Abstract
目的
探讨低氧诱导的长链非编码核内小RNA宿主基因14(long non‑coding small nucleolar RNA host gene 14,lncRNA SNHG14)在胶质瘤替莫唑胺(temozolomide,TMZ)耐药中的作用和潜在机制。
方法
根据不同处理将实验分为常氧组、低氧组、对照组(NC组)和TMZ组,利用real-time PCR和蛋白质印迹法分别检测胶质瘤细胞SNB19和U251中lncRNA SNHG14和O6-甲基鸟嘌呤DNA甲基转移酶(O6-methylguanine DNA methyltransferase,MGMT)的表达水平,分析lncRNA SNHG14表达水平与低氧和TMZ处理的关系。利用siRNA干扰胶质瘤细胞中lncRNA SNHG14表达,将转染后的胶质瘤细胞分为si-对照组(si-NC组)和si-SNHG14组,利用real-time PCR检测干扰效率,并采用蛋白质印迹法检测TMZ敏感性调控关键因子MGMT的表达变化,采用流式细胞术检测细胞凋亡;此外,增设常氧组和低氧组,应用MTT法检测不同TMZ浓度梯度下各组胶质瘤的细胞活性,分析lncRNA SNHG14对胶质瘤TMZ敏感性的影响。利用在线工具针对性地预测与lncRNA SNHG14和MGMT结合的miRNAs。应用real-time PCR观察不同环境下si-NC组、si-SNHG14组、常氧组和低氧组miR-143的丰度变化。利用miR-143拟似剂(mimics)和抑制剂(inhibitor)改变胶质瘤细胞中miR-143水平,将实验设置为NC inhibitor组、miR-143 inhibitor组、NC mimics组和miR-143 mimics组,采用real-time PCR检测干扰效率,应用蛋白质印迹法检测MGMT的表达水平,分析miR-143对MGMT水平的影响。对NC inhibitor组、miR-143 inhibitor组、NC mimics组和miR-143 mimics组进行不同干预,采用双荧光素酶报告实验观察lncRNA SNHG14和MGMT荧光素酶的活性变化,验证lncRNA SNHG14、miR-143和MGMT之间的靶向调控关系。最后,设置NC组和lncRNA SNHG14过表达组,通过RNA结合蛋白免疫沉淀实验检测各组中miR-143和MGMT的丰度变化,分析lncRNA SNHG14、miR-143和MGMT间的竞争结合关系。
结果
与常氧组相比,低氧组可以促进胶质瘤细胞中lncRNA SNHG14表达;与NC组相比,TMZ组能显著抑制lncRNA SNHG14的表达(均P<0.05)。与si-NC组相比,si-SNHG14组可有效抑制lncRNA SNHG14表达,且MGMT表达水平呈现显著下降,细胞凋亡率显著升高(均P<0.05)。随着TMZ浓度的升高,si-SNHG14组胶质瘤细胞活力显著低于si-NC组,低氧组的细胞活力显著高于常氧组(均P<0.05)。在线工具预测发现:miR-143与lncRNA SNHG14和MGMT存在结合位点,低氧组miR-143丰度显著低于常氧组,si-SNHG14组miR-143丰度显著高于si-NC组(均P<0.05)。miR-143 mimics组或miR-143 inhibitor组可显著过表达或低表达miR-143(均P<0.05),但NC mimics组或NC inhibitor组与NC组相比,差异均无统计学意义(均P>0.05);miR-143 inhibitor组MGMT蛋白水平显著上调(P<0.01),miR-143 mimics组则反之(P<0.01)。双荧光素酶报告实验显示:NC mimics组或NC inhibitor组与各自的对照组相比,差异均无统计学意义(均P>0.05);miR-143 mimics组野生型SNHG14和MGMT的荧光素酶活性显著下调,而miR-143 inhibitor组野生型SNHG14和MGMT的荧光素酶活性显著上调(分别P<0.01和P<0.05);但突变型SNHG14和MGMT的荧光素酶活性均无明显变化(均P>0.05)。RNA结合蛋白免疫沉淀实验结果显示:与NC组相比,过表达lncRNA SNHG14组细胞中沉淀的argonaute 2蛋白上结合有更多的lncRNA SNHG14,但MGMT mRNA丰度却出现显著下降,差异均有统计学意义(均P<0.01),lncRNA SNHG14、miR-143和MGMT之间存在靶向调控关系。
结论
低氧下胶质瘤细胞中表达上调的lncRNA SNHG14靶向miR-143,解除miR-143对MGMT的抑制,从而促进胶质瘤细胞产生TMZ耐药。
Keywords: 胶质瘤, 低氧, 替莫唑胺, 耐药, 长链非编码RNA
Abstract
Objective
This study aims to investigate the role of hypoxia-induced long non-coding small nucleolar RNA host gene 14 (lncRNA SNHG14) in glioma temozolomide (TMZ) resistance and underlying mechanisms.
