they had concomitant brain metastases (unless successfully treated) or history of another malignancy within the past 5 years, apart from basal cell carcinoma of the skin or intraepithelial carcinoma of the cervix. Participants with performance status 2 were excluded because this stage of disease is usually treated with single‐drug chemotherapy regimens. Age: median 58 years (interquartile range from 35 to 79). Sex: 107 men and 41 women. N randomised: 148; 74 to TG4010 and 74 to placebo. N analysed: 148. |
|
| Interventions |
Intervention group: TG4010 plus chemotherapy. TG4010 as a subcutaneous injection at a dose of 10⁸ plaque forming units once a week for 6 weeks, then once every 3 weeks, first in combination with chemotherapy and then as monotherapy until documentation of progression. Comparison: chemotherapy alone. Chemotherapy: cisplatin (75 mg/m² on day 1) and gemcitabine (1250 mg/m² on days 1 and 8) were initiated in all participants and administered every 3 weeks for up to six cycles or until progressive disease. |
|
| Outcomes | 1. Overall survival. 2. Progression‐free survival. 3. Safety. 4. Quality of life. 5. Response rate. 6. Time to progression. |
|
| Notes |
Funding for trial: Transgene SA, Advanced Diagnostics for New Therapeutic Approaches (ADNA)/OSEO. Conflicts of interest: BA, BB, NB, J‐YB, GL, J‐ML, and AT are Transgene employees and own stock in the company. MB is chairman at IDDI consultants and owns stock in the company. EQ has an advisory role for Transgene, Eli Lilly & Co, and Roche, received honoraria from AstraZeneca, Roche, Eli Lilly & Co, and Transgene, received research funding from Eli Lilly & Co, and received other remuneration from AstraZeneca, Roche, and Eli Lilly & Co. M‐PC has an advisory role for Roche and received honoraria from Transgene. VW received honoraria from Roche and Eli Lilly & Co, research funding from Roche, and other remuneration from AstraZeneca, Roche, and Eli Lilly & Co. All other authors declared no conflicts of interest. Registered: NCT00415818, in ClinicalTrials.gov. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated one‐to‐one through a web‐based system to one of the two treatment groups, using a dynamic minimisation procedure. |
| Allocation concealment (selection bias) | Low risk | Participants allocated one‐to‐one through a web‐based system. |
| Blinding of participants and personnel (performance bias) Survival; Serious adverse effects | Low risk | Unblinded study, but it is unlikely that lack of blinding could bias the analysis of the impact of treatments on outcomes such as mortality or severe adverse events. |
| Blinding of participants and personnel (performance bias) Progression‐free survival; Health‐related quality of life | High risk | Unblinded study. |
| Blinding of outcome assessment (detection bias) Survival; Serious adverse effects | Low risk | Open‐label, unblinded study, but important outcomes for this review are objective outcomes: overall survival, death rates at 3 or 5 years, and serious adverse events. |
| Blinding of outcome assessment (detection bias) Progression‐free survival; Health‐related quality of life | High risk | Unblinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one participant lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Authors present results on all outcome measures that were pre‐specified as relevant in ClinicalTrials.gov registry. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Quoix 2016.
| Study characteristics | ||
| Methods |
Study design: parallel, phase IIb, blinded RCT (patients, site staff, monitors, the study funder, data managers, and the statistician). Recruitment period: from April 2012 to September 2014. Duration of study: from April 2012 to April 2016. Objective: to evaluate the effects of TG4010 in combination with first‐line chemotherapy and the clinical usefulness of the "TrPAL biomarker", in previously untreated participants with advanced stage non‐small‐cell lung cancer. |
|
| Participants |
Countries: France, Belgium, the UK, Italy, Spain, Hungary, Poland, Israel, and the USA. Setting: hospital. Inclusion criteria: histologically confirmed, stage IV non‐small‐cell lung cancer without a known "activating EGFR mutation. MUC1 [mucin‐1] expression", analysed by immunohistochemistry of a tumour specimen, had to be present in at least 50% of the tumoural cells. Participants had to be aged at least 18 years, be previously untreated for the advanced stage of the disease, have a good general status (performance status 0 or 1 according to the Eastern Cooperative Oncology Group), have adequate haematological and biochemical characteristics, including albuminaemia at 30 g/L or higher, and had to have at least one measurable site of disease with a computed tomography (CT) scan or magnetic resonance imaging (MRI) as defined by Response Evaluation Criteria In Solid Tumors (RECIST [version 1.1]). Exclusion criteria: participants with central nervous system metastases unless they were surgically removed or irradiated with no residual disease. Previous history of any malignancy (except for basal‐cell carcinoma of the skin or cervical intraepithelial neoplasia) within 5 years. Age: median 61 years (interquartile range 54 to 68) Sex: 142 men, 80 women N randomised: 111 to TG4010; 111 to placebo. N analysed: 111 in each group (ITT analysis); in safety analysis: 110 in TG4010 group and 107 in the placebo group. |
|
| Interventions |
Intervention group: TG4010 + first line therapy. TG4010 (Transgene, Illkirch, France) consists of a suspension of a recombinant modified vaccinia Ankara that codes for the MUC1 tumour‐associated antigen and interleukin‐2. Administered starting on Day 1 (D1) of Cycle 1 of chemotherapy and administered weekly for 6 weeks by subcutaneous (SC) injections and then once every 3 weeks until progression or discontinuation due to any reason. Chemotherapy (and bevacizumab if prescribed), given as 21‐day cycles for a minimum of 4 cycles and up to 6 cycles. First line therapy: ‐ Non‐squamous carcinoma: pemetrexed + cisplatin or paclitaxel + carboplatin +/‐ bevacizumab. ‐ Squamous carcinoma: gemcitabine + cisplatin or paclitaxel + carboplatin. Maintenance therapy: ‐ Pemetrexed or erlotinib for eligible patients and according to labelling. Comparison: placebo + first line therapy. Placebo administered starting on D1 of Cycle 1 of chemotherapy and will be administered weekly for 6 weeks by SC injections and then once every 3 weeks until progression or discontinuation due to any reason. First line therapy and maintenance therapy as in vaccine group. |
|
| Outcomes | 1. Overall survival. 2. Progression‐free survival. 3. Adverse events. 4. Overall response rate. 5. Duration of response. |
|
| Notes |
