Abstract
目的
长链非编码RNA(long non-coding RNA,LncRNA)是机体重要的转录及转录后调控分子,近年其与肿瘤细胞恶性表型的关系逐步被揭示,本研究旨在探究垂体特异性转录因子-3相关长链非编码RNA(pit-oct-unc class 3 homeobox 3 related long non-coding RNA,Linc-POU3F3)在食管癌中的表达特征及其与食管癌细胞放射治疗(以下简称放疗)抵抗(radiation resistance,IR)及肿瘤干细胞(cancer stem cells,CSCs)标志物表达的关系。
方法
采用生物信息学方法检索公共数据库中Linc-POU3F3在食管癌中的表达特征及潜在的相互作用分子。收集42例食管癌组织样本及相应的癌旁组织,培养人正常食管上皮细胞HEEC及人食管癌细胞ECA109、TE-1、TE-2、TE-13。采用实时荧光定量PCR(qPCR)技术检测食管癌组织及细胞中Linc-POU3F3的表达水平。以梯度递增剂量的射线诱导食管癌细胞系TE-13 IR的形成作为IR组细胞,同等条件以0 Gy剂量处理TE-13细胞系作为对照组(Control)细胞;同时采用细胞转染技术,以实验组细胞为模型构建随机干扰序列组(siControl)细胞及靶向Linc-POU3F3的干扰组(siLinc-POU3F3)细胞,在ECA109细胞中转染空白及过表达Linc-POU3F3载体作为空白组(Vector)及过表达组(oeLinc-POU3F3)。采用qPCR技术及蛋白质印迹法分别检测CSCs标志物CD44、CD133及CD90 mRNA及蛋白质的表达水平;四唑化合物MTS[3-(4,5-dimenthylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,inner salt]检测不同放射剂量下细胞活力;以克隆形成实验验证IR细胞的抵抗能力。
结果
生物信息分析结果显示Linc-POU3F3的表达与食管癌患者肿瘤进展及预后不良相关;Linc-POU3F3 mRNA在食管癌组织及细胞系中的表达均显著高于正常癌旁组织及正常细胞(均P<0.01),IR细胞中Linc-POU3F3、CD44、CD133及CD90 mRNA及蛋白质表达均显著高于Control细胞(均P<0.01);siLinc-POU3F3细胞Linc-POU3F3表达显著低于siControl细胞(P<0.01),抑制率为87.21%,siLinc-POU3F3细胞CD44、CD133及CD90 mRNA及蛋白质表达均显著低于siControl细胞(均P<0.01);oeLinc-POU3F3细胞中Linc-POU3F3、CD44、CD133及CD90 mRNA及蛋白质表达均显著高于Vector细胞;IR细胞相对活力及克隆形成能力在2、4及8 Gy剂量下显著高于Control细胞(均P<0.01);siLinc-POU3F3细胞相对活力在4及8 Gy剂量下显著低于siControl细胞(均P<0.01);oeLinc-POU3F3细胞相对活力在4及8 Gy剂量下显著高于Vector细胞(均P<0.01)。
结论
Linc-POU3F3在食管癌组织中表达上调,并可促进食管癌细胞IR及CSCs标志物的表达。
Keywords: 食管癌, 放射治疗抵抗, 肿瘤干细胞, 长链非编码RNA
Abstract
Objective
Long non-coding RNA (LncRNA) is an important transcriptional and post-transcriptional regulatory molecule in the body. In recent years, relationship between LncRNA and malignant phenotype of tumor cells has been revealed gradually. This study aims to investigate the expression characteristics of pit-oct-unc class 3 homeobox 3 related long non-coding RNA (Linc-POU3F3) in esophageal cancer and its relationship with radiation resistance (IR) as well as the expressions of cancer stem cell (CSC) markers in esophageal cancer cells.
