Abstract
目的
胃癌是消化系统的常见癌症,患者晚期预后较差。长链非编码RNA(long non-coding RNA,lncRNA)在胃癌的发生、发展中起重要调控作用。本研究主要探讨lncRNA 114227对胃癌细胞生物学行为的影响。
方法
实验分为4组:阴性对照(NC)组、lncRNA 114227小干扰(si-lncRNA 114227)组、空载体(Vector)组、过表达载体(OE-lncRNA 114227)组。采用实时反转录聚合酶链反应(real-time reverse transcription PCR,real-time RT-PCR)检测lncRNA 114227在胃黏膜组织及胃癌组织、胃黏膜上皮细胞及不同胃癌细胞株中的表达情况;采用CCK-8实验检测lncRNA 114227对胃癌细胞增殖的影响;采用Transwell实验、划痕愈合实验和蛋白质印迹法检测lncRNA 114227对胃癌细胞迁移能力和上皮-间充质转化(epithelial-mesenchymal transformation,EMT)相关分子表达的影响;采用裸鼠皮下荷瘤实验检测lncRNA 114227对胃癌细胞体内增殖的影响。
结果
LncRNA 114227在胃癌组织的表达水平明显低于胃黏膜组织,在4种胃癌细胞中的表达水平明显低于胃黏膜上皮细胞(均P<0.01)。过表达lncRNA 114227后细胞的增殖和迁移能力明显减弱;沉默lncRNA 114227后细胞的增殖和迁移能力增强(均P<0.05)。体内裸鼠皮下荷瘤实验显示:OE-lncRNA 114227 组过荷瘤小鼠的成瘤体积明显小于Vector 组,成瘤质量低于Vector组(P<0.05),表明lncRNA 114227抑制肿瘤生成。
结论
LncRNA 114227在胃癌组织和胃癌细胞中低表达,可能通过EMT抑制胃癌细胞的增殖和迁移能力。
Keywords: 胃癌, lncRNA 114227, 细胞增殖, 上皮-间充质转化
Abstract
Objective
Gastric cancer is a common cancer of the digestive system. Long non-coding RNA (lncRNA) plays an important role in the formation and development of gastric cancer. This study aims to investigate the effect of long non-coding lncRNA 114227 on biologic behaviors in gastric cancer cells.
Methods
The experiment was divided into 4 groups: a negative control (NC) group, a lncRNA 114227 small interference (si-lncRNA 114227) group, an empty vector (Vector) group, and an overexpression vector (OE-lncRNA 114227) group. The expressions of lncRNA 114227 in gastric mucosa and gastric cancer tissues, gastric mucosal epithelial cells and different gastric cancer strains were determined by real-time reverse transcription PCR (real-time RT-PCR).The proliferation were detected by CCK-8 assay in gastric cancer cells. The epithelial-mesenchymal transformation (EMT) was utilized by Transwell assay, scratch healing assay, and Western blotting in gastric cancer cells. The effect of lncRNA 114227 on proliferation of gastric cancer cells was detected by tumor bearing experiment in nude mice in vivo.
Results
The expression level of lncRNA 114227 in the gastric cancer tissues was significantly lower than that in the gastric mucosa tissues, and in 4 kinds of gastric cancer strains was all significantly lower than that in gastric mucosal epithelial cells (all P<0.01). In vitro, the proliferation and migration abilities of gastric cells were significantly reduced after overexpressing lncRNA 114227, and cell proliferation and migration were enhanced after silencing lncRNA 114227 (all P<0.05). The results of in vivo subcutaneous tumorigenesis in nude mice showed that the tumorigenic volume of the tumor-bearing mice in the OE-lncRNA 114227 group was significantly smaller than that of the Vector group, and the tumorigenic quality was lower than that of the Vector group (P<0.05), indicating that lncRNA 114227 inhibited tumorigenesis.
Conclusion
The expression of lncRNA 114227 is downregulated in gastric cancer gastric cancer tissues and cell lines. LncRNA 114227 may inhibit the proliferation and migration of gastric cancer cells through EMT process.
