Abstract
OBJECTIVE:
Examine physical function and T-cell phenotype in patients with chronic lymphocytic leukemia (CLL) before and after a physical activity (PA) intervention.
METHODS:
Physical function measures and blood samples were collected from CLL patients (Rai stage 0–4, 50% receiving targeted therapy, N=24) enrolled in a 16-week intervention of at-home aerobic and/or resistance exercise. Flow cytometry characterized T-cells in cryopreserved peripheral blood cells. Wilcoxon signed-rank test compared physical function and T-cell phenotype at baseline and 16-weeks; Kendall’s Tau assessed associations between variables.
RESULTS:
Godin leisure-time PA score increased from baseline to 16-weeks (mean difference: 14.61, p<0.01) and fatigue decreased (mean difference: 6.71, p<0.001). At baseline, lower fatigue correlated with a lower proportion of CD8+T-cells (τ= 0.32, p=0.03) and cardiorespiratory fitness (CRF) inversely correlated with the percentage of PD-1+CD8+T-cells (τ −0.31, p=0.03). At 16-weeks, CRF inversely correlated with the proportion of PD-1+CD4+T-cells (τ −0.34, p=0.02). Reduced fatigue at 16-weeks correlated with an increased CD4:CD8 ratio (τ=0.36, p=0.02) and lower percentage of HLA-DR+PD-1+ CD4+ T-cells (τ =−0.37, p=0.01).
CONCLUSIONS:
This intervention increased leisure-time PA and decreased fatigue in CLL patients. These changes correlated with an increased CD4:CD8 T-cell ratio and reduced proportion of T-cells subsets previously associated with poor outcomes in CLL patients.
Trial registration:
ClinicalTrials.gov Identifier: NCT02194387
Keywords: cancer, lymphoma, T cell, immune, exercise, physical activity, fatigue
1. Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent form of adult leukemia in the United States, accounting for approximately one-third of the over 60,000 new annual leukemia cases (1). Although the disease affects mature B-lymphocytes, patients with CLL often exhibit altered T-cell phenotypes (2). Compared to healthy individuals, patients with CLL have an increased frequency of CD4+ T-cells with an antigen-experienced phenotype, especially cells expressing the activation marker human leukocyte antigen (HLA)-DR, as well as cells expressing the inhibitory receptor programmed cell death protein 1 (PD-1) (2). Such HLA-DR+PD-1+CD4+ T-cells have also been described in patients infected with human immunodeficiency virus (HIV) (3). In patients with CLL, the frequency of HLA-DR+PD-1+CD4+ T-cells is negatively associated with progression-free survival (4). Cytotoxic CD8+T-cells are also affected by CLL. These cells have an important anti-tumor role, but CD8+ T-cells in CLL patients often exhibit diminished cytotoxic function (2). An inverted CD4:CD8 T-cell ratio arising from an expansion of the CD8+ T-cell population is associated with faster rates of disease progression and decreased overall survival (4).
Healthy diet and regular physical activity are recommended for all adults, including patients with cancer, and positively associated with physical fitness and physical function (5). CLL patients with higher physical fitness and greater levels of physical activity (PA) have been shown to have fewer treatment side effects and slower in vitro tumor expansion (6). Patients with CLL experience no survival benefit from early treatment and thus undergo extended periods of post-diagnosis observation before treatment is warranted (7–9). During this time, improving physical fitness and function through improved diet and increased PA may be means to slow disease progression and increase time to first treatment. Whether increases in PA or physical fitness impact T-cell phenotype in patients with CLL is unknown. However, PA has been shown to influence immune markers in other cancer populations. A meta-analysis of 26 exercise interventions reported decreased pro-inflammatory mediators in post-intervention cancer survivors, with the largest effect sizes detected when aerobic and resistance training were implemented concurrently (10). In another example, total lymphocytes and effector memory CD8+ T-cells were found to increase in women at-risk of breast cancer after 12 weeks of moderate intensity aerobic training (11). Recent studies have established a relationship between PA and some aspects of immune function in patients with CLL. Higher physical fitness is associated with reduced chronic inflammation in patients with CLL (6). Further, 12 weeks of high-intensity interval training (HIIT) with muscle endurance-based resistance training in treatment-naïve adults with CLL resulted in improved immune function, as represented by increased NK-cell cytotoxicity (12). Although the researchers noted a small decrease in the proportion of CD4+ T-cells and a moderate increase in the proportion CD8+ T-cells, subsets of T-cells associated with CLL disease progression were not considered. Additionally, this study excluded patients exhibiting comorbidities. The presence of comorbidities is common in the CLL population. Patients exhibit a median of two comorbidities and 46% have one or more major comorbidities such as coronary artery disease, diabetes mellitus, respiratory disease, or second malignancy (13). Patients with two or more comorbidities have reduced overall and progression-free survival (14–17). Due to the prevalence and meaningful impact of comorbidities, it is warranted to examine the effects of PA in a CLL population which includes such factors.
The present investigation is a sub-study of a single arm 16-week feasibility trial that provided a PA and dietary counseling intervention in patients with CLL (HEALTH4CLL). Patients across Rai stage 0–4 CLL were included, as were patients receiving targeted therapies. The aim of the current study was to investigate the relationship of T-cells and physical function parameters before and after the intervention. We hypothesized that there would be an inverse relationship between physical function and T-cells, such that CLL patients with lower physical function would have a greater proportion of higher differentiated and activated T-cells and a lower CD4:CD8 ratio. We also hypothesized that improvements in physical function over the course of the intervention would correlate with a decrease in higher differentiated and activated T-cells and an increase in CD4:CD8 ratio. If this hypothesis is supported, our results would suggest additional benefit of PA interventions beyond improvement in physical function.
2. Materials and Methods
2.1. Participants
The current investigation included 24 (8 male, 16 female) patients with CLL recruited from the Leukemia Center at MD Anderson Cancer Center. These were a subset of participants from a larger trial testing the efficacy of a lifestyle intervention for patients with CLL (enrolled N=37, completed N=31) (ClinicalTrials.gov Identifier: NCT02194387). The subset included all participants from whom cryopreserved peripheral blood mononuclear cells (PBMCs) were available at baseline and at the end of the 16-week intervention. Recruitment was conducted in-person at the MD Anderson Leukemia Center. Interested patients were screened via Physical Activity Readiness Questionnaire (PARQ) (18). This study was approved by the Institutional Review Board at MD Anderson cancer center and was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The patients provided their written informed consent to participate in this study.
