ABSTRACT
Autoimmune liver diseases are rare serious diseases causing chronic inflammation and fibrosis in the liver parenchyma and bile ducts. Yet, the prevalence and burden of autoimmune liver diseases are largely unexplored in Arctic native populations. We investigated the prevalence and management of autoimmune liver diseases in Greenland using nationwide cross-sectional register data and subsequent medical chart reviews validating diagnoses and extracting liver histology examinations and medical treatments. The overall prevalence of autoimmune liver diseases in Greenland was 24.6 per 100,000 (95% CI: 14.7–41.3). This was based on 7 patients with autoimmune hepatitis (AIH) (12.3 per 100,000), 3 patients with primary biliary cholangitis (PBC) (5.3 per 100,000), 4 patients with AIH/PBC overlap disease (7.0 per 100,000), and no patients with primary sclerosing cholangitis. All diagnoses were confirmed by liver histology examinations. Medical treatments adhered to internal recommendations and induced complete remission in most patients with AIH, and complete or partial remission in 1 patient with PBC and 3 patients with AIH/PBC overlap disease. One patient had established cirrhosis at the time of diagnosis, while 2 patients progressed to cirrhosis. In conclusion, the prevalence of autoimmune liver diseases was lower in Greenland than in Scandinavia and among Alaska Inuit.
KEYWORDS: Greenland, Inuit, Arctic, autoimmune liver disease, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis
Introduction
Autoimmune liver disease represents a spectrum of rare diseases characterised by chronic inflammation in the liver parenchyma and bile ducts [1,2]. Based on clinical, serological, and histopathological characteristics, the three main categories of autoimmune liver disease include autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC). However, the diagnosis and treatment may be both challenging and complex as the diseases may overlap and require different therapeutic approaches [1].
Even though these autoimmune liver diseases are rare, their prevalence, incidence, phenotype, and outcome vary between geographical regions and ethnic groups; thus, genetic, environmental, and societal factors may influence the susceptibility to disease development and treatment response [3,4]. The indigenous population in Greenland was largely isolated from Westernisation until the 1950s and the unique genetic profile and traditional Inuit lifestyle might protect against autoimmune diseases as reported for the prevalence of autoimmune thyroid and diabetes antibodies [5–8]. However, as urbanisation developed, the lifestyle in Greenland has transitioned to resemble that of Western countries. Therefore, the burden of autoimmune diseases may also resemble that of Western countries, as recently reported for autoimmune inflammatory bowel diseases [9]. Hepatologists practicing in Circumpolar areas refer to studies investigating AIH, PBC, PSC, and AIH with overlap disease in Alaska natives [10,11]. These studies reported a high and rising prevalence of AIH and AIH with overlap disease and a similar prevalence of PBC compared to Scandinavian countries, while no patients with PSC were identified [10–14].
We aimed to investigate the burden of autoimmune liver diseases in Greenland, uncovering their prevalence, treatment regimens, treatment responses, histopathological descriptions, and clinical courses. We hypothesise that the prevalence and management of autoimmune liver diseases are similar to Scandinavian countries.
Methods
In Greenland, healthcare is tax-funded and all prescribed medication is free of charge [15]. The electronic medical record (EMR) system in Greenland includes comprehensive healthcare data on patients with a permanent address in Greenland. Correct registration in the EMR system requires a diagnosis code assignment which is achieved by 74% of patients in Nuuk but less for patients outside Nuuk [16,17]. Patients with rare liver diseases are predominantly followed at the largest hospital in Nuuk although very long travel distances across the country can limit the number of clinical and biochemistry control visits at a specialist department. However, remote biochemistry monitoring is possible with blood samples drawn at healthcare centres and stations along the coastline [9].
