Abstract
目的
绿原酸具有抗菌、抗炎、抗病毒等多种生理活性,研究显示绿原酸可缓解急性肺损伤(acute lung injury,ALI)小鼠机体炎症反应,但具体作用机制尚不明确。本研究旨在探讨绿原酸是否通过调控微RNA-223(microRNA-223,miR-223)/核苷酸结合寡聚化结构域样受体蛋白3(nucleotide-binding oligomerization domain-like receptor protein 3,NLRP3)轴来减轻脂多糖(lipopolysaccharide,LPS)诱导的小鼠ALI。
方法
将SPF级BALBc雄性小鼠随机分为对照组、模型组、绿原酸组、绿原酸+miR-223阴性对照(miR-223 NC)组、绿原酸+miR-223抑制物(miR-223 antagomir)组,每组10只,除对照组,其余各组均通过气道滴入4 mg/kg的LPS制备ALI小鼠模型,造模成功后绿原酸组小鼠使用绿原酸(100 mg/kg)连续灌胃7 d。绿原酸+miR-223 NC组、绿原酸+miR-223 antagomir组每天灌胃100 mg/kg的绿原酸后分别经尾静脉注射10 μL的miR-223 NC(0.5 nmol/μL)、miR-223 antagomir(0.5 nmol/μL),连续 7 d。对照组和模型组用生理盐水替代。取小鼠肺组织,测定肺湿/干重比(W/D);收集小鼠肺泡灌洗液,采用ELISA试剂盒测定炎症因子TNF-α、IL-6、IL-1β的水平;于光学显微镜下统计嗜酸性粒细胞(eosinophils,EOS)、淋巴细胞、中性粒细胞的数量;行HE染色后观察小鼠肺组织病理学变化并进行肺损伤评分;采用qRT-PCR法测定小鼠肺组织中miR-223的表达水平,蛋白质印迹法测定小鼠肺组织中NLRP3蛋白质的表达水平;采用荧光素酶报告实验分析miR-223与NLRP3的靶向关系。
结果
与对照组相比,模型组小鼠肺W/D值、肺损伤评分、肺泡灌洗液中炎症因子水平显著升高(均P<0.05),肺组织炎症细胞浸润严重,肺泡空间明显增加,肺泡壁显著增厚,肺泡灌洗液中EOS、淋巴细胞、中性粒细胞数量显著增加(均P<0.05),肺组织中miR-223表达水平显著降低、NLRP3蛋白质表达水平显著升高(均P<0.05)。与模型组相比,绿原酸组、绿原酸+miR-223 NC组、绿原酸+miR-223 antagomir组小鼠肺W/D值、肺损伤评分、肺泡灌洗液中炎症因子水平显著降低(均P<0.05),肺组织损伤减轻,肺泡灌洗液中EOS、淋巴细胞、中性粒细胞数量显著减少(均P<0.05),肺组织中miR-223表达水平显著升高,NLRP3蛋白质表达水平显著降低(均P<0.05)。与绿原酸组相比,绿原酸+miR-223 antagomir组小鼠肺W/D值、肺损伤评分、肺泡灌洗液中炎症因子水平显著升高(均P<0.05),肺组织损伤加重,肺泡灌洗液中EOS、淋巴细胞、中性粒细胞数量显著增加(均P<0.05),肺组织中miR-223表达水平显著降低,NLRP3蛋白质表达水平显著升高(均P<0.05)。荧光素酶报告实验结果显示miR-223与NLRP3存在靶向关系。
结论
绿原酸可能通过提高miR-223水平,靶向抑制NLRP3表达,降低LPS诱导的ALI小鼠炎症反应,缓解肺组织病理损伤。
Keywords: 绿原酸, 急性肺损伤, 微RNA-233, 核苷酸结合寡聚化结构域样受体蛋白3, 脂多糖
Abstract
Objective
Chlorogenic acid has various physiological activities such as antibacterial, anti-inflammatory, and antiviral activities. Studies have shown that chlorogenic acid can alleviate the inflammatory response of mice with acute lung injury (ALI), but the specific mechanism is still unclear. This study aims to investigate whether chlorogenic acid attenuates lipopolysaccharide (LPS)-induced ALI in mice by regulating the microRNA-223 (miR-223)/nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) axis.
Methods
SPF grade BALBc male mice were randomly divided into a control group, a model group, a chlorogenic acid group, a chlorogenic acid+miR-223 negative control (miR-223 NC) group, and a chlorogenic acid+miR-223 inhibitor (miR-223 antagomir) group, 10 mice in each group. Except the control group, the other groups were instilled with 4 mg/kg LPS through the airway to establish the ALI mouse model. After the modeling, the mice in the chlorogenic acid group were continuously given chlorogenic acid (100 mg/kg) by gavage for 7 d. The chlorogenic acid+miR-223 NC group and the chlorogenic acid+miR-223 antagomir group were given 100 mg/kg chlorogenic acid by gavage every day, and then were injected with 10 μL of miR-223 NC (0.5 nmol/μL) and miR-223 antagomir (0.5 nmol/μL) respectively for 7 consecutive days.The control group and the model group were replaced with normal saline. The lung tissues of mice were taken to measure the ratios of lung wet to dry weight (W/D). The bronchoalveolar lavage fluid of mice was collected to measure the levels of TNF-α, IL-6, and IL-1β by ELISA kit and to count the number of eosinophils (EOS), lymphocytes, neutrophils under light microscope. After HE staining, the pathological changes of lung tissues were observed and lung injury was scored. qRT-PCR method were used to determine the expression levels of miR-223 in lung tissues. Western blotting was used to determine the expression levels of NLRP3 protein in mouse lung tissues. Luciferase reporter assay was used to analyze the targeting relationship of miR-223 to NLRP3.
