Abstract
DNA supercoiling in the chloroplast of the unicellular green alga Chlamydomonas reinhardtii was found to change with a diurnal rhythm in cells growing in alternating 12-h dark–12-h light periods. Highest and lowest DNA superhelicities occurred at the beginning and towards the end of the 12-h light periods, respectively. The fluctuations in DNA supercoiling occurred concurrently and in the same direction in two separate parts of the chloroplast genome, one containing the genes psaB, rbcL, and atpA and the other containing the atpB gene. Fluctuations were not confined to transcribed DNA regions, indicating simultaneous changes in DNA conformation all over the chloroplast genome. Because the diurnal fluctuations persisted in cells kept in continuous light, DNA supercoiling is judged to be under endogenous control. The endogenous fluctuations in chloroplast DNA topology correlated tightly with the endogenous fluctuations of overall chloroplast gene transcription and with those of the pool sizes of most chloroplast transcripts analyzed. This result suggests that DNA superhelical changes have a role in the regulation of chloroplast gene expression in Chlamydomonas.
Recurrent diurnal fluctuations of molecular, biochemical, and physiological processes are common in organisms living in daily light-dark regimens (6). In many cases these fluctuations persist, at least for a few cycles, under constant conditions, suggesting that they are under the control of an endogenous circadian timing mechanism. The nature of the circadian pacemaker remains elusive, despite considerable progress in recent years in pinpointing the components of rhythmicity at the molecular level in eukaryotic and prokaryotic organisms (11).
Various analyses of a number of plant and algal circadian systems indicate that regulation of transcription can account, at least in part, for endogenous fluctuations of transcript levels (13, 27, 29, 30, 39). For example, a strong correlation has been found between variations in pool sizes of individual RNAs and variations in transcription rates, measured by run-on transcriptional assays or by in vivo labeling techniques (16, 34). 5′ sequences of genes, whose expression is known to be circadian regulated, imposed circadian fluctuations on levels of reporter gene transcripts (12, 19, 22, 27–29), and cis-acting sequences have been delineated upstream of the wheat and Arabidopsis cab-1 and cab-2 genes that conferred circadian rhythmicity onto levels of transcripts of chloramphenicol acetyltransferase (CAT) and β-glucuronidase (GUS) reporter genes in transgenic tobacco (12, 27, 29).
In cells of the unicellular green alga Chlamydomonas reinhardtii growing in 12-h light–12-h dark cycles, the abundance of a number of nuclear and chloroplast transcripts has been found to fluctuate diurnally, including transcripts of the nuclear cab-2 gene, which encodes a member of the family of chlorophyll a/b binding proteins (17), and transcripts of the chloroplast genes psaB, atpB, atpA, and tufA, encoding a photosystem I reaction center protein, the α and β subunits of the chloroplast ATPase complex, and elongation factor Tu, respectively (16, 23, 34). Expression of the cab, atpA, atpB, and tufA genes followed an endogenous rhythm, whereas levels of psaB gene transcripts were found to be regulated primarily by light (17, 34). A detailed analysis of tufA gene expression showed that levels of tufA transcripts exhibit robust circadian oscillations in Chlamydomonas cells grown in daily light-dark cycles (16).
The molecular mechanisms involved in controlling endogenous fluctuations of chloroplast transcript levels are not known. Because changes in DNA conformation have been shown to play a role in the control of bacterial gene expression (4, 26, 31, 33) and in transcription by maize chloroplast RNA polymerase in vitro (20, 37), we monitored relative DNA supercoiling in the chloroplast of Chlamydomonas cells grown in light-dark cycles in order to evaluate its importance for endogenous regulation of chloroplast transcript levels. We found fluctuations of DNA superhelicity in two separate regions of the chloroplast chromosome in cells growing in 12-h dark–12-h light cycles and 12-h dark–24-h light cycles. The superhelical changes correlated with changes in rates of chloroplast gene transcription, suggesting a contribution of DNA conformation to the control of chloroplast gene expression in Chlamydomonas.
MATERIALS AND METHODS
Growth of algae.
C. reinhardtii, atpB-defective mutant strain CC-373 (ac-uc-2-21), obtained from the Chlamydomonas Genetics Center at Duke University, Durham, N.C., and photosynthetic transformants of that mutant were grown on high-salt (HS) minimal medium (38) or HS minimal medium supplemented with 2.5 g of potassium acetate per liter (for the mutant) as described previously (21). Wild-type and transformant cells were grown in 12-h dark–12-h light cycles (followed in some experiments by a 12-h dark–24-h light cycle) (light intensity, 500 W/m2), with daily dilutions to approximately 2 × 106 cells/ml at the beginning of each light period. Cell density was monitored by counting with a hemocytometer.
Cross-linking assay.
Changes in relative superhelicity in the atpB, psaB, rbcL, and atpA gene regions of the Chlamydomonas chloroplast chromosome were measured by in vivo cross-linking of the two strands of the DNA helix with 4′-hydroxymethyl-4,5′,8-trimethylpsoralen (HMT; HRI Associates, Berkeley, Calif.) or 4,5′,8-trimethylpsoralen (trioxsalen; Sigma) essentially as described previously (42). Cross-linking was done for 90 s in 20 ml of HS minimal medium with 2 × 107 cells/ml at an HMT or trioxsalen concentration of 6 μg/ml with black UV-A-emitting (366 nm) lightbulbs. The number of cells per milliliter, the concentration of the psoralen reagent, and the UV-A dose were adjusted so that cross-linking efficiency was approximately 50 to 90% (as seen by the Southern analysis described below) in the atpB 5′ region in cells harvested at the end of the dark period. After cross-linking, DNA was isolated as described previously (2). For determining the relative degree of cross-linking in different DNA sequences, 1.5 μg of the isolated DNA was digested with 15 U each of the restriction enzymes BamHI and PstI (for the atpB gene 5′ region), SpeI and PvuI (for the psaB gene 5′ region), PstI (for the rbcL gene region), AccI (for the atpA gene region), and BamHI (for the GUS reporter gene sequence), and after sodium acetate precipitation and alkali denaturation, the DNA sequences were separated in a neutral 1% agarose gel at 75 V. DNA was transferred from the gel onto a nylon membrane (Zetaprobe; Bio-Rad) by alkaline transfer according to the manufacturer’s instructions. The approximately 700-bp EcoRI-HpaI restriction fragment from the Chlamydomonas chloroplast atpB structural gene (atpB gene probe [2]), the approximately 1.1-kb BamHI 16 fragment from the Chlamydomonas chloroplast genome (psaB gene probe), the approximately 890-bp HindIII restriction fragment from the rbcL structural gene (rbcL probe [2]), the approximately 740-bp EcoRI-AccI restriction fragment from the 5′ region of the atpA gene (atpA probe [2]), and the approximately 1.9-kb BamHI-SacI restriction fragment from pBI221 (GUS probe [2]), were labeled with 32P-labeled random primer and hybridized to the DNA blots for 24 h at 65°C (8). Membranes were washed as described previously (8) and exposed to X-ray film with an intensifying screen at −80°C for 24 h.
