Abstract
目的
脑白质高信号(white matter hyperintensity,WMH)是导致认知功能障碍的重要因素,机制目前仍未阐明。近年来研究发现环状RNA(circular RNA,circRNA)在脑血管疾病中存在差异性表达。本研究旨在分析circRNA在WMH伴认知功能障碍患者外周血单个核细胞中的表达谱,筛选其中差异性表达的circRNA,探讨circRNA在WMH伴认知功能障碍中的作用。
方法
利用circRNA基因芯片检测WMH伴认知障碍者、WMH不伴认知障碍者及正常对照者(各纳入3例,男女比为2꞉1)外周血单个核细胞circRNA表达谱。筛选WMH伴认知障碍者的差异性表达circRNA,差异性表达circRNA的筛选标准为表达差异倍数变化(fold change,FC)≥2.0(|log2FC≥1)且P<0.05。采用TargetScan和miRanda靶基因分析软件预测相关靶miRNA,Genespring软件预测靶基因。
结果
与对照组比较,WMH伴认知障碍组显著上调的circRNA有5条,下调的有3条;WMH不伴认知障碍组显著上调的circRNA有8条,下调的有2条。WMH伴认知障碍组的circRNA表达谱分别与WMH不伴认知障碍组、对照组比较,未发现共同差异表达的circRNA;与对照组比较,WMH伴认知障碍组、WMH不伴认知障碍组的circRNA表达谱中hsa_circ_0092222均表达上调,hsa_circ_0000662和hsa_circ_0083773均表达下调,且它们在2组间的表达差异无统计学意义(均P>0.05)。预测到hsa_circ_0092222有2个靶miRNA(hsa-miR-19a-3p、hsa-miR-19b-3p),靶基因为核糖体蛋白S4,Y连锁1(ribosomal protein S4, Y-linked 1,RPS4Y1);hsa_circ_0000662有1个靶miRNA(hsa-miR-194),靶基因为轴抑制因子1(axis inhibitor 1,AXIN1);hsa_circ_0083773有7个靶miRNA,靶基因为重组清道夫受体A类成员3(recombinant scavenger receptor class A member 3,SCARA3)。
结论
WMH患者的circRNA表达谱发生了显著变化;差异性表达的circRNA可能是导致脑白质出现高信号的原因;hsa_circ_0092222、hsa_circ_0000662、hsa_circ_0083773可能靶向吸附靶miRNA,调节靶基因的表达,通过Janus激酶2(Janus kinase 2,JAK2)/信号转导与转录活化因子3(signal transducers and activators of transcription,STAT3)信号通路、Wnt信号通路导致脑白质的损伤。未发现circRNA表达谱在WMH伴与不伴认知障碍患者中的显著差异,WMH患者出现认知障碍可能有其他发病机制。
Keywords: 脑白质高信号, 认知功能障碍, 环状RNA, 差异性表达, 靶基因预测
Abstract
Objective
White matter hyperintensity (WMH) is an important factor leading to cognitive impairment, and the mechanism has not been clarified. In recent years, studies have found that circular RNA (circRNA) has differential expression in cerebrovascular diseases. This study aims to analyze the expression profile of circRNA in peripheral blood mononuclear cell (PBMC) of patients with WMH with cognitive impairment, to screen the differentially expressed circRNA, and to explore the possible role of circRNA in WMH with cognitive impairment.
Methods
CircRNA microarray was used to detect the circRNA expression profile of PBMC in patients with WMH with cognitive impairment, and in patients with WMH without cognitive impairment as well as in normal controls (3 cases each, male to female ratio of 2꞉1). The differentially expressed circRNA in patients with WMH with cognitive impairment was screened. The screening criteria for differentially expressed circRNA was fold change (FC) ≥2.0 (|log2FC ≥1) and P<0.05. TargetScan and miRanda target gene analysis software were used to predict the relevant target miRNA, and Genespring software was used to predict the target genes.
Results
Compared with the control group, there were 5 significantly up-regulated circRNA and 3 down-regulated circRNA in the WMH with cognitive impairment group; 8 circRNA were significantly up-regulated and 2 were down-regulated in the WMH without cognitive impairment group. When compared with the WMH with cognitive impairment group, no co-differentially expressed circRNA was found in WMH without cognitive impairment group and control group. Compared with the control group, the expression of hsa_circ_0092222 was up-regulated and the expressions of hsa_circ_0000662 and hsa_circ_0083773 were down-regulated in the WMH with cognitive impairment group and the WMH without cognitive impairment group, and there was no significant difference between the 2 groups (all P>0.05). Two target miRNA (hsa-miR-19a-3p and hsa-miR-19b-3p) of hsa_circ_0092222 were predicted, and the target gene was ribosomal protein S4, Y-linked 1 (RPS4Y1). Hsa_circ_0000662 predicted a target miRNA (hsa-miR-194) with axis inhibitor 1 (AXIN1) as the target gene. Hsa_circ_0083773 predicted 7 target miRNA, and the target gene was recombinant scavenger receptor class A member 3 (SCARA3).