Methods
According to different treatments, the experiment was divided into a normoxia group and a hypoxia group, a control group and a TMZ group. The lncRNA SNHG14 and O6-methylguanine DNA methyltransferase (MGMT) levels in glioma SNB19 and U251 cell line were detected by real-time PCR and Western blotting, respectively, and the association of lncRNA SNHG14 level with hypoxia and TMZ treatment was analyzed. siRNA was used to knockdown the lncRNA SNHG14 expression in glioma cells, and the transfected glioma cells were divided into a negative control group (si-NC group) and a si-SNHG14 group. The interference efficiency was examined by real-time PCR, the key factor MGMT of lncRNA SNHG14 sensitivity regulation was detected by Western blotting, and the cell apoptosis was detected by cytometry. In addition, MTT method was used to detect the cell viability of gliomas in the different groups under the different TMZ concentrations, and the effect of lncRNA SNHG14 on TMZ sensitivity of gliomas was analyzed. Online tools were used to predict miRNAs that could specifically bind to lncRNAs SNHG14 and MGMT. A si-NC group, a si-SNHG14 group, a normoxia group and a hypoxia group were set up, and the changes of miR-143 abundance in different environments were observed by real-time PCR. miR-143 mimics and inhibitor were used to change the level of miR-143 in glioma cells. A NC inhibitor group, a miR-143 inhibitor group, a NC mimics group and a miR-143 mimics group were set up, the interference efficiency was detected by real-time PCR, the expression level of MGMT was detected by Western blotting, and the effect of miR-143 on the level of MGMT were analyzed. The NC inhibitor group, the miR-143 inhibitor group, the NC mimics group and the miR-143 mimics group were treated with different interventions, and the dual luciferase reporter assay was used to observe the changes of lncRNA SNHG14 and MGMT luciferase activities, and to verify the relationship among lncRNA SNHG14, miR-143 and MGMT. Finally, a NC group and a lncRNA SNHG14 overexpression group were set up, and the changes in the abundance of miR-143 and MGMT in each group were detected by RNA-binding protein immunoprecipitation experiments, and the competitive binding relationship among lncRNA SNHG14, miR-143 and MGMT was analyzed.
Results
Compared with the normoxia group, the hypoxia group could promote the expression of lncRNA SNHG14 in glioma cells. Compared with the control group, the expression of lncRNA SNHG14 could be significantly inhibited in the TMZ group (P<0.05). Compared with the si-NC group, the expression of lncRNA SNHG14 in the si-SNHG14 group could be effectively inhibited, and the expression level of MGMT was significantly decreased, and the apoptosis rate was significantly increased (all P<0.05). With the increase of TMZ concentrations, the glioma cell viability in the si-SNHG14 group was significantly lower than that in the si-NC group, and the cell viability in the hypoxia group was significantly higher than that in the normoxia group (both P<0.05). Online tool prediction found that miR-143 had binding sites with lncRNA SNHG14 and MGMT. The abundance of miR-143 in the hypoxia group was significantly lower than that in the normoxic group, and the abundance of miR-143 in the si-SNHG14 group was significantly higher than that in the si-NC group (both P<0.05). The miR-143 mimics group or the miR-143 inhibitor group could significantly over-express or under-express miR-143 (both P<0.05). But there was no significant difference between the NC mimics group (or the NC inhibitor group) and the control group (both P>0.05). The level of MGMT protein could significantly up-regulate in the miR-143 inhibitor group, and on the contrary which could significantly down-regulate in the miR-143 mimics group (both P<0.01). The dual luciferase reporter assay showed that there was no significant difference between the NC mimics group (or the NC inhibitor group) and the control group (both P>0.05). The wild-type SNHG14 and MGMT luciferase activities were significantly down-regulated in the miR-143 mimics group, which were significantly up-regulated in the miR-143 inhibitor group (P<0.01 and P<0.05, respectively), but there was no significant change in the luciferase activities of mutant SNHG14 and MGMT (both P>0.05). The results of the RNA-binding protein immunoprecipitation experiment showed that: compared with the NC group, more lncRNA SNHG14 was bound to the precipitated argonaute 2 protein in the cells in the lncRNA SNHG14 overexpression group, but the abundance of MGMT mRNA was decreased significantly, and there were significant differences (both P<0.01). There was a targeting regulatory relationship among lncRNA SNHG14, miR-143 and MGMT.