Chloroplast transformation.
The chloroplast of the nonphotosynthetic mutant CC-373 was stably transformed by bombarding cells spread on agar plates with tungsten particles that were coated with chimeric DNA constructs essentially as described previously (1, 5). Photosynthetic transformants were selected for their ability to grow on HS minimal medium under high-light conditions (5). Transgenic cell lines were maintained on agar plates and, when needed for analysis, grown in liquid HS minimal medium. Transformants were screened repeatedly for the presence of the introduced reporter gene constructs.
RNA isolation and RNA gel blot analyses.
Total RNA was isolated from about 1.2 × 108 cells by sodium dodecyl sulfate-phenol extractions and LiCl purification (2). RNA gel blots were prepared as described previously (34). Blots were hybridized at 65°C to specific random primer 32P-radiolabeled DNA probes for 24 h and washed following the protocol of Church and Gilbert (8). The approximately 700-bp EcoRI-HpaI restriction fragment from the Chlamydomonas chloroplast atpB structural gene (2), the approximately 1.9-kb BamHI-SacI restriction fragment from plasmid pBI221, containing the complete GUS coding region (18), and the 1.1-kb BamHI 16 restriction fragment from Chlamydomonas chloroplast DNA were used as probes to detect atpB, GUS, and psaB gene transcripts, respectively. The washed membranes were exposed overnight at −80°C to X-ray film with an intensifying screen.
Plasmids.
The basic transformation vector into which all chimeric GUS reporter genes were cloned for stable introduction into the chloroplast genome of mutant CC-373 consisted of the 5.3-kb EcoRI-BamHI Chlamydomonas chloroplast DNA restriction fragment, originally isolated from the chloroplast BamHI 10 restriction fragment (47), ligated into pUC8 as described previously (2, 21). The algal fragment contains the atpB gene and extends to within the inverted repeat; DNA intended for insertion into the chloroplast genome was inserted into the first KpnI site beyond the terminus of the endogenous atpB gene (2) (see Fig. 2 for location of the chimeric GUS gene on the chloroplast chromosomes).
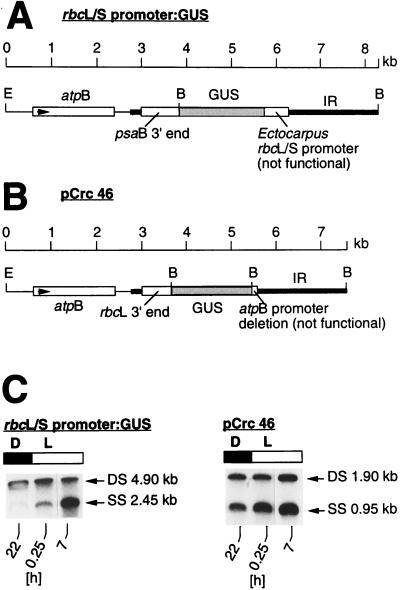
FIG. 2.
Changes in DNA superhelicities in untranscribed regions of the Chlamydomonas chloroplast genome. (A) Location of the chimeric rbcL/S promoter:GUS gene on the chromosome of transgenic Chlamydomonas. The gene is not transcribed, because the rbcL/S promoter from Ectocarpus does not function in Chlamydomonas. The ∼4.9-kb BamHI fragment, containing a portion of the inverted repeat (IR), the 580-bp rbcL/S sequence, and the coding region of the GUS gene (shaded dark gray), were examined for changes in superhelicity (shown in panel C). E, EcoRI; B, BamHI. (B) Location of the chimeric atpB promoter:GUS gene (pCrc46) with a deletion of the nonfunctional atpB promoter sequence (2) on the chloroplast chromosome of transgenic Chlamydomonas. Relative superhelicity was determined in the ∼1.9-kb BamHI fragment comprising the entire GUS coding region (shaded dark gray). Abbreviations are as defined for panel A. (C) DNA gel blot analyses of cross-linked DNA isolated from transgenic cells carrying the rbcL/S promoter:GUS gene construct (left) or the atpB promoter:GUS construct (right). Cells growing in 12-h light–12-h dark cycles were harvested at time points 22 h (dark), 0.25 h (light), and 7 h (light) and treated with trimethylpsoralen as described in Materials and Methods. Isolated cross-linked DNA was digested with BamHI and, after separation onto a 1% agarose gel, was transferred onto a nylon membrane. Both blots were hybridized to the ∼1.9-kb random primer 32P-labeled sequence of the GUS coding region. The double- and single-stranded DNA bands were visualized by autoradiography. Abbreviations are as defined in the legend to Fig. 1.
Plasmid pCrc34, containing the GUS structural gene under transcriptional control of the Chlamydomonas chloroplast atpB gene promoter region (224 bp) and terminated by 3′-flanking sequences from the Chlamydomonas chloroplast rbcL gene, was the starting plasmid for making deletions from both ends into the atpB promoter region. The construction of pCrc34 has been described previously (2). All chimeric atpB promoter:GUS deletion constructs used in this study were derived from pCrc34 as described earlier (2, 21).
Plasmid rbcL/S promoter:GUS, containing the putative promoter sequence of the plastidic rbcL/S gene of the brown alga Ectocarpus siliculosus fused to the coding region of the GUS gene, was constructed by first cloning the 580-bp BstBI-EcoRV restriction fragment from the Ectocarpus rbcL/S gene into the ClaI/EcoRV sites of pBluescript SK+ (Stratagene) followed by insertion of the XhoI-EcoRV fragment from the new plasmid into XhoI/SmaI-cut pCrc32 (3).