Results
Compared with the control group, the lung W/D value, the lung injury score and the level of inflammatory factors in the bronchoalveolar lavage fluid were significantly increased in the model group (all P<0.05); the infiltration of inflammatory cells in the lung tissue was severe; the alveolar space was significantly increased; the alveolar wall was significantly thickened; the number of EOS, lymphocytes, and neutrophils in the bronchoalveolar lavage fluid was significantly increased (all P<0.05); the expression levels of miR-223 in lung tissue were significantly decreased (P<0.05); and the protein expression levels of NLRP3 were significantly increased (P<0.05). Compared with the model group, the W/D value of lungs, lung injury score, and levels of inflammatory factors in bronchoalveolar lavage fluid were significantly decreased in the chlorogenic acid group, the chlorogenic acid+miR-223 NC group, and the chlorogenic acid+miR-223 antagomir group (all P<0.05); lung tissues damage was alleviated; the numbers of EOS, lymphocytes, and neutrophils in bronchoalveolar lavage fluid were significantly decreased (all P<0.05); the expression levels of miR-223 in lung tissues were significantly increased (P<0.05); and the expression levels of NLRP3 protein were significantly decreased (P<0.05). Compared with the chlorogenic acid group, the lung W/D value, lung injury score, and inflammatory factor levels in the bronchoalveolar lavage fluid were significantly increased in the chlorogenic acid+miR-223 antagomir group (all P<0.05); lung tissue damage was aggravated; the number of EOS, lymphocytes and neutrophils in bronchoalveolar lavage fluid significantly increased (all P<0.05); the expression levels of miR-223 in lung tissues were significantly decreased (P<0.05); and the expression levels of NLRP3 protein were significantly increased (P<0.05). The results of luciferase reporter assay showed that miR-223 had a targeting relationship with NLRP3.
Conclusion
Chlorogenic acid may increase the level of miR-223, target the inhibition of NLRP3 expression, reduce LPS-induced inflammatory response in ALI mice, and alleviate pathological damage of lung tissues.
Keywords: chlorogenic acid, acute lung injury, microRNA-233, nucleotide-binding oligomerization domain-like receptor protein 3, lipopolysaccharide