Conclusion
The up-regulated lncRNA SNHG14 can target miR-143, relieve the inhibition of miR-143 on MGMT, and promote the TMZ resistance in the hypoxia-induced glioma cells.
Keywords: glioma, hypoxia, temozolomide, drug resistance, long non-coding RNA
胶质瘤是中枢神经系统肿瘤中最常见的恶性肿瘤,占所有脑肿瘤的一半以上[1]。胶质瘤具有发病率高、复发率高、病死率高和治愈率低的特点,高度恶性脑胶质瘤患者的生存期不足1年[2]。替莫唑胺(temozolomide,TMZ)对胶质瘤具有一定的治疗作用,TMZ联合放射治疗(以下简称放疗)可显著延长胶质瘤患者的存活率[3]。虽然目前TMZ是临床治疗神经胶质细胞瘤的一线化学治疗(以下简称化疗)药物,但胶质瘤的高复发率和病死率依然威胁着人类健康。因此,寻找可以抑制胶质瘤浸润生长和提高TMZ敏感性以抵抗化疗耐受是目前胶质瘤防治的热点研究之一。
低氧是胶质瘤发展过程中所必须经历的环境条件之一[4],低氧可促进脑胶质瘤侵袭和预后不良表型的发展,对胶质瘤产生TMZ耐药起重要作用[5],而低氧下DNA修复酶O6-甲基鸟嘌呤DNA甲基转移酶(O6-methylguanine DNA methyltransferase,MGMT)是胶质瘤细胞对TMZ耐药的关键酶[6],MGMT的活性在胶质瘤对TMZ的耐药机制中是一个重要因素,能降低MGMT的表达活性,而降低胶质瘤细胞的DNA修复能力可用来增强TMZ对胶质瘤细胞的疗效,故可认为是提高TMZ疗效的一个重要途径[7]。随着高通量测序和芯片技术的发展,越来越多的胶质瘤中表达紊乱的长链非编码RNA(long non-coding RNA,lncRNA)已被发现,这些lncRNA可作为内源竞争RNA(competing endogenous RNA,ceRNA)靶向miRNA,调控相关基因表达,参与胶质瘤的病理、生理过程[8]。本研究通过芯片和在线工具分析发现:低氧下胶质瘤SNB19细胞中高表达的长链非编码核内小RNA宿主基因14(long non‑coding small nucleolar RNA host gene 14,lncRNA SNHG14)与miR-143、miR-143和MGMT mRNA之间均存在结合位点,提示lncRNA SNHG14、miR-143和MGMT之间可能存在相互调控关系,因此本研究拟探讨lncRNA SNHG14/miR-143/MGMT轴在低氧下胶质瘤TMZ耐药中的作用。
1. 材料与方法
1.1. 材料
人脑胶质瘤细胞株U251、SNB19均购自湖南丰晖生物科技有限公司;胎牛血清、细胞培养基购自美国Gibco公司;MTT检测试剂盒购自美国Sigma公司;TRIzol试剂购自美国Thermo Fisher公司;反转录试剂盒及荧光素酶报告载体Renilla psiCHECK2均购自美国Promega公司;real-time PCR试剂购自瑞士Roche公司,引物由北京擎科生物科技有限公司合成;BCA蛋白浓度检测试剂盒购自美国Bio-Rad公司;MGMT(抗体编号ab39253)、GAPDH(抗体编号ab8245)、argonaute 2(AGO2)(抗体编号ab57113)抗体均购自英国Abcam公司。
1.2. 方法
1.2.1. 细胞培养
胶质瘤SNB19和U251细胞株用RPMI-1640培养基(含15%小牛血清、100 U/mL青霉素、100 μg/mL链霉素)培养于37 ℃、5%CO2、20% O2(常氧)或1% O2(低氧)、饱和湿度95%的培养箱中,每隔2~3 d换液,胰蛋白酶消化、传代,显微镜下观察细胞形态,当细胞生长到对数期时,取细胞进行后续实验。实验分为常氧组、低氧组。
