Abstract
BACKGROUND AND AIMS:
Information is limited regarding HBV genotype and the outcome of chronic HBV (CHB) infection. We examined the effect of HBV genotype on HCC occurrence in Alaska Native (AN) persons with CHB, where five HBV genotypes are found: A2, B6, C2, D, and F1.
APPROACH AND RESULTS:
We calculated HCC incidence per 1,000 person-years of follow-up to determine which groups by age, sex, and genotype met current American Association for the Study of Liver Diseases (AASLD) HCC surveillance criteria. We used Poisson regression to compare HCC risk by genotype, age, sex, and Alaska region. Incidence of HCC was calculated using the sex-specific AASLD cutoff recommended for the Asian population of 50 years for women and 40 years for men. HCC screening was conducted semiannually using alpha-fetoprotein levels and abdominal ultrasound. Among 1,185 AN persons, median follow-up was 35.1 years; 667 (63%) were male. The HBV genotype distribution was 49% D, 18% F, 13% A, 6% C, 3% B, 0.1% H, and 12% undetermined. Sixty-three cases of HCC occurred. HCC incidence for genotype F was 5.73 per 1,000 person-years of follow-up, followed by 4.77 for C, 1.28 for A, 0.47 for D, and 0.00 for B. The HCC risk was higher for genotypes F (relative rate [RR], 12.7; 95% CI, 6.1–26.4), C (RR, 10.6; 95% CI, 4.3–26.0), and A (RR, 2.9; 95% CI, 1.0–8.0) compared to genotypes B and D. Among men < 40 years of age and women < 50 years of age, genotype F had the highest incidence (4.79/1,000 person-years).
CONCLUSIONS:
HBV genotype was strongly associated with HCC. HBV genotype should be considered in risk factor stratification.
Chronic HBV infection is the main etiology of HCC worldwide. Long-term survival after diagnosis of HCC depends on finding tumors at an early stage. While tumors found at advanced stages are rarely curable, small tumors detected early can be cured, leading to long-term survival. Regular surveillance for HCC with liver ultrasound, with or without alpha-fetoprotein (AFP) every 6 months, leads to the detection of tumors at an earlier stage, allowing more potentially curable treatment to be employed, including radiofrequency and microwave ablation, surgical resection, or transplantation. Current chemotherapeutic agents or other treatments, such as transhepatic arterial embolization techniques or radiation therapy, may offer additional short-term survival benefit but are rarely curative.(1) A cost–benefit analysis suggested that screening by liver ultrasound every 4–6 months is cost-effective in populations with an annual HCC incidence of ≥2 cases per 1,000 person-years of follow-up.(2) HCC surveillance should be considered based on sex-specific age cutoffs (men ≥ 40 years and women ≥ 50 years), presence of cirrhosis, and a family history of HCC.(3) Previous studies from Asia have found that the incidence of HCC is significantly higher in persons >40 years of age infected with HBV genotype C compared to those with genotype B(4); however, few studies have examined the risk of HCC in persons infected with other HBV genotypes. Therefore, there has not been enough evidence to include HBV genotype as one of the criteria in models examining cost-effectiveness of HCC surveillance in persons living with chronic HBV (CHB) infection.
Alaska Native (AN) people are the only US population born in an area endemic for HBV infection.(5) HBV is endemic in communities along the Bering Sea coast from Kodiak Island, through the Yukon-Kuskokwim delta in southwest Alaska, the Norton Sound region, and up to the northwest Bering Sea communities. From 1983 to 1987, 53,000 AN persons of all ages were screened for HBV seromarkers, and 40,000 seronegative individuals received hepatitis B vaccination, stopping transmission of HBV in this population.(6) During this program, 1,560 AN persons were found to have CHB infection. Ninety percent of these chronically infected individuals were from the Bering Sea coast region stretching from Kodiak Island to the Arctic Ocean and share common ancestry (Yupik and Inupiaq). These individuals have been followed clinically and for HCC surveillance. Beginning in 1983, we invited them to join a long-term study, the Hepatitis B Alaska (HEP-B-AK) cohort, to follow outcomes in persons with CHB infection. In addition to identifying persons who would benefit from antiviral therapy based on national guidelines,(7) two of the objectives of this cohort were to identify risk factors associated with HCC and to determine the most effective methods for HCC surveillance.(8) Participants in the HEP-B-AK cohort have been followed for an average of 32 years, and >90% of them have had HBV genotype testing performed. Five distinct HBV genotypes have been identified: A2, B6, C2, D2 and D3, and F1b.(9) The B6 in this population is a newly identified subtype of B (that does not have any recombination of genotype C), which is similar to the B1 found in Japan, differing by >4% sequence diversity.(10) Previous studies conducted in this cohort have shown that the predominant mode of transmission differs by HBV genotype. In regions where genotype C is the predominant genotype, HBV transmission is primarily perinatal, whereas horizontal transmission dominates in regions where the other genotypes are found.(11–13) We previously have shown an association between HCC and HBV genotype but have not examined incidence and which genotypes and age groups in this population meet the American Association for the Study of Liver Diseases (AASLD) guidelines for surveillance for HCC.(14,15)
We report on the relationship between HCC risk and HBV genotype in HEP-B-AK participants using data collected prospectively between 1983 and 2018 and retrospectively from 1973 to 1982. Using this long-term cohort, we evaluate if HBV genotype can be used as a risk factor in algorithms for HCC surveillance.