To construct plasmid psaB:GUS, containing the putative promoter of the Chlamydomonas chloroplast psaB gene fused to the GUS structural gene and terminated by the 3′ region of the Chlamydomonas chloroplast rbcL gene, a 618-bp DNA sequence from the 5′ region of the psaB gene was amplified from the Chlamydomonas chloroplast BamHI 8 fragment by the PCR with the 5′ and 3′ primers 5′-TCGCAGGTTCGAATCCTTC-3′ and 5′-TATCTTTCGAAGGGTGTTG-3′, respectively, both containing a BstBI restriction enzyme site. The PCR fragment was digested with BstBI and cloned into the ClaI site of pBluescript SK+ such that the 5′ end of the fragment was adjacent to the HindIII site of the Bluescript polylinker. A 351-bp psaB fragment was released by cutting the construct with NdeI (this site was filled in with the Klenow fragment of DNA polymerase) and XhoI and subcloned into EcoRV/XhoI-cut pBluescript SK+. After being cut with XhoI (the site was filled in with DNA polymerase) and EcoRI, the resulting fragment was inserted into EcoRI/SmaI-cut pCrc44 (2).
RESULTS
Chloroplast DNA conformation changes diurnally in Chlamydomonas cells growing in light-dark cycles.
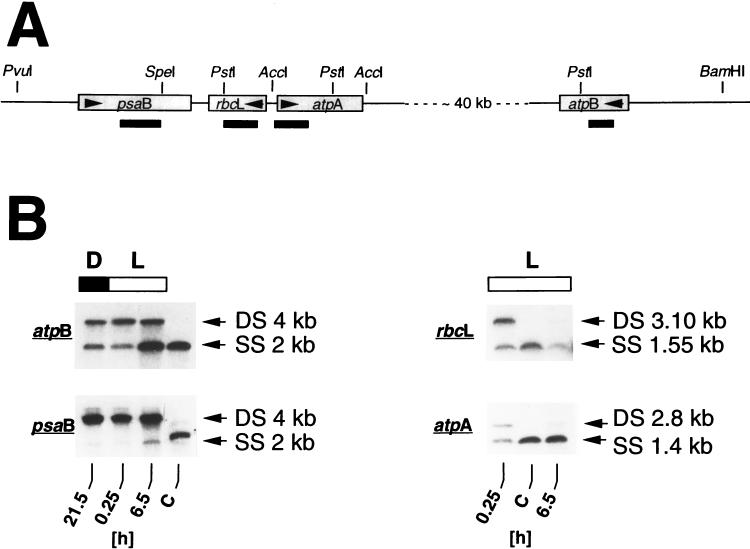
It had been determined previously (42) that the DNA conformation in at least three different regions of the Chlamydomonas chloroplast chromosome is more relaxed in cells growing in light than in cells growing in darkness. To find out whether the topology of Chlamydomonas chloroplast DNA actually fluctuates in cells growing in 12-h light–12-h dark cycles, we determined the relative degree of DNA supercoiling in different regions of the chloroplast genome at different time points in a 12-h dark–12-h light cycle (Fig. 1). The four DNA sequences studied were located in two different regions of the chloroplast chromosome separated by about 40 kb of DNA (Fig. 1A); one region contains the genes psaB, rbcL, and atpA, and the other region contains the atpB gene. Relative superhelicity was measured by the cross-linking assay developed by Vos and Hanawalt (44), as modified for Chlamydomonas by Thompson and Mosig (42). In this assay, the relative degree of superhelicity in a DNA sequence is probed by cross-linking the two strands of the DNA helix with psoralen in the presence of UV-A light. Cross-linking efficiency is proportional to the degree of supercoiling (36) and is visualized on Southern blots by the ratio of DNA in double- to single-stranded bands (the higher the ratio, the higher the superhelicity).
FIG. 1.
Changes in DNA superhelicity around Chlamydomonas chloroplast genes atpB, psaB, rbcL, and atpA in cells growing in a 12-h dark–12-h light regimen, as determined by the cross-linking assay. (A) Map of the DNA regions and location of the restriction fragments that were examined for relative changes of superhelicity with the cross-linking assay (for details of the cross-linking procedure, see Materials and Methods and reference 42). Genes are shown as shaded boxes. Arrows within the boxes indicate the direction of transcription. Restriction sites used in digestion of genomic DNA for the DNA gel (Southern) blots shown in panel B are indicated above the gene map, and the approximate sizes and locations of the probes used are indicated below the gene map. (B) DNA gel blot (Southern) analyses of cross-linked DNA to detect conformational changes in the DNA regions around the chloroplast atpB, psaB, rbcL, and atpA genes. Algae grown in light-dark cycles were harvested at the time points indicated below the autoradiograms. DNA was cross-linked in vivo and, after isolation, digested with BamHI/PstI (atpB), PvuI/SpeI (psaB), PstI (rbcL), and AccI (atpA), alkali denatured, and separated in a 1% agarose gel as described in Materials and Methods. Blots were hybridized to double-stranded random primer 32P-labeled DNA probes from the coding regions of the Chlamydomonas chloroplast genes (see Materials and Methods) as indicated in panel A. D, dark period (also indicated by a filled bar); L, light period (also indicated by an open bar); C, control DNA isolated at time point 6.5 h of the light period from algae that were not treated with psoralen; DS and SS, double-stranded and single-stranded restriction fragments, respectively. Fragment sizes are given in kilobase pairs (kb) for double-stranded DNA and in kilobases (kb) for single-stranded DNA. Ratios of double-stranded to single-stranded bands at time points 0.25 and 6.5 h in the light period, as calculated from laser densitometric readings of autoradiograms, respectively, are as follows: for atpB, 2.3 and 0.25; for psaB, 21.7 and 4.7; for rbcL, 1.6 and 0.2; for atpA, 0.6 and 0.06. The higher the ratio of DNA in double-stranded to single-stranded bands on the autoradiograms, the higher the degree of DNA supercoiling.
Relative superhelicity within a 4-kb sequence in the 5′ region of the atpB gene, as visualized by the ratio of double- to single-stranded DNA bands on Southern blots (Fig. 1B), was highest at time point 0.25 h (double strand/single strand ratio, about 2.3) near the beginning of the light period and lowest at time point 6.5 h (double strand/single strand ratio, about 0.25) in the middle of the light period. Similarly, in the region of the Chlamydomonas chloroplast chromosome in which the psaB, rbcL, and atpA genes are located, relative superhelicities changed from more supercoiling at the beginning of the light period to less supercoiling in the middle of the light period. Superhelicities decreased about 4.6-fold, 8-fold, and 10-fold in the psaB, rbcL, and atpA gene regions, respectively. Although the magnitude of the conformational changes was different in the four DNA sequences, which can be explained by different cross-linking efficiencies (see Discussion), the results of these analyses show that diurnal alterations in chloroplast DNA topology are not confined to specific regions of the chloroplast chromosome but occur in the same direction at widely separate DNA loci. The diurnal alterations do not seem to be causally linked to the replication of chloroplast DNA in the course of daily cell division because, under our growth conditions, chloroplast DNA replication is synchronized and takes place in a relatively small 2- to 3-h time window at the end of the light period. Thus, it does not correlate in time with the changes found in DNA supercoiling.
