Abstract
目的
肝脏X受体(liver X receptor,LXR)是核受体超家族中的一员,其中LXR-β是脑细胞内胆固醇含量的重要感受器,而LXR-β/类视黄醇X受体(retinoic X receptor,RXR)-α/ATP结合盒转运子A1(ATP binding cassette transporter A1,ABCA1)胆固醇跨膜转运体系与阿尔茨海默病(Alzheimer’s disease,AD)的发生、发展密切相关。LXR激动剂TO901317能影响APP/PS1双转基因小鼠脑组织中β淀粉样蛋白(β-amyloid protein,Aβ)的生成,但其具体机制尚未阐明。本研究旨在观察LXR激动剂TO901317对高胆固醇饲料饲喂的AD小鼠的认知功能的影响,并从胆固醇代谢的角度探讨其可能的机制。
方法
选取24只雄性6月龄APP/PS1双转基因AD小鼠,并随机分为4组,即普通饲料(control)组、高胆固醇饮食(cholesterol rich diet,CRD)组、CRD联合LXR激动剂TO901317饲喂组(TO901317组)以及CRD、TO901317联合LXR拮抗剂GSK2033饲喂组(GSK2033组),每组各6只。以CRD为基础饲料混合猪油、蛋黄粉、鱼肝油等加工制成的颗粒饲料,按每天两次饲喂小鼠;药物组在CRD的同时给予药物,TO901317及GSK2033溶解后均稀释至最终浓度0.03%,并按照小鼠体重通过胃管每日灌胃给药,各组总治疗时间为3个月。饲喂3个月后,采用Morris水迷宫实验观察各组小鼠空间探索和记忆能力的变化;酶标仪比色法检测各组小鼠血清中TC、LDL和HDL的含量;ELISA法检测各组AD小鼠脑组织中Aβ42的表达情况;蛋白质印迹法检测各组小鼠脑组织LXR-β、RXR-α、ABCA1和Caveolin-1蛋白质水平的变化。
结果
Morris水迷宫实验结果显示:与control组相比,CRD组小鼠穿越平台的次数、距离和时间均显著降低(均P<0.05);相较于CRD组,TO901317组小鼠穿越平台的次数、距离和时间显著增加(均P<0.05);而相较于TO901317组,GSK2033组上述指标均降低(均P<0.05)。酶标比色仪检测结果显示:CRD组小鼠血清中TC和LDL含量较control组显著增加,而HDL却显著减少(均P<0.001);TO901317组中TC和LDL含量较CRD组减少,HDL含量增加(均P<0.001);GSK2033组小鼠血清中TC和LDL含量较TO901317组却显著增加,HDL含量显著减少(均P<0.001)。ELISA结果显示:4组中CRD组小鼠脑内的Aβ42生成量最多;与CRD组比较,TO901317组小鼠脑内的Aβ42量显著减少(P<0.001),4组中含量最低;control组与GSK2033组含量接近。蛋白质印迹法结果显示:CRD组中LXR-β、RXR-α、ABCA1蛋白质水平较control组显著降低,Caveolin-1蛋白质水平却明显升高(均P<0.01)。而经TO901317处理后,AD小鼠脑组织中LXR-β、RXR-α、ABCA1蛋白质水平明显升高,Caveolin-1蛋白质水平降低(均P<0.001);在GSK2033组中,TO901317对AD小鼠的影响被GSK2033部分逆转,相较于TO901317组,小鼠脑内的LXR-β、RXR-α、ABCA1蛋白质水平降低,Caveolin-1蛋白质水平升高(均P<0.05)。
结论
CRD导致转基因AD小鼠产生更加严重的空间探索障碍和学习记忆能力障碍,而LXR激动剂TO901317减轻了这一效应。其机制可能为TO901317通过激活LXR-β/RXR-α/ABCA1跨膜转运体系促进胆固醇外排,降低Caveolin-1的表达并改善脂筏的构成,最终降低脑内Aβ42的生成。
Keywords: 肝脏X受体, TO901317, 阿尔茨海默病, ABCA1跨膜转运体系, 脂筏, Aβ42
Abstract
Objective
The liver X receptors (LXRs) are members of the nuclear hormone receptor superfamily, and LXR-β is an important receptor for cholesterol content in brain cells. LXR-β/retinoic X receptor (RXR-α)/ATP binding cassette transporter A1 (ABCA1) cholesterol transmembrane transport system is closely related to the occurrence and development of Alzheimer’s disease (AD). LXR agonist TO901317 can affect the accumulation of β- amyloid protein in the brain tissue of APP/PS1 double transgenic AD mice. However, the molecular mechanism is not clarified in detail. This study aims to evaluate the effects of LXR agonist TO901317 on the cognitive function of AD mice fed with high cholesterol diet, and to explore its possible mechanism from the perspective of cholesterol metabolism.