Keywords: gastric cancer, lncRNA 114227, cell proliferation, epithelial-mesenchymal transformation
胃癌是全球癌症病死率较高的疾病。中国胃癌呈现高发病率、高病死率、高年龄段高发的特点[1-2]。目前临床现有的肿瘤标志物尚不能完全揭示早期胃癌的发生及复发[3]。长链非编码RNA(long non-coding RNA,lncRNA)是一类长度大于200 nt,极少数具有编码短肽能力的RNA[4],在肿瘤发生、发展过程中通过调控转录、转录后和表观遗传水平发挥重要作用[5]。研究[6]显示恶性肿瘤中lncRNAs异常表达与患者预后密切相关。LncRNA 114227是新发现的一种RNA,在胃癌中的表达及其功能尚未明确。本研究通过实时反转录聚合酶链反应(real-time reverse transcription PCR,real-time RT-PCR)、蛋白质印迹法等方法探讨沉默和过表达lncRNA 114227后对胃癌细胞增殖与迁移能力的影响,为进一步阐述胃癌的发病机制提供实验依据。
1. 材料与方法
1.1. 主要材料
反转录试剂盒购于南京诺唯赞公司,RNA FISH试剂盒购于苏州吉玛基因股份公司,lncRNA 114227小干扰序列由苏州吉玛基因股份公司合成,E-Cadherin、N-Cadherin、MMP2、Vimentin、GAPDH兔源单克隆抗体购于美国Abcam公司。BALB/C(nu/nu)雌性裸鼠(4周龄)购于常州卡文斯实验动物有限公司(实验动物合格证号:202019236)。
1.2. 方法
1.2.1. 临床组织标本的收集
收集2019年至2021年镇江康复医院的25例正常胃黏膜组织和25例胃癌组织标本。本研究已通过江苏大学医学伦理委员会批准。
1.2.2. 细胞培养及转染
人胃癌细胞株(BGC-823、MGC-803、SGC-7901 和HGC-27)及人胃黏膜上皮细胞(GES-1)培养于含10%胎牛血清的DMEM中,置于37 ℃、5% CO2细胞培养箱。实验分为4组:阴性对照(NC)组、lncRNA 114227小干扰(si-lncRNA 114227)组、空载体(Vector)组、过表达载体(OE-lncRNA 114227)组。按照转染说明书进行转染,转染6 h后更换为新鲜完全培养基进行后续实验。siRNA序列如表1所示。
表1.
siRNA序列
Table 1 Sequences of siRNAs
| 名称 | 序列(5'-3') |
|---|---|
| si-lncRNA 114227正向 | GGACAAAGAAUUUCCACUUTT |
| si-NC正向 | UUCUCCGAACGUGUCACGUTT |
1.2.3. LncRNA 114227在胃癌组织及细胞系中的表达
采用real-time RT-PCR进行检测。按照TRIzol试剂说明书提取组织和细胞的总RNA,检测RNA的浓度和纯度,其中组织RNA采用超声破碎裂解;根据反转录说明书将样本反转录为cDNA模板。反转录程序:50 ℃ 15 min,85 ℃ 2 min。扩增后,采用2-ΔΔCt法分别计算GAPDH和lncRNA 114227的相对表达量。Real-time RT-PCR反应条件:首先95 ℃预变性5 min,然后95 ℃变性10 s,60 ℃退火30 s,40个循环。Real-time RT-PCR引物序列如表2所示。
表2.
