Abstract
OBJECTIVES:
Nonpharmacologic delirium management is recommended by current guidelines, but studies on the impact of ICU design are still limited. The study’s primary purpose was to determine if a multicomponent change in room design prevents ICU delirium. Second, the influence of lighting conditions on serum melatonin was assessed.
DESIGN:
Prospective observational cohort pilot study.
SETTING:
The new design concept was established in two two-bed ICU rooms of a university hospital. Besides modifications aimed at stress relief, it includes a new dynamic lighting system.
PATIENTS:
Seventy-four adult critically ill patients on mechanical ventilation with an expected ICU length of stay of at least 48 hours, treated in modified or standard rooms.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The clinical examination included a prospective assessment for depth of sedation, delirium, and pain every 8 hours using validated scores. Blood samples for serum melatonin profiles were collected every 4 hours for a maximum of three 24-hour periods. Seventy-four patients were included in the analysis. Seventy-six percent (n = 28) of patients in the standard rooms developed delirium compared with 46% of patients (n = 17) in the modified rooms (p = 0.017). Patients in standard rooms (vs. modified rooms) had a 2.3-fold higher delirium severity (odds ratio = 2.292; 95% CI, 1.582–3.321; p < 0.0001). Light intensity, calculated using the measure of circadian effective irradiance, significantly influenced the course of serum melatonin (p < 0.0001). Significant interactions (p < 0.001) revealed that differences in serum melatonin between patients in standard and modified rooms were not the same over time but varied in specific periods of time.
CONCLUSIONS:
Modifications in ICU room design may influence the incidence and severity of delirium. Dedicated light therapy could potentially influence delirium outcomes by modulating circadian melatonin levels.
Keywords: architecture, circadian rhythm, critical care, delirium, melatonin, nonpharmacological
KEY POINTS.
Question: Does a multicomponent change in ICU room design, including light therapy, prevent delirium in mechanically ventilated patients?
Findings: With a frequency of 76% versus 46% and a 2.3-fold higher severity, the delirium burden was significantly higher for patients in standard rooms than those treated in the modified rooms. The different light intensities between standard and modified rooms significantly influenced patients’ circadian melatonin patterns.
Meanings: Modifications in ICU room design may reduce delirium, where dedicated light potentially contributed to differences in outcome.
Delirium is the most frequent psychiatric syndrome in critically ill patients. This manifestation of acute brain dysfunction is independently associated with a three-fold higher risk for 6-month mortality (1). About one-third of ICU survivors have worse long-term cognitive scores similar in severity to that of patients with mild Alzheimer disease (2). Recent clinical trials investigating pharmacologic strategies for managing ICU delirium have failed to demonstrate beneficial effects on delirium incidence, symptom severity, or clinical outcomes (3, 4). Hence, the focus has moved toward nonpharmacologic approaches (5). The Society of Critical Care Medicine recommends a comprehensive intervention bundle to manage pain, agitation, delirium, immobility, and sleep disruption. This includes reorientation, cognitive stimulation, regulated lighting and sound, and minimized sedation and immobility (6). Being unable to sleep is among the top 10 ICU stressors among critically ill patients (7). ICU patients frequently develop circadian rhythm disruption and sleep disturbances (8, 9). Our research group developed a new ICU room concept, including a dynamic lighting system (DLS), that is supposed to reduce patients’ stress and promote circadian alignment, thus minimizing the time for sedation and allowing patients to receive early cognitive stimulation and mobilization more frequently (10). The concept is intended to facilitate the implementation of delirium prevention bundles. We hypothesized that mechanically ventilated critically ill patients treated in modified rooms have less delirium compared with patients treated in the standard rooms in the same ICU. Second, we postulated a significant association between lighting conditions and circadian serum melatonin levels, which were supposed to behave differently for patients in modified and standard rooms. Preliminary results of this study have previously been presented in abstract form (11).
MATERIALS AND METHODS
The “Evaluation of the All-New Environment for Critically Ill Patients” (VITALITY) prospective cohort study was conducted at the Charité—Universitätsmedizin Berlin Hospital and registered at ClinicalTrials.gov (NCT02143661). The study was approved by the Charité’s Ethics Committee (number EA1/019/14; approval date September 10, 2014) and the institutional data protection officer. After ICU admission, written informed consent was obtained from all patients or their authorized surrogates; if consent was initially obtained from a surrogate, we obtained consent from the patients once they were deemed legally competent. All procedures were followed under the ethical standards of Charité’s Ethics Committee on humans and with the Helsinki Declaration of 1975.
Study Population
The study participants were mechanically ventilated patients admitted to the ICU 8i at Campus Virchow-Klinikum, 18 years old or older, and had an expected ICU length of stay (LOS) of at least 48 hours.
Exclusion criteria were as follows: substantial recent ICU exposure (2), ICU readmission during the current hospital stay, psychiatric or sleep disorders, delirium at admission, analphabetism, amaurosis, anacusis or severe hypoacusis, history of stroke and known residual cognitive deficits, history of cardiopulmonary arrest or pulseless electric activity with cardiopulmonary resuscitation followed by therapeutic hypothermia during the current hospital stay, history or suspicion of hypoxic brain damage, intracranial bleeding (ICB) or elevated intracranial pressure (ICP) 7 days before study inclusion, open chest after cardiac surgery, patients who were unlikely to survive for 24 hour, liver-cirrhosis, non-German speaking patients, informed consent could not be obtained or was refused, patients with participation in other clinical trials and patients with accommodation in an institution due to an official or judicial order.
