Abstract
We have previously identified a transcriptional silencer that is critical for proper expression of the CD4 gene during T-cell development. Here we report that the Hairy/Enhancer of Split homologue HES-1, a transcription factor in the lin12/Notch signaling pathway, binds to an important functional site in the CD4 silencer. Overexpression of HES-1 leads to the silencer site-dependent repression of CD4 promoter and enhancer function as well as the downregulation of endogenous CD4 expression in CD4+ CD8− TH cells. Interestingly, overexpression of an activated form of Notch1 (NotchIC) leads to the repression of CD4 promoter and enhancer function both in the presence and absence of the silencer. NotchIC-mediated CD4 silencer function is not affected by the deletion of the HES-1-binding site, indicating that multiple factors binding to CD4 transcriptional control elements are responsive to signaling from this pathway, including other silencer-binding factors. Taken together, these data are consistent with the hypothesis that the lin12/Notch signaling pathway is important in thymic development and provide a molecular mechanism via the control of CD4 gene expression in which the lin12/Notch pathway affects T-cell developmental fate.
The expression of the CD4 coreceptor on developing T lymphocytes is tightly regulated and linked to the molecular events that drive repertoire selection (16, 32, 40). Expression of the CD4 gene is controlled by at least five distinct transcriptional control elements, including the promoter, three enhancers, and a silencer (1, 9–11, 42, 43, 45, 46, 50, 51, 57, 65). The silencer is the critical controlling element that downregulates CD4 transcription at several stages of T-cell development. First, the silencer represses CD4 transcription in the most immature T cells in the thymus, the CD4− CD8− double-negative cells. The CD4 silencer then ceases function in these cells, leading to the expression of CD4. CD8, a similar coreceptor molecule, is also expressed at this stage; the resulting thymocyte, referred to as the CD4+ CD8+ double-positive (DP) thymocyte, then undergoes the positive and negative selection processes required to ensure a properly restricted T-cell repertoire. T cells that survive this process have two potential developmental fates. Maturing DP thymocytes can maintain expression of CD4 and become CD4+ CD8− major histocompatibility complex class II-restricted T cells, which are primarily helper T cells. Alternatively, surviving thymocytes can downregulate CD4 and become CD4− CD8+ T cells, which are primarily cytotoxic T cells. The latter developmental fate decision requires the reinitiation of CD4 silencer function, leading to the repression of transcription of the CD4 gene and permitting the development of the mature CD4− CD8+ T cells from its CD4+ CD8+ DP precursor. Thus, CD4 silencer function correlates with both the developmental fate of the T cell and its mature functional subclass. An analysis of the factors that mediate CD4 silencer function will therefore provide insight into the molecular mechanisms that drive these processes. Using molecular, cellular, and transgenic techniques, we have identified three factor-binding sites in the CD4 silencer, all of which are required for full silencer function (10). To date, the factors that bind to these regions and mediate silencer function, however, have not been identified.
The lin12/Notch signaling pathway plays a critical role in developmental cell fate decisions in many different systems (2, 19, 20). In Caenorhabditis elegans, lin12 is a transmembrane protein that mediates intracellular signals specifying cell fate in vulval development. In Drosophila melanogaster, the homologous pathway is activated when the Notch receptor binds to its ligands, resulting in the translocation of the DNA-binding protein Suppressor of Hairless [Su(H)] to the nucleus (3, 31, 55). Once there, Su(H) binds to the transcriptional control regions of many genes that are important in the control of development, including the Enhancer of Split [E(spl)] genes, and induce their expression (3, 23, 24). Both epistasy tests and cell transplantation experiments place the E(spl) locus as an end point for the Notch pathway (17, 23, 31, 64). In Drosophila, the E(spl) locus is a complex of at least eight genes: seven basic helix-loop-helix (bHLH) proteins (referred to as mδ, mγ, mβ, m3, m5, m7, and m8) and an eighth nuclear protein (groucho) (8, 26, 38). The E(spl) genes themselves are believed to control the transcription of genes important in development (25, 36, 38, 56).
The involvement of the lin12/Notch pathway in the mammalian immune system has recently been characterized. Most of the mammalian homologues of the lin12/Notch pathway are expressed at high levels in the thymus, including Notch1, Su(H), and at least one E(spl) homologue (22, 44, 62). As in D. melanogaster, expression and function of the mammalian E(spl) homologue HES-1 is directly induced by Notch signaling and is thus an end point in the mammalian lin12/Notch pathway (23). There are at least four different mammalian homologues of Notch, referred to as Notch1, -2, -3, and -4 (7, 28–30, 58, 62, 63). Experiments by several groups have indicated that Notch signaling plays an important role in T-cell development and oncogenesis, although the precise mechanism has yet to be established conclusively (13, 18, 39, 41, 60). Interestingly, Robey and coworkers have demonstrated that the overexpression of an activated form of murine Notch1 biases T-cell development toward CD8 single-positive (SP) T cells, indicating that Notch1 is involved in T-cell fate determination (41, 60). The precise molecular mechanism in which Notch1 functions to drive development of mature CD8 SP T lymphocytes is unclear. Here we describe the characterization of one of the nuclear factors binding to the CD4 silencer in an important functional region. We determine that the mammalian E(spl) homologue HES-1 binds to S1 of the CD4 silencer. In transient-transfection analyses, overexpression of HES-1 leads to the repression of CD4 promoter and enhancer function that is dependent on the presence of both the CD4 silencer and an intact S1. In addition, the overexpression of HES-1 leads to the downregulation of endogenous CD4 expression in CD4+ CD8− TH cells. Interestingly, an activated form of Notch1 can repress CD4 promoter and enhancer both in the presence and absence of the silencer; in addition, deletion of S1 does not affect the ability of activated Notch to stimulate silencer function. These data indicate that signaling from the lin12/Notch pathway can affect multiple factors that control CD4 gene expression, including other silencer-binding factors. Our results support the hypothesis that the lin12/Notch pathway plays a role in thymic development and provides a mechanism in which it may affect T-cell lineage fate decisions.
MATERIALS AND METHODS
Nuclear extract purification.
Nuclear extracts were purified from the different cell lines as described previously (61). Cells were washed once in 1× phosphate-buffered saline with 5 mM MgCl2 and resuspended in 4 packed-cell volumes of buffer H (10 mM Tris HCl [pH 7.9], 10 mM KCl, 0.75 mM spermidine, 0.15 mM spermine, 0.1 mM EDTA, 0.1 mM EGTA, 2 mM dithiothreitol (DTT), 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 2 mg of benzamidine per ml, 1 μg of pepstatin per ml, 4 μg of leupeptin per ml, 10 μg of aprotinin per ml). The cells were allowed to swell on ice for 15 min and lysed with 10 to 15 strokes (or greater, as needed) of a Dounce homogenizer. The nuclei were pelleted at 3,000 × g for 8 min, washed once in buffer H, and resuspended in 4 packed-cell volumes of buffer D (50 mM Tris HCl [pH 7.5], 10% sucrose, 0.42 M KCl, 5 mM MgCl2, 0.1 mM EDTA, 20% glycerol, 2 mM DTT, 0.1 mM PMSF, 2 mg of benzamidine per ml, 1 μg of pepstatin per ml, 4 μg of leupeptin per ml, 10 μg of aprotinin per ml). The suspension was stirred on ice in the cold room for 30 min and centrifuged at 25,000 rpm for 1 h in a SW28 rotor. The supernatant was recovered, proteins were precipitated with 53% (NH4)2SO4 (0.33 g/ml of supernatant), and centrifuged at 16,000 rpm for 20 min. Pellets were recovered and resuspended in a solution containing 50 mM Tris HCl (pH 7.6), 12.5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 20% glycerol, and 0.1 M KCl and desalted on a Biogel P10 filtration column. Protein concentration of the resulting fractions was determined by the Coomassie blue assay (Pierce). Fractions containing protein were then pooled, realiquoted, and stored at −70°C.
Biochemical experiments.
