Abstract
Purpose
Heparanase (HPSE) cleaves heparan sulfate proteoglycans during herpes simplex virus-1 (HSV-1) infection, aiding in viral egress and disease progression. Its action has been well established in in vitro and in vivo models, but its relevance in human patients remains unclear. This study aimed to specifically evaluate tear HPSE levels of patients with herpes simplex keratitis (HSK) and to correlate these findings with a commonly used murine model.
Methods
Tear samples from patient and mice samples were collected at LV Prasad Eye Institute, Hyderabad, India, and at the University of Illinois, Chicago, IL, respectively. Tears were collected from HSV-1 patients, bacterial/fungal keratitis cases, and healthy individuals. For in vivo study, C57BL/6 mice were infected with HSV-1 (McKrae strain) followed by tear fluid collection at various time points (0–10 days).
Results
The HSV-1, bacterial keratitis, fungal keratitis, and healthy control groups each had 30 patients. There was a significant difference in HPSE expression in the HSV-1 infected eyes (1.55 ± 0.19 units/mL) compared to HSV-1 contralateral eyes (1.23 ± 0.13 units/mL; P = 0.82), bacterial keratitis eyes (0.87 ± 0.15 units/mL; P = 0.0078), fungal keratitis eyes (0.64 ± 0.09 units/mL; P < 0.00001), and normal controls (0.53 ± 0.06 units/mL; P < 0.00001). C57BL/6 mice tear HPSE expression in infected eyes was 0.66 to 5.57 ng heparan sulfate (HS) removed per minute when compared to non-infected eye (range, 0.70–3.67 ng HS removed per minute).
Conclusions
To the best of our knowledge, this study is the first to report elevated HPSE levels in the tears of patients with different forms of HSV-1 keratitis, and it confirms similar findings in a murine model, providing a valuable basis for future in vivo and clinical research on HSV-1 ocular infection.
Keywords: heparanase, tear fluids, clinical and in vivo correlation, herpes simplex virus-1 keratitis, ELISA
Herpes simplex virus-1 (HSV-1) is a neurotropic double-stranded DNA virus that has the potential to cause severe corneal manifestations such as blepharitis, conjunctivitis, iritis, retinitis, and keratitis. Herpes simplex keratitis (HSK) is a potentially blinding condition of the cornea. Although the seroprevalence rate of HSV-1 is declining in developed countries (currently at 64%), it is increasing in developing countries, reaching levels of approximately 90%.1–3 In Western countries, HSK is one of the leading causes of corneal infection when compared with other etiologies such as fungal and bacterial infections that are sporadic.4–6 A recent study reported by our group showed that immune stromal keratitis, which is a form of HSK, is prevalent in India, followed by epithelial keratitis, and it predominantly affects males between the third and fifth decades of life.7 In comparison with other viral infections, the unique features of HSV-1 keratitis are its chronicity and recurrent nature.8–10 An average of two or three recurrences of infection is found in more than 40% of individuals.11,12 The severity of viral infection depends on the interaction of its glycoproteins with host factors. Initial viral infection initiates with the interaction of viral glycoproteins (gB, gC, gH, gD, and gL) and host cell receptors, including heparan sulfate proteoglycans. This binding not only activates intracellular signaling pathways involved in innate immune responses but also activates a couple of host enzymes facilitate viral egress.13 Heparanase (HPSE) is one such host enzyme that is considered a hallmark of progression of viral infection within the host cell. It is an endo-β-d-glycosidase that acts upon the glycosidic bonds of heparan sulfate (HS), cleaving it and bringing about structural modifications at the extracellular matrix. Elevation in the level of HPSE during viral infections causes a dramatic decrease in HS levels.14 This decrease in HS levels allows the viral progenies to leave the infected cell and invade the neighboring uninfected cells.15–17
The literature indicates that HPSE expression increases with disease severity in in vitro studies, and reducing HPSE activity inhibits viral shedding and disease outcome in animal models.18–22 However, there is no information available on the role of HPSE in patients diagnosed with HSK or the tears of animal models of HSV keratitis. Taking into consideration that HPSE levels are significantly upregulated in HSV-1 infections, it is hypothesized to be a potential marker of HSV-1–related ocular infections. Identifying such a marker that can predict the occurrence of HSK in the subclinical stage is of utmost importance for improved disease prognosis. To understand the role of HPSE in viral pathogenesis we conducted a prospective study in which we evaluated the tear fluid HPSE levels in individuals with HSV-1 keratitis and correlated our findings in C57BL/6 mice tear fluids.
Methods
Ethics Statement
Patients clinically diagnosed with HSV-1 keratitis were included in the study. The study was performed at LV Prasad Eye Institute, Hyderabad, India, and was approved by the Institutional Review Board (LEC-BHR-P-04-21-618). The study adhered to the tenets of the Declaration of Helsinki. The nature of the study and its possible consequences were explained before individuals were included in the study, and written consent was obtained from each individual. The animal study was reviewed and approved by the animal care committee of the University of Illinois, Chicago (IRB No. 21-050).