To qualify for inclusion, patients were required to meet the following criteria: 1) patients had a confirmed diagnosis of CLL; 2) body mass index (BMI) ≥25 or completion of <150 minutes of moderate or vigorous exercise per week or <5 servings of fruit and vegetables per day; (3) able to read and write English; (4) 18 years of age or older; (5) have a cellular telephone and are able and willing to send and receive text messages; (6) have access to internet; (7) capable of participating in moderate-vigorous unsupervised exercise; and (8) experienced fatigue within the last seven days. Exclusion criteria included: (1) inability to walk without crutches, walker, cane, or other assistive device; (2) pregnancy (by self-report); (3) currently receiving radiation therapy or chemotherapy (targeted therapies were allowed); (4) surgery in the previous 3 months.
2.2. Experimental design and intervention
The primary aim of the parent HEALTH4CLL study was to explore the feasibility of using the Multiphase Optimization Strategy (MOST) approach to optimize behavioral interventions in CLL patients. The parent study also aimed to develop preliminary estimates of effect size and outcome variability in fatigue, readiness to change, beliefs related to diet, physical activity, weight management, and behavioral changes. The HEALH4CLL study provided weekly educational materials about exercise, diet, and weight management to all participants.
Participants were randomly assigned to one of 16 combinations of intervention components based on four categories: 1) aerobic exercise only (AE) or aerobic exercise with resistance training (AERT); 2) telephone coaching vs. no telephone coaching; 3) text messages vs. no text messages; 4) self-monitoring 4 – 7 days/week vs. 1 day/week. All participants were assigned an online account to self-report diet and weight. Participants in AE were instructed to increase their step count and PA levels. In the current study, 15 participants were in AE. Participants in AERT were instructed to increase their step count and PA and also issued resistance bands and instructional videos on how to properly perform resistance band exercises. In the current study, nine participants were in AERT. Resistance training was monitored via weekly self-reported training logs.
2.3. Physical function data collection
Physical function data and blood samples were collected on-site (MD Anderson Cancer Center, Houston TX) at baseline and 16 weeks. During both visits, height, weight, waist and hip circumference, and blood pressure were measured. Participants also completed a series of physical fitness assessments including: six-minute walk test, 30-second sit-to-stand test, 8-foot timed up-and-go test, 30-second arm curls for maximum repetitions on both arms, and handgrip strength via hydraulic grip dynamometer (reported as the higher score between hands).
Measurements of height, weight, and hip/waist circumference as well as perception of well-being were self-reported and measured by the participants at home after instruction. Questionnaires were completed online at baseline, 8-weeks, and at 16-weeks. Participants also had the option of completing paper questionnaires and returning by mail. Measures reported in the current study include: weight via personal scale measured after an overnight fast, leisure-time physical activity via The Godin Leisure Time Physical Activity Questionnaire, physical function via the Patient-Reported Outcome Measurement Information System-Physical Functioning (PROMIS PF) and fatigue via the Functional Assessment of Cancer Therapy-Fatigue (FACT-F) subscale (19–21).
The Godin Leisure Time Physical Activity Questionnaire has been used extensively in research with cancer survivors, has good test-retest reliability (.81 for total score) and has significant correlations with VO2max (20). The PROMIS-PF 10-Item Short Form questionnaire was used to assess participants’ physical capabilities ranging from activities of daily living to vigorous activity (21). The FACT-F Fatigue subscale is a 13-item widely used measure in research that has a clinically relevant change score (22).
2.4. Chart review (comorbidities and treatment)
Electronic medical records were reviewed in their entirety to identify medical comorbidities, concomitant medications, CLL directed therapies and disease characteristics.
2.5. PBMC isolation and phenotyping
At baseline and at the end of the 16-week intervention, peripheral blood samples were collected by venipuncture following a 12-hour fast and transferred to the Laboratory of Integrated Physiology at the University of Houston for immediate processing. PBMCs were isolated from an 8.5 ml collection tube treated with acid citrate dextrose (ACD) by density gradient centrifugation (Histopaque-1077, Millipore Sigma), cryopreserved, and stored at −80°C. For phenotyping, cells were quickly thawed in a 37° water bath, washed in PBS, resuspended in RPMI with 10% FBS and incubated two hours at 37° C with 5% CO2. Cells were washed in PBS and stained with Vioblue-conjugated anti-HLA-DR (mouse IgG2aκ, clone AC122), Viogreen-conjugated anti-CD3 (IgG1, clone REA613), FITC-conjugated anti-CD8 (mouse IgG2aκ, clone BW135/80), PE-Vio770-conjugated anti-CD4 (mouse IgG2aκ, clone VIT4), and APC-conjugated anti-PD-1 (mouse IgG2bκ, clone PD-1.3.1.3) (all antibodies purchased from Miltenyi Biotec Inc.). Antibodies were previously titrated to determine optimal dilutions. Propidium iodide was added to each sample immediately prior to analysis by flow cytometry (MACSQuant 10, Miltenyi Biotec Inc.) to identify dead cells. Compensation beads were used to compensate for spectral overlap. CD4+ and CD8+ T-cell subsets defined by HLA-DR and PD-1 expression were identified within viable CD3+ lymphocytes. Florescence minus one (FMO) controls were used for gating. Representative plots demonstrating the gating strategy are provided in Figure 1.
Figure 1.