Interventions
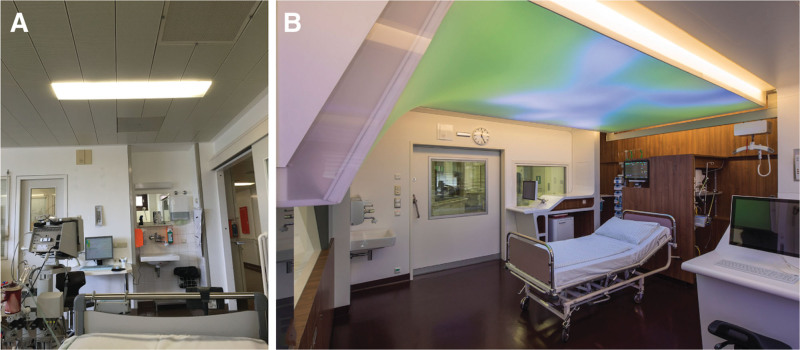
From April to October 2013, two of the seven double bedrooms received extensive changes in architectural room and interior design (10). Besides modifications for stress relief, noise reduction, cognitive training, early mobilization, and workflow optimization, we developed a new DLS for patient-individualized lighting therapy (12, 13). The terms “standard rooms” and “modified rooms” refer to patient rooms without (Fig. 1A) and with (Fig. 1B) modifications, respectively.
Figure 1.
Pictures from one of the two-bed-rooms on ICU-8i at Charité Campus Virchow-Klinikum before (A) and after (B) the architectural and design modifications. The light-emitting area of each dynamic lighting system covers a surface of up to 6.1 m × 2.4 m for one ICU bed.
Dynamic Lighting System
Each bed within the modified rooms is equipped with the newly developed DLS (10, 13, 14). The term DLS refers to a feature set that allows controlling and timing of both the illuminance (EV, Lux [㏓]) and the correlated color temperature (CCT, kelvin [K]). EV and CCT values for the lighting schedule were calculated to secure maximal melatonin suppression. As EV and CCT exert distinct melanopic effects, circadian effective irradiance (EC measured in 𝑊 × 𝑚−2), a measure of correlated data effective irradiance for melatonin suppression, was calculated from data of spectral irradiance of lighting at the patient’s eye level, which was weighted by the action spectrum of melatonin suppression, and integrated above the whole range of effective wavelengths (15, 16). Thus, a maximum EC of 2.5 𝑊 × 𝑚−2 was achieved between 12 pm and 2 pm. To apply these high EC levels safely, luminous intensity (LV, candela [㏅]), a measure of glare, was quantified at the eye level: LV reached its maximum at noon with 385 ㏅ × 𝑚−2, which is below the threshold of relative glare (LVtt = 500 ㏅ × 𝑚−2) (17). Standard rooms are equipped with white-light fluorescent tube ceiling lamps (FT-CL). As EV and CCT for the FT-CLs cannot be changed, patients were exposed to a static EC of 0.3263 𝑊 × 𝑚−2 (Supplementary Fig. 1, http://links.lww.com/CCM/H465).
Scheduled Assessments and Procedures
Study measures began the morning after ICU admission. The clinical examination incorporated a prospective assessment for depth of sedation, delirium, and pain, conducted at least every 8 hours. Delirium was assessed using the Confusion Assessment Method for the ICU (CAM-ICU) (18). Delirium severity was calculated daily using the Intensive Care Delirium Screening Checklist (ICDSC) (19, 20). The delirium assessment could not be conducted for patients with a Richmond Agitation Sedation Scale (RASS) of −3 or lower at the time of examination (21). Pain was assessed using the Numeric Rating Scale – Visualized (22). For patients with delirium or a RASS below −3, we used the Behavioral Pain Scale (BPS) or the BPS for Non-Intubated (BPS-NI) (23). Analgesia was considered sufficient if BPS (-NI) was below 5 or NRS-V was below 4.
The study protocol comprised three serum melatonin assessment periods (SMAP-A, -B, and -C). All included patients completed the SMAP-A, which began the morning after ICU admission. If a patient was prepared for ICU discharge upon completing either SMAP-A or SMAP-B, no further assessments were conducted. Every morning at 06:00 am, the patient’s depth of sedation was evaluated (Sedation Checkup Period [SCP]). SMAP-B and SMAP-C were only initiated if the patient had a RASS score of –3 or higher. Depending on the patient’s sedation status, multiple days (SCPs) could pass before starting either SMAP-B or SMAP-C. During every SMAP, blood samples were taken from the arterial line every 4 hours, starting at 08:00 am and ending at 08:00 am the following day, for a total of seven samples (Supplementary Fig. 2, http://links.lww.com/CCM/H465).
Assessment of Circadian Melatonin Secretion
Blood samples of 10 mL each were taken from the arterial cannula and immediately centrifuged to extract serum. Melatonin concentrations were determined using a 125-J radioimmunoassay (type BA 3300, Labor Diagnostika Nord GmbH, Nordhorn, Germany; measurement range: 0/3.0–300 pg/mL and sensitivity: 1.6 pg/mL according to information by the manufacturer).