The in vivo study included 6- to 8-week-old C57BL/6 mice. The experiments were conducted and animals housed in a Biosafety Level 2 rodent facility in the Biologic Resources Laboratory at the University of Illinois at Chicago with a standard 12-hour light, 12-hour dark cycle. Ambient temperatures and relative humidity at this facility were maintained at between 20°C and 26°C and between 45% and 65%, respectively. During the project, trained veterinarians provided care to the mice. All protocols were reviewed and certified by the Institutional Animal Care and Use Committee of the University of Illinois, Chicago (IACUC No. 23-027).
Clinical Specimens
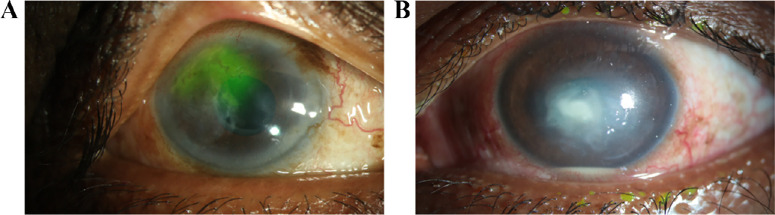
This was a prospective, consecutive, and comparative study conducted between January 2021 and November 2022. All patients during this time period who were clinically diagnosed with any form of HSV keratitis (Fig. 1) were recruited. All patients were examined in detail using a slit-lamp microscope (Haag-Streit, Köniz, Switzerland) for clinical features. All patients were managed as per the standard treatment protocol for the management of HSV-1 keratitis.23 The clinically identified corneal signs for the study cohort are described in Supplementary File S1.
Figure 1.
Slit-lamp imaging of the different clinically confirmed forms of HSV-1 keratitis: (A) recurrent epithelial keratitis showing dendritic defects, and (B) necrotizing stromal keratitis.
For comparison, patients with microbiologically confirmed bacterial and fungal keratitis were also recruited. Microbiologically confirmed cases of mixed infections were excluded from the analysis. Similarly, healthy individuals having no ocular or systemic comorbidities served as controls in the study. Tear fluids (approximately 25–30 µL) were collected from the infected and contralateral eyes of patients with HSV-1 keratitis, from the infected eyes of patients with bacterial and fungal keratitis, and from the left eyes of healthy controls. The samples were transferred into 0.6-mL centrifuge tubes after collection and given a short spin for about 12 to 20 seconds before being stored at −80°C until further use.
HSV-1 Ocular Infection in C57BL/6 Mice
Prior to the corneal epithelial debridement, the mice were anesthetized using a mixture of ketamine (100 mg/kg) and xylazine (5 mg/kg) followed by the addition of topical anesthesia (0.5% proparacaine). Mice were divided into two groups: infected (n = 3) and healthy controls (n = 3). Three horizontal scratches were made on the corneal epithelium using a 30-gauge needle. HSV-1 (McKrae strain) containing 5 × 105 plaque forming units in a volume of 5 µL was placed onto the corneal surface of the right eye, and the classical clinical signs of HSV-1 keratitis were evaluated for a span of 10 days. Tear fluids were collected using 10-µL tips in a total 100-µL volume of 1× PBS at various time points (0–10 days) from both the infected and contralateral eyes of the infected group. Additionally, tears were also collected from the right eyes of the non-infected mice (n = 3) which served as controls.
HPSE Activity in Clinically Diagnosed HSV-1 Keratitis Patients
Prior to the HPSE level assessment, the samples were brought down to room temperature and the total protein concentration was estimated in the cryopreserved samples using the bicinchoninic acid assay, followed by protein normalization. The HPSE levels in the tear fluids were assayed using the Heparan Sulfate Degrading Enzyme Assay Kit (MK412; Takara Bio, Kyoto, Japan) as per the manufacturer's instructions. Briefly, 50 µL of diluted samples and standard were added to 96-well plates (167008; Thermo Fisher Scientific, Waltham, MA, USA) along with 50 µL of biotinylated HS and were incubated at 37°C for 45 minutes. After incubation, the mixture of biotinylated HS was transferred to chitin binding domain (CBD)–fibroblast growth factor (FGF) immobilized on a microtiter plate (Takara Bio) and further incubated at 37°C for 15 minutes. Following the incubation, 100 µL of avidin peroxidase (POD) conjugate was added and incubated for 30 minutes at 37°C. Finally, 100 µL of POD substrate was added followed by 5 to 15 minutes of incubation at room temperature in the dark. After the microplate reader (SpectraMax M3; Molecular Devices, San Jose, CA, USA) was zeroed using distilled water, the samples were read at 450 nm. The detection range of the kit was 0 to 4.20 units/mL. A standard curve was generated using MyCurve Fit (four-parameter analysis). The values of a, b, c, and d were substituted into the formula: y = –d + [(a – b)/(1 + (x/c)a)], where y is the absorbance measured at 450 nm and x is the HPSE activity.
