ABSTRACT
BACKGROUND:
Cognitive impairment is a common multiple sclerosis (MS)-related symptom that impacts quality of life (QOL). Diet interventions are shown to be beneficial in managing QOL, and the intake of essential fatty acids is linked with improved cognitive function. However, the effect of diets on serum fatty acid profiles and cognitive function is unknown.
METHODS:
A previous randomized, parallel-arm trial recruited participants with relapsing-remitting MS (N = 77). Study visits included 4 time points: run-in, baseline, 12 weeks, and 24 weeks. During the run-in phase, participants followed their usual diet and were then randomly assigned to either a modified paleolithic (Wahls) or a low saturated fat (Swank) diet at baseline. Assessments at study visits included cognitive function assessed by Symbol Digit Modalities Test-Oral (SDMT-O) and Perceived Deficits Questionnaire (PDQ), and serum fatty acids, including eicosapentaenoic (EPA), docosahexaenoic (DHA), and arachidonic (ARA) acids
RESULTS:
Both groups had significant improvements in all serum fatty acids (P < .01), except for ARA, as well as SDMT-O at 24-weeks (P < .05), total PDQ at 12 and 24 weeks (P < .01) compared with baseline values. The 12-week changes in ω-3 (EPA + DHA) index and EPA serum fatty acids were associated with SDMT-O changes (P ≤ .05); however, the changes in fatty acid levels did not mediate the effect of the diets on SDMT-O or PDQ (P > .05).
CONCLUSIONS:
Both diets led to improvements in serum fatty acid profiles and cognitive function, with associations between the 12-week ω-3 (EPA + DHA) index and EPA changes with SDMT-O.
Keywords: multiple sclerosis, diet, cognitive function, omega-3 fatty acids
Multiple sclerosis (MS) is one of the most common causes of neurologic disability in young adults, with its peak incidence occurring in 20- to 40-year-olds.1 Among the deficits, impaired cognitive function, which affects up to 70% of individuals with MS,2 can be uniquely devastating. Population surveys show that cognitive dysfunction leads to a loss of self-esteem, increased social isolation, and decreased standards of living, all resulting in a lower quality of life (QOL).3 Cognitive impairment in MS most frequently presents as slowed information processing speed, as well as a decline in episodic memory.2 However, difficulties in executive function, verbal fluency, and visuospatial skills also have been observed.2 While impaired cognitive function is a well-recognized consequence of MS, it continues to be a prevalent result of disease progression, in part due to incompletely understood disease pathogenesis.
While the complexities of the relationship between MS and cognitive dysfunction are not fully understood, the connection between blood biomarkers and cognition is emerging.4 Specifically, serum fatty acid levels have been associated with MS disease severity, including its impact on cognitive function.4 Fatty acids in both systemic and central nervous system (CNS) circulation fluctuate during demyelinating events in people with MS.4 Therefore, abundant peripheral fatty acids may play an important role in remyelination and are essential materials in reconstruction and healing. As such, fatty acid serum levels may serve as potential biomarkers of cognitive function in individuals with MS.4
Fatty acids are consumed primarily from diet or supplementation. Flax, walnut, hemp, sesame, and olive oils are sources of monounsaturated fatty acids, and ω-3 fatty acids can be found in the aforementioned foods, as well as in oily marine fish, grass-fed meats, and high-fat vegetables, such as olives.5 Two popular diets in the MS community, the low-saturated fat diet developed by Roy Swank, MD, PhD, and the modified paleolithic diet developed by Terry Wahls, MD, have been shown to reduce perceived fatigue and improve QOL.6 However, these diets differ in recommended consumption quantities of high-fat foods5 with the Wahls Protocol diet averaging around 3.5 g per day and the Swank diet averaging around 2.5 g per day of ω-3 fatty acids intake.7,8
A clearer understanding of the relationship between fatty acid serum levels and cognition in MS may elucidate the role of specific dietary interventions in modifying this common and debilitating symptom. This secondary analysis of the WAVES trial (NCT02914964) aims to compare the effects of the Wahls and Swank diets (with supplemental cod liver oil included for both diets) on cognitive function and assess the association with serum fatty acid levels among people with relapsing-remitting MS (RRMS).
METHODS
Participants
This is a secondary analysis of a 36-week, randomized, parallel-group, single-blinded trial conducted at the University of Iowa Preventive Intervention Center. The University of Iowa Institutional Review Board 01 Biomedical approved the trial following the Consolidated Standards of Reporting Trials reporting guidelines.9 Written informed consent was obtained from all study participants.
