Abstract
目的
肝癌是全球第6大常见的恶性肿瘤,肝细胞癌(hepatocellular carcinoma,HCC)占所有肝癌患者的85%~90%。HCC具有起病隐匿、进展快、复发早、易耐药和预后差的临床特点。中心体相关激酶2(NIMA related kinase 2,NEK2)是细胞周期调控蛋白激酶,在有丝分裂中调节细胞周期。细胞衰老是一个复杂的异质过程,是一种稳定的细胞周期停滞形式,可限制细胞的增殖潜能。本研究旨在分析NEK2表达水平与肝癌细胞衰老的关系,探究NEK2对肝癌细胞衰老的影响及其分子机制。
方法
从GenAge获得581个衰老相关基因,从癌症基因组图谱数据库下载370例HCC患者肿瘤组织的基因表达数据,利用R包分析NEK2与衰老相关基因的共表达情况。采用KEGG分析NEK2过表达的HEK293细胞差异基因的富集通路。在肝癌细胞SMMC-7721及HepG2中构建NEK2过表达及敲低的稳定转染细胞株,采用衰老相关的β-半乳糖苷酶染色检测衰老细胞,CCK-8法和克隆形成实验检测细胞增殖,流式细胞仪分析细胞周期,蛋白质印迹法检测p53/p21、p16/Rb和第10号染色体上缺失与张力蛋白同源的磷酸酯酶(phosphatase and tensin homolog deleted on chromosome ten,PTEN)/Akt信号转导通路相关蛋白质的表达情况。
结果
与NEK2共表达的衰老相关基因有320个。KEGG分析显示细胞衰老信号通路在NEK2过表达的HEK293细胞中存在显著富集。与未敲低NEK2的SMMC-7721或HepG2相比,敲低NEK2的SMMC-7721和HepG2衰老细胞增加,细胞增殖和克隆形成明显减少,G0/G1期细胞的百分比增加,磷酸化的Akt(phospho-Akt,p-Akt)和磷酸化的Rb(phospho-Rb,p-Rb)蛋白质表达水平明显降低,p16蛋白质表达水平明显升高(均P<0.05);与转染空白质粒的SMMC-7721或HepG2相比,过表达NEK2的SMMC-7721和HepG2衰老细胞减少,细胞增殖和克隆形成明显增加,G0/G1期细胞的百分比减少,p-Akt和p-Rb蛋白质表达水平明显升高,p16蛋白质表达水平明显降低(均P<0.05)。
结论
NEK2可能通过p16/Rb及PTEN/Akt信号转导通路介导肝癌细胞的抗衰老效应,为NEK2促进肝癌进展提供了新的实验依据,也为肝癌的靶向治疗提供了新思路。
Keywords: 肝癌, 中心体相关激酶2, 细胞衰老, 细胞增殖, 细胞周期
Abstract
Objective
Liver cancer is the sixth most common malignant tumor in the world. Hepatocellular carcinoma (HCC) accounts for 85%-90% of all patients with liver cancer. It possesses the characteristics of insidious onset, rapid progression, early recurrence, easy drug resistance, and poor prognosis. NIMA related kinase 2 (NEK2) is a cell cycle regulating kinases, which regulates cell cycle in mitosis. Cellular senescence is a complex heterogeneous process, and is a stable form of cell cycle arrest that limits the proliferative potential of cells. This study aims to investigate the relationship between the expression level of NEK2 and the senescence in hepatoma cells, and to explore the effect of NEK2 expression on hepatoma cell senescence and the underlying molecular mechanism.
Methods
A total of 581 senescence-relevant genes were obtained from the GenAge website. The gene expression data of tumor tissues of 370 HCC patients were downloaded from the Cancer Genome Atlas database. The co-expression of NEK2 and aging-related genes was analyzed by R-package. KEGG was used to analyze the significant gene enrichment pathway of differentially expressed genes in NEK2 overexpression HEK293. The stable transfected cell lines with overexpression and knockdown of NEK2 were constructed in hepatoma cell line SMMC-7721 and HepG2, and senescence-associated β-galactosidase (SA-β-gal) staining was used to detect senescence, the cell proliferation was detected by CCK-8 method and clone formation experiment, the cell cycle was analyzed by flow cytometry, and the expression of proteins related to p53/p21, p16/Rb, and phosphatase and tensin homolog deleted on chromosome ten (PTEN)/Akt signal transduction pathway was detected by Western blotting.
Results
There were 320 senescence related genes co-expressed with NEK2. KEGG analysis showed that the senescence signaling pathway was significantly enriched in HEK293 cells with overexpression of NEK2.Compared with SMMC-7721 or HepG2 without knockdown of NEK2, the senescent cells of SMMC-7721 and HepG2 with knockdown of NEK2 were increased, cell proliferation and clone formation were decreased significantly, the percentage of cells in G0/G1 phase was increased, the expression levels of phospho-Akt (p-Akt) and phospho-Rb (p-Rb) protein were decreased significantly, and the expression level of p16 protein was increased significantly (all P<0.05). Compared with SMMC-7721 or HepG2 transfected with blank plasmid, the senescent cells of SMMC-7721 and HepG2 overexpressing NEK2 were decreased, the cell proliferation and clone formation were increased significantly, the percentage of cells in G0/G1 phase were decreased, the expression levels of p-Akt and p-Rb protein were increased significantly, and the expression level of p16 protein was decreased significantly (all P<0.05).
Conclusion
NEK2 may mediate the anti-aging effect of hepatoma cells through p16/Rb and PTEN/Akt signal transduction pathways, which provides a new theoretical basis for NEK2 to promote the progress of liver cancer and a new idea for the targeting treatment for liver cancer.
