Abstract
BACKGROUND:
Disparities in cancer incidence have not been described for urban American Indian/Alaska Native (AI/AN) populations. The purpose of the present study was to examine incidence rates (2008–2017) and trends (1999–2017) for leading cancers in urban non-Hispanic AI/AN (NH AI/AN) compared to non-Hispanic White (NHW) populations living in the same urban areas.
METHODS:
Incident cases from population-based cancer registries were linked with the Indian Health Service patient registration database for improved racial classification of NH AI/AN populations. This study was limited to counties in Urban Indian Health Organization service areas. Analyses were conducted by geographic region. Age-adjusted rates (per 100,000) and trends (joinpoint regression) were calculated for leading cancers.
RESULTS:
Rates of colorectal, liver, and kidney cancers were higher overall for urban NH AI/AN compared to urban NHW populations. By region, rates of these cancers were 10% to nearly 4 times higher in NH AI/AN compared to NHW populations. Rates for breast, prostate, and lung cancer were lower in urban NH AI/AN compared to urban NHW populations. Incidence rates for kidney, liver, pancreatic, and breast cancers increased from 2% to nearly 7% annually between 1999 to 2017 in urban NH AI/AN populations.
CONCLUSIONS:
This study presents cancer incidence rates and trends for the leading cancers among urban NH AI/AN compared to urban NHW populations for the first time, by region, in the United States. Elevated risk of certain cancers among urban NH AI/AN populations and widening cancer disparities highlight important health inequities and missed opportunities for cancer prevention in this population.
Keywords: Alaska Native, American Indian, cancer incidence, health disparity, trends, urban
INTRODUCTION
Previous studies have documented substantial disparities in cancer incidence between American Indian and Alaska Native (AI/AN) populations compared to White populations. These studies highlighted the disproportionate burden of certain cancers in AI/AN populations as well as the importance of disaggregating cancer incidence data by geography and cancer type. Largely, research has focused on cancer disparities in AI/AN populations living on or near federally recognized tribal lands, which are most often rural.1–3 Patterns in cancer incidence among urban AI/AN populations may be different. To date, there has not been a comprehensive examination of cancer incidence rates and trends for urban AI/AN populations using cancer registry data corrected for racial misclassification. Understanding these cancer disparities in urban AI/AN populations is a critical first step toward identifying the areas of cancer prevention and control of greatest importance for this population.
The purpose of the present study was to 1) characterize the leading cancer sites among urban non-Hispanic AI/AN (NH AI/AN) populations, 2) assess the trends of these cancers over time, and 3) describe the regional variation in cancer disparities between urban NH AI/AN and non-Hispanic White (NHW) populations.
MATERIALS AND METHODS
Cancer incidence data came from the National Program of Cancer Registries of the Centers for Disease Control and Prevention (CDC) and the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute.4,5 Incidence data from registries meeting rigorous quality control standards were combined to create the US Cancer Statistics AI/AN Incidence Analytic Database (USCS AIAD; the analytic database used for this study). By combining data from the 2 programs, we have 100% coverage of the US population, including AI/AN populations.
During the period covered by this study (2008–2017 for rates and 1999– 2017 for trends), tumor histology, tumor behavior, and primary cancer site were coded according to the Third Edition of the International Classification of Disease for Oncology (ICD-O-3) and classified according to SEER site categories.6
To reduce racial misclassification of AI/AN populations, cancer registry data were linked with the Indian Health Service (IHS) patient registration database using previously established and validated techniques that improve accuracy of cancer incidence estimates among AI/AN populations.1,2
Urban Indian health organizations (UIHOs) are a network of 72 independent health agencies in 133 counties that serve urban AI/AN peoples in 50 cities, also known as UIHO service areas. Many UIHOs are found in cities where AI/AN individuals both originally lived or migrated to as incentivized by the Indian Relocation Act of 1956.7 In this study, the counties containing UIHOs were grouped according to city and then geographic region and are shown in Figure 1. According to the population file used for the analytic database, approximately 35.4% of AI/AN individuals live in UIHO service areas used for this study (Fig. 1).
Figure 1.
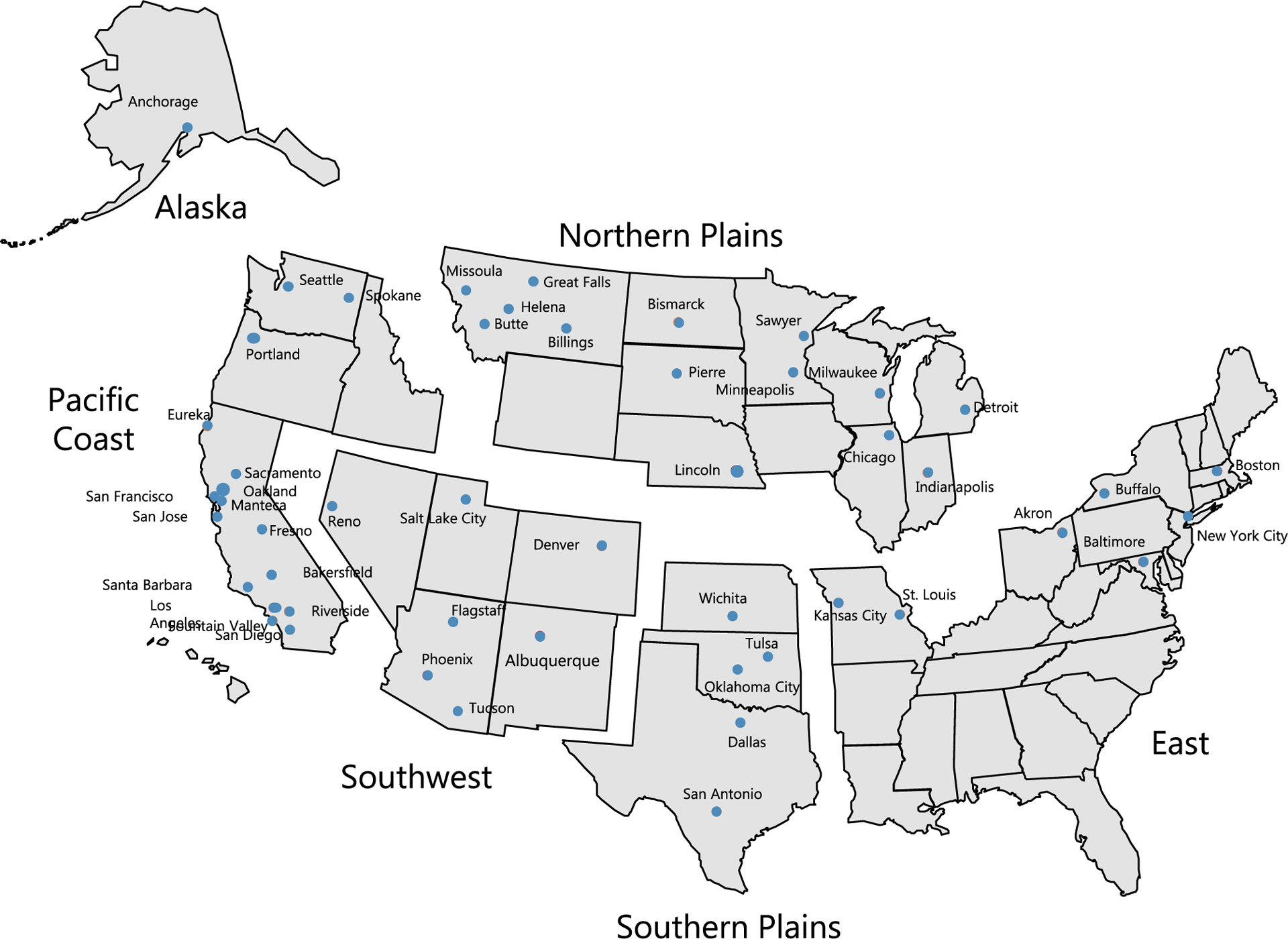
Cities containing UIHOs by geographic region. This figure presents an image of the 6 geographic regions used for this study as well as the cities within those regions containing UIHOs. UIHOs were grouped according to previously defined regions: Northern Plains (Billings, Bismarck, Butte, Chicago, Detroit, Great Falls, Helena, Indianapolis, Lincoln, Milwaukee, Minneapolis, Missoula, Pierre/Sioux Falls, and Sawyer), Alaska (Anchorage), Southern Plains (Dallas, Oklahoma City, San Antonio, Tulsa, and Wichita), Pacific Coast (Bakersfield, Eureka, Fountain Valley, Fresno, Los Angeles, Manteca, Oakland, Portland, Riverside, Sacramento, San Diego, San Francisco, San Jose, Santa Barbara, Seattle, and Spokane), East (Akron, Baltimore, Boston, Buffalo, Kansas City, New York City, and St. Louis) ,and Southwest (Albuquerque, Denver, Flagstaff, Phoenix, Reno, Salt Lake City, and Tuscon). The data include only the counties in which the 72 UIHOs are located and that have been identified as their service areas (Source: National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: US Cancer Statistics American Indian and Alaska Native Incidence Analytic Database—1998–2017. United States Department of Health and Human Services, Centers for Disease Control and Prevention. Released June 2020, based on the 2019 submission). IHS indicates Indian Health Service; UIHO, urban Indian health organization.
