Abstract
Objective
Laparoscopic surgery for cervical cancer has the advantages of little blood loss and rapid recovery, but its therapeutic effect is still controversial. This study aims to analyze the surgical procedure and clinical efficacy of tumor-free laparoscopic radical hysterectomy without a uterine manipulator for early-stage cervical cancer, and to explore the indications of laparoscopic surgery for cervical cancer.
Methods
This study was a retrospective study. The data of patients who underwent radical hysterectomy for early-stage cervical cancer admitted to Hunan Provincial Maternal and Child Health Care Hospital from July 2019 to December 2021 were collected. According to 2018 the International Federation of Gynecology and Obstetrics (FIGO) clinical staging, all patients were in IA1 with lymphovascular invasion, IA2, and IB1 stage. Among them, 45 patients underwent tumor-free laparoscopic radical hysterectomy without a uterine manipulator (laparoscopy group) and 16 patients underwent open surgery (open surgery group). Patients were followed up for 12-41 months. The differences between the 2 groups in terms of operative time, bleeding volume, extent of surgical resection, surgical complications, and prognosis were compared and analyzed.
Results
Compared to the open surgery group, the laparoscopy group had significantly shorter operation time and less intraoperative blood loss (both P<0.001). There were no significant differences between the 2 groups in terms of the length of excised uterosacral ligaments, cardinal ligaments, vagina, and the number of excised lymph nodes (all P>0.05). The incidence of postoperative complications did not differ significantly between the groups (P>0.05). No death or recurrence occurred in the 2 groups during the follow-up period. The overall survival rate and disease-free survival rate were both 100%.
Conclusion
For early-stage cervical cancer with a diameter ≤2 cm, tumor-free laparoscopic radical hysterectomy without a uterine manipulator is safe and feasible,and the short-term outcomes is no less than that of open surgery.
Keywords: radical hysterectomy, cervical cancer, laparoscopy, tumor-free, without a uterine manipulator
Abstract
目的
腹腔镜手术治疗宫颈癌具有出血少、恢复快等优点,但对其治疗效果目前仍有争议。本研究旨在分析早期宫颈癌腹腔镜手术的无瘤化无举宫手术操作方式及临床效果,探讨腹腔镜宫颈癌手术的适应证。
方法
本研究为回顾性研究。收集2019年7月至2021年12月湖南省妇幼保健院收治的早期宫颈癌行广泛性子宫切除术患者的资料。按2018年国际妇产科学联合会(International Federation of Gynecology and Obstetrics,FIGO)临床分期,患者均为IA1期伴脉管阳性、IA2期、IB1期,随访12~41个月。其中行无瘤化无举宫腹腔镜广泛性子宫切除术(腹腔镜手术组)患者45例,行开腹广泛性子宫切除术(开腹手术组)患者16例。比较分析2组患者在手术时间、出血量、手术切除范围、手术并发症、预后等方面的差异。
结果
与开腹手术组相比,腹腔镜手术组的手术时间明显更短,术中出血量更少(均P<0.001)。2组在切子宫骶韧带、主韧带、阴道切除长度,淋巴结切除数量,并发症发生率方面的差异均无统计学意义(均P>0.05)。随访期间2组患者无复发和死亡,总生存率和无瘤生存率均为100%。
结论
对于直径≤2 cm的早期宫颈癌,无瘤化无举宫腹腔镜广泛性子宫切除术安全可行,短期疗效与开腹手术接近。
Keywords: 广泛性子宫切除术, 早期宫颈癌, 腹腔镜, 无瘤化, 无举宫
Cervical cancer is a common malignant tumor of the female reproductive system, and in China, it has the highest incidence among malignant tumors of the female reproductive tract. With the development of minimally invasive surgery, minimally invasive treatment of malignant tumors has become a trend. Cervical cancer laparoscopic surgery has been widely accepted by doctors and patients in China due to its minimally invasive nature. However, in 2018, 2 studies[1-2] published in the New England Journal of Medicine compared minimally invasive surgery with open surgery for cervical cancer and pointed out that the outcomes of tumor groups treated with minimally invasive surgery were significantly worse than those of the open surgery group. Multiple guidelines such as the National Comprehensive Cancer Network (NCCN), the European Society of Gynaecological Oncology (ESGO), the Gynecological Oncology Working Group (AGO), et al have considered open surgery as the standard and recommended surgical approach for cervical cancer treatment. However, laparoscopic surgery has advantages such as clear visibility, clear anatomy, minimal bleeding, rapid recovery, and short hospital stay. Therefore, we are considering what factors are affecting the oncological outcomes of patients undergoing laparoscopic surgery for cervical cancer and whether there are ways to continue this approach.
