Abstract
Objective
To analyze the expressions and distributions of hypoxia-inducible factor-1α (HIF-1α), CD147, and glucose transporter 1 (GLUT1) in epidermis from psoriasis vulgaris and normal people, and to explore the associations among these proteins and their roles in hypoxic HaCaT cell line.
Methods
The expression levels of HIF-1α, CD147, and GLUT1 were determined by immunohistochemistry staining in skin biopsies from 48 psoriasis vularis patients and 33 healthy subjects. Cobalt chloride (CoCl2) was added into the culture media of HaCaT cells to mimic hypoxia while RNA interference and transfection technologies were used to explore the association among these proteins by quantitative real-time polymerase chain reaction and Western blotting. Glycolytic capacity was detected by ATP and lactate measurements.
Results
HIF-1α, CD147, and GLUT1 were highly expressed and the glycolytic capacity was increased in lesions of psoriasis vulgaris; HIF-1α upregulated the expression of CD147 and GLUT1, increased the lactate production and decreased the ATP level in CoCl2-treated HaCaT cells, while CD147 and GLUT1 directly or indirectly bound to each other.
Conclusion
Glycolytic capacity increases in the injured keratinocytes of psoriasis vulgaris, suggesting that HIF-1α, CD147, and GLUT1 are associated with glycolysis, which can be considered as the promising targets for psoriasis therapy.
Keywords: hypoxia-inducible factor-1α, glycolysis, psoriasis, CD147, glucose transporter 1
Abstract
目的
分析缺氧诱导因子1α(hypoxia-inducible factor-1α,HIF-1α)、白细胞分化抗原147(CD147)及葡萄糖转运蛋白1(glucose transporter 1,GLUT1)在寻常型银屑病皮损和正常人表皮中的分布与表达,并探索这些蛋白质之间的关联及其在缺氧的人永生化表皮细胞(HaCaT细胞)中的作用。
方法
采用免疫组织化学法分析48例寻常型银屑病患者及33例正常人皮肤活检组织中HIF-1α、CD147和GLUT1蛋白质的表达;在HaCaT细胞系中运用氯化钴诱导缺氧、RNA干扰和转染技术后,通过实时RT-PCR和蛋白质印迹法探索蛋白质间的关联;通过检测ATP和乳酸浓度反映皮肤组织和HaCaT细胞的糖酵解能力。
结果
寻常型银屑病皮损中HIF-1α,CD147,GLUT1的表达增加及糖酵解能力增强;在诱导HaCaT细胞缺氧的过程中,HIF-1α使CD147和GLUT1的表达增加,同时乳酸浓度升高及ATP浓度降低,而CD147和GLUT1之间也存在直接或间接的结合。
结论
糖酵解能力在寻常型银屑病皮损中增强,提示与其有关的HIF-1α,CD147,GLUT1可作为银屑病潜在的治疗靶点。
Keywords: 缺氧诱导因子1α, 糖酵解, 银屑病, CD147, 葡萄糖转运蛋白1
Psoriasis is a commom chronic skin disease with a incidence rate between 2% and 3%. It is characterized by hyperproliferation, abnormal differentiation, inflam-matory cell infiltration, and dilated microvessels. Its pathomechanism is complicated and still being explored. Gradually increased epidemiological studies suggest psoriasis is associated with serious comorbidities, including malignant tumors[1-2] like lymphoma, digestive system carcinoma, and so on. Molecules closely related with tumors such as tumor necrosis factor-α (TNF-α), matrix metalloproteinases (MMPs), and intercellular adhesion molecule-1 (ICAM-1) are also highly expressed in psoriasis. Antineoplastic drugs such as methotrexate and bimolane can be used for psoriasis. These evidences imply that the growth microenvironment of keratinocytes in psoriatic lesions is similar to that of tumor cells, and other organs might be disfunction in the microenvironment affected by psoriasis.
Warburg effect, a metabolic phenomenon best studied in a majority of cancer cells, exibits elevated glucose uptake and lactate production, regardless of oxygen availability, also known as aerobic glycolysis. It not only produces ATP faster than oxidative phosphorylation (OXPHOS), but also provides intermediates for cell growth, and becomes a well-recognized hallmark of cancer metabolism. During this process, glycolysis related proteins―glucose transporters (GLUTs) play important roles. It’s well known that GLUTs facilitate the transport of glucose over a plasma membrane. One member of GLUTs is GLUT1, which is induced by hypoxia-inducible factor-1α (HIF-1α)[3], highly expresses in various cancer cells[4-6]. HIF-1α is an oxygen sensitive subunit of HIF-1 (an α/β heterodimeric protein complex) and its expression is mainly induced under hypoxic conditions. It plays a vital role in metabolism, invasiveness, and angiogenesis in cancers. In Warburg effect, HIF-1α can increase the expressions of the transporters necessary for the entry of glucose into the cell, active the most enzymes involved in glycolytic pathway[7], decrease mitochondrial function[8], and turn into glycolysis for energy production[9-11].
It has been demonstrated that HIF-1α[12-15] and GLUT1[16] are also highly expressed in psoriatic lesions, so we suppose that in early stage of psoriasis, keratinocytes excessively proliferate under mutiple factors. Just like most cancer cells, keratinocytes may reprogram metabolism in the process of survival of the fittest.
CD147, also named extracellular matrix metalloproteinase inducer (EMMPRIN), is a multifunctional transmembrane glycoprotein belongs to immunoglobulin superfamily. It can interact with various regulators such as integrins[17], no obesity diabetes 2 (NOD2)[18], and EGF receptor[19], and is suggested to be used to determine the different cellular functions involved in a wide range of other biological activities including invasiveness, proliferation, and immunological responses, etc[20]. Our group previously confirmed that CD147 is one of psoriasis susceptibility genes[21]. It is highly expressed in psoriatic lesions and peripheral blood cells[22], which is closely related with disease activity[23-24], and may become a therapeutic target for psoriasis. CD147 is also found over-expressed in a number of cancerous specimens, and its expression gradually increases from benign tumors to malignancy in solid tumors[25-26]. Ke et al[27] demonstrated the upregulation of CD147 could be induced under hypoxia, which is mainly mediated by a combined effect of transcription factor HIF-1 and specificity protein 1 (Sp1) on the activation of CD147 promoter in epithelial solid tumors. It also has been demonstrated that the silencing of CD147 can decrease glycolytic rate and lactate efflux in carcinoma cell line, indicating that CD147 is essential in tumor glycolysis[28-30].