Population estimates used as denominators in the rate calculations are produced by the US Census Bureau. In a previous report, the updated, bridged, intercensal population estimates overestimated AI/AN populations of Hispanic origin.8 In this study, all analyses were limited to urban non-Hispanic AI/AN populations to avoid underestimation of incidence rates among AI/AN populations. Percentage of AI/AN populations that are of Hispanic origin (excluded in this study) for each region are as follows; total US = 8.9%, Northern Plains = 7.5%, Alaska = 1.0%, Southern Plains = 3.3%, Pacific Coast = 11.8%, East = 6.4%, Southwest = 15.1%. The NHW population was chosen as the reference population.
Statistical Analysis
All cancer incidence rates are expressed per 100,000 population and were directly age-adjusted using 19 age groups to the 2000 US standard population using SEER*Stat software version 8.3.2.9 Using the age-adjusted incidence rates, we calculated standardized rate ratios (RRs) for the years 2008 to 2017 for urban NH AI/AN populations using urban NHW populations as reference. RRs were rounded for presentation in the tables. Corresponding 95% confidence intervals (CIs) were calculated as modified gamma intervals based on the methods described by Tiwari et al10 to allow for the comparison of rates. The top 15 cancers were ranked according to rates for the urban NH AI/AN and urban NHW populations in the United States overall and by region. Information regarding regions and UIHO service areas are shown in Figure 1.
To determine the best fitting model for long-term trends, we evaluated trends in cancer incidence rates for the time period of 1999 to 2017. Annual percent change (APC) and average annual percent change (AAPC) were calculated using the Joinpoint Regression Program version 4.3.10.11 Joinpoint analyses with up to 4 joinpoints were allowed. Total percent change in incidence rates between 1999 and 2017 were also calculated. Two-sided P values <.05 were considered statistically significant.
RESULTS
Age-adjusted incidence rates (per 100,000) for the 15 most common cancers in urban NH AI/AN males are shown in Table 1 (95% CIs shown in Supporting Table 1). Overall, cancer incidence rates were significantly higher among urban NH AI/AN than among urban NHW males for colorectal (RR, 1.09), kidney (kidney and renal pelvis) (RR, 1.33), and liver (RR, 2.51) cancers. Colorectal cancer incidence rates were significantly higher in urban NH AI/AN than among urban NHW males in 4 of the 6 regions (RRs, 1.18–2.48), but lower in the East (RR, 0.43). Kidney cancer incidence rates were significantly higher among urban NH AI/AN males than among urban NHW males in 3 (Alaska, Southern Plains, and Southwest) of the 6 regions (RRs, 1.45–2.07). Liver cancer incidence rates (RRs, 1.93–3.49) were significantly higher among urban NH AI/AN than urban NH White males in all regions except the East. Lung cancer incidence rates were significantly higher in the Northern Plains, Alaska, and Southern Plains (RRs, 1.34–1.92) but significantly lower in the East and Southwest (RRs, 0.43, 0.68, respectively) among urban NH AI/AN males compared to NHW males.
TABLE 1.
Leading Cancer Sites for Urban Non-Hispanic American Indian/Alaska Native Males Versus Urban Non-Hispanic White Males: All Ages, UIHO Service Areas, United States, 2008–2017
| Site (in Order Based on Overall US Rank in AI/AN) | Overall US | Northern Plains | Alaska | Southern Plains | Pacific Coast | East | Southwest | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | |
| All Sites | 431.5 (508.9) | 0.85d | 538.0 (527.3) | 1.02 | 636.6 (485.1) | 1.31 | 585.4 (515.0) | 1.14d | 401.6 (504.0) | 0.80d | 225.8 (534.4) | 0.42d | 374.8 (452.9) | 0.83d | |||||||
| Prostate | 1 (1) | 82.9 (113.1) | 0.73d | 2 (1) | 103.4 (121.4) | 0.85d | 3 (1) | 90.9 (118.4) | 0.77 | 1 (1) | 121.9 (111.2) | 1.10d | 1 (1) | 73.8 (111.5) | 0.66d | 1 (1) | 56.7 (116.3) | 0.49d | 1 (1) | 66.0 (102.5) | 0.64d |
| Lung | 2 (2) | 62.1 (63.8) | 0.97 | 1 (2) | 109.8 (72.2) | 1.52d | 1 (2) | 123.0 (64.1) | 1.92d | 2 (2) | 97.7 (72.8) | 1.34d | 2 (2) | 52.4 (55.8) | 0.94 | 2 (2) | 31.0 (72.4) | 0.43d | 4 (2) | 34.6 (51.1) | 0.68d |
| Colorectal | 3 (3) | 47.4 (43.3) | 1,09d | 3 (3) | 55.8 (46.4) | 1.20d | 2 (4) | 99.2 (39.9) | 2.48d | 3 (3) | 62.2 (45.0) | 1.38d | 3 (4) | 43.4 (42.1) | 1.03 | 3 (3) | 19.3 (45.3) | 0.43d | 2 (4) | 44.5 (37.8) | 1.18d |
| Kidney | 4 (7) | 28.7 (21.5) | 1,33d | 5 (7) | 27.6 (21.8) | 1.27 | 4 (7) | 41.7 (21.3) | 1.96d | 4 (6) | 36.1 (24.9) | 1,45d | 6 (7) | 21.5 (20.5) | 1.05 | 6 (7) | 11.1 (22.9) | 0.48d | 3 (7) | 39.2 (18.9) | 2.07d |
| Liver | 5 (11) | 25.8 (10.3) | 2.51d | 4 (11) | 33.0 (9.5) | 3.49d | 8 (11) | 25.7 (11.9) | 2.15d | 7 (11) | 22.3 (11.6) | 1.93d | 4 (11) | 28.5 (10.6) | 2.70d | 5 (11) | 11.6 (11.1) | 1.05 | 5 (13) | 27.6 (8.4) | 3.27d |