Methods
Serosurvey studies for hepatitis B were performed beginning in 1973.(16) All persons identified with CHB were followed in a clinical registry to monitor for the need for antiviral therapy and to find HCC tumors at a treatable stage. In addition, those who consented were enrolled in the HEP-B-AK cohort starting in 1983. Greater than 90% of persons identified with CHB consented to be in the HEP-B-AK cohort by 1987. None had received HBV vaccine. All HBV serologic results obtained before this date were entered retrospectively into the cohort database. The Hep-B-AK cohort study has been approved by both the AN and the Centers for Disease Control and Prevention’s (CDC’s) institutional review boards, and approval has been obtained from all tribal health organizations in Alaska.
The Hep-B-AK cohort has been described previously, but in brief, patients in the cohort were sent letters by mail every 6 months reminding them to have a blood draw for liver function tests (LFTs) and AFP.(17) Over 75% of these patients lived in rural communities, most of which are isolated and not connected to the road system. Lists of patients due for blood draw were sent to the local community and/or regional clinic accompanied by barcoded lab slips. The local laboratory or community health aide/practitioner drew the blood, spun it down, and separated the sera, which was shipped off to the Alaska Native Medical Center (ANMC) and subjected to LFTs and tests for AFP, HBV DNA, as well as hepatitis B serologic markers. HBeAg is measured yearly. Results were downloaded into the ANMC hospital electronic health record and reviewed by a hepatologist and a clinical/research nurse. If LFTs and AFP levels were within normal limits and HBV DNA was <2,000 IU/mL, the patient was sent a letter informing them that their tests were not elevated, and they received another reminder in 6 months. If any LFT value was elevated, the patients were scheduled for further evaluation, which could have included one or more of the following: vibration-controlled transient elastography (FibroScan), imaging studies, or a liver biopsy. Persons with AFP values >10 μg/mL were urged to get a liver ultrasound at the nearest hospital or regional clinic facility, regardless of age. Beginning in 1990, all patients within the Hep-B-AK cohort had HBV genotype testing done, either at the CDC Division of Viral Hepatitis in Atlanta or at the molecular biology laboratory at ANMC using described methods.(14) Historical serum was used for genotyping in persons who had expired or were HBV DNA–negative in 1990 and who also were HBeAg-negative and in the inactive phase of CHB or had cleared HBsAg after many years of being HBsAg-positive. Five HBV genotypes have been identified: A2, B6, C2, two subtypes of genotype D (D2 and D3), and F1b.(9)
Every 6 months all patients who met the current AASLD guideline recommendations for HCC surveillance are sent a second letter urging them to have a liver ultrasound. More than 50% of the cohort live in a community where ultrasound is not available. Air travel to a hospital or clinic facility where ultrasound is available could have been cost-prohibitive, and consequently, of those recommended for ultrasound, 60% underwent at least one radiographic screening (ultrasound, MRI, or CT) on a yearly basis. Cases of HCC were identified in two ways. First, most cases were detected by the above surveillance system. Second, the Alaska Native Tribal Health Corporation National Cancer Institute–funded Surveillance, Epidemiology, and End Results Registry was regularly queried for persons who developed HCC to assure that no HCC cases were missed within the Hep-B-AK cohort.
STATISTICAL METHODS
We calculated HCC incidence per 1,000 person-years of follow-up. The start of follow-up was defined as the date of a person’s first positive HBsAg result or January 1, 1982, whichever came later. End of follow-up was defined as the person’s date of death, date of HCC diagnosis, or December 31, 2018. We calculated HCC incidence by age group (<40 years, 40–59 years, or ≥60 years) using the person’s age at the first time point they entered the cohort and their age at the time of follow-up. We used Poisson regression to compare HCC incidence by gender, age, HBV genotype, HBV DNA level at the follow-up midpoint, HBeAg status at cohort entry, and region of residence. The relative rate and 95% CI were used as effect measures. In order to evaluate HCC risk according to AASLD screening guidelines, we additionally calculated HCC incidence according to age and HBV genotype. Because of small sample sizes, only two age classes were used, and the cut-point was sex-specific (≥40 years of age in men, ≥50 years of age in women) in accordance with previous AASLD HCC screening guidelines.