急性肺损伤(acute lung injury,ALI)是一种肺部急性炎症损伤,由各种直接或间接的因素(如脓毒症、肺炎、肺泡出血、有毒气体等)引起肺泡上皮细胞、毛细血管内皮细胞损伤,进而造成弥漫性肺间质及肺泡水肿,主要有低氧血症、呼吸窘迫等不良表现,严重时可导致急性低氧性呼吸功能不全,极大地威胁着患者的生命安全[1-2]。绿原酸是杜仲、金银花等植物中常见的活性成分,具有抗菌、抗炎、抗病毒等多种生理活性。Liu等[3]研究发现:绿原酸、迷迭香酸、异黄酮联用可有效缓解脂多糖(lipopolysaccharide,LPS)诱导的小鼠ALI,并显著降低肺组织中炎症因子的表达水平,且呈现一定的剂量效应关系。郭静等[4]研究发现:单独使用绿原酸可显著降低ALI小鼠机体炎症反应,对于补体旁路激活诱导的小鼠ALI具有一定的保护作用,但其作用机制尚未阐明。微RNA-233(microRNA-233,miR-223)与许多炎症相关疾病联系密切,可抑制核苷酸结合寡聚化结构域样受体蛋白3(nucleotide-binding oligomerization domain-like receptor protein 3,NLRP3)炎症复合体的表达,进而发挥对线粒体损伤相关分子诱导的ALI[5]及放射性ALI的保护作用[6]。本研究拟对绿原酸是否通过调控miR-223/NLRP3轴减轻LPS诱导的小鼠ALI进行探讨,以期为临床治疗ALI提供实验依据。
1. 材料与方法
1.1. 材料
SPF级6~8周龄BALBc雄性小鼠由北京生物制品研究所有限责任公司提供,生产许可证号为SCXK(京)2016-0012,体质量约20 g。所有小鼠均于动物房(温度约25 ℃、相对湿度约50%)中饲养,实验期间自由进食、饮水,保持动物房环境及鼠笼清洁、透气。在实验过程中使用“3R”原则给予动物人道主义关怀。本研究经郑州大学附属郑州中心医院动物伦理委员会批准同意(审批号:20190017)。
小鼠肺成纤维细胞(货号:CP-M006)购于武汉普诺赛生物公司。绿原酸、LPS购于上海陶素生化科技有限公司;miR-223抑制物(miR-223 antagomir)、miR-223模拟物(miR-223 mimic)及miR-223阴性对照(miR-223 NC)购于广州市锐博生物科技有限公司;HE染色试剂盒、RNA提取试剂盒、反转录试剂盒、高效RIPA裂解液、BCA蛋白质测定试剂盒购于北京索莱宝科技有限公司;TNF-α、IL-6、IL-1β ELISA试剂盒购于上海碧云天生物技术有限公司;兔源NLRP3、GAPDH一抗、羊抗兔二抗购于英国Abcam公司。
MK-3Ex型全自动酶标仪购于美国Clover公司;ASP200S全封闭组织脱水机购于德国Leica公司;Mcllwain组织切片机购于美国Stoelting;SMZ745光学显微镜购于日本尼康公司;1659001蛋白电泳仪、Trans-Blot SD半干转膜仪购于美国Bio-Rad公司;GIS-500凝胶成像仪购于杭州米欧仪器有限公司。
1.2. 方法
1.2.1. 动物模型的制备及分组
ALI小鼠模型的制备参考文献[7]的方法并进行了改进。麻醉小鼠后,采用静脉留置针行气管插管,按4 mg/kg的剂量将LPS(50 μL)经气道滴入。观察到小鼠出现毛色暗淡、精神情况较差、进食量减少、呼吸急促、鼻唇区发绀、活动缓慢等现象时,认为ALI小鼠模型构建成功。整个实验期间出现小鼠死亡或不符合条件的现象,及时选取备用小鼠进行补充。
将造模成功的ALI小鼠按照随机数字法分为模型组、绿原酸组、绿原酸+miR-223 NC组、绿原酸+miR-223 antagomir组。绿原酸组小鼠按照100 mg/kg的剂量,使用绿原酸(溶解于0.2%的羧甲基纤维素钠)连续灌胃7 d。绿原酸+miR-223 NC组、绿原酸+miR-223 antagomir组每天灌胃100 mg/kg的绿原酸后分别经尾静脉注射10 μL 的miR-223 NC(0.5 nmol/μL)、miR-223 antagomir(0.5 nmol/μL),连续7 d。另设对照组,对照组及模型组均用生理盐水替代药物进行处理。各组小鼠均为10只。
1.2.2. 小鼠肺泡灌洗液、肺组织的采集
末次给药结束后24 h,将各组小鼠麻醉后处死,剪开胸部皮肤,暴露胸腔,结扎小鼠的右侧主支气管,将磷酸盐缓冲液经左侧主气管注入左侧肺泡,观察到小鼠肺部膨隆后抽回缓冲液,重复3次,合并缓冲液并以1 500 r/min离心10 min,取上清液用于炎症因子水平的测定,取沉淀物用于炎症细胞的分类计数。
取小鼠右侧肺组织、上叶肺组织用于HE染色,中叶肺组织用于肺湿/干重比(W/D)的测定,下叶肺组织用于miR-223、NLRP3等相关指标的测定。
1.2.3. 小鼠肺W/D值的计算
取小鼠肺组织,用预冷的生理盐水冲洗干净,用滤纸拭干后进行称重,记为湿重,接着将称过重的肺组织放入恒温箱中干燥至恒重,记为干重,计算W/D值。
1.2.4. 小鼠肺组织HE染色、肺损伤评分
取小鼠肺组织,用4%甲醛溶液固定约48 h,洗掉甲醛溶液,用自动组织脱水机进行脱水、透明处理,用融化的石蜡包埋肺组织,待其凝固后切成约 4 μm厚的薄片并贴附在载玻片上,按照HE染色试剂盒的要求进行脱蜡、水化、苏木精染色、分化、返蓝液返蓝、伊红染色、中性树胶封片等操作,在光学显微镜下观察。
观察各组小鼠肺组织病理学变化并参考文献[8]中的评分标准进行肺损伤评分,评分项目包括肺泡水肿、肺泡出血、炎症细胞浸润、中性粒细胞死亡情况,各评分项目正常记0分,轻度、中度、重度、极重度病变分别记1、2、3、4分,各项目评分之和为肺损伤评分。
