Abstract
目的
高同型半胱氨酸(homocysteine,Hcy)水平是心脑血管疾病的独立危险因素。MiR-18a-5p是微RNA(microRNA,miR)家族中的重要成员,与心血管疾病密切相关。本研究旨在探讨miR-18a-5p对Hcy损伤心肌细胞的影响。
方法
对H9c2细胞进行miR-18a-5p模拟物(mimic)/miR-18a-5p mimic阴性对照(negative control,NC)转染或与Hcy联合干预,另设未处理细胞作为对照组。采用实时反转录聚合酶链反应(real-time reverse transcription PCR,real-time RT-PCR)验证转染效率,细胞计数试剂盒-8(cell counting kit-8,CCK-8)法检测细胞活力,流式细胞术检测细胞凋亡率和细胞中活性氧(reactive oxygen species,ROS)水平,蛋白质印迹法检测微管相关蛋白1轻链3(microtubule-associated protein 1 light chain 3,LC3)-I、LC3-II、Beclin1、p62、Bax、Bcl-2和Notch2的蛋白质表达水平,双荧光素酶报告分析检测miR-18a-5p与Notch2的相互作用。
结果
与对照组相比,Hcy或转染miR-18a-5p mimic单独处理,或转染miR-18a-5p mimic/miR-18a-5p mimic NC与Hcy联合干预均显著降低H9c2细胞的活力,增加H9c2细胞的凋亡率和ROS的生成,上调Bax和Beclin1的蛋白质表达水平,下调Bcl-2、p62和Notch2的蛋白质表达水平,同时使LC3-II/LC3-I值增加(均P<0.05)。与miR-18a-5p mimic NC和Hcy联合干预相比,miR-18a-5p mimic和Hcy联合干预的H9c2细胞上述指标的改变更显著,且2组之间差异有统计学意义(均P<0.05)。Notch2与miR-18a-5p存在靶向结合。
结论
MiR-18a-5p通过增加心肌细胞内ROS的生成,诱导自噬和凋亡,加重Hcy诱导的心肌损伤。Notch2是miR-18a-5p的作用靶点。
Keywords: miR-18a-5p, 心肌损伤, 同型半胱氨酸, 活性氧, 自噬, Notch2
Abstract
Objective
Hyperhomocysteinaemia (Hcy) is an independent risk factor for cardiovascular and cerebrovascular diseases. MicroRNA (miR)-18a-5p is closely related to cardiovascular diseases. This study aims to investigate the effects of miR-18a-5p on homocysteine (Hcy)-induced myocardial cells injury.
Methods
H9c2 cells were transfected with miR-18a-5p mimic/miR-18a-5p mimic negative control (NC) or combined with Hcy for intervention, and untreated cells were set as a control group. The transfection efficiency was verified by real-time RT-PCR, and cell counting kit-8 (CCK-8) assay was used to determine cell viability. Flow cytometry was used to detect apoptosis and reactive oxygen species (ROS) levels. Western blotting was performed to measure the protein levels of microtubule-associated protein 1 light chain 3 (LC3)-I, LC3-II, Beclin1, p62, Bax, Bcl-2, and Notch2. Dual luciferase reporter assay was used to detect the interaction of miR-18a-5p with Notch2.
Results
Compared with the control, treatment with Hcy or transfection with miR-18a-5p mimic alone, or combined treatment with Hcy and miR-18a-5p mimic/miR-18a-5p mimic NC significantly reduced the H9c2 cell viability, promoted apoptosis and ROS production, up-regulated the expressions of Bax and Beclin, down-regulated the expressions of Bcl-2, p62, and Notch2, and increased the ratio of LC3-II/LC3-I (all P<0.05). Compared with the combined intervention of miR-18a-5p mimic NC and Hcy group, the above indexes were more significantly changed in the combined intervention of miR-18a-5p mimic and Hcy group, and the difference between the 2 groups was statistically significant (all P<0.05). There is a targeted binding between Notch2 and miR-18a-5p.
Conclusion
MiR-18a-5p could induce autophagy and apoptosis via increasing ROS production in cardiomyocytes, and aggravate Hcy-induced myocardial injury. Notch2 is a target of miR-18a-5p.