Currently, experts generally believe that the possible reasons for the inferior oncologic outcomes of laparoscopic surgery in cervical cancer patients mainly focus on the implementation of the minimally invasive surgery, issues related to CO2 pneumoperitoneum, specific details involving the use of uterine manipulators during surgery, relevant issues in pelvic lymph node dissection, approaches and methods for vaginal resection, and handling details before and after vaginal stump closure[3]. Based on extensive clinical discussions, the academic community has developed various modified approaches for laparoscopic surgery for cervical cancer, improving traditional laparoscopic techniques. However, there is currently no consensus in the industry on the specific standards for the execution of modified laparoscopic surgery. How laparoscopic surgery should be applied in the treatment of cervical cancer, the specific tumor-free measures involved, whether these tumor-free measures can be achieved during the clinical operation, and whether they can improve the oncological outcomes of laparoscopic surgery patients all need to be clinically validated. Therefore, in this study, we retrospectively analyzed the modified tumor-free laparoscopic radical hysterectomy versus open surgery, comparing intraoperative, postoperative, and follow-up data between the 2 types of surgery in order to assess the safety and short-term efficacy of laparoscopic surgery for early-stage cervical cancer, to explore tumor-free measures of surgery and the indications for laparoscopic surgery for cervical cancer.
1. Patients and methods
1.1. General information
We retrospectively analyzed cervical cancer patients with IA1 with lymphovascular invasion, IA2, and IB1 stage according to 2018 the International Federation of Gynecology and Obstetrics (FIGO) staging in Hunan Provincial Maternal and Child Health Care Hospital from July 2019 to December 2021. Patients were informed about the results of 2 studies published in the New England Journal of Medicine in 2018[1-2] and the modified tumor-free laparoscopic radical hysterectomy, and the choice of surgical approach was based on the patient preference. Among them, 45 patients underwent modified tumor-free laparoscopic radical hysterectomy (laparoscopy group) and 16 patients underwent open radical hysterectomy (open surgery group).
The inclusion criteria were as follows: Confirmed diagnosis of squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma of the cervix by pathological examination; no prior immunotherapy, chemotherapy, radiotherapy; preoperative imaging evaluations (MRI, CT, et al) confirming tumor diameter ≤2 cm without suspicion of lymph node metastasis or parametrial involvement; no contraindications for surgery and meeting surgical indications; patients were informed about the procedure and provided informed consent.
The exclusion criteria were as follows: Special types of cervical cancer; severe organ dysfunction; inability to comply with follow-up; concurrent malignancies; postoperative pathological staging upgrade due to factors such as lymph node metastasis. This study was approved by the Ethics Committee of Hunan Provincial Maternal and Child Health Care Hospital (Approval No. KUAI202036).
1.2. Methods
The operating surgeons were familiar with the relevant anatomical knowledge of cervical cancer surgery and obtained the qualification for performing level 4 laparoscopic surgeries. Open surgery: A midline incision approximately 3 cm above the umbilicus and slightly to the left was made, and under general anesthesia, a wide hysterectomy (Type B1 or Type C2 depending on the stage) was performed following the standard procedure.
Procedures of modified tumor-free laparoscopic radical hysterectomy were as follows:
1) Trocars were inserted, and CO2 gas was insufflated to establish pneumoperitoneum, maintaining intra-abdominal pressure at ≤12 mmHg (1 mmHg= 0.133 kPa), with a flow rate of <5 L/min.
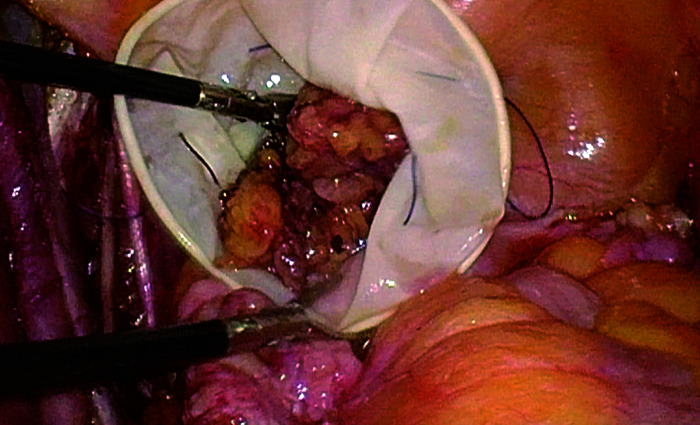
2) Pelvic lymphadenectomy was performed first, following a sequence of sharp dissection from superficial to deep, from lateral to medial, removing lymph nodes as a continuous whole piece, to avoid blunt tearing. Compression of enlarged lymph nodes was minimized, and excised lymph nodes were immediately placed into a specimen bag and sealed the bag. Potential open lymphatic vessels were promptly ligated (Figure 1).
Figure 1. Whole piece resection of lymph nodes and immediate isolation within a specimen bag.
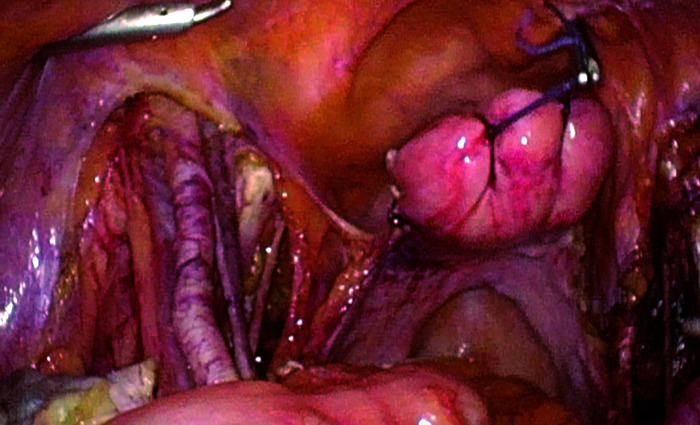
3) Suspension of the uterus under laparoscopy (Figure 2): The uterine fundus was sutured with absorbable suture in a figure-of-eight pattern, creating a loop at the distal end. The uterus was manipulated and adjusted according to intraoperative needs to expose the surgical field, followed by tumor-free radical hysterectomy (Type B1 or Type C2 depending on the stage).