Funding for trial: Transgene, Avancées Diagnostiques pour de Nouvelles Approches Thérapeutiques (ADNA), and OSEO. Conflicts of interest: Quote: "GLa, BB, AT, CH, TP, and J‐ML are employees of Transgene, the funder of the study. EQ and ZP report advisory board participation for Transgene. JTB reports grants from AstraZeneca, Eli Lilly, Novartis, Abbvie, Amgen, Genentech, and Incyte, outside the submitted work. EF reports personal fees from Eli Lilly, Pfizer, Roche, Boehringer Ingelheim, AstraZeneca, Bristol‐Myers Squibb, Merck Sharp & Dohme, and Novartis, outside the submitted work. All other authors declare no competing interests." Registered: NCT01383148, in ClinicalTrials.gov. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated (1:1) by an external service provider (Cenduit) to receive TG4010 or placebo through a web‐based system. We stratified randomisation according to baseline value of TrPAL (≤ or > the upper limit of normal [ULN]) and, in addition, used a dynamic minimisation procedure taking into account chemotherapy regimen (cisplatin‐based or carboplatin‐based), histology, addition or not of bevacizumab (squamous, non‐squamous without bevacizumab, or non‐squamous with bevacizumab), Eastern Cooperative Oncology Group performance status (0 or 1), and centre. The dynamic minimisation used a stochastic treatment allocation algorithm based on the variance method; to minimise imbalance, treatment was assigned with a probability of 0·8, or 0·5 in case of a tie. The minimisation algorithm used a random number sequence to allocate the treatment, including a random factor for allocation, with a probability of 80%." |
| Allocation concealment (selection bias) | Low risk | Done. See above. |
| Blinding of participants and personnel (performance bias) Survival; Serious adverse effects | Low risk | Blinded study: participant, care provider, investigator, outcomes assessor. |
| Blinding of participants and personnel (performance bias) Progression‐free survival; Health‐related quality of life | Low risk | Blinded study: participant, care provider, investigator, outcomes assessor. |
| Blinding of outcome assessment (detection bias) Survival; Serious adverse effects | Low risk | Blinded study: participant, care provider, investigator, outcomes assessor. |
| Blinding of outcome assessment (detection bias) Progression‐free survival; Health‐related quality of life | Low risk | Blinded study: participant, care provider, investigator, outcomes assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in ITT analysis for the main outcome. For safety analysis, one was lost in the TG4010 group and 4 in the placebo group. |
| Selective reporting (reporting bias) | Low risk | Outcomes pre‐specified in clinicaltrials.gov (NCT01383148) were reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Rodriguez 2016.
| Study characteristics | ||
| Methods |
Study design: parallel, open‐label, phase III RCT. Recruitment period: from July 2006 to January 2012. Duration of study: information not available. Objective: to evaluate the effects of CIMAvax‐EGF in patients with advanced NSCLC. |
|
| Participants |
Country: Cuba. Setting: Hospital. Inclusion criteria: participants 18 years or older with histologically or cytological proven stage IIIB/IV NSCLC and an Eastern Cooperative Cooperative Oncology Group (ECOG) performance status of 0 to 2. Participants with all histologic NSCLC subtypes and life expectancy of at least 3 months were trial candidates. Other inclusion criteria included haemoglobin values above 90 g/L, leukocytes count ≥ 3.0 x 109/L, platelets count ≥ 150 x 109/L, aspartate aminotransferase, and alanine aminotransferase up to 2.5 times the upper institutional limit, and creatinine, up to 2 times the upper institutional reference value. All participants received four to six cycles of platinum‐based chemotherapy (mostly cisplatin/carboplatin in combination with vinblastine, etoposide, or paclitaxel) and had stable disease or objective response. Exclusion criteria: participants enroled in other investigational drug trials, or with a history of any severe or life‐threatening hypersensitivity reaction or an unstable systemic disease (including active infection, uncontrolled hypertension, unstable angina, congestive heart failure, myocardial infarction within the previous year, serious cardiac arrhythmia requiring medication, hepatic, renal, and metabolic disease). Pregnant woman. Another active concurrent malignant disease except non‐melanoma skin lesions or cervix cancer. Brain metastases, only if detectable by radiological image scan of previous signs or symptoms. Age: not detailed. Sex: 264 men, 141 women. N randomised: 405 participants; 270 vaccine group and 135 control group. N analysed: 246 vaccine group and 132 control group. |
|
| Interventions |
Intervention group: CIMAvax‐EGF vaccine plus best supportive care. CIMAvax‐EGF vaccine is composed of human recombinant EGF manufactured in yeast (hu‐recEGF), and it is chemically conjugated to the" P64K Neisseria meningitides recombinant protein (recP64k)" manufactured in Escherichia coli. The final formulation of the cancer vaccine (0.6 mg "hu‐recEGF/ recP64k") is then mixed in a water‐in‐oil emulsion with Montanide (Seppic) immediately before injection. At each immunization, patients received 2.4 mg of "hu‐recEGF/recP64k"/Montanide. Treatment schedule: "4 to 6 weeks after finishing first‐line chemotherapy, patients received the EGF cancer vaccine plus the best supportive care. They were given a low dose of cyclophosphamide (200 mg/m2) intravenously 72 hours before the first immunization." Each vaccine dose was administered at four injection sites (two deltoid and two gluteus regions) every 2 weeks for four doses (induction period) and then monthly. Comparison: best supportive care alone. |
|
| Outcomes | 1. Overall survival. 2. Adverse events. 3. Quality of life. |
|
| Notes | The trial was stopped before reaching the intended sample size, after the marketing approval of CIMAvax‐ EGF by the Cuban National Regulatory Agency, at the second interim analysis. Funding for trial: not reported. Conflicts of interest: Quote:"No potential conflicts of interest were disclosed". Registered: RPCEC00000161, in the Cuban Registry of Clinical Trials. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure was not detailed. Quote: "patients were randomly assigned (2:1) to the vaccine group". |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) Survival; Serious adverse effects | Low risk | Unblinded study, but unlikely that lack of blinding could bias the analysis of the impact of treatments on outcomes such as mortality or severe adverse events. |
| Blinding of participants and personnel (performance bias) Progression‐free survival; Health‐related quality of life | High risk | Unblinded study. |
| Blinding of outcome assessment (detection bias) Survival; Serious adverse effects | Low risk | Open‐label, unblinded study, but important outcomes for this review are objective outcomes: overall survival, death rates at 3 or 5 years, and serious adverse events. |
| Blinding of outcome assessment (detection bias) Progression‐free survival; Health‐related quality of life | High risk | Unblinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses in follow‐up explained. Quote: "Twenty‐four patients (8.8%) from the vaccine arm did not receive any vaccine dose, while 27 patients (10.9%) who started vaccination did not complete CIMAvax‐EGF induction (four doses). The main causes of early dropout were rapid worsening of the performance status, consent withdrawal, uncompensated comorbidities, schedule violations, and rapid unset of death. Eighteen patients, 10 vaccinated (3.7%) and 8 controls (5.9%), died before day 45 (time needed to complete induction vaccination). No significant differences were found between both arms regarding early death." |
| Selective reporting (reporting bias) | Low risk | Protocol available and all pre‐specified outcomes had been reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Takayama 2016.