Adults aged between 18 and 70 years were recruited from Iowa City, Iowa, and the surrounding 500-mile area. Participants were eligible for enrollment in the study if they (1) had neurologist-confirmed RRMS consistent with the 2010 McDonald Criteria,10 (2) had moderate to severe fatigue as assessed by the Fatigue Severity Scale (FSS),11 (3) possessed the ability to walk 25 ft with minimal support, (4) were not pregnant nor planning to become pregnant, and (5) were willing to adhere to all study procedures. Primary exclusion criteria included (1) MS relapse or change in disease-modifying drug therapy within the 12 weeks prior to the start of the study, (2) any change in medication for management of MS-related symptoms within the prior 12 weeks, (3) BMI less than 19 kg/m2, (4) moderate to severe mental impairment as measured by the Short Portable Mental Status Questionnaire (SPMSQ),12 (5) self-reported adverse reactions to gluten-containing foods, (6) diagnosis of certain comorbidities (eg, celiac disease, severe psychiatric disorders, eating disorders, kidney stones, heart failure, angina, or cirrhosis), (7) taking insulin or warfarin, and (8) undergoing radiation or chemotherapy. Complete inclusion and exclusion criteria are listed in the trial protocol.13
Study Procedure
Eligible participants started a 12-week observational run-in phase adhering to their usual diet. At the baseline visit, participants were randomly assigned 1:1 to the low-saturated fat (Swank) diet or the modified Paleolithic elimination (Wahls) diet. The study registered dietitians (RDs) provided dietary education regarding their respective dietary interventions. Throughout the 12-week intervention period, study RDs provided in-person and telephone-based nutrition counseling. In the 12-week follow-up period, participants continued their allocated study diet without active RD support, though they were permitted to contact an RD for assistance. Participant visits occurred at 4 time points, spaced out by 12 weeks: run-in, baseline, 12 weeks, and 24 weeks.
Study Diets
Participants were randomly assigned to receive either the low-saturated fat (Swank) diet or the modified Paleolithic elimination (Wahls) diet and instructed to follow their respective diet as desired during the 24-week period. A detailed composition of both diets has been outlined elsewhere.5
In brief, the Swank diet limits saturated fat to less than or equal to 15 g per day and provides 20 to 50 g of unsaturated fat, 4 servings of grains, and 4 servings of fruits and vegetables per day. Depending on sex, the Wahls diet recommends 6 to 9 servings of fruits and vegetables and 6 to 12 oz of meat daily and excludes all grains, legumes, eggs, and dairy (except clarified butter and ghee). Between the baseline and 12-week time points, participants were instructed to exclude nightshade vegetables (ie, tomatoes, white potatoes, eggplant, peppers); subsequently, they were guided to reintroduce nightshades between the 12-week and 24-week points.
Regardless of diet, all study participants followed the same daily supplement regimen that included 1 tsp of cod liver oil per day.13 Every 4 weeks, participants received personalized feedback based on their diet checklists.
Outcomes
Dietary characteristics and primary outcomes of the initial trial are published elsewhere.13,14 Cognitive function was evaluated using the Symbol Digit Modalities Test-Oral (SDMT-O) and the Perceived Deficits Questionnaire (PDQ). The SDMT-O is a cognitive test that measures cognitive information processing speed, and it has been validated for use in individuals with MS.15 The test uses a coding key with 9 different abstract symbols paired with a respective number. Below the key, a series of abstract symbols is presented, and the participants are asked to recite the corresponding number for each symbol. The number of correct responses within a 90-second frame is recorded as the total score.
The PDQ is a validated 20-item self-report questionnaire designed to measure perceived cognitive dysfunction in individuals with MS.16 The PDQ has a total score as well as 4 separate scores in 4 cognitive subscales: attention, retrospective memory, prospective memory, and planning/organization. The total PDQ score is calculated by adding raw scores for all PDQ items, ranging from 0 to 80. Domain-specific subscores are calculated as the sum of the respective items on the subscale, with scores ranging from 0 to 20. Higher scores indicate greater perceived cognitive impairment.