Statistical Analysis
The sample size calculation for patients was done a priori. Supposing a difference in events of delirium of 70% (standard room) versus 35% (modified room) with ɑ = 5% (two-sided) and a power of 80%, 37 patients for each group were required. Results were expressed as medians with percentile range (25th–75th percentile) in case of continuous variables; absolute and relative frequencies were used for categorical and dichotomous variables. Because of limited sample sizes and nonsymmetrically distributed observations, we preferably applied nonparametric statistics. ICU delirium was univariately tested using the nonparametric (exact) Mann-Whitney and Fisher exact tests for independent groups. Furthermore, a Generalized Linear Model (GLM) with Poisson log-linear distribution was applied for a more complex analysis of the delirium counts (24). Delirium severity was analyzed, considering the observations’ dependency over time. Generalized Estimating Equations (GEE) with a Poisson log-linear model were applied to analyze the impact of different rooms on delirium severity over time (25). Differences in delirium severity between standard and modified rooms with respect to the whole-time course were also analyzed using multivariate nonparametric analysis of longitudinal data in a two-factorial design (MANOVA) (first factor [independent], groups (rooms); second factor [dependent], study visits) (26). This cumulates in three tests: differences in groups, significant changes in time, and interactions between groups and time. When appropriate, multivariate nonparametric covariance analysis of longitudinal data (MANCOVA) using baseline values and other influencing factors as covariates was complemented (27). The same procedure has been applied to further interesting time-dependent secondary endpoints such as serum melatonin levels. We considered a p value of less than 0.05 (two-sided) statistically significant. All tests should be understood as constituting exploratory data analysis in that no adjustments for multiple testing were made. The p values achieved are to be interpreted in the sense of a pilot study, that is, do not allow any confirmatory generalization. Numerical calculations were performed using SAS, Version 9.4 (TS1M3) (SAS Institute, Cary, NC), Stata Statistical Software: Release 17 (StataCorp LLC, College Station, TX), IBM SPSS Statistics, Version 28 (SPSS, an IBM Company, Chicago, IL), and the R Project for Statistical Computing, Version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Illustration of Relative Effects and Relative Effect Sizes
The results in the MANOVA and MANCOVA can be illustrated by so-called relative effects and relative effect sizes. Regarding different groups of a study in the MANOVA, the relative effect RE (X) of a clinical parameter X in a certain group corresponds to the treatment effect of that group (on a scale between 0 and 1 according to probability) relative to all groups; in other words, with respect to an average treatment effect. X has a greater relative effect in a group relative to all groups if RE (X) greater than 1 (middle of the scale). RE (X) is greater than 1 means no relative effect of X. The analogous effect RE (X|C) in the MANCOVA, henceforth referred to as relative effect size, results from a linear combination of the relative effect RE (X) of the target variable X and the relative effect RE (C) of the covariate according to RE (X|C) = RE (X) + r × RE (C) (r, regression parameter of the covariate).
RESULTS
We screened 1165 patients between June 28, 2014, and June 1, 2017. Ninety-nine patients were enrolled. The most frequent reasons for screening failure included an anticipated ICU-LOS less than 48 hours (n = 267), an ICB or elevated ICP (n = 157), and patients with substantial recent ICU exposure (n = 143). A total of 12 patients or their representatives withdrew IC during study assessments. Three patients in the standard rooms and four in the modified rooms died before obtaining the first valid delirium assessment. Six patients were discharged before completion of the study period due to internal capacity allocation decisions made by the leading ICU physician in charge (Supplementary Fig. 3, http://links.lww.com/CCM/H465). General patient characteristics and outcome parameters did not reveal statistical differences between groups (Table 1). Because sedation intensity within the first 48 hours has been shown to increase the hazard of developing delirium (28), we compared early sedation status between groups: The median RASS on the first study day for patients in standard rooms was lower compared with patients in modified rooms but did not reach statistical significance (–4 [–5 to 0] vs. 0 [–5 to 0], p = 0.0626) (Fig. 2). The relative frequency of assessments with sufficient analgesia was 81% versus 80% for standard and modified rooms, respectively.
TABLE 1.
Demographics and General Outcome Parameters of the Patients
| Parameter | Standard Room (n = 37) | Modified Room (n = 37) | p |
|---|---|---|---|
| Age, yr | 59 (43–71)a | 57 (42–67)a | 0.5665b |
| Male, n | 14 | 16 | 0.8130c |
| Emergency admission, n | 27 | 25 | 0.8000c |
| Acute Physiology and Chronic Health Evaluation II at admission | 24 (15–29)a | 22 (12–16)a | 0.3866b |
| Simplified Acute Physiology Score II at admission | 42 (31–61)a | 38 (25–51)a | 0.1784b |
| Sepsis-related Organ Failure assessment score at admission | 9 (6–11)a | 7 (4–10)a | 0.1023b |
| Surgical Intervention, n | 21 | 21 | 1.0000c |
| ICU length of stay, d | 14 (9–23)a | 11 (5–20)a | 0.3408b |
| Mechanical ventilation, hr | 235 (68–508)a | 211 (46–434)a | 0.6670b |
| Hospital length of stay, d | 23 (15–38)a | 23 (18–38)a | 0.6495b |
| Discharge to home, n | 14 | 20 | 0.2430c |
| In hospital mortality, n | 4 | 3 | 1.0000c |
Values are presented as medians with an interquartile range (25th–75th) in parentheses.
Exact Mann-Whitney U test.
Fisher exact test.
A p < 0.05 was considered significant.