The glutathione S-transferase (GST)–HES-1 fusion protein and the nuclear extracts from the 293T cells were provided by Kevin Fitzgerald and Jan Kitajewski and GuangYu Wu, respectively. The polyclonal rabbit anti-HES-1 antibody was generated against the GST–HES-1 fusion protein by BABCO (Berkeley, Calif.). Serum was purified by using protein A-Sepharose (Pharmacia) and used at 1:1,000 dilution. Electrophoretic mobility shift assay (EMSA) analyses were conducted as described previously (10). For the cold competition experiments, 50 or 250 molar excess of nonradioactive oligonucleotides were added to the binding mix without the radioactive probe and incubated at room temperature for 20 min. The radioactive probe was then added, and the EMSA was then performed. For UV cross-linking, the normal EMSA binding reaction was performed and subsequently exposed for 15 min to short-wave UV light by using the Stratalinker (Stratagene). Immunoprecipitations of the UV cross-linking reactions were precleared with the preimmune sera before immunoprecipitating with the anti-HES-1 antiserum, resolved on a sodium dodecyl sulfate–10% polyacrylamide gel, dried, and exposed to X-ray film for 3 to 7 days. For the Western blot analyses, the serum was first purified using a protein A-Sepharose (Pharmacia) and used at 1:1,000 dilution. The blots were developed with the BM chemiluminescence kit (Boehringer Mannheim) as described by the manufacturer.
Construct synthesis.
The pT vector was constructed in the pGL2 reporter vector and contains a 1.3-kb BglII fragment encompassing the CD4 distal enhancer (DH site 1), a 600-bp PstI-BglII fragment containing DH site 2, a 1.1-kb BstXI-SacI fragment containing the proximal enhancer (DH site 3), and a 3-kb XhoI fragment containing DH site 4 and the promoter region (for a complete map of the murine CD4 locus, see reference 50). A fourfold multimerization of a 1.6-kb SacI-SpeI restriction fragment containing the silencer was cloned downstream of the luciferase gene. The pTS4mS1 construct contains a fourfold multimerization of the silencer containing a site-specific mutation changing the S1 sequence from CCACAAGG to CCATGGGG.
Transient transfections and flow cytometric analyses.
Transfections and luciferase assays were conducted as described previously (11). Reporter transfections were conducted by cotransfecting test constructs into D10 CD4 SP TH cells with the pRLtk construct containing the Renilla luciferase reporter gene under the control of the tk promoter (Promega). All data were then controlled for different transfection efficiencies by normalizing the data to the Renilla luciferase values. For the analysis of endogenous CD4 expression, 5 μg of cytomegalovirus (CMV) green fluorescent protein (GFP) expression vector was cotransfected into CD4 SP D10 TH cells with increasing amounts of expression vectors containing the NotchIC and full-length HES-1 cDNAs under the transcriptional control of the CMV and the EF-1α promoter and enhancer, respectively. For NotchIC, the cDNA contained the portion of the gene encoding the intracellular domain of Notch starting at amino acid 1810; this portion contains the intact cdc10 repeats as well as the PEST/opa domains (23). Total DNA content per transfection point was kept constant with the addition of pKs plasmid. Cells were harvested and analyzed 24 h after transfection. To analyze the transfected T cells, we utilized allophycocyanin-conjugated GK1.5 (CD4) and phycoerythrin-conjugated 2C11 (CD3) monoclonal antibodies as described previously (10). Transfected cells were identified for analysis by gating on GFP-positive cells, and dead and dying cells were excluded from analysis using propidium iodide and forward and side scatter analysis. All cells were analyzed on a FACStarPlus flow cytometer (Becton Dickinson) at the Flow Cytometry Facility of the Herbert Irving Cancer Center of Columbia University. Data are presented as 5% probability plots.
RESULTS
The S1-binding factor binds to the S1 N box with the same sequence specificity as that of HES-1.
Using EMSA techniques, we previously determined that each functional site in the silencer is recognized by one major factor complex (10). To determine if the same set of factors could be binding sites 1 (S1) and 2 (S2), we conducted cold competition EMSA analyses using all three silencer site probes (Fig. 1 and 2A). As can be seen in Fig. 2, we can detect one major and one minor factor-probe complex using T-cell extracts and the S1 radioactive probe. Both complexes can be competed away using excess nonradioactive S1 but not linker, indicating that both complexes are specific. We can also inhibit S1 complex formation by using excess nonradioactive S2 and CD4 enhancer E-box probe, both of which contain consensus E recognition sites. These results are surprising for several reasons. The S1, S2, and E-box probes do not share significant sequence similarity (Fig. 1); the only significant sequence similarity between any of these probes is the consensus E recognition site in the S2 and E-box oligonucleotides. In addition, should the same factor be binding both S1 and S2, we would have predicted that we would have been able to inhibit S2 probe and E-box complex formation with excess nonradioactive S1, but this was not the case (data not shown). These data implied that different factors were binding to S1 and S2. In addition, the S1-binding factor is capable of recognizing sequences in the S2 and E-box probes, whereas the S2- and E-box-binding factors cannot recognize sequences in the S1 probe. A more detailed sequence analysis of the first functional site (S1) revealed the presence of a consensus N box (Fig. 1). This site is recognized by HES-1, the mammalian homologue of the D. melanogaster E(spl) m8 protein (44). This factor is a bHLH protein; this class of DNA-binding factors in general recognize the E consensus site (CANNTG). However, HES-1 contains a critical proline amino acid in its DNA-binding domain that alters its recognition site specificity. Although HES-1 can still recognize a consensus E box, it binds with highest affinity to an altered version of the E box, the N box (CACNAG). The binding specificity of HES-1 is completely consistent with the hypothesis that HES-1 is binding to S1. At high competitor concentrations, the E boxes in the S2 and E probes would be expected to successfully inhibit HES-1 binding to S1, whereas the N box in the S1 probe would not be expected to prevent E-box recognition by a bHLH protein.
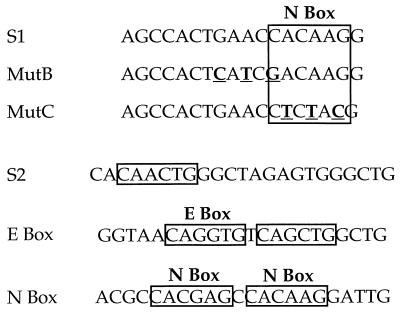
FIG. 1.
Sequences of oligonucleotide probes used in the EMSA experiments. The top box indicates consensus N-box sequence in the S1 probe; mutations in the mB (Mut B) and mC (Mut C) probes are in bold type and are underlined. S2 and E-box sequences are from the CD4 silencer and proximal enhancer, respectively (10, 45); the N-box sequence is from reference 44.
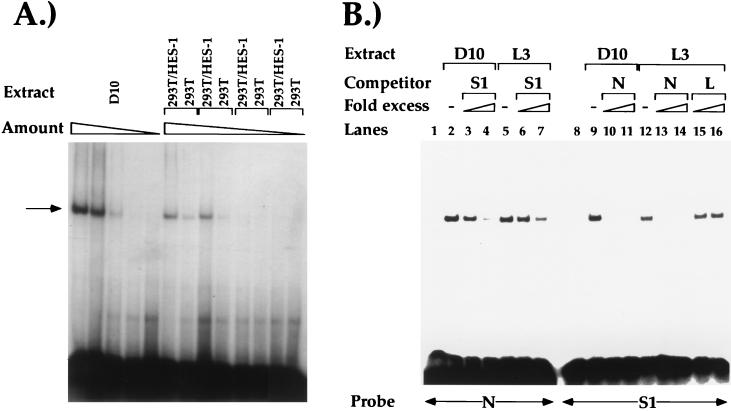
FIG. 2.