实时反转录聚合酶链反应引物序列
Table 2 Sequences of real-time RT-PCR primers
| 名称 | 序列(5'-3') | |
|---|---|---|
| GAPDH | 正向引物 | GCACCGTCAAGGCTGAGAAC |
| 反向引物 | TGGTGAAGACGCCAGTGGAC | |
| LncRNA 114227 | 正向引物 | CTCTGCTGTAACTATGGTTGTGT |
| 反向引物 | CTGGGTAAAGGGTAAGTGGAAAT |
1.2.4. RNA FISH检测lncRNA 114227细胞系定位
根据RNA FISH试剂盒说明书进行操作。Cy3标记lncRNA 114227探针,通过共聚焦荧光显微镜检测细胞内荧光信号的表达差异。
LncRNA 114227探针序列:5'-GGTAAACCTGG-GTAAAGGGTAAGTG-3'。
1.2.5. 蛋白质印迹法检测相关蛋白质的表达
用RIPA裂解液提取细胞总蛋白质,于-20 ℃保存备用。采用超声破碎后提取组织蛋白质。用12% SDS-PAGE分离蛋白质后,用300 mA电流转膜 120 min,5%脱脂牛奶封闭1 h,一抗于4 ℃孵育过夜。使用TBST洗膜,每次15 min,洗涤4次。用二抗孵育1 h后,TBST洗膜同上。使用ECL试剂呈现图像,Image J软件灰度扫描并计算蛋白质的相对表达量。
1.2.6. CCK-8实验检测胃癌细胞增殖能力
收集转染48 h后的细胞,以1 000/孔的密度种于96孔板,每组设3个复孔。各孔加入10 μL CCK-8溶液,置于37 ℃、5% CO2细胞培养箱培养1 h,用酶标仪检测450 nm波长处各孔的吸光度值。
1.2.7. Transwell实验和划痕愈合实验检测胃癌细胞 迁移能力
收集转染后48 h的细胞,用无血清培养基重悬细胞后,将20 000个/孔细胞种于Transwell小室上室,600 μL完全培养基置于下室。16 h后取出小室,用4%多聚甲醛于室温固定30 min,结晶紫染色 30 min,蒸馏水清洗2次,风干后于显微镜下拍摄。
待转染48 h的细胞长满后,用枪头笔直划线,PBS洗涤后加入无血清培养基置于细胞培养箱中孵育。分别在划线后0、24 h于镜下观察细胞的迁移情况。
1.2.8. 裸鼠皮下荷瘤实验检测胃癌细胞体内增殖和迁移能力
取对数生长期的HGC-27细胞分别转染空载质粒和过表达质粒,48 h后消化,用PBS重悬。注射细胞悬液于裸鼠背部,1个月后观察小鼠的荷瘤情况。安乐死处理裸鼠后,称量裸鼠的质量和体积。
1.3. 统计学处理
采用Graphpad Prism 8.0进行数据处理。计量资料用均数±标准差( ±s)表示。两组间比较采用t检验,多组间比较采用单因素方差分析。以P<0.05为差异有统计学意义。
2. 结 果
2.1. LncRNA 114227在胃组织和细胞系中的表达 及定位情况
Real-time RT-PCR检测结果显示:lncRNA 114227在胃癌组织表达相对较低于正常胃黏膜组织[(1.119 0±0.296 3) vs (0.643 4±0.463 9),P<0.01;图1A];lncRNA 114227在4种胃癌株BGC-823、SGC-7901、MGC-803及HGC-27中相对表达量分别为0.005 8±0.001 6、0.046 3±0.005 0、0.132 6±0.005 4、0.007 9±0.001 6,均低于胃黏膜上皮细胞GES-1相对表达量(1.007 0±0.147 5),差异均有统计学意义(均P<0.01,图1B)。RNA FISH结果显示:Cy3标记的lncRNA RNA探针主要位于细胞核中(图1C)。
图1.
LncRNA114227在胃癌组织及细胞中的表达及定位
Figure 1 Expression and location of lncRNA114227 in gastric tissues and cells
A: Expression levels of lncRNA 114227 in gastric mucosal tissues and gastric cancer tissues; B: Expression levels of lncRNA 114227 in gastric epithelial cells and gastric cancer strains; C: Localization of lncRNA 114227 in GES-1 and MGC-803 (×600). **P<0.01 vs the GES-1. DAPI: 4',6-diamidino-2-phenylindole.
2.2. LncRNA 114227对胃癌细胞增殖能力的影响
Real-time RT-PCR检测结果显示:与NC组相比,沉默后lncRNA 114227的RNA表达量降低[(0.166 0±0.038 0) vs (0.771 6±0.112 3),P<0.01;图2A];过表达后RNA表达量明显升高[(75.200 0±17.310 0) vs (1.004 0±0.034 6),P<0.01;图2B]。提示模型构建成功。CCK-8实验结果显示:沉默lncRNA 114227后的细胞吸光度值高于NC组(P<0.05,图2C),过表达后的细胞吸光度值低于Vector组(P<0.01,图2D)。表明lncRNA 114227具有抑制胃癌细胞增殖的能力。
图2.