Methods
The expression characteristics and potential interaction molecules of Linc-POU3F3 in esophageal cancer were collected from the public database via bioinformatics retrieval. Forty-two pair samples of esophageal cancer tissues and corresponding adjacent tissues were collected. Human normal esophageal epithelial cells (HEEC) and human esophageal cancer cell lines (ECA109, TE-1, TE-2, TE-13) were cultured. Real-time quantitative PCR (qPCR) was used to detect the expression level of Linc-POU3F3 in clinical tissues and cells. The formation of TE-13 IR cell line induced by different doses of radiation served as IR group cells, and the same condition treated with 0 Gy dose was set as control group (control) cells. Meanwhile, we used cell transfection technology to construct random interference sequence (siControl) cells and interference (siLinc-POU3F3) cells. In ECA109 cells, we transfected blank and over expressed Linc-POU3F3 plasmids as vector and over-expressed group (oeLinc-POU3F3). The mRNA and protein expressions of CD44, CD133 and CD90 were detected by qPCR and Western blotting, respectively. MTS [3-(4,5-dimenthylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] was used to detect the cell viability under different radiation doses, and the resistance of IR cells was verified by clone formation experiment.
Results
The expression of Linc-POU3F3 was correlated with the tumor progression and poor prognosis of esophageal cancer. The level of Linc-POU3F3 mRNA expression was significantly higher in esophageal cancer tissues and cell lines than that in normal adjacent tissues and cell lines (all P<0.01). The expressions of Linc-POU3F3 mRNA and protein expressions of CD44, CD133, and CD90 in IR cells were significantly higher than those in control cells (all P<0.01). The expression of Linc-POU3F3 in siLinc-POU3F3 cell was significantly lower than that in the siControl cells (P<0.01), and the inhibition rate was 87.21%. The mRNA and protein expressions of CD44, CD133, and CD90 in the siLinc-POU3F3 cells were significantly lower than those in the siControl cells (all P<0.05). The expressions of linc-POU3F3, CD44, CD133, and CD90 mRNA and protein in the oeLinc-POU3F3 cells were significantly higher than those in the vector cells. The relative activity and clone formation ability in the IR cells were significantly higher than those in the control cells at 2, 4, and 8 Gy doses (all P<0.01). The relative activity in the siLinc-POU3F3 cells was significantly lower than that in the siControl cells at 4 and 8 Gy doses (P<0.01). The relative activity in the oeLinc-POU3F3 cells was significantly higher than that in the vector cells at 4 and 8 Gy doses (P<0.01).