The aim of the present study is to analyze the expressions and distributions of key proteins mentioned above that related to psoriasis and glycolysis in psoriasis vulgaris or normal human epidermis, and to explore the relationship among these proteins and their roles in hypoxic HaCaT cell line.
1. Materials and methods
1.1. Cell culture
The spontaneously immortalized HaCaT cell line was purchased from American Type Culture Collection (ATCC, USA). Cells were maintained in DMEM medium (Thermo Scientific, MA, USA) with 10% fetal bovin serum (Thermo Scientific, MA, USA) and 100 U/mL penicillin/streptomycin (Dingguo, Beijing, China) at 37 ℃ in a humidified incubator (Thermo Scientific, MA, USA) under 5% CO2. When the cells were at 80%-90% confluence, they were washed three times with phosphate-buffered saline (PBS) and exchanged with fresh medium. Then 250 μmol/L CoCl2 (Sigma, CA, USA) or dimethyl sulfoxide (New England Biolabs, MA, USA) was added to induce hypoxia or be a negative control. The cells were harvested for further experiments.
1.2. Immunohistochemistry and immunofluorescence
Paraffin-embeded tissue blocks of primary lesions and normal skins were sectioned at a thickness of 5 μm, heated at 60 ℃ for 30 min, dewaxed in turpentine, rehydrated through graded concentrations of alcohol, and incubated in 3% hydrogen peroxide for 10 min to block endogenous peroxidase. Antigen retrieval was achived by microwaving tissue sections in 0.01 mol/L citrate buffer solution (pH 6.0) for 8 min (Galanz micro-oven, 800 W), and cooled for 20 min at room temperature. The sections were incubated overnight at 4 ℃ with primary antibodies, including HIF-1α (1꞉100, Epitomics, CA, USA), CD147 (1꞉300, Abcam, MA, USA), and GLUT1 (1꞉400, Abcam, MA, USA). Negative controls were incubated with PBS instead of primary antibodies. A 2-step plus poly-HRP anti-mouse/rabbit IgG detection system (Zhongshan Co., Beijing, China) was performed according to manufacturer’s instructions, using diaminobenzidine chromogen (Zhongshan Co., Beijing, China) as substrate for visualization. Sections were counterstained with hematoxylin and mounted in neutral balsam.
For immunofluorescence, paraffin-embeded tissue blocks from primary lesions were incubated overnight with primary rabbit anti-CD147 (1꞉100, Santa Cruz, California, USA) and mouse anti-GLUT1 (1꞉300, Abcam, MA, USA) at 4 ℃, visualized by incubation in Alexa Fluor® conjugated secondary antibodies (1꞉150, Invitrogen, CA, USA) for 2 h at room temperature,and covered with VECTASHIELDR mounting medium containing DAPI (Vector Laboratories, CA, USA) to preserve the fluorescence and counterstain nuclei. Images were acquired through an inverted fluorescence microscope (Leica, Solms, Germany).
1.3. Immunohistochemical scoring
Two independent dermatological pathologists performed microscopic examination without any prior knowledge of each patient’s clinical information. The staining cells were mainly in basal layer and spinous layer in epidermis. Both layers were evaluated for the proportion and intensity of staining cells. Staining intensity was graded as follows: 0 point was negative, 1 point was light, 2 points was moderate, and 3 points was intensive. The proportion of stained cells in each layer was graded as follows: 0 point was non-staining cells, 1 point was less than 25% stained cells, 2 points was 25%-50% stained cells, and 3 points was more than 50% stained cells. An immunostaining-intensity-distribution index was then computed for each specimen as follows: the score for the overall staining intensity was multiplied by the score of the proportion of staining cells in each layer, and the result scores of both layers then added. The average score (0-18) was classified into 0 (0), 1 (<6), 2 (6-12), and 3 (>12). 0 and 1 were considered as weak expression and 2 and 3 were as high expression.
1.4. Western blotting
At the end of treatments, HaCaT cells were harvested and solubilized in lysis buffer (containing 1% Triton X-100, 50 mmol/L Tris balanced to pH 7.4, 150 mmol/L NaCl, 10 mmol/L EDTA, 100 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L phenylmethylsulfonyl fluoride, and 2 mg/L aprotinin) at approximately 80% confluence, left on ice for 30 min, centrifuged at 12 000 g for 15 min at 4 ℃. The supernatant protein concentration was relatively quantified by the BCA protein assay (Santa Cruz, CA, USA). Cell lysates were mixed with lysis buffer and loading buffer, and boiled at 95 ℃ for 10 min, loaded equal mount proteins into 10% polyacrylamide gel for seperation and transferred to polyvinylidene difluoride membrane (Millipore, MA, USA). The membrane was blocked in 5% fat-free milk, incubated with indicating primary antibodies: HIF-1α (1꞉1 000), CD147 (1꞉1 000), GLUT1 (1꞉800, Santa Cruz, USA), washed three times and incubated with HRP-linked secondary antibodies (1꞉10 000, Sigma, USA), visulized by chemiluminescence detection system (Bio-Rad, CA, USA). Protein levels in each lane were normalized to that of β-actin (1꞉10 000, Sigma, USA).
1.5. Immunoprecipitation
Hypoxia HaCaT cells were harvested and solubilized in a special immunoprecipitation (IP) lysis buffer on ice for 30 min, centrifuged at 6 000 r/min for 4 min at 4 ℃. Supernatant containing 100 μg of protein was added to 1 μg of CD147, GLUT1 or control IgG and then with protein A+G agarose beads. The specimen was incubated overnight at 4 ℃ with shaking, washed three times with lysis buffer, added lysis buffer and 5×loading buffer, boiled in SDS sample buffer and loaded into a 10% SDS-polyacrylamide gel. The immuno-precipitates were analyzed by Western blotting.