| Study characteristics | ||
| Methods |
Study design: parallel, double‐blind RCT (participants and professionals). Recruitment period: from January 2010 to September 2013. Duration of study: from April 2014 to April 2015. Objective: to evaluate the efficacy and safety of personalised peptide vaccination (PPV) combined with chemotherapy for participants with previously treated advanced non‐small‐cell lung cancer (NSCLC). |
|
| Participants |
Country: Japan. Setting: hospital. Inclusion criteria: aged 20 years or older with histologically or cytologically confirmed inoperable stage IIIB/IV or recurrent NSCLC with epidermal growth factor receptor (EGFR) wild genotype were eligible for enrollment. Other eligibility criteria included measurable disease, ECOG (Eastern Cooperative Oncology Group) PS (performance status) of 0‐1, life expectancy of at least 12 weeks, and adequate haematologic (haemoglobin > 8.0 g/dL, neutrophil count > 2000/μL, lymphocyte count > 1200/μL, and platelet count > 100,000/μL), hepatic (bilirubin < 1.5 mg/dL, aspartate transaminase/alanine transaminase < 2.5x upper limit of normal), and renal (serum creatinine > 1.5 mg/dL) function. Participants who received prior one or two chemotherapy regimens were eligible. Prior radiation treatment or chemotherapy was to be completed at least 4 weeks before enrollment, and the participants were required to completely recover from all adverse effects due to prior treatment. All participants must be of positive status for human leukocyte antigen (HLA) ‐A2, HLA‐A24, HLA‐A26, or HLA‐A3 supertype (HLA‐A3, HLA‐A11, HLA‐A31, and HLA‐A33) and have at least two or more peptide‐specificimmunoglobulin Gs(IgGs) in the preregistration immunological screening Exclusion criteria: participants with active serious infection or other serious underlying medical conditions were ineligible. Immunocompromised participants or participants with systemic administration of corticosteroid was excluded. Age: median 64, ranging from 62.5 to 66 years. Sex: 41 men, 9 women. N randomised: 50 participants: 26 vaccine group; 24 control group. N analysed: 47. |
|
| Interventions |
Intervention group: PPV + docetaxel Comparison: docetaxel + placebo |
|
| Outcomes | 1. Overall survival. 2. Progression‐free survival. 3. Adverse effects. 4. Evaluation of immunological responses. |
|
| Notes |
Funding for trial: supported by grants from the Regional Innovation Strategy Support Program Global Type of the Ministry of Education, Science, Sports, and Culture in Japan (Masanori Noguchi). Conflicts of interest: Akira Yamada is a part‐time Executive of the Green Peptide Co. Ltd. Kyogo Itoh received consultancy fee and grant from Taiho Pharmaceutical Co., Japan. The other authors declare that they have no competing interests. Registered: UMIN 000003521, in the Japanese registry of clinical trials. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Detailed information not available. Quote: "patients were randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | Detailed information not available. |
| Blinding of participants and personnel (performance bias) Survival; Serious adverse effects | Low risk | Only participants were blinded, but unlikely that lack of blinding could bias the analysis of the impact of treatments on outcomes such as mortality or severe adverse events. |
| Blinding of participants and personnel (performance bias) Progression‐free survival; Health‐related quality of life | High risk | Only participants were blinded. High risk for progression‐free survival. |
| Blinding of outcome assessment (detection bias) Survival; Serious adverse effects | Low risk | Only participants were blinded, but important outcomes for this review are objective outcomes: overall survival, death rates at 3 or 5 years, and serious adverse events. |
| Blinding of outcome assessment (detection bias) Progression‐free survival; Health‐related quality of life | High risk | Single blind: only participants blinded. High risk for progression‐free survival. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | Study protocol or registry information was not provided, but the authors present results on all outcome measures that were pre‐specified as relevant. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
ALK: anaplastic lymphoma kinase; CT: computed tomography; ECOG (PS): Eastern Cooperative Oncology Group performance status; EGF: epidermal growth factor; EGFR: epidermal growth factor receptor; HLA: human leukocyte antigen; HRQoL: health‐related quality of life; ICG: immune checkpoint blockers; ITT: intention‐to‐treat; NSCLC: non‐small cell lung cancer; QLQ‐C30: quality of life questionnaire; RCT: randomised controlled trial; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Butts 2014 | Ineligible intervention: vaccine withdrawn by manufacturers. |
| Cohen 2014 | Ineligible comparison. The vaccine was part of a multicomponent intervention, vaccine plus cyclophosphamide. Not possible to assess the separate effect of the vaccine. |
| Govindan 2014 | Unpublished results. |
| Gray 2018 | Ineligible comparison: comparison of two vaccine regimens. |
| Katakami 2017 | Ineligible intervention: vaccine withdrawn by manufacturers. |
| Ramalingam 2014 | Ineligible comparison. The vaccine was part of a multicomponent intervention: vaccine plus cyclophosphamide versus placebo. Not possible to assess the separate effect of the vaccine. |
| Ramlau 2008 | Ineligible comparison: comparison of two vaccine regimens. Compares two schedules of combining TG4010 with first‐line chemotherapy. |
| Saavedra 2017 | Ineligible study design. Single‐arm study. |
| Saavedra 2021 | Ineligible comparison: comparison of different vaccine regimens. Three groups of patients received CIMAvax‐EGF in different maintenance schemes. |
| Sebastian 2014 | Ineligible study design. Single group study. |
| Wu 2011 | Ineligible population: planned to include people with stage IIIA cancer (as well as higher stages). Results not published. |
Differences between protocol and review
Background. We updated the Background to include references to relevant studies published after the publication of the protocol.