Methods
Twenty four male 6-month-old APP/PS1 double transgenic AD mice were randomly divided into 4 groups, 6 mice in each group: a control group (fed with normal diet), a cholesterol rich diet (CRD) group, a TO901317 group (fed with CRD combined with TO901317), and a GSK2033 group (fed with CRD combined with TO901317 and LXR antagonist GSK2033). The mice were fed with pellet feed made of high cholesterol feed, mixed with lard, egg yolk powder, and cod liver oil twice a day. TO901317 and GSK2033 were dissolved and diluted to a final concentration at 0.03%. The drugs were given to the mice daily through gastric tube according to their body weight. Meanwhile, the mice in the drug group were fed with high cholesterol diet. After feeding for 3 months, Morris water maze was used to observe the changes of spatial exploration and memory ability of AD mice in each group. The contents of TC, LDL, and HDL in serum of mice in each group were detected by cholesterol enzyme colorimetry, and the differences among the groups were compared. The expression of Aβ42 in the brain of AD mice was detected by ELISA. Western blotting was used to detect the protein levels of LXR-β, RXR-α, ABCA1, and Caveolin-1 in the brain of each group.
Results
Morris water maze results showed that the times, distance and the duration of mice crossing the platform in the CRD group were significantly decreased compared with the control group (all P<0.05), while these three figures in TO901317 group were significantly increased compared with the CRD group (all P<0.05). Compared with the TO901317 group, there was a decrease of these figures in the GSK2033 group (all P<0.05). The serum TC and LDL levels in the CRD group were significantly higher than those in the control group, while HDL levels were significantly lower (all P<0.001). The figures of the TC and LDL contents level in the TO901317 group were lower than those in the CRD group, while HDL levels were higher (all P<0.001). Compared with TO901317 group, the contents of the TC and LDL in GSK2033 group were significantly increased, while HDL content was significantly decreased (all P<0.001). ELISA results showed that the production of Aβ42 peptides in the brain of CRD group was the highest while the content in the TO901317 group was significantly decreased (P<0.001), which was the lowest among the groups. The figure in the control group was close to the GSK2033 group. Western blotting results showed that the protein levels of LXR-β, RXR-α, and ABCA1 in the CRD group were significantly decreased compared with the control group, but the protein level of Caveolin-1 was increased (all P<0.01). After TO901317 treatment, the protein levels of LXR-β, RXR-α and ABCA1 were significantly increased, while the protein level of Caveolin-1 was decreased partially (all P<0.001). In the GSK2033 group, the effect of TO901317 on AD mice was partially reversed by GSK2033. Compared to TO901317 group, the protein levels of LXR-β, RXR-α, and ABCA1 showed a decrease trend, while the protein level of Caveolin-1 showed an increase state (all P<0.05).
Conclusion
High cholesterol diet leads to severer spatial exploration, learning and memory impairment in transgenic AD mice, while the LXR agonist TO901317 attenuates this effect. The mechanism may be that TO901317 promotes cholesterol efflux by activating LXR-β/RXR-α/ABCA1 transmembrane transport system, reduces the expression of Caveolin-1, improves the composition of lipid raft, and ultimately reduces the production of Aβ42 in the brain.