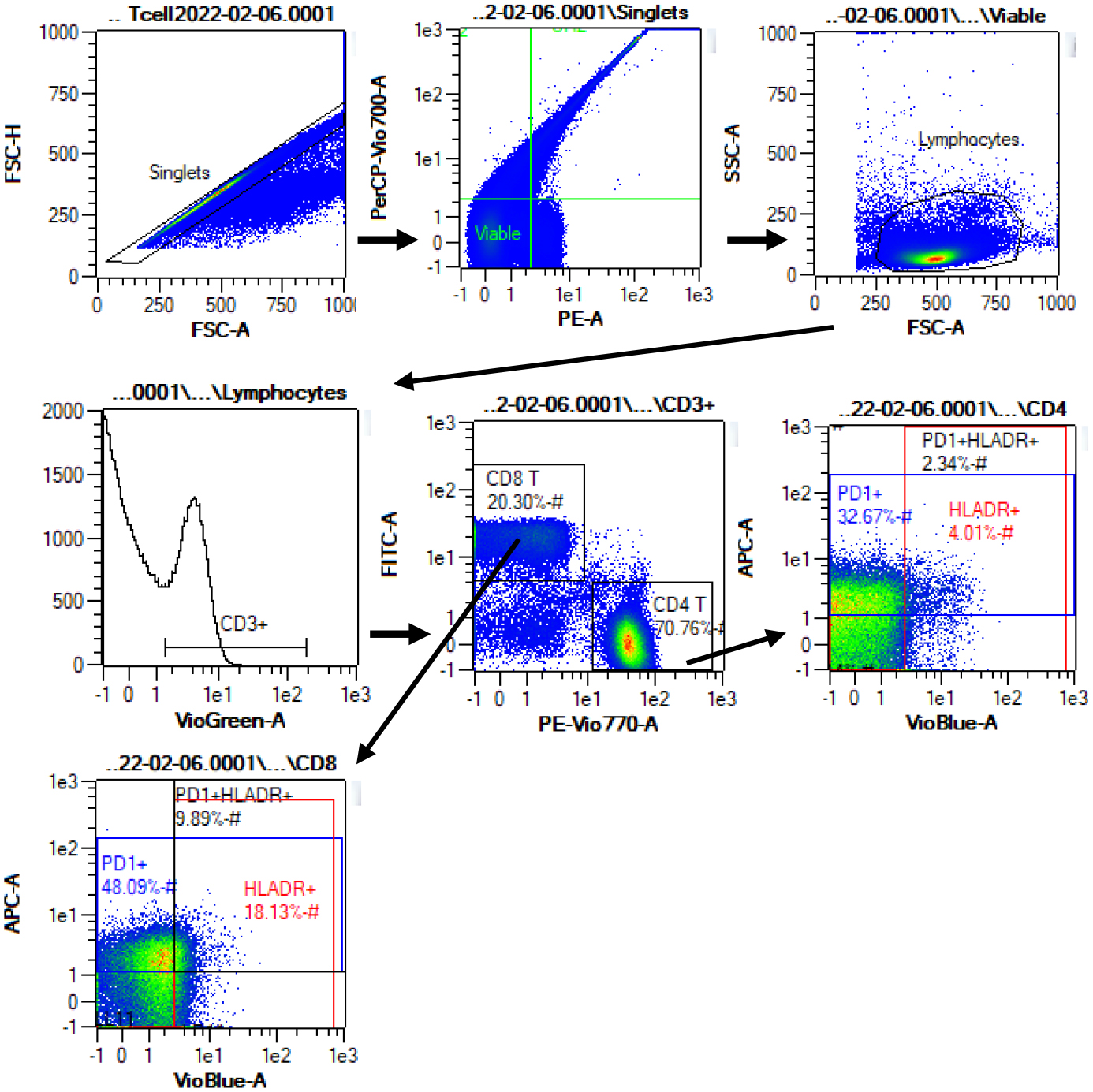
Representative flow cytometry data to illustrate gating strategy. Plots demonstrate sequential gating of singlets, viable (propidium iodide negative) cells, lymphocytes, T cells (CD3+), CD4+ and CD8+ T cells, and selection of HLA-DR+, PD-1+, and HLA-DR+PD-1+ subsets within CD4+ and CD8+ T cells. HLA-DR and PD-1 gates were set using fluorescence minus one controls.
2.6. Statistical analysis
Data were screened for normality by visual inspection. Mean values of physical function metrics and T-cell phenotypes were compared at baseline and post-intervention via Wilcoxon signed-rank test. Correlations between physical function measures and T-cell phenotypes were assessed by Kendall’s Tau. Differences in physical function and T-cell phenotype between participants receiving cancer therapy and not on cancer therapy were investigated via two-sample Kolmogorov-Smirnov Z tests. These statistical tests were selected due to the presence of non-parametric data and the small sample size. P values <0.05 were accepted as significant. Analyses were performed using IBM SPSS Statistics for Windows, version 28.0 (IBM Corp.).
3. Results
3.1. Participants
Table 1 provides participant demographics and clinical characteristics at intervention baseline. Fifty-four percent of the patients had Rai stage 3–4 CLL, 46% had unmutated IGHV, and 4% had a TP53 aberrancy. Also, 38% received prior CLL treatment (median number of prior lines of therapy, one), and 50% were currently receiving treatment (83% with a Bruton tyrosine kinase inhibitor.
Table 1.
Baseline participant demographics and clinical characteristics. Data are shown as mean ± standard deviation (range).
| Total | Female | Male | ||
|---|---|---|---|---|
| Participants (n) | 24 | 16 | 8 | |
| Age, years | 63.04±8.67 (49–81) | 63.88±9.86 (49–81) | 61.38±5.81 (51–68) | |
| Baseline weight, kg | 78.36±20.00 (54.50–131.00) | 68.79±9.94 (54.50–85.40) | 97.49±21.81 (76.30–131.00) | |
| 16 weeks weight, kg | 76.70±20.61 (54.10–131.00) | 67.01±9.27 (54.10–80.80) | 96.10±23.81 (74.10–131.00) | |
| Baseline BMI | 27.80±4.83 (18.50–38.90) | 26.40±4.23 (18.50–33.10) | 30.62±4.98 (24.70–38.90) | |
| 16 weeks BMI | 27.40±5.07 (18.50–38.90) | 25.83±3.92 (18.50–31.90) | 30.56±5.85 (23.60–38.90) | |
| Race | Black | 0 | 0 | 0 |
| White | 23 | 8 | 15 | |
| Other | 1 | 1 | 0 | |
| LDH units | 543 (199–1178) | 483 (199–1178) | 695 (415–684) | |
| B2-microgobulin ug/ml | 2.4 (1.4–6.4) | 2.6 (1.4–6.4) | 2.1 (1.8–2.9) | |
| WBC × 109/L | 37.0 (5.4–167.4) | 34.3 (5.4–121.2) | 42.6 (8.1–167.4) | |
| Hemoglobin g/dL | 13.4 (9.7–15.4) | 12.8 (9.7–14.1) | 14.5 (13.2–15.4) | |
| Platelets × 109/L | 196 (59–280) | 206 (59–280) | 176 (65–274) | |
| Albumin g/dL | 4.5 (4.0–5.1) | 4.5 (4.0–5.0) | 4.5 (4.0–4.9) | |
| Rai stage (n) | 0 | 7 | 5 | 2 |
| I | 4 | 3 | 1 | |
| II | 0 | 0 | 0 | |
| II | 4 | 3 | 1 | |
| IV | 9 | 5 | 4 | |
| Prior lines of therapy | ||||
| 0 | 15 | 10 | 5 | |
| 1 | 7 | 5 | 2 | |
| 2 | 2 | 1 | 1 | |
| Treatment (n) | None | 12 | 7 | 5 |
| Acalabrutinib | 2 | 2 | 0 | |
| Ibrutinib | 6 | 3 | 3 | |
| Obinutuzumab | 1 | 1 | 0 | |
| Venetoclax | 4 | 3 | 1 | |
| Zanubrutinib | 2 | 2 | 0 | |
| Combination | 3 | 2 | 1 | |
| Duration of current treatment, months | 16.4 (5–36) | 12.6 (5–24) | 21.4 (5–36) | |
| CLL comorbidity index score | 0 | 9 | 6 | 3 |
| 1 | 8 | 7 | 1 | |
| 2–3 | 7 | 3 | 4 | |
| del 11q† | 1 | 0 | 1 | |
| del 13q† | 12 | 8 | 4 | |
| del 17p† | 1 | 1 | 0 | |
| Tri 12† | 4 | 2 | 2 | |
| mutated TP 53 | 1 | 0 | 1 |
indicates fluorescence in situ hybridization (FISH) BMI: body mass index, CLL: chronic lymphatic leukemia LDH: lactate dehydrogenase, WBC: white blood cell count
3.2. Effects of intervention on physical function and T-cell composition
Several measures of patient physical function changed over the course of the four-month intervention (Table 2). Compared to baseline, body weight and fatigue were lower at intervention completion, and the number of repetitions performed during the 30 second sit-to-stand test was higher. Grip strength trended towards an increase (p=.05). Participants also increased reported leisure time exercise, on average moving from moderately active classification to active classification on the Godin Questionnaire. PROMIS-PF T scores increased over the intervention, suggesting an increase in the self-perception of current physical ability.