Objectives. We changed the wording of the objectives (in the protocol, the wording was: 'To evaluate the effectiveness and safety of therapeutic vaccines for people with advanced non‐small cell lung cancer (non‐irradiatable stage IIIB and stage IV NSCLC) who have either not received treatment, or have received treatment with chemotherapy, or radiotherapy, or both.' The new wording is: 'To evaluate the effectiveness and safety of different types of therapeutic vaccines for people with advanced non‐small cell lung cancer"). We made this change as radiotherapy is not medically relevant to metastatic lung cancer. We also removed the mention of radiotherapy from the Types of interventions section for the same reason.
Review criteria
We clarified that we would exclude studies examining vaccines that have been withdrawn by their manufacturers and are not available for use in clinical practice.
New text in the methods section: 'Type of interventions: Therapeutic cancer vaccines alone or in combination with chemotherapy...'
We removed the following text: 'If a study included more than 10% of patients with NSCLC stage IIIA cancer, it would be excluded from the review. Studies in which there were less than 10% of patients with NSCLC stage IIIA cancer would be included and certainty of the evidence would be downgraded by one level for indirectness.'
New text in the methods section: 'Primary outcome: Progression‐free survival: the interval between the date of randomisation and the appearance of new lesions, or the progression of the primary tumour, preferably according to RECIST criteria for studies done after the year 2009.'
We made the adverse events outcome a primary outcome (rather than a secondary outcome) and adjusted the wording to: 'Serious treatment‐related adverse events, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0'.
Search strategies. The search strategies were redesigned for CENTRAL, MEDLINE, and Embase. We added bibliographic searches in Wanfang Data and China Journal Net (often referred to as CNKI), two Chinese databases not included in the protocol, after finding studies in published reviews not included in the prespecified databases. We did not search in Clinical Key (www.clinicalkey.com/), International Cancer Immunotherapy Conference (www.cancerimmunotherapyconference.org/), and Google Scholar (https://scholar.google.com/), because ClinicalKey is intended to facilitate access to clinical answers and Google Scholar includes articles published in MEDLINE and Embase.
Methods: Assessment of risk of bias in included studies. New text added: "We considered that lack of blinding of participants and personnel could be a source of risk of performance bias and detection bias for subjective outcomes (progression‐free survival and health‐related quality of life), but not for objective outcomes (overall survival, survival rates at three and five years, and severe adverse events)."
Methods: Unit of analysis issues. We added: "If trials included several intervention comparisons, we followed standard methodological approaches, as recommended in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2023c)."
Methods:Summary of findings and assessment of the certainty of the evidence.
We reiterated that when assessing the certainty of the evidence regarding imprecision, a clinically relevant improvement for specific HRQoL scales considered was a 10‐point or greater increase in functional scores or a 10‐point or greater decrease in symptom scores of the EORTC QOL‐C30 Questionnaire. For the Functional Assessment of Cancer Therapy‐Lung (FACT‐L) Questionnaire, a minimally important difference is considered as a 2‐ to 3‐point change in the Lung Cancer Subscale (LCS) and 5‐ to 6‐point change for the Trial Outcome Index (TOI).
We clarified our approach to GRADE assessment: "For the summary of findings tables, we used the five GRADE considerations (study limitations (i.e. risk of bias); consistency of the effect (heterogeneity if I2 was higher than 50%); imprecision (if the confidence interval contains the threshold of clinical relevance and no clear conclusions can be drawn); indirectness; and publication bias) to assess the certainty of the body of evidence."
Summary of findings tables. We planned to create one SoF table but we ultimately presented two since we considered that it was more sensible to present separately the results of vaccines with different mechanisms of action. We only presented SoF tables for comparisons with data from at least two studies.
References. We updated some references to the Cochrane Handbook, including specific chapters on methodological issues, from the 2011 version to the 2023 version.
Contributions of authors
Conceiving, coordinating, and designing the review (MC‐J).
Screening of results of the literature searches (MC‐J, MR‐E, JRR).
Extraction of information for the characteristics of the studies (MC‐J, MR‐E, JRR, EJT).
Numerical data extraction for outcomes (MR‐E, JRR).
Evaluation of the risk of bias of the included studies (MC‐J, MR‐E, JRR).
Meta‐analyses (MR‐E, JRR).
Writing and clinical advice on the review (EO). All authors contributed to the drafting of the review text.
Sources of support
Internal sources
-
Universidad Católica de la Santísima Concepción, Chile
Employs review author, Marcela Cortés‐Jofré
-
University of the Basque Country., Spain
Employs Jose‐Ramón Rueda.
External sources
-
None, Other
No sources of support provided
Declarations of interest
Marcela Cortés‐Jofré: none known.
Mikel Rueda‐Etxebarria: none known.
Emeline Orillard: none known.
Elena Jimenez Tejero: none known.
José‐Ramón Rueda: none known.