Participant data (eg, height, weight, blood pressure) were collected at each visit using standardized protocols by trained staff. Phlebotomists collected blood biospecimens, which were sent to the Iowa City Veterans Affairs Medical Center Department of Pathology for analysis, including eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), arachidonic acid (ARA), ω-3 index (EPA + DHA), ω-6/ω-3 ratio, and the EPA/ARA ratio. These measures were chosen to capture the range of essential fatty acids that humans cannot synthesize.
Statistical Analysis
Enrollment variables were assessed using descriptive statistics to calculate frequencies, means plus or minus standard error of the mean (SEM), and medians (IQR). Outlier data were evaluated for correctness as well as potential entry errors. Continuous variables were analyzed as distributions for normality through graphical observation. Participants who completed the 12- and 24-week assessments were included in the primary analysis. Assessments of treatment group baseline differences in collected measures were made using 2-sample t and Pearson χ2 tests for continuous and categorical measures, respectively.
The dietary effect interactions and 6 outcome measures (ie, SDMT-O score, PDQ Total Score, 4 PDQ subscales) were tested using generalized linear mixed models17 with participant repeated time measures incorporated into the analysis. The identity link function was used for normally distributed outcomes. Variables of interest included age, sex, BMI, smoking status, alcohol consumption, walking assistance, years since MS diagnosis, disease-modifying drug therapy, baseline vitamin D, and a baseline 6-minute walk distance. Measures were modeled individually to assess their relationships with each outcome, as well as whether they modified the estimates for the diet and time interaction. Additionally, given that fatigue16 and education18 affect the perception of cognitive ability, FSS scores and education were also included as variables of interest. Model fits within each outcome were compared among all predictor sets. The model with the smallest Akaike information criterion (AIC)19 for each respective outcome model was considered to be the optimal predictor set, with point estimates, 95% CIs, and P values of the within- and between-group mean changes also generated.
Linear mixed models controlling for diet and baseline values were used to evaluate the association of 12-week changes in serum fatty acid profiles and cognitive function (PDQ, SDMT-O test), with slope estimates, 95% CIs, and P values generated for each serum fatty acid. Causal mediation analyses20 assessed the mediation effect for the interaction between serum fatty acid profiles and cognitive function from baseline to 12 weeks, with diet assignment controlled.
All analyses were conducted as 2-sided tests, α less than or equal to .05, using SAS software (version 9.4, SAS Institute, Inc).
RESULTS
This secondary analysis included 77 participants (39 Wahls and 38 Swank) who completed the primary study end point at 12 weeks and 72 participants (35 Wahls and 37 Swank) that completed 24-week follow-ups. At baseline, there were no differences in characteristics between groups (TABLE S1).
Participants in both the Swank and Wahls diet groups exhibited statistically significant standard error of the mean (SEM) increases in all serum fatty acids, except ARA, at 12 and 24 weeks (P < .05 for all; TABLE 1), as compared with baseline values. Both groups exhibited significant SEM increases in SDMT-O at 24 weeks (P < .05 for both), and SEM decreases for PDQ total score at 12 and 24 weeks (P < .01; Table 1), when compared with baseline values. No serum or cognitive function outcome exhibited statistically significant differences between groups at any point (P > .05 for all; Table 1).
TABLE 1.
Fatty Acid Values and Cognitive Function Assessment Outcomes
| Study visit | ||||
|---|---|---|---|---|
| Run-in | Baseline | 12 weeks | 24 weeks | |
| Swank diet | ||||
| Fatty acid biomarkers | ||||
| ω-3 (EPA + DHA) Index | 2.42 ± 0.16a | 2.55 ± 0.20 | 4.53 ± 0.31‡ | 4.10 ± 0.28‡ |