Keywords: liver cancer, NIMA related kinase 2, cellular senescence, cell proliferation, cell cycle
肝癌是常见的恶性肿瘤,病死率高,极大地威胁着人类的生命健康,每年全球新发肝癌超过100万例,其中中国约占50%[1-2]。由肝细胞恶变引起的肝细胞癌(hepatocellular carcinoma,HCC)占所有肝癌患者的85%~90%[3-4],它的临床特点是起病隐匿、进展快、复发早、易耐药和预后差,常在中晚期阶段才被发现[5]。
细胞衰老是生物体内潜在的肿瘤抑制机制,虽然细胞衰老与肿瘤发生的关系越来越受到研究者的重视[6],但肝细胞衰老抑制肝癌发生的机制仍不明确。细胞衰老是一个复杂的、异质性的过程,细胞形态扁平胀大、衰老相关的β-半乳糖苷酶(senescen- ce-associated β-galactosidase,SA-β-gal)活性升高、细胞的增殖活性受到抑制、细胞周期停滞在G0或G1期等都是细胞衰老的表现[7]。研究[8]表明衰老在肿瘤的发生和发展过程中发挥着关键的作用。阐明细胞衰老与肝癌发生和发展的关系,对于肝癌发生机制与靶向治疗的研究具有重要意义。
中心体相关激酶2(NIMA related kinase 2,NEK2)是在丝状真菌-构巢曲霉中发现的细胞周期调控蛋白激酶,在有丝分裂中调控纺锤体、中心体、染色体,推动细胞周期的正常进行。NEK2的表达量在整个细胞周期中动态变化,G1期表达较低,S期和G2期表达增加且活性更强,在G2/M后期表达达到峰值[9]。过表达的NEK2具有致癌作用,表现为使细胞有丝分裂异常、染色体不稳定或产生非整倍体细胞。研究[10]已证实在多种肿瘤中NEK2的表达水平上调。同时,NEK2在肝癌发生、耐药及不良预后中均起重要的作用[11-13],但是NEK2调控肝癌发生和发展的机制尚不明确。本研究以肝癌细胞系SMMC-7721及HepG2为体外模型,通过建立NEK2敲低及过表达细胞系,对NEK2与肝癌细胞衰老的关系进行初步探讨。
1. 材料与方法
1.1. 材料
人胚胎肾细胞293(HEK293),肝癌细胞SMMC-7721、HepG2,pLVX-Puro(空白质粒),pLVX-Puro-NEK2(过表达质粒)及连接有靶向NEK2 shRNA序列的pLKO-Tet-On均由中南大学肿瘤研究所肿瘤免疫室留存;胎牛血清、RPMI 1640培养基、Opti-MEM无血清培养基、胰蛋白酶购自美国Gibco公司;双抗(含100 U/mL青霉素,100 μg/mL链霉素)购自美国HyClone公司;Lipofectamine 3000转染试剂购自美国Invitrogen公司;CCK-8购自美国Bimake公司;BCA试剂盒、蛋白质裂解液和蛋白酶抑制剂购自上海碧云天生物技术有限公司;0.2 μm PVDF膜购自美国Milipore公司;磷酸化的Akt(phospho-Akt,p-Akt)抗体(S473)和磷酸化的Rb(phospho-Rb,p-Rb)抗体(S780)购自武汉ABclonal公司;NEK2抗体购自美国Santa Cruz公司;GAPDH、p16、p53及p21抗体购自美国Proteintech公司。
1.2. 方法
1.2.1. 细胞培养
HEK293及其建系细胞系采用含有10%的胎牛血清和1%双抗的DMEM培养基,SMMC-7721及其建系细胞、HepG2及建系细胞采用含有10%的胎牛血清和1%双抗的RPMI 1640培养基,置于37 ℃,含5% CO2的培养箱中培养。待细胞生长至约90%的融合时,用胰蛋白酶消化,传代培养。
1.2.2. 细胞转染及分组
构建pLVX-Puro(空白质粒)、pLVX-Puro-NEK2(过表达质粒)及连接有靶向NEK2 shRNA序列的pLKO-Tet-On(NEK2 shRNA)慢病毒表达载体,测序鉴定后使用Lipofectamine 3000将重组质粒和包装质粒共转染293T细胞,48 h后收集上清液,获取慢病毒滤液。
取对数生长期的HEK293细胞及肝癌细胞(SMMC-7721、HepG2),在细胞达到70%~80%融合时感染病毒。培养10 h后更换新鲜培养基,继续培养24~72 h,根据细胞状态使用嘌呤连续筛选7 d,建立稳定转染的细胞系。进一步将NEK2 shRNA慢病毒感染细胞分为2部分:一部分使用强力霉素Dox 72 h以诱导NEK2 shRNA的表达,另一部分不做诱导。
将HEK293分为2组:HEK293 EV(转染空白质粒)及HEK293 NEK2 OE(转染过表达质粒)。根据转染质粒的不同和是否诱导表达,将肝癌细胞SMMC-7721和HepG2各分为6组:1)SMMC-7721 EV和HepG2 EV,转染空白质粒;2)SMMC-7721 NEK2 OE和HepG2 NEK2 OE,转染过表达质粒;3)SMMC-7721 NEK2 shRNA1和HepG2 NEK2 shRNA1,转染NEK2 shRNA1并诱导表达;4)SMMC-7721 NEK2 shRNA2和HepG2 NEK2 shRNA2,转染NEK2 shRNA2并诱导表达;5)SMMC-7721 Ctrl1和HepG2 Ctrl1,转染NEK2 shRNA1但未诱导表达;6)SMMC-7721 Ctrl2和HepG2 Ctrl2,转染NEK2 shRNA2但未诱导表达。
1.2.3. 生物信息学分析