This study was approved by the Ethics Committee in Greenland (no. 2022–22081) and the Agency for Health and Prevention in Greenland. We extracted cross-sectional data from the electronic medical register in Greenland on 9 September 2023. Eligible patients from all five healthcare regions were identified using the International Classification of Diseases – Tenth Revision (ICD-10) codes DK73.2 (chronic active hepatitis, not elsewhere classified [NEC]), DK73.8 (other chronic hepatitis, NEC), DK74.1 (hepatic sclerosis), DK74.2 (hepatic fibrosis with hepatic sclerosis), DK74.3 (primary biliary cirrhosis), DK74.4 (secondary biliary cirrhosis), DK74.5 (biliary cirrhosis, unspecified), DK75.3 (granulomatous hepatitis, NEC), DK75.4 (autoimmune hepatitis), DK75.8 (other specified inflammatory liver diseases), DK75.9 (inflammatory liver disease, unspecified), DK83.0 (cholangitis), and DK83.9 (disease of biliary tract, unspecified), and the Anatomical Therapeutic Chemical (ATC) classification code for ursodeoxycholic acid (A05AA02) [18,19]. The ICD-10 codes used here adhered to standards for epidemiological studies of autoimmune liver diseases [12,14]. The most recent demographic and anthropometric data including place of birth, place of residence, age, sex, weight, and height were drawn from the electronic medical register. Furthermore, we extracted the most recent biochemistry results from the register. Next, the Greenlandic healthcare authorities allowed us to review the medical records of all patients identified by the relevant ICD-10 and ATC codes to validate their diagnosis of autoimmune liver disease and to extract descriptions of liver histology examinations as well as information on medical treatments and treatment responses. Patients with PBC were considered treatment responders when alkaline phosphatase levels dropped below 170 U/L and patients with AIH were treatment responders when alanine aminotransferase (ALT) stabilised within the normal range [1,20]. In Greenland, ALT is considered normal, when ≤35 U/L in women and ≤50 U/L in men aged 50 years or younger, and ≤70 U/L in all individuals more than 50 years [21]. We were unable to access and extract data from the national death registry. Therefore, only patients alive on 9 September 2023, were identified and included in this study.
According to Statistics Greenland, updated on 1 July 2023, the total Greenlandic population is 56,865 [22]. This number was used for prevalence calculations, and the corresponding 95% confidence intervals were estimated using Wilson’s method [23].
Results
In total, 70 patients were identified with a relevant ICD-10 code for autoimmune liver disease or the ATC code for ursodeoxycholic acid prescription. The most recent anthropometric and biochemistry cross-sectional data were collected between 1 January 2019, and 31 May 2023. The medical chart reviews confirmed the diagnosis of autoimmune liver disease in 14 patients, while 56 patients were misclassified, and therefore excluded, mainly due to cholestasis in pregnancy and non-autoimmune biliary diseases (Figure 1).
Figure 1.
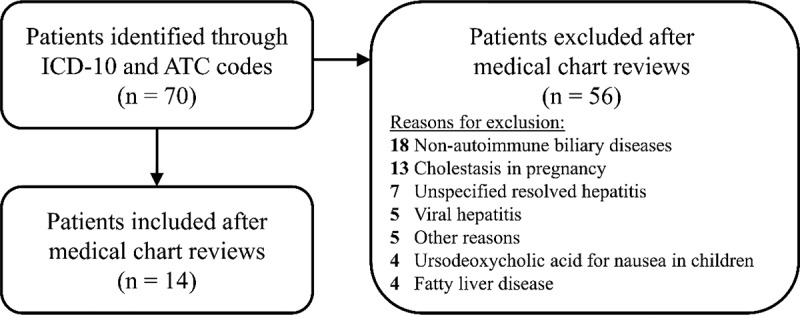
Flowchart of in- and exclusions.
Abbreviations: ICD-10, International Classification of Diseases – Tenth Revision; ATC, Anatomical Therapeutic Chemical.
The overall prevalence of autoimmune liver disease in Greenland was 24.6 per 100,000 (95% CI: 14.7–41.3). AIH was the most prevalent autoimmune liver disease with 7 patients and a prevalence of 12.3 per 100,000 (95% CI: 6.0–25.4). Three patients suffered from PBC equal to a prevalence of 5.3 per 100,000 (95% CI: 1.8–15.5) while 4 patients had AIH/PBC overlap disease and a prevalence of 7.0 per 100,000 (95% CI: 2.7–18.1). No patients with PSC were identified.
The background characteristics are provided in Tables 1 and 2. The age at the most recent contact ranged between 48 and 71 years and most patients were female. All patients had their diagnosis confirmed by liver histology examinations and 5 patients underwent liver biopsy twice, mainly those with AIH/PBC overlap disease. Hence, the patients with overlap disease showed the most abnormal liver biochemistry results, which might have contributed to the decision for repeating liver biopsy collections (Tables 1 and 2).