| Bladder | 6 (4) | 21.9 (39.4) | 0.55d | 6 (4) | 27.5 (41.3) | 0.67d | 9 (3) | 22.3 (40.9) | 0.55d | 5 (4) | 28.3 (37.1) | 0.76d | 5 (5) | 24.4 (38.6) | 0.66d | 4 (4) | 15.7 (43) | 0.36d | 8 (5) | 13.6 (34.5) | 0.39d |
| Non-Hodgkin Lymphoma | 7 (6) | 17.2 (25.2) | 0.68d | 8 (6) | 16.2 (26.2) | 0.62d | 12 (5) | 15.5 (25.3) | 0.61 | 6 (7) | 26.9 (24.4) | 1.10 | 7 (6) | 17.7 (25.4) | 0.70d | 7 (6) | 8.0 (26.8) | 0.30d | 7 (6) | 14.3 (21.7) | 0.66d |
| Oropharyngeal | 8 (9) | 15.7 (18.3) | 0.85d | 7 (9) | 20.1 (18.6) | 1.08 | 6 (8) | 25.9 (16.5) | 1.57 | 9 (9) | 19.4 (20.2) | 0.96 | 8 (8) | 17.8 (19.0) | 0.93 | 8 (9) | 6.6 (17.9) | 0.37d | 11 (9) | 10.8 (15.6) | 0.69d |
| Leukemia | 9 (8) | 14.5 (19.0) | 0.77d | 9 (8) | 14.5 (20.0) | 0.73 | 7 (9) | 25.9 (16.5) | 1.57 | 8 (8) | 20.1 (20.5) | 0.98 | 9 (9) | 14.0 (18.5) | 0.76d | 9 (8) | 6.6 (19.7) | 0.33d | 9 (8) | 12.7 (16.6) | 0.77d |
| Pancreas | 10 (10) | 13.2 (14.7) | 0.89 | 10 (10) | 11.8 (11.5) | 0.80 | 5 (10) | 36.5 (13.4) | 2.73d | 11 (10) | 16.8 (14.2) | 1.18 | 10 (10) | 11.2 (14.3) | 0.79d | 10 (10) | 6.5 (15.9) | 0.41d | 6 (10) | 14.5 (13.4) | 1.08 |
| Melanoma | 11 (5) | 10.7 (37.4) | 0.28d | 13 (5) | 11.4 (30.4) | 0.37d | 18 (6) | ~ (23.2) | 0.08d | 10 (5) | 18.0 (30.8) | 0.59d | 11 (3) | 10.1 (45.4) | 0.22d | 11 (5) | 5.3 (32.8) | 0.16d | 12 (3) | 9.4 (40.6) | 0.23d |
| Stomach | 12 (15) | 8.7 (7.8) | 1.12 | 12 (13) | 11.7 (8.7) | 1.35 | 10 (14) | 20.6 (7.0) | 2.96d | 14 (16) | 9.1 (6.7) | 1.35 | 15 (13) | 6.2 (7.7) | 0.81 | 13 (14) | 3.9 (8.9) | 0.44d | 10 (17) | 11.3 (6.2) | 1.83d |
| Myeloma | 13 (16) | 8.1 (7.7) | 1.06 | 11 (15) | 12.3 (7.9) | 1.56d | 19 (17) | ‘ (6.1) | 0.17 | 12 (14) | 11.5 (8.4) | 1.37 | 13 (16) | 6.7 (7.5) | 0.90 | 15 (16) | 3.3 (8.1) | 0.41d | 13 (15) | 8.5 (6.8) | 1.25 |
| Esophagus | 14 (14) | 7.7 (8.2) | 0.94 | 14 (12) | 11.2 (8.9) | 1.26 | 11 (13) | 19.8 (7.4) | 2.68d | 15 (15) | 8.1 (8.3) | 0.98 | 14 (17) | 7.1 (7.4) | 0.95 | 18 (13) | 2.2 (9) | 0.25d | 14 (14) | 7.8 (7.6) | 1.02 |
| Brain and other nervous system | 15 (12) | 6.1 (8.9) | 0.69d | 15 (14) | 6.4 (8.7) | 0.73 | 14 (12) | 8.9 (9.3) | 0.96 | 13 (12) | 9.1 (9.0) | 1.00 | 16 (12) | 5.4 (9.2) | 0.59d | 12 (15) | 4.7 (8.9) | 0.53d | 16 (11) | 5.2 (8.6) | 0.61d |
Abbreviations: APC, annual percent change; AI/AN, non-Hispanic American Indians/Alaska Natives living in UIHO service areas; NPCR, National Program of Cancer Registries; RR, rate ratio; SEER, Surveillance, Epidemiology and End Results; UIHO, urban Indian health organization; W, urban non-Hispanic Whites.
Rank is based on AI/AN rates overall. White rank is shown in parentheses.
Rates are per 100,000 persons and are age-adjusted to the 2000 US standard population (19 age groups, Census P25–1130).
RRs are for AI/AN versus White and are calculated in SEER*Stat before the rounding of rates, and they may not equal RRs calculated from rates presented in the table.
RR is statistically significant (P < .05).
Source: NPCR and SEER*Stat Database: US Cancer Statistics American Indian and Alaska Native Incidence Analytic Database—1998–2017 (US Department of Health and Human Services; released June 2020 and based on the 2019 submission). the Percent regional coverage of AI/AN in UIHO counties to AI/AN in all counties was as follows: Northern Plains, 28.1%; Alaska, 23.5%; Southern Plains, 30.7%; Pacific Coast, 55.0%; Nashville, 19.3%; Southwest, 33.6%; and total, 35.4%. Comparisons for AI/AN and White populations were made for the same geographic areas. AI/AN race is reported by NPCR and SEER registries or through linkage with the Indian Health Services patient registration database. Only AI/AN of non-Hispanic origin are included.
Overall, certain cancers had significantly lower incidence rates among urban NH AI/AN males than among urban NHW males, including prostate, bladder, non-Hodgkin lymphoma, oropharyngeal (oral cavity and pharynx), leukemias, melanomas of the skin, and brain and other nervous system (RRs, 0.28–0.85) (Table 1). These findings were largely consistent across regions except in the Southern Plains where rates of prostate cancer were higher in urban NH AI/AN males (RR, 1.10).
Urban NH AI/AN females had significantly higher rates of colorectal cancer, kidney cancer, stomach cancer, cervical cancer, myeloma, and liver cancer (RRs, 1.08–2.80) (Table 2, Supporting Table 2). Colorectal cancer incidence rates were significantly higher among urban NH AI/AN females than among urban NHW females in Alaska (RR, 2.40) and the Southern Plains (RR, 1.54). Incidence rates for kidney cancer, liver cancer, and cervical cancer were nearly 1.4 to 4.3 times higher among urban NH AI/AN females than among urban NHW females in all regions except the East. Stomach cancer incidence rates (RRs, 1.79–4.46) were significantly higher among urban NH AI/AN females than among urban NHW females in 4 (Northern Plains, Southern Plains, Alaska, and Southwest) of the 6 regions.
TABLE 2.