从GenAge(https://genomics.se5nescence.info/genes/ index.html)获得581个衰老相关基因,从癌症基因组图谱(the Cancer Genome Atlas,TCGA)数据库(https://www.cancer.gov/aboutnci/organization/ccg/research/structural-genomics/tcga)下载370例HCC患者肿瘤组织的基因表达数据,利用R包分析NEK2与衰老相关基因的共表达情况。
取对数生长期的HEK293 EV组和HEK293 NEK2 OE组细胞,按照TRIzol试剂盒说明书提取细胞总RNA。用NanoDrop2000检测RNA的浓度和纯度。将提取的RNA送华大基因公司测序,将测序结果导入R包,筛选差异倍数(fold change,FC)>1.5的基因,并进行京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)通路富集分析。
1.2.4. 衰老细胞染色
为探究NEK2对肝癌细胞衰老的影响,在肝癌细胞SMMC-7721和HepG2中分别敲低和过表达NEK2,并利用SA-β-gal对细胞进行染色。消化各组肝癌细胞,以1 000 r/min离心5 min,吹悬计数后在12孔板中每孔铺2.5×105个细胞;培养1 d后倒掉培养液,用PBS洗1次,加入固定液1 mL,在室温下固定15 min;倒弃固定液,用PBS洗3 min,重复3次;倒掉PBS,每孔加入SA-β-gal染色液1mL;在37 ℃培养箱中孵育过夜,注意保持孔板湿度;在光学显微镜下观察染色情况并拍照,每个孔计数大于500个细胞并统计结果。
1.2.5. CCK-8法
消化各组肝癌细胞并计数;加细胞悬液100 μL于96孔板(1 000个/孔)中,每组包括3个平行复孔,铺板6~7块,放细胞培养箱中孵育,在试验孔周围的孔中加入适量PBS,以维持湿度;每日固定时间取板1块,加入CCK-8试剂10 μL后再孵育2 h;使用酶标仪测定各孔在450 nm处的吸光度值,绘制各组细胞的生长曲线。
1.2.6. 克隆形成实验
消化各组肝癌细胞并计数;加细胞悬液于6孔板中,每孔加SMMC-7721细胞1 000个或HepG2细胞2 000个;加完全培养基培养1~2周;倒掉上清液后用PBS洗3次,用甲醇固定15 min;倒掉甲醇后用PBS洗3次;加适量结晶紫,染色15 min;用双蒸水洗掉多余染液,倒扣6孔板使其干燥;对形成的克隆进行计数,克隆形成率=克隆数÷接种细胞数×100%。
1.2.7. 细胞周期实验
消化生长状态良好的各组肝癌细胞,离心后用培养基重悬细胞并计数、铺板;当细胞生长到70%~80%的融合时,饥饿12 h,换新鲜培养基,培养12 h后消化、离心并收集细胞;加预冷PBS 250 μL混匀细胞,缓慢滴入无水乙醇750 μL,混匀,放入-20 ℃冰箱中固定过夜,以1 000 r/min离心5 min后弃上清液,用PBS重悬细胞,加10 ng RNA酶,置37 ℃水浴中30 min后消化细胞,以1 000 r/min离心5 min后弃上清液,加入PI染色液5 μL及1×分析缓冲液 95 μL,混匀后避光孵育30 min;采用流式细胞仪行细胞周期分析。
1.2.8. 蛋白质印迹法
为进一步探讨NEK2影响肝癌细胞衰老的机制,采用蛋白质印迹法检测敲低和过表达NEK2组衰老通路中相关基因的蛋白质表达水平。收集各组肝癌细胞,用PBS洗细胞后加入适量细胞裂解液,充分裂解后于4 ℃下,以12 000 r/min离心10 min,收集上清液至1.5 mL EP管。采用BCA法测定蛋白质浓度后进行蛋白质变性。用SDS-PAGE电泳分离蛋白质,转膜2 h,用5%脱脂牛奶于室温封闭1 h,加入一抗(稀释比例p-Akt、p-Rb、p16、p53、p21均为1꞉1 000,GAPDH为1꞉5 000,NEK2为1꞉500)于4 ℃下孵育过夜,用TBST缓冲液漂洗3次,每次10 min,加入二抗(稀释比例均为1꞉5 000)于室温下孵育90 min,用TBST缓冲液漂洗3次,每次10 min。采用ECL发光试剂显影,Image J软件分析条带灰度值,以GAPDH为内对照,计算各目的基因蛋白质的相对表达水平。
1.3. 统计学处理
使用SPSS 20.0软件进行统计学分析;计量资料采用均数±标准差( ±s)表示,多组比较采用单因素方差分析,t检验用于两两比较;P<0.05表示差异具有统计学意义。
2. 结 果
2.1. HCC肿瘤组织中NEK2与衰老基因共表达
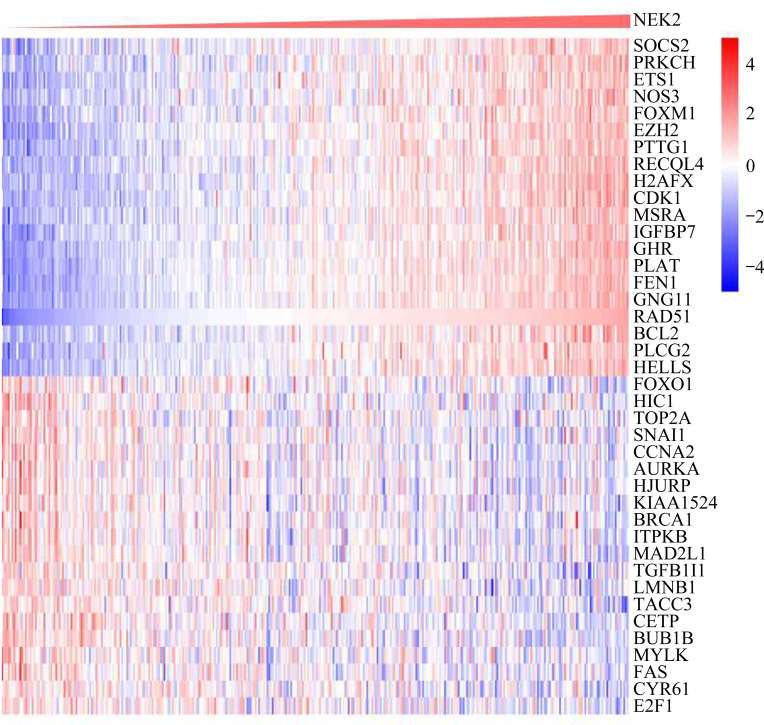
在从GenAge获得的581个衰老相关基因中,与NEK2共表达的基因有320个。热图(图1)显示与NEK2正相关和负相关的排名前20的衰老相关基因。
图1.
TCGA数据库中肝癌患者NEK2与衰老基因共表达情况的分析
Figure 1 Analysis of the co-expression of NEK2 and aging genes in patients with HCC in TCGA database NEK2: NIMA related kinase 2; HCC: hepatocellular carcinoma; TCGA: the Cancer Genome Atlas.
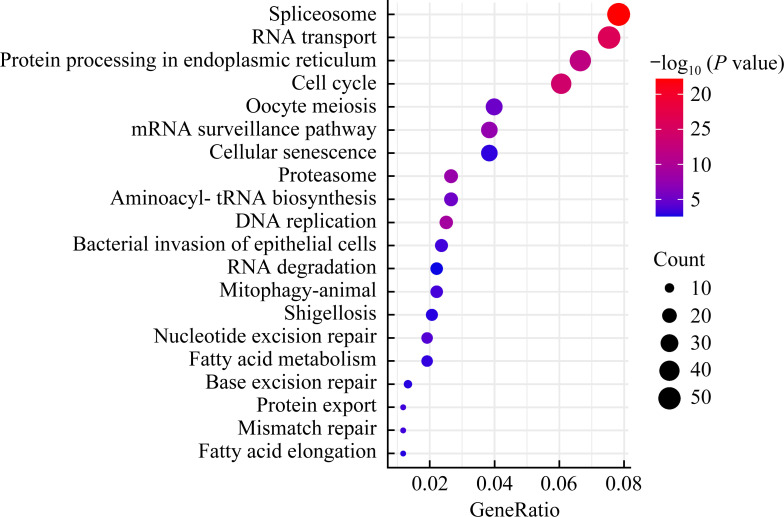
2.2. 差异表达基因富集到细胞周期和细胞衰老信号通路
KEGG结果显示:HEK293 EV组与HEK293 NEK2 OE组细胞差异表达基因富集在细胞周期、细胞衰老相关的信号通路(图2)。
图2.