| ω-6/ω-3 ratio | 11.7 ± 0.59 | 11.7 ± 0.67 | 6.39 ± 0.45‡ | 7.36 ± 0.59‡ |
| EPA | 0.53 ± 0.07 | 0.58 ± 0.10 | 1.21 ± 0.14‡ | 1.02 ± 0.10† |
| DHA | 1.87 ± 0.11 | 1.98 ± 0.13 | 3.37 ± 0.18‡ | 3.16 ± 0.20‡ |
| EPA/ARA ratio | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.15 ± 0.02‡ | 0.13 ± 0.01‡ |
| ARA | 8.36 ± 0.27 | 8.86 ± 0.26 | 8.54 ± 0.35 | 8.81 ± 0.40 |
| Cognitive function outcomes | ||||
| SDMT-O | 57.8 ± 1.61 | 60.4 ± 1.51 | 63.3 ± 1.52‡ | 62.9 ± 1.60† |
| PDQ total | 33.1 ± 2.50 | 32.4 ± 2.36 | 25.1 ± 2.00‡ | 26.1 ± 2.14‡ |
| PDQ planning | 9.43 ± 0.69 | 8.50 ± 0.65 | 6.81 ± 0.66† | 6.84 ± 0.63‡ |
| PDQ prospective memory | 6.62 ± 0.66 | 6.63 ± 0.57 | 5.03 ± 0.46† | 5.57 ± 0.54† |
| PDQ retrospective memory | 7.73 ± 0.78 | 8.11 ± 0.71 | 6.16 ± 0.58‡ | 6.41 ± 0.63‡ |
| PDQ attention | 9.35 ± 0.64 | 9.20 ± 0.64 | 7.08 ± 0.54* | 7.32 ± 0.58† |
| Wahls diet | ||||
| Fatty acid biomarkers | ||||
| ω-3 (EPA + DHA) Index | 2.34 ± 0.13 | 2.23 ± 0.14 | 3.75 ± 0.27‡ | 3.53 ± 0.30‡ |
| ω-6/ω-3 ratio | 11.5 ± 0.56 | 12.3 ± 0.50 | 8.25 ± 0.56‡ | 8.97 ± 0.63‡ |
| EPA | 0.54 ± 0.04 | 0.49 ± 0.04 | 0.95 ± 0.10‡ | 0.87 ± 0.13† |
| DHA | 1.79 ± 0.11 | 1.76 ± 0.11 | 2.82 ± 0.17‡ | 2.66 ± 0.19‡ |
| EPA/ARA ratio | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.11 ± 0.02† | 0.10 ± 0.02† |
| ARA | 8.38 ± 0.25 | 8.85 ± 0.29 | 9.34 ± 0.34 | 9.65 ± 0.42 |
| Cognitive function outcomes | ||||
| SDMT-O | 60.2 ± 1.43 | 61.2 ± 1.53 | 62.0 ± 1.55 | 63.5 ± 1.83* |
| PDQ total | 35.1 ± 2.24 | 25.9 ±2.16 | 29.1 ± 2.71† | 25.1 ± 3.03‡ |
| PDQ planning | 9.31 ± 0.62 | 9.46 ± 0.67 | 7.24 ± 0.79† | 6.20 ± 0.88‡ |
| PDQ prospective memory | 7.07 ± 0.54 | 7.33 ± 0.53 | 6.37 ± 0.57‡ | 5.54 ± 0.69‡ |
| PDQ retrospective memory | 9.00 ± 0.62 | 9.33 ± 0.60 | 7.63 ± 0.80‡ | 6.57 ± 0.82‡ |
| PDQ attention | 9.75 ± 0.67 | 9.79 ± 0.57 | 7.87 ± 0.71‡ | 6.77 ± 0.83‡ |
ARA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; PDQ, Perceived Deficits Questionnaire; SDMT-O, Symbol Digit Modalities Test-Oral. Note: Within-group statistical significance compared to baseline values indicated by
= P ≤ .05;
= P ≤ .01;
= P ≤ .001.
All values shown in mean ± SEM.
Cognitive function, as assessed by SDMT-O and PDQ, showed mean change improvements at 12 and 24 weeks with both groups. Total PDQ scores showed statistically significant SEM change decreases at 12 and 24 weeks within the Swank group (–7.37 ± 1.72 and –6.30 ± 1.68, respectively; P≤ .0002 for both) and the Wahls group (–6.81 ± 2.04 and –10.8 ± 2.25, respectively; P≤ .0002 for both; FIGURE 1A). Scores on the PDQ attention subscale also increased, showing statistically significant SEM change at these intervals in the Swank (4.54 ± 2.22 and 7.20 ± 2.31, respectively; P≤ .04 for both) and Wahls (8.11 ± 2.03 and 12.4 ± 2.70, respectively; P < .0001 for both) groups (FIGURE 1B). The Swank group had increased values in the PDQ retrospective memory subscale of –2.09 ± 0.53 (P < .0001) and –1.87 ± 0.54 (P = 0.0005) at 12- and 24-week intervals, respectively, and the Wahls group had statistically significant SEM increases of –2.42 ± 0.60 (P < .0001) and –3.41 ± 0.72 (P < .0001) at the same check-in intervals (FIGURE 1C). Similarly, the PDQ prospective memory subscale had SEM increases at 12 and 24 weeks for Swank (–1.82 ± 0.56 and –1.63 ± 0.54, respectively; P ≤ .003 for both) and Wahls (–2.19 ± 0.69 and –3.39 ± 0.72, respectively; P ≤ .002 for both) groups (FIGURE 1D). Scores on the PDQ planning subscale also presented SEM decreases of 1.30 ± 0.44 (P = 0.003) in the Swank group at 12 weeks, although this was not statistically significant at 24 weeks (–0.84 ± 0.50; P = .09), and –1.39 ± 0.51 and –2.29 ± 0.61 (P ≤ .007) for the Wahls group at 12 and 24 weeks, respectively (FIGURE 1E). SDMT-O values had SEM increases at 12- and 24-week intervals for both the Swank (2.93 ± 0.89 and 2.56 ± 0.97, respectively; P ≤ .009 for both) and the Wahls (0.87 ± 0.78 and 2.32 ± 0.91, respectively; P ≤ .02 for both) groups (FIGURE 1F). All cognitive function outcomes did not exhibit statistically significant differences between groups at any point (P > .05 for all).