1.2.5. 小鼠肺泡灌洗液中炎症因子水平的检测和炎症细胞计数
取小鼠肺泡灌洗液,用TNF-α、IL-6、IL-1β ELISA试剂盒测定炎症因子水平,具体操作按照相关试剂盒中的要求进行。取小鼠肺泡灌洗液下层沉淀,用生理盐水重悬,均匀涂抹于载玻片中央,待其干燥后行瑞氏染色,染色3 min后进行水洗,干燥后用中性树胶行封片,于光学显微镜下观察约200个白细胞,由经验丰富的细胞形态学检测人员统计其中嗜酸性粒细胞(eosinophils,EOS)、淋巴细胞、中性粒细胞的数量。
1.2.6. 小鼠肺组织中miR-223表达水平的检测
取小鼠肺组织,使用RNA提取试剂盒提取总RNA,用反转录试剂盒合成cDNA,以U6作为内参,行PCR扩增。MiR-223正向引物为5'-GGCTGTCA-GTTTGTCAAATACC-3',反向引物为5'-CGCGAGC-ACAGAATTAATACG-3';U6正向引物为5'-CTCGC-TTCGGCAGCACA-3',反向引物为5'-AACGCTTCA-CGAATTTGCGT-3'。反应体系主要有2.5 µL SYBR Mix、正向及反向引物各0.1 µL、cDNA 1 µL、去核糖核酸酶水1.3 µL等。反应条件:95 ℃预变性3 min、95 ℃变性15 s、65 ℃退火20 s、75 ℃延伸15 s,共40个循环。实验重复3次,采用2-∆∆Ct法计算小鼠肺组织中miR-223的相对表达水平。
1.2.7. 小鼠肺组织中NLRP3蛋白质表达水平的检测
取小鼠肺组织,用预冷的生理盐水冲洗3次后拭干,用高效RIPA裂解液提取总蛋白质,离心后取上清液,采用BCA蛋白质测定试剂盒测定上清液中蛋白质含量,用上样缓冲液将各组蛋白质浓度调整至相同,加热至蛋白质变性,然后进行SDS-聚丙烯酰胺凝胶电泳以分离蛋白质,将分离的蛋白质采用湿转法转移至硝酸纤维素(nitrocellulose,NC)膜上,用5%的脱脂奶粉进行封闭处理,加入兔源NLRP3、GAPDH一抗后在低温下孵育过夜,加入HRP标记的羊抗兔二抗,在室温下孵育1 h,经TBST缓冲液冲洗后进行显色、定影等步骤,采用凝胶成像仪进行观察和分析。
1.2.8. MiR-223与NLRP3靶向关系的验证
查询TargetScan数据库,结果显示miR-223在NLRP3的3'-UTR区存在相应结合位点。取小鼠肺成纤维细胞,构建突变型、野生型基因靶点的NLRP3 3'-UTR荧光素酶表达载体NLRP3-MUT、NLRP3-WT,将NLRP3-MUT、NLRP3-WT分别与miR-223 mimic NC、miR-223 mimic转染至小鼠肺成纤维细胞中,在37 ℃、5%CO2培养箱中培养24 h后测定各组细胞的相对荧光素酶活性,实验重复3次。
1.3. 统计学处理
采用GraphPad Prism 8软件对实验数据进行统计分析,计量资料以均数±标准差( ±s)表示,组间比较行单因素方差分析,进一步两组间比较行LSD-t检验,P<0.05为差异有统计学意义。
2. 结 果
2.1. 绿原酸对小鼠肺W/D值的影响
与对照组相比,模型组小鼠肺W/D值显著升高(P<0.05);与模型组相比,绿原酸组、绿原酸+miR-223 NC组、绿原酸+miR-223 antagomir组小鼠肺W/D值均显著降低(均P<0.05);与绿原酸组相比,绿原酸+miR-223 antagomir组小鼠肺W/D值显著升高(均 P<0.05,表1)。
表1.
各组小鼠肺W/D值比较(n=10, ±s)
Table 1 Comparison of W/D value of mice in each group
| 组别 | W/D |
|---|---|
| 对照组 | 4.02±0.41 |
| 模型组 | 6.59±0.87* |
| 绿原酸组 | 4.78±0.43† |
| 绿原酸+miR-223 NC组 | 4.83±0.44† |
| 绿原酸+miR-223 antagomir组 | 5.71±0.76†‡§ |
W/D:湿/干重比。与对照组比较,*P<0.05;与模型组比较,†P<0.05;与绿原酸组比较,‡P<0.05;与绿原酸+miR-223 NC组比较,§P<0.05。
n=10, ±s
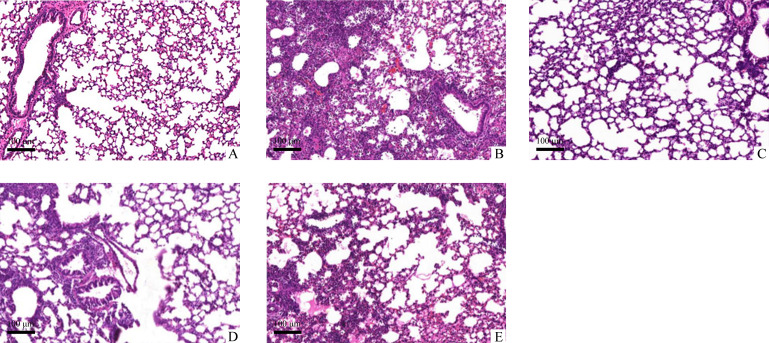
2.2. 绿原酸对小鼠肺组织病理学的影响
各组小鼠肺组织病理学改变见图1。对照组小鼠肺组织形态及结构基本正常;与对照组相比,模型组小鼠肺组织炎症细胞浸润严重,肺泡空间明显增加,肺泡壁显著增厚,肺损伤评分显著升高(P<0.05);与模型组相比,绿原酸组小鼠肺组织损伤减轻,肺损伤评分显著降低(P<0.05);与绿原酸组相比,绿原酸+miR-223 antagomir组小鼠肺损伤加重,肺损伤评分显著升高(P<0.05);绿原酸+miR-223 NC组与绿原酸组肺损伤评分差异无统计学意义(P>0.05,表2)。
图1.
各组小鼠肺组织病理学改变(HE,×400)
Figure 1 Pathological change of lung tissues in each group of mice (HE, ×400)
A: Control group; B: Model group; C: Chlorogenic acid group; D: Chlorogenic acid+miR-223 NC group; E: Chlorogenic acid+miR-223 antagomir group.
表2.