HPSE Activity in C57BL/6 Mice
HPSE activity assays for tear fluids collected from mice were performed using the AMSBIO Heparanase Assay Kit (Ra001-BE-K; AMSBIO, Cambridge, MA, USA). The samples were processed following the instructions provided by the manufacturer. Briefly, wells were covalently coated with biotinylated HS. The media of cells treated as indicated were then added to the wells and allowed to incubate for 1 hour at 37°C. The HS fragments were then washed away followed by the addition of horseradish peroxidase–conjugated streptavidin and the 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. The absorbance was read at 450 nm. Decreases in absorbance are directly proportional to HPSE activity. The activity of the samples (ng HS removed per minute) was calculated. Briefly, R was calculated using the formula: R = (OD/Max OD) × 500, where Max optical density (OD) is the maximum reading possible and OD is the reading obtained from the test well. Then, to obtain the HPSE activity, R was substituted into the following formula: Activity = (500 – R)/60 ng HS removed per minute.
Statistical Analysis
Correlation between the tear HPSE levels in different forms of keratitis patients was determined using Prism (GraphPad, Boston, MA, USA). Descriptive statistics using mean ± standard error (SE) were used to elucidate the demographic data. Student's t-test was used to compare parametric data between two groups with an equal variance, one-way ANOVA was used to compare among three groups, and the χ2 test was used to investigate the relationship between two categorical variables For in vivo assays, two-way ANOVA was used to make comparisons among the means of three or more groups of data where two independent variables were considered. P < 0.05 was considered statistically significant.
Results
Demographic Details
The study cohort was comprised of 120 individuals. Tear fluids were collected from 30 individuals diagnosed with infectious keratitis, which included HSV-1, bacterial, and fungal, along with healthy controls having no other ocular comorbidity. The overall mean age of the infectious keratitis was 49.41 ± 1.79 years (range, 10–83) and was dominant in males (70%, n = 63). For the healthy individuals (n = 30), the mean age was 26.87 ± 0.42 years (range, 22–32 years), and the group included 11 males (36.67%). HSV-1 keratitis included epithelial keratitis (n = 8), stromal keratitis (n = 11), and endotheliitis (n = 8), as well as a combination of epithelial and stromal keratitis (n = 3). Five of the 30 HSV-1 keratitis patients were suspected to have bilateral infections, and 11 out of thirty cases had recurrent HSV-1 infection. The demographic details for the patients clinically diagnosed with infectious keratitis are included in Table 1.
Table 1.
Demographic Characteristics of Patients Clinically Diagnosed With Infectious Keratitis Included in the Study Cohort
| Demographic Features | HSV-1 Keratitis (n = 30) | Bacterial Keratitis (n = 30) | Fungal Keratitis (n = 30) | Significance |
|---|---|---|---|---|
| Age (y), mean ± SE | 48.27 ± 4.14 (10–76) | 50.9 ± 3.88 (19–83) | 49.07 ± 3.43 (19–80) | 0.83a |
| Gender (male:female), n | 19:11 | 23:7 | 21:9 | 0.52b |
| Initial visual acuity, n | — | |||
| >20/200 | 15 | 23 | 21 | |
| >20/20–<20/200 | 14 | 7 | 9 | |
| 20/20 | 1 | — | — | |
| Diagnosis (n) | Epithelial keratitis (8) | — | ||
| Stromal keratitis (13) | ||||
| Endotheliitis (7) | ||||
| Epithelial and stromal keratitis (2) |
One-way ANOVA.
χ2 test (P > 0.05 not significant).
Microbiology Details
Corneal scrapings were collected from patients diagnosed with clinically suspected microbial keratitis for a complete microbiological workup as per the institutional protocol described previously.19 Tear fluids were additionally collected using capillary tubes prior to corneal scraping from the infected eye only. Among the cases of bacterial keratitis, 23 of 30 were smear positive, 19 of 30 were culture positive, and 10 of 30 were positive for both smear and culture. Twenty-nine of 30 clinically suspected fungal keratitis patients were smear positive, and 18 of 30 were positive for both smear and culture. The microbiological details for bacterial and fungal keratitis are provided in Table 2. Corneal scrapings were collected for nine of 30 HSV-1 keratitis individuals for PCR and two of 30 for immunofluorescence assay (IFA); they were positive for HSV-1 DNA (by conventional PCR) and HSV-1 antigen (by IFA), respectively.
Table 2.
