Abstract
Objective
This study examines the effectiveness of MIP-3alpha and severity scores in determining the prognosis of elderly sepsis patients.
Methods
From October 2020 to April 2021, a total of 171 elderly sepsis patients were admitted to the Emergency Department of the Shijingshan Branch of Beijing Chaoyang Hospital, Capital Medical University. According to the 28-day mortality rate, they were divided into two groups: survivors (48 cases) and deaths (123 cases). At admission, severity scores which are the Sequential Organ Failure Assessment (SOFA) and the Acute Physiology and Chronic Health Evaluation II (APACHE II) were calculated. The logistic regression was used to analyze the independent risk factors associated with 28-day mortality in elderly sepsis patients. The receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to evaluate the value of MIP-3alpha, SOFA, and APACHE II in the evaluation of 28-day mortality in elderly sepsis patients.
Results
MIP-3alpha, SOFA and APACHE II of the death group were significantly higher than those of the survival group (P < 0.05). Multivariate logistic regression analysis showed that MIP-3alpha, SOFA, APACHE II, and systolic blood pressure (SBP) were independent risk factors for 28-day mortality of senile sepsis (P < 0.05). Analysis of the ROC curve revealed that MIP-3alpha, SOFA, APACHE II had predictive value for the 28-day prognosis of senile sepsis (all P < 0.01). Combing with MIP-3alpha and SOFA showed better predictive ability (Z1 = 3.733, Z2 = 2.996, both P < 0.01), compared to detecting MIP-3alpha and SOFA alone.
Conclusion
In senile sepsis, MIP-3alpha, SOFA, APACHE II and SBP are independent risk factors for 28-day mortality. The combination of MIP-3alpha and SOFA can further enhance the predictive value of 28-day mortality in patients with senile sepsis and provide some reference value for the evaluation and treatment of senile sepsis.
Keywords: sepsis, the elderly, MIP-3alpha, prognosis
Introduction
Sepsis is an infection-induced systemic inflammatory response syndrome that progresses rapidly and can result in the failure of multiple vital organs, endangering the lives of patients in severe cases. The combination of low immune function and sepsis mortality in the elderly is frequently fatal.1 In a meta-analysis, the average 30-day mortality rate for sepsis was 24.4%.2 Consequently, early assessment of the severity of senile sepsis and aggressive treatment is of great value for enhancing patients’ prognoses. There are numerous studies on C-reactive protein and procalcitonin for assessing the severity of sepsis,3 but their diagnostic specificity and predictive value are inadequate.There are also many studies indicating that many biomarkers are associated with sepsis, but their sensitivity is insufficient.4–6 MIP3-alpha plays an important role in immune response and inflammatory damage in a variety of diseases,7 but relatively few studies have examined its prognostic value in elderly patients with sepsis. This study aims to investigate the prognostic value of MIP-3alpha, SOFA score, and APACHE II score in elderly sepsis patients. The results are summarized below.
Materials and Methods
Research Subjects
From October 2020 to April 2021, a retrospective analysis was conducted on 171 elderly patients with sepsis admitted to the Emergency Department of Beijing Chaoyang Hospital affiliated with Capital Medical University. The following were the criteria for inclusion: 1) Diagnostic criteria applicable to the 2021 International Guidelines for the Management of Sepsis and Septic Shock;8 2) Age > 65 years old; 3) Complete clinical data. Exclusion criteria: 1) Admission less than 24 hours; 2) Combined with autoimmune diseases, blood system diseases; 3) Complicated with malignant tumor; 4) Long-term use of immunosuppressants or hormones. This study adhered to medical ethics standards, and the hospital’s Ethics Committee approved the research protocol (approval number: 2021-Sector-636). Patients or their families provided informed consent for all treatments and procedures.