1.2.2. 细胞转染及分组
将处于对数生长期的胶质瘤SNB19和U251细胞株接种在6孔细胞培养板上,当细胞生长至60~70%的密度时,根据转染试剂产品Lipofectamine 2000的使用要求,按照实验步骤转染miRNA拟似剂(mimics)/抑制剂(inhibitor)或siRNA。实验分为si-正常对照组(si-NC组)、si-SNHG14组、mimics对照组(NC mimics组)、miR-143 mimics组、inhibitor对照组(NC inhibitor组)、miR-143 inhibitor组。
1.2.3. Real-time PCR实验
使用TRIzol一步法裂解SNB19和U251细胞,离心,获取RNA,通过核算定量仪及琼脂糖凝胶电泳,检测RNA的纯度和浓度,将获得的RNA反转录成cDNA,随后加入real-time PCR反应混合物Syber mix体系,上机,加入引物,PCR扩增,以GAPDH为内参,使用2-ΔΔCt方法(首先计算样品和校正样品的平均Ct值,然后用目的基因的平均Ct值减去内参基因的平均Ct值,计算各个样品的ΔCt值;样品的ΔCt值减去校正样品的ΔCt值,计算各个样品的ΔΔCt,最后计算相对表达比率2-ΔΔCt)。计算相对表达水平,所用引物序列信息见表1。实验分为常氧组、低氧组、NC组、TMZ组、si-NC组、si-SNHG14组。
表1.
Real-time PCR引物
Table 1 Primer of real-time PCR
| 引物 | 序列 | |
|---|---|---|
| SNHG14 | 正向 | 5'-TCACAGAGGCAAGTGCTACCG-3' |
| 反向 | 5'-ACTGATGGTAAAGTGGACTGGATG-3' | |
| MGMT | 正向 | 5'-ACCGTTTGCGACTTGGTACTT-3' |
| 反向 | 5'-GGAGCTTTATTTCGTGCAGACC-3' | |
| miR-143 | 正向 | 5'-GCCTGAGATGAAGCACTG-3' |
| 反向 | 5'-CAGTGCGTGTCGTGGA-3' | |
| GAPDH | 正向 | 5'-ACAGCCTCAAGATCATCAGC-3' |
| 反向 | 5'-GGTCATGAGTCCTTCCACGAT-3' |
1.2.4. 蛋白质印迹法检测
提取待测SNP19和U251细胞中的总蛋白,通过BCA法测定蛋白浓度,随后进行SDS-PAGE电泳,再将蛋白转模至PVDF膜上,并用脱脂牛奶进行封闭,封闭后加一抗孵育,4 ℃孵育12 h,孵育结束后洗膜,加入二抗孵育,室温孵育2 h,孵育结束后洗膜,利用ECL显影,各组样品用GAPDH作内源性对照。实验分为NC组、TMZ组、si-NC组、si-SNHG14组、NC mimics组、miR-143 mimics组、NC inhibitor组、miR-143 inhibitor组。
1.2.5. TMZ化疗敏感性检测
MTT检测细胞活力:以2×105个/孔细胞接种于96孔板中,向细胞中添加不同浓度的TMZ(7.5、15.0、30.0、60.0、120.0、240.0、480.0 μmol/L)处理72 h后,每孔加入20 μLMTT溶液,继续孵育4 h,终止培养,弃去上清液,每个孔加入150 μL DMSO,振荡10 min,充分溶解后在酶标仪读取490 nm处的吸光度值[抑制率=(1-A实验组/A对照组)×100%],以抑制率为纵坐标,药物浓度(lnX)为横坐标,再根据拟合曲线公式[Y=A×lnX+B(A为直线的斜率,B为截距)]求出半抑制率和细胞抑制率50%时的TMZ浓度(half maximal inhibitory concentration,IC50)。根据TMZ浓度分共7组:7.5、15.0、30.0、60.0、120.0、240.0及480.0 mmol/L组。