差异表达基因富集到细胞周期和细胞衰老信号通路
Figure 2 Differentially expressed genes enriches in signaling pathway of cell cycle and cellular senescence
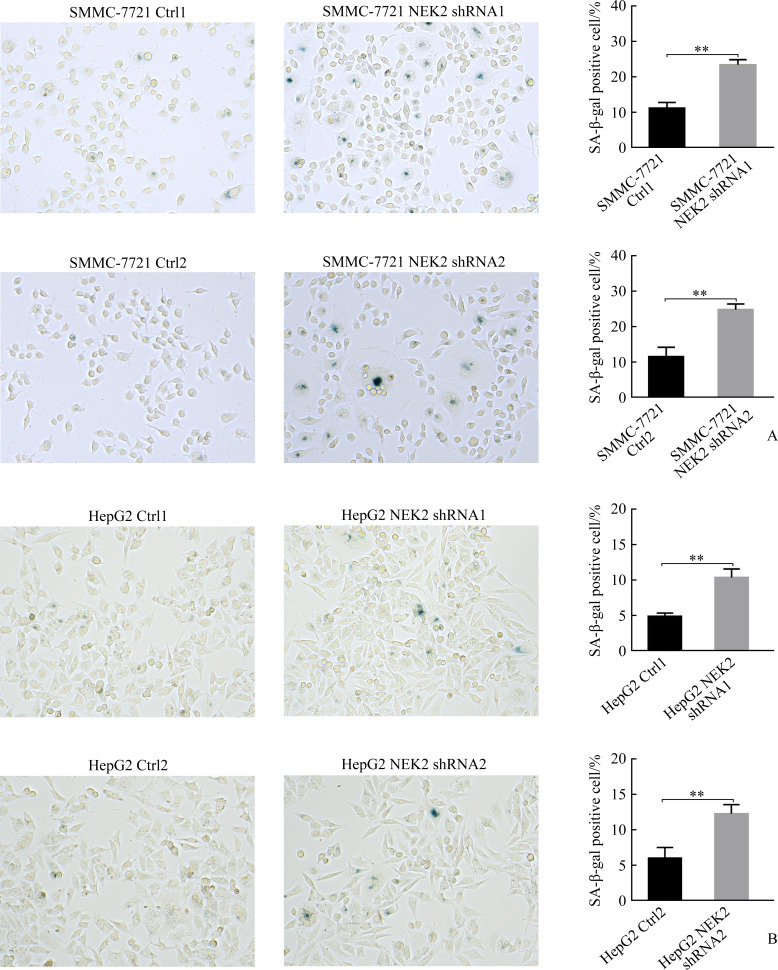
2.3. NEK2抑制肝癌细胞衰老
SMMC-7721 NEK2 shRNA1组与SMMC-7721 Ctrl1组相比,SMMC-7721 NEK2 shRNA2组与SMMC-7721 Ctrl2组相比,SA-β-gal染色细胞明显增加(均P<0.05,图3A);HepG2 NEK2 shRNA1组与HepG2 Ctrl1组相比,HepG2 NEK2 shRNA2组与HepG2 Ctrl2组相比,SA-β-gal染色细胞明显增加(均P<0.05,图3B),表明敲低NEK2表达可促进肝癌细胞的衰老。
图3.
敲低NEK2促进肝癌细胞SMMC-7721(A)和HepG2(B)的衰老
Figure 3 Knockdown of NEK2 promotes cell senescence in hepatoma cells SMMC-7721 (A) and HepG2 (B) NEK2: NIMA related kinase 2. **P<0.01.
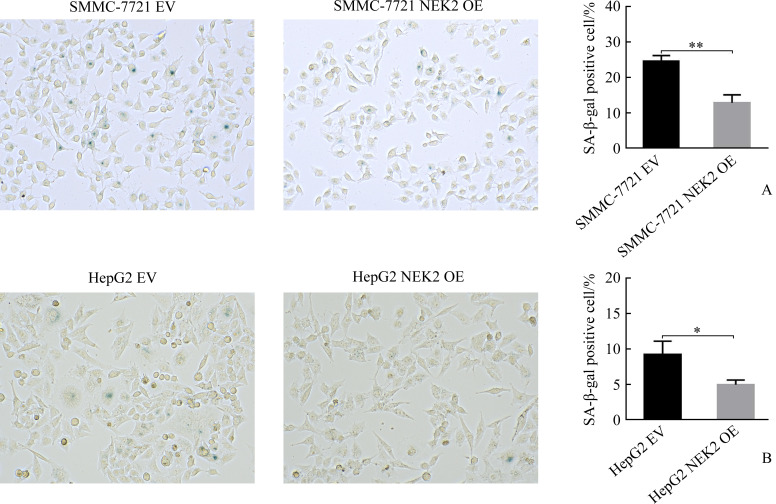
SMMC-7721 NEK2 OE组与SMMC-7721 EV组相比,HepG2 NEK2 OE组与HepG2 EV组相比,SA-β-gal染色细胞明显减少(均P<0.05,图4),表明过表达NEK2可抑制肝癌细胞的衰老。
图4.
过表达NEK2抑制肝癌细胞SMMC-7721(A)和HepG2(B)的衰老
Figure 4 Overexpression of NEK2 inhibits cell senescence in hepatoma cells SMMC-7721 (A) and HepG2 (B) NEK2: NIMA related kinase 2. *P<0.05, **P<0.01.