Methods
Research Methods
After patients were admitted to the emergency department, general information such as age, gender, basic diseases, infection site, etc. was collected, along with vital signs such as blood pressure, heart rate, and body temperature. Measure MIP-3alpha, albumin (ALB), serum creatinine (SCR), white blood cell (WBC), hemoglobin (HB), platelet (PLT), lactic acid (LAC), Procalcitonin (PCT), C-reactive protein (CRP), total bilirubin (TBIL), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Blood urea nitrogen (BUN) and such from immediately drawn 5mL of elbow venous blood at admission. Circulating levels of marker were measured in plasma samples by using Human XL Cytokine Luminex® Performance Assay 46-plex Fixed Panel (LKTM014B, R&D) according to the manufacturer’s instructions. At admission, the SOFA score, APACHE II score, and Glasgow coma score (GCS) were recorded. After admission, all patients received active fluid resuscitation, nutritional support, prevention of stress ulcers, anti-infection therapy, respiratory support, correction of acid-base balance, organ support, and other comprehensive treatments. The patients were monitored, and the 28-day mortality rate was recorded.Our study complies with the Declaration of Helsink.
Statistical Methods
Statistical Package for the Social Sciences (SPSS) version 23.0 was used to process the data. Normally distributed measurement data were expressed as mean ± standard deviation (x ± s). An independent sample t-test was used to compare two groups, a non-parametric test was used for normally distributed measurement data, and an x2 test was used for counting data. A multivariate logistic regression model was used to assess the predictive ability of MIP-3alpha, SOFA score, and APACHE II score on 28-day mortality using the receiver operating characteristic curve (ROC). The area under the curve (AUC) of 0.7 ~ 0.9 was deemed appropriate. AUC > 0.9 indicates a high degree of accuracy. The area under the ROC curve (AUC) was compared utilizing MedCalc’s Z test. P < 0.05 indicated a statistically significant difference.
Results
Comparison of General Data of Patients
There were 123 patients in the death group, and the overall mortality rate was 71.93% (123/171) in this study. According to Table 1, age, sex, Diastolic blood pressure (DBP), WBC, HB, PLT, BUN, PCT, CRP, TBIL, ALT, AST, and SCr did not differ significantly between the two groups (P > 0.05). MIP-3alpha (41.48 (14.61, 121.14) vs.10.77 (7.12, 28.18)), SOFA (9 (6, 10) vs. 5 (3, 5.75)) and APACHE II (22 (17, 27) vs.15 (12, 18)) of the death group were statistically significantly higher than those of the survival group (P < 0.05), whereas the SBP (132 (115, 145) vs.143 (128.25, 153)) and albumin (ALB) (32.8 (24.7, 37.1)vs.36.3 (27.68, 39.4)) of the death group were lower than those of the survival group (P < 0.05). The difference was statistically significant. As shown in Table 1, there was no statistical significance in the comparison of underlying diseases (diabetes, hypertension, and coronary heart disease) between the two groups (P > 0.05).
Table 1.
Comparison of Patient Baseline Data
| Survivor Group (n = 48) | Nonsurvivor group (n = 123) | P | |