Keywords: liver X receptor, TO901317, Alzheimer’s disease, ABCA1 transmembrane transport system, lipid rafts, Aβ42
阿尔茨海默病(Alzheimer’s disease,AD)是最常见的进行性退行性变疾病,是老年人群痴呆的主要类型,其病理改变主要表现为β淀粉样蛋白(β- amyloid protein,Aβ)在脑组织中的过度生成并沉积而形成老年斑、Tau蛋白过度磷酸化形成神经纤维缠结以及神经元死亡等。AD的发病机制不清,是多因素、多机制共同作用的结果[1],高胆固醇在AD的发生、发展等过程中发挥了关键作用[2],已有研究[3]证实他汀类降胆固醇药物可延缓AD的发展过程,并改善患者的认知功能。脑内富含胆固醇,但其自身不能降解,过多的胆固醇主要是通过ATP结合盒转运子跨膜转运体系从神经细胞内排出。在此转运过程中,首先肝脏X受体(liver X receptor,LXR)和类视黄醇X受体(retinoic X receptor,RXR)结合,生成异二聚体,然后与靶基因上增强子区域中的LXR反应原件结合,从而调控靶基因ATP结合盒转运子A1(ATP binding cassette transporter A1,ABCA1)的表达,促进胆固醇的外排,进而降低细胞内的胆固醇。ABCA1跨膜胆固醇转运体系的激活通过降低胆固醇的生成,在Aβ的沉淀及清除中发挥重要作用,这一机制为防治AD药物的开发提供了新的靶点[4]。本研究以APP/PS1双转基因AD小鼠为研究对象,分别给予普通饲料、高脂饲料、LXR激动剂TO901317或/和LXR抑制剂GSK2033饲喂,旨在观察AD小鼠空间探索和学习记忆能力,小鼠血液中TC、LDL和HDL的含量及脑内Aβ42生成的变化,探讨其可能的机制,以期为寻找新的AD治疗药物和靶点提供更多的理论基础和实验证据。
1. 材料与方法
1.1. 材料
1.1.1. 实验动物
24只雄性双转基因小鼠AD小鼠Tg2576(APPswe/PSEN1dE9)购自南京大学模式动物研究所。所有小鼠饲养于重庆医科大学动物实验中心,室内温度维持在(25±1) ℃,每天给予12 h光照、SPF级饲料和水。
1.1.2. 主要试剂
LXR激动剂TO901317和LXR拮抗剂GSK2033购自美国Medicine Chemistry Express公司;Aβ42的ELISA检测试剂盒购自北京博奥森生物技术有限公司;总蛋白质抽提试剂盒、BCA蛋白浓度检测试剂盒和RIPA蛋白质裂解液购自上海碧云天生物技术有限公司;所有的抗体均购自英国Abcam公司;TC、LDL和HDL检测试剂盒购自南京建成生物研究所。
1.2. 方法
1.2.1. 实验分组
将所有AD小鼠随机分为4组,即普通饲料(control)组、高胆固醇饮食(cholesterol rich diet,CRD)组、TO901317组(CRD联合LXR激动剂TO901317)以及GSK2033组(CRD、TO901317联合LXR拮抗剂GSK2033),每组各6只。
CRD的成分及饲喂:基础饲料(70%,包含玉米、豆粕、鱼粉、面粉、酵母粉、植物油、食盐、多种维生素和矿物元素等)、奶粉(10%)、猪油(10%)、蛋黄粉(10%)、鱼肝油(10滴)等成分混合后,加工制成颗粒饲料,使用前在冰箱中保存,按每天2次饲喂。
TO901317组和GSK2033组的饲喂:药物组同时给予CRD和相应药物。将TO901317溶解在二甲基亚砜(dimethyl sulfoxide,DMSO)中,并用双蒸馏水稀释至最终浓度0.03%。给药剂量为每只小鼠每天 15 mg/kg;GSK2033的饲喂方法与TO901317相同,剂量为每只小鼠每天10 mg/kg[5]。所有药物均通过胃管灌胃给药,各组总治疗时间为3个月。所有小鼠均顺利完成实验。
1.2.2. Morris 水迷宫实验
饲喂3个月后,采用Morris水迷宫实验观察各组小鼠空间探索和记忆能力的变化。实验第1天,将插有小红旗的平台固定于水迷宫装置的第2象限,并且保证平台漏出水面约1 cm。任意选择一个象限,将AD小鼠依次放入水中,并开始计时,记录小鼠找到并登上平台的时间。若小鼠在1 min内成功登上平台,则准确记录其时间(称作逃避潜伏期);若小鼠在1 min后仍未找到平台,记录其潜伏期为60 s,则手动引导AD小鼠至平台附近使之爬上平台并停留 10 s。实验第2至第5天,使水面略高于平台约1 cm,按照第一天的顺序放入小鼠,每只小鼠每天训练5次,每一轮的训练至少间隔1 h,记录逃避潜伏期。实验第6天,将平台撤掉,任意选择一象限(第3象限)放入小鼠,使之在池内游泳1 min,观察小鼠的游泳轨迹,记录小鼠穿越原平台所在象限(第3象限)的次数、游泳的时间和距离。