2.4. NEK2促进肝癌细胞增殖和细胞周期进程
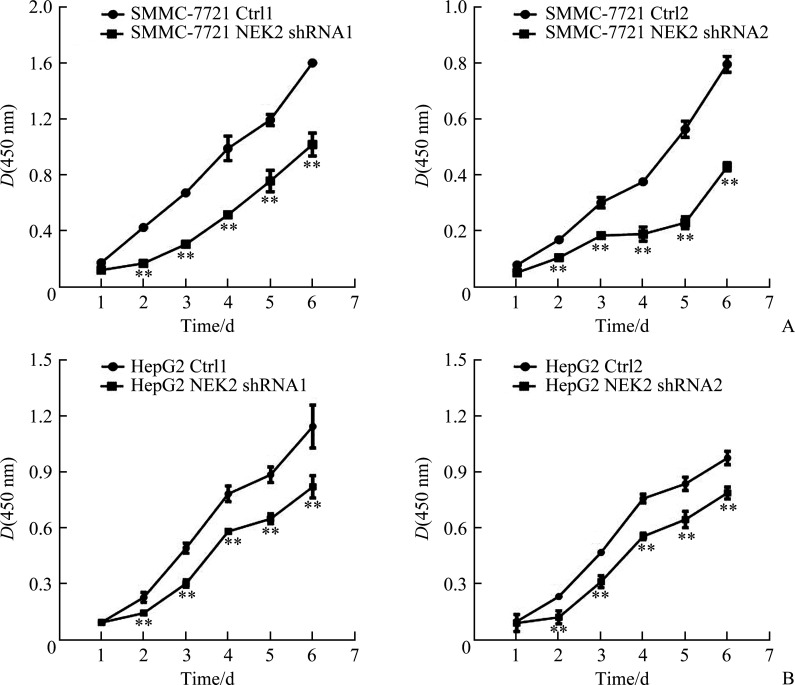
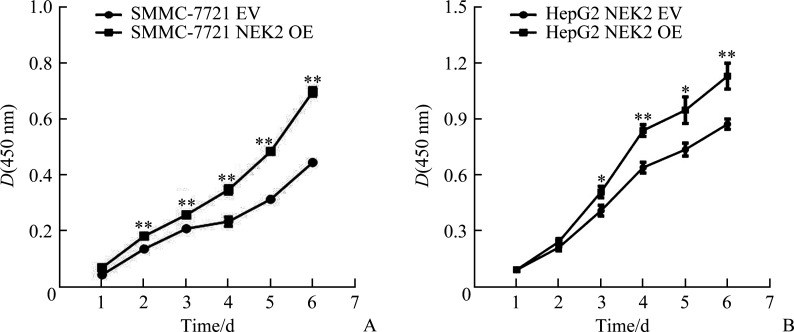
CCK-8法结果显示:SMMC-7721 NEK2 shRNA1组与SMMC-7721 Ctrl1组相比,SMMC-7721 NEK2 shRNA2组与SMMC-7721 Ctrl2组相比,细胞增殖明显减少(均P<0.05,图5A);HepG2 NEK2 shRNA1组与HepG2 Ctrl1组相比,HepG2 NEK2 shRNA2组与HepG2 Ctrl2组相比,细胞增殖亦明显减少(均P<0.05,图5B),表明敲低NEK2表达可抑制肝癌细胞的增殖。SMMC-7721 NEK2 OE组与SMMC-7721 EV组相比,HepG2 NEK2 OE组与HepG2 EV组相比,细胞增殖明显增加(均P<0.05,图6),表明过表达NEK2可促进肝癌细胞的增殖。
图5.
敲低NEK2抑制肝癌细胞SMMC-7721(A)和HepG2(B)的增殖
Figure 5 Knockdown of NEK2 inhibits cell proliferation in hepatoma cells SMMC-7721 (A) and HepG2 (B)
NEK2: NIMA related kinase 2. **P<0.01.
图6.
过表达NEK2促进肝癌细胞SMMC-7721(A)和HepG2(B)的增殖
Figure 6 Overexpression of NEK2 promotes cell proliferation in hepatoma cells SMMC-7721 (A) and HepG2 (B)
NEK2: NIMA related kinase 2. *P<0.05, **P<0.01.
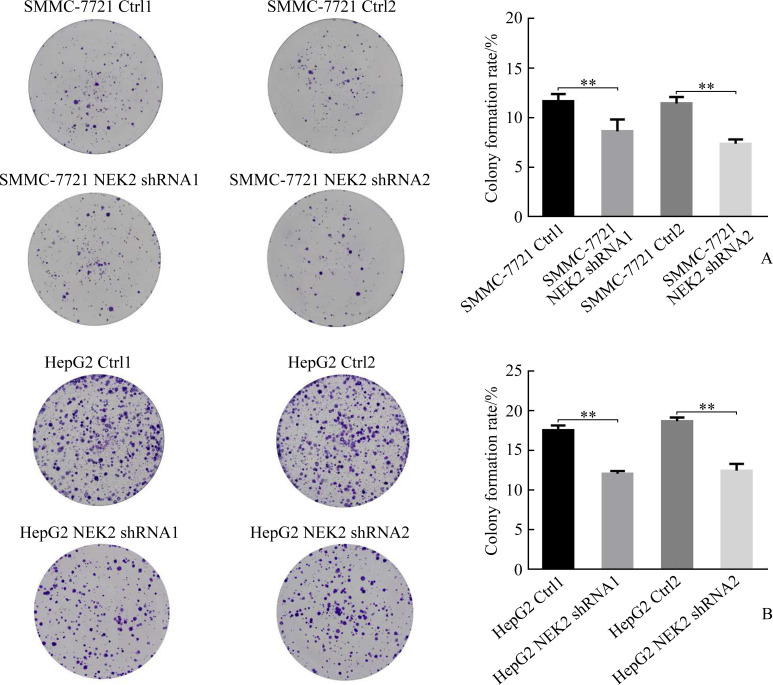
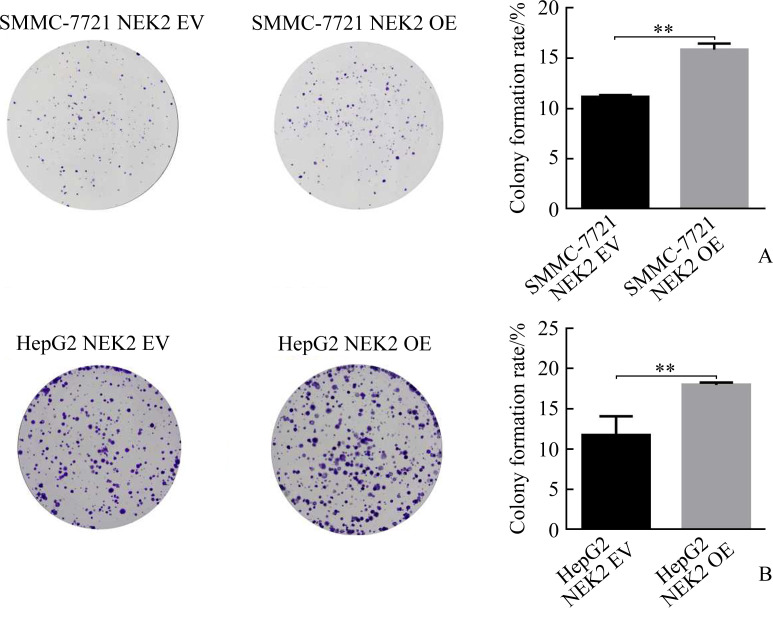
克隆形成实验结果显示:SMMC-7721 NEK2 shRNA1组与SMMC-7721 Ctrl1组相比,SMMC-7721 NEK2 shRNA2组与SMMC-7721 Ctrl2组相比,克隆形成明显减少(均P<0.05,图7A);HepG2 NEK2 shRNA1组与HepG2 Ctrl1组相比,HepG2 NEK2 shRNA2组与HepG2 Ctrl2组相比,克隆形成亦明显减少(均P<0.05,图7B),表明敲低NEK2表达可抑制肝癌细胞克隆形成。SMMC-7721 NEK2 OE组与SMMC-7721 EV组相比,HepG2 NEK2 OE组与HepG2 EV组相比,克隆形成亦明显增加(均P<0.05,图8),表明过表达NEK2可促进肝癌细胞克隆形成。
图7.