Figure 2. Suspension of the uterus with a figure-of-eight suture at the uterine cervix.
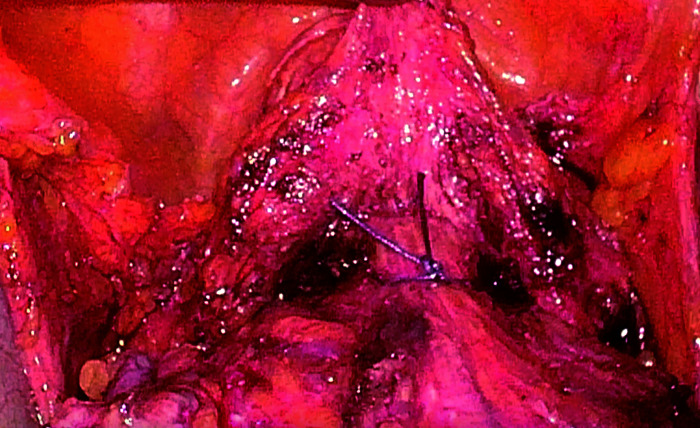
4) Method of vaginal dissection (Figure 3 and 4): The upper part of the vagina was ligated and closed under laparoscopy. The vaginal transection was performed under laparoscopy, and the specimen was removed vaginally. The stump was sutured under laparoscopy.
Figure 3. Ligation and closure of the upper part of the vagina.
Figure 4. Vaginal transection under laparoscopy.
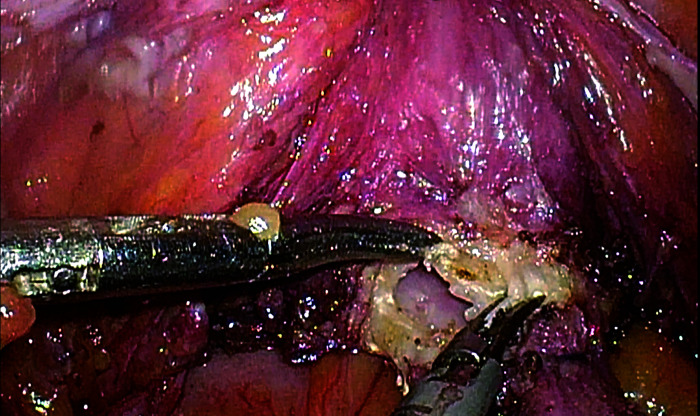
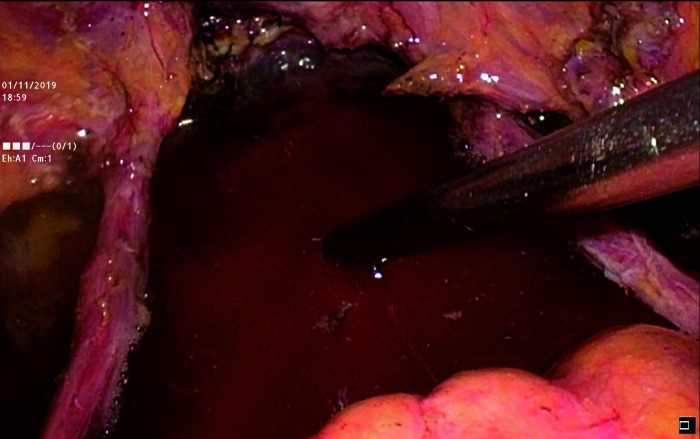
5) The pelvic and abdominal cavities were repeatedly irrigated with distilled water at 43 ℃ to reduce the presence of free cancer cells (Figure 5).
Figure 5. Repeated irrigation of the pelvic and abdominal cavities with distilled water at 43 ℃.
1.3. Postoperative adjuvant therapy
The choice of postoperative adjuvant therapy for both groups of patients depended on intraoperative findings and pathological staging. “High-risk factors” included positive lymph nodes, positive surgical margins, and parametrial involvement. Patients with any of these “high-risk factors” were recommended to receive postoperative adjuvant pelvic external beam radiotherapy plus concurrent cisplatin-based chemotherapy and vaginal brachytherapy. Patients whose surgical pathological staging was upgraded due to exclusion criteria were excluded from the study. Patients with any of “Intermediate-risk factors” (tumor size, stromal invasion, lymphovascular space invasion) were treated with pelvic external beam radiotherapy and cisplatin-based chemotherapy according to the “Sedlis criteria”. For patients with positive vaginal margins or margins <5 mm, vaginal brachytherapy was performed[4].