Microbiological Details for Clinically Suspected Patients With Bacterial and Fungal Keratitis
| Microbiology | Bacterial Keratitis (n = 30) | Fungal Keratitis (n = 30) |
|---|---|---|
| Culture positive, n | 19 | 18 |
| Acinetobacter baumannii (2) | Aspergillus flavus (4) | |
| Corynebacterium amycolatum (2) | Aspergillus fumigatus (2) | |
| Moraxells lacunata (1) | Enterococcus casseliflavus (1) | |
| Mycobacterium spp. (2) | Fusarium spp. (6) | |
| Nocardia spp. (2) | Lasiodiplodia theobromae (1) | |
| Pseudomonas aeruginosa (2) | Paecilomyces lilacinus (1) | |
| Staphylococcus aureus (2) | Phoma spp. (1) | |
| Staphylococcus epidermidis (2) | Unidentified dematiaceous fungus (1) | |
| Staphylococcus xylosus (1) | ||
| Stenotrophomanas maltophilia (1) | ||
| Streptococcus pneumoniae (1) |
HPSE Levels in Patients Diagnosed With Keratitis
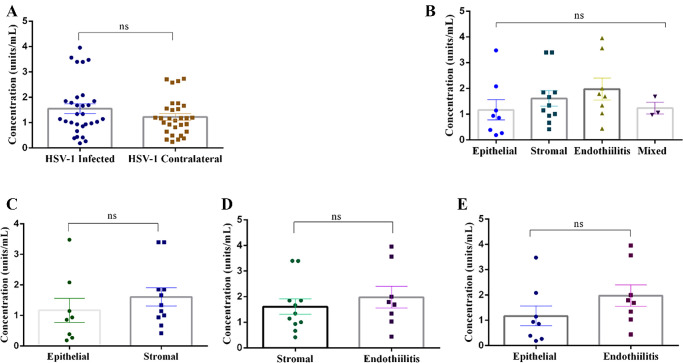
The average HPSE levels from the tears procured from the HSV-1 keratitis and contralateral eyes were 1.55 ± 0.19 units/mL and 1.23 ± 0.13 units/mL contralateral, respectively. Surprisingly, there was no significant difference between the tear HPSE levels when the two groups were compared (Fig. 2A). Similarly, no statistical significance was found when the HPSE levels were compared among the HSV-1 groups: epithelial keratitis (1.17 ± 0.39 units/mL, n = 8), stromal keratitis (1.61 ± 0.3 units/mL, n = 11), endotheliitis (1.98 ± 0.43 units/mL, n = 8), and the combination of epithelial and stromal HSV-1 keratitis (1.24 ± 0.22 units/mL, n = 3) (Fig. 2B). The HPSE levels of individual patients with each form of HSV-1 keratitis are provided in Supplementary File S2.
Figure 2.
Scatterplot comparing tear HPSE levels. (A) HSV-1 contralateral eye. (B) The Various forms of HSV-1 keratitis. (C) Epithelial versus stromal keratitis. (D) Stromal versus endotheliitis. (E) Epithelial versus endotheliitis. Statistical analysis was done using Student's t-test. *P < 0.05.
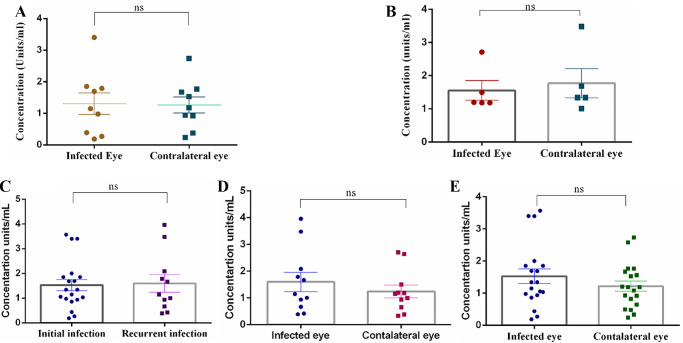
PCR was positive for the HSV-1 DNA for nine of 30 patients, but no statistical difference was observed in the HPSE expression between the infected eye (1.32 ± 0.34 units/mL) and the contralateral eye (1.26 ± 0.26 units/mL) (Fig. 3A). The tear HPSE levels were also calculated for bilateral infections (Fig. 3B) and initial versus recurrent infections (only in the infected eye) (Fig. 3C), but there was no significant difference in the expression of the enzyme. Moreover, there was no expression of tear HPSE among the initially infected HSV-1 versus contralateral eye (Fig. 3D) and the recurrent infected HSV-1 eye versus contralateral eye (Fig. 3E). The details regarding the subanalysis are provided in Table 3.
Figure 3.
Scatterplot comparing tear HPSE levels. (A) PCR-positive patients (9/30). (B) Bilateral infections (5/30). (C) Initial (19/30) versus recurrent (11/30) HSV-1 keratitis. (D) Recurrent HSV-1 keratitis (11/30). (E) Initial HSV-1 keratitis (19/30). Statistical analysis was done using Student's t-test for graphs. *P < 0.05.
Table 3.
Comparison of HPSE Levels Within HSV-1 Keratitis Individuals
| HSV-1 Keratitis Individuals | HPSE (units/mL) Mean ± SE | Significance |
|---|---|---|
| HSV-1 keratitis | 0.82a | |
| Infected eye (30) | 1.55 ± 0.19 | |
| Contralateral eye (30) | 1.23 ± 0.13 | |
| PCR positive | 0.46a | |
| Infected eye (9) | 1.30 ± 0.34 | |
| Contralateral eye (9) | 1.26 ± 0.25 | |
| HSV-1 keratitis stage (only infected eye) | 0.43a | |
| Initial HSV-1 keratitis (19) | 1.59 ± 0.35 | |
| Recurrent HSV-1 keratitis (11) | 1.52 ± 0.22 | |
| Initial HSV-1 keratitis | 0.13a | |
| Infected eye (19) | 1.59 ± 0.35 | |
| Contralateral eye (19) | 1.22 ± 0.16 | |
| Recurrent HSV-1 keratitis | 0.21a | |
| Infected eye (11) | 1.59 ± 0.35 | |
| Contralateral eye (11) | 1.25 ± 0.24 | |
| Bilateral infections | 0.34a | |
| Infected eye (5) | 1.77 ± 0.44 | |
| Contralateral eye (5) | 1.55 ± 0.29 | |
| HSV-1 characterization | 0.27b | |
| Epithelial keratitis (8) | 1.17 ± 0.39 | |
| Stromal keratitis (11) | 1.61 ± 0.3 | |
| Endothelial keratitis (8) | 1.98 ± 0.43 | |
| Mixed HSV-1 keratitis (3) | 1.24 ± 0.22 |
Student's t-test.