1.2.6. 双荧光素酶报告实验
将对数生长期的HEK293T细胞接种于24孔细胞培养板中,培养12 h,在细胞中分别转染野生型(wild type,wt)/突变型(mutant type,mut) MGMT、wt/mut SNHG14荧光素酶报告质粒,同时向不同组细胞中转染miR-143 mimics、mimics NC、miR143 inhibitor、inhibitor NC,放入培养箱中继续培养48 h,后利用试剂盒测定各组中荧光素酶的相对活性。实验分为NC mimics组、miR-143 mimics组、NC inhibitor组、miR-143 inhibitor组。
1.2.7. RNA结合蛋白免疫沉淀
将空载质粒和lncRNA SNHG14过表达质粒分别转染HEK 293T细胞,利用AGO2抗体将AGO2蛋白上结合的lncRNA SNHG14和MGMT mRNA一起进行沉淀,洗去未结合的物质,利用real-time PCR检测沉淀复合物中是否存在lncRNA SNHG14和MGMT mRNA以及各自的表达量。实验分为有NC组和过表达lncRNA SNHG14组。
1.3. 统计学处理
采用SPSS 22.0统计学软件进行数据分析,每项实验均平行重复3次,数据最后以均数±标准差( ±s)表示,并且在方差分析前,根据格拉布斯准则去除逸出值,两两比较使用LSD-t检验,P<0.05为差异有统计学意义。绘制图表采用Graphpad Prim 7.0软件。
2. 结 果
2.1. 低氧或TMZ处理下胶质瘤细胞系中lncRNA SNHG14的表达
Real-time PCR结果表明:低氧组lncRNA SNHG14的表达显著高于常氧组,TMZ组lncRNA SNHG14表达水平显著低于NC组(均P<0.01,图1A)。此外,相比于NC组,TMZ组中MGMT的蛋白水平显著下降(P<0.01;图1B,1C)。
图1.
胶质瘤细胞中低氧和TMZ对SNHG14表达的影响
Figure 1 Effect of hypoxic and TMZ on the expression of SNHG14 in glioma cells
A: Expression of SNHG14 in SNB19 and U251 cell lines in normoxic and hypoxic environment. **P<0.01 vs the normoxia group. B: Expression of SNHG14 in SNB19 and U251 cell line under TMZ treatment. **P<0.01 vs the NC group. C: Expression of MGMT in SNB19 and U251 cell lines under TMZ treatment. **P<0.01 vs the NC group.
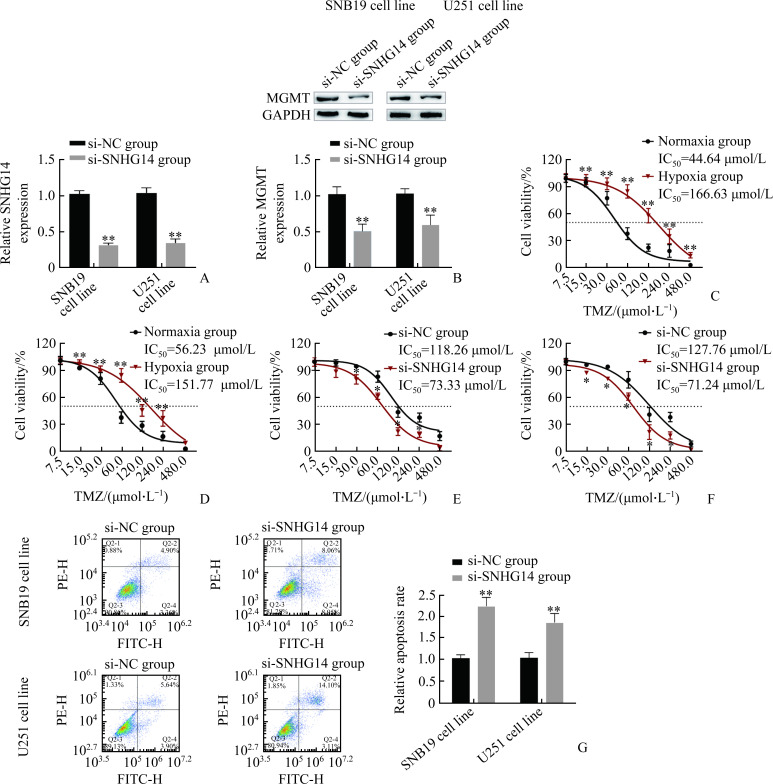
2.2. LncRNA SNHG14表达变化对低氧下人脑胶质瘤细胞TMZ敏感性的影响
相比于si-NC组,SNB19和U251细胞株si-SNHG14组的lncRNA SNHG14表达均被有效抑制(均P<0.01,图2A);si-SNHG14组MGMT的表达水平显著低于Si-NC组(均P<0.01,图2B)。MTT结果显示:相比于常氧组,胶质瘤SNB19和U251细胞株低氧组对TMZ的耐药性均明显提高(均P<0.01;图2C,2D);而si-SNHG14组细胞对TMZ的耐药性明显低于si-NC组(图2E,2F;均P<0.05)。流式细胞术检测显示:与si-NC组相比,胶质瘤SNB19和U251细胞株si-SNHG14组凋亡率均上升(均P<0.01,图2G)。
图2.