Table 2.
Physical function before and after intervention. Data are shown as median (interquartile range).
| Baseline Median (IQR) | 16-week Median (IQR) | Difference (95% CI) | T (p) | |
|---|---|---|---|---|
| Weight (kg) | 75.85 | 74.55 | −1.65 | −3.12 |
| (63.2, 84.13) | (61.25, 80.53) | (−2.54, −0.77) | (<0.01)* | |
| Godin PA | 16.00 | 24.00 | 14.61 | 3.83 |
| (6.00, 25.00) | (16.00, 43.00) | (6.73, 22.49) | (<0.01)* | |
| PROMIS PF | 49.10 | 49.10 | 2.92 | 2.05 |
| T-score | (45.65, 50.80) | (46.40, 61.70) | (0.48, 5.36) | (0.04)* |
| FACT Fatigue | 37.00 | 45.00 | 6.71 | 3.70 |
| (27.75, 45.75) | (39.50, 47.75) | (3.68, 9.74) | (<0.01)* | |
| 6min Walk Test (m) | 500.15 | 525.08 | 7.47 | 1.31 |
| (449.00, 581.21) | (467.15, 612.92) | (−23.64, 38.59) | (0.19) | |
| 30sec Sit-to-stand | 13.50 | 13.50 | 1.04 | 2.12 |
| (reps) | (10.25, 15.75) | (10.25, 17.75) | (0.03, 2.05) | (0.03)* |
| 30sec Arm Curl R | 16.00 | 17.00 | 0.92 | 1.52 |
| (reps) | (13.25, 19.00) | (13.25, 20.75) | (−0.18, 2.02) | (0.13) |
| 30sec Arm Curl L | 16.00 | 18.00 | 0.92 | 1.79 |
| (reps) | (15.00, 20.00) | (14.25, 21.00) | (−0.32, 2.15) | (0.07) |
| Eight Foot Timed | 5.58 | 5.67 | −0.09 | −0.77 |
| Up & Go (sec) | (5.21, 6.73) | (4.82, 6.53) | (−0.45, 0.27) | (0.44) |
| Grip Strength (kg) | 9.67 (4.83, 19.50) | 14.00 (6.84, 18.08) | 2.35 (0.17, 4.53) | 1.98 (0.05) |
indicates significant difference between Baseline and 16-week (p<0.05). CI: confidence interval, FACT: Functional Assessment of Cancer Therapy, IQR: interquartile range, L: left, PA: physical activity, PROMIS PF: Patient-Reported Outcome Measurement Information System physical function, R: right
No changes in total cell counts or proportions of CD4+ and CD8+ T-cell subsets were noted over the course of the intervention (Table 3).
Table 3.
T-cell subset proportions before and after intervention. Data are shown as median (interquartile range).
| Baseline Median (IQR) | 16-week Median (IQR) | Absolute Difference (95% CI) | T (p) | |
|---|---|---|---|---|
| CD4+ T-cells | 62.84 (44.33, 70.67) | 63.84 (43.98, 72.16) | 0.80 (−4.29, 5.89) | 0.89 (0.38) |
| HLADR+ CD4+ | 6.33 (3.54, 7.94) | 5.94 (3.72, 7.84) | 0.71 (−1.17, 2.59) | 0.03 (0.98) |
| PD1+ CD4+ | 39.65 (14.27, 50.08) | 41.64 (19.59, 48.78) | 0.46 (−1.20, 2.13) | 0.06 (0.95) |
| HLADR+PD1+ CD4+ | 3.67 (1.96, 4.87) | 3.35 (2.31, 5.31) | 0.38 (−0.66, 1.41) | 0.24 (0.81) |
| CD8+ T-cells | 20.13 (13.85, 33.82) | 19.07 (15.6, 32.43) | 0.13 (−2.99, 3.25) | 0.49 (0.63) |
| HLADR+ CD8+ | 17.44 (13.54, 26.31) | 18.64 (12.25, 27.87) | 2.32 (−1.47, 6.10) | 1.14 (0.25) |
| PD1+ CD8+ | 46.43 (16.32, 57.57) | 48.02 (24.43, 56.55) | 1.10 (−0.88, 3.08) | 0.60 (0.55) |
| HLADR+PD1+ CD8+ | 7.37 (5.73, 10.24) | 8.00 (5.76, 11.67) | 0.82 (−0.70, 2.33) | 0.71 (0.48) |
| CD4:CD8 Ratio | 2.97 (1.64, 4.23) | 2.72 (1.53, 4.55) | −0.02 (−0.43, 0.39) | −0.40 (0.69) |
Data are proportion of total CD3+ T-cells (CD4+ T-cells and CD8+ T-cells), proportion of total CD4+T-cells (HLA-DR+CD4+ T-cells, PD-1+CD4+T-cells, HLA-DR+PD-1+CD4+ T-cells), and proportion of total CD8+ T-cells (HLA-DR+CD8+ T-cells, PD-1+CD8+T-cells, HLA-DR+PD-1+CD8+T-cells).
CI: confidence interval, IQR: interquartile range.
No differences at baseline or post-intervention were found in physical function or T-cell phenotype between participants receiving cancer therapy and those not on treatment (Tables S1 and S2). In addition, groups did not differ in the change in physical function or T cell phenotype over course of the intervention (Table S3).
3.3. Relationships between physical function and T-cell subsets
Table 4 displays the relationship between baseline measures of physical function and the proportions of T-cell subsets, as well as the relationship between physical function and T-cell subsets at 16-weeks. At baseline, patients reporting more fatigue had a greater proportion of CD8+ T-cells and trended towards a lower CD4:CD8 T-cell ratio (p=.05). Those who were able to walk a greater distance in the six-min walk test had a lower proportion of PD-1+ CD8+ T-cells. Similarly, those who performed better in the 8-foot timed up-and-go test had a lower proportion of PD-1+CD4+ T-cells and a lower proportion of HLA-DR+PD-1+CD8+ T-cells. Grip strength was greater in those with a greater proportion of HLA-DR+CD4+ T-cells (Table 4).