(A) Competition EMSA experiments with the S1 probe. Radioactive S1 probe was incubated with CD4 SP D10 TH clone nuclear extracts alone (lane 2) or with 50- and 250-fold molar excess nonradioactive S1 (lanes 3 and 4), S2 (lanes 5 and 6), E box (lanes 7 and 8), and pKs linker (9 and 10). (B) Competition EMSAs with the S1 probe and MutB and MutC oligonucleotides. Radioactive S1 probe was incubated with CD4 SP D10 TH clone nuclear extracts alone (lane 2) or with 50- and 250-fold excess nonradioactive S1 (lanes 3 and 4), MutB (mB) (lanes 5 and 6), and MutC (mC) (lanes 7 and 8), or with pKs linker (lanes 9 and 10). (C) Competition EMSAs with the GST–HES-1 fusion protein. Competitions were conducted with 50- and 250-fold excess nonradioactive S1 (lanes 3 and 4), MutB (mB) (lanes 5 and 6), and MutC (mC) (lanes 7 and 8) or with pKs linker (lanes 9 and 10).
To determine if the S1-binding factor in T-cell nuclear extracts recognizes the N box in S1 specifically, we performed cold-competition EMSA analyses with a radioactive S1 probe (Fig. 2B and C). Formation of the factor-S1-probe complexes can be inhibited by an excess nonradioactive S1, but not with excess pKs linker (Fig. 2B, lanes 2 to 4, 9, and 10). In addition, S1 complex formation cannot be inhibited with excess nonradioactive oligonucleotides that contain point mutations within the N-box region that abrogate HES-1 binding (Fig. 1 and 2B, lanes 5 to 8). These data indicate that the endogenous S1-binding factor binds to the N box in the S1 probe. The factors that bind to the S1 probe to form the slow- and fast-migrating complexes appear to have the same sequence specificity (Fig. 2A and B); these data indicate that these complexes may contain the same DNA-binding factor. It is possible that the slow-migrating complex consists of a homodimer of HES-1 or a heterodimer of HES-1 and a second protein (see below). To determine if the S1 N box can be recognized by HES-1, we conducted EMSA analyses with S1 and a GST–HES-1 fusion protein. As can be seen in Fig. 2C, we can detect a single factor-probe complex whose formation can be inhibited by excess nonradioactive S1 and not linker, indicating that the GST–HES-1–probe interaction is S1 sequence specific (lanes 2 to 4, 9, and 10). We cannot detect complex formation with GST protein alone (data not shown). To determine if the GST–HES-1 fusion protein is recognizing the N box in the S1 probe, we conducted cold-competition EMSA analyses with nonradioactive excesses of the S1 oligonucleotides containing the N-box-specific point mutations. As can be seen in Fig. 2C (lanes 5 to 8), we cannot inhibit GST–HES-1–probe complex formation by using nonradioactive mB or mC probe as a competitor. These data indicate that mammalian HES-1 can bind to the S1 probe specifically at the N-box site and does so with a similar sequence specificity as the endogenous S1-binding factor.
In addition, we used a mammalian transfection system to overexpress HES-1 and study its ability to bind S1. A CMV-HES-1 expression vector was transfected into 293T kidney cells, and nuclear extracts were prepared from both transfected and mock-transfected cells. Both the transfected and mock-transfected cells express endogenous HES-1; however, the transfected cells express much higher levels of HES-1 as determined by Western blot analysis, due to the expression of HES-1 from the transfected plasmid (see below). These nuclear extracts were then used in EMSA experiments using S1 as a radioactive probe (Fig. 3A). As described above, we can detect one major protein-DNA complex when using D10 and L3 nuclear extracts; when the nuclear extract is diluted, we see a gradual diminution of this band (Fig. 3A and data not shown). When we use nuclear extracts purified from 293T cells transfected with HES-1 expression vectors (lanes labelled 293T/HES-1), we can detect a single strong protein-DNA complex; in mock-transfected 293T cells, the protein-DNA complex is much weaker in intensity. As for the T-cell nuclear extracts, when the 293T/HES-1 and 293T nuclear extracts are diluted, we see a gradual diminution of the protein-DNA complex. Interestingly, the protein-DNA complex formed by the HES-1 overexpressed in the 293T cells is identical in size to that of the protein-DNA complex formed by the S1-binding factor in the T-cell nuclear extracts. These data are further evidence that the T-cell S1-binding factor is HES-1.
FIG. 3.
(A) EMSAs using the S1 probe and decreasing amounts of nuclear extracts from D10 (1, 0.5, 0.3, 0.1, and 0 μg), 293T cells transfected with CMV-HES-1 (1, 0.5, 0.3, and 0 μg), or mock-transfected 293T cells (1, 0.5, 0.3, and 0 μg). The major complex is indicated by the arrow. (B) Competition EMSAs using either D10 or CD8 SP L3 TC clone nuclear extracts alone or with either the N box (lanes 1 to 7) or the S1 probe (lanes 8 to 16). Competitor oligonucleotides were added at 50- and 250-fold excess.
HES-1 in T-cell nuclear extracts binds to S1.
To test directly whether the S1-binding factor is HES-1, we conducted cold competition EMSA analyses using both the S1 probe and an N-box probe. The N-box probe contains the HES-1 recognition site that was used initially to characterize HES-1 binding (Fig. 1). As can be seen in Fig. 3A, we can detect a single nuclear factor binding specifically to the N-box probe using T-cell nuclear extracts from both the CD4+ CD8− TH clone D10 and the CD4− CD8+ TC clone L3. Using antisera against HES-1 in UV cross-linking–immunoprecipitation experiments, we determined that the T-cell nuclear factor binding to the N-box probe is in fact HES-1 (see below). Interestingly, the HES-1–N-box probe complex that we detect is identical in size and mobility to the protein-S1 probe complex, supporting the hypothesis that HES-1 is binding to both probes. We can successfully inhibit HES-1 binding to the N-box probe using excess nonradioactive S1 oligonucleotide; similarly, we can inhibit endogenous HES-1 binding to the S1 probe using excess nonradioactive N-box oligonucleotide (Fig. 3B). These cross competition experiments provide additional evidence that the S1-binding factor is endogenous HES-1.
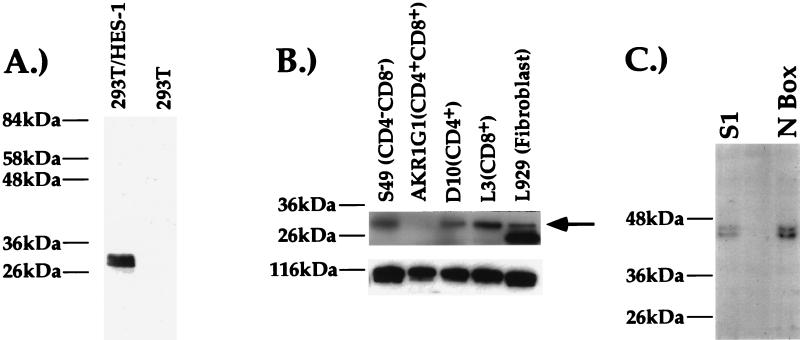
To determine conclusively if the S1-binding factor is HES-1, we generated a rabbit antiserum against the GST–HES-1 fusion protein. In Western blot analysis, this antiserum can recognize a 29-kDa band in 293T cells transfected with HES-1 expression vectors but not untransfected 293T cells, indicating that it indeed recognizes HES-1 specifically (Fig. 4A). A Western blot analysis of nuclear extracts purified from T-cell clones using this antiserum indicates that HES-1 is expressed in T cells of all developmental phenotypes, consistent with our EMSA data (Fig. 3 and 4B and data not shown). None of the available antibody reagents to HES-1 are able to supershift HES-1 in control experiments (data not shown). Thus, as an alternative, we used our antisera in UV cross-linking–immunoprecipitation experiments. An EMSA reaction was exposed to UV light, inducing the formation of covalent bonds between the DNA probe and bound nuclear proteins. After immunoprecipitation, the DNA-protein complexes were sized on a sodium dodecyl sulfate-polyacrylamide gel. We conducted this experiment with the anti-HES-1 antiserum and nuclear extracts purified from the D10 CD4 SP TH and the L3 CD8 SP TC cell clones (Fig. 4B and data not shown). Using either the S1 or N-box probes, we can immunoprecipitate a 43-kDa protein-DNA complex, indicating an apparent molecular mass for the immunoprecipitated factor of 29 kDa, which is the mass of HES-1 (44). The intensity of the HES-1–N-box complex is similar to that of the HES-1–S1 complex, indicating that HES-1 binds with a similar efficiency to the N box and the S1 probes. Thus, a factor that shares antigenic epitopes with and is the same molecular mass as HES-1 is binding to the S1 probe. As HES-1 is the only known member of the HES family of factors that is expressed in T cells and is 29 kDa in size, these data are strong evidence that murine HES-1 is binding to the CD4 silencer.