Table 1.
Cohort background characteristics at the disease level.
| Variable | AIH n = 7 |
PBC n = 3 |
AIH/PBC overlap n = 4 |
|---|---|---|---|
| Age (years), median (range) | 64 (60–70) | 69 (56–71) | 61 (48–69) |
| Female sex, n (%) | 4 (57%) | 3 (100%) | 3 (75%) |
| Time since diagnosis (years), median (range) | 5.8 (2.1–8.2) | 10.7 (2.5–15.4) | 14.7 (14.6–18.8) |
| Liver histopathology, n (%) Interphase hepatitis Lymphocytic inflammation Rosette formation Portal inflammation or granulomas Fibrosis Steatosis |
4 (57%) 5 (71%) 4 (57%) 1 (14%) 4 (57%) 3 (43%) |
1 (33%) 2 (67%) 0 (0%) 3 (100%) 2 (67%) 0 (0%) |
4 (100%) 4 (100%) 1 (25%) 3 (75%) 4 (100%) 2 (50%) |
| More than one liver biopsy, n (%) | 1 (14%) | 1 (33%) | 3 (75%) |
| Cirrhosis at diagnosis, n (%) | 1 (14%) | 0%) | 0 (0%) |
| Cirrhosis at present, n (%) | 1 (14%) | 1 (33%) | 1 (25%) |
| Pharmacotherapies, n (%) Prednisolone Azathioprine Mycophenolic acid Ursodeoxycholic acid |
7 (100%) 5 (71%) 1 (14%) 0 (0%) |
0 (0%) 0 (0%) 0 (0%) 3 (100%) |
2 (50%) 4 (100%) 0 (0%) 4 (100%) |
| Disease course, n (%) Complete treatment response Partial treatment response Relapsing Non-response |
6 (86%) 0 (0%) 1 (14%) 0 (0%) |
1 (33%) 1 (33%) 0 (0%) 1 (33%) |
1 (25%) 2 (50%) 1 (25%) 0 (0%) |
| Anthropometrics, median (range) Height (cm) Weight (kg) BMI (kg/m^2) |
158 (154–179) 87 (64–110) 31.5 (25.0–45.2) |
153 (146–160) 50 (48–53) 21.8 (18.7–24.9) |
169 (151–182) 83 (52–104) 28.9 (22.8–31.4) |
| Biochemistry, median (range) ALT (U/l) AST (U/l) Alkaline phosphatase (U/l) Bilirubin (µmol/l) FIB4 score |
33 (28–53) 30 (25–43) 91 (35–114) 27 (7–34) 1.34 (1.12–2.69) |
58 (12–111) 52 (21–176) 318 (70–521) 9 (7–155) 2.91 (1.39–4.43) |
80 (52–125) 50 (42–81) 155 (129–175) 21 (8–32) 1.94 (1.00–2.41) |
| Birthplace, n (%) Greenland Denmark Unknown |
6 (86%) 0 (0%) 1 (14%) |
3 (100%) 0 (0%) 0 (0%) |
3 (75%) 1 (25%) 0%) |
Abbreviations: AIH, autoimmune hepatitis; PBC, primary biliary cholangitis; BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FIB4, fibrosis 4.
Table 2.
Cohort characteristics at the individual level. The cross-sectional anthropometric and biochemistry background information were extracted from the most recent hospital contact, i.e. between January 1, 2019, and May 31, 2023. Subsequently, the pharmacotherapy data were extracted through the medical chart reviews. The term present treatment states that the treatment was ongoing at the most recent hospital contact.