Results
We followed 1,185 persons in the cohort for a median of 35 years, totaling 37,138 person-years of follow-up. The demographics of those in the cohort are shown in Table 1. At the time of diagnosis with HBV infection, the mean age for the cohort was 21.2 years; and at the end of follow-up, the mean age was 53.9 years. Patients with HBV genotype D constituted 49% (n = 583) of the cohort, genotype F comprised 18% (n = 209), followed by genotypes A, C, and then B. Over the course of follow-up, 123 patients were given antiviral treatment for HBV who met AASLD practice guideline recommendations. Because this treatment was targeted toward persons with advanced liver disease, it is not evaluated as a risk factor. One patient was found to be infected with genotype H, which is phylogenetically close to genotype F (Fig. 1). In 137 persons genotype was not determined, usually due to very low levels of HBV DNA in their sera. By the end of 2018, 313 persons (26%) had died and 63 (5%) had developed HCC (Table 2). The proportion of patients who developed HCC during the follow-up period by genotype in descending order was genotype F (17%), C (14%), A2 (4%), D (1.5%), and B6 (0%).
TABLE 1.
Characteristics of the 1,185 persons chronically infected with HBV in Alaska (1982–2018)
| Characteristic | Level | n (%) |
|---|---|---|
| Gender | Male | 667 (56%) |
| Female | 518 (44%) | |
| HBV genotype | D | 583 (49%) |
| F | 209 (18%) | |
| A | 150 (13%) | |
| C | 73 (6%) | |
| B | 32 (3%) | |
| H | 1 (0.1%) | |
| Unknown | 137 (12%) | |
| Deceased | Yes | 313 (26%) |
| No | 872 (74%) | |
| Age at the time of HBV diagnosis | <10 years | 370 (31%) |
| 10–19 years | 253 (21 %) | |
| 20–34 years | 314 (27%) | |
| 35–49 years | 156 (13%) | |
| ≥50 years | 92 (8%) |
FIG. 1.
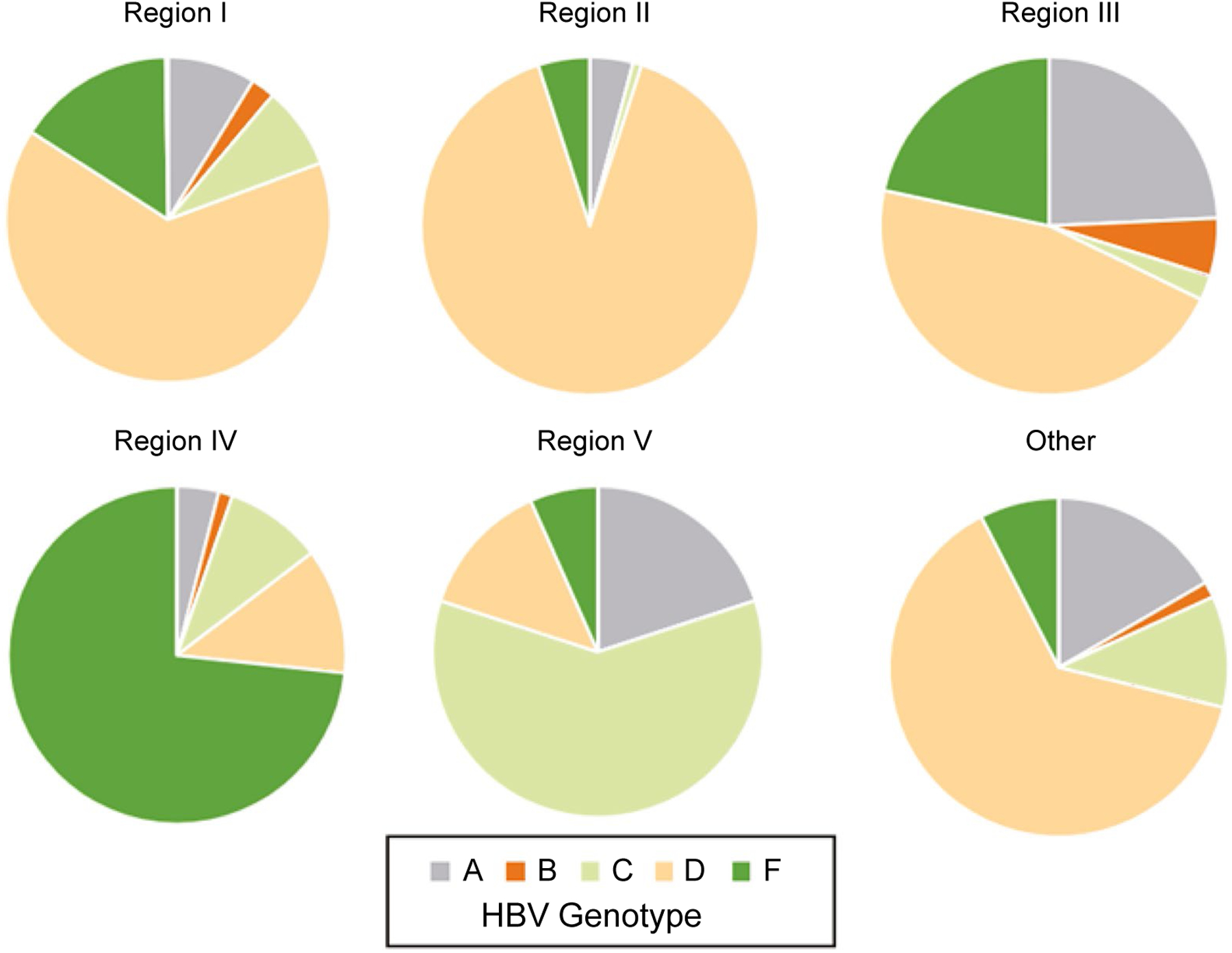
Distribution of Five HBV Genotypes in 1,185 Alaska Native Persons by Alaska Region of Residence.