|---|---|---|---|
| Age (years) | 71.5 (64.25, 83) | 76 (65, 83) | 0.505 |
| Gender (male/female) | 32/16 | 69/54 | 0.207 |
| SBP (mmHg) | 143 (128.25, 153) | 132 (115, 145) | 0.01 |
| DBP (mmHg) | 71.5 (65, 79.75) | 72 (64, 81) | 0.838 |
| Underlying disease | |||
| Hypertension (yes/no) | 23/25 | 55/68 | 0.706 |
| Diabetes (yes/no) | 17/31 | 39/84 | 0.642 |
| Coronary heart disease (yes/no) | 15/33 | 30/93 | 0.360 |
| GCS | 15 (15, 15) | 15 (15, 15) | 0.590 |
| SOFA | 5 (3, 5.75) | 9 (6, 10) | 0.000 |
| APACHE II | 15 (12, 18) | 22 (17, 27) | 0.000 |
| MIP3-alpha (ng/mL) | 10.77 (7.12, 28.18) | 41.48 (14.61, 121.14) | 0.000 |
| WBC (109/L) | 8.4 (7.2, 11.93) | 9.0 (7.3, 11.8) | 0.783 |
| HB (g/L) | 123.5 (97, 135) | 119 (106, 135) | 0.942 |
| PLT (109/L) | 218 (148, 303) | 175 (135.75, 247.25) | 0.071 |
| LAC (mmol/L) | 1.2 (0.9, 1.5) | 1.3 (1, 2) | 0.077 |
| SCr (umol/L) | 70.35 (52.7, 83.9) | 63.7 (46.8, 85.1) | 0.515 |
| ALB (g/L) | 36.3 (27.68, 39.4) | 32.8 (24.7, 37.1) | 0.044 |
| BUN (mmol/L) | 6.5 (5.03, 9.36) | 6.21 (4.69, 9.51) | 0.655 |
| TBIL (umol/L) | 14.8 (10.33, 24.28) | 14.5 (9.3, 24.2) | 0.666 |
| PCT (ng/mL) | 0.05 (0.05, 1.16) | 0.05 (0.05, 0.62) | 0.903 |
| CRP (mg/L) | 22.5 (8, 75.75) | 146 (8, 67.9) | 0.575 |
| AST (U/L) | 23.7 (18.25, 34.13) | 28.5 (18.6, 41.5) | 0.172 |
| ALT (U/L) | 18.7 (16.53, 26.03) | 22 (17, 31.4) | 0.380 |
Note: The differences were statistically significant when P < 0.05.
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment; GCS, Glasgow coma scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; PCT, procalcitonin; MIP3-alpha, Macrophage Inflammatory Protein-3α; WBC, white blood cell, HB, hemoglobin; PLT, platelet; LAC, lactate; CRP, C-reactive protein; BUN, blood urea nitrogen; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, Aspartate aminotransferase; Scr, serum creatinine; CRP, C-reactive protein; ALB, albumin.
Analysis of the Impact of Binary Logistic Regression on the Prognosis of Elderly Sepsis Patients
With the 28-day prognosis of patients as the dependent variable (assigned: 0 = survival, 1 = death), multiple clinical indicators with statistically significant differences in Table 1 were included as covariables in the binary multi-factor logistic regression model, while other confounding factors were excluded. MIP-3alpha, SOFA score, APACHE II score, and systolic blood pressure were identified as independent risk factors for senile sepsis-related 28-day mortality (all P < 0.05) (Table 2).
Table 2.
Multivariate Regression Analysis of MIP3-Alpha, SOFA Scores, APACHE II Scores, and 28-Day Prognosis in Critically Ill Patients
| β | SE | Wald | P | OR | (95% CI) | |
|---|---|---|---|---|---|---|
| MIP3-alpha | 0.027 | 0.007 | 13.925 | 0.000 | 1.028 | (0.013–1.043) |
| SOFA | 0.826 | 0.178 | 21.536 | 0.000 | 2.284 | (1.611–3.237) |
| APACHE II | 0.201 | 0.055 | 13.351 | 0.000 | 1.223 | (1.098–1.362) |
| SBP | −0.034 | 0.013 | 6.598 | 0.010 | 0.967 | (0.942–0.992) |
Note: The differences were statistically significant when P < 0.05.
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment; SBP, systolic blood pressure; MIP3-alpha, Macrophage Inflammatory Protein-3α.