One-way ANOVA (Kruskal–Wallis test); P < 0.05 considered significant.
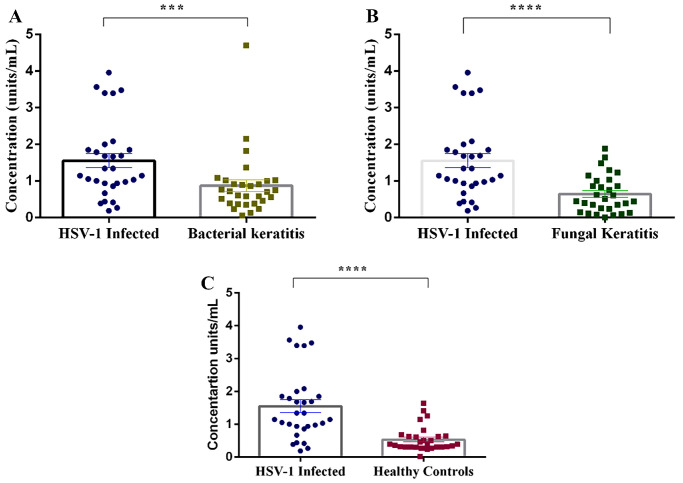
In comparison to HSV-1 keratitis, tear HPSE levels were lower for cases of bacterial keratitis (0.87 ± 0.15 units/mL) (Fig. 4A) and fungal keratitis (0.64 ± 0.09 units/mL) (Fig. 4B). Further, these levels were significantly lower in healthy individuals (0.53 ± 0.06 units/mL; P < 0.00001) (Fig. 4C). The levels of tear HPSE across the study population are shown in Table 4. The level of HPSE in each patient diagnosed with bacterial and fungal keratitis is provided in Supplementary File S3.
Figure 4.
Scatterplot depicting the elevation of tear HPSE levels in HSV-1 keratitis patients when compared to clinically and microbiologically confirmed patients with bacterial keratitis (A) or fungal keratitis (B). Tear HPSE levels were also compared between HSV-1 keratitis individuals and healthy controls (C). Statistical analysis was done using Student's t-test for graphs. *P < 0.05.
Table 4.
HPSE Levels Among the Study Population and Control
| Study Population | HPSE (Units/mL) Mean ± SE | Significancea |
|---|---|---|
| Bacterial keratitis (n = 30) | 0.87 ± 0.15 | 0.004 |
| Fungal keratitis (n = 30) | 0.64 ± 0.09 | <0.00001 |
| Healthy controls (n = 30) | 0.53 ± 0.06 | <0.00001 |
Student's t-test; P < 0.05 considered significant.
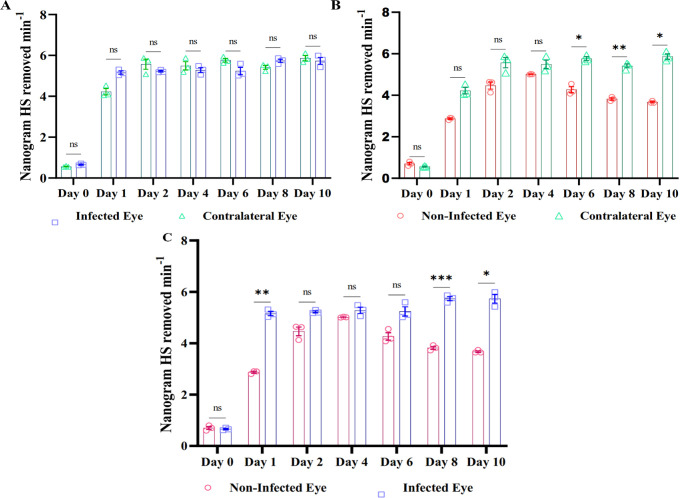
HPSE Activity in HSV-1–Infected C57BL/6 Mice
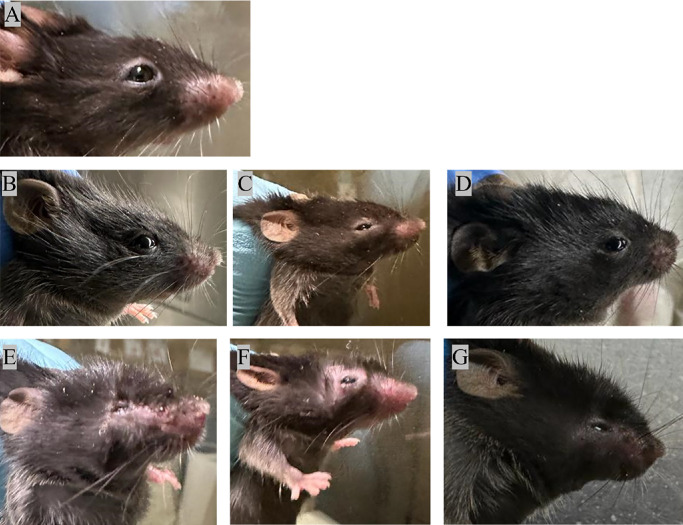
To investigate HPSE expression in the tears of HSV-1–infected mice, the right eyes of C57BL/6 mice were scratched and infected with HSV-1 (Fig. 5). Prior to the infection, tear fluid was collected from the entire study population. After HSV-1 corneal infection, tears were collected until 10 days post-infection from HSV-1 infected, contralateral, and healthy mice eyes. HPSE activity assays in the tear samples indicated that there was an initial increase in the tear HPSE levels in HSV-1–infected mice eye (scratched right eyes; 0.66–5.73 ng HS removed per minute) when compared to the contralateral eye (unscratched left eyes; 0.56–5.87 ng HS removed per minute); however, the difference was not statistically significant (Fig. 6A). The scratched right eyes of healthy mice also showed an increase in tear HPSE levels from baseline, but it was not significantly different when compared to contralateral non-scratched eyes until 4 days after epithelial debridement. Surprisingly, 4 days post-infection, a significantly higher amount of HPSE was found in the contralateral eyes of the infected mice (Fig. 6B). Finally, when HPSE activity was compared between the infected eyes (scratched right eyes) and non-infected eyes (unscratched left eyes), we observed that, with the progression in days, HPSE expression was elevated in the infected eyes (0.66–5.73 ng HS removed per minute) of mice when compared to their healthy counterparts (0.70–3.67 ng HS removed per minute). The levels of tear HPSE from animal eyes are provided in Supplementary File S4.