Conclusion
The circRNA expression profile of patients with WMH is changed significantly. The differentially expressed circRNA may be the cause of WMH; Hsa_circ_0092222, hsa_circ_0000662, and hsa_circ_0083773 may regulate the expression of target genes by targeting adsorption of the target miRNA, leading to brain white matter damage through Janus kinase 2 (JAK2)/signal transducers and activators of transcription (STAT3) signal pathway and Wnt signal pathway.There is no significant difference in circRNA expression profile between WMH with or without cognitive impairment. Cognitive impairment in patients with WMH may have other reasons.
Keywords: white matter hyperintensity, cognitive impairment, circular RNA, differential expression, target gene prediction
脑白质高信号(white matter hyperintensity,WMH)是弥漫性脑小血管病和脑萎缩的MRI影像学特征。WMH在颅脑MRI T2加权像(T2 weighted image,T2WI)及磁共振成像液体衰减反转恢复序列(fluid attenuated inversion recovery,FLAIR)上表现为深部白质及脑室旁点状、斑片状或融合性高信号,而在T1WI上呈等信号或稍低信号的表现[1]。WMH在MRI中非常常见,在健康人和痴呆患者中都能观察到WMH[2]。WMH与年龄的增长有关,在60~90岁的普通人群中WMH的患病率高达70%[3]。WMH的确切形成机制尚不清楚,目前普遍认为WMH对认知功能有不利影响,且临床上WMH与认知障碍的相关性存在个体差异[4]。
环状RNA(circular RNA,circRNA)是最近发现的一类新型非编码RNA分子,具有微RNA(microRNA,miRNA)的分子海绵功能,参与调控基因的转录与表达,细胞间信号通路的活性,影响蛋白质的翻译和功能[5]。此外,circRNA的表达主要集中在哺乳动物脑内,与其他组织相比,表达量的差异显著,提示circRNA在中枢神经系统中可能发挥重要的作用[6]。目前国内外有关circRNA的研究较多,研究[7]结果亦表明circRNA在脑血管病、帕金森病、癫痫、阿尔茨海默病等中枢神经系统疾病中存在差异性表达,circRNA有望成为相关疾病的生物学标志物,用于诊断及判断预后。研究[8-9]表明circRNA与阿尔茨海默病的发病相关,而阿尔茨海默病患者存在WMH,因此我们推测在WMH伴认知障碍患者中circRNA亦可能存在差异性表达。本研究探讨circRNA在WMH伴认知功能障碍中的差异性表达,旨在为circRNA成为WMH伴认知功能障碍早期干预的靶点提供研究基础。
1. 对象与方法
1.1. 对象
收集2018年12月至2019年6月就诊于内蒙古科技大学包头医学院第一附属医院(以下简称我院)神经内科的WMH患者,WMH伴认知功能障碍的诊断标准参照脑小血管病相关认知功能障碍中国诊疗指南(2019)[10]。WMH伴认知障碍组(A组)、WMH不伴认知障碍组(B组)、正常对照组(C组)。每组纳入3例,3组男女比均为2꞉1。C组来源于我院体检健康人群,年龄、性别、受教育程度等与其他2组相匹配,无认知下降的主诉且神经心理量表评分正常。受试者排除标准:1)存在头颅MRI禁忌证者;2)因伴有意识障碍、失语、耳聋等疾病,不能完善神经心理量表评估者;3)存在中枢神经系统感染性疾病、脑外伤、脑肿瘤、正常颅压脑积水、癫痫、帕金森病等神经系统疾病同时伴认知功能障碍者;4)颅内脱髓鞘病变是由于免疫性、遗传性、缺血缺氧性脑病、放射性等引起者;5)颅内大血管狭窄、有脑卒中病史者;6)可能由于重度贫血、严重甲状腺功能低下、重度感染、严重心肝肾功能不全等内科系统疾病导致认知功能障碍者。本研究遵循自愿参加并签署书面知情同意书,符合人体实验伦理学标准,并经过我院伦理委员会的批准。
1.2. 方法
1.2.1. 实验试剂及仪器
Ficoll淋巴细胞分离液购于天津市血研协康科技有限公司;TRIzol试剂盒和NanoDrop ND-2000型分光光度计购于美国Thermo公司;AffinityScriptRT试剂盒购于美国Stratagene公司;RNeasy试剂盒购于德国Qiagen公司;人竞争性内源RNA(competing endogenous RNA,ceRNA)芯片、Agilent Bioanalyzer 2100和微阵列芯片扫描仪G5761A购于购于安捷伦科技(中国)有限公司。
1.2.2. 临床资料及标本收集
收集所有受试者的年龄、性别、受教育程度、头颅MRI影像学资料、反映脑白质病变分级的Fazekas量表评分、蒙特利尔认知评估量表(Montreal Cognitive Assessment,MoCA)评分、简易精神状态检查量表(Mini-mental State Examination,MMSE)评分等。
1.2.3. 外周血单个核细胞的分离与总RNA的提取
抽取受试者清晨空腹8 h的肘静脉血4 mL于EDTA抗凝管中。依据Ficoll密度梯度离心法使用Ficoll淋巴细胞分离液分离每组外周血单个核细胞(peripheral blood mononuclear cell,PBMC),按照TRIzol试剂盒说明书提取样品的总RNA,利用NanoDrop ND-2000型分光光度计行RNA定量,并经Agilent Bioanalyzer 2100检测RNA完整性。
1.2.4. 基因芯片检测
应用基因芯片鉴定3组RNA中差异性表达的circRNA,使用RNeasy试剂盒纯化总RNA。取纯化后的总RNA 250 ng进行扩增、荧光标记、芯片杂交,芯片实验流程图详见附图(https://doi.org/10.11817/j.issn.1672-7347.2021.200692F1)。
1.2.5. 数据的处理和分析
应用Feature Extraction软件(version 12.0.3.1,Agilent Technologies)处理原始图像,提取原始数据,利用Genespring软件(version14.8,Agilent Techno-logies)进行数据的标准化和log2转换。
使用标准化的数据采用t检验进行组间比较,计算circRNA在2组(A组与C组,B组与C组)间表达差异倍数变化(fold change,FC)和P值;差异性表达circRNA的筛选标准为FC≥2.0(|log2FC≥1)且P<0.05,按照筛选标准得到差异性表达的circRNA。使用R语言对circRNA的表达FC进行log2转换后,利用转换后的数据绘制火山图,实现2组间的差异性表达的circRNA的可视化,并进行分层聚类分析。采用TargetScan和miRanda靶基因分析软件预测相关miRNA,Genespring软件预测靶基因信息。
2. 结 果
2.1. 临床一般资料
3组年龄、性别、受教育程度、MoCA和MMSE评分情况等见表1。
表1.