Keywords: miR-18a-5p, myocardial injury, homocysteine, reactive oxygen species, autophagy, Notch2
研究[1-2]表明高同型半胱氨酸(homocysteine,Hcy)水平与冠心病、心肌梗死等心脑血管疾病有着密不可分的关系。用Hcy干预血压正常的大鼠后会引起心肌细胞损伤,从而导致心肌肥厚和心力衰竭[3]。研究[4-6]发现:高Hcy可诱导氧化应激,增加活性氧(reactive oxygen species,ROS)的生成,使Bcl-2家族蛋白和自噬相关蛋白表达失衡,促进心肌细胞凋亡,最终导致心肌细胞损伤和心功能障碍。然而,Hcy细胞毒性的分子机制尚未完全阐明。
微RNA(microRNA,miRNA)是一类非编码小分子RNA,通过与靶基因的信使RNA(messenger RNA,mRNA)特异地结合,抑制或者促进mRNA的转录表达[7-9],在多种疾病的发生和发展中发挥重要作用。MiR-18a-5p是miRNA家族中的重要成员,与心血管疾病密切相关[10-12]。异氟醚通过下调miR-18a-5p的表达缓解缺氧/复氧引起的心肌细胞损伤[10]。交感神经兴奋性增强可抑制miR-18a-5p/缺氧诱导因子1α(hypoxia-inducible factor-1alpha,HIF-1α)信号通路,降低线粒体未折叠蛋白反应,升高ROS水平,并导致心肌细胞肥大[11]。在高糖孵育的人类主动脉瓣内皮细胞中,miR-18a-5p靶向Notch2,抑制内皮细胞向间质转化并改善心脏纤维化[12]。然而,miR-18a-5p是否在高Hcy诱导的心肌损伤中发挥作用鲜见报道。本研究在心肌细胞中过表达miR-18a-5p,研究其对Hcy处理的心肌细胞损伤的影响,并探讨其作用机制。
1. 材料与方法
1.1. 材料
1.1.1. 细胞
大鼠心肌细胞H9c2细胞(货号:BNCC100061)购自北京北纳创联生物技术研究院,并在含有10%胎牛血清(fetal bovine serum,FBS)的OPTI‑MEM®I培养基中,于37 ℃的5% CO2培养箱中培养。
1.1.2. 试剂和仪器
Hcy(货号:HY-W040821)购自美国MedChemExpress生物科技公司;双荧光素酶报告基因检测试剂盒(货号:RG027)购自上海碧云天生物技术有限公司;转染试剂Lipofectamine®3000(货号:L3000015)、OPTI‑MEM®I培养基(货号:31985-062)购自赛默飞世尔科技(中国)有限公司;细胞计数试剂盒-8(cell counting kit-8,CCK-8)(货号:KGT010-1)和ROS检测试剂盒(货号:KGT010-1)购自上海金畔生物科技有限公司;miRNA cDNA合成试剂盒(货号:CW2141S)、miRNA real-time PCR试剂盒(货号:CW2142S)和TRIzol(货号:CW0580S)购自北京康维生物科技有限公司;膜联蛋白V(Annexin V)-异硫氰酸荧光素(fluorescein isothiocyanate,FITC)/碘化丙啶(propidium iodide,PI)凋亡检测试剂盒(货号:AP101)购自生物谷公司;一抗GAPDH小鼠单克隆抗体(货号:TA-08,1꞉2 000)和二抗辣根过氧化物酶标记的山羊抗小鼠IgG(H+L;货号:ZB2305;1꞉2 000)购自北京傲瑞东源生物科技有限公司;一抗LC3-I/LC3-II(货号:ab48394;1꞉2 000)、Beclin1(货号:ab207612, 1꞉2 000)、p62(货号:ab109012,1꞉10 000)的兔抗体,Bcl-2小鼠单克隆抗体(货号:ab692;1꞉500)均购自艾博抗(上海)贸易有限公司;一抗Notch2兔抗体(货号BS2378T,1꞉1 000)购自北京奥博森生物技术有限公司;一抗Bax兔抗体(货号:50599-2-lg,1꞉5 000)购自派普泰克生物科技(苏州)有限公司。
1.2. 方法
1.2.1. 细胞转染
对数生长期H9c2细胞在6孔板生长至约80%的融合时,使用Lipofectamine®3000以5 µg/mL的终浓度转染质粒。操作完成后将细胞放回37 ℃培养箱中培养。转染6 h后,更换培养基,向6孔板中加入 1 mL含20%血清的OPTI‑MEM®I培养基,转染24 h后进行PCR检测。hsa-miR-18a-5p和miR-18a-5p mimic的序列见表1。
表1.