New
References
References to studies included in this review
Alfonso 2014 {published data only}RPCEC00000009
- Alfonso S, Valdés-Zayas A, Santiesteban ER, Flores Y, Areces F, Hernandez M, et al. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clinical Cancer Research 2014;20(14):3660‐71. [DOI: 10.1158/1078-0432.CCR-13-1674] [DOI] [PubMed] [Google Scholar]
Besse 2023 {published data only}
- Besse B, Felip E, Garcia Campelo R, Cobo M, Mascaux C, Madroszyk A, et al on behalf of the ATALANTE-1 study group. Randomized open-label controlled study of cancer vaccine OSE2101 versus chemotherapy in HLA-A2-positive patients with advanced non-small-cell lung cancer with resistance to immunotherapy: ATALANTE-1. Annals of Oncology 2023;34(9):1-14. [DOI: ] [DOI] [PubMed] [Google Scholar]
Gridelli 2020 {published data only}
- Gridelli C, Ciuleanu T, Domine M, Szczesna A, Bover I, Cobo M, et al. Clinical activity of a htert (vx-001) cancer vaccine as post-chemotherapy maintenance immunotherapy in patients with stage IV non-small cell lung cancer: final results of a randomised phase 2 clinical trial. British Journal of Cancer 2020;122(10):1461-6. [DOI: 10.1038/s41416-020-0785-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateras IS, Kotsakis A, Avgeris M, Baliou E, Kouroupakis P, Patsea E, et al. Clinical activity of an hTERT-specific cancer vaccine (Vx-001) in "immune desert" NSCLC. Cancers (Basel) 2021;13(7):1658. [DOI: 10.3390/cancers13071658] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez 2021 {published and unpublished data}
- Hernandez M, Neninger E, Ortiz RA, Camacho K, Amador RM, Bello L, et al. Switch maintenance therapy with racotumomab or nimotuzumab versus docetaxel for NSCLC patients. In: Annals of Oncology. Vol. 27. 2016. [DOI: 10.1093/annonc/mdw378.47] [DOI]
- Hernandez M, Neninger E, Santiesteban E, Camacho K, Hernandez N, Amador R, et al. Efficacy of racotumomab or nimotuzumab versus docetaxel as secondline therapy for advanced non-small cell lung cancer patients. In: Annals of Oncology. Vol. 29 - suppl 8. 2018:viii415. [DOI: 10.1093/annonc/mdy288.037] [DOI]
- Hernández M, Neninger E, Ortiz RA, Santiesteban E, Camacho K, Amador RM, et al on behalf of RANIDO Trial Research Group. Safety and efficacy of racotumomab-alum orimotuzumab versus docetaxel as switch maintenance therapy for advanced non-small cell lung cancer patients: a phase III open label randomized non-inferiority trial. Global Surgery 2021;7:1-8. [DOI: 10.15761/GOS.1000230] [DOI] [Google Scholar]
Neninger 2008 {published data only}
- Crombet T, Neninger E, Osorio M, Catala M, Torre A, Leonard I, et al. Vaccination with epidermal growth factor (EGF) for non-small cell lung cancer (NSCLC) therapy: preliminary results from a randomized phase II clinical trial. In: American Society of Clinical Oncology. Vol. 22 suppl 4. 2004:166.
- García B, Neninger E, la Torre A, Leonard I, Martinez R, Viada C, et al. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clinical Cancer Research 2008;14(3):840‐6. [DOI: 10.1158/1078-0432.CCR-07-1050] [DOI] [PubMed] [Google Scholar]
- Neninger E, la Torre A, Osorio M, Catalá M, Bravo I, Mendoza del Pino M, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. Journal of Clinical Oncology 2008;26(9):1452-8. [DOI: 10.1200/JCO.2007.11.5980] [DOI] [PubMed] [Google Scholar]
O'Brien 2004 {published data only}
- O'Brien ME, Anderson H, Kaukel E, O'Byrne K, Pawlichi M, et al. SRL172 (killed Mycobacterium vaccae) in addition to standard chemotherapy improves quality of life without affecting survival, in patients with advanced non-small-cell lung cancer: phase III results. Annals of Oncology 2004;15(6):906‐14. [DOI: 10.1093/annonc/mdh220] [DOI] [PubMed] [Google Scholar]
Quoix 2011 {published data only}
- Quoix E, Ramlau R, Westeel V, Papai Z, Madroszik A, Riviere et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncology 2011;12(12):1125‐33. [DOI: 10.1016/S1470-2045(11)70259-5] [DOI] [PubMed] [Google Scholar]
- Rotonda C, Anota A, Mercier M, Bastien B, Lacoste G, Limacher JM, et al. Impact of TG4010 vaccine on health-related quality of life in advanced non-small-cell lung cancer: results of a phase IIB clinical trial. PLoS One 2015;10(7):e0132568. [DOI: 10.1371/journal.pone.0132568] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quoix 2016 {published data only}
- Quoix E, Lena H, Losonczy G, Forget F, Chouaid C, Papai Z, et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncology 2016;17(2):212‐23. [DOI: 10.1016/S1470-2045(15)00483-0] [DOI] [PubMed] [Google Scholar]
- Quoix E, Nemunaitis J, Papai Z, Lena H, Genet D, Louis C, et al. TG4010 immunotherapy combined with first-line chemotherapy in advanced non-small cell lung cancer (NSCLC). Phase 2b results of the time study. In: Annals of Oncology. Vol. 26 suppl 1. 2015:i29. [DOI: 10.1093/annonc/mdv050.9] [NCT01383148] [DOI]
- Quoix E, Sequist L, Nemunaitis J, Beck T, Jaskiewicz P, Oster JP, et al. TG4010 immunotherapy combined with first-line therapy in advanced non-small cell lung cancer (NSCLC): phase IIb results of the TIME study. In: Journal of ImmunoTherapy of Cancer. Vol. 2. 2014. [DOI: 10.1002/central/CN-01472266/full] [NCT01383148] [DOI]