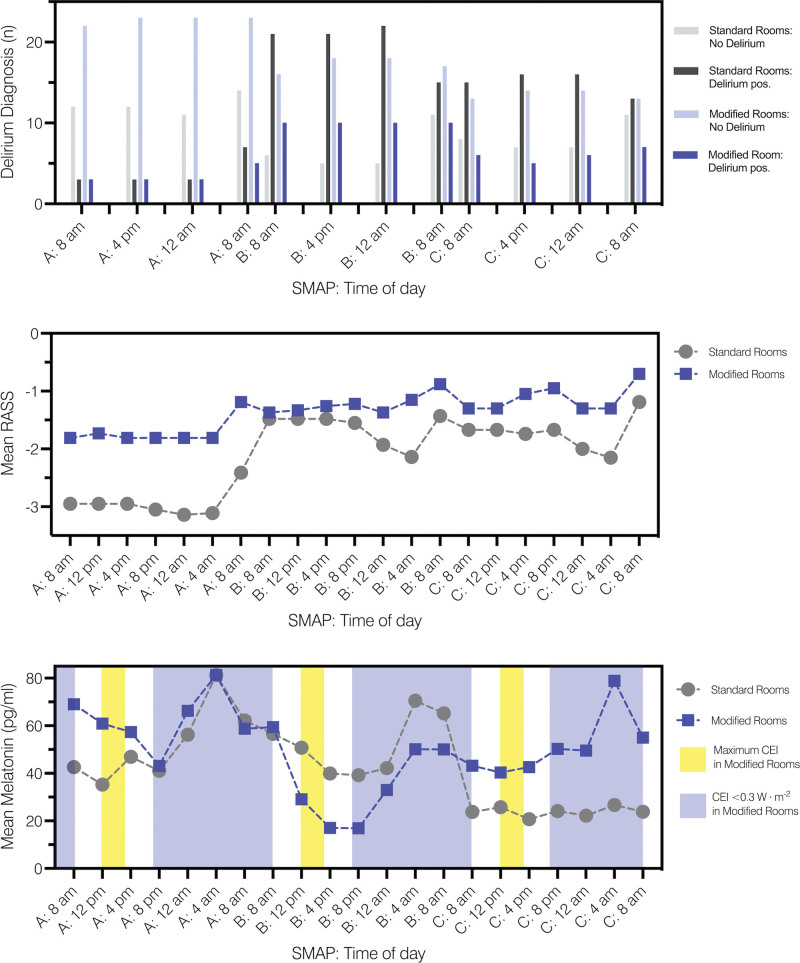
Figure 2.
Delirium diagnosis, mean Richmond Agitation Sedation Scale (RASS) values, and mean serum melatonin levels during serum melatonin assessment periods (SMAP) A, B, and C. CEI = circadian effective irradiance.
Prevalence and Severity of Delirium
Seventy-six percent (n = 28) of patients in the standard rooms developed delirium compared with 46% of patients (n = 17) in the modified rooms (p = 0.017) (Fig. 2). The GLM with a Poisson log-linear distribution revealed a significant association between the prevalence of delirium and the type of ICU room (p = 0.011): Patients treated in the standard rooms had a 1.7-fold increased risk for developing delirium compared with patients treated in the modified rooms (odds ratio [OR] = 1.65; 95% CI, 1.12–2.41).
Delirium severity was significantly lower for patients treated in modified compared with patients in standard rooms (0 [interquartile range, IQR, 0–2] vs. 3 [IQR, 1–4], p < 0.0001). The GEE with a Poisson log-linear model revealed a significant association between the ICDSC score and the ICU rooms: Patients in standard rooms had a 2.3-fold higher delirium severity than those in modified rooms (OR = 2.292; 95% CI, 1.582–3.321). Furthermore, MANOVA for delirium severity showed a statistically significant difference between groups (p < 0.0001) with higher ICDSC scores for patients in standard rooms. In addition, MANOVA revealed significant systematic changes in ICDSC scores over time (p < 0.0001). Finally, MANCOVA, adjusted for baseline (first ICDSC assessment), revealed not only a significant influence of the baseline ICDSC scores over time (p = 0.0243) but also significant differences between rooms over the entire assessment period (p = 0.0183).
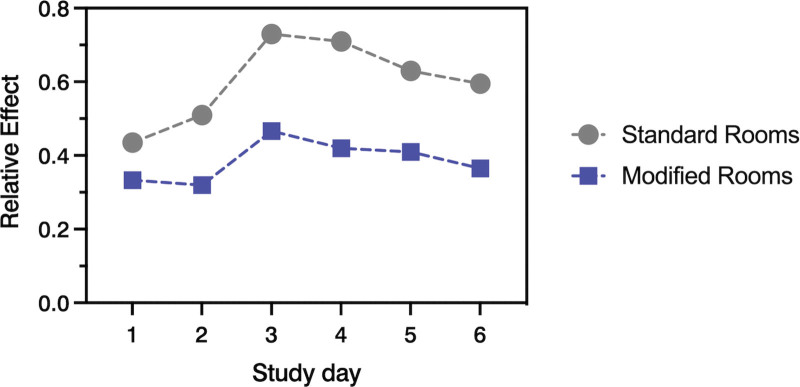
Because the depth of sedation is an independent risk factor for ICU delirium (28), we included baseline ICDSC values and RASS as covariates in the MANCOVA. Statistics confirmed the influence of baseline (p = 0.0243) but revealed that the sedation status of patients had no significant effect on the course of delirium severity during the study intervention (p = 0.2262). Delirium severity, adjusted for both covariates, significantly differed between patients in standard and modified rooms during study days (p = 0.0162) (Fig. 3).
Figure 3.
Delirium severity: relative effects from scoring results with the intensive care delirium screening checklist (ICDSC) between patients in standard and modified rooms. ICDSC scorings were conducted once daily at the beginning and end of each serum melatonin assessment period (SMAP).