Leading Cancer Sites for Urban Non-Hispanic American Indian/Alaska Native Females Versus Urban Non-Hispanic White Females: All Ages, UIHO Service Areas, United States, 2008–2017
| Site (in Order Based on Overall US Rank in AI/AN) | Overall US | Northern Plains | Alaska | Southern Plains | Pacific Coast | East | Southwest | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | Rank, AI/AN (W)a | AI/AN (W) Rateb | RRc | |
| All sites | 389.5 (444.9) | 0.88d | 456.5 (451.0) | 1.01 | 615.0 (431.5) | 1,43d | 528.2 (429.0) | 1.23d | 371.2 (443.4) | 0.84d | 192.4 (474.0) | 0.41d | 337.5 (404.6) | 0.83d | |||||||
| Breast | 1 (1) | 103.5 (138.2) | 0.75d | 1 (1) | 107.7 (135.1) | 0.80d | 1 (1) | 172.8 (132.4) | 1.31d | 1 (1) | 156.4 (132.5) | 1.18d | 1 (1) | 106.0 (142.6) | 0.74d | 1 (1) | 52.1 (142.9) | 0.36d | 1 (1) | 74.2 (129.6) | 0.57d |
| Lung | 2 (2) | 50.3 (53.2) | 0.94d | 2 (2) | 86.6 (58.7) | 1,48d | 2 (2) | 102.0 (51.4) | 1.98d | 2 (2) | 74.4 (53.7) | 1.39d | 2 (2) | 45.7 (48.3) | 0.95 | 2 (2) | 23.2 (61.2) | 0.38d | 4 (2) | 27.0 (43.6) | 0.62d |
| Colorectal | 3 (3) | 36.5 (33.8) | 1.08d | 3 (3) | 39.4 (35.6) | 1.11 | 3 (3) | 81.4 (33.9) | 2.40d | 3 (3) | 51.2 (33.3) | 1.54d | 3 (3) | 32.8 (33.7) | 0.97 | 3 (3) | 17.0 (34.9) | 0.49d | 2 (3) | 32.2 (29.8) | 1.08 |
| Uterine | 4 (4) | 25.1 (27.4) | 0.91d | 4 (4) | 24.2 (30.2) | 0.80d | 6 (4) | 21.1 (27.4) | 0.77 | 4 (4) | 23.2 (22.0) | 1.05 | 4 (5) | 27.0 (26.9) | 0.99 | 4 (4) | 12.9 (30.8) | 0.42d | 3 (6) | 30.0 (23.3) | 1.29d |
| Thyroid | 5 (6) | 17.5 (23.2) | 0.76d | 6 (6) | 15.9 (21.3) | 0.75d | 5 (5) | 23.0 (17.9) | 1.28 | 6 (5) | 21.8 (21.3) | 1.03 | 5 (6) | 14.3 (20.5) | 0.70d | 5 (5) | 11.2 (28.5) | 0.39d | 5 (4) | 20.2 (24.9) | 0.81d |
| Kidney | 6 (11) | 17.2 (10.6) | 1.62d | 5 (11) | 18.4 (11.0) | 1.68d | 4 (9) | 24.9 (12.3) | 2.03d | 5 (8) | 22.2 (13.1) | 1.69d | 6 (11) | 14.5 (9.5) | 1.52d | 9 (12) | 6.4 (11.0) | 0.58d | 6 (11) | 19.4 (9.5) | 2.04d |
| Non-Hodgkin lymphoma | 7 (7) | 13.5 (16.9) | 0.80d | 7 (7) | 15.8 (18.1) | 0.87 | 8 (7) | 14.6 (16.7) | 0.87 | 7 (7) | 18.8 (16.8) | 1.12 | 7 (7) | 13.8 (16.7) | 0.83 | 7 (7) | 6.9 (17.9) | 0.38d | 9 (7) | 11.0 (14.6) | 0.76d |
| Ovary | 8 (8) | 12.3 (12.5) | 0.98 | 8 (8) | 12.4 (12.6) | 0.99 | 13 (8) | 10.8 (14.0) | 0.77 | 8 (10) | 16.7 (11.9) | 1,40d | 11 (8) | 9.1 (12.6) | 0.74d | 6 (8) | 7.8 (12.8) | 0.61d | 7 (8) | 15.1 (11.6) | 1.30d |
| Liver | 9 (17) | 10.1 (3.6) | 2.80d | 13 (18) | 9.8 (3.5) | 2.79d | 9 (17) | 13.9 (3.6) | 3.84d | 13 (17) | 10.1 (4.1) | 2.45d | 12 (17) | 9.0 (3.7) | 2.45d | 13 (18) | 3.5 (3.5) | 1.00 | 8 (17) | 14.1 (3.3) | 4.31d |
| Pancreas | 10 (10) | 9.8 (11.0) | 0.89 | 9 (9) | 11.8 (11.5) | 1.03 | 7 (10) | 18.9 (11.8) | 1.59 | 10 (11) | 13.2 (10.5) | 1.26 | 13 (10) | 7.9 (10.7) | 0.74 | 11 (9) | 4.2 (11.9) | 0.35d | 10 (9) | 10.3 (10.2) | 1.01 |
| Leukemia | 11 (9) | 9.9 (11.2) | 0.88d | 12 (10) | 10.1 (11.5) | 0.87 | 16 (11) | 6.9 (11.1) | 0.62 | 9 (9) | 15.7 (12.3) | 1.28d | 8 (9) | 10.1 (10.9) | 0.92 | 12 (10) | 4.1 (11.7) | 0.35d | 11 (10) | 8.3 (10.0) | 0.83 |
| Cervix uteri | 12 (15) | 9.6 (6.4) | 1.50d | 10 (14) | 11.6 (6.5) | 1.80d | 10 (14) | 13.9 (7.0) | 1.99d | 11 (13) | 11.7 (7.7) | 1.51d | 10 (15) | 10.1 (6.4) | 1.59d | 8 (15) | 6.6 (6.1) | 1.07 | 12 (15) | 7.6 (5.6) | 1.36d |
| Melanoma | 13 (5) | 7.5 (23.8) | 0.32d | 17 (5) | 6.3 (21.3) | 0.30d | 19 (6) | 4.4 (17.6) | 0.25d | 12 (6) | 10.2 (17.8) | 0.58d | 9 (4) | 10.6 (28.2) | 0.36d | 15 (6) | 2.1 (22.4) | 0.09d | 14 (5) | 5.7 (24.2) | 0.23d |
| Myeloma | 14 (16) | 5.5 (4.6) | 1.20d | 18 (16) | 6.2 (4.7) | 1.33 | 18 (16) | ~ (5.8) | 0.78 | 14 (16) | 7.2 (5.3) | 1.35 | 14 (16) | 5.6 (4.4) | 1.26 | 17 (16) | 1.8 (4.8) | 0.37d | 15 (16) | 5.6 (4.1) | 1.36 |
| Stomach | 15 (18) | 5.4 (3.6) | 1.50d | 15 (17) | 6.9 (3.8) | 1.79d | 11 (18) | 13.5 (3.0) | 4.46d | 16 (18) | 5.5 (3.1) | 1.78d | 18 (18) | 4.1 (3.5) | 1.19 | 14 (17) | 2.2 (4.3) | 0.50 | 13 (18) | 6.6 (2.7) | 2.41d |
Abbreviations: APC, annual percent change; AI/AN, non-Hispanic American Indians/Alaska Natives living in UIHO service areas; NPCR, National Program of Cancer Registries; RR, rate ratio; SEER, Surveillance, Epidemiology and End Results; UIHO, urban Indian health organization; W, urban non-Hispanic Whites.
Rank is based on AI/AN rates overall. White rank is shown in parentheses.