LncRNA 114227对胃癌细胞增殖能力的影响
Figure 2 Effect of lncRNA 114227 on proliferation of gastirc cancer cells
A: LncRNA 114227 expression levels of MGC-803 after NC or si-lncRNA 114227 transfection; B: Expression levels of lncRNA 114227 of HGC-27 after Vector or OE-lncRNA 114227 transfection; C: Growth curves of MGC-803 transfected NC and si-lncRNA 114227; D: Growth curves of HGC-27 transfected with Vector and OE-lncRNA 114227. *P<0.05, **P<0.01.
2.3. LncRNA 114227对胃癌细胞迁移能力的影响
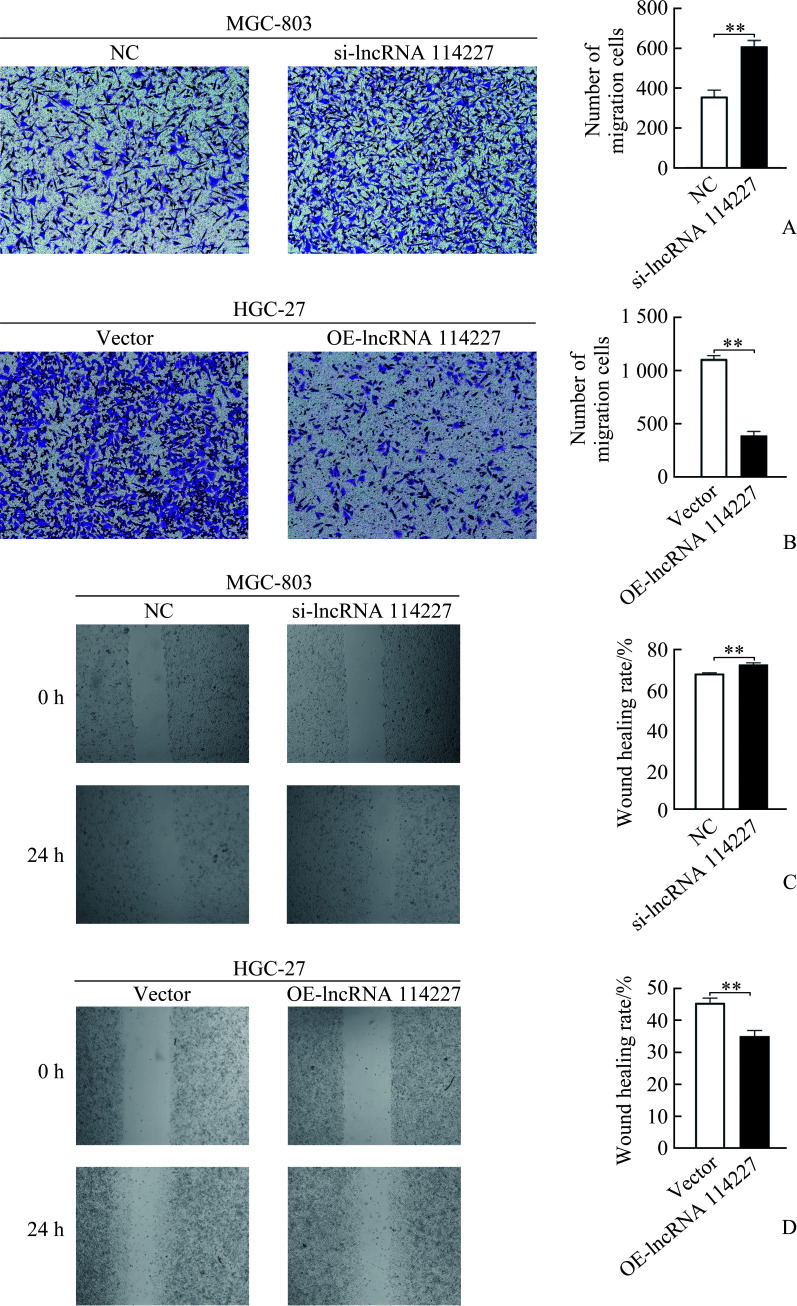
Transwell实验结果显示:lncRNA 114227沉默后MGC-803细胞数目明显多于NC组[(608.7±30.5)个 vs (357.0±33.4)个,P<0.01;图3A],过表达后HGC-27细胞数目明显少于Vector组[(390.3±39.3)个 vs (1 109.0±32.9)个,P<0.01;图3B]。划痕愈合实验结果显示:相比于NC组,lncRNA 114227沉默后MGC-803的愈合速度明显加快[(72.49±0.84)% vs (67.96±0.38)%,P<0.01;图3C],过表达后HGC-27细胞的愈合速度明显减慢[(35.28±1.75)% vs (45.63±1.50)%,P<0.01;图3D]。表明lncRNA 114227具有抑制胃癌细胞迁移的能力。
图3.