FIG. 4.
Western blot and UV cross-linking–immunoprecipitation analyses with the anti-HES-1 antiserum (31). Positions of protein molecular mass markers are indicated to the left of the blot. (A) Western blot analysis on nuclear extracts from 293T cells transfected with the CMV-HES-1 antiserum (left lane) or mock-transfected 293T cells. At much longer exposures we can detect endogenous HES-1 expression in the mock-transfected cells (data not shown). (B) Western analyses on nuclear extracts purified from different cell lines. The nitrocellulose blot probed with the anti-HES-1 antiserum (top) and the same blot probed with an antiserum against the transcription factor sp3 as a loading control (bottom) are shown. Molecular mass markers are indicated. On longer exposure, the HES-1 band in the AKR1G1 cell line is more evident. The arrow indicates HES-1 band. (C) UV-cross-link–immunoprecipitations with the anti-HES-1 antiserum and the S1 probe or the N-box probe. These experiments were conducted with the CD8 SP L3 TC clone; similar results were obtained with the CD4 SP D10 TH clone (data not shown).
HES-1 represses CD4 transcription by binding to S1 of the CD4 silencer.
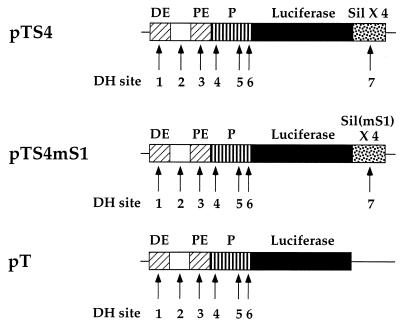
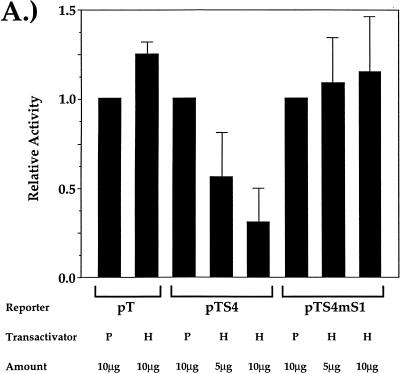
The biochemical data presented above indicate that HES-1 is binding to the CD4 silencer at a site that is important for silencer function. We can therefore predict that the overexpressed HES-1 should be able to recognize S1 in the CD4 silencer and repress CD4 promoter and enhancer function. To test this, we conducted a series of transient-transfection experiments (Fig. 5 and Fig. 6A). Transient-transfection reporter constructs that contain the luciferase gene under the control of the CD4 promoter, enhancers, and the silencer were generated (Fig. 5). The pT construct contains all six DNase-hypersensitive sites located in the 5′-flanking region of the CD4 gene, including both the proximal and distal enhancers and the complete promoter region. The pTS4 construct is identical to pT, except that it also contains a fourfold multimerization of the CD4 silencer. The pTS4mS1 construct contains a fourfold multimerization of the minimal silencer with a site-specific mutation in S1 that specifically abrogates HES-1 binding. These constructs were cotransfected along with the CMV-HES-1 expression construct into the D10 CD4 SP TH clone (Fig. 6A). We can detect high levels of activity when the pT, pTS4, or pTS4m1 construct is transfected into D10 alone (data not shown). We cannot detect differences in reporter gene activity in populations of cells transfected with each of these constructs alone (data not shown). This is to be expected, as the differences between these constructs are limited solely to the presence or absence of the silencer; since D10 is a CD4 SP T cell, the silencer would not be expected to function in these experiments. Overexpression of HES-1 has no repressive effect on the pT construct; indeed, we can even detect a small but reproducible increase in luciferase activity in these experiments. However, there is a significant dose-dependent decrease in luciferase activity when HES-1 is overexpressed with the pTS4 construct containing the unmutated silencer. In contrast, there is no significant repression when HES-1 is overexpressed with the pTS4mS1 construct containing the silencer with a site-specific mutation in the S1 N box. These data indicate that the binding of HES-1 to S1 of the CD4 silencer can lead to the silencer-dependent repression of CD4 promoter and enhancer function and thus transcription of the CD4 gene.
FIG. 5.
Reporter constructs used in transient-transfection experiments. Transcriptional control elements in each construct (CD4 promoter [P], distal enhancer [DE], and proximal enhancer [PE]), silencer (Sil) elements, and CD4 locus DH sites are indicated.
FIG. 6.
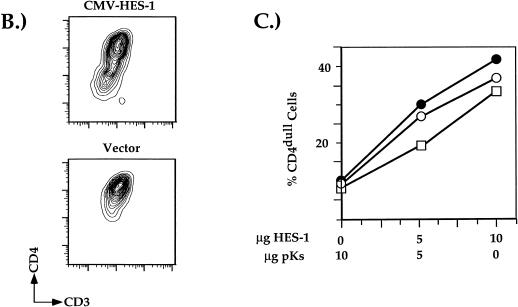
HES-1 binding to S1 of the CD4 silencer leads to repression of CD4 promoter and enhancer function and endogenous gene expression. (A) Transfection of the pT-based vectors into the D10 TH clone. Transfection of the CMV-HES-1 expression plasmid (H) and of the empty CMV expression plasmid (P) is indicated. Data from each reporter construct set are presented normalized to the results obtained from the transfection with empty plasmid. Data were also normalized for transfection efficiency using Renilla luciferase values as described in Materials and Methods. Total amount of DNA added to all transfections was kept constant with the addition of empty plasmid DNA as needed. Multiple transfection experiments were conducted; presented are averages of data from three independent transfections. (B) Representative two-color flow cytometric plot of D10 cells cotransfected with the CMV-GFP and CMV-HES-1 expression plasmids. For this experiment, D10 TH cells were cotransfected with (top) or without (bottom) the CMV-HES-1 expression plasmid and the CMV-GFP plasmid; GFP-positive cells were gated on and analyzed for surface CD4 and CD3 expression. (C) Percentage of transfected CD4dull SP D10 TH cells plotted against the amount of EF-1α-HES-1 plasmid added to the transfection. Total amounts of DNA added to the transfections was kept constant with the addition of irrelevant plasmid DNA as needed. Multiple experiments were conducted; shown are three representative experiments.
Expression of HES-1 represses endogenous CD4 expression in CD4+ SP TH cells.
From the transient-transfection data, we predict that the overexpression of HES-1 in a CD4+ CD8− TH cell would repress endogenous CD4 promoter and enhancer activity, thus leading to a decrease in endogenous expression of CD4. To test this hypothesis, a CMV-HES-1 expression vector was transfected into the CD4+ CD8− TH cell clone D10 and the transfectants analyzed for surface CD4 expression by flow cytometry. As can be seen in Fig. 6B, we observed a 20-fold decrease in endogenous CD4 expression with the addition of the CMV-HES-1 expression vector. In contrast, addition of the empty CMV expression plasmid did not appreciably affect CD4 expression. Addition of the CMV-HES-1 expression vector led to significantly smaller decrease in surface CD3 expression, indicating that the downregulation of CD4 was not the result of a general inhibition of gene expression (Fig. 6B). In addition, we conducted a dose-response transient-transfection experiment using both the CMV-HES-1 and the EF-1α-HES-1 expression vectors (Fig. 6C and data not shown). Increases in the amount of HES-1 expression plasmid added to the transfection led to dose-dependent decreases in endogenous CD4 gene expression; CD3 expression remained high at all doses, indicating again that the effect was specific for CD4 expression (data not shown). These transfection data are consistent with the hypothesis that HES-1 is binding to the silencer of the endogenous CD4 gene, resulting in the repression of transcription and expression of CD4.