Conclusion
Linc-POU3F3 is up-regulated in esophageal cancer and can promote IR and the expression of CSC markers in esophageal cancer cells.
Keywords: esophageal cancer, radiotherapy resistance, cancer stem cells, long non-coding RNA
放射治疗(以下简称放疗)是食管癌的主要治疗手段之一,对于手术难度较大的颈段及上胸段的食管癌尤为重要,但治疗过程中食管癌细胞逐渐累积形成的放疗抵抗(radiation resistance,IR)极大地影响了放疗疗效。IR的形成涉及复杂的调控机制,包括细胞周期阻滞、DNA损伤修复、肿瘤微环境及干细胞特性等多种机制[1]。肿瘤干细胞(cancer stem cells,CSCs)是导致食管癌细胞IR形成的关键因素之一[2],但其确切机制仍未完全阐明。长链非编码RNA(long non-coding RNA,LncRNA)在机体内广泛表达,并作为表观调控因子参与各类生物学过程[3-4],LncRNA Linc-POU3F3基因是定位于垂体特异性转录因子-3(pit-oct-unc class 3 homeobox 3,POU3F3)基因附近的反向LncRNA,参与多种恶性肿瘤的发生和发展[4-5]。本研究通过生物信息学方法及分子生物技术探究LncRNA Linc-POU3F3在食管癌中的表达特征及其对食管癌细胞IR及CSCs标志物的影响。
1. 材料与方法
1.1. 临床样本来源
收集2018年5月至2019年1月中南大学湘雅二医院胸外科手术切除后,经病理诊断为食管癌的40例标本及相应的40例癌旁组织。患者男性29例,女性11例;年龄42~71(51.8±9.2)岁;鳞癌31例,腺鳞癌4例,腺癌5例;高分化11例,中分化17例,低分化12例;按第七版国际食管癌TNM分期标准,I期2例,IIa期13例,IIb期15例,III期10例。手术后将部分组织用于病理诊断,另保存于液氮中,用于Linc-POU3F3的检测。本研究获得中南大学湘雅二医院伦理委员会的批准(S385)。
1.2. 方法
1.2.1. 细胞培养及细胞系的构建
用含10%胎牛血清(fetal bovine serum,FBS,美国Gibco公司)的RPMI 1640培养基(美国Hyclone公司)培养人正常食管上皮细胞HEEC及人食管癌细胞ECA109、TE-1、TE-2、TE-13。培养条件:37 ℃,5% CO2。对照(Control)组及抵抗(IR)组细胞的诱导:用1、2、4、8 Gy梯度剂量的射线处理TE-13,每2 d进行1次照射,每次照射完成后更换新鲜培养基,待TE-13细胞在当前剂量中可维持生长,采用下一个剂量进行处理,直到细胞在8 Gy剂量下维持生长,作为本研究中的IR细胞。同时采用0 Gy进行空白处理TE-13,作为本研究中的Control细胞。随机干扰序列组(siControl)及靶向Linc-POU3F3的干扰组(siLinc-POU3F3)细胞的构建:采用Lipofectamine 2000TM(美国Invitrogen公司)介导随机干扰序列及靶向Linc-POU3F3载体转染进入IR细胞中,转染24 h后收集细胞,作为本研究中的随机干扰序列组(siControl)及靶向Linc-POU3F3的干扰组(siLinc-POU3F3)细胞。空白组(Vector)及过表达组(oeLinc-POU3F3)细胞的构建:与siControl及siLinc-POU3F3细胞构建方法相同,在2组ECA109细胞中分别转染空白对照载体(Vector)及携带Linc-POU3F3的全长载体,作为本研究中的Vector及oeLincPOU3F3组细胞。
1.2.2. 实时荧光定量PCR
采用TRIzol(美国Invitrogen公司)试剂提取组织及细胞总RNA,保证组织重量>1 mg,细胞数量>106。用Nanodrop2000超微量紫外分光光度计(美国Thermo Fisher Scientific公司)检测总RNA浓度,取 1 μg RNA采用反转录试剂盒(美国Fermentas公司)进行反转录,稀释反转录产物至5 μmol/L,稀释引物(上海生工生物工程技术公司合成)至0.1 μmol/L工作浓度,Linc-POU3F3、CD44、CD133、CD90及内参基因GAPDH引物序列见表1。10 μL SYBR GreenI核酸荧光染料(日本Takara公司)体系进行实时荧光定量PCR(Real-time fluorescence quantitative PCR,qPCR)反应(美国ABI 7500型PCR系统),反应采用三步法:预变性,95 ℃,3 min;扩增,95 ℃,10 s,60 ℃,30 s,72 ℃,30 s,40次循环;熔解,55 ℃,30 s。以GAPDH mRNA表达作为参照,计算Linc-POU3F3、CD44、CD133及CD90 mRNA的相对表达水平。