1.4. Observation indices
1) General clinical data: Age, 2018 FIGO staging, pathological type, presence of intravascular tumor embolism, stromal invasion (invasion depth ≥ 1/2 is considered positive), and postoperative adjuvant treatment.
2) Surgical parameters: Operation time, blood loss, length of excised uterosacral ligaments, length of excised cardinal ligaments, length of excised vagina, and surgical complications.
3) Postoperative survival and recurrence outcomes: rate of disease-free survival, rate of overall survival, recurrence rate, and disease-specific mortality.
1.5. Follow-up
All patients underwent regular follow-up, which included outpatient visits, telephone follow-ups, gynecological examinations, imaging evaluations (including ultrasound, MRI, CT scans), ThinPrep cytology test (TCT), and human papilloma virus (HPV) testing. Colposcopy and pathological biopsies were performed when necessary to assess tumor recurrence. Follow-up visits were scheduled every 3 to 6 months within the first 2 years and every 6 to 12 months during the 3rd to 5th years. The follow-up period ranged from 12 to 41 months, with a median follow-up of 23 months.
1.6. Statistical analysis
SPSS 20.0 software was used for statistical analysis. Continuous variables were expressed as mean± standard deviation ( ±s), while categorical variables were presented as counts (n) and percentages (%). T-test or rank-sum test was used for continuous variables, and chi-square test (chi-squared test with continuity correction or Fisher’s exact test) was used for categorical variables. A P-value of less than 0.05 was considered statistically significant.
2. Results
2.1. General characteristics
The age of the laparoscopy group and the open surgery group was 33-70 (49.06±8.94) years and 36-65 (49.37±7.83) years, respectively. There were no statistically significant differences in age, 2018 FIGO staging, pathological type, lymphovascular space invasion, stromal invasion, and postoperative adjuvant treatment between the laparoscopy group and the open surgery group (all P>0.05, Table 1).
Table 1.
Comparison of clinical data between the laparoscopy group and the open surgery group
| Groups | n | Age*/years | 2018 FIGO staging†/[No.(%)] | Pathological type†/[No.(%)] | ||||
|---|---|---|---|---|---|---|---|---|
| IA1 with lymphovascular invasion | IA2 | IB1 | Squamous carcinoma | Adenocarcinoma | Adenosquamous carcinoma | |||
| Laparoscopy | 45 | 49.06±8.94 | 5(11.1) | 4(8.9) | 36(80.0) | 34(75.6) | 9(20.0) | 2(4.4) |
| Open surgery | 16 | 49.38±7.83 | 0(0.0) | 1(6.2) | 15(93.8) | 13(81.2) | 3(18.8) | 0(0.0) |
| t/χ 2 | 0.122 | 2.145 | 0.770 | |||||
| P | 0.610 | 0.342 | 0.680 | |||||
| Groups | Lymphovascular space invasion‡/[No.(%)] | Stromal invasion‡/[No.(%)] | Postoperative adjuvant treatment‡/[No.(%)] | Type of surgery‡/[No.(%)] | ||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | None | Radiotherapy and chemotherapy | QM-B type | QM-C type | |
| Laparoscopy | 10(22.2) | 35(77.8) | 10(22.2) | 35(77.8) | 38(84.4) | 7(15.6) | 9(20.0) | 36(80.0) |
| Open surgery | 3(18.8) | 13(81.2) | 2(12.5) | 14(87.5) | 15(93.8) | 1(6.2) | 1(6.2) | 15(93.8) |
| t | 0.085 | 0.706 | 0.897 | 1.628 | ||||
| P | 1.000 | 0.635 | 0.606 | 0.267 | ||||
*Data are expressed as the mean±standard deviation and T-test is used. †Chi-squared test is used. ‡Fisher’s exact test is used. FIGO: International Federation of Gynecology and Obstetrics.
2.2. Comparison of surgical parameters between the 2 groups
The laparoscopy group had significantly shorter operation time and less intraoperative blood loss compared to the open surgery group (both P<0.001, Table 2). There were no significant differences between the 2 groups in terms of the length of excised uterosacral ligaments, cardinal ligaments, vagina, and the number of excised lymph nodes (all P>0.05, Table 2). The incidence of postoperative complications did not differ significantly between the groups (P>0.05, Table 2). There were 6 and 2 cases of postoperative urinary retention, 3 and 2 cases of postoperative lymphocysts in the laparoscopy group and the open surgery group, respectively. Only 1 case of poor postoperative abdominal wound healing occurred in the open surgery group. Intraoperative organ injury (urinary and gastrointestinal), intraoperative vascular injury, and venous thromboembolism did not occur in the 2 groups.
Table 2.