- Quoix EA, Forget F, Papai-Szekely Z, Ottensmeier CH, Nemunaitis JJ, FElip E, et al. Results of the phase IIb part of TIME study evaluating TG4010 immunotherapy in stage IV non-small cell lung cancer (NSCLC) patients receiving first line chemotherapy. In: Journal of Clinical Oncology. Vol. 33. 2015. [DOI: 10.1002/central/CN-01130326/full] [NCT01383148] [DOI]
- Tosch, C, Bastien, B, Barraud, L, et al. Viral based vaccine TG4010 induces broadening of specific immune response and improves outcome in advanced NSCLC. Journal for ImmunoTherapy of Cancer 2017;5(1):7070. [DOI: 10.1186/s40425-017-0274-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez 2016 {published data only}
- Rodriguez PC, Popa X, Martinez O, Mendoza S, Santiesteban E, Crespo T, et al. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clinical Cancer Research 2016;22(15):3782‐90. [DOI: 10.1158/1078-0432.CCR-15-0855] [DOI] [PubMed] [Google Scholar]
- Viada-González C, Lorenzo-Monteagudo G, Ramos-Suzarte M, Álvarez-Cardona M, Frías-Blanco A, Neninger-Vinagera E, et al. Quality of life assessment in patients with non-small cell lung cancer treated with CIMAvaxEGF vaccine [Evaluación de la calidad de vida de pacientes con cáncer de pulmón de células no pequeñas tratados con la vacuna CIMAvaxEGF®]. VacciMonitor 2021;30(2):69-80. [Google Scholar]
Takayama 2016 {published data only}
- Takayama K, Sugawara S, Saijo Y, Maemondo M, Sato A, Takamori S, et al. Randomized phase II study of docetaxel plus personalized peptide vaccination versus docetaxel plus placebo for patients with previously treated advanced wild type EGFR non-small-cell lung cancer. Journal of Immunological Research 2016;2016:1745108. [DOI: 10.1155/2016/1745108] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Butts 2014 {published data only}
- Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncology 2014;15(1):59‐68. [DOI: 10.1016/S1470-2045(13)70510-2] [DOI] [PubMed] [Google Scholar]
- Mitchell P, Thatcher N, Socinski MA, Wasilewska-Tesluk E, Horwood K, Szczesna A, et al. Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Annals of Oncology 2015;26(6):1134-42. [DOI: 10.1093/annonc/mdv104] [DOI] [PubMed] [Google Scholar]
Cohen 2014 {published data only}
- Cohen RB, Nemunaitis J, Gabrail N, Bazhenova L, Schreiber TH, Price M. A phase II study of viagenpumatucel-l (HS-110) in combination with low-dose cyclophosphamide versus physician's choice in patients with advanced non-small cell lung cancer. Journal for ImmunoTherapy of Cancer 2014;6(2 Suppl 3):P82. [DOI: doi: 10.1186/2051-1426-2-S3-P82] [Google Scholar]
Govindan 2014 {published data only}
- Govindan R, Morris JC, Rossi GR, Vahanian NN, Link CJ. NLG-0301: an open-label, randomized phase 2B active control study of second-line tergenpumatucel-L immunotherapy versus docetaxel in patients with progressive or relapsed non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2014;32(15 Suppl):1. [Google Scholar]
Gray 2018 {published data only}
- Gray JE, Chiappori A, Williams CC, Tanvetyanon T, Haura EB, Creelan BC, et al. Phase I/randomized phase II study of GM.CD40L vaccine in combination with CCL21 in patients with advanced lung adenocarcinoma. Cancer Immunology Immunotherapy 2018;67(12):1853-62. [DOI: 10.1007/s00262-018-2236-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Katakami 2017 {published data only}
- Katakami N, Hida T, Nokihara H, Imamura F, Sakai H, Atagi S, et al. Phase I/II study of tecemotide cancer immunotherapy for Japanese patients with unresectable stage III non-small cell lung cancer (NSCLC). Lung Cancer 2017;105:23-30. [DOI] [PubMed] [Google Scholar]
Ramalingam 2014 {published data only}
- Ramalingam SS, Mitchell P, Vansteenkiste JF, Debus J, Curran WJ, Socinski MA. START2: tecemotide in unresectable stage III NSCLC after first-line concurrent chemoradiotherapy. Journal of Clinical Oncology 2013;32(15 suppl):TPS7608. [DOI: 10.1200/jco.2014.32.15_suppl.tps7608] [DOI] [Google Scholar]
Ramlau 2008 {published data only}
- Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Lévy E, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. Journal of Thoracic Oncology 2008;3(7):735-44. [DOI: 10.1097/JTO.0b013e31817c6b4f] [DOI] [PubMed] [Google Scholar]
Saavedra 2017 {published data only}
- Saavedra D, Crombet T. CIMAvax-EGF: a new therapeutic vaccine for advanced non-small cell lung cancer patients. Frontiers in Immunology 2017;8:269. [DOI: 10.3389/fimmu.2017.00269] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saavedra 2021 {published data only}
- https://trialsearchwhoint/?TrialID=RPCEC00000368. Evaluation of the immune response generated after vaccination with CIMAvax-EGF using different maintenance schedules [Evaluation of the immune response generated after vaccination with CIMAvax-EGF using different maintenance schedules]. Cuban registry of clinical trials Date of registration:12/05/2021.