1.2.3. ELISA法
采用ELISA法检测各组小鼠脑组织中Aβ42的浓度。严格按照试剂盒说明进行操作。收集各组脑组织,并加入蛋白裂解液,充分裂解离心后获得待测样品悬液。取出96孔板,加入标准品、待测样品,同时设空白对照组。采用酶标仪在450 nm波长下读取吸光度值,根据标准曲线计算出Aβ42的浓度。
1.2.4. 血清TC、LDL和HDL检测
按照说明书进行酶标比色法测量。获得各组血清后按照空白管(2.5 µL蒸馏水、250 µL试剂)、标准管(2.5 µL标准品、250 µL试剂)、测定管(2.5 µL血清、250 µL试剂)的方法进行加样,分别混匀后于37 ℃水浴5 min,以空白管调零,分别在510 nm、546 nm波长下比色,读取各管吸光度值,测定TC、LDL和HDL的浓度。样本TC(LDL或HDL)含量=(样本管吸光度值-空白管吸光度值)/(标准管吸光度值-空白管吸光度值)×校准品浓度。
1.2.5. 蛋白质印迹法
收集各组脑组织,加入1 mL RIPA蛋白裂解液,充分裂解后于4 ℃,以13 000 r/min离心15 min,用Bradford法测定每组蛋白质样品浓度并分装保存。配置8%~10%的Page胶,取50 μg经SDS-PAGE电泳,将蛋白质电转至PVDF膜上,用5%脱脂奶粉在室温下封闭2 h,然后加入TBST缓冲液稀释的LXR-β、RXR-α、ABCA1、Caveolin-1和内参β-actin抗体,于4 ℃孵育过夜;充分洗涤后加入1꞉5 000的HRP标记的羊抗兔二抗,室温下孵育2 h,最后用化学发光试剂盒行曝光显影,经Bio-rad凝胶成像系统进行条带分析。
1.3. 统计学处理
应用SPSS 22.0软件进行统计学分析,计量资料均采用均数±标准差( ±s)表示。两样本均数比较采用t检验,多组间均数比较采用单因素方差分析,以P<0.05为差异有统计学意义。
2. 结 果
2.1. LXR激动剂TO901317改善小鼠的空间探索和学习记忆能力
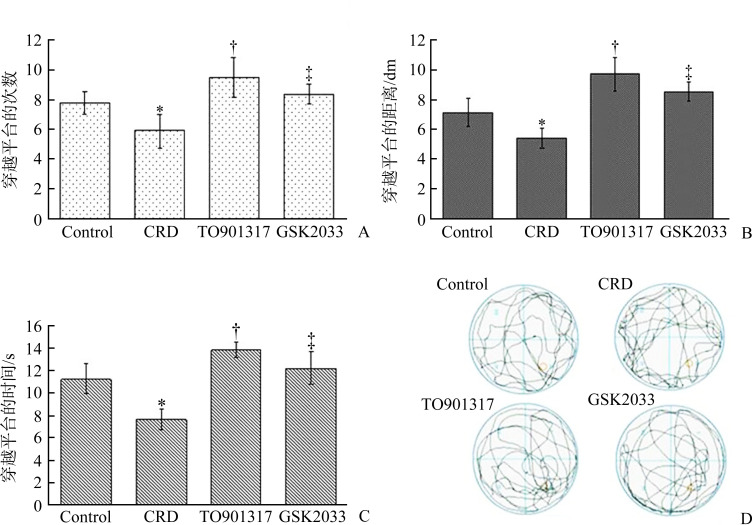
在实验的第6天撤掉平台后,对小鼠游泳运动的轨迹分析结果显示:与control组相比,CRD组在原固定平台所在第3象限内穿越平台的次数、穿越的距离和穿越的时间均显著减少(均P<0.05);与CRD组相比,TO901317组小鼠穿越平台的次数、距离和时间均明显增加(均P<0.05);与TO901317组相比,GSK2033组小鼠穿越平台的次数、距离和时间又显著减少(均P<0.05,图1)。
图 1.
LXR激动剂TO901317改善双转基因AD小鼠的空间探索和学习记忆能力
Figure 1 LXR agonist TO901317 improved spatial exploration and ability of learning and memory in double transgenic AD mice
A: Number of times of the AD mice crossed the quadrant of the original platform in each group; B: Distance of the AD mice acrossed the quadrant of the original platform in each group; C: Time of the AD mice acrossed the quadrant of the original platform in each group; D: Movement trajectory diagram of AD mice in each group in the water maze. *P<0.05 vs the control group; †P<0.05 vs the CRD group; ‡P<0.05 vs the TO901317 group. AD: Alzheimer’s disease; CRD: Cholesterol rich diet.