SNHG14对低氧下人脑胶质瘤细胞TMZ敏感性的影响
Figure 2 Effect of SNHG14 on TMZ sensitivity in human glioma cells under hypoxia
A: Efficiency of SNHG14 interference. **P<0.01 vs the si-NC group. B: Effect of SNHG14 on MGMT expression in SNB19 and U251 cell lines. **P<0.01 vs the si-NC group. C, D: Sensitivity of SNB19 (C) and U251 (D) cell lines to TMZ in normoxic and hypoxic environments.**P<0.01 vs the normoxia group. E, F: Effect of SNHG14 on the sensitivity to TMZ in SNB19 (E) and U251 cell lines (F). *P<0.05 vs the si-NC group. G: Effect of SNHG14 on apoptosis of SNB19 and U251 cell lines. **P<0.01 vs the si-NC group.
2.3. LncRNA SNHG14、miR-143、MGMT之间的靶向结果
在线工具预测发现miR-143与lncRNA SNHG14和MGMT分别存在结合位点。real-time PCR结果表明:与常氧组相比,低氧组miR-143的表达显著下调;相较于si-NC组,si-SNHG14组可显著提高细胞中miR-143的丰度(均P<0.01)。Real-time PCR结果显示:NC mimics组与NC inhibitor组相比,miR-143丰度无明显变化(均P>0.05),miR-143 mimics组或miR-143 inhibitor组细胞内miR-143丰度显著上调或下调(均P<0.001)。蛋白质印迹法检测结果显示:在常氧环境下,与miR-143丰度无明显变化的NC mimics组或NC inhibitor组相比,miR-143 inhibitor组SNB19细胞中MGMT表达上调(P<0.01),而miR-143 mimics组SNB19细胞中MGMT表达下调(P<0.05);U251细胞中呈现相同趋势,且差异均具有统计学意义(均P<0.01;图3)。
图 3.
SNHG14、miR-143、MGMT之间的靶向结果
Figure 3 Result of targeting among SNHG14、miR-143、MGMT
A: Binding sites of miR-143 with SNHG14 and MGMT. B: MiR-143 levels in SNB19 and U251 cell line under normoxia and hypoxia. **P<0.01 vs the normoxia group. C: Effect of SNHG14 on miR-143 levels in SNB19 and U251 cell line. **P<0.01 vs the si-NC group. D: Efficiency of miR-143 mimics or inhibitor. ***P<0.001 vs the NC mimics group; †††P<0.001 vs the NC inhibitor group. E: Effect of miR-143 on MGMT protein levels in SNB19 and U251 cell line. *P<0.05, **P<0.01 the NC inhibitor group; †P<0.05, ††P<0.01 vs the NC mimics group.
2.4. LncRNA SNHG14、miR-143和MGMT间调控范式的验证
荧光素酶报告实验显示:与荧光素酶活性无明显差异的NC mimics组和NC inhibitor组相比,miR-143 mimics组野生型SNHG14和MGMT荧光素酶活性显著下调(均P<0.01),而miR-143 inhibitor组野生型SNHG14(P<0.01)和MGMT(P<0.05)荧光素酶活性均显著上调;突变型SNHG14和MGMT的荧光素酶活性均无明显变化(均P>0.05,图4)。
图 4.
双荧光素酶活性分析lncRNA SNHG14、miR-143和MGMT间的调控范式
Figure 4 Regulatory paradigms among lncRNA SNHG14, miR-143 and MGMT by dual luciferase activity analysis
A: Verification of regulatory relationship between lncRNA SNHG14 and miR-143; B: Verification of regulatory relationship between miR-143 and MGMT. **P<0.01 vs the NC mimics group; †P<0.05, ††P<0.01 vs the NC inhibitor group.