受试者一般临床资料
Table 1 General clinical data of the subjects
| 组别 | 性别 | 年龄/岁 | 受教育程度 | 头颅MRI | Fazekas评分 | MoCA评分 | MMSE评分 | 危险因素 |
|---|---|---|---|---|---|---|---|---|
| A组 | 男 | 79 | 大学 | 髓质区缺血性脱髓鞘改变 | 3 | 11 | 16 | 高血压3级 |
| 男 | 76 | 小学 | 髓质区缺血性脱髓鞘改变 | 3 | 19 | 20 | 无 | |
| 女 | 82 | 小学 |
髓质区缺血性脱髓鞘改变、 老年性脑萎缩 |
3 | 21 | 22 | 无 | |
| B组 | 男 | 79 | 初中 | 髓质区缺血性脱髓鞘改变 | 3 | 28 | 29 | 无 |
| 男 | 78 | 小学 | 髓质区缺血性脱髓鞘改变 | 3 | 29 | 28 | 高血压3级 | |
| 女 | 79 | 小学 | 髓质区缺血性脱髓鞘改变 | 3 | 28 | 28 | 无 | |
| C组 | 男 | 71 | 初中 | 髓质区缺血性脱髓鞘改变 | 1 | 28 | 29 | 无 |
| 男 | 75 | 小学 | 未见明显异常 | 0 | 29 | 29 | 无 | |
| 女 | 69 | 小学 | 髓质区缺血性脱髓鞘改变 | 1 | 28 | 28 | 高血压3级 |
A组:WMH伴认知障碍组;B组:WMH不伴认知障碍组;C组:对照组;MoCA:蒙特利尔认知评估量表;MMSE:简易精神状态检查量表。
2.2. 总RNA质量检测
A260/A280在1.8~2.1、浓度>200 ng/μL的总RNA样品符合circRNA芯片实验要求。A260/A280<1.80代表总RNA样品存在蛋白质污染,需要重新用氯仿抽提;A260/A280>2.1代表总RNA样品可能部分降解。样品RNA完整值(RNA integrity number,RIN)≥7提示总RNA样品完整性良好。总RNA质量检测结果(表2)提示各组总RNA质量符合circRNA芯片实验的要求。
表2.
各组总RNA质量(n=3)
Table 2 Quality of total RNA in each group (n=3)
| 组别 | 浓度/(ng·μL-1) | A260/A280 | RIN | 结果 |
|---|---|---|---|---|
| A组 | 772.02 | 2.017 | 9 | 合格 |
| B组 | 2 200.01 | 2.098 | 10 | 合格 |
| C组 | 1 224.84 | 2.038 | 9.5 | 合格 |
A组:WMH伴认知障碍组;B组:WMH不伴认知障碍组;C组:对照组;RIN:RNA完整值。
2.3. CircRNA表达谱分析
2.3.1. 火山图分析
火山图(图1,2)的横坐标代表circRNA在各组间表达FC,纵坐标代表circRNA表达量变化的统计学显著程度。散点代表各个circRNA,灰色圆点表示表达差异无统计学意义的circRNA,红色圆点表示表达明显上调的circRNA,蓝色圆点表示表达明显下调的circRNA。与C组比较,B组和C组存在较多差异性表达的circRNA。
图1.

WMH伴认知障碍组与对照组的火山图
Figure 1 Volcano plot of the white matter hyperintense with cognitive impairment group and the control group
图2.

WMH不伴认知障碍组与对照组的火山图
Figure 2 Volcano plot of the white matter hyperintensity without cognitive impairment group and the control group
2.3.2. 聚类分析
对A、C组和B、C组的circRNA分别进行聚类分析。图中红色代表相对高的表达量,绿色代表相对低的表达量。与对照组比较,A组和B组中均存在特征性表达的circRNA(图3,4)。
图3.

WMH伴认知障碍组(A组)与对照组(C组)circRNA的聚类分析图
Figure 3 Cluster analysis of circRNA in the WMH with cognitive impairment group (Group A) and the control group (Group C)
图4.