Comparison of surgical parameters between the laparoscopy group and the open surgery group
| Groups | n | Operation time*/min | Estimated blood loss*/mL | Length of excised uterosacral ligaments*/cm |
|---|---|---|---|---|
| Laparoscopy | 45 | 248±36 | 238.67±229.43 | 3.1±0.6 |
| Open surgery | 16 | 294±39 | 893.75±449.03 | 2.9±0.3 |
| Z | -3.702 | -4.758 | -1.102 | |
| P | <0.001 | <0.001 | 0.309 |
| Groups | Length of excised cardinal ligaments*/cm | Length of excised vagina*/cm | Number of excised lymph nodes* | Surgical complications†/[No.(%)] |
|---|---|---|---|---|
| Laparoscopy | 3.1±0.5 | 2.93±0.579 | 29±7 | 9(20.0) |
| Open surgery | 3.0±0.4 | 3.16±0.569 | 27±8 | 5(31.3) |
| Z/χ 2 | -0.517 | -0.720 | -0.570 | 0.845 |
| P | 0.605 | 0.472 | 0.569 | 0.490 |
*Data are expressed as the mean±standard deviation and Rank-sum test is used. †Fisher’s exact test is used.
2.3. Rate of disease-free survival, rate of overall survival, recurrence rate, and disease-specific mortality
No death or recurrence occurred during the follow-up period. The overall survival rate and the disease-free survival rate were both 100%. In the open surgery group, 1 patient had persistent HPV16/18 infection, while in the laparoscopy group, 1 patient had persistent high-risk HPV infection.
3. Discussion
With the progress of time and technological advancements, minimally invasive surgery has been widely adopted in various surgical fields. Laparoscopic radical hysterectomy for cervical cancer has been performed for nearly 30 years[5]. Previous retrospective studies and meta-analyses[6-8] have shown no significant differences in recurrence rates and mortality rates between laparoscopic and open surgery. However, in November 2018, 2 studies[1-2] published in the New England Journal of Medicine reported shorter overall survival for minimally invasive cervical cancer surgery compared to open surgery. In 2020, the LACC research team published 2 additional secondary endpoints of the LACC study: 1) There is no difference in the overall incidence of adverse events during and within 6 months after surgery between the minimally invasive surgery group and the open surgery group[9]; 2) postoperative quality of life doesn’t differ between cervical cancer patients who underwent open surgery and those who underwent laparoscopic surgery[10]. Multiple guidelines such as NCCN, ESGO, AGO, etc. have considered open surgery as the standard and recommended surgical approach for cervical cancer treatment[11].
However, laparoscopic surgery as a minimally invasive technique has been used in gynecological clinical practice for many years and has advantages such as clear visualization, precise anatomical dissection, minimal blood loss, rapid recovery, and short hospital stay. What factors have influenced the oncological outcomes of cervical cancer patients undergoing laparoscopic surgery? Is it possible to improve surgical techniques and select appropriate indications for laparoscopic surgery to facilitate its application in the treatment of cervical cancer? Ramirez, the designer, implementer, and author of the LACC study, analyzed the reasons for the poorer oncological outcomes of laparoscopic surgery in cervical cancer and identified the most likely primary reason as the routine use of a uterine manipulator, with other potential factors including CO2 pneumoperitoneum and issues related to vaginal cuff closure through the abdominal cavity[1]. Our team conducted a comprehensive analysis based on the opinions of domestic[3] and international scholars. In July 2019, we started implementing modified tumor-free laparoscopic radical hysterectomy with selective small lesions (diameter ≤2 cm) for early-stage cervical cancer patients. The main tumor-free measures include: 1) Abandon the use of a uterine manipulator. This surgical approach eliminates the routine use of a uterine manipulator (particularly cup-shaped a manipulator) in traditional laparoscopic hysterectomy. Traditional uterine manipulators are used to elevate the uterus during laparoscopic surgery and provide manipulation to expose the surgical field by moving the uterus in various directions according to surgical needs. Additionally, the use of a uterine manipulator helps fill the vagina and provide tension to the vaginal wall, playing a crucial role in dissecting the cervix and surrounding areas, particularly the ureteral region. However, during the surgical process, we discovered that both uterine manipulator and cup could potentially cause rotational cutting, compression, and damage to the tumor. Studies have also confirmed that uterine manipulators can cause parametrial migration[12], lymphovascular space invasion[13-14], pelvic metastasis, and distant metastasis[15]. The NCD-SEER-RWS study demonstrated a significantly increased risk of recurrence and death in cervical cancer patients with tumor diameter >2 cm who underwent minimally invasive surgery[2], suggesting that large tumor lesions are susceptible to the influence of a uterine cup. Several modified methods of uterine traction without a uterine manipulator including figure-eight suturing of the uterine fundus, bilateral uterine cornual suturing, bilateral round ligament suturing, and lower uterine segment ligation. Among various traction methods, we considered figure-eight suturing of the uterine fundus to be the simplest and most feasible traction method used in this study. It is worth noting that the traction suture should be thick to avoid bleeding and tissue tearing during the traction process. The traction line can be inserted through a periumbilical puncture or operated by an assistant through a contralateral operating port to assist in exposing the surgical field. 2) Improve the vaginal cuff closure. In traditional open surgery for cervical cancer, the vagina is closed at the proximal end using 2 large right-angled clamps, effectively preventing the leakage of tumor tissue and achieving a tumor-free principle. However, in the majority of traditional laparoscopic cervical cancer surgeries, the vaginal cuff is dissected within the abdominal cavity using laparoscopy, followed by the removal of the uterus through the vagina. Subsequently, the vaginal cuff is sutured using laparoscopy, which may lead to direct exposure of cancer tissue in the abdominal cavity, violating the tumor-free principle. Previous study[16] has also indicated a significantly higher recurrence rate with abdominal cuff closure compared to vaginal cuff closure. In this study, we performed laparoscopic ligation and closure of the upper part of the vagina and cut off the vagina, followed by vaginal removal of the specimen, and then sutured the stump under laparoscopy. This method fully simulated the process of first sealing the tumor and then dissecting the vagina in open surgery, and it was simple and feasible. Another approach used by other researchers involved first sealing the cervical tumor, then separating and suturing the lower edge of the vagina through the vagina during the operation to close the cervical cancer lesion[17]. Additionally, multiple sterile water irrigations of the vagina were performed before suturing to ensure the absence of residual tumor. 3) Follow the consistent principles of complete, monoblock, and sequential lymph node dissection, preserving and storing specimens in a self-made sterile latex glove specimen bag, which was simple and easy to use. In September 2020, Chinese experts released the “Expert consensus of laparoscopic surgery for cervical cancer in China”[18], which aligns with the surgical techniques employed in this study.