Rates are per 100,000 persons and are age-adjusted to the 2000 US standard population (19 age groups, Census P25–1130).
RRs are for AI/AN versus White and are calculated in SEER*Stat before the rounding of rates, and they may not equal RRs calculated from rates presented in the table.
RR is statistically significant (P < .05).
Source: NPCR and SEER*Stat Database: US Cancer Statistics American Indian and Alaska Native Incidence Analytic Database—1998–2017 (US Department of Health and Human Services; released June 2020 and based on the 2019 submission). the Percent regional coverage of AI/AN in UIHO counties to AI/AN in all counties was as follows: Northern Plains, 28.1%; Alaska, 23.5%; Southern Plains, 30.7%; Pacific Coast, 55.0%; Nashville, 19.3%; Southwest, 33.6%; and total, 35.4%. Comparisons for AI/AN and White populations were made for the same geographic areas. AI/AN race is reported by the NPCR and SEER registries or through linkage with the Indian Health Services patient registration database. Only AI/AN of non-Hispanic origin are included.
Lung cancer incidence rates varied largely by region, with higher rates among urban NH AI/AN females than among urban NHW females in the Northern Plains, Alaska, and Southern Plains (RRs, 1.39–1.98) (Table 2) but lower rates in the East and Southwest (RRs, 0.38, 0.62, respectively). Rates of breast cancer were higher among urban NH AI/AN females than among urban NHW females only in Alaska (RR, 1.31) and the Southern Plains (RR, 1.18).
Long-term trends (APC) for leading cancer sites for urban NH AI/AN males and females are shown in Table 3 and Supporting Table 3 (AAPC). In urban NH AI/AN males, incidence rates for all cancer sites combined increased significantly between 1999 to 2015 (APC, 1.4), with nonsignificant change between 2015 to 2017 (APC, −7.4). Over the entire time period, rates in urban NH AI/AN males remained stable (AAPC, 0.4) but decreased significantly in urban NHW males (AAPC, −1.3). By site, kidney (AAPC, 2.8), liver (AAPC, 6.7), and pancreatic (AAPC, 3.3) cancer incidence rates increased significantly from 1999 to 2017 in urban NH AI/AN males (Table 3, Supporting Table 1). Trends for lung cancer increased significantly for urban NH AI/AN males until 2014 (APC, 2.2) , after which rates decreased significantly between 2014 to 2017 (APC, −13.2). Although rates of lung cancer declined in NH AI/AN males in recent years, rates were stable over the entire time period (AAPC, −0.5), but decreased significantly for urban NHW males (APC, −2.4). Colorectal cancer incidence rates plateaued in urban NH AI/AN males (AAPC, 0.4) but decreased significantly in urban NHW males (AAPC, −2.9), with the most significant changes occurring between 1999 to 2013 (APC, −3.3). Prostate cancer incidence rates decreased significantly in urban NH AI/AN in more recent years (2007–2017 APC, −5.0) and over the entire time period (AAPC, −2.0). Similar trends were seen in urban NHW (AAPC, −3.0) males.
TABLE 3.
Cancer Incidence Rate Trends With Joinpoint Analyses for Selected Cancers for Urban Non-Hispanic AI/AN Persons Versus Urban Non-Hispanic White Persons by Sex: UIHO Service Areas, United States, 1999–2017
| Trend 1 | Trend 2 | Trend 3 | Trend 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer Site | Race/Ethnicity and Sex | Years | APCa | Years | APCa | Years | APCa | Years | APCa |
| All sites | AI/AN | ||||||||
| Male | 1999–2015 | 1.4c | 2015–2017 | −7.4 | |||||
| Female | 1999–2015 | 2.1c | |||||||
| White | |||||||||
| Male | 1999–2007 | −0.4 | 2007–2017 | −1.9c | |||||
| Female | 1999–2015 | −0.1 | 2015–2017 | −1.5 | |||||
| Prostate | AI/AN | ||||||||
| Male | 1999–2007 | 1.8 | 2007–2017 | −5.0c | |||||
| White | |||||||||
| Male | 1999–2008 | −1.4c | 2008–2014 | −7.2c | 2014–2017 | 0.9 | |||
| Female breast | AI/AN | ||||||||
| Female | 1999–2017 | 1.9c | |||||||
| White | |||||||||
| Female | 1999–2004 | −2.4c | 2004–2017 | 0.3c | |||||
| Lung | AI/AN | ||||||||
| Male | 1999–2014 | 2.2c | 2014–2017 | −13.2c | |||||
| Female | 1999–2017 | 1.2c | |||||||
| White | |||||||||
| Male | 1999–2007 | −1.6c | 2007–2015 | −3.1c | |||||
| Female | 1999–2007 | 0.0 | 2007–2017 | −1.7c | |||||
| Colorectal | AI/AN | ||||||||
| Male | 1999–2017 | 0.4 | |||||||
| Female | 1999–2017 | 0.7 | |||||||
| White | |||||||||
| Male | 1999–2013 | −3.3c | 2013–2017 | −1.4 | |||||
| Female | 1999–2008 | −2.2c | 2008–2012 | −3.8c | 2012–2017 | −1.0c | |||
| Kidney | AI/AN | ||||||||
| Male | 1999–2017 | 2.8c | |||||||
| Female | 1999–2017 | 3.0c | |||||||
| White | |||||||||
| Male | 1999–2006 | 3.4c | 2006–2017 | 0.1 | |||||
| Female | 1999–2006 | 3.9c | 2006–2017 | −0.3 | |||||
| Uterine | AI/AN | ||||||||
| Female | 1999–2017 | 3.3c | |||||||
| White | |||||||||
| Female | 1999–2017 | 0.6c | |||||||
| Thyroidb | AI/AN | ||||||||
| Female | 1999–2017 | 7.5c | |||||||
| White | |||||||||
| Female | 1999–2009 | 7.9c | 2009–2012 | 2.4c | 2012–2015 | −0.8 | 2015–2017 | −7.4c | |
| Liver | AI/AN | ||||||||
| Male | 1999–2017 | 6.7c | |||||||
| Female | 1999–2017 | 4.5c | |||||||
| White | |||||||||
| Male | 1999–2009 | 4.4c | 2009–2015 | 1.6c | 2015–2017 | −2.6 | |||
| Female | 1999–2017 | 2.8c | |||||||
| Pancreas | AI/AN | ||||||||
| Male | 1999–2017 | 3.3c | |||||||
| Female | 1999–2017 | 2.3c | |||||||
| White | |||||||||
| Male | 1999–2017 | 0.9c | |||||||
| Female | 1999–2006 | 1.5c | 2006–2017 | 0.3 | |||||
| Melanoma | AI/AN | ||||||||
| Male | 1999–2017 | 2.6 | |||||||
| Female | 1999–2017 | 4.2c | |||||||
| White | |||||||||
| Male | 1999–2017 | 2.2c | |||||||
| Female | 1999–2005 | 4.1c | 2005–2012 | 0.6 | 2012–2017 | 3.4c | |||
| Stomach | AI/AN | ||||||||
| Male | 1999–2017 | −1.0 | |||||||
| Female | 1999–2017 | 2.4c | |||||||
| White | |||||||||
| Male | 1999–2008 | −2.4c | 2008–2014 | 0.0 | 2014–2017 | −5.7c | |||
| Female | 1999–2017 | −1.3c | |||||||
Abbreviations: APC, annual percent change; AI/AN, non-Hispanic American Indians/Alaska Natives living in UIHO service areas; UIHO, urban Indian health organization; White, urban non-Hispanic Whites.