Lighting Intervention and Serum Melatonin Levels
The multivariate, nonparametric covariance analysis of longitudinal data revealed a significant influence of EC on the course of serum melatonin for SMAP-A, -B, and -C (p < 0.0001) (Fig. 2). Serum melatonin levels under the influence of EC were significantly different between patients in standard and modified rooms during the whole-time course of SMAP-A (p = 0.0002) but not for SMAP-B and SMAP-C (p = 0.0998 and p = 0.5904, respectively). Patients in both groups showed systematic time effects for SMAP-A, -B, and -C, referring to significant changes in serum melatonin levels in time (p < 0.001). Furthermore, there were significant interactions for all assessment periods (p < 0.001), revealing that differences between rooms were not the same over time but varied during specific periods (Table 2).
TABLE 2.
Multivariate Test Statistics of Serum Melatonin Under the Influence of Circadian Effective Irradiance
| Hypothesis | p | ||
|---|---|---|---|
| SMAP-A | SMAP-B | SMAP-C | |
| Difference between groups during entire SMAP | 0.0002 | 0.0998 | 0.5904 |
| Systematic time effect | < 0.0001 | < 0.0001 | < 0.0001 |
| Interactions: Differences do change during SMAP between groups | < 0.0001 | < 0.0001 | 0.0002 |
SMAP = serum melatonin assessment period, SMAP-A = first day of intervention, SMAP-B = third day of intervention or later, SMAP-C = fifth day of intervention or later.
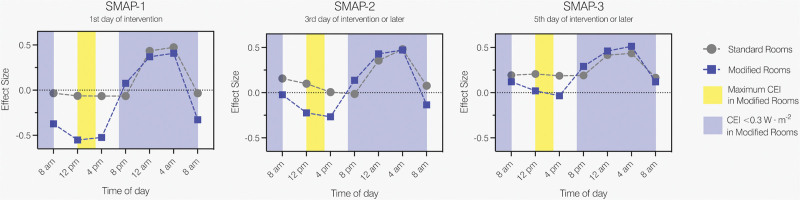
Figure 4 plots the relative effect sizes of serum melatonin levels under the influence of EC for patients in standard and modified rooms during SMAP-A, -B, and -C: Differences in relative effect sizes between rooms are clearly recognizable between 08:00 am and 04:00 pm, which disappear during evening and night, representing different deviations between rooms in time or interactions.
Figure 4.
Relative effect sizes of serum melatonin levels under the influence of circadian effective irradiance (EC). CEI = circadian effective irradiance, SMAP = serum melatonin assessment period, SMAP-A = first day of intervention, SMAP-B = third day of intervention or later, SMAP-C = fifth day of intervention or later.
DISCUSSION
Reshaping the ICU environment using architectural interventions and targeting patient-specific outcomes has drawn increasing interest among researchers within the last decade. The shift toward less sedation enables caregivers, patients, and relatives to use room design features to actively support the healing process (6, 10, 29). Zaal et al (30) were among the first to study the effect of comprehensive change in ICU room design on delirium. They considered the patients’ perceptions of the bedside environment as the primary concern, with a key critical focus on the formation of a physiologic day–night rhythm. Besides providing single-bed rooms, the design featured large windows to ensure appropriate lighting conditions. The authors found a nonsignificant decrease of 0.4 days in delirium duration. Major limitations of the study led by Zaal et al (30) were the before-and-after design and the potential alterations in adherence to guidelines and treatment protocols, which might have changed during the 16-month study period. Conversely, the strength of our study is that we analyzed delirium outcomes for patients between rooms in a two-armed parallel group design. Furthermore, we monitored delirium severity, revealing significantly lower scores for patients in the modified rooms. This difference in symptom severity remained significant in the longitudinal analysis even after adjustment for baseline ICDSC and depth of sedation. With respect to study data that prove the prediction of outcomes depending on the duration and severity of delirium, our results become even more important (31, 32). It suggests that the design interventions may promote sustainable effects during the ICU stay. Besides others, our room modifications were intended to entrain and maintain circadian rhythmicity. Critically ill patients are especially susceptible to circadian disruption, mainly due to the high severity of illness, sensory deprivation, and exposure to environments with inadequate sound and lighting. Over two decades ago, research discovered disrupted circadian melatonin secretion in patients with sepsis (33). A link between inadequate sensory input, pathologic melatonin patterns, sleep disturbances, and delirium in the ICU has long been postulated (34). There is increasing evidence for a “loss of chronofitness” during critical care, which has also been shown on the molecular level (35). Interestingly, circadian clock gene alterations emerge within the first few days of ICU treatment rather than being evident upon admission (36). Hence, guidelines for ICU design do recommend adequate artificial lighting with a high-intensity light source that should feature adjustable illuminance levels (37). Compliance with distinct photometric parameters is mandatory to ensure the circadian efficacy of lighting (38, 39). A retrospective study revealed that mechanically ventilated patients with access to daylight and window views experienced shorter ICU-LOS than those in windowless rooms (40). Still, natural light that enters the room through windows is poorly controllable: EV shows an exponential decline with increasing distance from the window (41). This partially explains why a study comparing delirium among patients treated in rooms with and without windows failed to show significant results (42). Consequently, we decided on a highly customizable DLS with a large surface area. The DLS underwent extensive evaluation before clinical use to secure sufficient EC at the patients’ eye level (13). Our study results revealed different circadian melatonin patterns between patients in both groups, attributable to distinct photometric parameters of the DLS and the FT-CL. These findings contrast previous results from a randomized controlled trial (RCT), which showed no effect of bright-light therapy on ICU delirium (43). In this RCT, the DLS emitted up to 800 lux from a relatively small area, possibly increasing glare-related effects and potentially contributing to the onset of delirium. The DLS used in our study has been extensively evaluated before implementation and delivers up to fourfold higher EV values without crossing the threshold of relative glare. This could constitute a key factor explaining the distinct outcome differences observed between groups in our study. Considering that our study assesses the effects of a multicomponent design intervention, the observed changes in delirium cannot be solely attributed to differing levels of EC in rooms. The DLS offers features expanding beyond bright-light therapy, including visual aids for reorientation and modules for active cognitive training (10, 14). Furthermore, the modified rooms provide reduced sound pressure levels and permanently installed patient lifters at each bedside to foster early mobilization (12). Despite the absence of systematic data collection on the usage of mobility aids and cognitive training sessions, it does appear that these interventions may have contributed to the differences in delirium outcomes. From the perspective of study methodology, this poses a limitation, making it impossible to distinguish which intervention contributed to the observed differences in outcomes and to estimate their respective impacts. Conversely, the expansive multifaceted change in design may be considered a notable strength and could potentially elucidate the significant differences in delirium outcomes relative to previous investigations (30, 42, 43).