Table 4.
Correlation between physical function and T-cell subset proportion at baseline and at 16-weeks. Data are displayed as correlation coefficient τ (p).
| Baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CD4+ | HLADR+ CD4+ | PD1+ CD4+ | HLADR+ PD1+ CD4+ | CD8+ | HLADR+ CD8+ | PD1+ CD8+ | HLADR+ PD1+ CD8+ | CD4:CD8 Ratio | |
| FACT | 0.11 | −0.10 | −0.04 | −0.22 | −0.32 | −0.17 | 0.02 | −0.18 | 0.29 |
| Fatigue | (0.46) | (0.52) | (0.77) | (0.14) | (0.03)* | (0.25) | (0.92) | (0.21) | (0.05) |
| 6min Walk | −0.16 | 0.11 | −0.28 | −0.06 | 0.05 | 0.10 | −0.31 | −0.15 | −0.13 |
| Test (m) | (0.28) | (0.46) | (0.05) | (0.69) | (0.73) | (0.49) | (0.03)* | (0.32) | (0.37) |
| Eight Foot | |||||||||
| 0.01 | −0.04 | 0.37 | 0.13 | 0.07 | 0.06 | 0.27 | 0.30 | −0.01 | |
| Timed Up | |||||||||
| (0.92) | (0.80) | (0.01)* | (0.37) | (0.66) | (0.69) | (0.07) | (0.04)* | (0.92) | |
| &Go (sec) | |||||||||
| Grip | |||||||||
| 0.15 | 0.34 | −0.20 | 0.27 | −0.18 | 0.22 | −0.10 | −0.02 | 0.13 | |
| Strength | |||||||||
| 0.30) | (0.02)* | (0.18) | (0.07) | (0.23) | (0.14) | (0.49) | (0.92) | (0.37) | |
| (kg) | |||||||||
| 16-weeks | |||||||||
| CD4+ | HLADR+ CD4+ | PD1+ CD4+ | HLADR+ PD1+ CD4+ | CD8+ | HLADR+ CD8+ | PD1+ CD8+ | HLADR+ PD1+ CD8+ | CD4:CD8 Ratio | |
| 6min Walk | −0.07 | 0.15 | −0.34 | 0.10 | 0.02 | 0.20 | −0.25 | −0.05 | −0.12 |
| Test (m) | (0.66) | (0.3) | (0.02)* | (0.49) | (0.88) | (0.17) | (0.09) | (0.73) | (0.43) |
| 30sec Arm | |||||||||
| 0.05 | 0.01 | −0.38 | −0.09 | 0.05 | 0.15 | −0.22 | −0.07 | 0.02 | |
| Curl R | |||||||||
| (0.75) | (0.94) | (0.01)* | (0.53) | (0.83) | (0.33) | (0.14) | (0.64) | (0.90) | |
| (reps) | |||||||||
| 30sec Arm | |||||||||
| 0.09 | −0.04 | −0.30 | −0.14 | 0.01 | 0.14 | −0.12 | −0.01 | 0.00 | |
| Curl L | |||||||||
| (0.55) | (0.80) | (0.04)* | (0.34) | (0.96) | (0.34) | (0.43) | (0.96) | (1.00) | |
| (reps) | |||||||||
indicates significant correlation (p<0.05) (2-tail).
FACT: Functional Assessment of Cancer Therapy, L: left, R: right
After the intervention, those who were able to walk a greater distance in the six-min walk test had a lower proportion of PD-1+ CD4+ T-cells. Those who could perform a greater number of arm curls also had a lower proportion of PD-1+ CD4+ T-cells (Table 4).
Table 5 displays relationships between the change in measures of physical function and change in T-cell subsets (baseline to post-intervention). An increase in body weight at the end of the intervention relative to baseline significantly associated with a decrease in the proportion of HLA-DR+CD8+ T-cells. Both an increase in leisure time exercise and a decrease in fatigue associated with an increased CD4:CD8 T-cell ratio. A decrease in fatigue also associated with a decrease in HLA-DR+CD4+T-cells and HLA-DR+PD-1+CD4+T-cells (Table 5).
Table 5.
Correlation between change in physical function and change in T cell subsets. Data are displayed as correlation coefficient τ (p).
| Δ CD4+ | Δ HLADR+ CD4+ | Δ PD1+ CD4+ | Δ HLADR+ PD1+ CD4+ | Δ CD8+ | Δ HLADR+ CD8+ | Δ PD1+ CD8+ | Δ HLADR+ PD1+ CD8+ | Δ CD4:CD8 Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| Δ Weight | 0.17 | −0.04 | −0.25 | −0.13 | −0.21 | −0.41 | 0.03 | −0.19 | 0.17 |
| (kg) | (0.26) | (0.79) | (0.09) | (0.38) | (0.16) | (0.01)* | (0.86) | (0.21) | (0.25) |
| Δ Godin | 0.18 | −0.11 | 0.24 | 0.10 | 0.24 | 0.04 | 0.23 | 0.15 | 0.35 |
| PA | (0.24) | (0.46) | (0.12) | (0.52) | (0.11) | (0.79) | (0.14) | (0.34) | (0.02)* |
| Δ FACT | 0.16 | −0.37 | −0.21 | −0.37 | −0.22 | −0.07 | −0.16 | −0.06 | 0.36 |
| Fatigue | (0.30) | (0.01)* | (0.17 | (0.01)* | (0.15) | (0.63) | (0.30) | (0.67) | (0.02)* |
| Δ 6min Walk | −0.10 | 0.11 | 0.05 | 0.16 | 0.04 | 0.07 | 0.30 | 0.02 | −0.18 |
| Test (m) | (0.49) | (0.46) | (0.73) | (0.28) | (0.77) | (0.62) | (0.04)* | (0.88) | (0.22) |
indicates significant correlation (p<0.05) (2-tail)
FACT: Functional Assessment of Cancer Therapy, PA: physical activity
4. Discussion
This analysis of T-cell phenotypes in patients with CLL is to our knowledge the first to investigate the relationship between differentiated T-cells and physical function in this population. Data in the current study was derived from a larger intervention, the primary purpose of which was to determine the most effective strategies for increasing PA and maximizing patient adherence. Accordingly, the intervention did not seek to improve any one physical function metric specifically, but rather to increase overall PA and reduce fatigue. It should be noted that all 24 participants (mean age=63.04±8.67) included in this analysis completed the intervention. Importantly, participants post-intervention reported increased leisure time PA from baseline. The observed increase in PA correlated with an increase in the CD4:CD8 T-cell ratio. An inverted CD4:CD8 T-cell ratio in patients with CLL has been associated with reduced progression-free and overall survival (4). Although the inversion of the CD4:CD8 T-cell ratio is also associated with aging and cytomegalovirus infection, previous studies have demonstrated that this phenomenon in CLL patients is distinct from such factors (4). Although markers of senescence were not measured in this study, a low CD4:CD8 T-cell ratio is associated with increased CD57 expression (22) which has been identified as a predictive biomarker in many cancer treatments (23).