敲低NEK2抑制肝癌细胞SMMC-7721(A)和HepG2(B)的克隆形成
Figure 7 Knockdown of NEK2 inhibits clonogenesis in hepatoma cells SMMC-7721 (A) and HepG2 (B) NEK2: NIMA related kinase 2. **P<0.01.
图8.
过表达NEK2促进肝癌细胞SMMC-7721(A)和HepG2(B)的克隆形成
Figure 8 Overexpression of NEK2 promotes clonogenesis in hepatoma cells SMMC-7721 (A) and HepG2 (B)
NEK2: NIMA related kinase 2. **P<0.01.
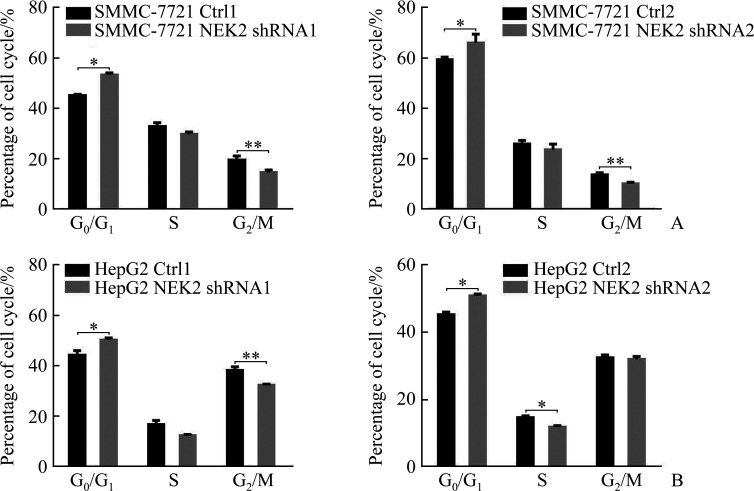
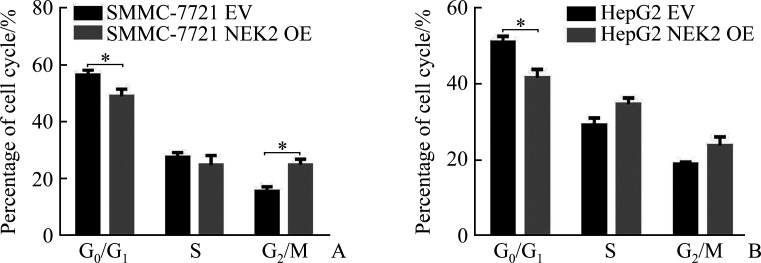
细胞周期实验结果显示:SMMC-7721 NEK2 shRNA1组与SMMC-7721 Ctrl1组相比,SMMC-7721 NEK2 shRNA2组与SMMC-7721 Ctrl2组相比,细胞G0/G1期阻滞,G0/G1期细胞的百分比增加(均P<0.05,图9A);HepG2 NEK2 shRNA1组与HepG2 Ctrl1组相比,HepG2 NEK2 shRNA2组与HepG2 Ctrl2组相比,细胞G0/G1期阻滞,G0/G1期细胞的百分比增加(均P<0.05,图9B),表明敲低NEK2表达可阻滞肝癌细胞周期进程。SMMC-7721NEK2 OE组与SMMC-7721EV组相比,HepG2 NEK2 OE组与HepG2 EV组相比,G0/G1期细胞的百分比减少(均P<0.05,图10),表明过表达NEK2可促进肝癌细胞周期进程。
图9.
敲低NEK2抑制肝癌细胞SMMC-7721(A)和HepG2(B)阻滞细胞周期进程
Figure 9 Knockdown of NEK2 inhibits cell cycle in hepatoma cells SMMC-7721 (A) and HepG2 (B) NEK2: NIMA related kinase 2. *P<0.05, **P<0.01.
图10.
过表达NEK2促进肝癌细胞SMMC-7721(A)和HepG2(B)细胞周期进程
Figure 10 Overexpression of NEK2 promotes cell cycle in hepatoma cells SMMC-7721 (A) and HepG2(B)
NEK2: NIMA related kinase 2. *P<0.05.
2.5. NEK2激活p16/Rb及PTEN/Akt信号通路
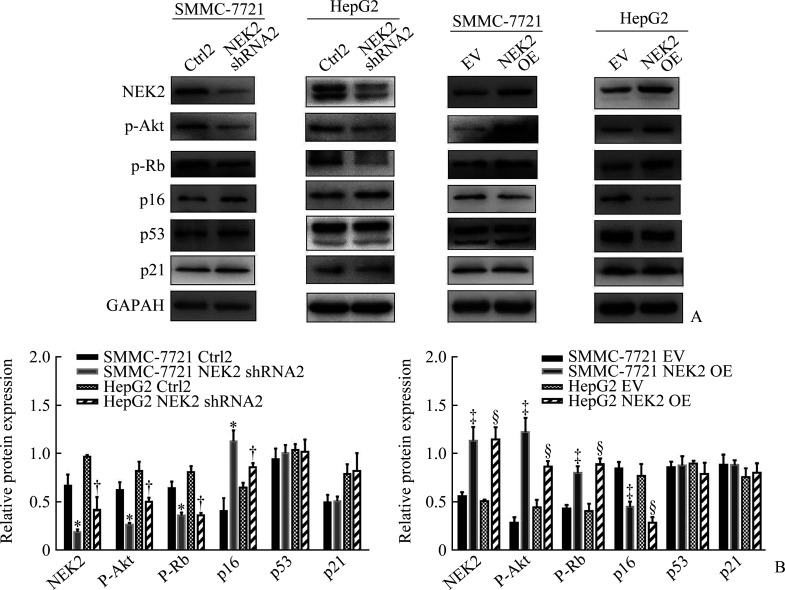
蛋白质印迹法结果显示:SMMC-7721 NEK2 shRNA2组与SMMC-7721 Ctrl2组相比,HepG2 NEK2 shRNA2组与HepG2 Ctrl2组相比,p-Akt及p-Rb蛋白质表达水平明显降低(均P<0.05),p16蛋白质表达水平明显升高(均P<0.05),p53及p21蛋白质表达水平差异无统计学意义(均P>0.05,图11)。SMMC-7721 NEK2 OE组与SMMC-7721 EV组相比,HepG2 NEK2 OE组与HepG2 EV组相比,p-Akt及p-Rb蛋白质表达水平明显升高(均P<0.05),p16蛋白质表达水平明显降低(均P<0.05),p53及p21蛋白质表达水平差异无统计学意义(均P>0.05,图11)。
图11.