TABLE 2.
Demographic characteristics, person-years of follow-up, and HCC incidence according to HBV genotype in Alaska (1982–2018)
| Genotype | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | A | B | C | D | F | H | Unknown | Overall |
| Number of participants | 150 | 32 | 73 | 583 | 209 | 1 | 137 | 1,185 |
| Median age at study entry (IQR) | 23 (15–34) | 46.5 (30.5–55.5) | 24 (15–45) | 18 (7–31) | 16 (9–27) | 25 (25–25) | 25 (15–37) | 20 (10–33) |
| Number male (%) | 88 (59%) | 19 (59%) | 32 (44%) | 329 (56%) | 122 (58%) | 1 (100%) | 76 (55%) | 667 (56%) |
| Number alive at end of study period (12/31/2018) | 111 | 16 | 40 | 459 | 147 | 0 | 99 | 872 |
| Median age of survivors (IQR) | 56 (51–63) | 67.5 (58–75) | 53 (46–63) | 50 (42–61) | 52 (45–59) | — | 56 (49–63) | 53 (44–62) |
| Number who died during study period | 39 | 16 | 33 | 124 | 62 | 1 | 38 | 313 |
| Median age at death (IQR) | 57 (47–67) | 78.5 (71.5–81) | 65 (45–77) | 63 (44–73) | 46.5 (35–63) | 55 (55–55) | 61.5 (49–72) | 53 (44–62) |
| Number treated for HBV during study period | 19 | 4 | 20 | 49 | 21 | 0 | 10 | 123 |
| Number with HCC during study period | 6 | 0 | 10 | 9 | 36 | 0 | 2 | 63 |
| Median age at HCC diagnosis (IQR) | 58.5 (45–76) | — | 58.5 (45–68) | 55 (45–57) | 28 (19–48.5) | — | 56 (52–60) | 45 (23–60) |
| Total person-years of follow-up | 4,680 | 931 | 2,098 | 19,048 | 6,285 | 30 | 4,067 | 37,138 |
| Median (IQR) | 35.2 (30–37) | 33.4 (21–36) | 32.7 (24–35) | 35.1 (32–37) | 35.7 (24–37) | 29.9 | 34.7 (25–36) | 35.1 (29–36) |
Abbreviation: IQR, interquartile range.
In univariable analysis, the HCC incidence per 1000 person-years was over 2.4 times higher in men (2.31 cases/1,000 person-years) than women (0.95) and >10 times higher in persons who were aged ≥60 years at the time they entered the cohort compared to persons <40 years of age (Table 3). For all ages, genotype F had the highest incidence of 5.73/1,000 person-years, followed by genotypes C (4.77/1,000), then A (1.28/1,000) (Table 3). When compared to genotypes B and D combined, the relative rate of HCC was 12.7 (95% CI, 6.1–26.4) for genotype F, 10.6 (95% CI, 4.3–26.0) for genotype C, and 2.9 (95% CI, 1.0–8.0) for genotype A. Among all ages, only genotypes A, C (for those persons >40 years of age), and F (at all ages) had a rate of HCC < 2 cases/1,000 person-years, the incident threshold determined to be cost-effective for screening recommended by the AASLD.(3) There were no cases of HCC found in persons infected with genotype B, despite the fact that people with this genotype had an overall higher average age (67.5, range 58–75) than those with other genotypes. However, there were very few people with genotype B (n = 32 persons), and the upper limit of the 95% CI for HCC incidence includes the threshold for screening. There were significant differences in HBV genotype distribution and HCC incidence according to the region of residence for Alaskan patients (Table 3 and Fig. 1). In multivariable analysis, age at entry, sex, HBeAg status, and HBV genotype were statistically significant (Table 3). As a sensitivity analysis, when we adjusted for HBV antiviral treatment, all P values remained similar in the multivariate model. After demographic and HBV genotype adjustment, region of residence was no longer a statistically significant factor in HCC risk.