2.2. LXR激动剂TO901317降低小鼠脑组织中Aβ42 的含量
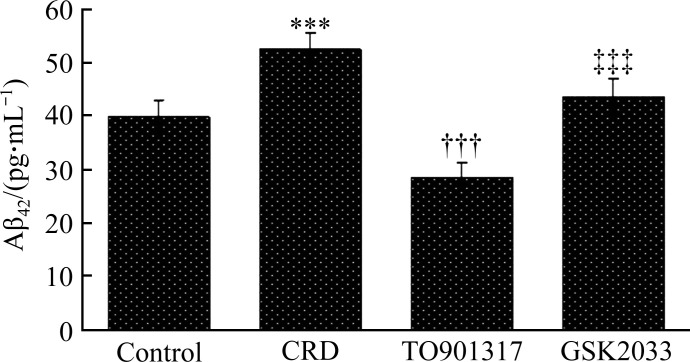
ELISA结果显示:4组中CRD组小鼠脑组织中的Aβ42生成量最多,TO901317组最少。与control组比较,CRD组AD小鼠脑组织中Aβ42的含量明显增多[(39.84±3.15) pg/mL vs (52.36±3.28) pg/mL,P<0.001];与CRD组比较,TO901317组Aβ42的含量显著减少[(28.51±2.74) pg/mL,P<0.001];而GSK2033部分抵消了TO901317的作用,与TO901317组相比,GSK2033组小鼠脑组织中Aβ42的含量显著增多[(43.48±3.56) pg/mL;P<0.001,图2]。
图2.
LXR激动剂TO901317降低转基因AD小鼠脑组织中Aβ42 的含量
Figure 2 LXR agonist TO901317 reduced the content of Aβ42 in the brains of transgenic AD mice
***P<0.001 vs the control group; †††P<0.001 vs the CRD group; ‡‡‡P<0.001 vs the TO901317 group.
2.3. LXR激动剂TO901317对小鼠血清TC、LDL 和HDL含量的影响
酶标仪比色法检测结果显示:与control组相比,CRD组小鼠血清中的TC和LDL含量增加,而HDL的含量却显著减少(均P<0.001);与CRD组相比,TO901317组小鼠血清中的TC和LDL含量减少,HDL含量增加(均P<0.001)。再给予LXR拮抗剂GSK2033处理后,与TO901317组相比,GSK2033组小鼠血清中的TC和LDL的含量显著增加,而HDL的含量显著减少(均P<0.001,表1)。
表1.
各组转基因AD小鼠血清中TC、LDL和HDL的含量(n=6, ±s)
Table 1 TC, LDL, and HDL levels in serum in each group of transgenic AD mice (n=6, ±s)
| 组别 | TC/(mmol·L-1) | LDL/(mmol·L-1) | HDL/(mmol·L-1) |
|---|---|---|---|
| Control | 2.49±0.11 | 0.76±0.14 | 1.55±0.09 |
| CRD | 3.57±0.17*** | 2.54±0.18*** | 0.66±0.07*** |
| TO901317 | 2.72±0.19††† | 1.03±0.23††† | 1.29±0.15††† |
| GSK2033 | 3.31±0.15‡‡‡ | 1.83±0.12‡‡‡ | 0.86±0.11‡‡‡ |
| F | 60.841 | 130.937 | 82.252 |
| P | <0.001 | <0.001 | <0.001 |
***P<0.001 vs the control group; †††P<0.001 vs the CRD group; ‡‡‡P<0.001 vs the TO901317 group. AD: Alzheimer’s disease; CRD: Cholesterol rich diet; TC: Total cholesteol; LDL: Low density lipoprotein; HDL: High density lipoprotein.
2.4. LXR激动剂TO901317对小鼠脑组织中LXR-β、RXR-α、ABCA1和Caveolin-1蛋白质水平的影响
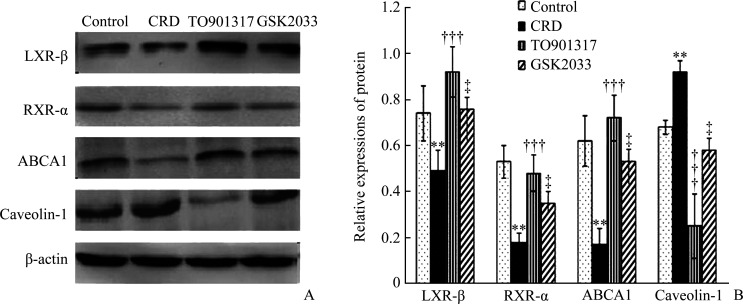
与control组比较,CRD组小鼠脑组织中LXR-β、RXR-α和ABCA1的蛋白质水平均显著降低,而Caveolin-1蛋白质水平显著升高(均P<0.01);与CRD组比较,TO901317组小鼠脑内的LXR-β、RXR-α和ABCA1蛋白质水平均有显著升高,而Caveolin-1蛋白质水平降低(均P<0.001)。再给予LXR拮抗剂GSK2033处理后,与TO901317组比较,GSK2033组小鼠脑内的LXR-β、RXR-α、ABCA1蛋白质水平又显著降低,而Caveolin-1蛋白质水平则升高(均P<0.05,图3)。
图3.