1.6. Tissue source
A total of 48 paraffin-embedded archival tissue blocks from patients (aged 18-60 years) with psoriasis vulgaris were obtained between 2012 and 2013, and 33 normal skin tissue blocks were derived from uninvolved skin of people under mole resection at Department of Dermatology in Xiangya Hospital of Central South University (Changsha, Hunan, China). The involved skin and uninvolved skin were biopsied for 5 mm according to the criterion that they had not received any treatment in the last 4 weeks before taking biopsies, and normal skin samples were derived from univolved skin of mole resection. Informed consent was obtained from each patient. All experimental procedures were approved by the Regional Committee for Medical Research Ethics (No. 201212087).
1.7. ATP assay
The concentration of ATP was measured by the luciferin-luciferase method following the protocol of ATP detection kit (Beyotime, Beijing, China). Treated HaCaT cells and skin epidermis [normal skin epidermis (N), psoriatic non-lesional epidermis (PN) and psoriatic lesional epidermis (PS)] were grinded with 200 μL lysis buffer on ice, centrifuged at 12 000 g for 5 min at 4 ℃. The supernatant protein concentration was relatively quantified by the BCA protein assay (Santa Cruz, USA), in 96-well plates, 100 μL of each supernatant was mixed with 100 μL ATP detection working dilution. Luminance was measured by a enzyme microplate reader (Beckman DTX880, CA, USA). The standard curve of ATP concentration was prepared from a known amount (1 nmol/L-1 μmol/L). Total ATP levels were expressed as nmol/mg protein. All the experiments were carried out with triplicates.
1.8. Lactate concentration measurements
Lactate was detected following the protocol of lactate colorimetric assay kit (Biovision, CA, USA). Equal culture medium of differently treated HaCaT cells and skin epidermis (N, PS, PN) were added to assay buffer, centrifuged three times to remove insoluble materials, and transferred 50 μL supernatant to 96-well plates, added 50 μL reaction mix to each well, incubated the reaction for 30 min at room temperature, measured at OD 450 nm in a microplate reader. Standard curves were also generated and the protein concentration of each group was determined using the BCA protein assay (Santa Cruz, USA). Total lactate concentration was expressed as nmol/mg protein. All the experiments were carried out with triplicates.
1.9. Cell transfection
For the transient transfection, cells were seeded in 3.5 cm culture dishes. At 50%-70% confluence, siRNA(HIF-1α siRNA: sense 5'-UAUUUGUUCACAUUAUC-AGAA-3', antisense 5'-CUGAUAAUGUGAACAAAU-ACA-3'; CD147 siRNA: sense 5'-CAUACACUUCC-UUCUUUUUUA-3', antisense 5'-AAAAAGAAGGAA-GUGUAUGAU-3'; GLUT1 siRNA: sense 5'-AUAGAA-GACAGCGUUGAUGCC-3', antisense 5'-CAUCAAC-GCUGUCUUCUAUUA-3'; or scramble siRNA: sense 5'-UUCUCCGAACGUGUCACGUTT-3', antisense 5'-ACGUGACACGUUCGGAGAATT-3' as negative controls) synthesized by GenePharma Company (Shanghai, China), was transfected with RNAi-max (Invitrogen, CA, USA) according to manufacturers’ instructions.
In same conditions, the HaCaT cell line was transfected with the pGL3-basic plasmids (Promega, WI, USA) containing the entire EMMPRIN or GLUT1 sequence using TurboFect Transfection Reagent (Thermo Scientfic, USA) according to manufacturers’ instructions. Control transfectants were generated by transfection with empty pGL3 vector.
1.10. Real-time RT-PCR
Total RNA was extracted using the TRIzol (Invitrogen, CA, USA) procedure. First-strand cDNA was synthesized from 2 μg RNA using Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, USA) according to manufacturers’ instructions. The sequences of PCR primers were as follows: GAPDH sense 5'-CTGCACCACCAACTGCTTAG-3'; antisense 5'-AG-GGAG-GGGAGCCGGCTGTC-3'; HIF-1α sense 5'-CCATTA-GAAAGCAGTTCCGC-3', antisense 5'-TG-GGTAGGA-GATGGAGATGC-3'; CD147 sense 5'-GCAGCGGTT-GGAGGTTGT-3', antisense 5'-AGCC-AGGGATGCCC-AGGAAGG-3'; GLUT1 sense 5'-CTTCTCTGTGGG-CCTTTTCGT-3', antisense 5'-CAA-AGGACTTGCCC-AGTTTCG-3'. Real-time PCR was performed using the SYBR Green PCR Master Mix kit (Takara Bio, Dalian, China) and ABI7500 real time system (Applied Biosystems, CA, USA). Each sample was run in triplicate and in 3 independent experiments. All data were normalized to GAPDH expression comparing different groups determined by the ddCt comparative threshold (ΔΔCt) method. For all real-time PCRs, the conditions were as follows: 40 cycles, 94 ℃ for 25 s, 60 ℃ for 25 s, and 72 ℃ for 25 s.
1.11. Statistical analysis
All statistical analyses were performed using the SPSS 17.0 statistical software package (SPSS, IL, USA) and the GraphPad Prism 5.01 (GraphPad Software, CA, USA) statistical packages. Spearman’s rank correlation coefficient test was used to evaluate the pairwise association between different markers. χ2 test was used to detect the relationship between each marker and psoriasis. Significance was determined by P<0.05.