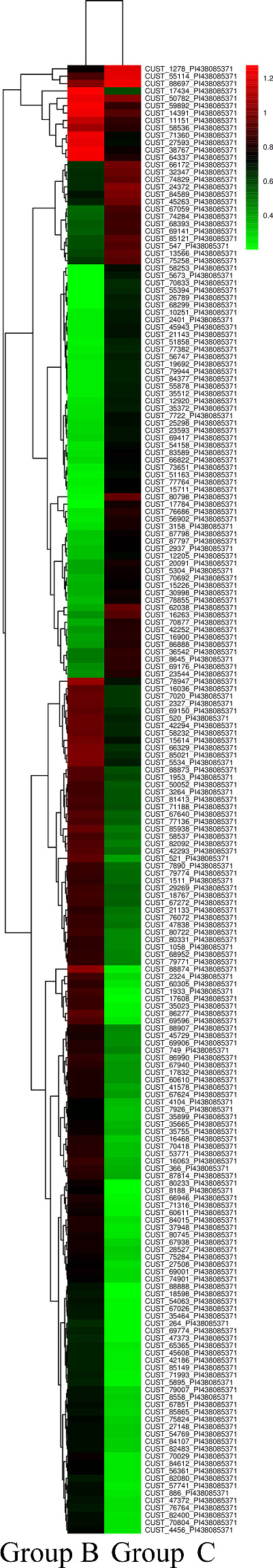
WMH不伴认知障碍组(B组)与对照组(C组)circRNA的聚类分析图
Figure 4 Cluster analysis of circRNA in the WMH without cognitive impairment group (Group B) and the control group (Group C)
2.3.3. CircRNA的差异性表达
与C组比较,A组存在28 530个circRNA表达失调,其中上调的circRNA共15 346个,下调的circRNA共13 184个。表达上调和下调的circRNA前10位分别见表3和表4。
表3.
WMH伴认知障碍组前10位表达上调circRNA
Table 3 Top 10 up-regulated circRNA in the white matter hyperintensity with cognitive impairment group
| 探针号 | circRNA名称 | Fold change | log2(fold change) | P | 基因定位 | 染色体定位 |
|---|---|---|---|---|---|---|
| CUST_17645_PI438085371 | hsa_circ_0017835 | 744.52 | 9.54 | 0.03 | FAM171A1 | Chr10 |
| CUST_32316_PI438085371 | hsa_circ_0033065 | 333.62 | 8.38 | 0.03 | IFI27 | Chr14 |
| CUST_88874_PI438085371 | hsa_circ_0092222 | 261.28 | 8.03 | 0.02 | RPS4Y1 | ChrY |
| CUST_78947_PI438085371 | hsa_circ_0081876 | 174.85 | 7.45 | 0.07 | NAMPT | Chr7 |
| CUST_42293_PI438085371 | hsa_circ_0043580 | 154.82 | 7.27 | 0.05 | KRT23 | Chr17 |
| CUST_2324_PI438085371 | hsa_circ_0001759 | 145.79 | 7.19 | 0.03 | MGAM | Chr7 |
| CUST_32314_PI438085371 | hsa_circ_0033063 | 145.61 | 7.19 | 0.04 | IFI27 | Chr14 |
| CUST_60122_PI438085371 | hsa_circ_0062195 | 121.2 | 6.92 | 0.05 | USP18 | Chr22 |
| CUST_76692_PI438085371 | hsa_circ_0079465 | 110.48 | 6.79 | 0.05 | VWDE | Chr7 |
| CUST_42294_PI438085371 | hsa_circ_0043581 | 104.7 | 6.71 | 0.09 | KRT23 | Chr17 |
表4.
WMH伴认知障碍组前10位表达下调circRNA
Table 4 Top 10 down-regulated circRNA in the white matter hyperintensity with cognitive impairment group
| 探针号 | circRNA名称 | Fold change | log2(fold change) | P | 基因定位 | 染色体定位 |
|---|---|---|---|---|---|---|
| CUST_1278_PI438085371 | hsa_circ_0000662 | 0 | -10.87 | 0.02 | AXIN1 | Chr16 |
| CUST_55114_PI438085371 | hsa_circ_0056984 | 0 | -9.03 | 0.07 | UBR3 | Chr2 |
| CUST_80798_PI438085371 | hsa_circ_0083773 | 0.01 | -7.04 | 0.03 | SCARA3 | Chr8 |
| CUST_75258_PI438085371 | hsa_circ_0077903 | 0.01 | -6.43 | 0.04 | MYB | Chr6 |
| CUST_16263_PI438085371 | hsa_circ_0016396 | 0.01 | -6.39 | 0.05 | PPP2R5A | Chr1 |
| CUST_78303_PI438085371 | hsa_circ_0081193 | 0.02 | -5.83 | 0.06 | ACN9 | Chr7 |
| CUST_46418_PI438085371 | hsa_circ_0047873 | 0.02 | -5.65 | 0.07 | KIAA1468 | Chr18 |
| CUST_2749_PI438085371 | hsa_circ_0002209 | 0.02 | -5.63 | 0.08 | ASPH | Chr8 |
| CUST_29774_PI438085371 | hsa_circ_0030443 | 0.02 | -5.60 | 0.07 | LMO7 | Chr13 |
| CUST_64394_PI438085371 | hsa_circ_0066614 | 0.02 | -5.54 | 0.06 | DCBLD2 | Chr3 |
依据差异性表达circRNA的筛选标准筛选出5个在A组中差异性表达上调的circRNA即hsa_circ_0092222、hsa_circ_0033065、hsa_circ_0001759、hsa_circ_0017835和hsa_circ_0033063,3个差异性表达下调的circRNA即hsa_circ_0000662、hsa_circ_0083773和hsa_circ_0077903。其余上调及下调的circRNA在A、C两组间表达的差异均无统计学意义(均P>0.05),不再进一步分析。
与C组比较,B组存在16 770个circRNA表达失调,其中上调的circRNA共9 814个,下调的circRNA共6 956个。表达上调和下调的circRNA前10位分别见表5和表6。
表5.