各组小鼠肺损伤评分比较(n=10, ±s)
Table 2 Comparison of lung injury score of mice in each group (n=10, ±s)
| 组别 | 肺损伤评分 |
|---|---|
| 对照组 | 0.28±0.05 |
| 模型组 | 11.83±2.46* |
| 绿原酸组 | 4.19±0.64† |
| 绿原酸+miR-223 NC组 | 4.22±0.78† |
| 绿原酸+miR-223 antagomir组 | 8.04±1.24†‡§ |
与对照组比较,*P<0.05;与模型组比较,†P<0.05;与绿原酸组比较,‡P<0.05;与绿原酸+miR-223 NC组比较,§P<0.05。
2.3. 绿原酸对小鼠肺泡灌洗液中炎症细胞、炎症因子的影响
与对照组相比,模型组小鼠肺泡灌洗液中EOS、淋巴细胞、中性粒细胞数量增加,炎症因子TNF-α、IL-6、IL-1β的水平均显著升高(均P<0.05);与模型组相比,绿原酸组小鼠肺泡灌洗液中EOS、淋巴细胞、中性粒细胞数量减少,炎症因子TNF-α、IL-6、IL-1β的水平均显著降低(均P<0.05);与绿原酸组相比,绿原酸+miR-223 antagomir组小鼠肺泡灌洗液中EOS、淋巴细胞、中性粒细胞数量增加,炎症因子TNF-α、IL-6、IL-1β的水平均显著升高(均P<0.05);绿原酸+miR-223 NC组与绿原酸组上述指标差异均无统计学意义(均P>0.05,表3)。
表3.
各组小鼠肺泡灌洗液中炎症细胞数、炎症因子水平比较(n=10, ±s)
Table 3 Comparison of the number of inflammatory cells and the level of inflammatory factors in the alveolar lavage fluid in each group of mice (n=10, ±s)
| 组别 | EOS/ (×106个/L) |
淋巴细胞/ (×106个/L) |
中性粒细胞/ (×106个/L) |
TNF-α/(ng·L-1) | IL-6/(ng·L-1) | IL-1β/(ng·L-1) |
|---|---|---|---|---|---|---|
| 对照组 | 0.12±0.02 | 2.16±0.45 | 1.44±0.29 | 55.41±10.16 | 15.80±3.29 | 31.02±6.46 |
| 模型组 | 4.22±0.88* | 7.69±1.61* | 5.43±1.15* | 138.71±20.29* | 54.14±11.30* | 129.60±21.05* |
| 绿原酸组 | 1.06±0.22† | 4.01±0.83† | 2.12±0.44† | 73.06±15.12† | 22.36±4.63† | 56.55±11.70† |
| 绿原酸+miR-223 NC组 | 1.09±0.26† | 3.98±0.93† | 2.15±0.51† | 72.49±17.06† | 22.57±5.29† | 55.98±13.14† |
| 绿原酸+miR-223 antagomir组 | 3.06±0.62†‡§ | 5.57±1.13†‡§ | 3.56±0.72†‡§ | 90.13±18.08†‡§ | 40.03±8.09†‡§ | 107.34±21.68†‡§ |
EOS:嗜酸性粒细胞。与对照组比较,*P<0.05;与模型组比较,†P<0.05;与绿原酸组比较,‡P<0.05;与绿原酸+miR-223 NC组比较,§P<0.05。
2.4. 绿原酸对小鼠肺组织中miR-223表达水平、NLRP3蛋白质表达水平的影响
与对照组相比,模型组小鼠肺组织中miR-223表达水平显著降低,NLRP3蛋白表达水平显著升高(均P<0.05);与模型组相比,绿原酸组小鼠肺组织中miR-223表达水平显著升高,NLRP3蛋白表达水平显著降低(均P<0.05);与绿原酸组相比,绿原酸+miR-223 antagomir组miR-223表达水平显著降低,NLRP3蛋白表达水平显著升高(均P<0.05);绿原酸组与绿原酸+miR-223 NC组小鼠肺组织中miR-223表达水平、NLRP3蛋白质表达水平差异无统计学意义(P>0.05;表4,图2)。
表4.
各组小鼠肺组织中miR-223表达水平、NLRP3蛋白质表达水平比较(n=10, ±s)
Table 4 Comparison of miR-223 expression levels and protein expression levels of NLRP3 in lung tissues of mice in each group (n=10, ±s)
| 组别 | MiR-223/U6 | NLRP3/GAPDH |
|---|---|---|
| 对照组 | 1.00±0.00 | 1.03±0.20 |
| 模型组 | 0.11±0.02* | 1.83±0.31* |
| 绿原酸组 | 0.76±0.14† | 1.28±0.22† |
| 绿原酸+miR-223 NC组 | 0.74±0.13† | 1.27±0.25† |
| 绿原酸+miR-223 antagomir组 | 0.48±0.09†‡§ | 1.52±0.28†‡§ |
MiR-223:微RNA-233;NLRP3:核苷酸结合寡聚化结构域样受体蛋白3。与对照组比较,*P<0.05;与模型组比较,†P<0.05;与绿原酸组比较,‡P<0.05;与绿原酸+miR-223 NC组比较,§P<0.05。
图2.