| Cross-sectional background anthropometric and biochemistry information |
Follow-up on pharmacotherapies |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Disease | Sex | Age group(yr) | Time sincediagnosis(yr) | Birth-place | ALT (U/l) |
AST (U/l) |
Alkaline phosphatase (U/L) | Bilirubin (µmol/l) |
FIB4 | BMI (kg/m2) |
prednisolone | azathioprine | mycophenolic acid | UDCA | Treatment response |
| 1 2 3 4 5 6 7 8 9 10 11 12 13 14 |
AIH AIH AIH AIH AIH AIH AIH AIH/PBC AIH/PBC AIH/PBC AIH/PBC PBC PBC PBC |
M M F F M F F M F F F F F F |
≥60 ≥60 ≥60 ≥60 ≥60 ≥60 ≥60 <60 ≥60 <60 ≥60 ≥60 ≥60 <60 |
6.6 5.4 7.5 8.4 2.3 5.9 2.8 18.9 14.8 14.7 14.8 15.5 2.7 10.8 |
GR GR GR GR GR N/R GR GR GR GR DK GR GR GR |
43 33 28 39 53 28 28 91 69 125 52 111 58 12 |
43 25 N/R 29 31 31 29 55 42 81 45 176 52 21 |
52 88 109 114 35 91 109 157 175 129 153 521 318 70 |
27 7 8 27 34 14 31 24 8 17 32 155 9 7 |
2.69 1.12 N/R 1.33 1.35 2.09 1.18 1.00 2.28 1.61 2.41 4.43 1.39 N/R |
29.1 25.0 45.2 N/R 32.3 44.0 30.8 31.4 22.8 28.9 N/R 24.9 18.7 N/R |
present present present previously present previously present present present N/R N/R never never never |
present present never present present previously never present present present present never never never |
never never never never never present never never never never never never never never |
never never never never never never never present present present present present present present |
Complete Relapsing Complete Complete Complete Complete Complete Relapsing Partial Complete Partial Non-resp. Non-resp. Responder |
Abbreviations: AIH, autoimmune hepatitis; PBC, primary biliary cholangitis; M, male; F, female; yr, years; GR, Greenland; DK, Denmark; N/R, not reported; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FIB4, fibrosis 4; BMI, body mass index; UDCA, ursodeoxycholic acid; Non-resp., non-responder.
One patient with PBC was a treatment responder with plasma alkaline phosphatase <170 U/L, while the other 2 patients with PBC were non-responders. All patients with AIH had normal ALT in their most recent biochemistry results, of whom 1 had had a relapsing disease course. Hence, the pharmacological treatments followed international guidelines and recommendations [1,2] and induced complete remission for most patients.
Liver histology examinations confirmed the clinical diagnosis for most patients (Table 3). Among 13 available descriptions of liver histology, 6 patients had no fibrosis and 6 patients had mild or moderate fibrosis. Steatosis was described in specimens from 4 patients. The patients with a second liver histology examination had it performed between 3.5 and 11.8 years later than their first biopsy, and 3 patients had progression in fibrosis stage, 1 remained unchanged, and 1 had resolution of fibrosis. One patient with AIH had established cirrhosis at the time of diagnosis, while 1 patient with AIH/PBC overlap and 1 patient with PBC progressed to cirrhosis during their disease course. High alcohol consumption and chronic viral hepatitis were excluded as contributors to liver disease in all included patients.
Table 3.
Liver histology examinations at the individual level.
| Histopathology at diagnosis |
Histopathology follow-up |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Date (yr) | Interphase hepatitis | lymphocyte infiltration | rosette formation | biliary changes | fibrosis | steatosis | Date (yr) | Interphase hepatitis | lymphocyte infiltration | rosette formation | biliary changes | fibrosis | steatosis |
| 1 2 3 4 5 6 7 8 9 10 11 12 13 14 |
2017 2018 2016 2015 2021 2017 2021 2004 2009 2011 2009 2008 2010 2013 |
yes no no yes N/R yes yes yes yes yes yes yes no no |
yes no yes yes N/R yes yes yes yes yes yes yes yes no |
no N/R yes yes N/R yes yes no N/R N/R N/R no no no |
no no no no N/R no yes no yes yes no yes no yes |
grade 1 no no grade 1 N/R no grade 4 grade 1 no grade 2 grade 1 grade 2 no no |
yes yes no no N/R no yes no no no yes no no no |
- - - - - 2020 - 2016 2017 2019 - - 2021 - |
- - - - - yes - yes yes no - - no - |
- - - - - yes - yes yes yes - - no - |
- - - - - no - no yes no - - no - |
- - - - - no - no no no - - yes - |
- - - - - grade 3 - grade 1 grade 4 grade 1 - - grade 2 - |
- - - - - no - no no yes - - no - |
Abbreviations: N/R, not reported; yr, year.