2. Results
2.1. Expressions of HIF-1α, CD147, and GLUT1 in normal and psoriatic epidermis
HIF-1α was predominantly expressed in the nuclear throughout the psoriatic epidermis, while mainly in the basal layer in normal epidermis (Figure 1A, 1D, 1G, 1J). CD147 (Figure 1B, 1E, 1H, 1K) and GLUT1 (Figure 1C, 1F, 1I, 1L) showed mainly membranous expression in basal and spinous layer in psoriatic epidermis and almost in basal layer in normal epidermis. The differences in the expressions of HIF-1α, CD147 and GLUT1 between normal and psoriatic human epidermis were significant (P<0.01, Table 1).
Figure 1. Expressions of HIF-1α, CD147, and GLUT1 protein in normal human skin (NHS) and psoriasis (immunohistochemistry, ×200, ×400, respectively) .
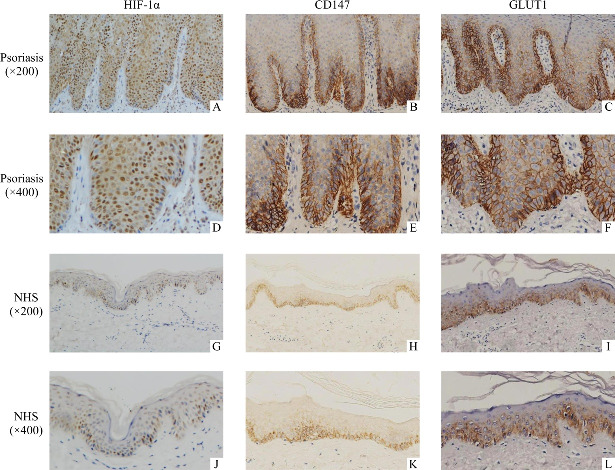
A, D, G, J: HIF-1α is expressed in nucleus; B, E, H, K: CD147 is expressed in membranes; C, F, I, L: GLUT1 is expressed in membranes. Positive signals appear brown; blue counter staining with hematoxylin.
Table 1.
Differences in expressions of HIF-1α, CD147, and GLUT1 between psoriatic and normal epithelium
| Groups | n | Expression | Protein level | ||
|---|---|---|---|---|---|
| HIF-1α | CD147 | GLUT1 | |||
| Psoriasis | 48 | Weak | 14 | 14 | 2 |
| High | 34 | 34 | 46 | ||
| Normal | 33 | Weak | 10 | 28 | 12 |
| High | 23 | 5 | 21 | ||
| P | <0.001 | 0.001 | <0.001 | ||
2.2. Correlations among expressions of HIF-1α,CD147 and GLUT1 in psoriatic epidermis
The result of Spearman’s rank test analyses for the immunohistochemical scores in psoriatic epithelium showed that HIF-1α expression was positively correlated with the expressions of CD147 and GLUT1 (r>0,P<0.05), and the CD147 expression was also positively correlated with the GLUT1 expression (r>0, P<0.05).
2.3. HIF-1α induces the expressions of CD147 and GLUT1 in HaCaT cells under hypoxia
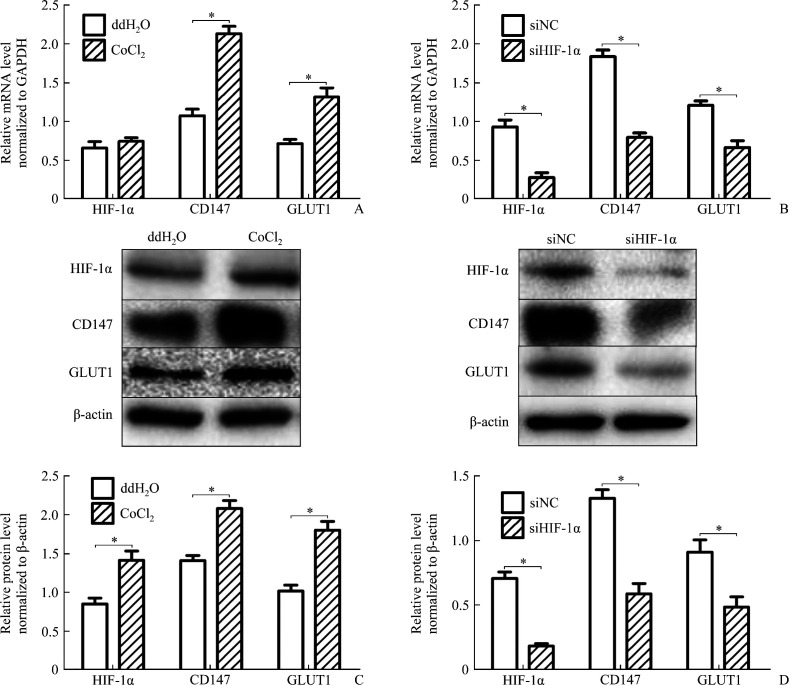
The results of real-time RT-PCR and Western blotting showed a clear induction of HIF-1α, CD147, and GLUT1 at both mRNA and protein level (Figure 2), while HIF-1α mRNA level had no significant difference between hypoxia and normoxia (Figure 2A, 2C). After siRNA against HIF-1α was used to specifically knock down HIF-1α, then treated cells with same hypoxia conditions, the results of real-time PCR and Western blotting showed a reduction of CD147 and GLUT1 (Figure 2B, 2D).
Figure 2. CD147 and GLUT1 were mediated by HIF-1α under hypoxia .
A: HaCaT cells were treated with 250 μmol/L CoCl2 for 12 h or ddH2O without previous siRNA transfection and transcripts of HIF-1α, CD147, and GLUT1 were quantified by real-time RT-PCR. B: HaCaT cells were treated with 250 μmol/L CoCl2 for 12 h or ddH2O with previous siRNA transfection [siRNA against human HIF-1α or scrambled siRNA (siNC) for 48 h], transcripts of HIF-1α, CD147, and GLUT1 were quantified by real-time RT-PCR. C: Western blotting was performed with indicated antibodies for HaCaT cells only treated with 250 μmol/L CoCl2 or ddH2O. D: Western blotting was performed with indicated antibodies for HaCaT cells transfected with siRNA in hypoxia induced by 250 μmol/L CoCl2. *P<0.05.