表1.
引物序列
Table 1 Sequence of primers
| Gene | Forward (5'→3') | Reverse (5'→3') |
|---|---|---|
| Linc-POU3F3 | AATCACTGCAATTGAAGGAAA | CCTTGTTTTCCAACCCTTAGA |
| CD44 | ATGGACAAGTTTTGGTGGCACGC | AAGATGTAACCTCCTGAAGTGCTG |
| CD133 | TGGATGCAGAACTTGACAACGT | ATACCTGCTACGACAGTCGTGGT |
| CD90 | GACCCGTGAGACAAAGAAGC | GCCCTCACACTTGACCAGTT |
| GAPDH | AATGAAGGGGTCATTGATGG | AAGGTGAAGGTCGGAGTCAA |
1.2.3. 蛋白质印迹法
采用RIPA裂解液(上海碧云天生物技术有限公司)抽提细胞总蛋白质,保证细胞数量>106。以BCA蛋白定量试剂盒检测蛋白质浓度,取50 μg与SDS上样缓冲液(上海碧云天生物技术有限公司)配制蛋白质体系,进行10%聚丙烯酰氨凝胶电泳,转PVDF膜,以丽春红染色液显示蛋白质条带并剪取CD44、CD133、CD90及GAPDH条带,用10%脱脂牛奶封闭2 h,加入1꞉1 000一抗[抗CD44、抗CD133及抗CD90抗体(美国Genetex公司)]于4 ℃下孵育过夜,洗膜后加入1꞉2 000二抗[羊抗兔IgG(美国proteintech公司)]于室温下孵育1 h。采用ECL化学发光试剂盒孵育条带,ChemiDoc XRS+化学发光成像分析仪(美国Bio-Rad公司)对条带进行曝光。
1.2.4. 克隆形成实验
接种Control及IR细胞于6孔板,每孔1 000个细胞,每组设置3个复孔,接种后24 h进行4 Gy剂量照射,更换培养基后继续培养12 d,每3 d更换培养基,以无水乙醇固定10 min。用0.1%结晶紫染色 15 min,扫描6孔板,用Image J软件进行计数。
1.2.5. MTS检测细胞活力
接种各组细胞于96孔板,每孔2×104个细胞,每组设置重复孔5个,并重复接种5块96孔板,培养过夜后,分别以0、1、2、4及8 Gy剂量处理5块96孔板,继续培养24 h。用1꞉5四唑化合物MTS[3-(4,5-dimenthylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,inner salt]试剂(美国Promega公司)与含10%胎牛血清的RPMI 1640培养基混匀后更换96孔板中的培养基,培养2 h。用酶标仪检测490 nm处的吸光度值,并计算各组相对吸光度值以表示细胞活力。
1.2.6. 生物信息学预测
基于生物信息Annolnc数据库(annolnc.cbi.pku.edu.cn/)对Linc-POU3F3可能的相互作用分子进行预测,筛选出具有统计学意义的分子。通过TCGA公共数据库(http://gepia.cancer-pku.cn/)分析Linc-POU3F3的表达与食管癌患者肿瘤进展及预后的关系。
1.3. 统计学处理
采用SPSS 18.0统计软件进行数据分析,计量资料以均数±标准差( ±s)表示。用Shapiro-Wilk检验衡量计量资料的正态性,配对资料t检验比较食管癌与癌旁组织中Linc-POU3F3的表达差异。服从正态分布的计量资料采用单因素方差检验分析差异,非正态分布的计量资料采用Mann-Whitney秩和检验。以P<0.05为差异有统计学意义。
2. 结 果
2.1. Linc-POU3F3在食管癌中的表达特征及其潜在的相互作用分子
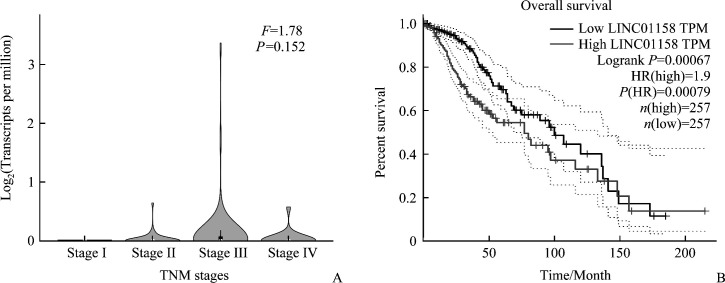
生物信息学分析结果显示:Linc-POU3F3在III~IV期食管癌患者肿瘤组织中的表达水平显著高于I~II期食管癌患者,差异具有统计学意义(P<0.01,图1A),同时Linc-POU3F3高表达患者总生存时间明显缩短(P<0.001,图1B)。通过Annolnc数据库分析发现Linc-POU3F3与CD44及CD133(PROM1)存在潜在的相互作用(均P<0.001)。
图1.
Linc-POU3F3与食管癌患者肿瘤进展及预后的关系
Figure 1 Relationship between Linc-POU3F3 and tumor progression or prognosis of esophageal cancer patients
A: Expression of Linc-POU3F3 in esophageal cancer tissues with different TNM stages was analyzed by bioinformatics database. B: Relationship between Linc-POU3F3 expression and prognosis of esophageal cancer patients was analyzed.