LXR激动剂TO901317对转基因AD小鼠脑组织中LXR-β、RXR-α、ABCA1和Caveolin-1蛋白质表达的影响
Figure 3 Effects of TO901317 on the expressions of LXR-β, RXR-α, ABCA1, and Caveolin-1 protein in the brains of transgenic AD mice
A: Protein expressions of LXR-β, RXR-α, ABCA1, and Caveolin-1 in the brain of rats in each group. B: Statistical analysis of expressions of LXR-β, RXR-α, ABCA1 and Caveolin-1 protein in the brain of mice in each group. **P<0.01 vs the control group; †††P<0.001 vs the CRD group; ‡P<0.05 vs the TO901317 group. AD: Alzheimer’s disease; CRD: Cholesterol rich diet; LXR-β: Liver X receptor-β; RXR-α: Retinoic X receptor-α; ABCA1: ATP binding cassette transporter A1.
3. 讨 论
虽然AD的发病机制尚不清楚,但Aβ在脑组织中的过度生成并积聚是各种原因诱发AD的共同通路,也是目前公认的关键的致病因素。Aβ是淀粉样前体蛋白(amyloid precursor protein,APP)经过体内多次分解形成的一段多肽。在正常神经元内,APP经β-和γ-分泌酶的切割分解后形成Aβ,Aβ又被降解,以维持在低浓度水平。Aβ的降解途径有3种:1)细胞外可溶性的Aβ单体直接由相应的蛋白酶分解;2)Aβ由ABCA1、ApoE多种因子等介导的细胞内吞作用吞噬,再与溶酶体融合后,被相应的蛋白酶水解;3)Aβ结合特定的分子(如HDL、LDL等)后,通过血脑屏障在外周组织内完成降解。在形成和降解过程中,任一环节出现异常,都会导致Aβ的生成增多并沉积,最终导致AD的发生;而干预其中任一环节,则会抑制Aβ的形成或促进其降解,降低其沉积的可能,进而抑制AD的发生、发展[6]。
脑是富含胆固醇的器官,其含量占人体总胆固醇的25%。由于血脑屏障的存在,一方面,脑组织不能直接从血液循环中获取胆固醇,而是靠星形胶质细胞和神经元合成胆固醇;另一方面,脑内过多的胆固醇也不能直接降解或直接排到血液中,而主要是通过ABCA1跨膜转运体系来促进胆固醇的外排,减少因过多胆固醇堆积造成的神经元损害[7]。与先前的报道[8-9]结果一致,本研究用富含胆固醇的食物饲喂转基因AD小鼠后发现:AD小鼠认知功能障碍明显加重,脑内Aβ的含量增多,血清中的TC和LDL含量也显著增高,而HDL的含量却减少。这都表明胆固醇代谢紊乱与AD的发病机制有着重要的关联。
ABCA1跨膜转运体系通常单向转运胆固醇,它是以ABCA1为核心,以贫脂的载脂蛋白(如apoA1、HDL)为受体,主要通过LXR和RXR结合形成二聚体,进而调控胞内游离胆固醇和磷脂偶联后向外转运,整个过程都需要ATP的供能。LXR是核受体超家族的一员,也是多种细胞内胆固醇含量的感受器。LXR有LXR-α和LXR-β两种亚型,其中LXR-β在机体内广泛分布,以脑组织含量最为丰富,是肝脏组织的2~5倍。RXR存在RXR-α、RXR-β和RXR-γ 3种亚型,其中RXR-α主要表达于脑组织的海马和新皮质区。当脑细胞内胆固醇含量增高时,激活的LXR-β配体进一步激活LXR-β,并与RXR-α形成异二聚体,接着结合靶基因上特异的脱氧核糖核酸元件,从而调节靶基因(如ABCA1)在转录水平上的表达,LXR-β/RXR-α/ABCA1胆固醇跨膜转运体系与AD的发生、发展过程有着密切联系。Lei等[10]研究显示:LXR激活剂TO901317可通过激活LXR-α/ABCA1轴减轻Aβ1-40诱导的人视网膜色素上皮细胞的炎症及衰老反应。Martens等[11]利用LXR激动剂24(S)-Saringosterol处理6月龄AD转基因鼠APPswe/PS1dE9后,发现小鼠脑内的Aβ沉积显著减少,记忆能力也增强。Fitz等[12]使用TO901317治疗APP23转基因小鼠时发现:TO901317可减少高脂饮食饲喂导致的脑组织中Aβ的沉积,促进Aβ清除,并改善认知功能。这些体内、体外实验都表明LXR激动剂能抑制Aβ生成以及减轻Aβ引起的各种不良反应,但其机制并未阐明。本研究也发现TO901317处理CRD饲喂的转基因AD小鼠后,小鼠损伤的学习记忆能力和空间探索能力得到提升,脑组织中Aβ42的含量也减少,同时血清中TC和LDL的含量减少,而HDL的含量却增加。此外,本研究结果显示:高脂饲料饲喂能引起AD小鼠脑组织中LXR-β、RXR-α、ABCA1蛋白质表达水平降低,但TO901317却能减弱这一效应;而在TO901317联合LXR拮抗剂GSK2033后,与单用TO901317相比,LXR-β、RXR-α、ABCA1蛋白质的表达水平降低,这提示TO901317可通过激活LXR-β/RXR-α/ABCA1胆固醇跨膜转运体系促进AD小鼠脑内Aβ的生成,进而改善认知功能。
本研究还发现:激活LXR-β/RXR-α/ABCA1通路影响Aβ生成与细胞膜上的脂筏结构相关。脂筏是质膜上富含胆固醇和神经鞘脂的微结构区域,分布着大量的膜蛋白受体,是一种动态结构。脂筏与信号转导 [13]、物质转运[14]以及神经突触的可塑性[15]均有密切的关系。越来越多的证据表明脂筏与Aβ生成有关,主要表现在以下3个方面:1)脂筏上含有Aβ降解所需的β-分泌酶和γ-分泌酶[16];2)脂筏上存在ABCA1、ApoE等,它们可结合Aβ[17-18];3)在AD的典型发病区,如大脑皮层和海马区域的神经元中脂筏含量要明显高于其他脑区[19]。作为细胞膜脂筏结构小凹的标志蛋白Caveolin-1,它不仅与AD发生时胆固醇稳态失调有关,还参与了APP的裂解和Aβ的生成[20-21]。因此,脂筏是神经元Aβ生成的关键场所,降低胆固醇的含量不仅会改变脂筏的构成,也会影响Aβ的生成过程。本研究结果显示:TO901317降低了AD小鼠脑中的脂筏标志蛋白Caveolin-1的表达,而GSK2033却能部分拮抗TO901317的作用。
综上所述,高胆固醇饮食能引起AD小鼠更加严重的认知功能损伤,其机制可能是神经元胆固醇的转运障碍导致脂筏的结构异常,从而引起Aβ的生成增加,进而促进AD的发生、发展。而LXR激动剂TO901317却能改善这一效应,其主要机制则可能是通过激活LXR-β/RXR-α/ABCA1通路,促进胆固醇的外排,调整脂筏的构成,从而最终减少Aβ的生成。