TABLE 3.
HCC Incidence per 1000 person-years of follow-up by HBV genotype and sex, Alaska, 1982–2018
| Characteristic | Level | Number with HBV (%) | Number with HCC (%) | HCC incidence (95% Cl) | Relative rate (95% Cl) | P | Multivariable relative rate (95% Cl) |
|---|---|---|---|---|---|---|---|
| Sex | Male | 667 (56%) | 47 (75%) | 2.31 (1.70–3.07) | 2.4 (1.4–4.3) | 0.002 | 2.3 (1.3–4.2) Ref |
| Female | 518 (44%) | 16 (25%) | 0.95 (0.54–1.55) | Ref | |||
| Age group at entry | <40 years | 997 (84%) | 41 (65%) | 1.24 (0.89–1.69) | Ref | Ref | |
| 40–60 years | 146 (12%) | 14 (22%) | 3.89 (2.12–6.52) | 3.1 (1.7–5.7) | <0.001 | 3.7 (1.8–7.5) | |
| ≥60 years | 42 (4%) | 8 (13%) | 13.08 (5.65–25.78) | 10.5 (4.9–22.4) | <0.001 | 13.7 (5.9–30.5) | |
| HBV genotype | A | 150 (13%) | 6 (10%) | 1.28 (0.47–2.79) | 2.9 (1.0–8.0) | 0.05 | 3.6 (1.3–10.3) |
| B | 32 (3%) | 0 (0%) | 0.00 (0.00–3.96) | Ref | Ref | ||
| C | 73 (6%) | 10 (16%) | 4.77 (2.29–8.76) | 10.6 (4.3–26.0) | <0.0001 | 8.3 (3.3–21.2) | |
| D | 583 (49%) | 9 (14%) | 0.47 (0.22, 0.90) | Ref | Ref | ||
| F | 209 (18%) | 36 (57%) | 5.73 (4.01–7.93) | 12.7 (6.1–26.4) | <0.0001 | 14.8 (7.1–30.8) | |
| H | 1 (0.1%) | 0 (0%) | 0.00 (0.00– 123.3) | ||||
| Unknown | 137 (12%) | 2 (3%) | 0.49 (0.06– 1.63) | ||||
| HBeAg status at entry | Positive | 455 (39%) | 29 (51%) | 1.96 (1.31–2.81) | 1.6 (0.9–2.6) | 0.10 | 2.5 (1.4–4.3) |
| Negative | 723 (61%) | 28 (49%) | 1.26 (0.84–1.82) | Ref | Ref | ||
| HBV DNA at follow-up midpoint | ≥2,000 mlU/mL | 191 (17%) | 9 (24%) | 1.48 (0.67–2.76) | 1.5 (0.72–3.21) | 0.27 | NS |
| <2 000 mlU/mL | 917 (83%) | 29 (76%) | 0.97 (0.65–1.40) | Ref | |||
| Region | I | 437 (37%) | 12 (19%) | 0.89 (0.46–1.55) | Ref | ||
| II | 138 (12%) | 2 (3%) | 0.45 (0.05–1.61) | 0.5 (0.1–2.2) | 0.37 | NS | |
| III | 414 (35%) | 24 (38%) | 1.78 (1.14–2.65) | 2.0 (1.0–4.0) | 0.05 | ||
| IV | 85 (7%) | 15 (24%) | 6.23 (3.51–10.34) | 7.1 (3.3–15.1) | <0.0001 | ||
| V | 33 (3%) | 7 (11%) | 7.73 (3.11–15.93) | 8.7 (3.4–22.1) | <0.0001 | ||
| Other/unknown | 78 (6%) | 3 (5%) | 1.30 (0.26–3.71) | 1.4 (0.4–5.1) | 0.58 |
Abbreviation: ND, removed, not significant in multivariate model after adjustment for age at entry, gender, HBeAg status, HBV genotype.
When we further broke down the HCC incidence rates by gender-specific age cut points (≥40 years of age in men, ≥50 years of age in women; Table 4), the age groups that are included in the AASLD screening recommendations, genotype C had the highest HCC incidence (15.35), followed by genotypes F (9.58), A (2.72), and D (1.47).
TABLE 4.