MiR-18a-5p和miR-18a-5p mimic序列
Table 1 Sequence of miR-18a-5p and miR-18a-5p mimic
| MiRNA | 序列(5'-3') |
|---|---|
| hsa-miR-18a-5p正向 | UAAGGUGCAUCUAGUGCAGAUAG |
| hsa-miR-18a-5p反向 | CUAUCUGCACUAGAUGCACCUUA |
| miR-18a-5p mimic正向 | CACAACCUCCUAGAAAGAGUAGA |
| miR-18a-5p mimic反向 | UCUACUCUUUCUAGGAGGUUGUGA |
1.2.2. 实时反转录聚合酶链反应
转染48 h后,使用实时反转录聚合酶链反应(real-time reverse transcription PCR,real-time RT-PCR)定量miR-18a-5p的表达。使用TRIzol提取H9c2细胞总RNA,用miRNA cDNA合成试剂盒将RNA反转录为cDNA,随后使用miRNA real-time PCR试剂盒进行实时PCR。U6为内对照正向引物为5'-GCTT-CGGCAGCACATATACTAAAAT-3',反向引物为 5'-CGCTTCACGAATTTGCGTGTCAT-3';miR-18a-5p的正向引物为5'-TAAGGTGCATCTAGTGCA-GATAG-3',反向引物为通用引物。实时PCR总体系为10 µL,包含1.5 µL cDNA、5 µL 2×SYBR-Green PCR Master Mix、1 µL荧光探针和2.5 µL无核酶水。反应条件为:首先50 ℃,2 min;95 ℃,10 min。随后进行45个循环,每个循环由95 ℃,10 s和58 ℃,30 s组成。目的基因的相对表达量以2-ΔΔCt法计算[13]。
1.2.3. 细胞分组与处理
MiR-18a-5p mimic阴性对照(negative control,NC)+Hcy组、miR-18a-5p mimic+Hcy组:在质粒miR-18a-5p mimic NC、miR-18a-5p mimic转染H9c2细胞6 h后,用终浓度4 mmol/L的Hcy处理细胞 24 h[14-15]。miR-18a-5p mimic NC组、miR-18a-5p mimic组H9c2细胞只转染相应质粒,不加Hcy。对照组为常规培养的H9c2细胞(不转染质粒且不用Hcy处理),Hcy组H9c2细胞用Hcy处理,但不转染质粒。
1.2.4. 细胞活性检测
使用CCK-8法测定miR-18a-5p mimic对H9c2细胞活性的影响。药物干预结束后,每孔加入CCK-8试剂20 µL,放回培养箱继续孵育2 h后,用酶标仪测定各孔在450 nm波长处的吸光度值。
1.2.5. 流式细胞术
为探讨miR-18a-5p mimic对H9c2细胞凋亡的影响,采用流式细胞术检测细胞的凋亡率。取1×106个细胞,在25 ℃下以500 g离心10 min沉淀后,使用PBS洗涤2次。将细胞重新悬浮在预冷的结合缓冲液中,并与Annexin V-FITC和PI在25 ℃下避光孵育 10 min,然后使用流式细胞仪分析细胞凋亡情况。
为探讨miR-18a-5p mimic对H9c2细胞中ROS的影响,采用流式细胞术检测细胞中ROS水平。用无血清培养基以1꞉1 000稀释ROS荧光探针2’,7’-二氯二氢荧光素二乙酸酯(2’,7’-dichlorodihydrofluorescein diacetate,DCFH-DA)至最终浓度10 µmol/L,每组加入DCFH-DA探针,于37 ℃孵育20 min后,用无血清培养基洗涤细胞3次以去除DCFH-DA,使用流式细胞仪分析细胞中ROS水平。
1.2.6. 蛋白质印迹法
使用含有蛋白酶和磷酸酶抑制剂的RIPA缓冲液(50 mmol/L Tris,pH 7.2;150 mmol/L NaCl;1% TritonX-100;0.1% SDS)裂解细胞,使用BCA试剂盒检测蛋白质的浓度。取50 µg蛋白质进行12% SDS聚丙烯酰胺凝胶电泳(SDS polyacrylamide gel electrophoresis,SDS-PAGE),并转移到聚偏二氟乙烯(polyvinylidenefluoride,PVDF)膜上,将膜在室温下用0.5%脱脂奶粉封闭2 h后,加入抗LC3-I、LC3-II、Beclin1、p62、Bax、Bcl-2和Notch2的抗体,并在4 ℃下孵育过夜。抗GAPDH的小鼠单克隆抗体用作内对照。用TBS缓冲液洗涤3次后,将膜与二抗辣根过氧化物酶标记的山羊抗小鼠IgG在室温下孵育 1 h。用凝胶成像系统捕获免疫反应条带,ImageLab软件(5.2版,Bio-Rad)分析灰度值。
1.2.7. 双荧光素酶报告分析
查询预测网站(https://starbase.sysu.edu.cn/),结果显示miR-18a-5p与Notch2存在结合位点(图1)。构建含有Notch2潜在结合位点的荧光素酶报告基因载体质粒,包括野生型(wild type,WT)-Notch2和突变型(mutant,Mut)-Notch2。WT-Notch2和Mut-Notch2的结合序列分别为AAGGUG和CGTGGAA。将WT-Notch2/Mut-Notch2分别与miR-18a-5p mimic NC、miR-18a-5p mimic共转染H9c2细胞(WT-Notch2+mimic NC组、WT-Notch2+mimic组、Mut-Notch2+mimic NC组、Mut-Notch2+mimic组)。对照组为常规培养的H9c2细胞,WT-Notch2组/Mut-Notch2组只转染WT-Notch2/Mut-Notch2。
图1.