2.4. Co-localization and interaction of CD147 and GLUT1
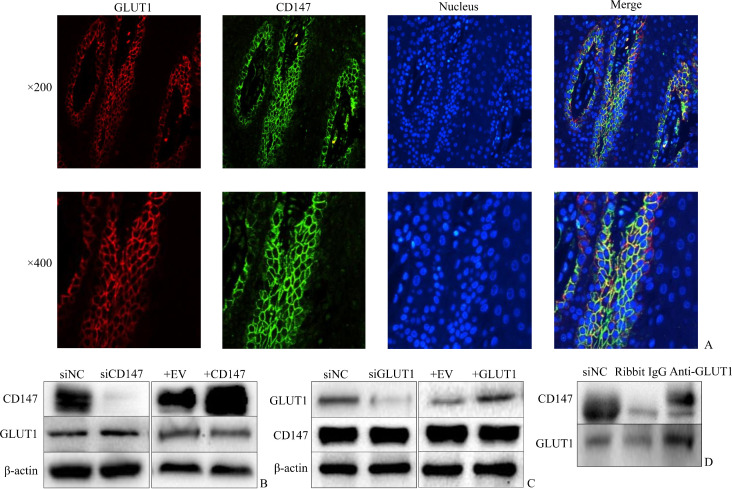
The immunohistochemical results of CD147 and GLUT1 in normal and psoriatic human epidermis showed that the expressions and locations of these two proteins were nearly the same. Then immuno-fluorescence was performed in psoriatic epidermis in order to show the location more clearly (Figure 3A). Co-localization of proteins, as revealed by yellow immunostaining of merged images, was evident in the epidermis.
Figure 3. Interaction between CD147 and GLUT1.
A: Co-localization of CD147 and GLUT1 in psoriatic epidermis (immunofluorescence, ×200, ×400, respectively). B: HaCaT cells were transfected with siRNA against human CD147 and scrambled siRNA as negative control (siNC), or CD147 cDNA plasmid and empty vector (EV), after 48 h, then treated with 250 μmol/L CoCl2 for 12 h. Protein expressions of GLUT1 and CD147 were analyzed by Western blotting. C: HaCaT cells were transfected with siRNA against human GLUT1 or GLUT1 cDNA plasmid (negative controls and hypoxia condition as described before). Western blotting analysis was performed. D: HaCaT cells were treated with CoCl2 for 12 h, and cell lysates were performed co-IP. Lysates were immunoprecipitated with antibodies of GLUT1 and rabbit IgG, and subjected to antibodies of CD147 and GLUT1 for immunoblotting. Anti-GLUT1 isolated CD147 while rabbit IgG did not.
The results of Western blotting showed no changes in expressions of CD147 and GLUT1 proteins (Figure 3B, 3C).
Next co-IP was performed in hypoxic HaCaT cells, cell lysates were then analyzed by co-IP using the polyclonal anti-GLUT1 antibody. As shown in Figure 3D, GLUT1 is co-IPed with CD147. However, it is failed to use the anti-CD147 antibody to pull down GLUT1, probably because monoclonal CD147 antibody only binds part of the mutiple epitopes of GLUT1, and the protein level isn’t enough to visulize as a band.
2.5. ATP level was associated with expression of HIF-1α in both HaCaT cells and human epidermis
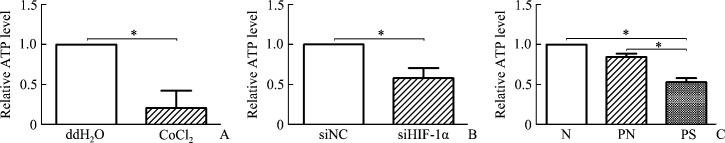
We have confirmed that HIF-1α induced expressions of CD147 and GLUT1 in HaCaT cells under hypoxia. Next, ATP level was detected in identically treated HaCaT cells. It showed that ATP level significantly decreased in hypoxic HaCaT cells induced by CoCl2 compared with negative control cells (P<0.05,Figure 4A), while in hypoxic siHIF-1α HaCaT cells, ATP level decreased in a greater degree compared with hypoxic siNC cells (P<0.05, Figure 4B). At last, ATP level was detected in equal weight and body surface area of human epidermis (P<0.05), the results showed ATP level significantly decreased in psoriatic epidermis, while no significant change was found between non-lesional and normal epidermis (P<0.05, Figure 4C).
Figure 4. Relative level of ATP in hypoxic HaCaT cells and different kinds of skin epidermis.
A: Cellular ATP level in hypoxic HaCaT cells induced by 250 μmol/L CoCl2 for 12 h, or in negative control with ddH2O. B: HaCaT cells were treated with 250 μmol/L CoCl2 for 12 h with previous siRNA transfection [siRNA against human HIF-1α or scrambled siRNA (siNC) for 48 h]. Cellular ATP level was detected. C: Cellular ATP level in normal skin epidermis (N), psoriatic non-lesional epidermis (PN), and psoriatic lesional epidermis (PS). Values present as mean±SD of 3 independent experiments. *P<0.05.
2.6. Lactate level was associated with expression of HIF-1α both in HaCaT cells and human epidermis
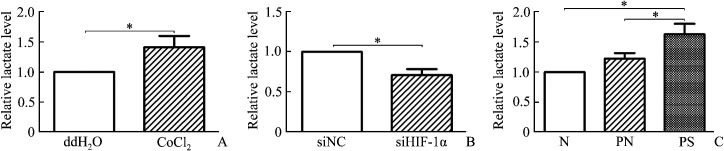
Under the same treatments with ATP detection, lactate level was detected in HaCaT cells and human epidermis. The results showed that lactate level significantly increased in medium of hypoxic HaCaT cells induced by CoCl2 compared with negative control cells (P<0.05, Figure 5A), and significantly decreased in medium of hypoxic siHIF-1α HaCaT cells than that of hypoxic siNC cells (P<0.05, Figure 5B). Also in human epidermis, lactate level significantly increased in psoriatic epidermis, and no significant change was found between non-lesional and normal epidermis (P<0.05, Figure 5C).