Diurnal changes in chloroplast DNA topology are independent of transcription.
In bacteria, supercoiling of the circular chromosome is under the control of several topoisomerases that cooperate to maintain the DNA conformation at the optimum conformation for processes like transcription and DNA replication (35). Chloroplasts contain topoisomerases (40), which presumably have the same function as those in bacteria. Because transcription is one of the processes that perturbs DNA supercoiling (movement of RNA polymerase along the DNA duplex relaxes the torsional tension in front of and creates negative supercoils behind the transcription complex [24, 31, 46]), the diurnal changes in superhelicity measured by the cross-linking assay (Fig. 1) could be due to diurnal variations of transcriptional activities along the chloroplast genome instead of to independent control. To be able to assess directly a potential influence of transcription on the fluctuations in chloroplast DNA conformation found in Chlamydomonas grown in the light-dark regimen, we determined the relative superhelicities in untranscribed regions of the chloroplast genome in cells growing in 12-h dark–12-h light cycles (Fig. 2). Two transgenic cell lines harboring GUS reporter genes fused to nonfunctional promoter sequences were used in these analyses (Fig. 2A and B). In one chimeric gene construct (designated rbcL/S promoter:GUS), the GUS coding region was fused to 580 bp of the 5′ region (including the putative promoter) of the plastid rbcL/S gene from the brown alga E. siliculosus (Fig. 2A). The other construct (designated pCrc46 [2]) contained extensive 5′ deletions of promoter sequences of the Chlamydomonas chloroplast atpB gene linked to the GUS coding region (Fig. 2B). Both constructs were inserted into the chloroplast chromosome near the 3′ end of the endogenous atpB gene (2) (Fig. 2A and B). No GUS transcripts could be detected by Northern analysis in transgenic Chlamydomonas cells harboring these constructs, showing that the two GUS sequences are not transcribed.
In chloroplast transformants growing in 12-h dark–12-h light cycles, the superhelicity within a 4.9-kb BamHI fragment containing the 2.5-kb rbcL/S promoter:GUS construct (Fig. 2A) and within a 1.9-kb BamHI fragment, the latter containing only GUS gene coding sequences (Fig. 2B), decreased in the light period (Fig. 2C), analogous to the decreases in superhelicity measured in the atpB, psaB, rbcL, and atpA sequences of the chloroplast chromosome (Fig. 1). Although the extent of the superhelical changes varied among the DNA sequences examined, the results show that endogenous changes of DNA topology in the Chlamydomonas chloroplast chromosome occur in the same direction and concurrently in transcribed and nontranscribed sequences. Thus, DNA supercoiling seems to be controlled independently of, but is not necessarily unaffected by, transcriptional activities along the DNA double helix.
Superhelicity of Chlamydomonas chloroplast DNA changes endogenously.
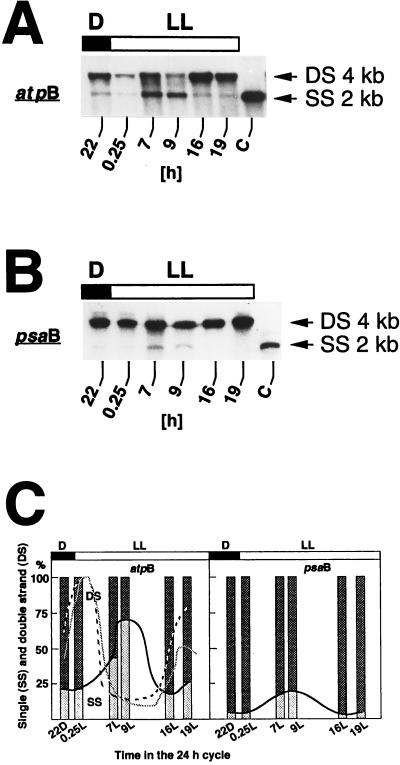
To find out whether the diurnal changes in chloroplast DNA topology are controlled by the light-dark regimen or regulated endogenously, the relative degrees of supercoiling in the 5′ regions of the atpB and psaB genes were determined in cells that were first grown in 12-h dark–12-h light cycles, followed by one 12-h dark–24-h light cycle. Samples were taken at time point 10 h dark and points 0.25, 7, 9, 16, and 19 h light of the latter dark-light cycle (Fig. 3). Time points 16 and 19 h light correspond to 4 and 7 h darkness in the subjective dark period of the 12-h dark–12-h light cycles. Over the 24 h of continuous illumination, the DNA conformation in 4 kb of sequences in the 5′ regions of the atpB and psaB regions fluctuated (Fig. 3); after being more supercoiled near the beginning of the light period than after 9 h of illumination, it reverted to a higher degree of supercoiling at 16 and 19 h in continuous light, i.e., in the middle of the subjective dark period, suggesting an endogenous control of chloroplast DNA topology, at least during the 12 h of extended illumination. Although the cross-linking assay indicated a relatively high degree of DNA supercoiling in the psaB 5′ region at all times of the light-dark cycle, there is clearly a decrease at time points 7 and 9 h light (Fig. 3B and C), showing that changes in DNA topology are qualitatively similar in the atpB and psaB regions of the chloroplast chromosome.
FIG. 3.
Endogenous changes of DNA conformation in the 5′ regions of the Chlamydomonas chloroplast atpB and psaB genes in cells grown in a 12-h dark–24-h light cycle, as determined by the cross-linking assay. (A and B) Cells were first grown in several 12-h dark–12-h light (D/L) cycles before being shifted to the 12-h dark–24-h light (D/LL) regimen. Samples were taken at the time points indicated below the autoradiograms, treated with psoralen, and processed as described in Materials and Methods. (A) Gel blot of DNA digested with BamHI/PstI. The DNA blot was hybridized to a probe specific for the atpB gene (see Fig. 1A for the locations of the probe and restriction fragment). (B) Gel blot of DNA digested with PvuI/SpeI and probed with the 1.1-kb BamHI 16 fragment of the Chlamydomonas chloroplast chromosome (see Fig. 1A for the locations of the probe and restriction fragment). All steps of sample preparation were identical to those described in the legend to Fig. 1. Abbreviations are as defined in the legend to Fig. 1. (C) Graphic representation of the changes in superhelicity within the 5′ regions of the Chlamydomonas chloroplast atpB and psaB genes. Autoradiograms of the DNA gel blots of panels A and B were scanned, and relative band intensities were determined with an image-analyzing computer program (NIH image). Ratios of double- to single-stranded band intensities are plotted as percent fractions of total DNA. To better visualize the changes in superhelicity measured during the time course of the experiments, a line is drawn that connects the single-stranded portions of the figure bars. For comparison, the dashed and shaded lines in the atpB panel show the corresponding changes in levels of atpB transcripts and in rates of atpB gene transcription, respectively, drawn with the data in Fig. 4 (transcript levels) and in Hwang et al. (16) (transcription rates). Abbreviations are as defined for panels A and B.