WMH不伴认知障碍组前10位表达上调circRNA
Table 5 Top 10 up-regulated circRNA in the white matter hyperintensity without cognitive impairment group
| 探针号 | circRNA名称 | Fold change | log2(fold change) | P | 基因定位 | 染色体定位 |
|---|---|---|---|---|---|---|
| CUST_17434_PI438085371 | hsa_circ_0017619 | 33 567.91 | 15.03 | 0 | SFMBT2 | Chr10 |
| CUST_38767_PI438085371 | hsa_circ_0039921 | 5 030.59 | 12.30 | 0.01 | DUS2 | Chr16 |
| CUST_71360_PI438085371 | hsa_circ_0073863 | 4 271.22 | 12.06 | 0.01 | FNIP1 | Chr5 |
| CUST_64337_PI438085371 | hsa_circ_0066553 | 2 914.60 | 11.51 | 0.02 | PDZRN3 | Chr3 |
| CUST_27593_PI438085371 | hsa_circ_0028170 | 2 761.20 | 11.43 | 0.01 | ATP2A2 | Chr12 |
| CUST_59892_PI438085371 | hsa_circ_0061949 | 2 207.98 | 11.11 | 0.03 | C21orf33 | Chr21 |
| CUST_14391_PI438085371 | hsa_circ_0014462 | 568.77 | 9.15 | 0.07 | SHC1 | Chr1 |
| CUST_88874_PI438085371 | hsa_circ_0092222 | 526.63 | 9.04 | 0.01 | RPS4Y1 | ChrY |
| CUST_86277_PI438085371 | hsa_circ_0089468 | 104.11 | 6.70 | 0.03 | PPP1R26 | Chr9 |
| CUST_11151_PI438085371 | hsa_circ_0011066 | 103.12 | 6.69 | 0.14 | WASF2 | Chr1 |
表6.
WMH不伴认知障碍组前10位表达下调circRNA
Table 6 Top 10 down-regulated circRNA in the white matter hyperintensity without cognitive impairment group
| 探针号 | circRNA名称 | Fold change | log2(fold change) | P | 基因定位 | 染色体定位 |
|---|---|---|---|---|---|---|
| CUST_1278_PI438085371 | hsa_circ_0000662 | 0 | -10.39 | 0.03 | AXIN1 | Chr16 |
| CUST_55114_PI438085371 | hsa_circ_0056984 | 0 | -8.70 | 0.08 | UBR3 | Chr2 |
| CUST_88697_PI438085371 | hsa_circ_0092031 | 0.01 | -7.41 | 0.16 | EMD | ChrX |
| CUST_80798_PI438085371 | hsa_circ_0083773 | 0.01 | -7.18 | 0.03 | SCARA3 | Chr8 |
| CUST_62038_PI438085371 | hsa_circ_0064217 | 0.01 | -6.55 | 0.05 | FANCD2 | Chr3 |
| CUST_24372_PI438085371 | hsa_circ_0024858 | 0.02 | -5.95 | 0.12 | PRDM10 | Chr11 |
| CUST_16263_PI438085371 | hsa_circ_0016396 | 0.02 | -5.56 | 0.09 | PPP2R5A | Chr1 |
| CUST_66172_PI438085371 | hsa_circ_0068455 | 0.03 | -5.21 | 0.16 | EIF4A2 | Chr3 |
| CUST_85121_PI438085371 | hsa_circ_0088262 | 0.04 | -4.75 | 0.18 | ASTN2 | Chr9 |
| CUST_76686_PI438085371 | hsa_circ_0079459 | 0.04 | -4.74 | 0.10 | VWDE | Chr7 |
依据差异性表达circRNA的筛选标准筛选出8个在B组中差异性表达上调的circRNA即hsa_circ_0017619、hsa_circ_0092222、hsa_circ_0073863、hsa_circ_ 0039921、hsa_circ_0028170、hsa_circ_0066553、hsa_circ_0061949和hsa_circ_0089468,2个差异性表达下调的circRNA即hsa_circ_0000662和hsa_circ_0083773。其余上调及下调的circRNA在B、C两组间表达的差异均无统计学意义(均P>0.05),不再进一步分析。
2.3.4. WMH伴或不伴认知障碍患者共同差异表达的circRNA
将A组的circRNA表达谱分别与B、C组比较,未发现共同差异表达的circRNA;与C组的circRNA表达谱比较,A、B组的hsa_circ_0092222表达均上调,hsa_circ_0000662和hsa_circ_0083773表达均下调,且它们在A组和B组中的表达差异均无统计学意义(P>0.05,表7)。
表7.