Figure 5. Relative level of lactate in hypoxic HaCaT cells and different kinds of skin epidermis.
A: Cellular lactate level in hypoxic HaCaT cells induced by 250 μmol/L CoCl2 for 12 h, or in negative control with ddH2O. B: HaCaT cells were treated with 250 μmol/L CoCl2 for 12 h with previous siRNA transfection [siRNA against human HIF-1α or scrambled siRNA (siNC) for 48 h]. Cellular lactate level was detected. C: Cellular lactate level in normal skin epidermis (N), psoriatic non-lesional epidermis (PN), and psoriatic lesional epidermis (PS). Values present as the mean±SD of 3 independent experiments. *P<0.05.
3. Discussion
In 4 classifications of psoriasis, psoriasis vulgaris is the most common form. Regardless of form, psoriasis is always recurrent and refractory, and brings an enormous physical and psychological distress to patients. Multiple factors such as genetic and environmental factors are involved in its complicated pathomechanism[31-33].
In psoriatic lesion, the proliferation of keratinocytes increases oxygen consumption and epidermal thickening could lead to impaired oxygen supply. It’s resonable to speculate that as the oxygen level decreases, the generation of ATP shifts from the OXPHOS pathway to the oxygen-independent pathway of glycolysis. HIF-1α and GLUT1 were significantly higher expressed in psoriasis vulgaris lesional epidermis than in normal epidermis. We also found that ATP level decreases and lactate production increases in psoriatic epidermis, indicating that psoriatic keratinocytes indeed mainly metabolize through glycolytic pathway. CD147, a multifaceted protein involved in the growth, survival, invasion, angiogenesis, and metastasis of tumors, mainly via MMP induction and interaction with various ligands involved in the neoplastic cell behavior, while recently Marchiq et al[34] proposed that the strongest pro-tumoural action of CD147 was mediated through the control of fermentative glycolysis, and CD147 could play a role as a cell recognition molecule in a cell dependant manner. The studied evidence for CD147 participation in glycolysis is that it controls tumor metabolism by facilitating lactic acid export through the two monocarboxylate transporters MCT1 and hypoxia-inducible MCT4, and serves as a chaperone required for the plasma membrane translocation of MCTs[35-37]. Ke et al[27, 30] also confirmed that hypoxia-induced CD147 upregulation significantly promoted glycolytic switch and tumor cell survival and invasion.We’ve already confirmed CD147 is closely related to psoriasis both in genetics and molecular mechanisms[22, 38-39]. In this study, we demonstrated that CD147 was strongly related to HIF-1α and GLUT1 in lesional epidermis and hypoxic HaCaT cell line, although not experimented in primary psoriatic keratinocytes, CD147 co-localized with GLUT1 in psoriatic cell membranes and interacted with each other in CoCl2 induced hypoxic HaCaT cells under HIF-1α control. Our study offers a new insight of CD147 involving in glycolysis, that may make contribution to psoriasis pathogenesis.
HIF-1α is found to drive the expressions of hundreds of genes involved in many biological processes, including glycolysis, angiogenesis, survival/apoptosis, migration, invasion, and metastasis[40-41]. In psoriasis, the main mechanism about HIF-1α is that it could differentially promote angiogenesis by upregulating vascular endothelial growth factor (VEGF)[42-46] and its receptor Flt-1[45], and VEGF upregulation has been demonstrated to be an early and important step in the pathophysiology of psoriasis[47]. In our study, we verified a significant increase of HIF-1α protein level after CoCl2 induction, probably CoCl2 reduces hydroxylase activity, allowing HIF-1α to escape from both destruction and blockade of coactivator recruitment, and enter the nucleus to heterodimerise with HIF-1β, further recruiting CBP/p300 co-activator, thereby upregulating downstream genes involved in cellular and systemic hypoxia responses[48]. We found that CD147 and GLUT1 were regulated by HIF-1α and interacted with each other, but we did not observe a notable change in protein expression of GLUT1 or CD147 after one another’s expression was changed. It remains unclear whether CD147 binds to GLUT1 directly or indirectly through other molecules and what the specific roles of this interaction in psoriasis are. Our team previously confirmed that CD147 interactes with GLUT1 at D105-199, CD147 silencing downregulates GLUT1 level via inhibiting PI3K/Akt signaling and decreases glucose uptake in melanoma[49]. From the above, we speculate that in psoriasis, the metabolism of proliferated cell mainly depends on glycolysis: regional hypoxia induces HIF-1α expression, increases GLUT1 and CD147 expressions, and they consequently dependently or independently participate in the highly complex network involving overlapping mechanisms contributed to pathogenesis of psoriasis.
Skin has been suggested to be a hypoxic tissue[12], and hypoxia is further intensified in psoriasis, that is most likely related to the metabolic switch. While in Warburg effect, cells show increased rates of glycolysis despite the presence of adequate O2 levels due to irreversible damages to OXPHOS[50-51], environmental acidosis[52], etc. Though the drive factors of glycolysis are possibly different in cancers and psoriasis, the most probable purpose is to generate ATP faster and provide carbon skeletons for biosyntheses[53]. Since active glycolysis may play a crucial role in psoriatic pathomechanisms, it provides new insights for therapies of psoriasis, but we should also concern on the side effects in normal tissues that also rely on glycolysis. However, to date, no selective HIF-1α inhibitor has been clinically approved, partially due to the requirements of targeting protein-protein interactions without affecting other pathways. Needless to say, highly selective GLUT1 and CD147 inhibitors are very promising in psoriasis therapy although no direct inhibitors have been found yet. Finally, combination strategies may be more effective in inhibiting metabolic changes as well as simultaneously inhibiting other important processes such as angiogenesis.