Value of Various Indices in Predicting the 28-Day Prognosis of Patients with Senile Sepsis
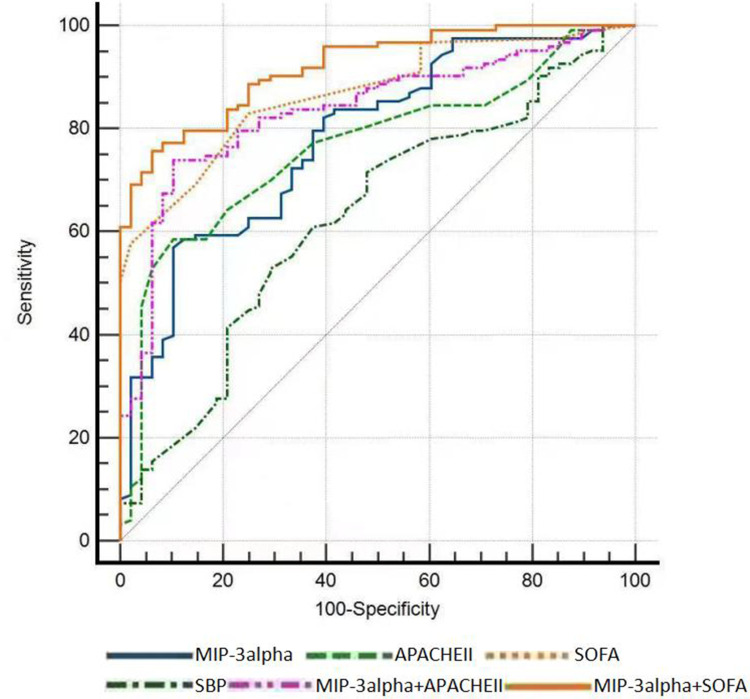
ROC curve analysis revealed that: MIP-3alpha, SOFA score, and APACHE II score can predict the prognosis of aged sepsis patients at 28 days, with MIP-3alpha (AUC = 0.781, sensitivity 56.9%, specificity 89.6%, cut-off 32.41); SOFA score (AUC = 0.867, sensitivity 82.9%, specificity 75%, cut-off 5.5) predicting the 28-day prognosis of patients with a higher AUC than APACHE II score (AUC = 0.764, sensitivity 58.5%, specificity 89.6%, cut-off 20.5). The combined detection of MIP-3alpha and SOFA demonstrated superior predictive ability (Z1 = 3.733, Z2 = 2.996, both P < 0.01), AUC = 0.919, and the sensitivity and specificity were 75.6% and 93.7%, respectively, then MIP-3alpha and SOFA alone. As shown in Table 3 and Figure 1, SBP was poor at predicting the 28-day prognosis of elderly patients with sepsis. The area under the curve was only 0.372.
Table 3.
Predictive Value of MIP3-α, APACHE II Score, SOFA Score, and 28-Day Prognosis in Critically Ill Patients
| AUC | Cut-off | 95% CI | P | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|
| MIP-3 alpha | 0.781 | 32.41 | 0.706–0.856 | 0.000 | 56.9 | 89.6 |
| APACHEII | 0.764 | 20.5 | 0.689–0.840 | 0.000 | 58.5 | 89.6 |
| SOFA | 0.867 | 5.5 | 0.814–0.920 | 0.000 | 82.9 | 75 |
| SBP | 0.372 | 142.5 | 0.280–0.465 | 0.01 | 28.5 | 47.9 |
| MIP-3alpha+APACHEII | 0.835 | 0.772–0.899 | 0.000 | 74 | 89.6 | |
| MIP-3 alpha + SOFA | 0.919 | 0.879–0.958 | 0.000 | 75.6 | 93.7 |
Note: The differences were statistically significant when P < 0.05.
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment; SBP, systolic blood pressure; MIP3-alpha, Macrophage Inflammatory Protein-3α.
Figure 1.
ROC curve of MIP-3 alpha, APACHE II score, SOFA score, and SBP for predicting 28-day prognosis in senile sepsis patients.