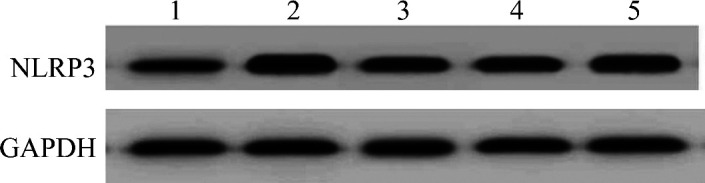
各组小鼠肺组织中NLRP3蛋白质的表达情况
Figure 2 NLRP3 protein expression in lung tissues of mice in each group
1: Control group; 2: Model group; 3: Chlorogenic acid group; 4: Chlorogenic acid+miR-223 NC group; 5: Chlorogenic acid+miR-223 antagomir group. NLRP3: Nucleotide-binding oligomerization domain-like receptor protein 3.
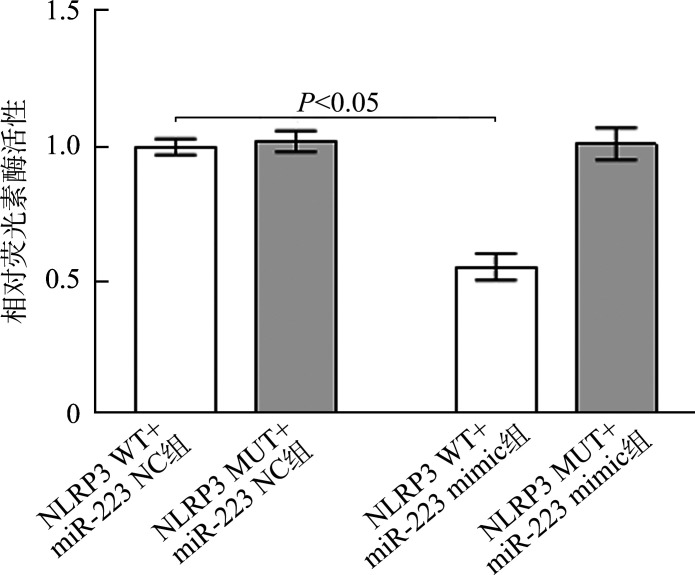
2.5. MiR-223与NLRP3靶向关系验证
TargetScan数据库预测结果显示miR-223与NLRP3存在相应结合位点(图3)。荧光素酶报告实验结果(图4)显示:与NLRP3 WT+miR-223 NC组相比,NLRP3 WT+miR-223 mimic组小鼠肺成纤维细胞荧光素酶活性显著降低(0.97±0.02 vs 0.53±0.01,P<0.05),NLRP3 MUT+miR-223 NC组与NLRP3 MUT+miR-223 mimic组荧光素酶活性差异无统计学意义(0.96±0.02 vs 0.97±0.03,P>0.05)。
图3.
MiR-223与NLRP3的3'-UTR区序列相互结合
Figure 3 MiR-223 and sequence of NLRP3 3'-UTR region mutually bind
图4.
荧光素酶报告实验检测结果(n=3, ±s)
Figure 4 Test results of luciferase report experiment (n=3, ±s)
3. 讨 论
ALI的发病机制复杂,主要是各种肺内外致伤因素引起的急性炎症反应,多种炎症细胞在肺内被激活、募集,并促进TNF-α、IL-6、IL-1β等炎症因子的大量释放,造成炎症细胞及炎症因子的互相作用和激活,形成失控性炎症反应。因此关于ALI的治疗,主要以抑制炎症因子的释放为出发点,抑制炎症反应的发生及发展对于降低ALI发病率及病死率有积极的作用[9-10]。
多项研究表明绿原酸对于ALI具有一定的缓解作用。葛媛媛等[11]研究发现绿原酸可部分缓解过氧化氢所致ALI,并能显著降低ALI大鼠肺泡灌洗液中炎症因子水平,认为绿原酸有望成为治疗ALI的药物;Zhang等[12]研究发现绿原酸可显著减轻LPS诱导的ALI小鼠肺水肿及肺泡结构损伤等症状。本研究通过气道滴入LPS成功建立ALI小鼠模型,发现模型组小鼠肺组织受损严重,肺W/D值、肺损伤评分、肺泡灌洗液中炎症因子水平均升高,肺泡灌洗液中炎症细胞EOS、淋巴细胞、中性粒细胞数量增加,提示模型构建成功;经过绿原酸干预后,ALI小鼠的肺组织损伤得到一定的缓解,肺W/D值、肺损伤评分、肺泡灌洗液中炎症因子水平均降低,肺泡灌洗液中炎症细胞EOS、淋巴细胞、中性粒细胞数量减少,与Zhang等[12]的研究结果一致,提示绿原酸对LPS所致ALI小鼠具有一定的保护作用。
MiRNA是一类内源性非编码小分子RNA,长度为22~25 nt,参与对细胞增殖、凋亡、分化等活动的调节,具有维持机体生理稳态等作用,与感染性疾病、心血管疾病、遗传性疾病、炎症性疾病等多种疾病的发生和发展联系密切。MiRNA主要通过与靶基因的3'-UTR配对结合,调控靶基因的翻译及表达,进而参与多种生物活动[13-14]。MiR-223是miRNA的一员,在多种疾病中表达异常,近年来的研究发现miR-223与ALI疾病的发生和发展联系密切。Neudecker等[15]研究发现miR-223在ALI小鼠肺组织中呈低表达,并认为低表达的miR-223会加重肺部细菌感染导致的ALI的病情,提高miR-223表达水平则有利于ALI病情的缓解。本研究结果显示模型组小鼠miR-223表达水平显著低于对照组,提示miR-223与ALI的发生、发展过程联系密切,经过绿原酸处理后,ALI小鼠肺组织中miR-223水平显著升高,提示绿原酸保护肺部的作用可能与其上调miR-223的表达有关。本研究通过生物信息学分析和荧光素酶报告实验发现miR-223与NLRP3存在靶向调节关系。NLRP3是与炎症相关的蛋白质之一,组织损伤、氧化应激、细菌等均可激活NLRP3,激活后的NLRP3可促进TNF-α、IL-6、IL-1β等炎症因子的释放,进而参与炎症相关疾病的发生与发展[16-17]。多项研究表明抑制NLRP3可缓解LPS诱导的ALI。阻断NLRP3激活后肺组织中炎症因子的释放显著减少,肺组织的损伤进而得到缓解[18-19];莫湘涛等[20]研究发现miR-223/NLRP3轴与LPS诱导的人单核细胞炎症反应联系密切,提高miR-223表达可降低NLRP3的表达水平,进而减轻LPS诱导的炎症反应。本研究结果显示:模型组ALI小鼠肺组织中NLRP3表达水平显著升高,经过绿原酸处理后NLRP3表达水平显著降低;与绿原酸组相比,绿原酸+miR-223 antagomir组小鼠肺组织中NLRP3表达水平显著升高。MiR-223 antagomir可减轻绿原酸对ALI小鼠的保护作用,绿原酸可能通过miR-223/NLRP3轴,减轻LPS诱导的ALI小鼠的肺组织病理损伤,提示绿原酸的肺保护作用可能与其增加miR-223表达,进而抑制NLRP3表达有关。绿原酸作用机制复杂,本研究初步探讨了其通过miR-223/NLRP3轴对LPS诱导的ALI小鼠的影响,但并未设置相应的NLRP3抑制剂组进行对比,这是本研究的不足,下一步工作可对其进行完善。
基金资助
河南省医学科技攻关计划联合共建项目(LHGJ20191051);河南省高等学校重点科研项目(20B320024)。
This work was supported by the Joint Construction Project of Medical Science and Technology Research Plan of Henan Province (LHGJ20191051) and the Key Scientific Research Project of Colleges and Universities of Henan Province (20B320024), China.
利益冲突声明
作者声称无任何利益冲突。
作者贡献
刘畅、张强 论文的构想、撰写和修改,实验的设计和实施;程晓丹、孙家安、张少华 数据的处理、统计分析。
原文网址
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/202203280.pdf
参考文献