Signaling from the lin12/Notch pathway represses endogenous CD4 expression by affecting the function of multiple CD4 transcriptional control elements.
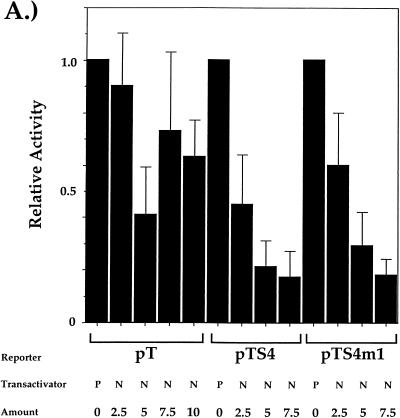
Since HES-1 is a target of the lin12/Notch pathway and is upregulated by Notch signaling, we predict from our HES-1 transfection data that constitutive signaling from Notch would also lead to repression of CD4 transcriptional control element function and endogenous gene expression. To test this hypothesis, we conducted transient-transfection experiments as described above using the CMV expression vectors and cDNAs encoding Notch1. Previous studies on D. melanogaster and C. elegans indicated that an N-terminal truncation of Notch functions as a constitutively active intrinsic signaling activity (15, 54, 55); similar results were subsequently obtained with truncations in mammalian Notch1 (27). We therefore used a cDNA encoding the carboxyl-terminal 721 amino acids (amino acids 1810 to 2531) of murine Notch1 for our expression studies. As can be seen in Fig. 7A, overexpression of NotchIC leads to a dose-dependent decrease in luciferase activity when cotransfected into the CD4 SP TH clone D10 with the pTS4 construct. Surprisingly, we can detect similar dose-dependent repression of luciferase activity with the pTS4mS1 construct (Fig. 7A), indicating that signaling via the Notch pathway is also capable of initiating CD4 silencer function in the absence of a functional HES-1 binding site. This observation indicates that signaling from the lin12/Notch pathway can affect the function of at least some of the other silencer-binding factors. In addition, we can see partial repression of luciferase activity with the pT construct, indicating that enhancer-binding factors can be directly repressed by lin12/Notch signaling. Thus, signaling through the lin12/Notch pathway represses CD4 expression by affecting at least two different control elements (see below for a full discussion of these issues).
FIG. 7.
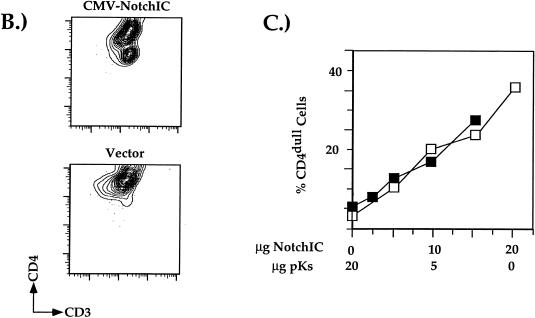
Notch signaling represses CD4 transcription element function and endogenous gene expression. (A) Transfection of the pT-based vectors into the D10 TH clone. Transfection of the CMV-NotchIC expression plasmid (N) and of the empty CMV expression plasmid (P) is indicated. Data are presented and normalized as described for the data in Fig. 6. Multiple transfection experiments were conducted; presented are averages of data from three independent transfections. (B) Representative two-color flow cytometric plot of D10 cells cotransfected with the CMV-GFP and CMV-NotchIC expression plasmids. Transfections were conducted and analyzed as described in the legend to Fig. 6. (C) Percentage of transfected CD4dull SP D10 TH cells plotted against the amount of CMV-NotchIC plasmid added to the transfection. Multiple experiments were conducted; shown are two representative experiments.
To determine if the overexpression of NotchIC would also lead to the repression of endogenous CD4 gene expression, the CMV-NotchIC construct was transfected into the CD4 SP TH clone D10 and the transfectants were analyzed by flow cytometry as described above for the HES-1 transfections. We can detect a 20-fold decrease in surface CD4 expression with the transfection of the CMV-NotchIC expression vector; surface CD3 expression is unchanged, indicating that the downregulation of CD4 is not part of a general overall downregulation of surface molecule expression (Fig. 7B). In addition, we can detect a dose-dependent decrease in endogenous CD4 expression with increasing amounts of NotchIC plasmid added to the transfection, similar to the results observed with the HES-1 transfection experiments described above (Fig. 7C). Taken together, our transfection data indicate that Notch pathway signaling represses endogenous CD4 gene expression by directly altering the function of multiple CD4 transcriptional control elements and that the induction of HES-1 and ultimately CD4 silencer function may be one of these mechanisms.
DISCUSSION
lin12/Notch pathway and repression of CD4 gene expression.
Using a variety of different biochemical and molecular techniques, we have determined that HES-1 binds a functional site in the CD4 silencer and mediates silencer function, supporting the hypothesis that HES-1 is playing an important role in the control of CD4 gene expression. As HES-1 is specifically activated by lin12/Notch pathway signaling (17, 23, 31, 64), these data indicate that signaling through this pathway leads to the repression of CD4 gene expression. We have determined that Notch signaling in fact can lead to both silencer-dependent and -independent repression of CD4 transcription by affecting the activity of both silencer-binding factors and enhancer-binding factors. Recently, Ordentlich and colleagues have reported that activated mammalian lin12/Notch receptor signaling directly inhibits the function of the bHLH transcription factor E47 (37). The CD4 proximal enhancer has two functional factor-binding sites, one of which is recognized by a heterodimer containing the E12 factor (45) which, like E47, is a protein product of the E2A gene (35). It is therefore possible that in addition to the activation of the silencer, lin12/Notch signaling is repressing CD4 transcription by repressing enhancer activity by inhibiting the function of E12. We are currently conducting experiments to address these issues in detail; nonetheless, the fact that signaling from the Notch pathway represses CD4 transcription by affecting the function of multiple transcriptional control elements underscores the importance of this signaling pathway in the control of CD4 gene expression.
Our data thus demonstrate that CD4 is a target gene for the mammalian lin12/Notch signaling pathway and indicate that this pathway affects T-cell development in part by altering CD4 expression. The lin12/Notch pathway has been implicated in developmental cell fate decisions in many different systems. Oncogenic forms of mammalian Notch that result in the generation of thymic lymphomas have been described (13, 39). Robey and coworkers have proposed that the mammalian lin12/Notch pathway plays a major role in T-cell development (41, 60). Our data provide a potential mechanism in which the lin12/Notch pathway may influence T-cell developmental fate. However, it is clear that induction of HES-1 function alone is not sufficient to explain the effects of activated Notch on T-cell development (41, 60). We have been unable to detect enhanced development of CD8 SP T cells in mice transgenic with HES-1 expression vectors, consistent with the hypothesis that the activation of multiple Notch-dependent pathways may be necessary to affect T-cell development (25a). There are several reports of lin12/Notch signaling pathways that are independent of E(spl), and thus it is possible that the full phenotype observed by groups using forms of activated Notch receptor is mediated by other pathways (6, 21, 27, 37, 48). Thus, full lineage commitment is likely to require signaling through at least some of these alternate pathways.
Mechanism of CD4 silencer function.
The CD4 silencer is a critical determining element in proper CD4 subclass-specific expression (10, 46, 50). In mediating CD4-specific expression, the silencer must perform two functions: it must inhibit the transcriptional machinery from functioning (the mechanism of action), and it must do so in a subclass-specific manner (the specificity of action). These two functions may be completely distinct from each other, requiring different sets of factors, or there may be significant functional overlap. Nonetheless, in constructing models for the role of the CD4 silencer-binding factors in the control of CD4 gene expression, it is useful to consider these concepts separately.