NEK2激活肝癌细胞p16/Rb及PTEN/Akt信号通路
Figure 11 NEK2 activates p16/Rb and PTEN/Akt signaling pathways in hepatoma cells
A: Electropherogram of Western blotting showing the protein expression of NEK2, p-Akt, p-Rb, p16, p53, and p21; B: Relative expression of NEK2, p-Akt, p-Rb, p16, p53, and p21 normalised by GAPDH was determined by densitometric analysis. NEK2: NIMA related kinase 2; PTEN: phosphatase and tensin homolog deleted on chromosome ten; p-Akt: phospho-Akt; p-Rb: phospho-Rb. *P<0.05 vs the SMMC-7721 Ctrl2 group; †P<0.05 vs the HepG2 Ctrl2 group; ‡P<0.05 vs the SMMC-7721 EV group; §P<0.05 vs the HepG2 EV group.
3. 讨 论
本研究通过分析TCGA数据库和GenAge网站的相关数据发现NEK2和衰老相关基因存在共表达。在HEK293中过表达NEK2,KEGG分析结果显示差异表达基因富集到细胞周期和细胞衰老的生物学过程。此外,在肝癌细胞中分别敲低和过表达NEK2,前者促进肝癌细胞衰老,后者抑制肝癌细胞衰老。进一步研究发现:敲低NEK2抑制肝癌细胞增殖和引起肝癌细胞周期阻滞,过表达NEK2促进肝癌细胞增殖和细胞周期进程。蛋白质印迹法检测结果显示NEK2可能通过p16/Rb及第10号染色体上缺失与张力蛋白同源的磷酸酯酶(phosphatase and tensin homolog deleted on chromosome ten,PTEN)/Akt信号通路调控肝癌细胞衰老。研究结果为NEK2抑制肝细胞衰老及相关机制提供了实验依据。
细胞衰老是细胞周期阻滞的稳定状态,是细胞对致癌应激或氧化应激等作出的反应。肿瘤基因被异常激活后,正常细胞进入细胞衰老程序,这是防止体内肿瘤发生的重要机制。诱导衰老、抑制有丝分裂和促进细胞凋亡是治疗肝癌的有效策略。研究[14]发现:TNF-α介导的有丝分裂缺陷会导致细胞衰老和死亡致敏,两者的协同作用可抑制肝癌。肝细胞免疫监视受损导致小鼠HCC的发生,衰老监测对抑制肿瘤有重要意义[15]。本课题组前期研究[16-18]发现:NEK2异常表达影响有丝分裂的正常进行,造成染色体不稳定、细胞周期失调,最终导致肿瘤发生。目前,在乳腺癌、膀胱癌、结直肠癌、卵巢癌等多种肿瘤中发现NEK2表达上调[19-22]。NEK2在肝癌中亦呈现高表达,且对肝癌的发生和发展、迁移和侵袭、耐药及预后具有重要影响[23-25]。本研究发现:敲低NEK2表达促进肝癌细胞衰老,抑制细胞增殖并且引起细胞周期阻滞,提示抑制NEK2的表达可以作为治疗肝癌的手段。
p53/p21、p16/Rb及PTEN/Akt信号通路是调控细胞周期和细胞衰老的重要途径。p21抑制细胞周期依赖性蛋白激酶(cyclin-dependent kinase,CDK)的活性,抑制Rb和E2F的磷酸化。p16通常在各种恶性肿瘤中失活,能够抑制Rb的磷酸化,阻止p-Rb与E2Fs的结合,从而导致细胞周期停滞和衰老[26]。为进一步探讨NEK2表达与肝癌细胞衰老相关信号通路蛋白质的关系,在NEK2过表达和敲低的肝癌细胞中检测p53/p21、p16/Rb及PTEN/Akt信号通路相关蛋白质的表达,结果发现:在敲低NEK2的肝癌细胞中,p-Akt及p-Rb蛋白质表达水平明显降低,p16蛋白质表达水平明显升高,差异均有统计学意义。既往研究[27]发现83%的肝癌患者Rb通路活化,这为p16/Rb衰老途径在肝癌发展中的作用提供了关键证据。Akt在细胞增殖中起着核心作用[28]。Akt抑制剂可抑制NEK2诱导的克隆形成[16],表明NEK2可能通过Akt信号通路诱导细胞增殖。本研究结果提示NEK2可能通过活化p16/Rb、PTEN/Akt信号通路抑制肝癌细胞衰老,促进肝癌细胞的增殖。
综上,本研究揭示了NEK2可能通过p16/Rb、PTEN/Akt信号通路参与调控细胞衰老,具体机制尚待进一步阐明。通过抑制NEK2诱导肿瘤细胞衰老有望成为肝癌的治疗靶点,本研究为治疗肝癌提供了新的思路。
基金资助
国家自然科学基金(81974010);中南大学中央高校基本科研业务费专项资金(2019zzts717)。
This work was supported by the National Natural Science Foundation (81974010) and the Fundamental Research Funds for the Central Universities of Central South University (2019zzts717), China.