预测miR-18a-5p在Notch2上的结合位点
Figure 1 Predicting the binding sites of miR-18a-5p on Notch2
转染后48 h,通过在4 ℃下以500 g离心10 min使细胞沉淀,并在4 ℃下用双荧光素酶报告基因检测试剂盒中的缓冲液(200 µL)裂解细胞30 min。为检测荧光素酶活性,将70 µL裂解液和100 µL荧光素酶检测溶液加入待测溶液中。使用多功能酶标仪检测萤火虫荧光素酶活性。每孔加入100 µL海肾荧光素酶检测试剂检测海肾荧光素酶活性。相对荧光素酶活性为萤火虫荧光素酶荧光值与海肾荧光素酶荧光值之比。
1.3. 统计学处理
使用GraphPad Prism 5.0进行统计分析。所有实验至少重复3次。数据表示为均数±标准误( ±SEM)。使用单因素方差分析,Tukey检验作为事后检验进行比较。P<0.05为差异有统计学意义。
2. 结 果
2.1. 转染成功
与对照组或miR-18a-5p mimic NC组的H9c2细胞相比,miR-18a-5p mimic组H9c2中miR-18a-5p的表达水平均明显上调(均P<0.05,图2),表明转染成功。
图2.
实时反转录PCR检测转染后miR-18a-5p的表达水平(n=3, ±SEM)
Figure 2 Expression levels of miR‑18a‑5p mRNA detected by real-time RT-PCR after transfection (n=3, ±SEM)
*P<0.05.
2.2. MiR‑18a‑5p和Hcy降低细胞活性并促进细胞凋亡
与对照组相比,miR-18a-5p mimic组、Hcy组、miR-18a-5p mimic NC+Hcy组、miR-18a-5p mimic+Hcy组H9c2细胞的活性均显著降低;与miR-18a-5p mimic NC+Hcy组相比,miR-18a-5p mimic+Hcy组H9c2细胞的活性进一步下降(均P<0.05,图3)。
图3.
CCK-8法检测各组H9c2细胞活性(n=3, ±SEM)
Figure 3 Viability of H9c2 cells in each group detected by CCK-8 assay (n=3, ±SEM)
*P<0.05 vs the control group or the miR-18a-5p mimic NC group; †P<0.05 vs the miR-18a-5p mimic NC+ Hcy group. CCK-8: Cell counting kit-8; Hcy: Homocysteine; NC: Negative control.
与对照组相比,miR-18a-5p mimic组、Hcy组、miR-18a-5p mimic NC+Hcy组、miR-18a-5p mimic+Hcy组H9c2细胞的凋亡率均显著增加;与miR-18a-5p mimic NC+Hcy组相比,miR-18a-5p mimic+Hcy组H9c2细胞的凋亡率进一步增加(均P<0.05,图4)。
图4.
流式细胞术检测各组H9c2细胞凋亡(n=3, ±SEM)
Figure 4 Apoptosis of H9c2 cells in each group detected by flow cytometry (n=3, ±SEM)
*P<0.05 vs the control group or the miR-18a-5p mimic NC group; †P<0.05 vs the miR-18a-5p mimic NC+Hcy group. Hcy: Homocysteine; NC: Negative control.
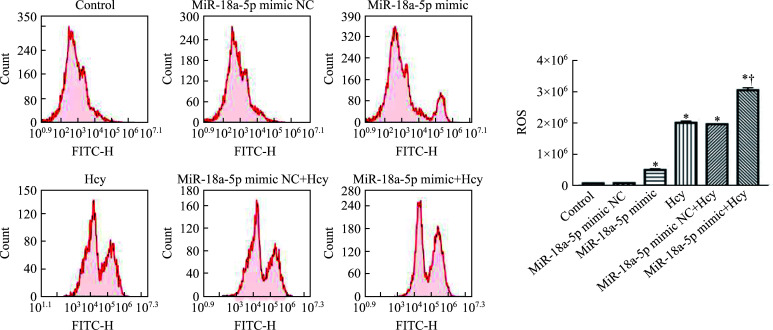
2.3. MiR‑18a‑5p和Hcy增加细胞中ROS的生成
与对照组相比,miR-18a-5p mimic组、Hcy组、miR-18a-5p mimic NC+Hcy组、miR-18a-5p mimic+Hcy组H9c2细胞中的ROS水平均显著升高;与miR-18a-5p mimic NC+Hcy组相比,miR-18a-5p mimic+Hcy组H9c2细胞中的ROS水平进一步升高(均P<0.05,图5)。
图5.