Severe fatigue is a commonly reported symptom among CLL patients and can interfere with daily activity (24). Consequently, cancer-related fatigue presents a challenge to maintaining physical function and can be detrimental to patient prognosis (25). Previous PA interventions have been successful in decreasing fatigue in cancer survivors and improving tolerance for exercise (26, 27). In the present study, reports of fatigue significantly decreased post-intervention as measured via the FACT-F. This change correlated with an increase in the CD4:CD8 T-cell ratio and a decrease in the proportion of HLA-DR+PD-1+CD4+ T-cells. Additionally, greater fatigue at baseline correlated to a higher percentage of CD8+ T-cells. Both a higher percentage of CD8+ T-cells and a greater proportion of HLA-DR+PD-1+CD4+ T-cells have been linked to reduced progression-free survival and more rapid disease progression in patients with CLL (4).
The HLA-DR+PD-1+CD4+ T-cell phenotype has been reported as constituting up to 50% of CD4+ T-cells in CLL patients as opposed to <7% in healthy populations (4). Although there were no significant changes in T-cell subsets in participants overall, these data suggest that some participants did reduce the proportion of HLA-DR+PD-1+CD4+ T-cells, and experienced reduced fatigue following the intervention.
Higher cardiorespiratory fitness (CRF), as indicated by 6-min walk test performance, was associated with fewer PD-1+CD8+T-cells at baseline, fewer PD-1+CD4+ T-cells at 16-weeks, and a trend for fewer PD-1+ CD8+ T-cells at 16-weeks (p=.09). As an inhibitory receptor, PD-1 expression on T-cells indicates T-cell exhaustion and in CLL patients has been associated with increased disease burden (2). These data suggest for the first time that higher CRF associates with a reduced proportion of PD-1+ T-cells in patients with CLL. In contrast, an improvement in CRF (that is, greater distance walked during the six-min walk test) at 16-weeks relative to baseline correlated with an increased proportion of PD-1+CD8+ T-cells. This result, however, was largely driven by two participants, and the relationship was no longer significant when these two participants were removed from analysis. Thus, these data require further study in a larger sample of patients with CLL.
The role of exercise in cancer treatment has been widely recognized. Numerous studies have demonstrated that PA interventions are effective at increasing physical function, reducing fatigue, and improving quality of life in lymphoma patients (28–31). Higher PA and physical fitness have also been linked to slower disease progression and reduced treatment side effects (6, 32). However, it is yet unclear what physiological changes resulting from PA are responsible for these improved outcomes. Recent studies have attempted to identify possible mechanisms by investigating the effect of PA interventions on immune function in cancer populations (10–12). Exercise is increasingly linked to improved immune function in healthy populations (33), but the altered immune profile of patients with CLL suggests these findings may not be directly applicable (4). Only one study to date has investigated the relationship between PA and immune function in patients with CLL. This study found that 12-weeks of HIIT exercise combined with resistance training improved immune function via increased NK-cell cytotoxicity. Researchers reported high adherence to the HIIT protocol which proved effective at increasing muscular strength and CRF; however, the improvement in CRF was higher in the control group. Although this study did not report T-cell ratios, they did report an increase in the percentage of CD8+ T-cells and a decrease in the percentage of CD4+ T-cells (12). This result is similar to a comparison of HIIT to moderate-intensity continuous training (MICT) in women at high risk of breast cancer which found a significant reduction in total CD4+ T-cells and CD4:CD8 T-cell ratio after HIIT training but not MICT (11). These studies indicate HIIT training may relate to an undesirable T-cell ratio in patients with CLL. The current study differed from that of MacDonald et al. (12), in both the modality of exercise and our inclusion of CLL patients with comorbidities. Although such conditions have known impacts on the immune system (34, 35) and may complicate the interpretation of these results, the prevalence of CLL patients with comorbidities suggests that the current data may be more indicative of the general CLL population than that of a study which excludes patients on this basis.
Implications of the study and its results
A limitation to the present study is the lack of a control group. Although this is standard during the optimization phase of a MOST intervention (36), it limits the interpretations which can be made from the present data. Further, the MOST design was used to compare four intervention components with two alternatives each, resulting in the formation of 16 unique treatment groups. Comparison between groups was not feasible due to an inadequate sample size in each.
Therefore, all participants were treated as a homogenous group and analysis was conducted accordingly. As a result, participants conducting aerobic exercise only and participants conducting aerobic and resistance training are not differentiated. Rather, this study instead linked physical function at baseline and after the intervention period with T-cell subsets present in the blood at each time point. Further, 12 of the 24 patients received CLL directed chronic targeted therapy (Table 1) while participating in the intervention. These medications have been found to alter T-cell phenotype and function in CLL patients (36–40). Specifically, BTK and BCL2 inhibitors are known to down regulate PD-1 expression in CD4+ T-cells (acalabrutinib, ibrutinib, zanubrutinib) and CD8+ T-cells (ibrutinib, venetoclax, zanubrutinib) (37, 39, 40). Treatment with ibrutinib may also increase the total number of T-cells, while obinutuzumab has been shown to decrease the total number of T-cells (38–41). However, participants who had received therapy did not differ in T cell phenotype from participants who had not received therapy in this study. Finally, participants in this study were not currently receiving radiation or chemotherapy, and were able to participate in unsupervised exercise. Thus, results from this study may not apply to patients with more advanced disease.
Despite these limitations, this study demonstrates that a PA intervention utilizing aerobic exercise can increase leisure time PA and decrease fatigue in older adult patients with CLL, and that these changes are correlated to an improved T-cell ratio and reduced proportion of T-cell subsets indicative of poor prognosis. These findings further support the use of exercise to improve physical fitness and quality of life in patients with cancer. Future exercise interventions involving CLL patients should utilize controlled interventions to investigate impacts on immune cell function including pro-inflammatory cytokines which have been associated with high levels of fatigue in other types of cancer (42).
Supplementary Material
Supplemental Table 1. Physical function and T-cell subset proportions by treatment at baseline.
Supplemental Table 2. Physical function and T-cell subset proportions by treatment post intervention.
Supplemental Table 3. Change in physical function and T-cell subset proportions by treatment.