2.5. LncRNA SNHG14、miR-143和MGMT间ceRNA的验证
RNA结合蛋白免疫沉淀实验显示:与转染空载质粒的NC组HEK 293T细胞相比,转染有SNHG14过表达质粒的过表达lncRNA SNHG14组细胞中沉淀的AGO2蛋白上结合有更多的lncRNA SNHG14,但MGMT mRNA丰度却出现显著下降(均P<0.01,图5)。
图5.
LncRNA SNHG14、miR-143和MGMT间ceRNA的验证
Figure 5 Verification of ceRNA among lncRNA SNHG14, miR-143 and MGMT
**P<0.01 vs the NC group.
3. 讨 论
随着全球环境的不断恶化,胶质瘤发病率呈逐年增长的趋势,因此现今胶质瘤也成为中枢神经系统肿瘤研究热点领域[9]。
低氧是胶质瘤发展过程中所必须经历的微环境条件之一[4],胶质瘤细胞的异常增殖导致耗氧较高,超过了微血管所能提供的耗氧量,肿瘤微循环被扰乱,从而使实体瘤发生缺氧[10]。研究[5]发现低氧可促进胶质瘤侵袭和向预后不良表型的发展,对胶质瘤细胞产生TMZ耐药也起重要作用。本研究通过生物信息分析和功能试验证明:与正常胶质细胞相比,lncRNA SNHG14在不用胶质瘤细胞中表达均出现上调,且低氧环境可以促进lncRNA SNHG14表达,低氧环境下敲低lncRNA SNHG14可以增加胶质瘤细胞的TMZ敏感性。通过在线预测工具,发现lncRNA SNHG14、miR-143和MGMT之间可能存在ceRNA调控范式关系,再通过转染si-SNHG14,发现敲低lncRNA SNHG14可以降低miR-143丰度,增加TMZ敏感性调控关键因子MGMT丰度;而miR-143具有降低MGMT丰度的作用。最后通过双荧光素酶报告实验证实了lncRNA SNHG14、miR-143和MGMT之间的靶向关系,进一步验证低氧下胶质瘤细胞中表达上调的lncRNA SNHG14可能介导miR-143/MGMT轴,因而可参与胶质瘤细胞产生TMZ耐药。
近年来随着测序技术的发展,越来越多的胶质瘤中表达失调的lncRNA被发现,这些lncRNA可通过沉默或激活基因,参与胶质瘤的发生和发展[11-12]。目前,lncRNA SNHG14的促癌作用在多种肿瘤中已被揭示,如外泌体lncRNA SNHG14会促进乳腺癌细胞产生曲妥珠单抗耐药。lncRNA-SNHG14/miR-5590-3p/锌指E-盒结合同源异形盒-1正反馈环通过激活PD-1/PD-L1而促进弥漫性大B细胞淋巴瘤的进展和免疫逃避[13]。在胶质瘤相关研究[14]中,lncRNA SNHG14也是胶质瘤进展的危险因素,增加lncRNA SNHG14稳定性可增加裸鼠体内异位移植瘤的生长,缩短裸鼠的存活时间。此外,有研究[15]发现DNA甲基化介导的lncRNA-SNHG12激活可以促进胶质母细胞瘤对TMZ的耐药。本研究在已有研究报道基础上,进一步探讨低氧下lncRNA SNHG14在胶质瘤TMZ耐药中的功能作用以及具体机制,明确低氧下lncRNA SNHG14/miR-143/MGMT轴是胶质瘤产生TMZ耐药的关键。而MGMT负责TMZ诱导的主要毒性DNA加合物O6-甲基鸟嘌呤损伤的直接修复,因此MGMT是胶质瘤TMZ耐药和复发的标志物[7, 16]。
结合本研究结果和上述研究报道,低氧下胶质瘤细胞中高表达的lncRNA SNHG14通过靶向miR-143,解除miR-143对MGMT的抑制,从而导致胶质瘤产生TMZ耐药。本研究通过明确lncRNA SNHG14/miR-143/MGMT轴在胶质瘤TMZ耐药中的功能,不但阐明了lncRNA SNHG14在促进胶质瘤TMZ耐药中的功能和机制,为胶质瘤的诊治提供了潜在靶点,而且为TMZ在胶质瘤治疗中的联合用药方法提供了理论指导。
Http://xbyxb.csu.edu.cn
《中南大学学报(医学版)》编辑部
基金资助
国家自然科学基金(81702490)。
This work was supported by the National Natural Science Foundation of China(81702490).