Incidence of HCC according to HBV genotype and sex-specific age category at time of follow-up, Alaska, 1982–2018
| HBV genotype (no. persons in follow-up) | Younger age (women < 50 years: men < 40 years) | Older age (women ≥ 50 years: men ≥ 40 years) | ||
|---|---|---|---|---|
| No. HCC cases | HCC incidence (95% CI) | No HCC cases | HCC incidence (95% CI) | |
| A (n = 150) | 2 | 0.62 (0.07–2.25) | 4 | 2.72 (0.74–7.00) |
| B (n = 32) | 0 | 0.00 (0.00–12.2) | 0 | 0.00 (0.00–5.87) |
| C (n = 73) | 0 | 0.00 (0.00–2.55) | 10 | 15.35 (7.36–28.24) |
| D (n = 583) | 2 | 0.14 (0.02–0.50) | 7 | 1.47 (0.59–3.05) |
| F (n = 209) | 24 | 4.79 (3.06–7.10) | 12 | 9.58 (4.95–16.74) |
| H (n = 1) | 0 | 0.00 (0.00–239.4) | 0 | 0.00 (0.00–253.9) |
| Unknown (n = 137) | 0 | 0.00 (0.06–1.38) | 2 | 1.43 (0.17–5.17) |
| All known genotypes | 28 | 1.15 (0.76–1.66) | 33 | 3.78 (2.60–5.24) |
| All persons | 28 | 1.01 (0.69–1.50) | 35 | 3.45 (2.40–4.80) |
Bold indicates significance.
There were 28 persons in the younger age group (<40 years of age for men; <50 for women) who developed HCC who would not have met the sex and age criteria for AASLD guidelines for HCC surveillance. Twenty-four individuals in this group were infected with genotype F and two each with genotypes A and D. The incidence of HCC in younger persons was highest for genotype F (4.79/1,000 person-years; Table 4). The incidence in this younger age group for genotype F carriers was 23.0 (95% CI, 8.0–66.3) times higher than in persons with other known genotypes combined.
Discussion
We report a strong association between HBV genotype and HCC risk. These data come from a large, long-standing prospective cohort of AN people with CHB infection. The racial, and thus the genetic, similarity in this population allows us to focus on the impact of HBV genotype on HCC rates, limiting the impact of differences in human ethnicity or racial characteristics.
We determined that the highest overall incidence of HCC in AN people, regardless of age, occurred in those infected with HBV genotype F1b. Importantly, we found that even young persons infected with genotype F had an HCC incidence rate that exceeded the recommended threshold for cost-effective HCC US surveillance. Among men 40 years and women 50 years of age and older, we found that the highest incidence of HCC occurred in people infected with HBV genotype C, similar to what has been found in Asia.(4) One possible reason for the high rate of HCC in persons with genotype C after age 40 years could be related to the duration of high levels of HBV DNA. We previously found that the mean age of HBeAg seroconversion to anti-HBe, which is associated with a fall in HBV DNA levels, occurs in those infected with genotype C at 45 years of age versus <20 years of age in the other four genotypes found in AN persons with CHB.(9) The rate of HCC among people with genotype F was above the recommended screening cutoff of 2/1,000 person-years irrespective of age. Our data suggest that persons infected with HBV genotype F1b might benefit from screening at any age and that those infected with HBV C could benefit from screening beginning at age 40 years if male and at age 50 years if female. For those with genotypes A and D in the older age group, we found an incidence near 2/1,000 person-years; the CIs overlapped 1. We only had a small number of patients infected with genotype B; thus, we are limited in our ability to evaluate HCC risk in persons infected with this genotype. Therefore, we cannot arrive at any conclusions regarding screening for HCC in AN persons infected with HBV genotype B6. Currently, we screen all men 40 years and over and women 50 years and over who are infected with any of the five genotypes; however, we have now included persons under age 40 years who have genotype F1b. In addition, we perform surveillance on those with a family history of HCC and those with cirrhosis. Data from this study have prompted us to change the surveillance recommendations in AN persons infected with HBV in light of our findings on HBV genotype. Our data suggest that HBV genotype should be included in the criteria for surveillance for HCC in HBV-infected persons in national and international guidelines and in algorithms to identify those at the highest risk for HCC.