Endogenous changes of DNA superhelicity correlate with changes in chloroplast gene transcription and changes in transcript pool sizes.
Because the degree of DNA supercoiling has been shown to be an important factor in transcription initiation in bacteria (4) and in chloroplasts (37, 41), we tried to assess the contribution of the endogenous fluctuations in DNA topology to the endogenous and circadian fluctuations found previously (16, 34) in chloroplast gene transcription and chloroplast transcript pool sizes in Chlamydomonas. There is a strong correlation between fluctuations of transcript levels and transcription rates, reported earlier (16, 34), and changes in DNA conformation found in the present study (Fig. 3). In all cases studied, transcription rates were highest at the beginning of the light period and lowest near the end of the 12-h light period, when DNA supercoiling is highest and lowest, respectively.
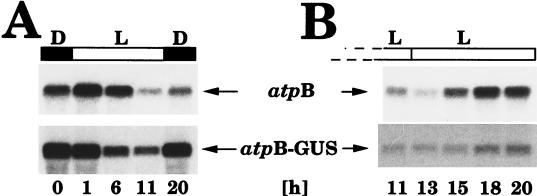
To evaluate the importance of promoter and cis-acting DNA sequences for fluctuations of transcript levels (Fig. 4), we analyzed the expression of chimeric atpB promoter:GUS constructs that had deletions in the 5′ region in the 5′→3′ and 3′→5′ directions down to the basic promoter sequence (see Materials and Methods). The starting construct for these analyses consisted of a 224-bp DNA fragment from the 5′ region of the Chlamydomonas chloroplast atpB gene (including the promoter) fused 5′ to the coding region of the bacterial uidA (GUS) gene. As were all other GUS constructs described in this report (see also Fig. 2), the construct was inserted into the Chlamydomonas chloroplast genome adjacent to the 3′ end of the atpB gene as described previously (2). The DNA conformation in this region alters in the same manner as in the other regions of the chloroplast genome we analyzed in cells grown in dark-light cycles (Fig. 2). Levels of GUS transcripts, measured in transgenic Chlamydomonas cells grown in light-dark cycles, by RNA gel blot (Northern) analysis, fluctuated in light-dark cycles with a pattern similar to that of transcripts from the endogenous atpB gene (Fig. 4A). Fluctuations of GUS transcript levels persisted in continuous light (Fig. 4B), as did fluctuations of transcript levels of the endogenous atpB gene, and occurred concurrently with changes in DNA conformation in the atpB gene region (Fig. 3A), suggesting a causal link between changes in DNA topology and changes in transcript levels.
FIG. 4.
RNA gel blot analyses to determine the abundance of atpB and atpB-GUS gene transcripts in a Chlamydomonas chloroplast transformant growing in 12-h light–12-h dark cycles (A) and in continuous light following growth in light-dark cycles (B). Total RNA was isolated at the indicated time points (time point 0 = onset of light = end of dark period) from a chloroplast transformant (designated 5/3 [21]; see Fig. 5 and Materials and Methods) carrying a chimeric atpB promoter:GUS:rbcL 3′-end gene. Four micrograms of total RNA was separated in a 1.3% agarose–formaldehyde gel, transferred onto a nylon membrane (Zetaprobe; Bio-Rad), and hybridized to gene-specific probes for the endogenous Chlamydomonas chloroplast atpB gene or the GUS gene (see Materials and Methods). Membranes were exposed for 24 h at −80°C to X-ray film with an intensifying screen. Light (L) and dark (D) periods are indicated by open and filled bars, respectively, above the autoradiograms.
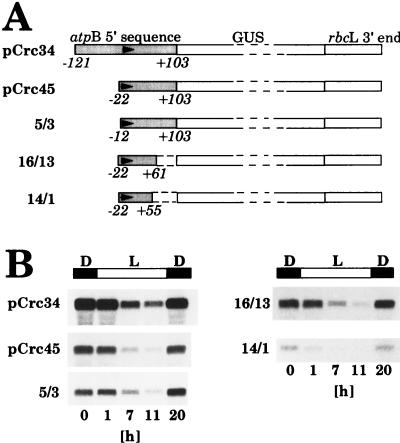
Deletions into the atpB promoter region (5′→3′ and 3′→5′) leaving a promoter fragment as short as 77 bp, extending from positions −22 to +55 relative to the start site of transcription, did not alter the pattern of GUS transcript fluctuations in light-dark cycles (Fig. 5), despite a 95% reduction in the rate of GUS gene transcription from this deleted atpB promoter:GUS construct (21). The results show that all elements required for transcript-level fluctuations lie within the basic atpB promoter sequence. Levels of transcripts of most other Chlamydomonas chloroplast genes were found to fluctuate with the same pattern as transcripts of the atpB gene (16, 34), and it is likely that in those cases only the basic promoter sequences are sufficient to direct endogenous fluctuations of transcript levels.
FIG. 5.
Abundance of GUS transcripts in Chlamydomonas chloroplast transformants grown in light-dark cycles carrying chimeric atpB promoter:GUS:rbcL 3′-end genes with 5′→3′- and 3′→5′-deleted atpB promoter sequences. (A) Schematic drawings of the constructs used in these analyses. Numbers below the construct drawings denote the end points of the atpB 5′ sequence deletions relative to the transcription start site (21). Arrows in the shaded atpB 5′ sequences indicate the start site and the direction of transcription. (B) RNA gel blot analyses of total RNA isolated during a 12-h light–12-h dark cycle at the time points (in hours of the 24-h cycle) indicated below the autoradiograms. Blots were made as described in the legend to Fig. 4 and in Materials and Methods.
A default pattern of chloroplast gene expression in Chlamydomonas?