FIGURE 1.
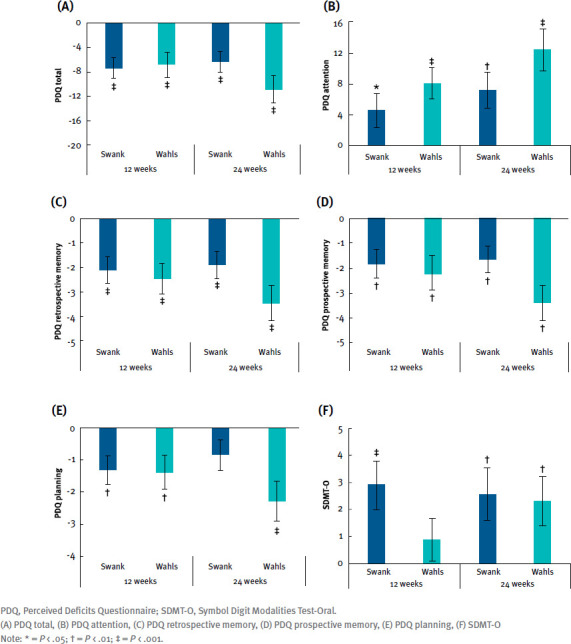
Mean Change From Baseline for Cognitive Function Outcomes
The 12-week changes in SDMT-O were significantly associated with changes in the ω-3 (EPA + DHA) index with a β-coefficient (95% CI) of 9.56 (0.60, 18.5; P = .04) and changes in EPA with a β-coefficient (95% CI) of 10.4 (1.98, 18.9; P = .02; TABLE 2). However, no significant mediation effects were identified (TABLE S2).
TABLE 2.
Association and Mediation Effect of 12-Week Serum Fatty Acid Changes With Perceived Cognitive Function Changes
| PDQ total | SDMT-O | |||||||
|---|---|---|---|---|---|---|---|---|
| β-coefficienta (95% CI) | P value | Percentage mediated (95% CI) | P value | β-coefficient (95% CI) | P value | Percentage mediated (95% CI) | P value | |
| ω-3 (EPA + DHA) index | −2.05 (−20.5, 16.4) |
.82 | 5.94 (−40.5, 52.4) |
.80 | 9.56 (0.60, 18.5) |
.04* | −145 (−454, 165) |
.36 |
| ω-6/ω-3 ratio | 2.52 (−6.93, 12.0) |
.60 | 22.6 (−46.9, 92.1) |
.52 | −2.15 (-6.17, 1.87) |
.29 | −170 (−590, 250) |
.43 |
| EPA | 1.76 (−37.7, 41.3) |
.93 | 2.17 (−31.2, 35.6) |
.90 | 10.4 (1.98, 18.9) |
.02* | −145 (−473, 183) |
.39 |
| DHA | −3.07 (−32.1, 25.9) |
.83 | 10.5 (−40.6, 61.6) |
.69 | 6.19 (−6.59, 19.0) |
.33 | −141 (−478, 196) |
.41 |
| EPA/ARA ratio | −78.4 (−369, 213) |
.59 | 13.5 (−16.0, 43.0) |
.37 | −42.1 (-174, 89.6) |
.53 | −14 (−345, 138) |
.40 |
| ARA | 1.21 (−14.0, 16.4) |
.87 | −0.40 (−5.09, 4.30) |
.87 | −0.24 (−6.58, 6.11) |
.94 | −2.54 (−19.6, 14.5) |
.77 |
ARA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; PDQ, Perceived Deficits Questionnaire; SDMT-O, Symbol Digit Modalities Test-Oral.