Studies, primarily from Asia, are limited and include comparison of usually only two HBV genotypes. These studies have shown that HBV genotype plays a role in the risk for HCC.(4,18) While these studies show that genotype C has the highest rate of development of HCC, agreeing with our current study, they also included a subgenotype not seen in Alaska, genotype Ba or B Asia. The Ba genotype virus contains mutations in the basal core promoter due to recombination from genotype C.(19) The REVEAL study from Taiwan, following over 3,000 persons with HBV infected with either genotype Ba or C, found that while HCC incidence was significantly higher in those with genotype C, incidence of HCC was high in persons >40 years of age with genotype Ba.(4) In Japan, where B1 is found, genotype C was again found to have the highest rate of HCC. In addition, investigators found that people with HBV genotype C had an average age of HCC presentation a decade younger than is seen in those with genotype B1 infection.(20,21) While the overall pattern of HCC risk by genotype from these studies matched ours, we found a lower absolute HCC risk. This difference may be due to a lower risk of HCC found in HBV-infected persons with genotype D. This genotype is present in nearly 50% of the HBV-infected cohort in the AN population compared to those in the east Asian populations (e.g., China, Taiwan, Korea, and Vietnam), where this genotype is uncommon. In addition, HBV genotype B6 in Alaska, similar to the subtype B1 found in Japan, has not yet been associated with a case of HCC.(22) It is possible that the Alaska subtype B6 is less carcinogenic than B1 or that other environmental factors not seen in Alaska affect HCC development in Japan. Conversely, we may begin to see HCC occur as those persons with B6 grow older, as has been seen with B1 in Japan.(21)
Our findings indicate that the highest incidence of HCC in young AN people occurred in those infected with HBV genotype F1b. Although other subgeno-types of F are found throughout Central and South America, genotype F1b has only been found in the Amazon basin. Previously, we showed that most young persons infected with this genotype who developed HCC did not have cirrhosis but had mild portal hepatitis with mild fibrosis.(23) We recently published data with collaborators from Japan which showed that replicons made from F1b virus from AN patients with HCC developed unique mutations in the viral core region between the time of infection and the time of cancer genesis.(19) In contrast, these unique mutations were not seen in the F1b strains from HBV-infected AN individuals who did not develop HCC. Strains of HBV genotype F1 virus from patients with HCC who had these unique mutations, when introduced into human hepatocyte tissue culture, resulted in more hepatocyte damage and replicated at higher rates compared to the wild-type F1 mutated virus.(24) Furthermore, when the replicons with the unique mutations were introduced into immunodeficient mice with humanized livers, five oncogenes associated with HCC were expressed. The fact that underlying viral mutations in genotype F1b can advance replicative and hepatocellular destructive properties in cell culture and generate oncogenes in vivo in an animal model shows how this HBV genotype could result in higher risk of HCC if these mutations were to occur over time.
Limitations in this study include the small number of patients infected with genotype B and the limited number of HCC cases. Also, 12% of participants did not have HBV genotype determination. Almost all of these patients did not have enough virus in their sera to perform HBV genotyping as most were in the inactive phase with undetected or low levels of HBV. However, because 88% did have enough sera to virus present in their sera to perform HBV genotype testing, we feel the results are valid. We were limited by sample size in assessing time-dependent covariates, but we hope to examine this in a future multicenter study.
In conclusion, this study provides evidence that HBV genotype is an important factor in the risk for developing HCC in HBV-infected individuals, along with cirrhosis and family history. Additional studies in other populations with more than one HBV genotype are needed to determine if HBV genotype should be added to guideline recommendations to assess risk factors for HCC surveillance. Our epidemiologic studies and the work from our colleagues in Japan have paved the way for investigators to initiate more HBV gene studies with both F1b and other genotypes to learn more about the specific HBV genotypes’ cellular destructive and replicative properties and identify and evaluate oncogene mutations that predispose to the development of HCC. Our extensive HBV serum bank offers the opportunities to continue work on oncogeneses and potential tumor markers of HBV genotypes in association with development of HCC. Our findings suggest that guidelines for HCC screening in AN persons with CHB take into consideration HBV genotype. While additional studies examining HBV genotype and its association with HCC in other populations are needed, these studies are difficult to establish as multiple genotypes, in a sufficiently sized cohort, followed long term would be required, and meeting these criteria is rare. In the meantime, HBV genotype status should be considered in future screening algorithms and HCC surveillance recommendations.
Acknowledgments
Supported by the Centers for Disease Control and Prevention (U01PS001097, U50/CCU022279).