Our pilot study used an open-labeled design without randomization, a major limitation. The room modifications may also have impacted caregivers’ behavior, who would have been biased toward keeping patients less sedated, rooted in the belief that the improved rooms inherently offer better conditions.
A study performed in a surgical ICU revealed disruptions in circadian melatonin only for delirious patients with pathologically elevated levels in the subgroup that developed postoperative infections. Delirious patients without further complications failed to show the typical nocturnal rise in melatonin present before surgery (44). Our data confirm the large variability in melatonin levels among patients. Hence, it is expected that a blanket approach to oral melatonin supplementation for all ICU patients does not prevent delirium (45). The future might be a bundle of interventions, including DLS and melatonin, which must be tailored depending on the patient’s clinical condition.
CONCLUSIONS
This proof-of-concept pilot study suggests that a comprehensive set of changes to ICU design may reduce the incidence and severity of delirium, enhancing the evidence for the effectiveness of nonpharmacologic strategies in delirium management. Integrating a DLS with adequate light intensity into the room could potentially impact delirium outcomes in critically ill patients by modulating circadian melatonin rhythms. Randomized multicenter studies are needed to prove the efficacy of these measures.
ACKNOWLEDGMENTS
The authors thank the nursing and medical staff for their efforts and the patient’s cooperation during the study. Furthermore, the authors want to thank the ICU Design Working Group for their precious and inspiring work during the development and rebuilding process of the ICU rooms. The authors thank Friedrich and Laura Bluemich, Paul Brauer, Anke Brockmann, Lisa-Maria Lochner, Annika Schaffrin, Carola Scholz, and Hannah Uhrlau for supporting the collection and analysis of the study data.
Supplementary Material
Footnotes
Drs. Spies and Luetz were involved in conception of the work. Dr. Spies, Piazena, Deja, Willemeit, and Luetz were involved in design of the work. Dr. Luetz contributed to the acquisition of data for the work. Drs. Piazena, Wernecke, and Luetz contributed to the analysis of data for the work. Drs. Spies, Piazena, Wernecke, and Luetz contributed to the interpretation of data for the work. Drs. Wernecke and Luetz contributed to the drafting the work critically for important intellectual content. Drs. Spies, Piazena, Deja, Wernecke, Willemeit, and Luetz were involved in revising the work critically for important intellectual content. Drs. Spies, Piazena, Deja, Wernecke, Willemeit, and Luetz were involved in final approval of the version to be published. Drs. Spies, Piazena, Deja, Wernecke, Willemeit, and Luetz were involved in agreement to be accountable for all aspects of the work. Drs. Wernecke and Luetz had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Dr. Luetz received funding from the Berlin Institute of Health Charité Clinician Scientist Program for conducting this study. Dr. Spies reports grants from the German Research Society, the German Aerospace Center, the Einstein Foundation Berlin, the Federal Joint Committee (G-BA), the Project Management Agency, the Non-Profit Society Promoting Science an Education, the European Society of Anaesthesiology and Intensive Care, Baxter Deutschland GmbH, Cytosorbents Europe GmbH, Edwards Lifesciences Germany GmbH, Fresenius Medical Care, Grünenthal GmbH, Masimo Europe Ltd., Pfizer Pharma PFE GmbH, Georg Thieme Verlag, Dr. F. Köhler Chemie GmbH, Sintetica GmbH, Philips Healthcare, Stiftung Charité, AGUETT ANT Deutschland GmbH, AbbVie Deutschland GmbH and KG, Amomed Pharma GmbH, lnTouch Health, Copra System GmbH, Correvio GmbH, Drägerwerk AG KGaA, the Max-Planck-Gesellschaft zur Förderung der Experiencing the risk of overutilizing opioids among patients with non-tumor chronic pain in ambulant care (ERONA) 2019–2021 Wissenschaften e.V., the Deutsche Gesellschaft für Anästhesiologie and lntensivmedizin and the Federal Ministry of Education and Research, outside the submitted work. Dr. Spies’ institution received funding from the Federal Ministry. Dr. Willemeit reports consulting fees from Trockland, Mercedes Benz, GSG Development, Hines Interests Limited Partnership and Signa Holding GmbH Development, outside the submitted work. Dr. Willemeit reports financial relationships with GRAFT Gesellschaft von Architekten mbH, GRAFT Designhaus GmbH and Graft GbR. Dr. Willemeit’s institution received funding from the German Ministry for Economic Research Funding (number KF2882602KJ2) and Charité Campus Virchow-Klinikum, Charité Facility Management; they disclosed relationships with Lighting Consultants LichtKunstLicht, Philips Lighting, and Art&Com; they received support for article research from the German Ministry for Economics Research Funding (number KF2882602KJ2). Dr. Luetz reports personal fees from Avanos Medical, Drägerwerk AG KGaA, Dr. Franz Köhler Chemie GmbH, Hill-Rom Holdings and Philips Healthcare outside the submitted work. He reports a grant from Philips Healthcare outside the submitted work which was paid into departmental funds and Charité Clinician Scientist Program. Drs. Spies (inventor), Willemeit (assignee), and Luetz (inventor) report issued patents related to the submitted work: European Patent 3 174 589 from July 31, 2015, European Patent 3 174 588 from July 31, 2015 and US Patent US 2017/0224950 A1 from August 10, 2017. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Written informed consent was obtained from all patients or their authorized surrogates to publish study results, which must not include information that may identify individual persons.