LncRNA 114227对胃癌细胞迁移的影响
Figure 3 Effect of lncRNA 114227 on migration of gastric cancer cells
A: Migration cell number of MGC-803 after NC or si-lncRNA 114227 transfection (×100); B: Migration cell number of HGC-27 after Vector or OE-lncRNA 114227 transfection (×100); C: Wound healing rate of MGC-803 after NC or si-lncRNA 114227 transfection (×40); D: Wound healing rate of HGC-27 after Vector or OE-lncRNA 114227 transfection (×40). **P<0.01.
2.4. LncRNA 114227对EMT相关分子表达的影响
蛋白质印迹法结果显示:lncRNA 114227沉默后MGC-803细胞的EMT相关蛋白E-Cadherin表达量降低,N-Cadherin、MMP2及Vimentin表达量升高(均 P<0.05,图4A),lncRNA 114227过表达HGC-27后呈现与此相反的结果(均P<0.01,图4B)。
图4.
LncRNA 114227对胃癌细胞EMT相关蛋白质表达的影响
Figure 4 Effect of lncRNA 114227 on EMT-related proteins expressions of gastric cancer cells
A: EMT-related protein expressions of MGC-803 transfected NC or si-lncRNA 114227; B: EMT-related protein expressions of HGC-27 transfected with Vector or OE-lncRNA 114227. *P<0.05, **P<0.01.
2.5. LncRNA 114227对胃癌细胞体内增殖和迁移的影响
裸鼠皮下荷瘤实验结果显示:OE-lncRNA 114227组荷瘤小鼠的成瘤体积明显小于Vector组 (图5A),成瘤质量低于Vector组(P<0.05,图5B)。在裸鼠组织中,过表达lncRNA 114227后E-Cadherin表达量升高,N-Cadherin、Vimentin和MMP2表达量降低(均P<0.05,图5C),与体外实验的结果一致。
图5.
LncRNA 114227对胃癌细胞体内增殖的影响
Figure 5 Effect of lncRNA 114227 on proliferation of gastric cancer cells in vivo
A: Tumor size of the Vector group and the OE-lncRNA group; B: Tumor weight of the Vector group and the OE-lncRNA group; C: EMT-related protein expressions of nude mice tumor tissues transfected Vector or OE-lncRNA 114227. *P<0.05, **P<0.01. EMT: Epithelial-mesenchymal transformation.