基金资助
重庆市自然科学基金(Cstc2019jcyj-msxmX0673)。
This work was supported by the Natural Science Foundation of Chongqing, China (Cstc2019jcyj-msxmX0673).
利益冲突声明
作者声称无任何利益冲突。
作者贡献
罗英茂 实验操作,论文撰写;李远、黄杰 动物饲喂及样品收集处理;谭小林、张雄 数据收集及统计分析;邓煜 研究设计,实验操作统筹及论文修改。所有作者阅读并同意最后的文本。
原文网址
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/2022101324.pdf
参考文献
- 1. Kent SA, Spires-Jones TL, Durrant CS. The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics[J]. Acta Neuropathol, 2020, 140(4): 417-447. 10.1007/s00401-020-02196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu CJ, Apostolova LG, Oblak AL, et al. Association of hypercholesterolemia with Alzheimer’s disease pathology and cerebral amyloid angiopathy[J]. J Alzheimers Dis, 2020, 73(4): 1305-1311. 10.3233/JAD-191023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oliveira FF, Chen ES, Smith MC, et al. Selected LDLR and APOE polymorphisms affect cognitive and functional response to lipophilic statins in Alzheimer’s disease[J]. J Mol Neurosci, 2020, 70(10): 1574-1588. 10.1007/s12031-020-01588-7. [DOI] [PubMed] [Google Scholar]
- 4. Behl T, Kaur I, Sehgal A, et al. The interplay of ABC transporters in Aβ translocation and cholesterol metabolism: implicating their roles in Alzheimer’s disease[J]. Mol Neurobiol, 2021, 58(4): 1564-1582. 10.1007/s12035-020-02211-x. [DOI] [PubMed] [Google Scholar]
- 5. Dou FF, Chen JL, Cao H, et al. Anti-atherosclerotic effects of LXRα agonist through induced conversion of M1 macrophage to M2[J]. Am J Transl Res, 2019, 11(6): 3825-3840. [PMC free article] [PubMed] [Google Scholar]
- 6. Kent SA, Spires-Jones TL, Durrant CS. The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics[J]. Acta Neuropathol, 2020, 140(4): 417-447. 10.1007/s00401-020-02196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teixeira V, Maciel P, Costa V. Leading the way in the nervous system: Lipid Droplets as new players in health and disease[J]. Biochim Biophys Acta Mol Cell Biol Lipids, 2021, 1866(1): 158820. 10.1016/j.bbalip.2020.158820. [DOI] [PubMed] [Google Scholar]
- 8. Cho KH. Structural and functional impairments of reconstituted high-density lipoprotein by incorporation of recombinant β-amyloid42[J]. Molecules, 2021, 26(14): 4317. 10.3390/molecules26144317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonaterra-Pastra A, Fernández-de-Retana S, Rivas-Urbina A, et al. Comparison of plasma lipoprotein composition and function in cerebral amyloid angiopathy and Alzheimer’s disease[J]. Biomedicines, 2021, 9(1): 72. 10.3390/biomedicines9010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lei CY, Lin R, Wang JM, et al. Amelioration of amyloid β- induced retinal inflammatory responses by a LXR agonist TO901317 is associated with inhibition of the NF-κB signaling and NLRP3 inflammasome[J]. Neuroscience, 2017, 360: 48-60. 10.1016/j.neuroscience.