ICU Design Working Group are as follows: Benjamin Albrecht, Claudia Denke, Kai Dolata, Ingo Fietze, Annette Finke, Jing he, Dennis Hegic, Susanne Kronfeld, Ingrid Masswig, Thomas Penzel, Joachim Quantz, Joachim Sauter, Andreas Sund, Norman Wassmuth, Steffen Weber-Carstens, Bjoern Weiss, Ute Winninghoff, and Susanne Zimmermann.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Contributor Information
Collaborators: Benjamin Albrecht, Claudia Denke, Kai Dolata, Ingo Fietze, Annette Finke, Jing he, Dennis Hegic, Susanne Kronfeld, Ingrid Masswig, Thomas Penzel, Joachim Quantz, Joachim Sauter, Andreas Sund, Norman Wassmuth, Steffen Weber-Carstens, Bjoern Weiss, Ute Winninghoff, and Susanne Zimmermann
REFERENCES
- 1.Ely E, Shintani A, Truman B, et al. : Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004; 291:1753–1762 [DOI] [PubMed] [Google Scholar]
- 2.Pandharipande PP, Girard TD, Jackson JC, et al. ; BRAIN-ICU Study Investigators: Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369:1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girard TD, Exline MC, Carson SS, et al. ; MIND-USA Investigators: Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med 2018; 379:2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smit L, Dijkstra-Kersten SMA, Zaal IJ, et al. : Haloperidol, clonidine and resolution of delirium in critically ill patients: A prospective cohort study. Intensive Care Med 2021; 47:316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotfis K, van Diem-Zaal I, Roberson SW, et al. : The future of intensive care: Delirium should no longer be an issue. Crit Care 2022; 26:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devlin JW, Skrobik Y, Gélinas C, et al. : Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 7.Krampe H, Denke C, Gülden J, et al. : Perceived severity of stressors in the intensive care unit: A systematic review and semi-quantitative analysis of the literature on the perspectives of patients, health care providers and relatives. J Clin Med 2021; 10:3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisani MA, Friese RS, Gehlbach BK, et al. : Sleep in the intensive care unit. Am J Respir Crit Care Med 2015; 191:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Telias I, Wilcox ME: Sleep and circadian rhythm in critical illness. Crit Care 2019; 23:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luetz A, Grunow J, Mörgeli R, et al. : Innovative ICU solutions to prevent and reduce delirium and post-intensive care unit syndrome. Semin Respir Crit Care Med 2019; 40:673–686 [DOI] [PubMed] [Google Scholar]
- 11.Luetz A, Uhrlau H, Piazena H, et al. : Modification of ICU environment is associated with reduced incidence of delirium—results from the VITALITY study. Intensive Care Med Exp 2018; 40:1305 [Google Scholar]
- 12.Luetz A, Weiss B, Penzel T, et al. : Feasibility of noise reduction by a modification in ICU environment. Physiol Meas 2016; 37:1041–1055 [DOI] [PubMed] [Google Scholar]
- 13.Luetz A, Piazena H, Weiss B, et al. : Patient-centered lighting environments to improve health care in the intensive care unit. Clin Health Promotion 2016; 6:5–12 [Google Scholar]
- 14.Luetz A, He J, Weiss B, et al. : 850: Parameterized visual content for symptom control in critically ill patients. Crit Care Med 2013; 41:A212 [Google Scholar]
- 15.Brainard GC, Hanifin JP, Greeson JM, et al. : Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci 2001; 21:6405–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thapan K, Arendt J, Skene DJ: An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol 2001; 535:261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Light and lighting: Basic terms and criteria for specifying lighting requirements, 2018. Available at: 10.3403/30326220. Accessed July 20, 2023 [DOI]
- 18.Ely EW, Margolin R, Francis J, et al. : Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001; 29:1370–1379 [DOI] [PubMed] [Google Scholar]
- 19.Ouimet S, Riker R, Bergeron N, et al. : Subsyndromal delirium in the ICU: Evidence for a disease spectrum. Intensive Care Med 2007; 33:1007–1013 [DOI] [PubMed] [Google Scholar]
- 20.Collet MO, Nielsen AH, Larsen LK, et al. : Delirium and delirium severity screening in the intensive care—correspondence of screenings tools. Aust Crit Care 2023. Jul 10. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Sessler CN, Gosnell MS, Grap MJ, et al. : The Richmond Agitation–Sedation Scale. Am J Respir Crit Care Med 2002; 166:1338–1344 [DOI] [PubMed] [Google Scholar]
- 22.Chanques G, Viel E, Constantin J, et al. : The measurement of pain in intensive care unit: Comparison of 5 self-report intensity scales. Pain 2010; 151:711–721 [DOI] [PubMed] [Google Scholar]