流式细胞术检测各组H9c2细胞中ROS水平(n=3, ±SEM)
Figure 5 ROS levels in H9c2 cells of each group detected by flow cytometry (n=3, ±SEM)
*P<0.05 vs the control group or the miR-18a-5p mimic NC group; †P<0.05 vs the miR-18a-5p mimic NC+Hcy group. ROS: Reactive oxygen species; Hcy: Homocysteine; NC: Negative control.
2.4. MiR‑18a‑5p和Hcy调节自噬和凋亡相关蛋白质的表达
与对照组相比,miR-18a-5p mimic组、Hcy组、miR-18a-5p mimic NC+Hcy组和miR-18a-5p mimic+Hcy组H9c2细胞中Beclin1和Bax的蛋白质表达水平均显著上调,而Notch2、p62和Bcl-2的表达水平显著下调,同时LC3-II/LC3-I值增加(均P<0.05,图6)。与miR-18a-5p mimic NC+Hcy组相比,miR-18a-5p mimic+Hcy组H9c2细胞中Beclin1和Bax的蛋白质表达水平均显著上调,而Notch2、p62和Bcl-2的表达水平显著下调,同时LC3-II/LC3-I值增加(均P<0.05,图6)。miR-18a-5p mimic NC组与对照组上述指标的差异均无统计学意义(P>0.05,图6)。
图6.
蛋白质印迹法检测各组H9c2细胞中自噬和凋亡相关蛋白质的表达(n=3, ±SEM)
Figure 6 Detection of autophagy and apoptosis-related protein expression in H9c2 cells of each group by Western blotting (n=3, ±SEM)
A: Protein expression of Notch2, Beclin1, p62, LC3-I, and LC3-II; B: Protein expression of Bax and Bcl-2. *P<0.05 vs the control group or the miR-18a-5p mimic NC group; †P<0.05 vs the miR-18a-5p mimic NC+Hcy group. Hcy: Homocysteine; NC: Negative control; LC3: Microtubule-associated protein 1 light chain 3.
2.5. MiR-18a-5p与Notch2的靶向关系
双荧光素酶报告分析结果显示:与WT-Notch2+mimic NC组相比,WT-Notch2+mimic组的荧光素酶活性显著下降(P<0.05,图7);而与Mut-Notch2+mimic NC组与Mut-Notch2+mimic组的荧光素酶活性差异无统计学意义(P>0.05,图7)。
图7.
双荧光素酶报告分析验证miR-18a-5p与Notch2之间的靶向关系(n=3, ±SEM)
Figure 7 Verify the targeting relationship between miR-18a-5p and Notch2 by double-luciferase reporter analysis (n=3, ±SEM)
*P<0.05. WT: Wild type; Mut: Mutant; NC: Negative control.
3. 讨 论
既往研究[16-17]表明细胞凋亡广泛参与动脉粥样硬化、心力衰竭和心律失常等多种心血管疾病的发生和发展。高Hcy水平是心脑血管疾病的独立危险因素[18]。Hcy能够在各种细胞模型中诱导细胞凋亡[19-20],并且可能是引发心肌细胞损伤的原因之一[21]。因此,Hcy可用于探索相关心血管疾病的发病机制[14]。本研究使用Hcy处理大鼠心肌细胞,诱导心肌细胞损伤,并研究miR-18a-5p对Hcy损伤细胞的影响。目前尚未发现Hcy和miR-18a-5p之间存在通路关联或互补关联。在既往研究[22]中,用Hcy处理心肌细胞后,心肌细胞线粒体中ROS生成增加,同时膜电位降低。ROS生成增加诱导细胞氧化应激,使细胞的氧化还原状态发生变化,从而导致心肌收缩减弱及心肌细胞存活率降低[23]。Bax和Bcl-2是细胞凋亡的经典生物标志物,在主动脉内皮细胞中,Hcy可上调Bax和下调Bcl-2的表达[5]。本研究结果也表明:Hcy抑制H9c2细胞的活性,增加ROS的生成并促进H9c2细胞凋亡。
MiRNA的异常表达会影响靶基因的表达,从而引起多种疾病[24]。MiR-18a-5p是miR-17-92家族的成员,位于染色体13q31.3上[25];在非小细胞肺癌、胰腺癌等多种恶性肿瘤细胞中表达[26]。MiR-18a-5p在人类主动脉瓣内皮细胞中通过Notch2途径抑制内皮-间充质转化和心脏纤维化[12]。Notch信号通路在心血管系统的发育及心血管疾病的发生和发展中发挥着重要作用[27]。抑制Notch信号通路可减少心肌纤维化,促进受损心肌组织中血管的生成,对心肌起着保护和促进再生的作用[28-29]。本研究构建了miR-18a-5p过表达载体,并通过转染过表达质粒增加H9c2细胞中miR-18a-5p的表达水平。结果表明:过表达miR-18a-5p抑制细胞活性,促进细胞凋亡,对Hcy处理的心肌细胞可进一步抑制其活性。我们的研究和既往研究存在差异。既往研究[10-11]发现miR-18a-5p可改善心肌损伤。其可能原因是miRNA的功能主要取决于其对靶基因的生物学效应[30]。据报道,miR-18a-5p通过抑制Notch2的表达来促进原发性肺动脉平滑肌细胞的增殖和迁移[31]。MiR-18a-5p-Notch2信号通路参与高糖诱导的内皮间质转化的调节[12]。本研究通过双荧光素酶分析发现:在Notch2荧光素酶报告基因中,上调miR-18a-5p表达后,荧光素酶活性显著降低;在Notch2突变荧光素酶报告基因中,上调miR-18a-5p表达后,荧光素酶活性无显著变化。这表明Notch2与miR-18a-5p存在靶向结合。
Chen等[15]发现在Hcy处理H9c2细胞后,裂解的caspase-3的表达水平上调和Bax/Bcl-2值增大。因此,miR-18a-5p和Hcy可能通过调节细胞凋亡相关基因Bax、Bcl-2的表达来诱导H9c2细胞凋亡。自噬在发育、衰老、感染、免疫、肿瘤发生等多种生理和病理过程中发挥关键作用[32-34],过度自噬可能导致自噬相关细胞死亡[35]。Beclin1是启动自噬并参与自噬小体形成的主要因子之一[36]。p62是最常见的自噬底物,它与泛素化蛋白质结合并在自噬小体降解过程中被消耗[37]。Beclin1介导自噬小体形成并参与可溶性LC3-I向自噬性囊泡相关LC3-II的转化[38]。因此,在miR-18a-5p和Hcy联合干预后,LC3-II/LC3-I值、Beclin1表达的增加和p62表达的降低表明miR-18a-5p和Hcy可能促进心肌细胞的自噬和并在诱导自噬方面具有协同作用。氧化应激反应广泛参与多种病理生理过程[39]。细胞的自噬和凋亡依赖ROS[40],ROS参与细胞凋亡的调节[41]。本研究结果表明miR-18a-5p和Hcy增加心肌细胞中ROS的生成,提示在心肌细胞中观察到的凋亡可能是ROS生成增加所致。
综上,miR-18a-5p通过增加心肌细胞内ROS的生成,促进自噬和凋亡,加重Hcy诱导的心肌损伤。Notch2是miR-18a-5p的作用靶点。本研究为Hcy诱导心血管疾病的分子机制提供了新线索。
基金资助