2.2. Linc-POU3F3在食管癌、正常组织及细胞系中的表达
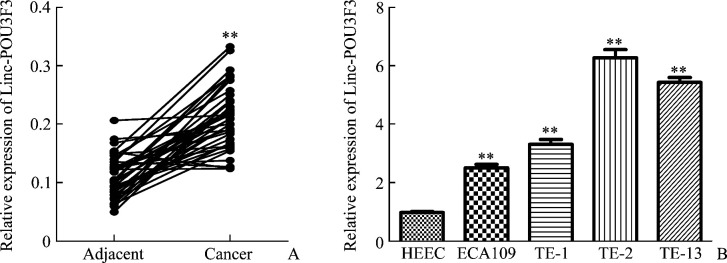
癌旁组织及食管癌组织中Linc-POU3F3表达水平分别为0.110±0.006及0.214±0.009,食管癌组织Linc-POU3F3表达显著高于癌旁组织(t=9.908,P<0.01;图2A)。HEEC、ECA109、TE-1、TE-2及TE-13细胞中Linc-POU3F3表达水平分别为1.000±0.070、2.533±0.201、3.349±0.281、6.296±0.484及5.457±0.287,各食管癌细胞系均高于HEEC细胞(均P<0.01,图2B)。
图2.
Linc-POU3F3在食管癌、正常组织及细胞系中的表达
Figure 2 Expression of Linc-POU3F3 in esophageal carcinoma, normal tissues, and cell lines
A: Expression of Linc-POU3F3 in esophageal carcinoma and adjacent normal tissues (**P<0.01 vs adjacent normal tissues); B: Expression of Linc-POU3F3 in esophageal carcinoma and normal esophageal epithelial cell lines (**P<0.01 vs HEEC).
2.3. 各组细胞中Linc-POU3F3、CD44、CD133及CD90 mRNA表达差异
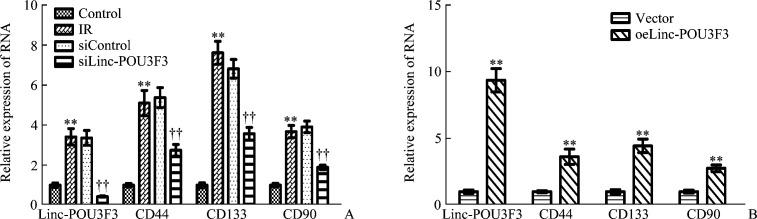
Control及IR细胞Linc-POU3F3表达分别为1.000±0.064及3.416±0.412,CD44表达分别为1.000±0.050及5.108±0.633,CD133表达分别为1.000±0.071及7.620±0.572,CD90表达分别为1.000±0.053及3.680±0.307,IR细胞Linc-POU3F3、CD44、CD133及CD90 mRNA表达均显著高于Control细胞;siControl及siLinc-POU3F3细胞Linc-POU3F3表达分别为3.364±0.372及0.430±0.030,抑制率为87.21%,CD44表达分别为5.379±0.501及2.748±0.290,CD133表达分别为6.818±0.467及3.572±0.319,CD90表达分别为3.915±0.286及1.892±0.116,siLinc-POU3F3细胞Linc-POU3F3、CD44、CD133及CD90 mRNA表达均显著低于siControl细胞(均P<0.01,图3A);Vector及oeLinc-POU3F3细胞Linc-POU3F3表达分别为1.000±0.065及9.357±0.871,CD44表达分别为1.000±0.048及3.620±0.572,CD133表达分别为1.000±0.092及4.436±0.508,CD90表达分别为1.000±0.066及2.750±0.254。oeLinc-POU3F3细胞Linc-POU3F3、CD44、CD133及CD90 mRNA表达均显著高于Vector细胞(均P<0.01,图3B)。
图3.
各组细胞Linc-POU3F3、CD44、CD133及CD90 mRNA表达及比较
Figure 3 mRNA expression and comparison of Linc-POU3F3, CD44, CD133 and CD90 in each group cells
A: mRNA expression of Linc-POU3F3, CD44, CD133, and CD90 in TE-13 control, IR, siControl, and siLinc-POU3F3 cell lines, **P<0.01 vs control; ††P<0.01 vs siControl. B: mRNA expression of Linc-POU3F3, CD44, CD133, and CD90 in ECA109 vector and oeLinc-POU3F3 cell lines. **P<0.01 vs Vector.