Novelty and Impact:
In this study, a physical activity intervention in an older adult population with CLL increased leisure time physical activity and decreased patient fatigue. These results were associated with improvements in immune markers previously linked to faster disease progression in patients with CLL. This study supports the use of physical activity to improve physical fitness and quality of life in patients with CLL.
Funding Information
The HEALTH4CLL study is supported by the Center for Energy Balance in Cancer Prevention and Survivorship at the University of Texas MD Anderson Cancer Center, Bionutrition Research Core, CLL Global Research Foundation, and generous philanthropic contributions to the University of Texas MD Anderson Cancer Center Moon Shots Program. Funding for this project also provided by a CLASS Research Project Grant from the University of Houston to EL, MM, and RS. MG is supported by a T32 training grant (5T32CA009666-27). Content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Footnotes
Declaration of Interest
Conflicts of interest: none
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
References
- 1.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019; 69(5):363–85. [DOI] [PubMed] [Google Scholar]
- 2.Roessner PM, Seiffert M. T-cells in chronic lymphocytic leukemia: Guardians or drivers of disease? Leukemia 2020; 34(8):2012–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eller MA, Blom KG, Gonzalez VD, Eller LA, Naluyima P, Laeyendecker O, Quinn TC, Kiwanuka N, Serwadda D, Sewankambo NK, Tasseneetrithep B, Wawer MJ, Gray RH, Marovich MA, Michael NL, de Souza MS, Wabwire-Mangen F, Robb ML, Currier JR, Sandberg JK. Innate and adaptive immune responses both contribute to pathological CD4 T cell activation in HIV-1 infected Ugandans. PLoS One 2011; 6(4):e18779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elston L, Fegan C, Hills R, Hashimdeen SS, Walsby E, Henley P, Pepper C, Man S. Increased frequency of CD4(+) PD-1(+) HLA-DR(+) T cells is associated with disease progression in CLL. Br J Haematol 2020; 188(6):872–80. [DOI] [PubMed] [Google Scholar]
- 5.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH, Morris GS, Patel AV, Hue TF, Perna FM, Schmitz KH. Exercise Guidelines for Cancer Survivors: Consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc 2019; 51(11):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sitlinger A, Deal MA, Garcia E, Thompson DK, Stewart T, MacDonald GA, Devos N, Corcoran D, Staats JS, Enzor J, Weinhold KJ, Brander DM, Weinberg JB, Bartlett DB. Physiological fitness and the pathophysiology of chronic lymphocytic leukemia (CLL). Cells 2021; 10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dighiero G, Maloum K, Desablens B, Cazin B, Navarro M, Leblay R, Leporrier M, Jaubert J, Lepeu G, Dreyfus B, Binet JL, Travade P. Chlorambucil in indolent chronic lymphocytic leukemia. French Cooperative Group on Chronic Lymphocytic Leukemia. N Engl J Med 1998; 338(21):1506–14. [DOI] [PubMed] [Google Scholar]
- 8.Shustik C, Mick R, Silver R, Sawitsky A, Rai K, Shapiro L. Treatment of early chronic lymphocytic leukemia: intermittent chlorambucil versus observation. Hematol Oncol 1988; 6(1):7–12. [DOI] [PubMed] [Google Scholar]
- 9.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating M, Montserrat E, Chiorazzi N, Stilgenbauer S, Rai KR, Byrd JC, Eichhorst B, O’Brien S, Robak T, Seymour JF, Kipps TJ. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018; 131(25):2745–60. [DOI] [PubMed] [Google Scholar]
- 10.Khosravi N, Stoner L, Farajivafa V, Hanson ED. Exercise training, circulating cytokine levels and immune function in cancer survivors: A meta-analysis. Brain Behav Immun 2019; 81:92–104. [DOI] [PubMed] [Google Scholar]
- 11.Niemiro GM, Coletta AM, Agha NH, Mylabathula PL, Baker FL, Brewster AM, Bevers TB, Fuentes-Mattei E, Basen-Engquist K, Katsanis E, Gilchrist SC, Simpson RJ. Salutary effects of moderate but not high intensity aerobic exercise training on the frequency of peripheral T-cells associated with immunosenescence in older women at high risk of breast cancer: a randomized controlled trial. Immun Ageing 2022; 19(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald G, Sitlinger A, Deal MA, Hanson ED, Ferraro S, Pieper CF, Weinberg JB, Brander DM, Bartlett DB. A pilot study of high-intensity interval training in older adults with treatment naive chronic lymphocytic leukemia. Sci Rep 2021; 11(1):23137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurmes P, Call T, Slager S, Zent C, Jenkins G, Schwager S, Bowen D, Kay N, Shanafelt T. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma 2008; 49(1):49–56. [DOI] [PubMed] [Google Scholar]
- 14.Goede V, Cramer P, Busch R, Bergmann M, Stauch M, Hopfinger G, Stilgenbauer S, Dohner H, Westermann A, Wendtner CM, Eichhorst B, Hallek M, German CLLSG. Interactions between comorbidity and treatment of chronic lymphocytic leukemia: results of German Chronic Lymphocytic Leukemia Study Group trials. Haematologica 2014; 99(6):1095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon MJ, Kaempf A, Sitlinger A, Shouse G, Mei M, Brander DM, Salous T, Hill BT, Alqahtani H, Choi M, Churnetski MC, Cohen JB, Stephens DM, Siddiqi T, Rivera X, Persky D, Wisniewski P, Patel K, Shadman M, Park B, Danilov AV. The Chronic Lymphocytic Leukemia Comorbidity Index (CLL-CI): A Three-Factor Comorbidity Model. Clin Cancer Res 2021; 27(17):4814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotbain EC, Niemann CU, Rostgaard K, da Cunha-Bang C, Hjalgrim H, Frederiksen H. Mapping comorbidity in chronic lymphocytic leukemia: impact of individual comorbidities on treatment, mortality, and causes of death. Leukemia 2021; 35(9):2570–80. [DOI] [PubMed] [Google Scholar]
- 17.Strati P, Parikh SA, Chaffee KG, Kay NE, Call TG, Achenbach SJ, Cerhan JR, Slager SL, Shanafelt TD. Relationship between co-morbidities at diagnosis, survival and ultimate cause of death in patients with chronic lymphocytic leukaemia (CLL): a prospective cohort study. Br J Haematol 2017; 178(3):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Canadian Journal of Sport Sciences 1992; 17(4):338–45. [PubMed] [Google Scholar]
- 19.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 1985; 10(3):141–6. [PubMed] [Google Scholar]