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 11. Martens N, Schepers M, Zhan N, et al. 24(S)-saringosterol prevents cognitive decline in a mouse model for Alzheimer’s disease[J]. Mar Drugs, 2021, 19(4): 190. 10.3390/md19040190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fitz NF, Cronican A, Pham T, et al. Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice[J]. J Neurosci, 2010, 30(20): 6862-6872. 10.1523/JNEUROSCI.1051-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ediriweera MK, Moon JY, Nguyen YT, et al. 10-gingerol targets lipid rafts associated PI3K/Akt signaling in radio-resistant triple negative breast cancer cells[J/OL]. Molecules, 2020, 25(14): E3164 [2020-07-10]. 10.3390/molecules25143164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu HM, Liu Y, Wang DS, et al. Shenmai injection maintains blood-brain barrier integrity following focal cerebral ischemia via modulating the expression and trafficking of occludin in lipid rafts[J]. J Ethnopharmacol, 2019, 237: 55-63. 10.1016/j.jep.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 15. Zhong W, Huang QY, Zeng LW, et al. Caveolin-1 and MLRs: a potential target for neuronal growth and neuroplasticity after ischemic stroke[J]. Int J Med Sci, 2019, 16(11): 1492-1503. 10.7150/ijms.35158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho YY, Kwon OH, Chung S. Preferred endocytosis of amyloid precursor protein from cholesterol-enriched lipid raft microdomains[J/OL]. Molecules, 2020, 25(23): E5490[2020-11-24]. 10.3390/molecules25235490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee HJ, Ryu JM, Jung YH, et al. High glucose upregulates BACE1-mediated Aβ production through ROS-dependent HIF-1α and LXRα/ABCA1-regulated lipid raft reorganization in SK-N-MC cells[J]. Sci Rep, 2016, 6: 36746. 10.1038/srep36746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee SI, Jeong W, Lim H, et al. APOE4-carrying human astrocytes oversupply cholesterol to promote neuronal lipid raft expansion and Aβ generation[J]. Stem Cell Reports, 2021, 16(9): 2128-2137. 10.1016/j.stemcr.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malchiodi-Albedi F, Contrusciere V, Raggi C, et al. Lipid raft disruption protects mature neurons against amyloid oligomer toxicity[J]. Biochim Biophys Acta, 2010, 1802(4): 406-415. 10.1016/j.bbadis.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 20. Tang WX, Li YS, Li Y, et al. Caveolin-1, a novel player in cognitive decline[J]. Neurosci Biobehav Rev, 2021, 129: 95-106. 10.1016/j.neubiorev.2021.06.044. [DOI] [PubMed] [Google Scholar]
- 21. Messiha BAS, Ali MRA, Khattab MM, et al. Perindopril ameliorates experimental Alzheimer’s disease progression: role of amyloid β degradation, central estrogen receptor and hyperlipidemic-lipid raft signaling[J]. Inflammopharmacology, 2020, 28(5): 1343-1364. 10.1007/s10787-020-00724-4. [DOI] [PubMed] [Google Scholar]