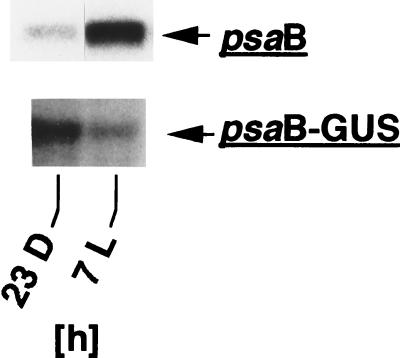
To substantiate the conclusion that basic promoter sequences alone are sufficient for typical diurnal fluctuations of chloroplast transcript levels in Chlamydomonas, we examined in more detail the expression of the psaB gene. DNA supercoiling in the 5′ region of the psaB gene changes in cells grown in light-dark cycles with the same endogenous pattern as does DNA supercoiling in the 5′ region of the atpB gene (Fig. 3), and changes in DNA supercoiling correlate strongly with changes in rates of psaB gene transcription determined earlier (34). However, unlike levels of transcripts of most other Chlamydomonas chloroplast genes, which peak in the beginning of the light period (16, 34), psaB transcript levels peak in the middle of the light period (34). Because the accumulation pattern of psaB gene transcripts in cells growing in light-dark cycles does not follow the changes in psaB gene transcription, psaB transcript accumulation appears to be controlled posttranscriptionally, most likely mediated by sequence elements in the mRNA outside of the basic psaB promoter sequences. Thus, transcripts of a chimeric gene construct consisting only of basic psaB promoter sequences fused to the GUS reporter gene would be expected to accumulate with a default pattern typical for the majority of chloroplast transcripts in cells growing in light-dark cycles. To test this notion, a 336-bp NdeI-BstI DNA fragment from the 5′ region of the psaB gene (see Materials and Methods), containing the putative psaB gene promoter, was fused to the GUS coding sequence and stably inserted into the Chlamydomonas chloroplast genome by biolistic particle transformation. Total RNA was isolated from a chloroplast transformant at time points 23 h dark and 7 h light, and GUS transcript levels were compared to levels of transcripts of the endogenous psaB gene on RNA gel blots (Fig. 6). Unlike psaB gene transcripts, which accumulate to relatively high levels in the middle of the light period (Fig. 6 [34]), levels of psaB promoter:GUS transcripts decreased during the light period (Fig. 6), as has been found for levels of most other chloroplast transcripts (16, 34). This result supports the idea that endogenous fluctuations of transcription and transcript accumulation in the Chlamydomonas chloroplast follow a default pattern that requires only basic promoter sequences, whereas a different pattern of gene expression requires additional sequence elements. The fact that the endogenous fluctuations in chloroplast DNA topology found in this study correlate tightly with a default pattern of transcription and transcript level accumulation again suggests a direct causal link between both processes.
FIG. 6.
Transcripts of a chimeric psaB promoter:GUS:rbcL 3′-end gene accumulate differently than transcripts of the endogenous psaB gene in Chlamydomonas transformants growing in 12-h dark–12-h light cycles. Total RNA was isolated at time points 23 h dark and 7 h light from a chloroplast transformant that had the psaB promoter:GUS:rbcL 3′-end construct inserted near the 3′ end of the endogenous atpB gene (see Materials and Methods), and RNA gel blots were made as described in Materials and Methods and in the legend to Fig. 4.
DISCUSSION
Torsional stress has been shown to play an important role in initiation of transcription of chloroplast and prokaryotic genes (20, 31, 33, 37), presumably by facilitating promoter element recognition and open complex formation by the RNA polymerase complex. Several topoisomerases have been identified that intricately cooperate to control DNA topology in bacterial cells (10, 35, 45). A number of reports implicate an influence of environmental conditions on DNA conformation and regulation of transcription (15, 26). Chloroplasts contain topoisomerases (40), and transcription of chloroplast genes has been found to be affected differentially by changes in DNA topology in vitro (37) and in vivo (41). Furthermore, it has been determined previously that the DNA conformation in at least three separate regions of the Chlamydomonas chloroplast chromosome is more relaxed in cells kept in light than in cells kept in darkness (42). These data show that DNA supercoiling in the Chlamydomonas chloroplast is controlled by topoisomerases and that it can be influenced by external conditions, e.g., light and dark.
In this study, we found a strong correlation between endogenous fluctuations of DNA topology in two different distant regions of the chloroplast chromosome and endogenous fluctuations of overall gene transcription in Chlamydomonas, suggesting a direct causal link between both processes. Because the conformational changes in chloroplast DNA also occur in DNA sequences that are not transcribed (Fig. 2), transcription itself can be ruled out as the sole cause for changes in DNA supercoiling. However, long-distance effects of conformational changes in actively transcribed regions of the chloroplast chromosome on DNA topology in nontranscribed regions cannot be totally excluded. The twin-supercoiled-domain model (24), which is supported by a number of experimental studies (4, 7, 25, 32), predicts the accumulation of positive and negative supercoils ahead of and behind an RNA polymerase elongation complex, respectively, provided rotation of the RNA polymerase transcription ensemble around the template DNA and superhelical diffusion are prevented, e.g., by anchoring the transcription-translation complex to a membrane (4, 25). In bacteria, transcription-induced supercoils seem to be restricted to less than 800 bp in the vicinity of RNA polymerase transcription units (32). The supercoils are removed by DNA topoisomerases, so that they do not build up on DNA templates in cells with normal topoisomerase I and DNA gyrase activities (7). We do not know how putative transcription-induced supercoils are dissipated in chloroplasts along the circular chromosomal DNA. Given the local appearance of transcription-induced supercoiling around the bacterial RNA polymerase transcription elongation complex and in view of the special conditions (high rates of transcription from a very strong promoter, topoisomerase-deficient mutants, anchoring of the RNA polymerase to a membrane) required in bacteria for the observation of any effect of transcription-induced supercoiling on neighboring DNA sequences (4, 25), it appears unlikely that, under our conditions, a long-distance effect of transcription on DNA topology in nontranscribed regions of chloroplast chromosomes can be visualized by the cross-linking assay. Therefore, the endogenous changes in superhelical densities seen in this study in nontranscribed regions of the Chlamydomonas chloroplast chromosomes are most likely independent of transcription elsewhere on the chromosome and indicative of overall endogenous changes in chloroplast DNA topology.