脑白质高信号伴或不伴认知障碍患者共同差异表达的circRNA的表达量
Table 7 Expression of co-differentially expressed circRNA in patients of high white matter hyperintense with or without cognitive impairment
| 组别 | hsa_circ_0092222 | hsa_circ_0000662 | hsa_circ_0083773 |
|---|---|---|---|
| A组 | 9.76* | 5.25* | 1.80* |
| B组 | 10.77*† | 5.73*† | 1.66*† |
| C组 | 1.73 | 16.11 | 8.84 |
A组:WMH伴认知障碍组;B组:WMH不伴认知障碍组;C组:对照组;与C组比较,*P<0.05;与A组比较, †P>0.05。
2.4. 预测的靶基因
hsa_circ_0092222、hsa_circ_0000662和hsa_circ_0083773分别预测到2、1和7个靶miRNA。预测到hsa_circ_0092222、hsa_circ_0000662和hsa_circ_0083773的靶基因分别为核糖体蛋白S4,Y连锁1(ribosomal protein S4, Y-linked 1,RPS4Y1)、轴抑制因子1(axis inhibitor 1,AXIN1)和重组清道夫受体A类成员3(recombinant scavenger receptor class A member 3,SCARA3),详见表8。
表8.
共同差异表达的circRNA的靶miRNA
Table 8 Target miRNAs of co-differentially expressed circRNA
| CircRNA名称 | 基因定位 | 靶miRNA | UTR起点 | UTR终点 |
|---|---|---|---|---|
| hsa_circ_0092222 | RPS4Y1 | hsa-miR-19a-3p | 288 | 294 |
| hsa_circ_0092222 | RPS4Y1 | hsa-miR-19b-3p | 288 | 294 |
| hsa_circ_0000662 | AXIN1 | hsa-miR-194 | 1 769 | 1 775 |
| hsa_circ_0083773 | SCARA3 | hsa-let-7a-5p | 2 402 | 2 408 |
| hsa_circ_0083773 | SCARA3 | hsa-let-7b-5p | 2 402 | 2 408 |
| hsa_circ_0083773 | SCARA3 | hsa-let-7c-5p | 2 402 | 2 408 |
| hsa_circ_0083773 | SCARA3 | hsa-let-7d-5p | 2 402 | 2 408 |
| hsa_circ_0083773 | SCARA3 | hsa-let-7e-5p | 2 402 | 2 408 |
| hsa_circ_0083773 | SCARA3 | hsa-let-7f-5p | 2 402 | 2 408 |
| hsa_circ_0083773 | SCARA3 | hsa-miR-17-3p | 2 937 | 2 943 |
| hsa_circ_0083773 | SCARA3 | hsa-miR-17-3p | 386 | 392 |
RPS4Y1:核糖体蛋白S4,Y连锁1;AXIN1:轴抑制因子1;SCARA3:重组清道夫受体A类成员3。
3. 讨 论
CircRNA是近年来发现的与人类疾病密切相关的非编码RNA。研究[11]表明circRNA的表达在神经元发育和衰老过程中呈动态变化,且在突触中表达水平高,提示circRNA在神经元的功能和活动中起重要作用。目前,越来越多的证据[9, 12]表明circRNA与阿尔茨海默病、帕金森病等疾病密切相关。然而,目前尚无WMH患者或WMH伴认知障碍患者外周血中circRNA差异性表达方面的研究报道。
WMH的发生率极高,其影像学表现及临床症状的异质性为早期发现、早期诊断带来了困难。目前对WMH的病理生理学仍知之甚少。高血压、糖尿病、高胆固醇血症、高同型半胱氨酸血症等心血管疾病的危险因素也是WMH的危险因素,小血管动脉硬化性疾病被认为是导致WMH的重要机制[13]。近年来,越来越多的证据[14-15]表明:慢性炎症、胶质增生、脱髓鞘病变、轴突损伤以及血脑屏障功能障碍也可能参与了WMH的发生。探讨WMH导致认知障碍的机制可为相关疾病的早期诊断、预后评估及治疗提供新的思路。
本研究采用基因芯片检测WMH伴认知障碍组、WMH不伴认知障碍组和对照组的外周血中circRNA的表达谱,并分析共同差异表达的circRNA,结果发现:WMH伴认知障碍组分别与WMH不伴认知障碍组、对照组比较,无共同差异表达的circRNA;与对照组比较,hsa_circ_0092222在WMH伴认知障碍组、WMH不伴认知障碍组中表达均上调,hsa_circ_0000662和hsa_circ_0083773表达均下调。