Sebastian 2014 {published data only}
- Sebastian M, Papachristofilou A, Weiss C, Früh M, Cathomas R, Hilbe W, et al. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive®) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC Cancer 2014;14:748. [DOI: 10.1186/1471-2407-14-748] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2011 {published data only}
- Wu Y-L, Park K, Soo RA, Sun Y, Tyroller K, Wages D, et al. INSPIRE: a phase III study of the BLP25 liposome vaccine (L-BLP25) in Asian patients with unresectable stage III non-small cell lung cancer. BMC Cancer 2011;11:430. [DOI: 10.1186/1471-2407-11-430] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85(5):365-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Antonia 2017
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R et al, PACIFIC Investigators. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. New England Journal of Medicine 2017;377(20):1919-29. [DOI: 10.1056/NEJMoa1709937] [DOI] [PubMed] [Google Scholar]
Antonia 2018
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. New England Journal of Medicine 2018;379(24):2342-50. [DOI: 10.1056/NEJMoa1809697] [DOI] [PubMed] [Google Scholar]
Ascarateil 2015
- Ascarateil S, Puget A, Koziol M-E. Safety data of montanide ISA 51 VG and montanide ISA 720 VG, two adjuvants dedicated to human therapeutic vaccines. Journal for Immunotherapy of Cancer 2015;3(Suppl 2):428. [Google Scholar]
Butts 2014
- Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncology 2014;15(1):59‐68. [DOI] [PubMed] [Google Scholar]
Cella 2002
- Cella D, Eton DT, Fairclough DL, Bonomi P, Heyes AE, Silberman C, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. Journal of Clinical Epidemiology 2002;55(3):285-95. [DOI: 10.1016/s0895-4356(01)00477-2.] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 23 June 2019. Melbourne, Australia: Veritas Health Innovation, 2019. Available at www.covidence.org.
Damm 2013
- Damm K, Roeske N, Jacob C. Health-related quality of life questionnaires in lung cancer trials: a systematic literature review. Health Economics Review 2013;3(1):15. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dammeijer 2016
- Dammeijer F, Lievense LA, Veerman GD, Hoogsteden HC, Hegmans JP, Arends LR, et al. The efficacy of tumor vaccines and cellular immunotherapies in non-small cell lung cancer: a systematic review and meta-analysis. Journal of Clinical Oncology 2016;34(26):3204-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Declerck 2014
- Declerck S, Vansteenkiste J. Immunotherapy for lung cancer: ongoing clinical trials. Future Oncology 2014;10(1):91-105. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2023
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Detterbeck 2018
- Detterbeck FC. The eighth edition TNM stage classification for lung cancer: what does it mean on main street? Journal of Thoracic and Cardiovascular Surgery 2018;15(5):356-9. [DOI] [PubMed] [Google Scholar]
Ding 2014
- Ding M, Yang J. Therapeutic vaccination for non-small-cell lung cancer: a meta-analysis. Medical Oncology 2014 apr;31(4):928. [DOI] [PubMed] [Google Scholar]
Domingues 2014
- Domingues D, Turner A, Silva MD, Marques DS, Mellidez JC, Wannesson L, et al. Immunotherapy and lung cancer: current developments and novel targeted therapies. Immunotherapy 2014;6(11):1221-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freites‐Martinez 2021
- Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 2021;112(1):90-2. [DOI] [PubMed] [Google Scholar]
Gandhi 2018
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al, KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. New England Journal of Medicine 2018;378(22):2078-92. [DOI: 10.1056/NEJMoa1801005] [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2024
- Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L, et al. Age-standardized rate (world) per 100 000, incidence, both sexes, in 2022: trachea, bronchus and lung. GLOBOCAN 2024.
Goldstraw 2016
- Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al, International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. Journal of Thoracic Oncology 2016 Jan;11(1):39-51. [DOI] [PubMed] [Google Scholar]
Govindan 2014
- Govindan R, Morris JC, Rossi GR, Vahanian NN, Link CJ. NLG-0301: an open-label, randomized phase 2B active control study of second-line tergenpumatucel-L immunotherapy versus docetaxel in patients with progressive or relapsed non-small cell lung cancer (NSCLC). Journal of Clinical Oncology 2014;32(15 Suppl):1. [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 23 June 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available from gradepro.org.
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analysis of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI] [PubMed] [Google Scholar]
Hernandez 2008
- Hernandez AM, Toledo D, Martinez D, Grinan T, Brito V, Macias A, et al. Characterization of the antibody response against NeuGcGM3 ganglioside elicited in non-small cell lung cancer patients immunized with an anti-idiotype antibody. Journal of Immunology (Baltimore, Md. 1950) 2008;181(9):6625-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hernandez 2011
- Hernandez AM, Rodriguez N, Gonzalez JE, Reyes E, Rondon T, Grinan T, et al. Anti-NeuGcGM3 antibodies, actively elicited by idiotypic vaccination in non-small cell lung cancer patients, induce tumor cell death by an oncosis-like mechanism. Journal of Immunology (Baltimore, Md. 1950) 2011;186(6):3735-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2023a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Higgins 2023b
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Higgins 2023c
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Hillman 2017
- Hillman GG, Reich LA, Rothstein SE, Abernathy LM, Fountain MD, Hankerd K, et al. Radiotherapy and MVA-MUC1-IL-2 vaccine act synergistically for inducing specific immunity to MUC-1 tumor antigen. Journal for Immunotherapy of Cancer 2017;5:4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Katakami 2017
- Katakami N, Hida T, Nokihara H, Imamura F, Sakai H, Atagi S, et al. Phase I/II study of tecemotide cancer immunotherapy for Japanese patients with unresectable stage III non-small cell lung cancer (NSCLC). Lung Cancer 2017;105:23-30. [DOI] [PubMed] [Google Scholar]
Lefebvre 2023
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated October 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Lemjabbar‐alaoui H 2015
- Lemjabbar-alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: biology and treatment options. Biochimica et Biophysica Acta 2015;1856(2):189-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leone 2013
- Leone P, Shin EC, Perosa F, Vacca A, Dammacco F, Racanelli V. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. Journal of the National Cancer Institute 2013;105(16):1172-87. [PMID: ] [DOI] [PubMed] [Google Scholar]
Maringwa 2011
- Maringwa JT, Quinten C, King M, Ringash J, Osoba D, Coens C, et al, EORTC PROBE project and the Lung Cancer Group. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Supportive Care in Cancer 2011;19(11):1753-60. [DOI: 10.1007/s00520-010-1016-5] [DOI] [PubMed] [Google Scholar]
Mittal 2014
- Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases – elimination, equilibrium and escape. Current Opinion in Immunology 2014;27:16-25. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Telzlaff J, Altman DG, the PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monteiro 2016
- Monteiro ID, Califano R, Mountzios G, Mello RA. Immunotherapy with checkpoint inhibitors for lung cancer: novel agents, biomarkers and paradigms. Future Oncology 2016;12(4):551-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mountzios 2016
- Mountzios G, Linardou H, Kosmidis P. Immunotherapy in non-small cell lung cancer: the clinical impact of immune response and targeting. Annals of Translational Medicine 2016;4(14):268. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oken 1982
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the eastern cooperative oncology group. American Journal of Clinical Oncology 1982;5:649-55. [PubMed] [Google Scholar]
Page 2023
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook.