Figure 5.
Representative images of eyes of C57BL/6 mice: (A) Prior to the infection and post-infection on days 1, 2, 4, 6, 8, and 10 (B–G).
Figure 6.
Scatterplot comparing tear HPSE expression between infected and contralateral eyes of C57BL/6 mice (A), between non-infected or healthy mice and contralateral eyes (B), and between healthy eyes and infected eyes (C). Statistical analysis was done using two-way-ANOVA for graphs. *P < 0.05.
Discussion
HSV-1 keratitis is a serious ocular manifestation, and understanding the underlying host mechanisms and potential markers, such as tear HPSE expression, can be beneficial to better diagnosis and prognosis of the eye disease. HPSE is an endo-β-d-glycosidase that is ubiquitously expressed and required for the removal of HS during infection. Moreover, the enzyme, being a pro-angigogenic factor, not only allows the virus to spread within the cornea but also causes angiogenesis of the cornea. Previous studies by our group indicated an increase in HPSE expression in the virally infected corneas of Balb/c mice and human corneal epithelial cells, and knocking down the HPSE expression helped to reduce spreading of the virus throughout the cornea.14 A study by Lobo et al.,24 who used host corneal buttons, showed that this mammalian host enzyme was upregulated in the corneal epithelium and stromal tissues of 10 patients diagnosed with HSV-1 keratitis. Various other groups have also predicted a role for HPSE in viral pathogenesis in vitro.25–27 The findings of these studies support a role for HPSE in viral pathogenesis in cell lines and animal models, but it is important to be able to predict its expression in clinical samples of patients diagnosed with presumed HSV-1 keratitis to routinely use it as a therapeutic. To confirm the expression of HPSE in tear fluids, we evaluated its expression in HSV-1 keratitis patients. We found significant upregulation of tear HPSE levels in HSV-1 keratitis individuals when compared to their counterparts (Fig. 4).
An increase in HPSE expression is not only specific to viral infections but also involved in various other inflammatory conditions such as cancer, neuroinflammation,28–30 bacterial infections, gastric cancer,31 inflammatory bowel disease,32 and diabetic retinopathy.33 When we compared the tear HPSE levels between the HSV-1 keratitis and bacterial keratitis patients, there was a significant difference in HPSE levels (0.87 ± 0.15 units/mL; P = 0.004) (Fig. 4A). Despite strict scrutiny before the samples were selected, four of the 30 patients with bacterial keratitis had higher HPSE levels. It might be possible that those individuals visited the clinic in the prodromal stage. Moreover, secondary bacterial infections are associated with HSV-1 keratitis, so it is quite possible that those groups of patients were exposed to bacterial infections at some stage of their life or the virus might have been in the latent phase.34 Furthermore, significant differences in tear HPSE levels were found between patients with fungal keratitis and those with HSV-1 keratitis (0.64 ± 0.09 units/mL; P < 0.00001) (Fig. 4B).
When the tear HPSE levels for healthy controls were compared with those of HSV-1 keratitis patients, significant expression was noted in the infected eyes (Fig. 4C). But, five of the 30 healthy controls who had no ocular or systemic comorbidity expressed HPSE in their tears, indicative of subclinical shedding of HSV-1. Kaufman et al.35 reported that around 70% (n = 50) of individuals having no ocular comorbidity were positive for HSV-1.36
It was also surprising to find that there was no statistical difference in the HPSE levels between contralateral eyes when compared to the infected eyes of the HSV-1 keratitis patients (Fig. 2A). This might be due to subclinical inflammation without having any signs of classical keratitis, but the exact cause behind the expression remains to be determined. It is presumed that there might be neutrophil infiltration to the site of the infection, which releases neutrophil extracellular traps (NETs) as a primary defense mechanism against the infection.37 Studies have shown the NETs play a role in fungal and bacterial ocular infections, as well as in viral infections such as influenza virus and SARS–CoV-2.38–41 However, their role in ocular HSV-1 infections remains a mystery. Further studies are necessary to provide insights into the role of NETosis in HSV-1 keratitis.