The main difficulties encountered during tumor-free laparoscopic radical hysterectomy were: 1) Overcoming the challenges of exposure caused by suture traction on the uterus. 2) Difficulty in separating soft tissue spaces such as the vesicovaginal and rectovaginal spaces after the vaginal wall loses support from uterine cups. However, with proficiency, surgeons were able to achieve similar surgical outcomes in terms of time, blood loss, and metrics such as ligament and lymph node resection.This study confirms that tumor-free laparoscopic radical hysterectomy can be safely performed in clinical practice. In comparison to open surgery, tumor-free laparoscopic surgery had shorter operative times and less intraoperative bleeding. The lengths of the cardinal and uterosacral ligaments resected, the length of vaginal tissue resected, and the number of lymph nodes removed were comparable to the open surgery group. There was also no increase in surgical complications. Some domestic researchers have also studied laparoscopic surgery for cervical cancer[19-20]. These studies have made tumor-free improvements to the surgical procedure. However, due to the relatively short follow-up period, these studies did not analyze tumor outcomes or explore the indications for laparoscopic surgery for cervical cancer.
This study also investigated the indications for laparoscopic surgery. In the studies conducted by Ramirez et al[1] and Melamed et al[2], the staging was based on the 2009 FIGO staging system. With the introduction of the new cervical cancer staging system, the staging criteria were revised in the 2018 system, which supplemented and modified the old staging system (FIGO 2009). The new staging system included the involvement of imaging and pathological examinations in staging, provided a more detailed stratification of tumor size, and incorporated lymph node involvement into the staging. For stage IB patients, in the 2009 system, IB1 referred to a visible lesion with a maximum diameter ≤4 cm, while in the new staging system, IB1 indicated invasive cancer with a depth of infiltration >5 mm and a maximum diameter of the lesion ≤2 cm. IB2 referred to an invasive cancer with a maximum diameter of the lesion >2 cm and ≤4 cm, while infiltrative cancers with a lesion diameter >4 cm were classified as stage IB3. The LACC study[1] did not evaluate the tumor prognosis for low-risk patients (tumor diameter <2 cm, depth of infiltration <10 mm, no lymph node involvement) using both surgical approaches. In the study by Melamed et al[2], there was insufficient evidence to accurately assess the association between minimally invasive surgery and the subgroup of tumors with a diameter <2 cm and mortality rate. Previous studies have also indicated that there is no statistically significant difference in tumor outcomes between laparoscopic and open radical surgeries for cervical cancer patients with a tumor diameter <2 cm or ≤2 cm[21-22]. Expert consensus of laparoscopic surgery for cervical cancer in China published in September 2020, also stated that laparoscopic surgery can be performed for patients with stage IB1 cervical cancer according to the 2009 FIGO staging system and a tumor diameter ≤2 cm[18]. In 2021, based on the 1 538 project database, LIU et al[23] compared the long-term tumor outcomes of laparoscopic and open approaches for FIGO 2018 stage IB1 cervical cancer using a comprehensive cohort study and propensity score matching. The results showed no statistically significant difference between the 2 approaches, and Cox analysis also revealed that the laparoscopic approach was not an independent risk factor for death, recurrence, or death from cervical cancer in 2018 FIGO stage IB1 patients[23].
Therefore, this study selected patients with early-stage cervical cancer (2018 FIGO staging: IA1 with lymphovascular space invasion, IA2, IB1) who underwent strict preoperative evaluation using imaging techniques such as MRI and CT to confirm tumor diameter ≤2 cm without suspicion of lymph node metastasis or parametrial involvement as the study subjects. This study implemented measures such as tracting the uterus instead of using uterine manipulator, tumor-free dissection of the vagina, modified pelvic and abdominal lymph node dissection techniques, and improved pelvic and abdominal cavity irrigation methods to achieve tumor-free principles as much as possible[3]. QM-B type radical hysterectomy was performed for stage IA1 with lymphovascular space invasion and stage IA2, while QM-C type radical hysterectomy was performed for stage IB1[24].