采用Genespring软件定位上述3条差异表达的circRNA的基因与染色体信息,TargetScan和miRanda软件预测其靶miRNA。预测到hsa_circ_0092222有2个相关的miRNA,分别为hsa-miR-19a-3p、hsa-miR-19b-3p;hsa_circ_0000662仅有1个相关miRNA即hsa-miR-194;hsa_circ_0083773有7个相关miRNA即hsa-let-7a-5p、hsa-let-7b-5p、hsa-let-7c-5p、hsa-let-7d-5p、hsa-let-7e-5p、hsa-let-7f-5p和hsa-miR-17-3p。
本研究发现表达上调的hsa_circ_0092222的来源基因为RPS4Y1。RPS4Y1是核糖体蛋白S4E家族的成员,可使信号转导与转录活化因子3(signal transducers and activators of transcription,STAT3)的磷酸化水平降低。STAT作为转录因子,在白细胞介素、干扰素、肿瘤坏死因子、生长因子等细胞因子的刺激下,可被Janus激酶(Janus kinase,JAK)、表皮生长因子受体(epithelial growth factor receptor,EGFR)等激活,进而入核介导下游靶基因的转录。Janus激酶2(Janus kinase 2,JAK2)/STAT3信号通路参与细胞的生存、增殖、分化及炎症等生理过程。JAK2/STAT3信号通路在中枢神经系统中也具有神经元特异性功能。Chen等[16]通过结扎双侧颈总动脉建立大鼠白质损伤模型,用免疫组织化学方法观察海马神经元JAK2和STAT3磷酸化的变化,发现JAK2/STAT3信号通路参与脑白质的损伤和神经元的凋亡。本研究预测到hsa_circ_0092222相关的miRNA为hsa-miR-19a-3p和hsa-miR-19b-3p。目前已有研究[17]表明hsa-miR-19a-3p可通过调节糖代谢和神经元凋亡预防缺血性中风;hsa-miR-19b-3p可能参与神经元分化、神经系统发育、突触传递和多巴胺分泌有关的过程,但是具体机制尚不明确。因此我们推测上调的hsa_circ_0092222可能靶向吸附hsa-miR-19a-3p/19b-3p,调节RPS4Y1基因的表达,通过JAK2/STAT3信号通路导致脑白质的损伤,出现WMH,进而引起认知功能障碍。
本研究发现表达下调的hsa_circ_0000662的来源基因为AXIN1。AXIN家族包括AXIN1和AXIN2。AXIN是一种多功能的构架蛋白,它可与Wnt信号通路、JNK信号通路、TGF-β信号通路上的因子结合形成复合物,调节信号转导系统,从而调节细胞的增殖、分化、凋亡和癌变等重要过程。AXIN1是Wnt信号通路的负调节因子,广泛表达的AXIN1参与胚胎神经轴的形成。通过巨噬细胞/小胶质细胞与星形胶质细胞之间的相互作用调节Wnt信号级联,Wnt信号通路在中枢神经系统疾病中具有多种作用,其介导的炎症和氧化应激是老年性脑病的关键病理基础[18]。另外,Wnt/β-catenin信号转导通路对血脑屏障的发育具有重要作用,在多种病理生理条件下参与损伤血脑屏障[19]。本研究预测到hsa_circ_0000662相关的miRNA为hsa-miR-194,hsa-miR-194可激活Wnt信号通路,促进相关疾病的发生。因此,我们推测下调的hsa_circ_0000662可能通过靶向吸附hsa-miR-194,调节AXIN1基因的表达,通过Wnt信号通路损伤脑白质,进而引起认知功能障碍。
本研究发现表达下调的hsa_circ_0083773的来源基因为SCARA3。A类清道夫受体由5种表面受体组成,其在哺乳动物体内的功能与先天免疫有关[20]。SCARA3可改变氧化应激,具有清除活性氧等有害氧化物质的功能。SCARA3还可以通过与多种阴离子配体结合获得脂肪酸,并且是肿瘤中树突状细胞和巨噬细胞发挥作用的主要途径[21]。此外,巨噬细胞中的A类清道夫受体具有促动脉粥样硬化作用[22]。目前SCARA3在多发性骨髓瘤、卵巢癌和乳腺癌方面已有研究,但尚未见与神经系统相关疾病的研究。本研究的hsa_circ_0083773预测到7个相关miRNA,其中hsa-let-7b-5p被认为是调控胶质瘤发展的候选基因的核心miRNA,过表达的hsa-let-7b-5p能抑制胶质瘤细胞的迁移、侵袭[23]。因此我们推测hsa_circ_0083773可能通过hsa-let-7b-5p调节SCARA3基因的表达,导致脑白质损伤,出现WMH,进而引起认知功能障碍。
综上,本研究结果表明hsa_circ_0092222、hsa_circ_0000662和hsa_circ_0083773是与WMH相关的circRNAs,它们可能参与WMH的发生,随着脑白质损伤的不断加重,最终导致认知功能障碍。WMH的出现和进展在认知功能下降过程中起着重要作用。然而,受限于纳入病例的病例数量,本研究的结论仍需要大样本研究的证实。未来还可进一步研究circRNA在WMH的发生及导致认知功能障碍中的分子机制。
附录.
附图1. 芯片实验流程图Supplementary Figure 1 Flow chart of chip experiment .
基金资助
包头市科技创新能力建设项目(2018C2007-1-22)。
This work was supported by the Baotou Science and Technology Innovation Capacity Construction Project, China (2018C2007-1-22).