It is possible that HES-1 is primarily involved in the mechanism as opposed to specificity of silencer function. HES-1 is a bHLH protein that functions primarily as a transcriptional repressor, which is consistent with this hypothesis. The mechanism of repression function of the HES proteins is complicated. Three protein domains are required for full function: the bHLH, Orange, and WRPW domains (5, 14, 25, 59). Transcriptional repression involves two different mechanisms: repression of specific transcriptional activators via the bHLH and Orange domains, and the repression of other activators through the interaction of the WRPW domain with other corepressors. It is possible that HES-1 is using both to mediate CD4 silencer function. Sasai and colleagues have shown that HES-1 is capable of directly repressing E-box-dependent gene transcription by interacting with E2A gene products (44). As discussed above, an HEB-E2A heterodimer binds to the CD4 enhancer and is critical for its function (45). It is possible that direct interactions between silencer-bound HES-1 and the enhancer-bound E12-HEB heterodimer lead to inhibition of CD4 enhancer function. Coupled with the direct negative effects of Notch signaling on E2A gene product function, this would lead to effective repression of CD4 transcription. Alternatively, HES-1 may function by binding directly to the N box and mediating silencer function via interactions with corepressors such as transducin-like enhancer of split, thus nucleating heterochromatin formation and inhibiting CD4 gene transcription (4, 12, 33, 34, 47, 52). We are currently conducting both molecular and transgenic experiments to test these models in detail.
Models for CD4 silencer functional mechanisms may be drawn from comparisons with the best-characterized eukaryotic transcriptional silencer, the Saccharomyces cerevisiae mating-type silencer (49). This silencer constitutively represses transcription of inactive genes at the mating-type (MAT) locus. Like the CD4 silencer, the yeast mating-type silencer has three factor-binding sites to which three different transcription factor complexes bind. Despite the fact that the factor-binding sites in the yeast mating-type silencer are recognized by different protein complexes, there is significant functional redundancy in these sites. Single mutations in any site had little effect on repression, but mutations in any two of three resulted in derepression, indicating that this silencer is composed of functionally redundant elements; thus, the yeast mating-type silencer shares striking functional similarities with the CD4 silencer. As for the CD4 silencer, the mechanism for the redundancy in yeast mating-type silencer function is unknown. However, many transcriptional enhancers also contain functionally redundant factor-binding sites (66), so this is not unprecedented. It is possible that the factors that bind to the silencer at the three sites may be binding as a complex; loss of any one site is not critical, but the alteration of two of the three sites decreases the affinity of the overall complex for the silencer sufficiently to prevent complex binding and thus silencer function. We are currently characterizing the other silencer-binding factors and thus will soon be able to address this issue directly.
Specificity of CD4 silencer function.
The specificity of CD4 silencer function is of particular interest. Although the lin-12/Notch pathway has been shown to have a role in T-cell development and oncogenesis (18, 39, 41, 60), the mechanism of its role in T-cell lineage decisions is still unknown. Any model for the specificity of silencer function must take into account the observation that although genetically the silencer is required for appropriate CD4 gene expression, biochemical experiments indicate that the factors that bind to the silencer are expressed in a much broader fashion (10). Although our data indicate that HES-1 is important in CD4 silencer function, we can detect HES-1 binding in both CD4 and CD8 SP T cells. We believe that the specificity of action of the Notch pathway on T-cell development is more likely to be the result of differential interaction between Notch expressed on the thymocyte and its ligand expressed by the thymic antigen-presenting cell (41). Experiments reported by Robey and coworkers have demonstrated that overexpression of activated Notch1 leads to increased development of CD4− CD8+ T cells independent of the specificity of the T-cell receptor itself (41). As Notch expression on thymocytes does not correlate to specific T-cell lineage fates and Notch ligands are present on a wide variety of cells (22), it is likely that specific combinations of Notch-Notch ligand interactions in conjunction with signaling from the T-cell receptor during selection mediates mature T-cell lineage determination. Signaling through the Notch pathway during this process activates HES-1 expression, leading to CD4 silencer function, the downregulation of CD4 expression, thus facilitating development of CD4− CD8+ T cells. In this model, HES-1 is serving primarily to transmit the signal from the cell surface to the target gene and is thus more important for the actual repression of target gene expression than the cell type specificity of action of the silencer itself.
It is nonetheless still possible that specificity of silencer function is mediated by HES-1 itself. For example, it is possible that posttranslational modification of HES-1 in CD4 SP T cells is important for the inhibition of repressive activity; overexpression of HES-1 may saturate the modification process, resulting in the production of unmodified HES-1 and thus endogenous CD4 silencing. Recently, work by Strom and coworkers have determined that HES-1 is inactivated by phosphorylation, leading to decreased HES-1 binding to control elements of target genes and a concomitant inhibition of nerve growth factor-induced neural differentiation (53). Mutations of the phosphorylation sites on HES-1 generated constitutively active protein that strongly blocked neuronal differentiation. However, we were unable to detect T-cell subclass-specific differences in the ability of HES-1 to bind DNA in the presence of phosphatase inhibitors (25b). In addition, we were unable to detect significant differences in the ability of the constitutively active HES-1 mutants to repress CD4 promoter or enhancer function or endogenous CD4 expression (25b). Thus, it is unlikely that modification of HES-1 at these specific phosphorylation sites is mediating subclass-specific function of HES-1 at the CD4 silencer. However, it is still possible that HES-1 is posttranslationally modified in a subclass-specific manner. There are other potential phosphorylation sites in the HES-1 protein; it is possible that subclass-specific phosphorylation at these other sites is affecting the ability of HES-1 to interact with cofactors and thus its ability to mediate silencer function. In addition, other subclass-specific posttranslational modifications such as acetylation are possible. It is also possible that subclass-specific cofactors are necessary for mediating HES-1-dependent effects on silencer function. For example, subclass-specific coactivators may mediate interaction of HES-1 and the other silencer-binding factors to the basal transcriptional machinery. Another possibility is that HES-1 is differentially compartmentalized within the cell. In this model, cells that express CD4 sequester HES-1 in specific subcellular compartments, making it inaccessible to the CD4 silencer. In cells that do not make CD4, HES-1 is released freely into the nucleus, thus permitting it to bind to the silencer and mediate its function. We are currently conducting experiments to distinguish between all of these models.
ACKNOWLEDGMENTS
We thank Sophia Sarafova and Dan Ng for advice and technical assistance, Ryoichiro Kageyama for the HES-1 cDNA clone, Kevin Fitzgerald for the GST–HES-1 fusion protein, Jan Kitajewski and GuangYu Wu for the HES-1-transfected 293T extracts, Chris Roman for the EF-1α expression plasmid, and Kathryn Calame, Kevin Fitzgerald, Max Gottesman, Iva Greenwald, Jan Kitajewski, Andrew Henderson, Gary Struhl, and the members of the Siu lab for helpful discussions and critical reading of the manuscript.
This work was supported by grants from the American Cancer Society (JFRA-484 and RPG-98-185-01-CIM) and the Irma T. Hirschl-Monique Caulier Weill Charitable Trust to G.S. H.K.K. is supported by NIH training grant T32AI07525.