The endogenously controlled overall change in DNA topology could provide a simple mechanism to regulate overall chloroplast transcription simultaneously. It could establish a default pattern of chloroplast gene transcription (for Chlamydomonas, the highest and lowest rates of transcription are at the beginning and end of the light period, respectively) on which other mechanisms that regulate the expression of individual genes, e.g., light-dependent mechanisms, could be superimposed. The finding that accumulation of psaB promoter:GUS gene transcripts differs from accumulation of transcripts of the endogenous psaB gene in Chlamydomonas cells grown in light-dark cycles (Fig. 6) and follows the default pattern of most other Chlamydomonas chloroplast transcripts supports the notion that in the Chlamydomonas chloroplast only basic promoter sequences are required for a basic pattern of gene expression (Fig. 5) and that additional sequences can alter the pattern (Fig. 6). It seems likely that both specific promoter sequences and fluctuations in the topology of the region of the chromosome in which a gene is located are important for gene expression but that timing could result from changes in DNA topology.
A change in DNA topology might be one, but not the only, mechanism involved in circadian control of chloroplast gene transcription in Chlamydomonas. In other systems, other factors have been found to produce daily changes of transcript levels. In Synechococcus, for example, circadian expression of the psbAI gene has been found to be influenced by a sigma70-like transcription factor (43). The loss of the factor resulted in a phenotype that was still rhythmic but one in which transcripts fluctuated with lower amplitudes than in wild-type cells (43). In our system, binding and dissociation of DNA binding proteins during the 12-h dark–12-h light cycles may, in addition to topoisomerases, contribute to changes in DNA superhelicities. These DNA binding proteins may be involved in control of transcription, DNA replication, or other processes occurring periodically.
Because psoralens photoreact preferentially with thymidine residues (9), the efficiency of DNA cross-linking with psoralens depends on the A+T content, the position of the thymidine residues relative to each other, and the size of the DNA sequence studied. The relatively high superhelicities determined for the 5′ region of the psaB gene (Fig. 3B) compared to the relatively low superhelicities found, for instance, in the GUS coding region (Fig. 2C) might in part reflect such differences in cross-linking efficiency in the two DNA segments (the 4-kb psaB gene fragment contains 65% A and T, whereas the A+T content in the 1.9-kb GUS coding region is only 48%). A decrease in cross-linking efficiency in short DNA sequences makes it difficult to determine relative superhelicities in DNA fragments smaller than ∼1.5 kb and did not permit us to examine superhelical changes in small segments, e.g., promoter sequences, of the chloroplast DNA.
ACKNOWLEDGMENTS
M. L. Salvador and U. Klein contributed equally to this work.
We thank Klaus Valentin, University of Gießen, Germany, for the gift of the plasmid containing the rbcL/S gene of E. siliculosus and Toril Håkestad, University of Oslo, Norway, for plasmid psaB:GUS.
The work was supported by grants from DGICYT (PB 95-1075) to M.L.S., the Norwegian Research Council (100946/410) to U.K., and the National Institute of General Medical Sciences of the N.I.H., U.S.P.S., to L.B.
REFERENCES
- 1.Blowers A D, Bogorad L, Shark K B, Sanford J C. Studies on Chlamydomonas chloroplast transformation: foreign DNA can be stably maintained in the chromosome. Plant Cell. 1989;1:123–132. doi: 10.1105/tpc.1.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blowers A D, Ellmore G S, Klein U, Bogorad L. Transcriptional analysis of endogenous and foreign genes in chloroplast transformants of Chlamydomonas. Plant Cell. 1990;2:1059–1070. doi: 10.1105/tpc.2.11.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blowers A D, Klein U, Ellmore G S, Bogorad L. Functional in vivo analyses of the 3′ flanking sequences of the Chlamydomonas chloroplast rbcL and psaB genes. Mol Gen Genet. 1993;238:339–349. doi: 10.1007/BF00291992. [DOI] [PubMed] [Google Scholar]
- 4.Bowater R P, Chen D, Lilley D M J. Modulation of tyrT promoter activity by template supercoiling in vivo. EMBO J. 1994;13:5647–5655. doi: 10.1002/j.1460-2075.1994.tb06903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boynton J E, Gillham N W, Harris E H, Hosler J P, Johnson A M, Jones A R, Randolph-Anderson B L, Robertson D, Klein T M, Shark K B, Sanford J C. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988;240:1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- 6.Bünning E. The physiological clock. 3rd ed. New York, N.Y: Springer-Verlag; 1973. [Google Scholar]
- 7.Chen D, Bowater R, Lilley D M J. Topological promoter coupling in Escherichia coli: ΔtopA-dependent activation of the leu-500 promoter on a plasmid. J Bacteriol. 1994;176:3757–3764. doi: 10.1128/jb.176.12.3757-3764.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church G, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimino G D, Gamper H B, Isaacs S T, Hearst J E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- 10.Drlica K. Bacterial topoisomerases and the control of supercoiling. Trends Genet. 1990;6:433–437. doi: 10.1016/0168-9525(90)90306-q. [DOI] [PubMed] [Google Scholar]
- 11.Dunlap J C. Genetic analysis of circadian clocks. Annu Rev Physiol. 1993;55:683–728. doi: 10.1146/annurev.ph.55.030193.003343. [DOI] [PubMed] [Google Scholar]
- 12.Fejes E, Pay A, Kanevsky I, Szell M, Adam E, Kay S, Nagy F. A 268 bp upstream sequence mediates the circadian clock-regulated transcription of the wheat Cab-1 gene in transgenic plants. Plant Mol Biol. 1990;15:921–932. doi: 10.1007/BF00039431. [DOI] [PubMed] [Google Scholar]
- 13.Giuliano G, Hoffman N E, Ko K, Scolnik P A, Cashmore A R. A light-entrained circadian clock controls transcription of several plant genes. EMBO J. 1988;7:3635–3642. doi: 10.1002/j.1460-2075.1988.tb03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris E H. The Chlamydomonas sourcebook. San Diego, Calif: Academic Press; 1989. [Google Scholar]
- 15.Hulton C S J, Seirafi A, Hinton J C D, Sidebotham J M, Waddell L, Pavitt G D, Owen-Hughes T, Spassky A, Buc H, Higgins C F. Histone-like protein H1 (H-NS), DNA supercoiling, and gene expression in bacteria. Cell. 1990;63:631–642. doi: 10.1016/0092-8674(90)90458-q. [DOI] [PubMed] [Google Scholar]