江西省教育厅科技计划项目(GJJ200101);江西省卫生健康委员会科技计划项目(20203006)。
This work was supported by the Science and Technology Plan Projects of Department of Education of Jiangxi Province (GJJ200101) and the Science and Technology Plan Projects of Health Commission of Jiangxi Province (20203006), China.
利益冲突声明
作者声称无任何利益冲突。
作者贡献
尹绢 实验设计和操作,统计分析,论文撰写与修订;胡龙龙、余玲玲 协助实验操作,统计分析;韩雪玲、陈露 协助实验操作;卢寅辉 实验指导,论文修改。所有作者阅读并同意最终的文本。
原文网址
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/20230124.pdf
参考文献
- 1. Han K, Lu Q, Zhu W J, et al. Correlations of degree of coronary artery stenosis with blood lipid, CRP, Hcy, GGT, SCD36 and fibrinogen levels in elderly patients with coronary heart disease[J]. Eur Rev Med Pharmacol Sci, 2019, 23(21): 9582-9589. 10.26355/eurrev_201911_19453. [DOI] [PubMed] [Google Scholar]
- 2. Liu W, Wang T, Sun P, et al. Expression of Hcy and blood lipid levels in serum of CHD patients and analysis of risk factors for CHD[J]. Exp Ther Med, 2019, 17(3): 1756-1760. 10.3892/etm.2018.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joseph J, Joseph L, Shekhawat NS, et al. Hyperhomo-cysteinemia leads to pathological ventricular hypertrophy in normotensive rats[J]. Am J Physiol Heart Circ Physiol, 2003, 285(2): H679-H686. 10.1152/ajpheart.00145.2003. [DOI] [PubMed] [Google Scholar]
- 4. Hung YC, Wang PW, Pan TL. Functional proteomics reveal the effect of Salvia miltiorrhiza aqueous extract against vascular atherosclerotic lesions[J]. Biochim Biophys Acta, 2010, 1804(6): 1310-1321. 10.1016/j.bbapap.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5. Hu H, Wang C, Jin Y, et al. Catalpol inhibits homocysteine-induced oxidation and inflammation via inhibiting Nox4/NF-kappaB and GRP78/PERK pathways in human aorta endothelial cells[J]. Inflammation, 2019, 42(1): 64-80. 10.1007/s10753-018-0873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fan CD, Sun JY, Fu XT, et al. Astaxanthin attenuates homocysteine-induced cardiotoxicity in vitro and in vivo by inhibiting mitochondrial dysfunction and oxidative damage[J]. Front Physiol, 2017, 8: 1041. 10.3389/fphys.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta A, Baltimore D. MicroRNAs as regulatory elements in immune system logic[J]. Nat Rev Immunol, 2016, 16(5): 279-294. 10.1038/nri.2016.40. [DOI] [PubMed] [Google Scholar]
- 8. Wang Y, Zou L, Wu T, et al. Identification of mRNA-miRNA crosstalk in human endothelial cells after exposure of PM2.5 through integrative transcriptome analysis[J]. Ecotoxicol Environ Saf, 2019, 169: 863-873. 10.1016/j.ecoenv.2018.11.114. [DOI] [PubMed] [Google Scholar]
- 9. Yao S, Hu M, Hao T, et al. MiRNA-891a-5p mediates HIV-1 Tat and KSHV Orf-K1 synergistic induction of angiogenesis by activating NF-kappaB signaling[J]. Nucleic Acids Res, 2019, 47(5): 2700. 10.1093/nar/gkz088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Su Y, Chen G, Zhang F, et al. Isoflurane alleviates myocardial injury induced by hypoxia/reoxygenation by regulating miR-18a-5p[J]. Cardiovasc Toxicol, 2021, 21(10): 800-807. 10.1007/s12012-021-09670-1. [DOI] [PubMed] [Google Scholar]
- 11. Nandi SS, Katsurada K, Mahata SK, et al. Neurogenic hypertension mediated mitochondrial abnormality leads to cardiomyopathy: contribution of UPR(mt) and norepinephrine-miR-18a-5p-HIF-1alpha axis[J]. Front Physiol, 2021, 12: 718982. 10.3389/fphys.2021.718982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geng H, Guan J. MiR-18a-5p inhibits endothelial-mesenchymal transition and cardiac fibrosis through the Notch2 pathway[J]. Biochem Biophys Res Commun, 2017, 491(2): 329-336. 10.1016/j.bbrc.2017.07.101. [DOI] [PubMed] [Google Scholar]