REFERENCES
- 1.Adlam M, Duncan D D, Ng D K, Siu G. Positive selection induces CD4 promoter and enhancer function. Int Immunol. 1997;9:877–887. doi: 10.1093/intimm/9.6.877. [DOI] [PubMed] [Google Scholar]
- 2.Artavanis-Tsakonas S, Matsuno K, Fortini M E. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 3.Bailey A M, Posakony J W. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- 4.Cooper J P, Roth S Y, Simpson R T. The global transcriptional regulators, SSN6 and TUP1, play distinct roles in the establishment of a repressive chromatin structure. Genes Dev. 1994;8:1400–1410. doi: 10.1101/gad.8.12.1400. [DOI] [PubMed] [Google Scholar]
- 5.Dawson S R, Turner D L, Weintraub H, Parkhurst S M. Specificity for the hairy/enhancer of split basic helix-loop-helix (bHLH) proteins maps outside the bHLH domain and suggests two separable modes of transcriptional repression. Mol Cell Biol. 1995;15:6923–6931. doi: 10.1128/mcb.15.12.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Celis J F, de Celis J, Ligoxygakis P, Preiss A, Delidakis C, Bray S. Functional relationships between Notch, Su(H) and the bHLH genes of the E(spl) complex: the E(spl) genes mediate only a subset of Notch activities during imaginal development. Development. 1996;122:2719–2728. doi: 10.1242/dev.122.9.2719. [DOI] [PubMed] [Google Scholar]
- 7.Del Amo F F, Smith D E, Swiatek P J, Gendron-Maguire M, Greenspan R J, McMahon A P, Gridley T. Expression pattern of Motch, a mouse homolog of Drosophila Notch, suggests an important role in early postimplantation mouse development. Development. 1992;115:737–744. doi: 10.1242/dev.115.3.737. [DOI] [PubMed] [Google Scholar]
- 8.Delidakis C, Artavanis-Tsakonas S. The Enhancer of split [E(spl)] locus of Drosophila encodes seven independent helix-loop-helix proteins. Proc Natl Acad Sci USA. 1992;89:8731–8735. doi: 10.1073/pnas.89.18.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donda A, Schulz M, Burki K, De Libero G, Uematsu Y. Identification and characterization of a human CD4 silencer. Eur J Immunol. 1996;26:493–500. doi: 10.1002/eji.1830260232. [DOI] [PubMed] [Google Scholar]
- 10.Duncan D D, Adlam M, Siu G. Asymmetric redundancy in CD4 silencer function. Immunity. 1996;4:301–311. doi: 10.1016/s1074-7613(00)80438-0. [DOI] [PubMed] [Google Scholar]
- 11.Duncan D D, Stupakoff A, Hedrick S M, Marcu K B, Siu G. A Myc-associated zinc-finger protein (MAZ) binding site is one of four important functional regions in the CD4 promoter. Mol Cell Biol. 1995;15:3179–3186. doi: 10.1128/mcb.15.6.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edmondson D G, Smith M M, Roth S Y. Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev. 1996;10:1247–1259. doi: 10.1101/gad.10.10.1247. [DOI] [PubMed] [Google Scholar]
- 13.Ellisen L W, Bird J, West D C, Soreng A L, Reynolds T C, Smith S D, Sklar J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 14.Fisher A L, Ohsako S, Caudy M. The WRPW motif of the Hairy-related basic-helix-loop-helix repressor proteins acts as a 4-amino-acid transcription repression and protein-protein interaction domain. Mol Cell Biol. 1996;16:2670–2677. doi: 10.1128/mcb.16.6.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortini M E, Rebay I, Caron L A, Artavanis-Tsakonas S. An activated Notch receptor blocks cell-fate commitment in the developing Drosophila eye. Nature. 1993;365:555–557. doi: 10.1038/365555a0. [DOI] [PubMed] [Google Scholar]
- 16.Fowlkes B J, Pardoll D. Molecular and cellular events of T cell development. Adv Immunol. 1989;44:207–264. doi: 10.1016/s0065-2776(08)60643-4. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa T, Kobayakawa Y, Tamura K, Kimura K, Kawaichi M, Tanimura T, Honjo T. Suppressor of hairless, the Drosophila homologue of RBP-J kappa, transactivates the neurogenic gene E(spl)m8. Jpn J Genet. 1995;70:505–524. doi: 10.1266/jjg.70.505. [DOI] [PubMed] [Google Scholar]
- 18.Girard L, Hanna Z, Beaulieu N, Hoemann C D, Simard C, Kozak C A, Jolicoeur P. Frequent provirus insertional mutagenesis of Notch1 in thymomas of MMTVD/myc transgenic mice suggests a collaboration of c-myc and Notch1 for oncogenesis. Genes Dev. 1996;10:1930–1944. doi: 10.1101/gad.10.15.1930. [DOI] [PubMed] [Google Scholar]
- 19.Greenwald I. Structure/function studies of lin-12/Notch proteins. Curr Opin Genet Dev. 1994;4:556–562. doi: 10.1016/0959-437x(94)90072-b. [DOI] [PubMed] [Google Scholar]
- 20.Greenwald I, Rubin G M. Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992;68:271–281. doi: 10.1016/0092-8674(92)90470-w. [DOI] [PubMed] [Google Scholar]
- 21.Guan E, Wang J, Laborda J, Norcross M, Baeuerle P A, Hoffman T. T cell leukemia-associated human Notch/translocation-associated Notch homologue has IκB-like activity and physically interacts with Nuclear Factor-κB proteins in T cells. J Exp Med. 1996;183:2025–2032. doi: 10.1084/jem.183.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasserjian R, Aster J, Davi D, Weinberg D, Sklar J. Modulated expression of Notch1 during thymocyte development. Blood. 1996;88:970–976. [PubMed] [Google Scholar]
- 23.Jarriault S, Brou C, Logeat F, Schroeter E H, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 24.Jennings B, Preiss A, Delidakis C, Bray S. The Notch signalling pathway is required for Enhancer of split bHLH protein expression during neurogenesis in the Drosophila embryo. Development. 1994;120:3537–3548. doi: 10.1242/dev.120.12.3537. [DOI] [PubMed] [Google Scholar]
- 25.Kageyama R, Sasai Y, Akazawa C, Ishibashi M, Takebayashi K, Shimizu C, Tomita K, Nakanishi S. Regulation of mammalian neural development by helix-loop-helix transcription factors. Crit Rev Neurobiol. 1995;9:177–188. [PubMed] [Google Scholar]
- 25a.Kim, H. K., and G. Siu. Unpublished data.
- 25b.Kim, H. K., M. Caudy, and G. Siu. Unpublished data.