Finally, to validate our findings we carried out an in vivo experiment in which it was found that there was no difference in the HPSE activity between the infected and contralateral eyes of the mice, which corroborated our human study (Fig. 6A). There was a significant increase in HPSE expression in the non-infected eyes when compared to the contralateral eyes (Fig. 6B), which coincides with the tear HPSE levels found in the healthy human controls. Interestingly, we found an increase in HPSE expression in the non-infected eyes of the mice until day 4 post-infection, when it decreased (Fig. 6C). Because HPSE expression is associated with inflammation, there could be cell migration to the scratch site for wound healing and inflammation reduction.
This study utilized patient samples to evaluate HPSE expression. All of the different forms of HSV-1 keratitis (epithelial, stromal, and endothelial) were evaluated in the study. The strictest scrutiny was applied to confirm the presence of bacterial keratitis or fungal keratitis. With regard to study limitations, the sample size was a major drawback. We could not derive any conclusions regarding differences in HPSE expression among initial and recurrent HSV-1 keratitis (Fig. 3C), bilateral infections (Fig. 3B), or different forms of HSV-1 keratitis (Fig. 2B).
To conclude, to the best of our knowledge, our study is the first to include patient samples to evaluate tear HPSE levels in HSV-1 keratitis. The results agree with reported tear HPSE expression in in vivo models and indicate that tear HPSE levels could help in distinguishing HSV-1 keratitis from non-viral pathogenesis. From the findings of this study, it can be predicted that identifying HPSE levels in the tears of presumed HSV-1 keratitis patients would significantly contribute to disease management. Further, studies using HPSE inhibitors might lead to pathbreaking therapeutic targets to better diagnosis and improve the prognosis of herpes simplex keratitis.
Supplementary Material
Acknowledgments
The authors thank the Lady Tata Junior Research Fellowship for funding Satyashree Gagan during the pursuit of her PhD.
Supported by grants from the Indian Council of Medical Research (35/8/2019-Nano/BMS), Ramoji Foundation Centre for Ocular Infections (16119), National Institutes of Health Research Project (R01 EY029426 to DS), and National Eye Institute, National Institutes of Health (P30 EY001792). Additional support was provided by the Hyderabad Eye Research Foundation.
Disclosure: S. Gagan, None; A. Khapuinamai, None; D. Kapoor, None; P. Sharma, None; T. Yadavalli, None; J. Joseph, None; D. Shukla, None; B. Bagga, None
References
- 1. Chaloulis SK, Mousteris G, Tsaousis KT.. Incidence and risk factors of bilateral herpetic keratitis: 2022 update. Trop Med Infect Dis. 2022; 7(6): 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Looker KJ, Magaret AS, May MT, et al.. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One. 2015; 10(10): e0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koganti R, Yadavalli T, Shukla D.. Current and emerging therapies for ocular herpes simplex virus type-1 infections. Microorganisms. 2019; 7(10): 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koujah L, Suryawanshi RK, Shukla D.. Pathological processes activated by herpes simplex virus-1 (HSV-1) infection in the cornea. Cell Mol Life Sci. 2019; 76(3): 405–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brady RC, Bernstein DI.. Treatment of herpes simplex virus infections. Antiviral Res. 2004; 61(2): 73–81. [DOI] [PubMed] [Google Scholar]
- 6. Farooq S, Farooq SD, Shah SD. The role of herpesviruses in ocular infections. Virus Adapt Treat. 2010; 2: 115–123. [Google Scholar]
- 7. Das AV, Satyashree G, Joseph J, et al.. Herpes simplex virus keratitis: electronic medical records driven big data analytics report from a tertiary eye institute of South India. Int Ophthalmol. 2023; 43(12): 4669–4676. [DOI] [PubMed] [Google Scholar]
- 8. Agelidis AM, Hadigal SR, Jaishankar D, Shukla D.. Viral activation of heparanase drives pathogenesis of herpes simplex virus-1. Cell Rep. 2017; 20(2): 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grinde B. Herpesviruses: latency and reactivation - viral strategies and host response. J Oral Microbiol. 2013; 5, 10.3402/jom.v5i0.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wishart MS, Darougar S, Viswalingam ND.. Recurrent herpes simplex virus ocular infection: epidemiological and clinical features. Br J Ophthalmol. 1987; 71(9): 669–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farooq AV, Shukla D.. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012; 57(5): 448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shuster JJ, Kaufman HE, Nesburn AB.. Statistical analysis of the rate of recurrence of herpesvirus ocular epithelial disease. Am J Ophthalmol. 1981; 91(3): 328–331. [DOI] [PubMed] [Google Scholar]