Pardoll 2015
- Pardoll D. Cancer and the immune system: basic concepts and targets for intervention. Seminars in Oncology 2015;42(4):523-38. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paz‐Ares 2018
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J et al, KEYNOTE-407 Investigators. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. New England Journal of Medicine 2018;379(21):2040-51. [DOI: doi: 10.1056/NEJMoa1810865] [DOI] [PubMed] [Google Scholar]
Prasad 2018
- Prasad KT, Kaur H, Muthu V, Aggarwal AN, Behera D, Singh N. Interconversion of two commonly used performance tools: An analysis of 5844 paired assessments in 1501 lung cancer patients. World Journal of Clinical Oncology 2018;9(7):140-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quoix 2017
- Quoix E, Lena H, Losonczy G, Forget F, Chouaid C, Papai Z, et al. T64010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncology 2017;2:212-23. [DOI] [PubMed] [Google Scholar]
Ramamurthy 2017
- Ramamurthy C, Godwin JL, Borghaei H. Immune checkpoint inhibitor therapy: what line of therapy and how to choose? Current Treatment Options in Oncology 2017;18(6):33. [PMID: ] [DOI] [PubMed] [Google Scholar]
RECIST 2009
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45:228-47. [DOI] [PubMed] [Google Scholar]
Reck 2016
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A et al, KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New England Journal of Medicine 2016;375(19):1823-33. [DOI: 10.1056/NEJMoa1606774] [DOI] [PubMed] [Google Scholar]
Remon 2017
- Remon J, Pardo N, Martinez-Marti A, Cedres S, Navarro A, Martinez de Castro AM, et al. Immune-checkpoint inhibition in first-line treatment of advanced non-small cell lung cancer patients: current status and future approaches. Lung Cancer 2017;106:70-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rosenberg 1999
- Rosenberg SA. A new era of cancer immunotherapy: converting theory to performance. CA: a Cancer Journal for Clinicians 1999;49(2):70-3, 65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Statistics in Medicine 2008;27(5):746-63. [DOI] [PubMed] [Google Scholar]
Saavedra 2016
- Saavedra D, Garcia B, Lorenzo-Luaces P, Gonzalez A, Popa X, Fuentes KP, et al. Biomarkers related to immunosenescence: relationships with therapy and survival in lung cancer patients. Cancer Immunology, Immunotherapy 2016;65(1):37-45. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saavedra 2017
- Saavedra D, Crombet T. CIMAvax-EGF: a new therapeutic vaccine for advanced non-small cell lung cancer patients. Frontiers in Immunology 2017;8:269. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaedler 2017
- Schaedler E, Remy-Ziller C, Hortelano J, Kehrer N, Claudepierre MC, Gatard T, et al. Sequential administration of a MVA-based MUC1 cancer vaccine and the TLR9 ligand Litenimod (Li28) improves local immune defence against tumors. Vaccine 2017;35(4):577-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schreiber 2011
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331(6024):1565-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Smith 2014
- Smith AB, Cocks K, Parry D, Taylor M. Reporting of health-related quality of life (HRQOL) data in oncology trials: a comparison of the European Organization for Research and Treatment of Cancer Quality of Life (EORTC QLQ-C30) and the Functional Assessment of Cancer Therapy–General (FACT-G). Quality of Life Research 2014;23(3):971-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Suzuki 2014
- Suzuki H, Owada Y, Watanabe Y, Inoue T, Fukuharav M, Yamaura T, et al. Recent advances in immunotherapy for non-small-cell lung cancer. Human Vaccines & Immunotherapeutics 2014;10(2):352-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tosch 2017
- Tosch C, Bastien B, Barraud L, Grellier B, Nourtier V, Gantzer M, et al. Viral based vaccine TG4010 induces broadening of specific immune response and improves outcome in advanced NSCLC. Journal for Immunotherapy of Cancer 2017;5(1):70. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vesely 2011
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annual Review of Immunology 2011;29:235-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vesely 2013
- Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Annals of the New York Academy of Sciences 2013;1284:1-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2015
- Wang M, Cao JX, Liu YS, Xu BL, Li D, Zhang XY, et al. Evaluation of tumour vaccine immunotherapy for the treatment of advanced non-small cell lung cancer: a systematic meta-analysis. BMJ Open 2015;5(4):e006321. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2016
- Yang L, Wang L, Zhang Y. Immunotherapy for lung cancer: advances and prospects. American Journal of Clinical and Experimental Immunology 2016;5(1):1-20. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Zhou 2016
- Zhou L, Wang XL, Deng QL, Du YQ, Zhao NQ. The efficacy and safety of immunotherapy in patients with advanced NSCLC: a systematic review and meta-analysis. Scientific Reports 2016;6:32020. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2017
- Zhu J, Li R, Tiselius E, Roudi R, Teghararian O, Suo C, et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD011300. [DOI: 10.1002/14651858.CD011300.pub2] [CD011300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2021
- Zhu J, Yuan Y, Wan X, Yin D, Li R, Chen W, Suo C, Song H. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non‐small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD011300. [DOI: 10.1002/14651858.CD011300.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cortés‐Jofré 2019
- Cortés‐Jofré M, Uranga R, Torres Pombert A, Arango Prado MD, Caballero Aguirrechu I, Pacheco C, et al. Therapeutic vaccines for advanced non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2019, Issue 8. Art. No: CD013377. [DOI: 10.1002/14651858.CD013377] [DOI] [PMC free article] [PubMed] [Google Scholar]