- 26.Knust E, Schrons H, Grawe F, Campos-Ortega J A. Seven genes of the Enhancer of split complex of Drosophila melanogaster encode helix-loop-helix proteins. Genetics. 1992;132:505–518. doi: 10.1093/genetics/132.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopan R, Nye J S, Weintraub H. The intracellular domain of mouse Notch: a constitutively activated repressor of myogenesis directed at the basic helix-loop-helix region of MyoD. Development. 1994;120:2385–2396. doi: 10.1242/dev.120.9.2385. [DOI] [PubMed] [Google Scholar]
- 28.Lardelli M, Dahlstrand J, Lendahl U. The novel Notch homologue mouse Notch 3 lacks specific epidermal growth factor-repeats and is expressed in proliferating neuroepithelium. Mech Dev. 1994;46:123–136. doi: 10.1016/0925-4773(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 29.Lardelli M, Lendahl U. Motch A and motch B—two mouse Notch homologues coexpressed in a wide variety of tissues. Exp Cell Res. 1993;204:364–372. doi: 10.1006/excr.1993.1044. [DOI] [PubMed] [Google Scholar]
- 30.Lardelli M, Williams R, Lendahl U. Notch-related genes in animal development. Int J Dev Biol. 1995;39:769–780. [PubMed] [Google Scholar]
- 31.Lecourtois M, Schweisguth F. The neurogenic suppressor of hairless DNA-binding protein mediates the transcriptional activation of the enhancer of split complex genes triggered by Notch signaling. Genes Dev. 1995;9:2598–2608. doi: 10.1101/gad.9.21.2598. [DOI] [PubMed] [Google Scholar]
- 32.Littman D R, Davis C B, Killeen N, Xu H. Signal transduction during T cell development. Adv Exp Med Biol. 1994;365:63–71. doi: 10.1007/978-1-4899-0987-9_7. [DOI] [PubMed] [Google Scholar]
- 33.Mallo M, Franco del Amo F, Gridley T. Cloning and developmental expression of Grg, a mouse gene related to the groucho transcript of the Drosophila Enhancer of split complex. Mech Dev. 1993;42:67–76. doi: 10.1016/0925-4773(93)90099-j. [DOI] [PubMed] [Google Scholar]
- 34.Miyasaka H, Choudhury B K, Hou E W, Li S S. Molecular cloning and expression of mouse and human cDNA encoding AES and ESG proteins with strong similarity to Drosophila enhancer of split groucho protein. Eur J Biochem. 1993;216:343–352. doi: 10.1111/j.1432-1033.1993.tb18151.x. [DOI] [PubMed] [Google Scholar]
- 35.Murre C, Bain G, van Dijk M A, Engel I, Furnari B A, Massari M E, Matthews J R, Quong M W, Rivera R R, Stuiver M H. Structure and function of helix-loop-helix proteins. Biochim Biophys Acta. 1994;1218:129–135. doi: 10.1016/0167-4781(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 36.Oellers N, Dehio M, Knust E. bHLH proteins encoded by the Enhancer of split complex of Drosophila negatively interfere with transcriptional activation mediated by proneural genes. Mol Gen Genet. 1994;244:465–473. doi: 10.1007/BF00583897. [DOI] [PubMed] [Google Scholar]
- 37.Ordentlich P, Lin A, Shen C P, Blaumueller C, Matsuno K, Artavanis-Tsakonis S, Kadesch T. Notch inhibition of E47 supports the existence of a novel signaling pathway. Mol Cell Biol. 1998;18:2230–2239. doi: 10.1128/mcb.18.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paroush Z, Finley R L, Jr, Kidd T, Wainwright S M, Ingham P W, Brent R, Ish-Horowicz D. Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell. 1994;79:805–815. doi: 10.1016/0092-8674(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 39.Pear W S, Aster J C, Scott M L, Hasserjian R P, Soffer B, Sklar J, Baltimore D. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med. 1996;183:2283–2291. doi: 10.1084/jem.183.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perlmutter R M, Marth J D, Ziegler S F, Garvin A M, Pawar S, Cooke M P, Abraham K M. Specialized protein tyrosine kinase proto-oncogenes in hematopoietic cells. Biochim Biophys Acta. 1988;948:245–262. doi: 10.1016/0304-419x(89)90001-2. [DOI] [PubMed] [Google Scholar]
- 41.Robey E, Chang D, Itano A, Cado D, Alexander H, Lane D, Weinmaster G, Salmon P. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- 42.Salmon P, Boyer O, Lores P, Jami J, Klatzmann D. Characterization of an intronless CD4 minigene expressed in mature CD4 and CD8 T cells, but not expressed in immature thymocytes. J Immunol. 1996;156:1873–1879. [PubMed] [Google Scholar]
- 43.Salmon P, Giovane A, Wasylyk B, Klatzmann D. Characterization of the human CD4 gene promoter: transcription from the CD4 gene core promoter is tissue-specific and is activated by Ets proteins. Proc Natl Acad Sci USA. 1993;90:7739–7743. doi: 10.1073/pnas.90.16.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992;6:2620–2634. doi: 10.1101/gad.6.12b.2620. [DOI] [PubMed] [Google Scholar]
- 45.Sawada S, Littman D R. A heterodimer of HEB and an E12-related protein interacts with the CD4 enhancer and regulates its activity in T-cell lines. Mol Cell Biol. 1993;13:5620–5628. doi: 10.1128/mcb.13.9.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawada S, Scarborough J D, Killeen N, Littman D R. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell. 1994;77:917–929. doi: 10.1016/0092-8674(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt C J, Sladek T E. A rat homolog of the Drosophila enhancer of split (groucho) locus lacking WD-40 repeats. J Biol Chem. 1993;268:25681–25686. [PubMed] [Google Scholar]
- 48.Shawber C, Nofziger D, Hsieh J J, Lindsell C, Bogler O, Hayward D, Weinmaster G. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development. 1996;122:3765–3773. doi: 10.1242/dev.122.12.3765. [DOI] [PubMed] [Google Scholar]
- 49.Shore D. Telomere position effects and transcriptional silencing in the yeast Saccharomyces cerevisiae. In: Blackburn E H, Greider C W, editors. Telomeres. Plainview, N.Y: Cold Spring Harbor Press; 1995. pp. 139–191. [Google Scholar]
- 50.Siu G, Wurster A L, Duncan D D, Soliman T M, Hedrick S M. A transcriptional silencer controls the developmental expression of the CD4 gene. EMBO J. 1994;13:3570–3579. doi: 10.1002/j.1460-2075.1994.tb06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siu G, Wurster A L, Lipsick J S, Hedrick S M. Expression of the CD4 gene requires a Myb transcription factor. Mol Cell Biol. 1992;12:1592–1604. doi: 10.1128/mcb.12.4.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stifani S, Blaumueller C M, Redhead N J, Hill R E, Artavanis-Tsakonas S. Human homologs of a Drosophila Enhancer of split gene product define a novel family of nuclear proteins. Nature Genet. 1992;2:119–127. doi: 10.1038/ng1092-119. [DOI] [PubMed] [Google Scholar]
- 53.Strom A, Castella P, Rockwood J, Wagner J, Caudy M. Mediation of NGF signaling by post-translational inhibition of HES-1, a basic helix-loop-helix repressor of neuronal differentiation. Genes Dev. 1997;11:3168–3181. doi: 10.1101/gad.11.23.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Struhl G, Adachi A. Nuclear access and action of Notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- 55.Struhl G, Fitzgerald K, Greenwald I. Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell. 1993;74:331–345. doi: 10.1016/0092-8674(93)90424-o. [DOI] [PubMed] [Google Scholar]
- 56.Tata, F., and D. A. Hartley. 1993. The role of the enhancer of split complex during cell fate determination in Drosophila. Development 1993(Suppl.):139–148. [PubMed]
- 57.Uematsu Y, Donda A, De Libero G. Thymocytes control the CD4 gene differently from mature T lymphocytes. Int Immunol. 1997;9:179–187. doi: 10.1093/intimm/9.1.179. [DOI] [PubMed] [Google Scholar]
- 58.Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development. 1996;122:2251–2259. doi: 10.1242/dev.122.7.2251. [DOI] [PubMed] [Google Scholar]
- 59.Wainwright S M, Ish-Horowicz D. Point mutations in the Drosophila hairy gene demonstrate in vivo requirements for basic, helix-loop-helix, and WRPW domains. Mol Cell Biol. 1992;12:2475–2483. doi: 10.1128/mcb.12.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Washburn T, Schweighoffer E, Gridley T, Chang D, Fowlkes B J, Cado D, Robey E. Notch activity influences the α/β versus γ/δ T cell lineage decision. Cell. 1997;88:833–843. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 61.Waterman M L, Jones K A. Purification of TCF-1α, a T-cell-specific transcription factor that activates the T-cell receptor Cα gene enhancer in a context-dependent manner. New Biol. 1990;2:621–636. [PubMed] [Google Scholar]
- 62.Weinmaster G, Roberts V J, Lemke G. A homolog of Drosophila Notch expressed during mammalian development. Development. 1991;113:199–205. doi: 10.1242/dev.113.1.199. [DOI] [PubMed] [Google Scholar]
- 63.Weinmaster G, Roberts V J, Lemke G. Notch2: a second mammalian Notch gene. Development. 1992;116:931–941. doi: 10.1242/dev.116.4.931. [DOI] [PubMed] [Google Scholar]
- 64.Wettstein D A, Turner D L, Kintner C. The Xenopus homolog of Drosophila Suppressor of Hairless mediates Notch signaling during primary neurogenesis. Development. 1997;124:693–702. doi: 10.1242/dev.124.3.693. [DOI] [PubMed] [Google Scholar]
- 65.Wurster A L, Siu G, Leiden J, Hedrick S M. Elf-1 binds to a critical element in a second CD4 enhancer. Mol Cell Biol. 1994;14:6452–6463. doi: 10.1128/mcb.14.10.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zenke M, Grundstrom T, Matthes H, Wintzerith M, Schatz C, Wildeman A, Chambon P. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986;5:387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]