In summary, tumor-free laparoscopic radical hysterectomy without a uterine manipulator for early-stage cervical cancer offers advantages such as clear visualization, minimal intraoperative bleeding, and short operation time. Compared to open surgery, it shows no significant difference in oncological outcomes. However, this is a retrospective study, and prospective larger data samples and long-term follow-up are needed to further evaluate the pros and cons of laparoscopic surgery.
Contributions: ZHAO Jing Study design, data analysis and interpretation, manuscript preparation; LIU Qiao Data analysis and interpretation, manuscript preparation; JIANG Dan, CHEN Tianmin, and MENG Shengjun Data collection; SHU Chuqiang Study design, manuscript preparation. All authors have approved the final version of the manuscript.
Funding Statement
This work was supported by the Science and Technology Innovation Program of Hunan Province, China (2020SK50605).
Conflict of Interest
The authors declare that they have no conflicts of interest to disclose.
Note
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/2023111686.pdf
References
- 1. Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer[J]. N Engl J Med, 2018, 379(20): 1895-1904. 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 2. Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer[J]. N Engl J Med, 2018, 379(20): 1905-1914. 10.1056/NEJMoa1804923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. 陈春林, 郎景和. 中国专家“关于宫颈癌腹腔镜手术相关问题”的几点意见[J]. 中国实用妇科与产科杂志, 2019, 35(2): 188-193. 10.19538/j.fk2019020115. [DOI] [Google Scholar]; CHEN Chunlin, LANG Jinghe. Opinions of Chinese experts on “some problem about laparoscopic surgery for cervical cancer”[J]. Chinese Journal of Practical Gynecology and Obstetrics, 2019, 35(2): 188-193. 10.19538/j.fk2019020115. [DOI] [Google Scholar]
- 4. Koh WJ, Abu-Rustum NR, Bean S, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2019, 17(1): 64-84. 10.6004/jnccn.2019.0001. [DOI] [PubMed] [Google Scholar]
- 5. Dueñas-González A, Campbell S. Global strategies for the treatment of early-stage and advanced cervical cancer[J]. Curr Opin Obstet Gynecol, 2016, 28(1): 11-17. 10.1097/GCO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 6. Nam JH, Park JY, Kim DY, et al. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study[J]. Ann Oncol, 2012, 23(4): 903-911. 10.1093/annonc/mdr360. [DOI] [PubMed] [Google Scholar]
- 7. Wang YZ, Deng L, Xu HC, et al. Laparoscopy versus laparotomy for the management of early stage cervical cancer[J]. BMC Cancer, 2015, 15: 928. 10.1186/s12885-015-1818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao TF, Feng YL, Huang QD, et al. Prognostic and safety roles in laparoscopic versus abdominal radical hysterectomy in cervical cancer: a meta-analysis[J]. J Laparoendosc Adv Surg Tech A, 2015, 25(12): 990-998. 10.1089/lap.2015.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Obermair A, Asher R, Pareja R, et al. Incidence of adverse events in minimally invasive vs open radical hysterectomy in early cervical cancer: results of a randomized controlled trial[J]. Am J Obstet Gynecol, 2020, 222(3): 249.e1-249249.e10. 10.1016/j.ajog.2019.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frumovitz M, Obermair A, Coleman RL, et al. Quality of life in patients with cervical cancer after open versus minimally invasive radical hysterectomy (LACC): a secondary outcome of a multicentre, randomised, open-label, phase 3, non-inferiority trial[J]. Lancet Oncol, 2020, 21(6): 851-860. 10.1016/S1470-2045(20)30081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. 向阳, 蒋芳. 子宫颈癌微创手术的未来——放弃还是改良[J]. 中国实用妇科与产科杂志, 2021, 37(1): 15-17. 10.19538/j.fk2021010104. [DOI] [Google Scholar]; XIANG Yang, JIANG Fang. Future of minimally invasive surgery for cervical cancer—to give up or to improve[J]. Chinese Journal of Practical Gynecology and Obstetrics, 2021, 37(1): 15-17. 10.19538/j.fk2021010104. [DOI] [Google Scholar]
- 12. Rakowski JA, TANTran, Ahmad S, et al. Does a uterine manipulator affect cervical cancer pathology or identification of lymphovascular space involvement?[J]. Gynecol Oncol, 2012, 127(1): 98-101. 10.1016/j.ygyno.2012.07.094. [DOI] [PubMed] [Google Scholar]