- 20.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, Thissen D, Revicki DA, Weiss DJ, Hambleton RK, Liu H, Gershon R, Reise SP, Lai JS, Cella D. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS). Med Care 2007; 45(5 Suppl 1):S22–31. [DOI] [PubMed] [Google Scholar]
- 21.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 1997; 13(2):63–74. [DOI] [PubMed] [Google Scholar]
- 22.Nunes C, Wong R, Mason M, Fegan C, Man S, Pepper C. Expansion of a CD8(+)PD-1(+) replicative senescence phenotype in early stage CLL patients is associated with inverted CD4:CD8 ratios and disease progression. Clin Cancer Res 2012; 18(3):678–87. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, He T, Xue L, Guo H. Senescent T cells: a potential biomarker and target for cancer therapy. EBioMedicine 2021; 68:103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pashos CL, Flowers CR, Kay NE, Weiss M, Lamanna N, Farber C, Lerner S, Sharman J, Grinblatt D, Flinn IW, Kozloff M, Swern AS, Street TK, Sullivan KA, Harding G, Khan ZM. Association of health-related quality of life with gender in patients with B-cell chronic lymphocytic leukemia. Support Care Cancer 2013; 21(10):2853–60. [DOI] [PubMed] [Google Scholar]
- 25.Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, Stewart KJ, Cameron L, Zawacki K, Podewils LJ, Cohen G, McCorkle R. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology 2005; 14(6):464–77. [DOI] [PubMed] [Google Scholar]
- 26.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012; 11:CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaVoy EC, Fagundes CP, Dantzer R. Exercise, inflammation, and fatigue in cancer survivors. Exerc Immunol Rev 2016; 22:82–93. [PMC free article] [PubMed] [Google Scholar]
- 28.Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, Tankel K, Basi S, Chua N, Mazurek A, Reiman T. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol 2009; 27(27):4605–12. [DOI] [PubMed] [Google Scholar]
- 29.Furzer BJ, Ackland TR, Wallman KE, Petterson AS, Gordon SM, Wright KE, Joske DJ. A randomised controlled trial comparing the effects of a 12-week supervised exercise versus usual care on outcomes in haematological cancer patients. Support Care Cancer 2016; 24(4):1697–707. [DOI] [PubMed] [Google Scholar]
- 30.Persoon S, Chin AMJM, Buffart LM, Liu RDK, Wijermans P, Koene HR, Minnema MC, Lugtenburg PJ, Marijt EWA, Brug J, Nollet F, Kersten MJ. Randomized controlled trial on the effects of a supervised high intensity exercise program in patients with a hematologic malignancy treated with autologous stem cell transplantation: Results from the EXIST study. PLoS One 2017; 12(7):e0181313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Streckmann F, Kneis S, Leifert JA, Baumann FT, Kleber M, Ihorst G, Herich L, Grussinger V, Gollhofer A, Bertz H. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol 2014; 25(2):493–9. [DOI] [PubMed] [Google Scholar]
- 32.Sitlinger A, Brander DM, Bartlett DB. Impact of exercise on the immune system and outcomes in hematologic malignancies. Blood Adv 2020; 4(8):1801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gleeson M Immune function in sport and exercise. J Appl Physiol (1985) 2007; 103(2):693–9. [DOI] [PubMed] [Google Scholar]
- 34.Ratter-Rieck JM, Maalmi H, Trenkamp S, Zaharia OP, Rathmann W, Schloot NC, Straßburger K, Szendroedi J, Herder C, Roden M. Leukocyte counts and T-cell frequencies differ between novel subgroups of diabetes and are associated with metabolic parameters and biomarkers of inflammation. Diabetes 2021; 70(11):2652–62. [DOI] [PubMed] [Google Scholar]
- 35.Welsh C, Welsh P, Mark PB, Celis-Morales CA, Lewsey J, Gray SR, Lyall DM, Iliodromiti S, Gill JMR, Pell J, Jhund PS, Sattar N. Association of total and differential leukocyte counts with cardiovascular disease and mortality in the UK Biobank. Arterioscler Thromb Vasc Biol 2018; 38(6):1415–23. [DOI] [PubMed] [Google Scholar]
- 36.Wyrick DL, Rulison KL, Fearnow-Kenney M, Milroy JJ, Collins LM. Moving beyond the treatment package approach to developing behavioral interventions: addressing questions that arose during an application of the Multiphase Optimization Strategy (MOST). Transl Behav Med 2014; 4(3):252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrd JC, Wierda WG, Schuh A, Devereux S, Chaves JM, Brown JR, Hillmen P, Martin P, Awan FT, Stephens DM, Ghia P, Barrientos J, Pagel JM, Woyach JA, Burke K, Covey T, Gulrajani M, Hamdy A, Izumi R, Frigault MM, Patel P, Rothbaum W, Wang MH, O’Brien S, Furman RR. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated phase 2 results. Blood 2020; 135(15):1204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Muñoz R, Aguinaga L, Feliu J, Anton-Remirez J, Jorge-Del-Val L, Casajús-Navasal A, Nebot-Villacampa MJ, Daroca-Fernandez I, Domínguez-Garrido E, Rabasa P, Panizo C. Obinutuzumab induces depletion of NK cells in patients with chronic lymphocytic leukemia. Immunotherapy 2018; 10(6):491–9. [DOI] [PubMed] [Google Scholar]
- 39.Kondo K, Shaim H, Thompson PA, Burger JA, Keating M, Estrov Z, Harris D, Kim E, Ferrajoli A, Daher M, Basar R, Muftuoglu M, Imahashi N, Alsuliman A, Sobieski C, Gokdemir E, Wierda W, Jain N, Liu E, Shpall EJ, Rezvani K. Ibrutinib modulates the immunosuppressive CLL microenvironment through STAT3-mediated suppression of regulatory B-cell function and inhibition of the PD-1/PD-L1 pathway. Leukemia 2018; 32(4):960–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM, Maddocks KJ, Cheney C, Jones JA, Flynn JM, Andritsos LA, Awan F, Fraietta JA, June CH, Maus MV, Woyach JA, Caligiuri MA, Johnson AJ, Muthusamy N, Byrd JC. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest 2017; 127(8):3052–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou YX, Zhu HY, Li XT, Xia Y, Miao KR, Zhao SS, Wu YJ, Wang L, Xu W, Li JY. The impacts of zanubrutinib on immune cells in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Hematol Oncol 2019; 37(4):392–400. [DOI] [PubMed] [Google Scholar]
- 42.Bower JE, Ganz PA, Aziz N, Fahey JL, Cole SW. T-cell homeostasis in breast cancer survivors with persistent fatigue. J Natl Cancer Inst 2003; 95(15):1165–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Physical function and T-cell subset proportions by treatment at baseline.
Supplemental Table 2. Physical function and T-cell subset proportions by treatment post intervention.
Supplemental Table 3. Change in physical function and T-cell subset proportions by treatment.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.