- 16.Hwang S, Kawazoe R, Herrin D L. Transcription of tufA and other chloroplast-encoded genes is controlled by a circadian clock in Chlamydomonas. Proc Natl Acad Sci USA. 1996;93:996–1000. doi: 10.1073/pnas.93.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobshagen S, Johnson C H. Circadian rhythms of gene expression in Chlamydomonas reinhardtii: circadian cycling of mRNA abundances of cabII, and possibly of β-tubulin and cytochrome c. Eur J Cell Biol. 1994;64:142–152. [PubMed] [Google Scholar]
- 18.Jefferson R A. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:307–405. [Google Scholar]
- 19.Johnson C H, Knight M R, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]
- 20.Jolly S O, Bogorad L. Preferential transcription of cloned chloroplast DNA sequences by maize chloroplast RNA polymerase. Proc Natl Acad Sci USA. 1980;77:822–826. doi: 10.1073/pnas.77.2.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein U, De Camp J D, Bogorad L. Two types of chloroplast gene promoters in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1992;89:3453–3457. doi: 10.1073/pnas.89.8.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo T, Strayer C A, Kulkarni R D, Taylor W, Ishiura M, Golden S S, Johnson C H. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proc Natl Acad Sci USA. 1993;90:5672–5676. doi: 10.1073/pnas.90.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leu S, White D, Michaels A. Cell cycle-dependent transcriptional and post-transcriptional regulation of chloroplast gene expression in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1990;1049:311–317. doi: 10.1016/0167-4781(90)90103-9. [DOI] [PubMed] [Google Scholar]
- 24.Liu L F, Wang J C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci USA. 1987;84:7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch A S, Wang J C. Anchoring of DNA to the bacterial cytoplasmic membrane through cotranscriptional synthesis of polypeptides encoding membrane proteins or proteins for export: a mechanism of plasmid hypernegative supercoiling in mutants deficient in DNA topoisomerase I. J Bacteriol. 1993;175:1645–1655. doi: 10.1128/jb.175.6.1645-1655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClellan J A, Boublíková P, Palecek E, Lilley D M J. Superhelical torsion in cellular DNA responds directly to environmental and genetic factors. Proc Natl Acad Sci USA. 1990;87:8373–8377. doi: 10.1073/pnas.87.21.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millar A J, Kay S A. Circadian control of cab gene transcription and mRNA accumulation in Arabidopsis. Plant Cell. 1991;3:541–550. doi: 10.1105/tpc.3.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millar A J, Short S R, Chua N-H, Kay S A. A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell. 1992;4:1075–1087. doi: 10.1105/tpc.4.9.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy F, Kay S A, Chua N-H. A circadian clock regulates transcription of the wheat cab-1 gene. Genes Dev. 1988;2:376–382. [Google Scholar]
- 30.Paulsen H, Bogorad L. Diurnal and circadian rhythms in the accumulation and synthesis of mRNA for the light-harvesting chlorophyll a/b-binding protein in tobacco. Plant Physiol. 1988;88:1104–1109. doi: 10.1104/pp.88.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruss G J, Drlica K. DNA supercoiling and prokaryotic transcription. Cell. 1989;56:521–523. doi: 10.1016/0092-8674(89)90574-6. [DOI] [PubMed] [Google Scholar]
- 32.Rahmouni A R, Wells R D. Direct evidence for the effect of transcription on local DNA supercoiling in vivo. J Mol Biol. 1992;223:131–144. doi: 10.1016/0022-2836(92)90721-u. [DOI] [PubMed] [Google Scholar]
- 33.Richet E, Raibaud O. Supercoiling is essential for the formation and stability of the initiation complex at the divergent malEp and malKp promoters. J Mol Biol. 1991;218:529–542. doi: 10.1016/0022-2836(91)90699-7. [DOI] [PubMed] [Google Scholar]
- 34.Salvador M L, Klein U, Bogorad L. Light-regulated and endogenous fluctuations of chloroplast transcript levels in Chlamydomonas. Regulation by transcription and RNA degradation. Plant J. 1993;3:213–219. doi: 10.1046/j.1365-313x.1993.t01-13-00999.x. [DOI] [PubMed] [Google Scholar]
- 35.Schmid M B, Sawitzke J A. Multiple bacterial topoisomerases: specialization or redundancy? Bioessays. 1993;15:445–449. doi: 10.1002/bies.950150703. [DOI] [PubMed] [Google Scholar]
- 36.Sinden R R, Carlson J O, Pettijohn D E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980;21:773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- 37.Stirdivant S M, Crossland L D, Bogorad L. DNA supercoiling affects in vitro transcription of two maize chloroplast genes differently. Proc Natl Acad Sci USA. 1985;82:4886–4890. doi: 10.1073/pnas.82.15.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sueoka N. Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardi. Proc Natl Acad Sci USA. 1960;46:83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor W C. Transcriptional regulation by a circadian rhythm. Plant Cell. 1989;1:259–264. doi: 10.1105/tpc.1.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson R J, Mosig G. An ATP-dependent supercoiling topoisomerase of Chlamydomonas reinhardtii affects accumulation of specific chloroplast transcripts. Nucleic Acids Res. 1985;13:873–891. doi: 10.1093/nar/13.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson R J, Mosig G. Stimulation of a chloroplast promoter by novobiocin in situ and in E. coli implies regulation by torsional stress in the chloroplast DNA. Cell. 1987;48:281–287. doi: 10.1016/0092-8674(87)90431-4. [DOI] [PubMed] [Google Scholar]
- 42.Thompson R J, Mosig G. Light affects the structure of Chlamydomonas chloroplast chromosomes. Nucleic Acids Res. 1990;18:2625–2631. doi: 10.1093/nar/18.9.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsinoremas N F, Ishiura M, Kondo T, Andersson C R, Tanaka K, Takahashi H, Johnson C H, Golden S S. A sigma factor that modifies the circadian expression of a subset of genes in cyanobacteria. EMBO J. 1996;15:2488–2495. [PMC free article] [PubMed] [Google Scholar]
- 44.Vos J-M H, Hanawalt P C. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987;50:789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- 45.Wang J C. DNA topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 46.Wang J C, Lynch A S. Transcription and DNA supercoiling. Curr Opin Genet Dev. 1993;3:764–768. doi: 10.1016/s0959-437x(05)80096-6. [DOI] [PubMed] [Google Scholar]
- 47.Woessner J P, Gillham N W, Boynton J E. The sequence of the chloroplast atpB gene and its flanking regions in Chlamydomonas reinhardtii. Gene. 1986;44:17–28. doi: 10.1016/0378-1119(86)90038-7. [DOI] [PubMed] [Google Scholar]