- 13. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T) method[J]. Methods, 2001, 25(4): 402-408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14. Aminzadeh A, Mehrzadi S. Cardioprotective effect of levosimendan against homocysteine-induced mitochondrial stress and apoptotic cell death in H9C2[J]. Biochem Biophys Res Commun, 2018, 507(1/4): 395-399. 10.1016/j.bbrc.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 15. Chen K, Chen L, Ouyang Y, et al. Pirfenidone attenuates homocysteineinduced apoptosis by regulating the connexin 43 pathway in H9C2 cells[J]. Int J Mol Med, 2020, 45(4): 1081-1090. 10.3892/ijmm.2020.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease[J]. Apoptosis, 2009, 14(4): 536-548. 10.1007/s10495-008-0302-x. [DOI] [PubMed] [Google Scholar]
- 17. Shekhar A, Heeger P, Reutelingsperger C, et al. Targeted imaging for cell death in cardiovascular disorders[J]. JACC Cardiovasc Imaging, 2018, 11(3): 476-493. 10.1016/j.jcmg.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 18. Fu Y, Wang X, Kong W. Hyperhomocysteinaemia and vascular injury: advances in mechanisms and drug targets[J]. Br J Pharmacol, 2018, 175(8): 1173-1189. 10.1111/bph.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Zhang Y, Tang J, et al. Oxymatrine inhibits homocysteine-mediated autophagy via MIF/mTOR signaling in human umbilical vein endothelial cells[J]. Cell Physiol Biochem, 2018, 45(5): 1893-1903. 10.1159/000487912. [DOI] [PubMed] [Google Scholar]
- 20. Majumder S, Ren L, Pushpakumar S, et al. Hydrogen sulphide mitigates homocysteine-induced apoptosis and matrix remodelling in mesangial cells through Akt/FOXO1 signalling cascade[J]. Cell Signal, 2019, 61: 66-77. 10.1016/j.cellsig.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Y, Zhang H, Liu H, et al. Homocysteine up-regulates ETB receptors via suppression of autophagy in vascular smooth muscle cells[J]. Microvasc Res, 2018, 119: 13-21. 10.1016/j.mvr.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 22. Kurauchi K, Nishikawa T, Miyahara E, et al. Role of metabolites of cyclophosphamide in cardiotoxicity[J]. BMC Res Notes, 2017, 10(1): 406. 10.1186/s13104-017-2726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishikawa T, Miyahara E, Kurauchi K, et al. Mechanisms of fatal cardiotoxicity following high-dose cyclophosphamide therapy and a method for its prevention[J/OL]. PLoS One, 2015, 10(6): e131394[2022-12-01]. 10.1371/journal.pone.0131394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong WH, Li Q, Zhang XY, et al. Deep sequencing identifies deregulation of microRNAs involved with vincristine drug-resistance of colon cancer cells[J]. Int J Clin Exp Pathol, 2015, 8(9): 11524-11530. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4637701/#. [PMC free article] [PubMed] [Google Scholar]