Joinpoint analyses with up to 4 joinpoints are based on rates per 100,000 persons and were age-adjusted to the 2000 US standard population (11 age groups, Census P25–1130; Joinpoint Regression Program, version 4.0.1). Analyses are limited to persons of non-Hispanic origin. AI/AN race is reported through linkage with the Indian Health Service patient registration database.
Based on rates that were age-adjusted to the 2000 US standard population (11 age groups, Census P25–1130).
Thyroid cancer was not one of the 15 leading cancers for AI/AN males and thus was excluded from this analysis.
Two-sided P < .05.
Incidence rates for all cancer sites combined increased significantly in urban NH AI/AN females through 2017 (APC, 2.1) but remained stable for NHW females (Table 3, Supporting Table 3). By site, breast, lung, kidney, corpus uteri (uterine), thyroid, liver, pancreas, melanoma, and stomach (AAPCs, 1.2–7.5) incidence rates increased significantly from 1999 to 2017 in urban NH AI/AN females. No cancers decreased significantly for urban NH AI/AN females between 1999 to 2017, whereas several cancers have decreased in urban NHW females, especially in recent years (Table 3).
The total percent change from 1999 to 2017 of incidence rates for leading cancers is shown in Figure 2. Incidence rate increases for these cancers were larger among urban NH AI/AN compared to urban NHW males: 53% versus 30% for kidney cancer, 194% versus 58% for liver cancer, 98% versus 54% for melanoma, and 26% versus 18% for pancreatic cancer (Fig. 2A, Supporting Table 4). Stomach cancer incidence rates increased for urban NH AI/AN males (29%) but decreased for urban NHW males (32%). Prostate cancer incidence rates among urban NH AI/AN males decreased by 29% compared to 42% among urban NHW males. In NH AI/AN females, breast and stomach cancer incidence rates increased by 36% and 76%, respectively, but decreased by 7% and 22%, respectively, in urban NHW females (Fig. 2B, Supporting Table 4). Incidence rate increases were larger among urban NH AI/AN than among urban NHW females for kidney, uterine, pancreas, melanoma, and thyroid cancer.
Figure 2.

Total percent changes in cancer incidence rates for urban non-Hispanic American Indian/Alaska Native persons versus urban non-Hispanic White persons by sex in UIHO service areas in the United States from 1999 to 2017: (A) total percent change for males and (B) total percent change for females. Rates were adjusted to the US Standard population (11 age groups; census P25–1130) have been used. Analyses are limited to persons of non-Hispanic origin. AI/AN race is reported through linkage with the Indian Health Service patient registration database. AI/AN indicates non-Hispanic American Indians/Alaska Natives living in UIHO service areas; UIHO, urban Indian health organization; White, urban non-Hispanic Whites.
DISCUSSION
Many previous studies focusing on AI/AN cancer disparities have not specifically described these disparities in urban AI/AN populations. Nearly 34% of AI/AN populations live in urban areas covered by our national analytic data set, highlighting the importance of addressing cancer disparities in urban AI/AN populations. National data sets are often challenged by small sample sizes, racial misclassification, and lack of specificity for urban AI/AN populations.12,13 The present study provides a comprehensive overview of cancer incidence rates and trends in urban NH AI/AN populations, using a unique database that has been linked with IHS records for the improvement of racial misclassification. This study also reveals distinct regional variation in cancer incidence rates for urban NH AI/AN populations consistent with prior findings in NH AI/AN populations living in purchased/referred care delivery area (PRCDA) counties.3,14 Additionally, this study reveals a disproportionate burden of certain cancers such as liver, kidney, colorectal, and cervical cancers for urban AI/AN populations in many regions, as well as significantly lower incidence rates of other cancers, including breast, prostate, and lung cancers.
The key findings of the present study are as follows: 1) the 3 leading cancers in urban NH AI/AN males and females are consistent, in most regions, with the 3 leading cancers in urban NHW populations; 2) disparities exist among urban NH AI/AN populations by region and between urban NH AI/AN and urban NHW populations for certain cancers, including liver, kidney, colorectal, stomach (female), and cervical; and 3) incidence rates for certain cancers among urban NH AI/AN populations have increased significantly and more rapidly than for urban NHW populations over the last 18 years, indicating growing disparities in cancer incidence rates for urban NH AI/AN populations. These findings provide further evidence that data aggregated across cancer sites and geographic region could mask important disparities in cancer incidence rates and trends for this highly heterogenous population.
Among urban NH AI/AN populations overall, rates of breast, prostate, and lung cancers were equal to or lower than among urban NHW populations, whereas rates of colorectal and cervical cancers were higher. Variation in the rates of these screening amenable cancers suggest regional and racial disparities that could be driven by differential access to cancer screening services. Although studies of cancer incidence are lacking for this population, previous studies of cancer-related outcomes in urban AI/AN populations suggest that geographic location, access and availability of cancer screening services, and use of cancer screening are interrelated.15
A prior study found that AI/AN women treated at an urban-based facility presented with later breast cancer stage, underused breast cancer screening, and experienced greater delays to treatment compared to other racial/ethnic subgroups.16 Poorer prostate cancer-related outcomes have been linked with lower screening use and inequities in access to care in certain AI/AN populations.17 Colorectal cancer screening rates also vary substantially by geographic region and are potentially impacted by proximity to IHS facilities.18 Our study is limited to counties that are served by UIHOs, and we were not able to evaluate barriers in access to care for urban AI/AN populations. It is possible that AI/AN populations residing in urban areas not served by UIHOs may have different challenges in accessing care and preventive health services. Future studies could potentially determine if lower incidence rates for screening amenable cancers in certain regions are in part being driven by barriers in access to care and preventive services for urban AI/AN populations.
Existing cancer control programs promote cancer awareness, prevention, and surveillance activities for AI/AN populations across the United States, aimed at targeting the observed disparities in screening amenable cancers highlighted in this study. Nationally, the IHS provides direct clinical and preventive services through its network of direct services clinics and IHS funded self-governance tribal health facilities. The CDC’s National Breast and Cervical Cancer Early Detection Program provides breast cancer screening for 33% of eligible AI/AN women.19 Other collaborations with tribal organizations in Alaska, Minnesota, Arizona, New Mexico, and various health facilities in the Northern Plains have also been established to improve colorectal cancer screening, implement screening navigator services, and develop patient educational resources to increase awareness of colorectal cancer screening.20–22
The CDC has also funded organizations such as the American Indian Cancer Foundation (AICAF) to support Urban Indian Health Programs nationally through screening, navigation, and evidence-based interventions (EBIs).23 These EBIs have been centrally focused on 5 specific topics—human papillomavirus, colorectal cancer, hepatitis C, tobacco cessation, and survivorship.23 Through this, the AICAF has created the first national cancer plan focusing on urban Indian communities that aims to reduce cancer burdens in Indigenous populations and promote health and well-being throughout Indian country.24 These programs are examples of ongoing efforts to address disparities in cancer-related risk factors and cancer care for urban AI/AN populations. Continuation and expansion of these types of efforts may play an important role in addressing health inequities for this population.