Discussion
Sepsis is a prevalent life-threatening condition among elderly patients in the emergency department. Despite significant advances in the treatment of sepsis,9 the mortality rate associated with sepsis remains high.10 Elderly sepsis patients are frequently complicated by organ failure, relatively poor nutritional and immune functions,11 and atypical clinical manifestations, making it difficult to attract clinical attention, and thus missing the optimal treatment prospect. Consequently, early diagnosis and prognostic evaluation of senile sepsis are crucial. In recent years, with the continuous exploration of the pathogenesis of sepsis, a large number of biomarkers have been validated for the assessment of sepsis conditions and prognosis,12 but the sensitivity and specificity are inadequate. Finding new accurate and effective biomarkers to assess the patient’s condition and prognosis is crucial for improving the prognosis of elderly sepsis patients. In previous research, PCT and CRP are the most widely used and studied biomarkers, and PCT is considered superior to CRP in many studies.13 However, PCT levels can also be increased in other conditions.14 Some studies think that PCT and CRP may be more useful to rule out sepsis rather than to diagnosing it.15,16
MIP-3 alpha, also known as C-C chemokine ligand 20 (CCL20), is primarily expressed in the liver, lungs, and peripheral blood cells, where it plays an important role in an inflammatory response. It is primarily secreted by neutrophils, Th17 cells, and monocytes in the peripheral blood. Under the influence of inflammatory mediators, secretion is increased.17 MIP-3 alpha binds exclusively to specific chemokine receptor 6 (CCR6).18–20 Most chemokines bind to a variety of receptors. CCR6 is a G-protein-coupled seven-transmembrane receptor superfamily, that is predominantly expressed on immature dendritic cells, B cells, T cells, and various tumor cells involved in intercellular signal transduction.21 After binding with CCR6, MIP-3 alpha participates in inflammation, stimulates an antigen-specific immune response, promotes the release of inflammatory mediators and cytokines, protects against microbial invasion, and mediates pathological tissue damage, thereby creating a vicious cycle of inflammation.22,23
MIP-3 alpha has been shown to play a detrimental role in the immune response of numerous chronic inflammatory diseases, mediating the distribution of immune cells through chemotaxis, recruitment of lymphocytes and dendritic cells, and participating in the pathophysiology of rheumatoid arthritis, atopic dermatitis, contact dermatitis, fungal infection, and other diseases.24,25 Several animal studies have confirmed that MIP-3 alpha is involved in the progression of severe inflammation,26–28 and a recent study demonstrated that the MIP-3 alpha-driven cascade promoted the development of systemic inflammation in patients with severe sepsis.29 They demonstrated plasma levels of MIP-3alpha in ICU patients with sepsis were elevated. Consistent with previous research, this study revealed that the concentration of MIP-3alpha in the death group was significantly higher than that in the survival group.
The SOFA score is widely used to indicate disease severity and predict mortality in sepsis30 and is regarded as the best indicator for the rapid diagnosis of sepsis.31,32 Studies have shown that the average mortality rate for sepsis will increase by 1.8% to 3.3% for each increase in the average SOFA score.2 In this study, the median SOFA score of the patients in the death group was significantly higher than that of the patients in the survival group. Consistent with the results of the preceding study, the SOFA score (AUC = 0.867, sensitivity 82.9%, specificity 75%, truncation value 5.5) predicted the 28-day prognosis of patients with higher AUC than the APACHEII score. This study confirmed that MIP-3alpha, the SOFA score, the APACHEII score, and systolic blood pressure were independent risk factors for 28-day mortality among elderly sepsis patients. Although MIP-3alpha can be used as an independent risk factor for the prognosis of patients with sepsis, it has a relatively low sensitivity (56.9%).
Numerous factors influence senile sepsis, and a single biomarker cannot accurately reflect the severity and prognosis of sepsis patients.6,12 Previous research has demonstrated that combining lactic acid and APACHEII score to assess the prognosis of sepsis patients is more effective than using a single biomarker.33 There are currently few studies on MIP-3alpha as a sepsis prognostic biomarker. This study confirmed that MIP-3alpha in conjunction with the SOFA score can better predict the prognosis of senile sepsis (Z1 = 3.733, Z2 = 2.996, both P < 0.01), AUC = 0.919. The SOFA score is simple to calculate in the clinic, and the MIP-3 alpha detection method is quick, straightforward, and simple to promote. The combination of the two tests can determine the severity of senile sepsis earlier and provide early intervention, which may significantly improve the prognosis of senile sepsis and decrease the mortality rate of severely ill patients.