2.4. 各组细胞中CD44、CD133及CD90蛋白质表达差异
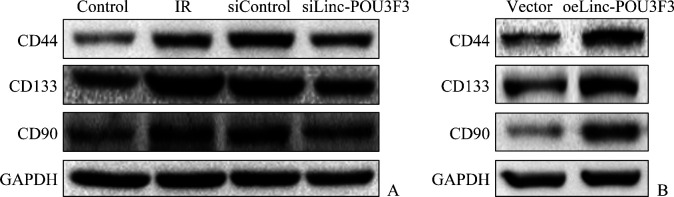
IR细胞CD44、CD133及CD90蛋白质表达均高于Control细胞,siLinc-POU3F3细胞CD44、CD133及CD90蛋白质表达均低于siControl细胞(图4A);oeLinc-POU3F3细胞CD44、CD133及CD90蛋白质表达均高于Vector细胞(图4B)。
图4.
蛋白质印迹法检测各组细胞CD44、CD133及CD90
Figure 4 Levels of CD44, CD133, and CD90 in each group detected by Western blotting
A: CD44, CD133, and CD90 in TE-13 control, IR, siControl, and siLinc-POU3F3 cells; B: CD44, CD133, and CD90 in ECA109 vector and oeLinc-POU3F3 cells.
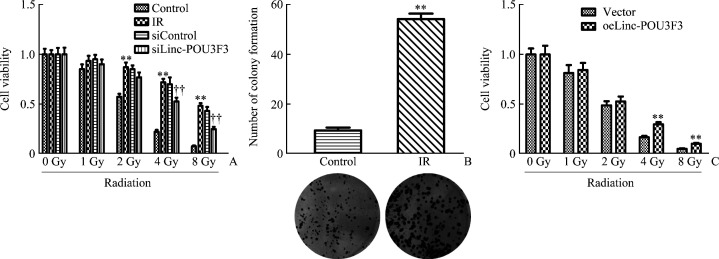
2.5. 不同放射剂量下各组细胞活力差异
IR细胞相对活力在2、4及8 Gy剂量下显著高于Control细胞(均P<0.01,图5A);4 Gy照射后,IR细胞克隆形成能力显著强于Control细胞(P<0.01,图5B);siLinc-POU3F3细胞相对活力在4及8 Gy剂量下显著低于siControl细胞(均P<0.01,图5A);oeLinc-POU3F3细胞相对活力在4及8 Gy剂量下显著高于Vector细胞(均P<0.01,图5C)。
图5.
不同放射剂量下各组细胞活力比较及放疗抵抗细胞系的验证
Figure 5 Comparison of cell viability and validation of radiation resistant cell lines under different radiation doses
A: Cell viability and comparison of TE-13 control and IR, siControl and siLinc-POU3F3 cell lines under different radiation doses. **P<0.01 vs Control, ††P<0.01 vs siControl; B: Comparison of clones forming cells between Control and IR cells treated with 4 Gy radiation. **P<0.01 vs Control; C: Cell viability and comparison of ECA109 vector and oeLinc-POU3F3 cells at different radiation doses. **P<0.01 vs Vector.
3. 讨 论
CSCs特性与肿瘤IR的形成密切相关:Zhao等[9]分离结直肠癌、肝癌、乳腺癌及前列腺癌细胞群中相应的CSCs,发现存在胰岛素样生长因子II(insulin-like growth factor II,IGF2)表达增高,同时具有较高的IR能力,表明CSCs存在IR特性。Kurth等[10]研究表明头颈鳞状细胞癌乙醛脱氢酶(acetaldehyde dehydrogenase,ALDH)阳性细胞具有CSCs特性,且有较强的IR能力;CD44阳性的鼻咽癌细胞同样具有CSCs特性,且其表达与Bim-1密切相关,敲除Bmi-1基因可延长细胞G1期,促进细胞G2/M阻滞,抑制DNA损伤修复,提高P16、P14和P53蛋白表达水平,导致CD44阳性细胞凋亡增加,进而增强细胞放疗敏感性[11]。研究[12-13]发现诱导食管癌细胞Eca109 IR形成后,细胞增殖、转移能力及CSCs特性显著增强。CD44、CD71、CD90、CD133、ATP结合盒家族G蛋白亚家族2(ATP binding cassette subfamily G member 2,ABCG2)及ALDH可作为食管癌CSCs的标志物;CSCs通过修复DNA损伤、影响细胞周期分布、抗氧化作用及调控肿瘤微环境促进食管癌细胞IR的形成[1, 14-16]。上述研究表明CSCs特征受到多种因子联合调控,且参与肿瘤细胞的IR。Linc-POU3F3作为LncRNA的成员之一,与多种恶性肿瘤的进展有关,其在结直肠癌[4]、食管癌[5]、胃癌[17]及脑胶质瘤[18-19]中表达显著增高,并在其中发挥重要的生物学作用:Linc-POU3F3可促进结直肠癌细胞中骨形成蛋白(bone morphogenetic protein,BMP)的表达,介导细胞增殖及转移能力增强,并抑制细胞凋亡[4];同时Linc-POU3F3抑制食管癌及肝细胞癌中抑癌基因POU3F3的表达,进而促进细胞增殖及转移等恶性行为[5, 20];Linc-POU3F3还可促进胃癌细胞转化生长因子-β的募集,介导细胞增殖能力增强[17];胶质瘤细胞中Linc-POU3F3过表达使细胞增殖能力及血管形成能力增强[18-19]。基于Linc-POU3F3在恶性肿瘤进展中的重要作用,笔者对Linc-POU3F3与食管癌干细胞特性及IR的关系进行了探究。