- 25. Hamilton MP, Rajapakshe K, Hartig SM, et al. Identification of a pan-cancer oncogenic microRNA superfamily anchored by a central core seed motif[J]. Nat Commun, 2013, 4: 2730. 10.1038/ncomms3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morimura R, Komatsu S, Ichikawa D, et al. Novel diagnostic value of circulating miR-18a in plasma of patients with pancreatic cancer[J]. Br J Cancer, 2011, 105(11): 1733-1740. 10.1038/bjc.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kokubo H, Miyagawa-Tomita S, Nakazawa M, et al. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system[J]. Dev Biol, 2005, 278(2): 301-309. 10.1016/j.ydbio.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 28. Zheng Y, Wang S, Xue X, et al. Notch signaling in regulating angiogenesis in a 3D biomimetic environment[J]. Lab Chip, 2017, 17(11): 1948-1959. 10.1039/c7lc00186j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woltje K, Jabs M, Fischer A. Serum induces transcription of Hey1 and Hey2 genes by Alk1 but not Notch signaling in endothelial cells[J/OL]. PLoS One, 2015, 10(3): e120547[2022-12-01]. 10.1371/journal.pone.0120547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barwari T, Joshi A, Mayr M. MicroRNAs in cardiovascular disease[J]. J Am Coll Cardiol, 2016, 68(23): 2577-2584. 10.1016/j.jacc.2016.09.945. [DOI] [PubMed] [Google Scholar]
- 31. Miao R, Liu W, Qi C, et al. MiR-18a-5p contributes to enhanced proliferation and migration of PASMCs via targeting Notch2 in pulmonary arterial hypertension[J]. Life Sci, 2020, 257: 117919. 10.1016/j.lfs.2020.117919. [DOI] [PubMed] [Google Scholar]
- 32. Hale AN, Ledbetter DJ, Gawriluk TR, et al. Autophagy: regulation and role in development[J]. Autophagy, 2013, 9(7): 951-972. 10.4161/auto.24273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levine B, Kroemer G. Autophagy in the pathogenesis of disease[J]. Cell, 2008, 132(1): 27-42. https:// 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sermersheim MA, Park KH, Gumpper K, et al. MicroRNA regulation of autophagy in cardiovascular disease[J]. Front Biosci (Landmark Ed), 2017, 22: 48-65. 10.2741/4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yonekawa T, Thorburn A. Autophagy and cell death[J]. Essays Biochem, 2013, 55: 105-117. 10.1042/bse0550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jin S, Tian S, Chen Y, et al. USP19 modulates autophagy and antiviral immune responses by deubiquitinating Beclin-1[J]. EMBO J, 2016, 35(8): 866-880. 10.15252/embj.201593596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee Y, Weihl CC. Regulation of SQSTM1/p62 via UBA domain ubiquitination and its role in disease[J]. Autophagy, 2017, 13(9): 1615-1616. 10.1080/15548627.2017.1339845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sciarretta S, Maejima Y, Zablocki D, et al. The role of autophagy in the heart[J]. Annu Rev Physiol, 2018, 80: 1-26. 10.1146/annurev-physiol-021317-121427. [DOI] [PubMed] [Google Scholar]
- 39. Snezhkina AV, Kudryavtseva AV, Kardymon OL, et al. ROS generation and antioxidant defense systems in normal and malignant cells[J]. Oxid Med Cell Longev, 2019, 2019: 6175804. 10.1155/2019/6175804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qian M, Tan HM, Yu N, et al. Inactivated sendai virus induces ROS-dependent apoptosis and autophagy in human prostate cancer cells[J]. Biomed Environ Sci, 2018, 31(4): 280-289. 10.3967/bes2018.036. [DOI] [PubMed] [Google Scholar]
- 41. Yang H, Xie Y, Yang D, et al. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma[J]. Oncotarget, 2017, 8(15): 25310-25322. 10.18632/oncotarget.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]