However, our study has certain limitations, it was a single center retrospective study with a small sample size, and the results may be biased; Further confirmation is needed from multicenter, large sample, prospective randomized controlled studies.
Conclusion
In summary, MIP-3alpha, SOFA, APACHE II and SBP can be used as independent risk factors for 28-day mortality of senile sepsis. Compared to the test alone, the combination of MIP-3 alpha and SOFA score could better predict the 28-day mortality risk. The early detection of MIP-3 alpha and calculation of the SOFA is of great importance for assessing the condition and improving the prognosis of sepsis in elderly patients.
Acknowledgment
Funding from the Shijingshan District medical key support specialty construction foundation is gratefully acknowledged.
Disclosure
The authors declare they have no conflicts of interest in this work.
References
- 1.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer M, Gerlach H, Vogelmann T, et al. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit Care. 2020;24(1):239. doi: 10.1186/s13054-020-02950-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hausfater P, Robert Boter N, Morales Indiano C, et al. Monocyte distribution width (MDW) performance as an early sepsis indicator in the emergency department: comparison with CRP and procalcitonin in a multicenter international European prospective study. Crit Care. 2021;25(1):227. doi: 10.1186/s13054-021-03622-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong HR. Pediatric sepsis biomarkers for prognostic and predictive enrichment. Pediatr Res. 2022;91(2):283–288. doi: 10.1038/s41390-021-01620-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barichello T, Generoso JS, Singer M, et al. Biomarkers for sepsis: more than just fever and leukocytosis-a narrative review. Crit Care. 2022;26(1):14. doi: 10.1186/s13054-021-03862-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci. 2013;50(1):23–36. doi: 10.3109/10408363.2013.764490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman L, Hubert P, Caberg J-H, et al. MIP3 alpha stimulates the migration of Langerhans cells in models of human papillomavirus (HPV)-associated (pre)neoplastic epithelium. Cancer Immunol Immunother. 2007;56(7):1087–1096. doi: 10.1007/s00262-006-0255-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–e1143. doi: 10.1097/CCM.0000000000005337 [DOI] [PubMed] [Google Scholar]
- 9.Uffen JW, Oosterheert JJ, Schweitzer VA, et al. Interventions for rapid recognition and treatment of sepsis in the emergency department: a narrative review. Clin Microbiol Infect. 2021;27(2):192–203. doi: 10.1016/j.cmi.2020.02.022 [DOI] [PubMed] [Google Scholar]
- 10.Grebenchikov OA, Kuzovlev AN. Long-term outcomes after sepsis. Biochemistry. 2021;86(5):563–567. doi: 10.1134/S0006297921050059 [DOI] [PubMed] [Google Scholar]
- 11.Shimazui T, Nakada T-A, Walley KR, et al. Significance of body temperature in elderly patients with sepsis. Crit Care. 2020;24(1):387. doi: 10.1186/s13054-020-02976-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierrakos C, Velissaris D, Bisdorff M, et al. Biomarkers of sepsis: time for a reappraisal. Crit Care. 2020;24(1):287. doi: 10.1186/s13054-020-02993-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luzzani A, Polati E, Dorizzi R, et al. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 2003;31(6):1737–1741. doi: 10.1097/01.CCM.0000063440.19188.ED [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Kim DH, Jang HR, et al. Clinical relevance of procalcitonin and C-reactive protein as infection markers in renal impairment: a cross-sectional study. Crit Care. 2014;18(6):640. doi: 10.1186/s13054-014-0640-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menon K, Schlapbach LJ, Akech S, et al. Criteria for pediatric sepsis-A systematic review and meta-analysis by the pediatric sepsis definition taskforce. Crit Care Med. 2022;50(1):21–36. doi: 10.1097/CCM.0000000000005294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan YL, Liao H-C, Tsay P-K, et al. C-reactive protein as an indicator of bacterial infection of adult patients in the emergency department. Chang Gung Med J. 2002;25(7):437–445. [PubMed] [Google Scholar]