利益冲突声明
作者声称无任何利益冲突。
作者贡献
雷倩 实验操作,统计分析,论文撰写与修订;郭姣姣 协助实验,统计分析;冯湘玲、夏机良 提供实验指导;李官成、周文 实验设计和指导,论文修改。
原文网址
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/202202153.pdf
参考文献
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015, 65(2): 87-108. 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2. 中华人民共和国国家卫生健康委员会医政医管局 . 原发性肝癌诊疗规范(2019年版)[J]. 传染病信息, 2020, 33(6): 481-500. 10.3760/cma.j.issn.1007-3418.2020.02.004. [DOI] [Google Scholar]; National Health Commission of the People’s Republic of China . Guidelines for diagnosis and treatment of primary liver cancer (Versoin 2019)[J]. Infectious Disease Information, 2020, 33(6): 481-500. 10.3760/cma.j.issn.1007-3418.2020.02.004. [DOI] [Google Scholar]
- 3. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population[J]. J Clin Gastroenterol, 2013, 47(Suppl): S2-S6. 10.1097/mcg.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis[J]. Lancet Oncol, 2012, 13(6): 607-615. 10.1016/s1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 5. Gravitz L. Liver cancer[J]. Nature, 2014, 516(7529): S1. 10.1038/516s1a. [DOI] [PubMed] [Google Scholar]
- 6. Zeng S, Shen WH, Liu L. Senescence and cancer[J]. Cancer Transl Med, 2018, 4(3): 70-74. 10.4103/ctm.ctm_22_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huda N, Liu G, Hong H, et al. Hepatic senescence, the good and the bad[J]. World J Gastroenterol, 2019, 25(34): 5069-5081. 10.3748/wjg.v25.i34.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calcinotto A, Kohli J, Zagato E, et al. Cellular senescence: aging, cancer, and injury[J]. Physiol Rev, 2019, 99(2): 1047-1078. 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 9. Xia J, Franqui Machin R, Gu Z, et al. Role of NEK2A in human cancer and its therapeutic Potentials[J]. Biomed Res Int, 2015, 2015(6): 1-12. 10.1155/2015/862461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayward DG, Fry AM. NEK2 kinase in chromosome instability and cancer[J]. Cancer Lett, 2006, 237(2): 155-166. 10.1016/j.canlet.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 11. Lai XB, Nie YQ, Huang HL, et al. NIMA-related kinase 2 regulates hepatocellular carcinoma cell growth and proliferation[J]. Oncol Lett, 2017, 13(3): 1587-1594. 10.3892/ol.2017.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Wang W, Wang Y, et al. NEK2 promotes hepatocellular carcinoma migration and invasion through modulation of the epithelial-mesenchymal transition[J]. Oncol Rep, 2018, 39(3): 1023-1033. 10.3892/or.2018.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng Y, Chen X, Ye L, et al. The prognostic significance of NEK2 in hepatocellular carcinoma: evidence from a meta-analysis and retrospective cohort study[J]. Cell Physiol Biochem, 2018, 51(6): 2746-2759. 10.1159/000495966. [DOI] [PubMed] [Google Scholar]
- 14. Narita M, Lowe SW. Senescence comes of age[J]. Nat Med, 2005, 11(9): 920-922. 10.1038/nm0905-920. [DOI] [PubMed] [Google Scholar]
- 15. Kang TW, Yevsa T, Woller N, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development[J]. Nature, 2011, 479(7374): 547-551. 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 16. Zhou W, Yang Y, Xia J, et al. NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancer[J]. Cancer Cell, 2013, 23(1): 48-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xia J, He Y, Meng B, et al. NEK2 induces autophagy-mediated bortezomib resistance by stabilizing Beclin-1 in multiple myeloma[J]. Mol Oncol, 2020, 14(4): 763-778. 10.1002/1878-0261.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu H, Zeng L, Guan Y, et al. High NEK2 confers to poor prognosis and contributes to cisplatin-based chemotherapy resistance in nasopharyngeal carcinoma[J]. J Cell Biochem, 2019, 120(3): 3547-3558. 10.1002/jcb.27632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cappello P, Blaser H, Gorrini C, et al. Role of NEK2 on centrosome duplication and aneuploidy in breast cancer cells[J]. Oncogene, 2014, 33(18): 2375-2384. 10.1038/onc.2013.183. [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto Y, Matsuyama H, Kawauchi S, et al. Overexpression of polo-like kinase 1 (PLK1) and chromosomal instability in bladder cancer[J]. Oncology, 2006, 70(3): 231-237. 10.1159/000094416. [DOI] [PubMed] [Google Scholar]
- 21. Neal CP, Fry AM, Moreman C, et al. Overexpression of the NEK2 kinase in colorectal cancer correlates with beta-catenin relocalization and shortened cancer-specific survival[J]. J Surg Oncol, 2015, 110(7): 828-838. 10.1002/jso.23717. [DOI] [PubMed] [Google Scholar]
- 22. Liu X, Gao Y, Lu Y, et al. Upregulation of NEK2 is associated with drug resistance in ovarian cancer[J]. Oncol Rep, 2014, 31(2): 745-754. 10.3892/or.2013.2910. [DOI] [PubMed] [Google Scholar]
- 23. Wen S, Liu Y, Yang M, et al. Increased NEK2 in hepatocellular carcinoma promotes cancer progression and drug resistance by promoting PP1/Akt and Wnt activation[J]. Oncol Rep, 2016, 36(4): 2193-2199. 10.3892/or.2016.5009. [DOI] [PubMed] [Google Scholar]
- 24. Deng L, Sun J, Chen X, et al. NEK2 augments sorafenib resistance by regulating the ubiquitination and localization of β-catenin in hepatocellular carcinoma[J]. J Exp Clin Cancer Res, 2019, 38(1): 316. 10.1186/s13046-019-1311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fu L, Liu S, Wang H, et al. Low expression of NEK2 is associated with hepatocellular carcinoma progression and poor prognosis[J]. Cancer Biomark, 2017, 20(1): 101-106. 10.3233/cbm-170586. [DOI] [PubMed] [Google Scholar]
- 26. 赵欢, 周斌. 细胞衰老研究现状[J]. 中国细胞生物学学报, 2017, 39(6): 687-694. 10.11844/cjcb.2017.06.9002. [DOI] [Google Scholar]; ZHAO Huan, ZHOU Bin. Research status of cell senescence[J]. Chinese Journal of Cell Biology, 2017, 39(6): 687-694. 10.11844/cjcb.2017.06.9002. [DOI] [Google Scholar]
- 27. Ozturk M, Arslan-Ergul A, Bagislar S, et al. Senescence and immortality in hepatocellular carcinoma[J]. Cancer Lett, 2009, 286(1): 103-113. 10.1016/j.canlet.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 28. Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor[J]. Cell, 1999, 96(6): 857-868. 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]