In addition to screening, observed geographic variation in cancer incidence rates could be affected by differences in the characteristics of the urban environments of AI/AN populations. Previous studies have shown higher prevalence of several cancer-related risk factors among AI/ AN populations compared to other racial/ethnic populations.25–28 The most recent age-adjusted prevalence data from the Behavioral Risk Factor Surveillance System indicates that 37.1% of AI/AN adults report obese body mass index compared to 30.8% in White populations.29 Tobacco use is also higher in AI/AN (49.4%) compared to White populations (42.3%).29 Similar differences are observed in prevalence of physical activity and fruit and vegetable consumption.29 The prevalence of these risk factors has increased over the previous decade,30 suggesting an increase in the number of AI/AN persons with comorbidities such as diabetes and obesity that put them at increased risk for various cancers.
Our data show that lung cancer is a leading cancer for urban NH AI/AN male and females in the Northern Plains, Southern Plains, and Alaska, with rates increasing for females. Despite ongoing efforts to reduce the use of commercial tobacco, the prevalence of cigarette smoking in AI/AN populations remains higher than all other racial/ethnic subgroups in the United States.31 Smoking prevalence also varies by geographic region28,31 and is higher in regions with the highest lung cancer mortality rates.32 In 2006, a new Government Performance and Results Act measure was established to track tobacco cessation service delivery among current smokers within the IHS and other tribal programs. This measure has been improving progressively each year (increasing from 12% to 50% between 2006 and 2016).17 Given the link between smoking and numerous cancers,33 the development of novel and culturally relevant tobacco control strategies continues to be an important aspect of cancer prevention in urban AI/AN populations.31
Liver cancer incidence rates are between 2.5 and 4.0 times higher among urban NH AI/AN than among urban NHW populations by region, and incidence rates of liver cancer have increased dramatically over the last 2 decades. Because of these growing disparities, the prevalence of viral hepatitis is an increasingly important risk factor to characterize in urban AI/AN populations.14,34 Previous studies have shown that compared to the national average, AI/AN persons have nearly a 2-fold higher rate of acute HCV incidence and HCV-associated mortality.34,35 Currently, there are efforts to increase screening for HCV among AI/AN populations in the Southern Plains through the Cherokee Nation Health Services. A clinical decision support tool implemented via health records for primary care physicians has led to an increase in the number of eligible individuals receiving HCV screening and treatment.36 Other similar efforts could potentially be initiated in urban areas to address HCV and other liver cancer risk factors for urban AI/AN populations.
Thyroid cancer incidence rates increased significantly for NH AI/AN females through 2012 and saw the largest percent change in incidence rates of any cancer between 1999 and 2017. Other authors have suggested that enhanced detection may contribute to the observed trends,37 as well as variations in thyroid cancer reporting38 and classification changes.39 Further research could help inform our understanding of the observed increase in incidence for this population and help inform interventions for thyroid cancer.
Other disparities in health-related risk factors, although unlikely to explain all the variation in other high incidence cancers such as kidney cancer, could potentially play a role in growing cancer disparities.40,41 Ongoing efforts to address the prevalence of chronic diseases and their risk factors include partnerships developed through the CDC’s National Center for Chronic Disease Prevention and Health Promotion’s “Good Health and Wellness in Indian Country” (GHWIC). The goal of GHWIC is to support coordinated, holistic approaches to healthy living and chronic disease prevention for tribes and UIHOs aimed at preventing obesity, commercial tobacco use, diabetes, heart disease and stroke. These novel programs have the potential to reduce cancer incidence rates through the reduction of related chronic disease risk factors. However, future efforts could aim to better understand and characterize impact of cancer risk factors across the lifespan for urban AI/AN populations.
This study the first comprehensive overview of cancer incidence rates and trends among urban NH AI/AN populations using the USCS AIAD covering the entire population. However, this study is subject to limitations. First, although racial misclassification was addressed, the problem may persist in the database, and could differentially impact AI/AN populations by region. Additionally, the linkages only address racial misclassification for persons who have previously accessed services through the Indian Health Services, thus AI/AN persons who are not members of federally recognized tribes are not included. These data were analyzed by geographic region, similar to previous studies.1–3,14 These geographic regions were based on previous work in AI/AN populations living in PRCDA counties. Many of these regions cover broad geographic areas and include heterogenous populations. The variation in social, ecological, and economic factors impacting cancer risk may be masked when combining smaller geographic areas into larger regions. Additionally, AI/AN individuals living outside these counties may not be well represented within the data. Although these regions may not be as informative for urban AI/AN populations, the states with the largest AI/AN populations42 are represented in this study and may improve the generaliz-ability of these results. These data do not include Hispanic populations and their exclusion could differentially impact cancer incidence rates in specific regions. Finally, this study uses data from the central cancer registries, which do not collect data on risk factors and social determinants of health that have been associated with cancer incidence.
Elevated incidence of certain cancers and widening cancer disparities in urban NH AI/AN compared to urban NHW populations highlight important health inequities. By comprehensively describing ongoing cancer disparities between urban NH AI/AN and NHW populations, we have identified several areas of focus for public health. Rates of certain cancers, including liver, kidney, and colorectal cancers have increased significantly over the past 2 decades and continue to widen the racial and geographic disparities for this population. Continued collaborations between public health and clinical care communities, health policy advocates, tribes and tribal organizations, and UIHOs are needed. These partnerships can aid in the development, initiation, and strengthening of culturally appropriate programs, policies and interventions to reduce cancer disparities and improve health in urban AI/AN populations.
Supplementary Material
FUNDING SUPPORT
This work was supported by the Centers for Disease Control and Prevention.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Additional supporting information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Espey DK, Wiggins CL, Jim MA, Miller BA, Johnson CJ, Becker TM. Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer. 2008;113(suppl 5):1120–1130. doi: 10.1002/cncr.23724 [DOI] [PubMed] [Google Scholar]
- 2.Jim MA, Arias E, Seneca DS, et al. Racial misclassification of American Indians and Alaska Natives by Indian Health Service Contract Health Service Delivery Area. Am J Public Health. 2014;104(suppl 3):S295–S302. doi: 10.2105/AJPH.2014.301933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melkonian SC, Jim MA, Haverkamp D, et al. Disparities in cancer incidence and trends among American Indians and Alaska Natives in the United States, 2010–2015. Cancer Epidemiol Biomarkers Prev. 2019;28:1604–1611. doi: 10.1158/1055-9965.EPI-19-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hankey BF, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–1121. [PubMed] [Google Scholar]
- 5.Thoburn KK, German RR, Lewis M, Nichols PJ, Ahmed F, Jackson-Thompson J. Case completeness and data accuracy in the Centers for Disease Control and Prevention’s National Program of Cancer Registries. Cancer. 2007;109:1607–1616. doi: 10.1002/cncr.22566 [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Site Recode. Accessed March 1, 2020. https://seer.cancer.gov/siterecode/ [Google Scholar]