利益冲突声明
作者声称无任何利益冲突。
原文网址
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/2021101080.pdf
参考文献
- 1. Ding T, Cohen AD, O'Connor EE, et al. An improved algorithm of white matter hyperintensity detection in elderly adults[J]. Neuroimage Clin, 2020, 25: 102151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Veldsman M, Kindalova P, Husain M, et al. Spatial distribution and cognitive impact of cerebrovascular risk-related white matter hyperintensities[J]. Neuroimage Clin, 2020, 28: 102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han F, Zhai FF, Wang Q, et al. Prevalence and risk factors of cerebral small vessel disease in a Chinese population-based sample[J]. J Stroke, 2018, 20(2): 239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lampe L, Kharabian-Masouleh S, Kynast J, et al. Lesion location matters: The relationships between white matter hyperintensities on cognition in the healthy elderly[J]. J Cereb Blood Flow Metab, 2019, 39(1): 36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meng SJ, Zhou HC, Feng ZY, et al. Epigenetics in neurodevelopment: emerging role of circular RNA[J]. Front Cell Neurosci, 2019, 13: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bu Q, Long H, Shao X, et al. Cocaine induces differential circular RNA expression in striatum[J]. Transl Psychiatry, 2019, 9(1): 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu J, Zhao K, Huang N, et al. Circular RNAs and human glioma[J]. Cancer Biol Med, 2019, 16(1): 11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soldan A, Pettigrew C, Zhu Y, et al. White matter hyperintensities and CSF Alzheimer disease biomarkers in preclinical Alzheimer disease[J]. Neurology, 2020, 94(9): e950-e960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akhter R. Circular RNA and Alzheimer's disease[J]. Adv Exp Med Biol, 2018, 1087: 239-243. [DOI] [PubMed] [Google Scholar]
- 10. 中华医学会老年医学分会老年神经病学组、脑小血管病认知功能障碍诊疗指南中国撰写专家组 . 脑小血管病相关认知功能障碍中国诊疗指南(2019)[J]. 中华老年医学杂志, 2019, 38(4): 345-354. [Google Scholar]; Geriatric Neuropathy Group of Geriatric Medicine Branch of Chinese Medical Association, Chinese Expert Group Writing Guidelines for the Diagnosis and Treatment of Cognitive Dysfunction of Cerebral Vascular Diseases . Clinical practice guideline for cognitive impairment of cerebral small vessel disease of China (2019)[J]. Chinese Journal of Geriatrics, 2019, 38(4): 345-354. [Google Scholar]
- 11. Hanan M, Soreq H, Kadener S. CircRNAs in the brain[J]. RNA Biol, 2017, 14(8): 1028-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanan M, Simchovitz A, Yayon N, et al. A Parkinson's disease CircRNAs Resource reveals a link between circSLC8A1 and oxidative stress[J]. EMBO Mol Med, 2020, 12(11): e13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kynast J, Lampe L, Luck T, et al. White matter hyperintensities associated with small vessel disease impair social cognition beside attention and memory[J]. J Cereb Blood Flow Metab, 2018, 38(6): 996-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woollacott IOC, Bocchetta M, Sudre CH, et al. Pathological correlates of white matter hyperintensities in a case of progranulin mutation associated frontotemporal dementia[J]. Neurocase, 2018, 24(3): 166-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Li M, Zhang XY, et al. Higher blood-brain barrier permeability is associated with higher white matter hyperintensities burden[J]. J Neurol, 2017, 264(7): 1474-1481. [DOI] [PubMed] [Google Scholar]
- 16. Chen XM, Yu YH, Wang L, et al. Effect of the JAK2/STAT3 signaling pathway on nerve cell apoptosis in rats with white matter injury[J]. Eur Rev Med Pharmacol Sci, 2019, 23(1): 321-327. [DOI] [PubMed] [Google Scholar]
- 17. Ge XL, Wang JL, Liu X, et al. Inhibition of miR-19a protects neurons against ischemic stroke through modulating glucose metabolism and neuronal apoptosis[J]. Cell Mol Biol Lett, 2019, 24: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marchetti B, Pluchino S. Wnt your brain be inflamed? Yes, it Wnt![J]. Trends Mol Med, 2013, 19(3): 144-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laksitorini MD, Yathindranath V, Xiong W, et al. Modulation of Wnt/β-catenin signaling promotes blood-brain barrier phenotype in cultured brain endothelial cells[J]. Sci Rep, 2019, 9(1): 19718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poynter SJ, Monjo AL, DeWitte-Orr SJ. Identification of three class A scavenger receptors from rainbow trout (Oncorhynchus mykiss): SCARA3, SCARA4, and SCARA5[J]. Fish Shellfish Immunol, 2018, 76: 121-125. [DOI] [PubMed] [Google Scholar]
- 21. Tian Y, Zhou K, Hu J, et al. Scavenger receptor class a, member 3 is associated with severity of hand, foot, and mouth disease in a case-control study[J]. Medicine (Baltimore), 2019, 98(40): e17471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown CO, Schibler J, Fitzgerald MP, et al. Scavenger receptor class A member 3 (SCARA3) in disease progression and therapy resistance in multiple myeloma[J]. Leuk Res, 2013, 37(8): 963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xi XN, Chu YH, Liu N, et al. Joint bioinformatics analysis of underlying potential functions of hsa-let-7b-5p and core genes in human glioma[J]. J Transl Med, 2019, 17(1): 129. [DOI] [PMC free article] [PubMed] [Google Scholar]