- 13. Shukla SD, Valyi-Nagy T.. Host molecules that promote pathophysiology of ocular herpes. Front Microbiol. 2022; 13: 818658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hadigal S, Koganti R, Yadavalli T, Agelidis A, Suryawanshi R, Shukla D.. Heparanase-regulated syndecan-1 shedding facilitates herpes simplex virus 1 egress. J Virol. 2020; 94(6): e01672–e01719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lobo AM, Agelidis AM, Shukla D.. Pathogenesis of herpes simplex keratitis: the host cell response and ocular surface sequelae to infection and inflammation. Ocul Surf. 2019; 17(1): 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Donnell CD, Shukla D.. The importance of heparan sulfate in herpesvirus infection. Virol Sin. 2008; 23(6): 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abboud-Jarrous G, Rangini-Guetta Z, Aingorn H, et al.. Site-directed mutagenesis, proteolytic cleavage, and activation of human proheparanase. J Biol Chem. 2005; 280(14): 13568–13575. [DOI] [PubMed] [Google Scholar]
- 18. Karasneh GA, Kapoor D, Bellamkonda N, Patil CD, Shukla D.. Protease, growth factor, and heparanase-mediated syndecan-1 shedding leads to enhanced HSV-1 egress. Viruses. 2021; 13(9): 1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hadigal SR, Agelidis AM, Karasneh GA, et al.. Heparanase is a host enzyme required for herpes simplex virus-1 release from cells. Nat Commun. 2015; 6(1): 6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma P, Kapoor D, Shukla D. Role of heparanase and syndecan-1 in HSV-1 release from infected cells. Viruses. 2022; 14: 2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tiwari V, Liu J, Valyi-Nagy T, Shukla D.. Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo. J Biol Chem. 2011; 286(28): 25406–25415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suryawanshi RK, Patil CD, Agelidis A, et al.. Pathophysiology of reinfection by exogenous HSV-1 is driven by heparanase dysfunction. Sci Adv. 2023; 9(17): eadf3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Labib BA, Chigbu DI.. Clinical management of herpes simplex virus keratitis. Diagnostics (Basel). 2022; 12(10): 2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lobo A-M, Shukla D. Heparanase upregulation in human corneal tissues infected with herpes simplex virus type 1. Invest Ophthalmol Vis Sci. 2018; 59(9): 513. [Google Scholar]
- 25. Shukla D, Spear PG.. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001; 108(4): 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thakkar N, Yadavalli T, Jaishankar D, Shukla D.. Emerging roles of heparanase in viral pathogenesis. Pathogens. 2017; 6(3): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bacsa S, Karasneh G, Dosa S, Liu J, Valyi-Nagy T, Shukla D.. Syndecan-1 and syndecan-2 play key roles in herpes simplex virus type-1 infection. J Gen Virol. 2011; 92(pt 4): 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanderson RD, Elkin M, Rapraeger AC, Ilan N, Vlodavsky I.. Heparanase regulation of cancer, autophagy and inflammation: new mechanisms and targets for therapy. FEBS J. 2017; 284(1): 42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldberg R, Meirovitz A, Hirshoren N, et al.. Versatile role of heparanase in inflammation. Matrix Biol. 2013; 32(5): 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang X, Wang B, Li JP. Implications of heparan sulfate and heparanase in neuroinflammation. Matrix Biol. 2014; 35: 174–181. [DOI] [PubMed] [Google Scholar]
- 31. Liu L, Zhao Y, Fan G, Shuai T, Li B, Li Y. Helicobacter pylori infection enhances heparanase leading to cell proliferation via mitogen‑activated protein kinase signalling in human gastric cancer cells. Mol Med Rep. 2018; 18(6): 5733–5741. [DOI] [PubMed] [Google Scholar]
- 32. Waterman M, Ben-Izhak O, Eliakim R, Groisman G, Vlodavsky I, Ilan N.. Heparanase upregulation by colonic epithelium in inflammatory bowel disease. Mod Pathol. 2007; 20(1): 8–14. [DOI] [PubMed] [Google Scholar]
- 33. Gil N, Goldberg R, Neuman T, et al.. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012; 61(1): 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kitzmann AS, Goins KM, Syed NA, Wagoner MD.. Bilateral herpes simplex keratitis with unilateral secondary bacterial keratitis and corneal perforation in a patient with pityriasis rubra pilaris. Cornea. 2008; 27(10): 1212–1214. [DOI] [PubMed] [Google Scholar]
- 35. Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM.. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005; 46(1): 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coyle PK, Sibony PA.. Viral antibodies in normal tears. Invest Ophthalmol Vis Sci. 1988; 29(10): 1552–1558. [PubMed] [Google Scholar]
- 37. Kapoor D, Shukla D.. Neutrophil extracellular traps and their possible implications in ocular herpes infection. Pathogens. 2023; 12(2): 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shan Q, Dwyer M, Rahman S, Gadjeva M.. Distinct susceptibilities of corneal Pseudomonas aeruginosa clinical isolates to neutrophil extracellular trap-mediated immunity. Infect Immun. 2014; 82(10): 4135–4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jin X, Zhao Y, Zhang F, et al.. Neutrophil extracellular traps involvement in corneal fungal infection. Mol Vis. 2016; 22: 944–952. [PMC free article] [PubMed] [Google Scholar]
- 40. Narasaraju T, Yang E, Samy RP, et al.. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011; 179(1): 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cortjens B, de Boer OJ, de Jong R, et al.. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J Pathol. 2016; 238(3): 401–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.