- 1. Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review[J]. Arch Pathol Lab Med, 2016, 140(4): 345-350. 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 2. 吴松林, 田小利, 徐陶, 等. microRNA-125b在脓毒症急性肺损伤中的表达及其与炎症因子水平相关性[J]. 天津医药, 2019, 47(8): 810-814, 898. 10.11958/20181913. 30873868 [DOI] [Google Scholar]; WU Songlin, TIAN Xiaoli, XU Tao, et al. Expression of microRNA-125b in acute lung injury induced by sepsis and its correlation with inflammatory factors[J]. Tianjin Medical Journal, 2019, 47(8): 810-814, 898. 10.11958/20181913. [DOI] [Google Scholar]
- 3. Liu CP, Liu JX, Gu J, et al. Combination effect of three main constituents from sarcandra glabra inhibits oxidative stress in the mice following acute lung injury: a role of MAPK-NF-κB pathway[J]. Front Pharmacol, 2021, 11(1): 580064. 10.3389/fphar.2020.580064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. 郭静, 李敏, 杨毅, 等. 绿原酸对小鼠急性肺损伤的保护作用研究[J]. 天然产物研究与开发, 2018, 30(7): 1214-1218, 1251. 10.16333/j.1001-6880.2018.7.020. [DOI] [Google Scholar]; GUO Jing, LI Min, YANG Yi, et al. Protective effect of chlorogenic acid on acute lung injury in mice[J]. Natural Product Research and Development, 2018, 30(7): 1214-1218, 1251. 10.16333/j.1001-6880.2018.7.020. [DOI] [Google Scholar]
- 5. Feng Z, Qi S, Zhang Y, et al. Ly6G+ neutrophil-derived miR-223 inhibits the NLRP3 inflammasome in mitochondrial DAMP-induced acute lung injury[J]. Cell Death Dis, 2017, 8(11): e3170 (2017-11-16)[2021-02-15]. 10.1038/cddis.2017.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. 王锃, 苏丽, 陈瑞庆, 等. miR-223通过抑制NLRP3防护小鼠急性放射性肺损伤[J]. 中华放射医学与防护杂志, 2019, 39(3): 166-171. 10.3760/cma.j.issn.0254-5098.2019.03.002. [DOI] [Google Scholar]; WANG Chen, SU Li, CHEN Ruiqing, et al. MiR-223 protects mice from acute radiation-induced lung injury by inhibiting NLRP3[J]. Chinese Journal of Radiological Medicine and Protection, 2019, 39(3): 166-171. 10.3760/cma.j.issn.0254-5098.2019.03.002. [DOI] [Google Scholar]
- 7. 董年, 王蓓蓓, 宋晨剑, 等. CCN1在脂多糖诱导急性肺损伤中的表达调控和在炎症反应中的作用[J]. 中国病理生理杂志, 2020, 36(3): 507-513. 10.3969/j.issn.1000-4718.2020.03.019. [DOI] [Google Scholar]; DONG Nian, WANG Beibei, SONG Chenjian, et al. Regulatory role of CCN1 in lipopolysaccharide-induced mouse acute lung injury[J]. Chinese Journal of Pathophysiology, 2020, 36(3): 507-513. 10.3969/j.issn.1000-4718.2020.03.019. [DOI] [Google Scholar]
- 8. 袁清红, 刘强胜, 刘安鹏, 等. 激活大麻素2型受体对脓毒症大鼠急性肺损伤的影响: 自噬在其中的作用[J]. 中华麻醉学杂志, 2018, 38(12): 1509-1512. 10.3760/cma.j.issn.0254-1416.2018.12.027. [DOI] [Google Scholar]; YUAN Qinghong, LIU Qiangsheng, LIU Anpeng, et al. Effect of activating cannabinoid receptor 2 on sepsis-induced acute lung injury in mice: the role of autophagy[J]. Chinese Journal of Anesthesiology, 2018, 38(12): 1509-1512. 10.3760/cma.j.issn.0254-1416.2018.12.027. [DOI] [Google Scholar]