3. 讨 论
全球每年约有100万人确诊为胃癌[7],且呈年轻化趋势[8],早期诊断与筛查有助于胃癌患者的临床诊断和治疗,因此寻找胃癌有效监测和检测标志物对于胃癌患者的诊断、治疗及预后有重要意义。近年来研究[9-11]表明lncRNAs可通过直接或间接与RNA、蛋白质等结合参与肿瘤细胞的增殖、转移、血管生成、耐药及免疫逃避等行为。据文献[12]报道:LEF1-AS1通过miR-5100/DEK轴促进胃癌自噬的发生;SNHG22在胃癌组织中的上调提示不良预后,其激活Wnt相关信号通路促进胃癌的增殖和迁移,且胃癌来源的SNHG22通过间接调控HMGA1进而促进血管生成[13]。
LncRNA 114227是新发现的一种RNA,在胃癌中的表达及其功能尚未十分明确。本研究主要探讨其对胃癌细胞增殖与迁移能力的影响,结果显示lncRNA 114227在胃癌组织及胃癌细胞系中表达量较低,且位于胞核中。比较其在4种胃癌细胞株(BGC-823、SGC-7901、HGC-27及MGC-803)中的表达水平发现,在HGC-27中表达水平最低,MGC-803中表达水平最高。因此,在HGC-27中构建过表达模型,在MGC-803中构建敲减模型。在敲减和过表达细胞模型成功构建的基础上,进一步研究lncRNA 114227在胃癌细胞中的生物学行为,发现lncRNA 114227过表达后胃癌细胞增殖能力明显减弱,沉默后胃癌细胞增殖能力增强,与lncRNA 114227在胃癌组织和胃癌细胞株相对表达量的结论相符。
正常细胞在遗传、化学、物理等多种因素作用下,经历增殖、迁移和侵袭、血管生成、淋巴结转移等过程发展为恶性肿瘤。EMT是其中的重要环节[14-15],其特点是细胞失去上皮细胞黏连功能,发展为转移能力较强类似间充质细胞功能的一类细胞。参与EMT进程的蛋白质中主要是E-Cadherin表达降低,N-Cadherin和Vimentin表达升高。研究[16]发现lncRNA DGCR5间接上调PTEN,进而抑制胃癌细胞的迁移和侵袭。LncRNA p4516通过上调Vimentin、Snail等EMT有关蛋白表达促进胃癌的进展[17]。本研究发现过表达lncRNA 114227后能明显抑制胃癌细胞的EMT进程,沉默后呈现与此相反的结果。提示lncRNA 114227可能通过EMT抑制胃癌的发生、发展。值得注意的是,lncRNAs在细胞中的定位决定了其可能的调控方式。细胞质中的lncRNAs主要与细胞质中的microRNA竞争结合,从而影响转录后水平的表达[18],而胞核中的lncRNAs主要参与核染色质重塑[19],通过与DNA、转录因子等结合,调节有关基因的表达[20]。lncRNA 114227位于细胞核,还需要通过RNA免疫共沉淀、染色质免疫共沉淀等技术确定与靶基因有关的调控机制,其作为肿瘤诊断标志物用于临床诊断还需进一步验证。
综上所述,lncRNA 11227在胃癌组织及胃癌细胞株中低表达,可能通过EMT进程延缓胃癌细胞的增殖和迁移。
基金资助
国家自然科学基金(81772157)。
This work was supported by the National Natural Science Foundation of China (81772157).
利益冲突声明
作者声称无任何利益冲突。
作者贡献
甘海宁、向惠英 实验操作,论文撰写与修改;奚悦、姚敏 数据采集及统计分析;邵晨 论文修改;邵世和 实验设计和指导,论文修改。所有作者阅读并同意最终的文本。
原文网址
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/202302157.pdf
参考文献
- 1. Ning FL, Lyu J, Pei JP, et al. The burden and trend of gastric cancer and possible risk factors in five Asian countries from 1990 to 2019[J]. Sci Rep, 2022, 12(1): 5980. 10.1038/s41598-022-10014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang SZ, Zhang L, Xie L. Cancer burden in China during 1990-2019: analysis of the global burden of disease[J]. Biomed Res Int, 2022, 2022: 3918045. 10.1155/2022/3918045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer[J]. Lancet, 2020, 396(10251): 635-648. 10.1016/s0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 4. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions[J]. Nat Rev Genet, 2009, 10(3): 155-159. 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 5. You BH, Yoon JH, Kang H, et al. HERES, a lncRNA that regulates canonical and noncanonical Wnt signaling pathways via interaction with EZH2[J]. Proc Natl Acad Sci USA, 2019, 116(49): 24620-24629. 10.1073/pnas.1912126116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. 李璐燕, 陈梦湘, 何国献, 等. 长链非编码RNA ZFAS1作为肿瘤预后因子的荟萃分析[J]. 临床检验杂志, 2021, 39(9): 705-710, 715. 10.13602/j.cnki.jcls.2021.09.14. [DOI] [Google Scholar]; LI Luyan, CHEN Mengxiang, HE Guoxian, et al. Long non-coding RNA ZFAS1 as a prognostic factor for tumors: a meta-analysis[J]. Chinese Journal of Clinical Laboratory Science, 2021, 39(9): 705-710, 715. 10.13602/j.cnki.jcls.2021.09.14. [DOI] [Google Scholar]