- 7.Straus T, Valentino D. Retribalization in urban Indian communities. Am Indian Cult Res J. 1998;22:103–115. [Google Scholar]
- 8.Arias E, Heron M, National Center for Health Statistics, Hakes J, US Census Bureau. The validity of race and Hispanic-origin reporting on death certificates in the United States: an update. Vital Health Stat. 2016;2:1–21. [PubMed] [Google Scholar]
- 9.Surveillance Research Program, National Cancer Institute. SEER*Stat Software, latest release: 8.3.2. Accessed January 2020. http://seer.cancer.gov/seerstat
- 10.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–569. doi: 10.1177/0962280206070621 [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. Joinpoint regression program, version 4.3.1.0 2016. Accessed January 1, 2020. http://surveillance.cancer.gov/joinpoint
- 12.Urban Indian Health Commission (2007). Invisible tribes: urban Indians and their health in a changing world. Accessed November 27, 2011. http://www.uihi.org/wp-content/uploads/
- 13.Rutman S, Taualii M, Ned D, Tetrick C. Reproductive health and sexual violence among urban American Indian and Alaska Native young women: select findings from the National Survey of Family Growth (2002). Matern Child Health J. 2012;16(suppl 2):347–352. doi: 10.1007/s10995-012-1100-1 [DOI] [PubMed] [Google Scholar]
- 14.Melkonian SC, Weir HK, Jim MA, Preikschat B, Haverkamp D, White MC. Incidence of and trends in the leading cancers with elevated incidence among American Indian and Alaska Native populations, 2012–2016. Am J Epidemiol. 2021;190:528–538. doi: 10.1093/aje/kwaa222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Towne SD Jr, Smith ML, Ory MG. Geographic variations in access and utilization of cancer screening services: examining disparities among American Indian and Alaska Native Elders. Int J Health Geogr. 2014;13:18. doi: 10.1186/1476-072X-13-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tillman L, Myers S, Pockaj B, Perry C, Bay RC, Al-kasspooles M. Breast cancer in Native American women treated at an urban-based Indian health referral center 1982–2003. Am J Surg. 2005;190:895–902. doi: 10.1016/j.amjsurg.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 17.Emerson MA, Banegas MP, Chawla N, et al. Disparities in prostate, lung, breast, and colorectal cancer survival and comorbidity status among urban American Indians and Alaskan Natives. Cancer Res. 2017;77:6770–6776. doi: 10.1158/0008-5472.CAN-17-0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacher MC, Slattery ML, Lanier AP, et al. Prevalence and predictors of cancer screening among American Indian and Alaska native people: the EARTH study. Cancer Causes Control. 2008;19:725–737. doi: 10.1007/s10552-008-9135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard DH, Tangka FK, Royalty J, et al. Breast cancer screening of underserved women in the USA: results from the National Breast and Cervical Cancer Early Detection Program, 1998–2012. Cancer Causes Control. 2015;26:657–668. doi: 10.1007/s10552-015-0553-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haverkamp D, English K, Jacobs-Wingo J, Tjemsland A, Espey D. Effectiveness of interventions to increase colorectal cancer screening among American Indians and Alaska Natives. Prev Chronic Dis. 2020;17:E62. doi: 10.5888/pcd17.200049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadeau M, Walaszek A, Perdue DG, Rhodes KL, Haverkamp D, Forster J. Influences and practices in colorectal cancer screening among health care providers serving Northern Plains American Indians, 2011–2012. Prev Chronic Dis. 2016;13:E167. doi: 10.5888/pcd13.160267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redwood D, Provost E, Perdue D, Haverkamp D, Espey D. The last frontier: innovative efforts to reduce colorectal cancer disparities among the remote Alaska Native population. Gastrointest Endosc. 2012;75:474–480. doi: 10.1016/j.gie.2011.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Indian Cancer Foundation. Urban cancer solutions. Accessed January 2021. https://www.americanindiancancer.org/urban-cancer-solutions/
- 24.American Indian Cancer Foundation. Urban cancer solutions. Comprehensive Cancer Control Plan. Accessed January 2021. https://www.americanindiancancer.org/wp-content/uploads/2020/07/Urban-Cancer-Solutions-Cancer-Plan_−2020-2022-Version_−6_16_2020.pdf
- 25.Jernigan VB, Duran B, Ahn D, Winkleby M. Changing patterns in health behaviors and risk factors related to cardiovascular disease among American Indians and Alaska Natives. Am J Public Health. 2010;100:677–683. doi: 10.2105/AJPH.2009.164285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adakai M, Sandoval-Rosario M, Xu F, et al. Health disparities among American Indians/Alaska Natives - Arizona, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1314–1318. doi: 10.15585/mmwr.mm6747a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson JS, Nobmann ED, Asay E, Lanier AP. Dietary intake of Alaska Native people in two regions and implications for health: the Alaska Native Dietary and Subsistence Food Assessment Project. Int J Circumpolar Health. 2009;68:109–122. doi: 10.3402/ijch.v68i2.18320 [DOI] [PubMed] [Google Scholar]
- 28.Cobb N, Espey D, King J. Health behaviors and risk factors among American Indians and Alaska Natives, 2000–2010. Am J Public Health. 2014;104(suppl 3):S481–S489. doi: 10.2105/AJPH.2014.301879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool, Based on 2020 Submission Data (1999–2018). US Department of Health and Human Services; 2021. [Google Scholar]
- 30.Breathett K, Sims M, Gross M, et al. Cardiovascular health in American Indians and Alaska Natives: a scientific statement from the American Heart Association. Circulation. 2020;141:e948–e959. doi: 10.1161/CIR.0000000000000773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odani S, Armour BS, Graffunder CM, Garrett BE, Agaku IT. Prevalence and disparities in tobacco product use among American Indians/Alaska Natives - United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2017;66:1374–1378. doi: 10.15585/mmwr.mm6650a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plescia M, Henley SJ, Pate A, Underwood JM, Rhodes K. Lung cancer deaths among American Indians and Alaska Natives, 1990–2009. Am J Public Health. 2014;104(suppl 3):S388–S395. doi: 10.2105/AJPH.2013.301609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang SS. Re: smoking cessation: a report of the Surgeon General. J Urol. 2020;204:384. doi: 10.1097/JU.0000000000001114 [DOI] [PubMed] [Google Scholar]
- 34.Hatcher SM, Joshi S, Robinson BF, Weiser T. Hepatitis C-related mortality among American Indian/Alaska Native persons in the Northwestern United States, 2006–2012. Public Health Rep. 2020;135: 66–73. doi: 10.1177/0033354919887748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rempel JD, Uhanova J. Hepatitis C virus in American Indian/Alaskan Native and Aboriginal peoples of North America. Viruses. 2012;4:3912–3931. doi: 10.3390/v4123912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momin B, Mera J, Essex W, et al. Implementation of liver cancer education among health care providers and community coalitions in the Cherokee Nation. Prev Chronic Dis. 2019;16:E112. doi: 10.5888/pcd16.180671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li N, Du XL, Reitzel LR, Xu L, Sturgis EM. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the Surveillance, Epidemiology, and End Results registry, 1980–2008. Thyroid. 2013;23:103–110. doi: 10.1089/thy.2012.0392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitahara CM, Sosa JA, Shiels MS. Influence of nomenclature changes on trends in papillary thyroid cancer incidence in the United States, 2000 to 2017. J Clin Endocrinol Metab. 2020;105:e4823–e4830. doi: 10.1210/clinem/dgaa690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paja M, Zafon C, Iglesias C, et al. Rate of non-invasive follicular thyroid neoplasms with papillary-like nuclear features depends on pathologist’s criteria: a multicenter retrospective Southern European study with prolonged follow-up. Endocrine. 2021;73:131–140. doi: 10.1007/s12020-021-02610-7 [DOI] [PubMed] [Google Scholar]
- 40.Sanfilippo KM, McTigue KM, Fidler CJ, et al. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension. 2014;63:934–941. doi: 10.1161/HYPERTENSIONAHA.113.02953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow WH, Gridley G, Fraumeni JF Jr, Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med. 2000;343:1305–1311. doi: 10.1056/NEJM200011023431804 [DOI] [PubMed] [Google Scholar]
- 42.Norris T, Vines PL, Hoeffel EM. The American Indian and Alaska Native Population: 2010. US Census Bureau; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