笔者在公共数据库中发现Linc-POU3F3的表达与食管癌患者高TNM分期及预后不良呈正相关,提示Linc-POU3F3可能在食管癌进展中发挥重要作用。因此,笔者进一步在食管癌及正常临床组织和细胞中检测Linc-POU3F3的表达水平,发现其高表达于食管癌组织及细胞,且在低分化的食管癌细胞中表达上调更显著,表明Linc-POU3F3的表达异常可能与食管癌的发生发展及细胞分化程度有关。为了明确Linc-POU3F3在食管癌中扮演着何种角色,我们通过生物信息学方法分析了Linc-POU3F3的潜在相互作用分子,发现Linc-POU3F3对CSCs标志物CD44及CD133可能存在调控作用。基于CSCs对IR形成的重要地位,Linc-POU3F3可能作为干性调节因子参与食管癌细胞IR的形成。因此,我们通过诱导低分化食管癌细胞TE-13放疗抵抗能力后发现,细胞Linc-POU3F3及食管癌干细胞标志物CD44、CD133及CD90表达显著上调,表明Linc-POU3F3的高表达与食管癌细胞IR的形成存在潜在联系,同时证明了IR的食管癌细胞株中存在CSCs特性,符合Da等[12]及Che等[13]的研究结果。为了进一步明确Linc-POU3F3与食管癌干细胞特性及其IR的关系,我们敲低了IR的食管癌细胞株中Linc-POU3F3的表达,发现随着Linc-POU3F3的下调,细胞对放疗的敏感性显著增强,且干细胞标志物CD44、CD133及CD90表达下调,而上调ECA109细胞Linc-POU3F3可促进CD44、CD133及CD90的表达,并使ECA109 IR能力增强。
综上,本研究结果表明:Linc-POU3F3在食管癌组织中高表达,可能与患者肿瘤的进展及预后相关,其在体外可促进食管癌CSCs标志物的表达及细胞IR。笔者将进一步通过RNA pull down及蛋白质组学分析手段探究Linc-POU3F3的相互作用分子,明确Linc-POU3F3在食管癌中的确切机制,以期开发新的食管癌标志物及相应的增敏药物。
利益冲突声明
作者声称无任何利益冲突。
原文网址
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/202106583.pdf
参考文献
- 1. McLaughlin M, Patin EC, Pedersen M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy[J]. Nat Rev Cancer, 2020, 20(4): 203-217. [DOI] [PubMed] [Google Scholar]
- 2. Chen GZ, Zhu HC, Dai WS, et al. The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity[J]. J Thorac Dis, 2017, 9(3): 849-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ali T, Grote P. Beyond the RNA-dependent function of LncRNA genes[J]. Elife, 2020, 23(9): e60583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chi HC, Tsai CY, Tsai MM, et al. Roles of long noncoding RNAs in recurrence and metastasis of radiotherapy-resistant cancer stem cells[J]. Int J Mol Sci, 2017, 18(9): E1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shan TD, Xu JH, Yu T, et al. Knockdown of linc-POU3F3 suppresses the proliferation, apoptosis, and migration resistance of colorectal cancer[J]. Oncotarget, 2016, 7(1): 961-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li W, Zheng J, Deng JQ, et al. Increased levels of the long intergenic non-protein coding RNA POU3F3 promote DNA methylation in esophageal squamous cell carcinoma cells[J]. Gastroenterology, 2014, 146(7): 1714-1726.e5. [DOI] [PubMed] [Google Scholar]
- 7. 孙伟杰,李建成,姚奇伟,等. CD44+/CD44-食管鳞癌细胞LncRNA表达谱差异分析[J] . 中华肿瘤防治杂志, 2019, 26(16): 1163-1168. [Google Scholar]; SUN Jiewei, LI Jiancheng, YAO Qiwei, et al. Screening and analysis of LncRNA on CD44+/CD44- esophageal squamous cancer cell[J]. Chinese Journal of Cancer Prevention and Treatment, 2019. ,26(16): 1163-1168. [Google Scholar]
- 8. Wang D, Plukker JTM, Coppes RP. Cancer stem cells with increased metastatic potential as a therapeutic target for esophageal cancer[J]. Semin Cancer Biol, 2017, 44: 60-66. [DOI] [PubMed] [Google Scholar]