Discussion
We here present the first prevalence estimates and disease course descriptions of autoimmune liver diseases in Greenland. The overall prevalence is 24.6 per 100,000 (95% CI: 14.7–41.3), based on 7 cases with AIH, 3 cases with PBC, and 4 cases with AIH/PBC overlap disease. The patient’s age and sex distributions were typical for autoimmune liver diseases [12].
Regarding AIH, the prevalence of 12.3 per 100,000 is lower than reported for most North European countries, including Denmark with a prevalence of 23.9 per 100,000 [12,24]. Notably, AIH is also less prevalent in Greenland than among Inuit in Alaska, who are included in the “Alaska Natives” minority population with a record-high AIH prevalence of 91.2 per 100,000 [11]. Hence, although Inuit in Greenland and Alaska share unique genetics, this seems to have little influence on the susceptibility to develop AIH. Also, the prevalence of AIH was considerably lower in Greenland compared with the Faroe Islands (71.8 per 100,000), which comprise a population genetically non-related to Inuit but share traditional lifestyle elements including a seafood diet [25].
The prevalence of PBC in Greenland was 5.3 per 100,000 which is also lower than most Nordic countries including Denmark (11.5 per 100,000) and the Faroe Islands (38.5 per 100,000) and among Inuit in Alaska (18 per 100,000) [10,25–27]. On the other hand, 29% of the patients with autoimmune liver disease in Greenland had clinically and histologically confirmed AIH/PBC overlap disease, which is a larger proportion than the approximately 10% usually reported [25,28,29]. No patients with PSC were identified, which is remarkable as the prevalence of inflammatory bowel diseases in Greenland is similar to Scandinavian countries [9]. In general epidemiological studies of PSC are hampered by the lack of a disease-specific ICD-10 code [30]. However, as PSC usually develops in approximately 8% of patients with inflammatory bowel disease, we would expect 20 cases of PSC in Greenland assuming the same prevalence as in Scandinavian countries [3]. As patients with PSC would undergo examinations at the hospital in Nuuk, it is unlikely that the authors are unaware of such a patient group. Therefore, Inuit with inflammatory bowel disease might be protected from developing the serious extraintestinal manifestation; PSC.
The current study supports the frequently held notion that Greenlanders are less prone to develop chronic liver disease than Caucasians [31]. However, as chronic liver disease progresses along a spectrum of disease severity, this concept may not apply to all liver diseases and therefore warrants continued research in chronic liver diseases in Inuit populations. At the mild end of the severity spectrum, the prevalence of non-alcoholic fatty liver disease (NAFLD) among Arctic native people follows the global trend [32], although the degree of liver fibrosis is lower in Greenland Inuit than Danes with type 2 diabetes living in Greenland [21]. At the severe end of the spectrum, the prevalence of patients with a hospital discharge diagnosis of cirrhosis was slightly lower in Greenland than in Denmark [31]. This aligns with the present study reporting only 1 patient with AIH and concurrent clinically and histologically evident cirrhosis at the time of diagnosis. The rate of disease progression to cirrhosis was low, including 1 patient with AIH/PBC overlap disease and 1 treatment non-responder patient with PBC. This underlines that patients with autoimmune liver disease in Greenland receive high awareness and are treated effectively following treatment algorithms tailored for an Arctic scenery.
A major strength of this study is the nationwide population-based setting with a medical chart review validation of all patients drawn from the electronic medical register allocated an autoimmune liver disease diagnosis. Furthermore, the diagnoses and treatment responses were affirmed through liver histology descriptions for all of the patients.
The small number of patients limits the possibility of subgroup analyses. Moreover, the large travel distances for specialised healthcare services may result in mild cases of these rare liver diseases living undetected. On the other hand, the lower prevalence of autoimmune liver diseases in Greenland could be a consequence of a higher mortality, although this seems unlikely as we in general observed disease remission on treatment and slow disease progressions. Moreover, the risk of compliance issues was reduced due to the de facto elimination of financial reasons for treatment non-compliance. Finally, there is a risk for underestimating the prevalence of autoimmune liver diseases as the EMR registration is not complete [17]. However, as national recommendations dictate patients with rare liver diseases being followed at the specialised hospital department in Nuuk, the likelihood for correct disease coding and registration in the EMR reaches the highest level for the country [17].