- 9. Zhu Y, Wang Y, Xing S, et al. Blocking SNHG14 antagonizes LPS-induced acute lung injury via SNHG14/miR-124-3p axis[J]. J Surg Res, 2021, 263(1): 140-150. 10.1016/j.jss.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 10. Zhang A, Cai Y, Wang PF, et al. Diagnosis and prognosis of neutrophil gelatinase-associated lipocalin for acute kidney injury with sepsis: a systematic review and meta-analysis[J]. Crit Care, 2016, 20(1): 41. 10.1186/s13054-016-1212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. 葛媛媛, 张桐桐, 张萌萌, 等. 肺吸入绿原酸β-环糊精包合物治疗急性肺损伤[J]. 国际药学研究杂志, 2018, 45(10): 772-777. 10.13220/j.cnki.jipr.2018.10.007. [DOI] [Google Scholar]; GE Yuanyuan, ZHANG Tongtong, ZHANG Mengmeng, et al. Pulmonary delivery of chlorogenic acid β-cyclodextrin inclusion complexes for therapy of acute lung injury[J]. Journal of International Pharmaceutical Research, 2018, 45(10): 772-777. 10.13220/j.cnki.jipr.2018.10.007. [DOI] [Google Scholar]
- 12. Zhang X, Huang H, Yang T, et al. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury[J]. Injury, 2010, 41(7): 746-752. 10.1016/j.injury.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 13. Jiang K, Yang J, Guo S, et al. Peripheral circulating exosome-mediated delivery of miR-155 as a novel mechanism for acute lung inflammation[J]. Mol Ther, 2019, 27(10): 1758-1771. 10.1016/j.ymthe.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee W, Kim I, Shin S, et al. Expression profiling of microRNAs in lipopolysaccharide-induced acute lung injury after hypothermia treatment[J]. Mol Cell Toxicol, 2016, 12(3): 243-253. 10.1007/s13273-016-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neudecker V, Brodsky KS, Clambey ET, et al. Neutrophil transfer of miR-223 to lung epithelial cells dampens acute lung injury in mice[J]. Sci Transl Med, 2017, 9(408): eaah5360. 10.1126/scitranslmed.aah5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Y, Yang F, Yu X, et al. miR-16 inhibits NLRP3 inflammasome activation by directly targeting TLR4 in acute lung injury[J]. Biomed Pharmacother, 2019, 112(1): 108664. 10.1016/j.biopha.2019.108664. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Q, Ran X, He Y, et al. Acetate downregulates the activation of NLRP3 inflammasomes and attenuates lung injury in neonatal mice with bronchopulmonary dysplasia[J]. Front Pediatr, 2021, 8(1): 595157. 10.3389/fped.2020.595157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meng M. Digitoflavone (DG) attenuates LPS-induced acute lung injury through reducing oxidative stress and inflammatory response dependent on the suppression of TXNIP/NLRP3 and NF-κB[J]. Biomed Pharmacother, 2017, 94(1): 712-725. 10.1016/j.biopha.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 19. Zhao B, Lu R, Chen J, et al. S100A9 blockade prevents lipopolysaccharide-induced lung injury via suppressing the NLRP3 pathway[J]. Respir Res, 2021, 22(1): 45. 10.1186/s12931-021-01641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. 莫湘涛, 张依山, 李勇军. 硼替佐米调节miR-223/NLRP3轴对脂多糖诱导的人单核细胞炎症反应的影响[J]. 中国病理生理杂志, 2020, 36(8): 1482-1486. 10.3969/j.issn.1000-4718.2020.08.021. 32615264 [DOI] [Google Scholar]; MO Xiangtao, ZHANG Yishan, LI Yongjun. Bortezomib attenuates LPS-induced inflammatory response of human monocytes through regulating miR-223/NLRP3 signaling pathway[J]. Chinese Journal of Pathophysiology, 2020, 36(8): 1482-1486. 10.3969/j.issn.1000-4718.2020.08.021. [DOI] [Google Scholar]