- 9. Zhao X, Liu XL, Wang GJ, et al. Loss of insulin-like growth factor II imprinting is a hallmark associated with enhanced chemo/radiotherapy resistance in cancer stem cells[J]. Oncotarget, 2016, 7(32): 51349-51364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurth I, Hein L, Mäbert K, et al. Cancer stem cell related markers of radioresistance in head and neck squamous cell carcinoma[J]. Oncotarget, 2015, 6(33): 34494-34509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu XH, Liu XY, Su J, et al. ShRNA targeting Bmi-1 sensitizes CD44⁺ nasopharyngeal cancer stem-like cells to radiotherapy[J]. Oncol Rep, 2014, 32(2): 764-770. [DOI] [PubMed] [Google Scholar]
- 12. Da CL, Wu L, Liu YT, et al. Effects of irradiation on radioresistance, HOTAIR and epithelial-mesenchymal transition/cancer stem cell marker expression in esophageal squamous cell carcinoma[J]. Oncol Lett, 2017, 13(4): 2751-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Che SM, Zhang XZ, Liu XL, et al. The radiosensitization effect of NS398 on esophageal cancer stem cell-like radioresistant cells[J]. Dis Esophagus, 2011, 24(4): 265-273. [DOI] [PubMed] [Google Scholar]
- 14. Schizas D, Moris D, Kanavidis P, et al. The prognostic value of CD44 expression in epithelial-mesenchymal transition: preliminary data from patients with gastric and esophageal cancer[J]. In Vivo, 2016, 30(6): 939-944. [DOI] [PubMed] [Google Scholar]
- 15. Okamoto H, Fujishima F, Nakamura Y, et al. Significance of CD133 expression in esophageal squamous cell carcinoma[J]. World J Surg Oncol, 2013, 11: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang KH, Dai YD, Tong M, et al. A CD90(+) tumor-initiating cell population with an aggressive signature and metastatic capacity in esophageal cancer[J]. Cancer Res, 2013, 73(7): 2322-2332. [DOI] [PubMed] [Google Scholar]
- 17. Xiong GY, Yang LH, Chen Y, et al. Linc-POU3F3 promotes cell proliferation in gastric cancer via increasing T-reg distribution[J]. Am J Transl Res, 2015, 7(11): 2262-2269. [PMC free article] [PubMed] [Google Scholar]
- 18. Guo H, Wu L, Yang Q, et al. Functional linc-POU3F3 is overexpressed and contributes to tumorigenesis in glioma[J]. Gene, 2015, 554(1): 114-119. [DOI] [PubMed] [Google Scholar]
- 19. Lang HL, Hu GW, Chen Y, et al. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3[J]. Eur Rev Med Pharmacol Sci, 2017, 21(5): 959-972. [PubMed] [Google Scholar]
- 20. Li YC, Li YN, Wang D, et al. Linc-POU3F3 is overexpressed in hepatocellular carcinoma and regulates cell proliferation, migration and invasion[J]. Biomedecine Pharmacother, 2018, 105: 683-689. [DOI] [PubMed] [Google Scholar]