In conclusion, we have uncovered the burden of autoimmune liver diseases in Greenland and report a prevalence of 24.6 per 100,000 inhabitants. This estimate emerges from 7 cases with AIH, 3 with PBC, 4 with AIH/PBC overlap disease, and none with PSC. The prevalence is lower than in Scandinavian countries as well as in related Arctic native populations. Finally, the autoimmune liver diseases were treated in accordance with international guidelines, in general resulting in satisfactory treatment responses.
Funding Statement
This project was financially supported by the Novo Nordisk Foundation [grant no. NNF20SA0064190].
Disclosure statement
Henning Grønbæk reports: Research grants (AbbVie, Intercept, ARLA Food for Health, ADS AIPHIA Development Services AG); Consulting Fees (Ipsen, NOVO, Pfizer); Lecturer (AstraZeneca, EISAI); Data Monitoring Committee (CAMURUS AB).
The other authors report there are no competing interests to declare.
Author contributions
All authors contributed to the conceptualisation of this study. MLP performed the chart reviews and data extraction. RHG drafted the first manuscript version. All authors contributed to the interpretation of data. All authors critically reviewed and approved the final manuscript.
References
- [1].EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63(4):971–7. doi: 10.1016/j.jhep.2015.06.030 [DOI] [PubMed] [Google Scholar]
- [2].EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51(2):237–267. doi: 10.1016/j.jhep.2009.04.009 [DOI] [PubMed] [Google Scholar]
- [3].Karlsen TH, Folseraas T, Thorburn D, et al. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67(6):1298–1323. doi: 10.1016/j.jhep.2017.07.022 [DOI] [PubMed] [Google Scholar]
- [4].Czaja AJ. Autoimmune hepatitis in diverse ethnic populations and geographical regions. Expert Rev Gastroenterol Hepatol. 2013;7(4):365–385. doi: 10.1586/egh.13.21 [DOI] [PubMed] [Google Scholar]
- [5].Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950-1974. Acta Med Scand. 1980;208(5):401–406. doi: 10.1111/j.0954-6820.1980.tb01221.x [DOI] [PubMed] [Google Scholar]
- [6].Harvald B. Genetic epidemiology of Greenland. Clin Genet. 1989;36(5):364–367. doi: 10.1111/j.1399-0004.1989.tb03214.x [DOI] [PubMed] [Google Scholar]
- [7].Noahsen P, Rex KF, Bülow Pedersen I, et al. Thyroid autoimmunity in Greenlandic Inuit. Eur Thyroid J. 2022;11(3). doi: 10.1530/ETJ-22-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pedersen ML, Bjerregaard P, Jørgensen ME. GAD65 antibodies among Greenland Inuit and its relation to glucose intolerance. Acta Diabetol. 2014;51(4):641–646. doi: 10.1007/s00592-014-0569-z [DOI] [PubMed] [Google Scholar]
- [9].Gantzel RH, Vesterdal JD, Haase A-M, et al. The prevalence of inflammatory bowel disease in Greenland. Inflamm Bowel Dis. Published online January 2023;29(12):1879–1885. doi: 10.1093/ibd/izad002 [DOI] [PubMed] [Google Scholar]
- [10].Hurlburt KJ, McMahon BJ, Deubner H, et al. Prevalence of autoimmune liver disease in Alaska Natives. Am J Gastroenterology. 2002;97(9):2402–2407. doi: 10.1111/j.1572-0241.2002.06019.x [DOI] [PubMed] [Google Scholar]
- [11].Johnston JM, McMahon B, Townshend-Bulson L, et al. Autoimmune hepatitis and overlap syndrome among Alaska native people: prevalence, clinical characteristics, and remission. JGH Open An Open Access J Gastroenterol Hepatol. 2023;7(8):545–552. doi: 10.1002/jgh3.12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60(3):612–617. doi: 10.1016/j.jhep.2013.10.020 [DOI] [PubMed] [Google Scholar]
- [13].Boberg KM, Aadland E, Jahnsen J, et al. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33(1):99–103. doi: 10.1080/00365529850166284 [DOI] [PubMed] [Google Scholar]