- 13. Krizova A, Clarke BA, Bernardini MQ, et al. Histologic artifacts in abdominal, vaginal, laparoscopic, and robotic hysterectomy specimens: a blinded, retrospective review[J]. Am J Surg Pathol, 2011, 35(1): 115-126. 10.1097/PAS.0b013e31820273dc. [DOI] [PubMed] [Google Scholar]
- 14. Logani S, Herdman AV, Little JV, et al. Vascular “pseudo invasion” in laparoscopic hysterectomy specimens: a diagnostic pitfall[J]. Am J Surg Pathol, 2008, 32(4): 560-565. 10.1097/pas.0b013e31816098f0. [DOI] [PubMed] [Google Scholar]
- 15. Uppal S, Gehrig P, Vetter MH, et al. Recurrence rates in cervical cancer patients treated with abdominal versus minimally invasive radical hysterectomy: a multi-institutional analysis of 700 cases[J]. J Clin Oncol, 2019, 37(15_suppl): 5504. 10.1200/jco.2019.37.15_suppl.5504. [DOI] [PubMed] [Google Scholar]
- 16. Kong TW, Chang SJ, Piao XL, et al. Patterns of recurrence and survival after abdominal versus laparoscopic/robotic radical hysterectomy in patients with early cervical cancer[J]. J Obstet Gynaecol Res, 2016, 42(1): 77-86. 10.1111/jog.12840. [DOI] [PubMed] [Google Scholar]
- 17. 赵福杰, 王升科, 苗欣欣, 等. 先封闭子宫颈癌瘤体法的无瘤化免举宫腹腔镜广泛性子宫切除改良手术20例分析[J]. 中国实用妇科与产科杂志, 2020, 36(7): 651-654. 10.19538/j.fk2020070117. [DOI] [Google Scholar]; ZHAO Fujie, WANG Shengke, MIAO Xinxin, et al. Clinical analysis of 20 cases of LRH for cervical cancer without tumor exposure by sealing the tumor[J]. Chinese Journal of Practical Gynecology and Obstetrics, 2020, 36(7): 651-654. 10.19538/j.fk2020070117. [DOI] [Google Scholar]
- 18. 陈春林, 郎景和, 向阳, 等. 子宫颈癌腹腔镜手术治疗的中国专家共识[J]. 中华妇产科杂志, 2020, 55(9): 579-585. 10.3760/cma.j.cn112141-20200310-00202. [DOI] [Google Scholar]; CHEN Chunlin, LANG Jinghe, XIANG Yang, et al. Expert consensus of laparoscopic surgery for cervical cancer in China[J]. Chinese Journal of Obstetrics and Gynecology, 2020, 55(9): 579-585. 10.3760/cma.j.cn112141-20200310-00202. [DOI] [Google Scholar]
- 19. 王璐, 赵新蕊, 朱琳. 25例早期宫颈癌无瘤化免举宫腹腔镜子宫切除术临床效果[J]. 山东大学学报(医学版), 2021, 59(6): 76-80. 10.6040/j.issn.1671-7554.0.2021.0328. [DOI] [Google Scholar]; WANG Lu, ZHAO Xinrui, ZHU Lin. Clinical efficacy of laparoscopic radical hysterectomy without lift and tumor spillage in 25 cases of early-stage cervical cancer[J]. Journal of Shandong University. Health Sciences, 2021, 59(6): 76-80. 10.6040/j.issn.1671-7554.0.2021.0328. [DOI] [Google Scholar]
- 20. 樊杨, 张娜, 李熳, 等. 无举宫腹腔镜广泛子宫切除术在早期宫颈癌的临床研究[J]. 宁夏医学杂志, 2020, 42(12): 1142-2243. 10.13621/j.1001-5949.2020.12.1142. 33183098 [DOI] [Google Scholar]; FAN Yang, ZHANG Na, LI Man, et al. Clinical study of tumor-free non-lifting laparoscopic radical hysterectomy for early cervical cancer[J]. Ningxia Medical Journal, 2020, 42(12): 1142-2243. 10.13621/j.1001-5949.2020.12.1142. [DOI] [Google Scholar]
- 21. Kim SI, Cho JH, Seol A, et al. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1-IIA2 cervical cancer[J]. Gynecol Oncol, 2019, 153(1): 3-12. 10.1016/j.ygyno.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 22. Kim SI, Lee M, Lee S, et al. Impact of laparoscopic radical hysterectomy on survival outcome in patients with FIGO stage IB cervical cancer: a matching study of two institutional hospitals in Korea[J]. Gynecol Oncol, 2019, 155(1): 75-82. 10.1016/j.ygyno.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 23. 柳攀, 龚时鹏, 黎志强, 等. 2018年FIGO子宫颈癌新分期中IB1期治疗策略的探讨[J]. 中国实用妇科与产科杂志, 2021, 37(8): 836-840. 10.19538/j.fk2021080112. [DOI] [Google Scholar]; LIU Pan, GONG Shipeng, LI Zhiqiang, et al. Exploration of treatment strategies for stage IB1 in the new 2018 FIGO cervical cancer staging[J]. Chinese Journal of Practical Gynecology and Obstetrics, 2021, 37(8): 836-840. 10.19538/j.fk2021080112. [DOI] [Google Scholar]
- 24. 陈春林, 黎志强. 子宫颈癌手术治疗质量控制需要注意的几个问题[J]. 中国实用妇科与产科杂志, 2022, 38(1): 22-24. 10.19538/j.fk2022010106. [DOI] [Google Scholar]; CHEN Chunlin, LI Zhiqiang. Several issues that need attention in quality control of surgical treatment for cervical cancer[J]. Chinese Journal of Practical Gynecology and Obstetrics, 2022, 38(1): 22-24. 10.19538/j.fk2022010106. [DOI] [Google Scholar]