利益冲突声明
作者声称无任何利益冲突。
作者贡献
赵海婷 论文构想、撰写;孟莉、廖新斌 实验相关试剂采购,数据采集;刘燚、莫鑫、龚梦琪 统计分析,绘图;廖艺玮 论文修改。所有作者阅读并同意最终的文本。
原文网址
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/202207829.pdf
参考文献
- 1. Le Rhun E, Preusser M, Roth P, et al. Molecular targeted therapy of glioblastoma[J]. Cancer Treat Rev, 2019, 80: 101896. 10.1016/j.ctrv.2019.101896. [DOI] [PubMed] [Google Scholar]
- 2. Campos B, Olsen LR, Urup T, et al. A comprehensive profile of recurrent glioblastoma[J]. Oncogene, 2016, 35(45): 5819-5825. 10.1038/onc.2016.85. [DOI] [PubMed] [Google Scholar]
- 3. Ulasov IV, Mijanovic O, Savchuk S, et al. TMZ regulates GBM stemness via MMP14-DLL4-Notch3 pathway[J]. Int J Cancer, 2020, 146(8): 2218-2228. 10.1002/ijc.32636. [DOI] [PubMed] [Google Scholar]
- 4. Chan N, Milosevic M, Bristow RG. Tumor hypoxia, DNA repair and prostate cancer progression: new targets and new therapies[J]. Future Oncol, 2007, 3(3): 329-341. 10.2217/14796694.3.3.329. [DOI] [PubMed] [Google Scholar]
- 5. Liu P, Zhang H, Wu X, et al. Tf-PEG-PLL-PLGA nanoparticles enhanced chemosensitivity for hypoxia-responsive tumor cells[J]. Onco Targets Ther, 2016, 9: 5049-5059. 10.2147/OTT.S108169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma[J]. N Engl J Med, 2005, 352(10): 997-1003. 10.1056/NEJMoa.043331. [DOI] [PubMed] [Google Scholar]
- 7. Oldrini B, Vaquero-Siguero N, Mu Q, et al. MGMT genomic rearrangements contribute to chemotherapy resistance in gliomas[J]. Nat Commun, 2020, 11(1): 3883. 10.1038/s41467-020-17717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beermann J, Piccoli MT, Viereck J, et al. Non-coding RNAs in development and disease: Background, mechanisms, and thera-peutic approaches[J]. Physiol Rev, 2016, 96(4): 1297-1325. 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 9. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions[J]. Neuro Oncol, 2020, 22(8): 1073-1113. 10.1093/neuonc/noaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome[J]. Cancer Metastasis Rev, 2007, 26(2): 225-239. 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 11. Liu Z, Wang X, Yang G, et al. Construction of lncRNA-associated ceRNA networks to identify prognostic lncRNA biomarkers for glioblastoma[J]. J Cell Biochem, 2020, 121(7): 3502-3515. 10.1002/jcb.29625. [DOI] [PubMed] [Google Scholar]
- 12. Liu B, Zhou J, Wang C, et al. LncRNA SOX2OT promotes temozolomide resistance by elevating SOX2 expression via ALKBH5-mediated epigenetic regulation in glioblastoma[J]. Cell Death Dis, 2020, 11(5): 384. 10.1038/s41419-020-2540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao L, Liu Y, Zhang J, et al. LncRNA SNHG14/miR-5590-3p/ZEB1 positive feedback loop promoted diffuse large B cell lymphoma progression and immune evasion through regulating PD-1/PD-L1 checkpoint[J]. Cell Death Dis, 2019, 10(10): 731. 10.1038/s41419-019-1886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu J, Liu X, Zheng J, et al. Lin28A promotes IRF6-regulated aerobic glycolysis in glioma cells by stabilizing SNHG14[J]. Cell Death Dis, 2020, 11(6): 447. 10.1038/s41419-020-2650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu C, Wei Y, Wang X, et al. DNA-methylation-mediated activating of lncRNA SNHG12 promotes temozolomide resistance in glioblastoma[J]. Mol Cancer, 2020, 19(1): 28. 10.1186/s12943-020-1137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen X, Zhang M, Gan H, et al. A novel enhancer regulates MGMT expression and promotes temozolomide resistance in glioblastoma[J]. Nat Commun, 2018, 9(1): 2949. 10.1038/s41467-018-05373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]