- 7. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 8. He YX, Wang YD, Luan FJ, et al. Chinese and global burdens of gastric cancer from 1990 to 2019[J]. Cancer Med, 2021, 10(10): 3461-3473. 10.1002/cam4.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma ZL, Liu G, Hao SH, et al. PITPNA-AS1/miR-98-5p to mediate the cisplatin resistance of gastric cancer[J]. J Oncol, 2022, 2022: 7981711. 10.1155/2022/7981711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pei LJ, Sun PJ, Ma K, et al. LncRNA-SNHG7 interferes with miR-34a to de-sensitize gastric cancer cells to cisplatin[J]. Cancer Biomark, 2021, 30(1): 127-137. 10.3233/CBM-201621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun LQ, Li JT, Yan WY, et al. H19 promotes aerobic glycolysis, proliferation, and immune escape of gastric cancer cells through the microRNA-519d-3p/lactate dehydrogenase A axis[J]. Cancer Sci, 2021, 112(6): 2245-2259. 10.1111/cas.14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang HM, Wang J, Wang YD, et al. Long non-coding LEF1-AS1 sponge miR-5100 regulates apoptosis and autophagy in gastric cancer cells via the miR-5100/DEK/AMPK-mTOR axis[J]. Int J Mol Sci, 2022, 23(9): 4787. 10.3390/ijms23094787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cui XF, Zhang HY, Chen T, et al. Long noncoding RNA SNHG22 induces cell migration, invasion, and angiogenesis of gastric cancer cells via microRNA-361-3p/HMGA1/Wnt/β- catenin axis[J]. Cancer Manag Res, 2020, 12: 12867-12883. 10.2147/CMAR.S281578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ou XD, Zhou XY, Li J, et al. p53-induced LINC00893 regulates RBFOX2 stability to suppress gastric cancer progression[J]. Front Cell Dev Biol, 2021, 9: 796451. 10.3389/fcell.2021.796451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang YM, Yuan Y, Zhang YW, et al. SNHG7 accelerates cell migration and invasion through regulating miR-34a-Snail-EMT axis in gastric cancer[J]. Cell Cycle, 2020, 19(1): 142-152. 10.1080/15384101.2019.1699753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu Y, Zhang GH, Zou C, et al. Long noncoding RNA DGCR5 suppresses gastric cancer progression by acting as a competing endogenous RNA of PTEN and BTG1[J]. J Cell Physiol, 2019, 234(7): 11999-12010. 10.1002/jcp.27861. [DOI] [PubMed] [Google Scholar]
- 17. Nie ML, Han J, Huang HC, et al. The novel lncRNA p4516 acts as a prognostic biomarker promoting gastric cancer cell proliferation and metastasis[J]. Cancer Manag Res, 2019, 11: 5375-5391. 10.2147/CMAR.S201793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cao Y, Xiong JB, Zhang GY, et al. Long noncoding RNA UCA1 regulates PRL-3 expression by sponging microRNA-495 to promote the progression of gastric cancer[J]. Mol Ther Nucleic Acids, 2020, 19: 853-864. 10.1016/j.omtn.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation[J]. Nat Struct Mol Biol, 2013, 20(3): 300-307. 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- 20. Qian M, Ling WW, Ruan ZY. Long non-coding RNA SNHG12 promotes immune escape of ovarian cancer cells through their crosstalk with M2 macrophages[J]. Aging, 2020, 12(17): 17122-17136. 10.18632/aging.103653. [DOI] [PMC free article] [PubMed] [Google Scholar]