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- AFP
alpha-fetoprotein
- AN
Alaska Native
- ANMC
Alaska Native Medical Center
- CHB
chronic HBV
- HEP-B-AK
Hepatitis B Alaska
- LFT
liver function test
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1).Sherman M, Bruix J, Porayko M, Tran T. Screening for hepatocellular carcinoma: the rationale for the American Association for the Study of Liver Diseases recommendations. Hepatology 2012;56:793–796. [DOI] [PubMed] [Google Scholar]
- 2).Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Terrault NA, Lok ASF, McMahon BJ, Chang K-M, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Chan HL, Hui AY, Wong ML, Tse AM, Hung LC, Wong VW, et al. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut 2004;53: 1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Schreeder MT, Bender TR, McMahon BJ, Moser MR, Murphy BL, Sheller MJ, et al. Prevalence of hepatitis B in selected Alaskan Eskimo villages. Am J Epidemiol 1983;118:543–549. [DOI] [PubMed] [Google Scholar]
- 6).McMohon BJ, Schoenberg S, Bulkow L, Wainwright RB, Fitzgerald MA, Parkinson AJ, et al. Seroprevalence of hepatitis B viral markers in 52,000 Alaska Natives. Am J Epidemiol 1993;138:544–549. [DOI] [PubMed] [Google Scholar]
- 7).Terrault NA, Lok ASF, McMahon BJ, Chang K-M, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Clin Liver Dis (Hoboken) 2018;12:33–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).McMahon BJB, Harpster L, Snowball A, Lanier M, Sacco A, Dunaway F, et al. Screening for hepatocellular carcinoma in Alaska Natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology 2000;32:842–846. [DOI] [PubMed] [Google Scholar]
- 9).Livingston S, Simonetti J, McMahon B, Bulkow L, Hurlburt K, Homan C, et al. Hepatitis B virus genotypes in Alaska Native people with hepatocellular carcinoma: preponderance of genotype F. J Infect Dis 2007;195:5–11. [DOI] [PubMed] [Google Scholar]
- 10).Osiowy C, Simons BC, Rempel JD. Distribution of viral hepatitis in indigenous populations of North America and the circumpolar Arctic. Antivir Ther 2013;18:467–473. [DOI] [PubMed] [Google Scholar]
- 11).Livingston SE, Simonetti JP, Bulkow LR, Homan CE, Snowball MM, Cagle HH, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C. D and F. Gastroenterology 2007;133:1452–1457. [DOI] [PubMed] [Google Scholar]
- 12).McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis 1985;151:599–603. [DOI] [PubMed] [Google Scholar]
- 13).Esteroff D, Greenman JA, Heyward HL, McMahon BJ, Hardison HH, Bender TR. Increased prevalence of hepatitis B surface antigen in pregnant Alaska Eskimos. Circumpolar Health 1985;84:206–208. [Google Scholar]
- 14).Livingston S, Simonetti J, McMahon B, Bulkow L, Hurlburt K, Homan C, et al. Hepatitis B virus genotypes in Alaska Native people with hepatocellular carcinoma: preponderance of genotype F. J Infect Dis 2007;195:5–11. [DOI] [PubMed] [Google Scholar]
- 15).Ching LK, Gounder PP, Bulkow L, Spradling PR, Bruce MG, Negus S, et al. Incidence of hepatocellular carcinoma according to hepatitis B virus genotype in Alaska Native people. Liver Int 2016;36:1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Barrett DH, Burks JM, McMahon B, Elliott S, Berquist KR, Bender TR, et al. Epidemiology of hepatitis B in two Alaska communities. Am J Epidemiol 1977;105:118–122. [DOI] [PubMed] [Google Scholar]
- 17).McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, et al. Screening for hepatocellular carcinoma in Alaska Natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology 2000;32:842–846. [DOI] [PubMed] [Google Scholar]
- 18).Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology 2000;118:554–559. [DOI] [PubMed] [Google Scholar]
- 19).McMahon BJ. The influence of hepatitis B virus genotype and subgenotype on the natural history of chronic hepatitis B. Hepatol Int 2009;3:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu S, et al. Epidemiologic and virologic characteristics of hepatitis B virus genotype B having the recombination with genotype C. Gastroenterology 2003;124:925–932. [DOI] [PubMed] [Google Scholar]
- 21).Orito E, Sugauchi F, Tanaka Y, Ichida T, Sata M, Tanaka E, et al. Differences of hepatocellular carcinoma patients with hepatitis B virus genotypes of Ba, Bj or C in Japan. Intervirology 2005;48:239–245. [DOI] [PubMed] [Google Scholar]
- 22).Sakamoto T, Tanaka Y, Simonetti J, Osiowy C, Børresen M, Koch A, et al. Classification of hepatitis B virus genotype B into 2 major types based on characterization of a novel subgenotype in Arctic indigenous populations. J Infect Dis 2007;196:1487–1492. [DOI] [PubMed] [Google Scholar]
- 23).Popper H, Thung SN, McMahon BJ, Lanier AP, Hawkins I, Alberts SR. Evolution of hepatocellular carcinoma associated with chronic hepatitis B virus infection in Alaskan Eskimos. Arch Pathol Lab Med 1988;112:498–504. [PubMed] [Google Scholar]
- 24).Hayashi S, Khan A, Simons BC, Homan C, Matsui T, Ogawa K, et al. An association between core mutations in hepatitis B virus genotype F1b and hepatocellular carcinoma in Alaskan Native people. Hepatology 2019;69:19–33. [DOI] [PubMed] [Google Scholar]