In summary, our study showed demonstrated that glycolytic capacity increases in lesional keratinocytes of psoriasis vulgaris, and confirms that CoCl2 -mimicked hypoxia in HaCaT cells leads to up-regulate HIF-1α protein expression, further increase CD147 and GLUT1 expressions, thereby elevate cell glycolytic capacity. These results indicate the potential of glycolysis associated proteins as promising targets in psoriasis therapy. Future studies are needed to elucidate the specific roles of these proteins participated in the metabolic change in psoriasis.
Funding Statement
This work was supported by the National Natural Science Foundation (81974478, 81830096) and the Department of Science and Technology of Hunan Province (2018SK2092), China.
Conflict of Interest
The authors declare that they have no conflicts of interest to disclose.
Note
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/202104333.pdf
References
- 1. Haider AS, Peters SB, Kaporis H, et al. Genomic analysis defines a cancer-specific gene expression signature for human squamous cell carcinoma and distinguishes malignant hyperproliferation from benign hyperplasia[J]. J Invest Dermatol, 2006, 126(4): 869-881. [DOI] [PubMed] [Google Scholar]
- 2. Boffetta P, Gridley G, Lindelöf B. Cancer risk in a population-based cohort of patients hospitalized for psoriasis in Sweden[J]. J Invest Dermatol, 2001, 117(6): 1531-1537. [DOI] [PubMed] [Google Scholar]
- 3. Chen C, Pore N, Behrooz A, et al. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia[J]. J Biol Chem, 2001, 276(12): 9519-9525. [DOI] [PubMed] [Google Scholar]
- 4. Fan JY, Yang Y, Xie JY, et al. MicroRNA-144 mediates metabolic shift in ovarian cancer cells by directly targeting Glut1[J]. Tumour Biol, 2016, 37(5): 6855-6860. [DOI] [PubMed] [Google Scholar]
- 5. Fan R, Hou WJ, Zhao YJ, et al. Overexpression of HPV16 E6/E7 mediated HIF-1α upregulation of GLUT1 expression in lung cancer cells[J]. Tumour Biol, 2016, 37(4): 4655-4663. [DOI] [PubMed] [Google Scholar]
- 6. Stewart PA, Parapatics K, Welsh EA, et al. A pilot proteogenomic study with data integration identifies MCT1 and GLUT1 as prognostic markers in lung adenocarcinoma[J]. PLoS One, 2015, 10(11): e0142162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha[J]. Genes Dev, 1998, 12(2): 149-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papandreou I, Cairns RA, Fontana L, et al. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption[J]. Cell Metab, 2006, 3(3): 187-197. [DOI] [PubMed] [Google Scholar]
- 9. Ong SG, Lee WH, Theodorou L, et al. HIF-1 reduces ischaemia-reperfusion injury in the heart by targeting the mitochondrial permeability transition pore[J]. Cardiovasc Res, 2014, 104(1): 24-36. [DOI] [PubMed] [Google Scholar]
- 10. Zhao T, Zhu Y, Morinibu A, et al. HIF-1-mediated metabolic reprogramming reduces ROS levels and facilitates the metastatic colonization of cancers in lungs[J]. Sci Rep, 2014, 4: 3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao L, Mao Y, Zhao Y, et al. Role of multifaceted regulators in cancer glucose metabolism and their clinical significance[J]. Oncotarget, 2016, 7(21): 31572-31585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenberger C, Solovan C, Rosenberger AD, et al. Upregulation of hypoxia-inducible factors in normal and psoriatic skin[J]. J Invest Dermatol, 2007, 127(10): 2445-2452. [DOI] [PubMed] [Google Scholar]
- 13. Tovar-Castillo LE, Cancino-Díaz JC, García-Vázquez F, et al. Under-expression of VHL and over-expression of HDAC-1, HIF-1alpha, LL-37, and IAP-2 in affected skin biopsies of patients with psoriasis[J]. Int J Dermatol, 2007, 46(3): 239-246. [DOI] [PubMed] [Google Scholar]
- 14. Vasilopoulos Y, Sourli F, Zafiriou E, et al. High serum levels of HIF-1α in psoriatic patients correlate with an over-expression of IL-6[J]. Cytokine, 2013, 62(1): 38-39. [DOI] [PubMed] [Google Scholar]
- 15. Ioannou M, Sourli F, Mylonis I, et al. Increased HIF-1 alpha immunostaining in psoriasis compared to psoriasiform dermatitides[J]. J Cutan Pathol, 2009, 36(12): 1255-1261. [DOI] [PubMed] [Google Scholar]
- 16. Abdou AG, Maraee AH, Eltahmoudy M, et al. Immunohistochemical expression of GLUT-1 and Ki-67 in chronic plaque psoriasis[J]. Am J Dermatopathol, 2013, 35(7): 731-737. [DOI] [PubMed] [Google Scholar]
- 17. Li Y, Wu J, Song F, et al. Extracellular membrane-proximal domain of HAb18G/CD147 binds to metal Ion-dependent adhesion site (MIDAS) motif of integrin β1 to modulate malignant properties of hepatoma cells[J]. J Biol Chem, 2012, 287(7): 4759-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Till A, Rosenstiel P, Bräutigam K, et al. A role for membrane-bound CD147 in NOD2-mediated recognition of bacterial cytoinvasion[J]. J Cell Sci, 2008, 121(Pt 4): 487-495. [DOI] [PubMed] [Google Scholar]
- 19. Grass GD, Tolliver LB, Bratoeva M, et al. CD147, CD44, and the epidermal growth factor receptor (EGFR) signaling pathway cooperate to regulate breast epithelial cell invasiveness[J]. J Biol Chem, 2013, 288(36): 26089-26104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners[J]. J Biochem, 2016, 159(5): 481-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression[J]. Thromb Haemost, 2005, 93(2): 199-204. [DOI] [PubMed] [Google Scholar]
- 22. Lu H, Kuang YH, Su J, et al. CD147 is highly expressed on peripheral blood neutrophils from patients with psoriasis and induces neutrophil chemotaxis[J]. J Dermatol, 2010, 37(12): 1053-1056. [DOI] [PubMed] [Google Scholar]