- 23.Chanques G, Payen J-F, Mercier G, et al. : Assessing pain in non-intubated critically ill patients unable to self report: An adaptation of the Behavioral Pain Scale. Intensive Care Med 2009; 35:2060–2067 [DOI] [PubMed] [Google Scholar]
- 24.McCullagh P, Nelder JA: Generalized Linear Models. Boca Raton, Chapman & Hall/CRC, 1997 [Google Scholar]
- 25.Wang Y: Generalized estimating equations. By Ziegler A.. New York, NY, Springer, 2011, 159 pages. €49.95 (hardback). ISBN 9781461404989. Aust N Zealand J Stat 2013; 55: 61–61 [Google Scholar]
- 26.Brunner E, Domhof S, Langer F: Nonparametric Analysis of Longitudinal Data in Factorial Experiments. New York, NY, J. Wiley, 2002 [Google Scholar]
- 27.Bathke A, Brunner E: Recent advances and trends in nonparametric statistics. In: Recent Advances and Trends in Nonparametric Statistics. Akritas MG, Politis Dimitris N. (Eds), Amsterdam, Elsevier, 2003, pp 109–120 [Google Scholar]
- 28.Shehabi Y, Bellomo R, Kadiman S, et al. ; Sedation Practice in Intensive Care Evaluation (SPICE) Study Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group: Sedation intensity in the first 48 hours of mechanical ventilation and 180-day mortality. Crit Care Med 2018; 46:850–859 [DOI] [PubMed] [Google Scholar]
- 29.Vincent J-L, Shehabi Y, Walsh TS, et al. : Comfort and patient-centred care without excessive sedation: The eCASH concept. Intensive Care Med 2016; 42:962–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaal IJ, Spruyt CF, Peelen LM, et al. : Intensive care unit environment may affect the course of delirium. Intensive Care Med 2013; 39:481–488 [DOI] [PubMed] [Google Scholar]
- 31.Lindroth H, Khan BA, Carpenter JS, et al. : Delirium severity trajectories and outcomes in ICU patients defining a dynamic symptom phenotype. Ann Am Thorac Soc 2020; 17:1094–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pisani MA, Kong SYJ, Kasl SV, et al. : Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med 2009; 180:1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mundigler G, Delle-Karth G, Koreny M, et al. : Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis*. Crit Care Med 2002; 30:536–540 [DOI] [PubMed] [Google Scholar]
- 34.Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, et al. : Sleep and delirium in ICU patients: A review of mechanisms and manifestations. Intensive Care Med 2009; 35:781–795 [DOI] [PubMed] [Google Scholar]
- 35.Lachmann G, Ananthasubramaniam B, Wünsch VA, et al. : Circadian rhythms in septic shock patients. Ann Intensive Care 2021; 11:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz E, Diaz I, Del Busto C, et al. : Clock genes disruption in the intensive care unit. J Intensive Care Med 2019; 35:1497–1504 [DOI] [PubMed] [Google Scholar]
- 37.Thompson DR, Hamilton DK, Cadenhead CD, et al. : Guidelines for intensive care unit design*. Crit Care Med 2012; 40:1586–1600 [DOI] [PubMed] [Google Scholar]
- 38.Nagare R, Rea MS, Plitnick B, et al. : Nocturnal melatonin suppression by adolescents and adults for different levels, spectra, and durations of light exposure. J Biol Rhythms 2019; 34:178–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss B, Spies C, Piazena H, et al. : Exposure to light and darkness and its influence on physiological measures of intensive care unit patients—a systematic literature review. Physiol Meas 2016; 37:R73–R87 [DOI] [PubMed] [Google Scholar]
- 40.Jafarifiroozabadi R, Joseph A, Bridges W, et al. : The impact of daylight and window views on length of stay among patients with heart disease: A retrospective study in a cardiac intensive care unit. J Intensive Med 2023; 3:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bullough J, Rea MS, Stevens RG: Light and magnetic fields in a neonatal intensive care unit. Bioelectromagnetics 1996; 17:396–405 [DOI] [PubMed] [Google Scholar]
- 42.Wunsch H, Gershengorn H, Mayer SA, et al. : The effect of window rooms on critically ill patients with subarachnoid hemorrhage admitted to intensive care. Crit Care 2011; 15:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simons KS, Laheij RJF, van den Boogaard M, et al. : Dynamic light application therapy to reduce the incidence and duration of delirium in intensive-care patients: A randomised controlled trial. Lancet Respir Med 2016; 4:194–202 [DOI] [PubMed] [Google Scholar]
- 44.Shigeta H, Yasui A, Nimura Y, et al. : Postoperative delirium and melatonin levels in elderly patients. Am J Surg 2001; 182:449–454 [DOI] [PubMed] [Google Scholar]
- 45.Wibrow B, Martinez FE, Myers E, et al. : Prophylactic melatonin for delirium in intensive care (Pro-MEDIC): A randomized controlled trial. Intensive Care Med 2022; 48:414–425 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.