- 17.Lee AY, Phan TK, Hulett MD, et al. The relationship between CCR6 and its binding partners: does the CCR6-CCL20 axis have to be extended? Cytokine. 2015;72(1):97–101. doi: 10.1016/j.cyto.2014.11.029 [DOI] [PubMed] [Google Scholar]
- 18.Ito T, Carson WF, Cavassani KA, et al. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011;317(5):613–619. doi: 10.1016/j.yexcr.2010.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee AYS, Körner H. The CCR6-CCL20 axis in humoral immunity and T-B cell immunobiology. Immunobiology. 2019;224(3):449–454. doi: 10.1016/j.imbio.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 20.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14(5):409–426. doi: 10.1016/S1359-6101(03)00049-2 [DOI] [PubMed] [Google Scholar]
- 21.Li L, Zhou J, Xu Y, et al. C-C chemokine receptor type 6 modulates the biological function of osteoblastogenesis by altering the expression levels of Osterix and OPG/RANKL. Biosci Trends. 2021;15(4):240–248. doi: 10.5582/bst.2021.01199 [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Li Y, Wang X, et al. Synergistic association of FOXP3+ tumor infiltrating lymphocytes with CCL20 expressions with poor prognosis of primary breast cancer: a retrospective cohort study. Medicine. 2019;98(50):e18403. doi: 10.1097/MD.0000000000018403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fennen M, Weinhage T, Kracke V, et al. A myostatin-CCL20-CCR6 axis regulates Th17 cell recruitment to inflamed joints in experimental arthritis. Sci Rep. 2021;11(1):14145. doi: 10.1038/s41598-021-93599-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitagawa Y, Kikuchi S, Arita Y, et al. Inhibition of CCL20 increases mortality in models of mouse sepsis with intestinal apoptosis. Surgery. 2013;154(1):78–88. doi: 10.1016/j.surg.2013.02.012 [DOI] [PubMed] [Google Scholar]
- 25.Tack BF, Malik ZA. Structure of human MIP‐3α chemokine. Acta Crystallographica Section F. 2006;62:631–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein M, Brouwer MC, Angele B, et al. Leukocyte attraction by CCL20 and its receptor CCR6 in humans and mice with pneumococcal meningitis. PLoS One. 2014;9(4):e93057. doi: 10.1371/journal.pone.0093057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crispo M, Van Maele L, Tabareau J, et al. Transgenic mouse model harboring the transcriptional fusion ccl20-luciferase as a novel reporter of pro-inflammatory response. PLoS One. 2013;8(11):e78447. doi: 10.1371/journal.pone.0078447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammerich L, Bangen JM, Govaere O, et al. Chemokine receptor CCR6-dependent accumulation of γδ T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology. 2014;59(2):630–642. doi: 10.1002/hep.26697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klaus DA, Seemann R, Roth-Walter F, et al. Plasma levels of chemokine ligand 20 and chemokine receptor 6 in patients with sepsis: a case control study. Eur J Anaesthesiol. 2016;33(5):348–355. doi: 10.1097/EJA.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 30.Gaini S, Relster MM, Pedersen C, et al. Prediction of 28-days mortality with sequential organ failure assessment (SOFA), quick SOFA (qSOFA) and systemic inflammatory response syndrome (SIRS) - A retrospective study of medical patients with acute infectious disease. Int J Infect Dis. 2019;78:1–7. doi: 10.1016/j.ijid.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 31.Karakike E, Kyriazopoulou E, Tsangaris I, et al. The early change of SOFA score as a prognostic marker of 28-day sepsis mortality: analysis through a derivation and a validation cohort. Crit Care. 2019;23(1):387. doi: 10.1186/s13054-019-2665-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA Score, SIRS Criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290–300. doi: 10.1001/jama.2016.20328 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Wang J, Yu B-Z, et al. Predictive value of heart-type fatty acid-binding protein for mortality risk in critically ill patients. Dis Markers. 2022;2022:1720414. doi: 10.1155/2022/1720414 [DOI] [PMC free article] [PubMed] [Google Scholar]