- 23. Arendt BK, Walters DK, Wu X, et al. Increased expression of extracellular matrix metalloproteinase inducer (CD147) in multiple myeloma: role in regulation of myeloma cell proliferation[J]. Leukemia, 2012, 26(10): 2286-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kanekura T, Chen X. CD147/basigin promotes progression of malignant melanoma and other cancers[J]. J Dermatol Sci, 2010, 57(3): 149-154. [DOI] [PubMed] [Google Scholar]
- 25. Agrawal SM, Silva C, Wang J, et al. A novel anti-EMMPRIN function-blocking antibody reduces T cell proliferation and neurotoxicity: relevance to multiple sclerosis[J]. J Neuroinflammation, 2012, 9: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng HC, Takahashi H, Murai Y, et al. Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis[J]. Br J Cancer, 2006, 95(10): 1371-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ke X, Fei F, Chen YK, et al. Hypoxia upregulates CD147 through a combined effect of HIF-1α and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors[J]. Carcinogenesis, 2012, 33(8): 1598-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slomiany MG, Grass GD, Robertson AD, et al. Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells[J]. Cancer Res, 2009, 69(4): 1293-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneiderhan W, Scheler M, Holzmann KH, et al. CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models[J]. Gut, 2009, 58(10): 1391-1398. [DOI] [PubMed] [Google Scholar]
- 30. Ke X, Chen YK, Wang P, et al. Upregulation of CD147 protects hepatocellular carcinoma cell from apoptosis through glycolytic switch via HIF-1 and MCT-4 under hypoxia[J]. Hepatol Int, 2014, 8(3): 405-414. [DOI] [PubMed] [Google Scholar]
- 31. Lebwohl M. Psoriasis[J]. Lancet, 2003, 361(9364): 1197-1204. [DOI] [PubMed] [Google Scholar]
- 32. Gudjonsson JE, Elder JT. Psoriasis[M]// Goldsmith LA, Katz SI, Gilchrest BA, et al. Fitzpatrick’s dermatology in general medicine. 8th ed. New York: The McGraw-Hill Companies, 2012: 197-231. [Google Scholar]
- 33. Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis[J]. Pediatr Dermatol, 2000, 17(3): 174-178. [DOI] [PubMed] [Google Scholar]
- 34. Marchiq I, Albrengues J, Granja S, et al. Knock out of the BASIGIN/CD147 chaperone of lactate/H+ symporters disproves its pro-tumour action via extracellular matrix metalloproteases (MMPs) induction[J]. Oncotarget, 2015, 6(28): 24636-24648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirk P, Wilson MC, Heddle C, et al. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression[J]. EMBO J, 2000, 19(15): 3896-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilson MC, Meredith D, Fox JE, et al. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70)[J]. J Biol Chem, 2005, 280(29): 27213-27221. [DOI] [PubMed] [Google Scholar]
- 37. Li XF, Yu XZ, Dai D, et al. The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters[J]. Oncotarget, 2016, 7(17): 23141-23155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao S, Chen C, Liu S, et al. CD147 promotes MTX resistance by immune cells through up-regulating ABCG2 expression and function[J]. J Dermatol Sci, 2013, 70(3): 182-189. [DOI] [PubMed] [Google Scholar]
- 39. Wu LS, Li FF, Sun LD, et al. A miRNA-492 binding-site polymorphism in BSG (basigin) confers risk to psoriasis in central south Chinese population[J]. Hum Genet, 2011, 130(6): 749-757. [DOI] [PubMed] [Google Scholar]
- 40. Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression[J]. Nature, 2006, 441(7092): 437-443. [DOI] [PubMed] [Google Scholar]
- 41. Semenza GL. Targeting HIF-1 for cancer therapy[J]. Nat Rev Cancer, 2003, 3(10): 721-732. [DOI] [PubMed] [Google Scholar]
- 42. Levy AP, Levy NS, Wegner S, et al. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia[J]. J Biol Chem, 1995, 270(22): 13333-13340. [DOI] [PubMed] [Google Scholar]
- 43. Liu Y, Cox SR, Morita T, et al. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5' enhancer[J]. Circ Res, 1995, 77(3): 638-643. [DOI] [PubMed] [Google Scholar]
- 44. Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1[J]. Mol Cell Biol, 1996, 16(9): 4604-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takeda N, Maemura K, Imai Y, et al. Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1[J]. Circ Res, 2004, 95(2): 146-153. [DOI] [PubMed] [Google Scholar]
- 46. Torales-Cardeña A, Martínez-Torres I, Rodríguez-Martínez S, et al. Cross talk between proliferative, angiogenic, and cellular mechanisms orchestred by HIF-1α in psoriasis[J]. Mediators Inflamm, 2015, 2015: 607363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Detmar M. Evidence for vascular endothelial growth factor (VEGF) as a modifier gene in psoriasis[J]. J Invest Dermatol, 2004, 122(1): xiv-xv. [DOI] [PubMed] [Google Scholar]
- 48. Webb JD, Coleman ML, Pugh CW. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing[J]. Cell Mol Life Sci, 2009, 66(22): 3539-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Su J, Gao TY, Jiang MH, et al. CD147 silencing inhibits tumor growth by suppressing glucose transport in melanoma[J]. Oncotarget, 2016, 7(40): 64778-64784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pelicano H, Martin DS, Xu RH, et al. Glycolysis inhibition for anticancer treatment[J]. Oncogene, 2006, 25(34): 4633-4646. [DOI] [PubMed] [Google Scholar]
- 51. Matoba S, Kang JG, Patino WD, et al. p53 regulates mitochondrial respiration[J]. Science, 2006, 312(5780): 1650-1653. [DOI] [PubMed] [Google Scholar]
- 52. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis?[J]. Nat Rev Cancer, 2004, 4(11): 891-899. [DOI] [PubMed] [Google Scholar]
- 53. López-Lázaro M. The Warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen?[J]. Anticancer Agents Med Chem, 2008, 8(3): 305-312. [DOI] [PubMed] [Google Scholar]