- [14].Marschall H-U, Henriksson I, Lindberg S, et al. Incidence, prevalence, and outcome of primary biliary cholangitis in a nationwide Swedish population-based cohort. Sci Rep. 2019;9(1):11525. doi: 10.1038/s41598-019-47890-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pedersen ML. Microvascular complications in Nuuk, Greenland, among greenlanders and non-greenlanders diagnosed with type 2 diabetes. Diabet Res Clin Pract. 2018;136:1–6. doi: 10.1016/j.diabres.2017.11.030 [DOI] [PubMed] [Google Scholar]
- [16].Nielsen MT, Hykkelbjerg Nielsen M, Andersen S, et al. Quality of care among patients diagnosed with atrial fibrillation in Greenland. International Journal Of Circumpolar Health. 2024;83(1):2311965. doi: 10.1080/22423982.2024.2311965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Botvid SHC, Storgaard Hove L, Sauer Mikkelsen C, et al. Patterns in contacts with primary health care centres in Greenland. International Journal Of Circumpolar Health. 2023;82(1):2217007. doi: 10.1080/22423982.2023.2217007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Okkes IM, Becker HW, Bernstein RM, et al. The March 2002 update of the electronic version of ICPC-2. A step forward to the use of ICD-10 as a nomenclature and a terminology for ICPC-2. Fam Pract. 2002;19(5):543–546. doi: 10.1093/fampra/19.5.543 [DOI] [PubMed] [Google Scholar]
- [19].World Health Organisation . 2004. ICD-10: international statistical classification of diseases and related health problems: tenth revision. https://apps.who.int/iris/handle/10665/42980 [PubMed]
- [20].Kumagi T, Guindi M, Fischer SE, et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105(10):2186–2194. doi: 10.1038/ajg.2010.216 [DOI] [PubMed] [Google Scholar]
- [21].Muhammad AG, Hansen FO, Gantzel RH, et al. Non-alcoholic fatty liver disease in patients with type 2 diabetes in Greenland: a register-based cross-sectional study. Int J Of Cir Health. 2022;81(1):2065755. doi: 10.1080/22423982.2022.2065755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Greenland Statistics . [cited 2023 Nov 8]. Available from: https://bank.stat.gl/pxweb/en/Greenland/
- [23].Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci. 2001;16(2):101–133. doi: 10.1214/ss/1009213286 [DOI] [Google Scholar]
- [24].Tanaka A. Autoimmune hepatitis: 2019 update. Gut Liver. 2020;14(4):430–438. doi: 10.5009/gnl19261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nielsen KR, Midjord J, Johannesen HL, et al. A nationwide study of autoimmune liver diseases in the Faroe Islands: Incidence, prevalence, and causes of death 2004 - 2021. Int J Of Cir Health. 2023;82(1):2221368. doi: 10.1080/22423982.2023.2221368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181–1188. doi: 10.1016/j.jhep.2011.10.025 [DOI] [PubMed] [Google Scholar]
- [27].Lleo A, Jepsen P, Morenghi E, et al. Evolving trends in female to male incidence and male mortality of primary biliary cholangitis. Sci Rep. 2016;6(1):25906. doi: 10.1038/srep25906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rust C, Beuers U. Overlap syndromes among autoimmune liver diseases. World J Gastroenterol. 2008;14(21):3368–3373. doi: 10.3748/wjg.14.3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chazouillères O, Wendum D, Serfaty L, et al. Long term outcome and response to therapy of primary biliary cirrhosis-autoimmune hepatitis overlap syndrome. J Hepatol. 2006;44(2):400–406. doi: 10.1016/j.jhep.2005.10.017 [DOI] [PubMed] [Google Scholar]
- [30].Molodecky NA, Myers RP, Barkema HW, et al. Validity of administrative data for the diagnosis of primary sclerosing cholangitis: a population-based study. Liver Int Off J Int Assoc Study Liver. 2011;31(5):712–720. doi: 10.1111/j.1478-3231.2011.02484.x [DOI] [PubMed] [Google Scholar]
- [31].Rokkjaer MS, Jensen MT, Grønbaek H, et al. Discharge diagnoses of liver diseases in Nuuk Greenland compared to a Danish county hospital. Int J Of Cir Health. 2006;65(2):162–168. doi: 10.3402/ijch.v65i2.18094 [DOI] [PubMed] [Google Scholar]
- [32].Gantzel RH, Villadsen GE, Rex KF, et al. Signs of non-alcoholic fatty liver disease in indigenous Arctic populations - a systematic review. Dan Med J. 2023;70(5). [PubMed] [Google Scholar]