Note: = P ≤ .05;
= P ≤ .01;
= P ≤ .001.
The β-coefficient is the degree of change in the outcome variable for every 1 unit of change in the independent variable. The t test assesses whether the β-coefficient is statistically and significantly different from the effects beyond the covariates.
DISCUSSION
At the 12- and 24-week follow-ups, when compared with the baseline, participants with MS who followed the Swank and Wahls diets showed considerable improvement in serum fatty acid profiles, which included ω-3 (EPA + DHA) index, ω-6/ω-3 ratio, EPA, DHA, and EPA/ARA ratio. Similarly, perceived cognitive difficulties, as assessed by the PDQ, and objectively assessed cognitive processing speed, as measured via the SDMT-O, also exhibited favorable improvements at 12 and 24 weeks when compared with the baseline. The changes for cognitive information processing speed (specifically SDMT-O) at 12 weeks were associated with serum fatty acid profiles, including the ω-3 (EPA + DHA) index and EPA, among participants with RRMS, although fatty acid changes did not mediate the effect of diet on cognitive function. This suggests that fatty acids may be associated with objectively assessed cognitive information processing speed, rather than perceived cognitive impairment. Perception of cognitive impairment may be impacted by other factors, necessitating further investigation that includes objective cognitive tests in addition to self-reported questionnaires.
Cognitive impairment is a common and substantial consequence of MS that negatively impacts QOL.2 Assessing cognitive function is an important component of monitoring MS development and progression.2 This secondary analysis revealed notable improvements in the scores on the PDQ total and PDQ subscales, as well as the SDMT-O, following the Swank and Wahls diets. These findings corroborate the growing evidence showing improved cognitive function following dietary modifications, such as the Mediterranean diet,21-23 the DASH diet (Dietary Approaches to Stop Hypertension),23 ketogenic diet,24 and paleolithic diet,25 or supplementation with polyunsaturated fatty acids (PUFAs).26 Participants with progressive MS who followed a multimodal intervention that included a modified paleolithic diet showed improved cognitive function after 12 months.25 While improvements were seen in cognitive function, the mechanisms by which diet and/or supplements improve cognitive function among people with MS are not well understood and warrant additional research.
Dietary PUFAs are thought to influence cognitive function in MS.27 In the present study, all serum fatty acids, except for ARA, improved over the study period. Given that PUFAs, such as ω-3 and ω-6, are not synthesized by the human body, improvement in levels of serum fatty acid profiles in this study can be attributed to the dietary intervention that included cod liver oil supplementation. The Swank diet recommends restricting dietary saturated fat to less than 15 g per day and replacing it with 20 to 50 g per day of oils rich in mono- and polyunsaturated fatty acids.5 In contrast, the Wahls diet encourages monounsaturated fats from sources such as avocado, olive, sesame, and sunflower oils, as well as ω-3 fatty acids from sources such as fatty fish, grass-fed beef, flax, walnut, and hemp oils.5 Additionally, participants in both groups were instructed to supplement with 5 g (ie, 1.13 tsp) per day of cod liver oil, a rich source of ω-3 fatty acids, especially EPA and DHA.28 Therefore, the PUFA sources from the diets and supplements likely had a direct impact on the serum fatty acid profiles of the participants for the duration of the study intervention, which in turn, was associated with the improvement of cognitive function in the present study.
Emerging evidence links improved cognitive function with consumption of PUFAs, especially in people with MS.29 This is corroborated by the present analysis that found an association between SDMT-O, a sensitive objective tool assessing cognitive functions of processing speed and visual scanning,2 with the 12-week serum level changes of the ω-3 (EPA + DHA) index and EPA following intervention with the Swank or Wahls diets. This association may be because ω-3 long-chain PUFAs, including DHAs, are major components of neuronal membranes, which are essential to membrane fluidity,29 as well as the fatty acids utilized to form myelin sheaths.27 Proper remyelination is vital to ensure neuronal function and protect axons from degeneration.27 In particular, fatty acid synthesis is found to be essential for both myelination and remyelination, as well as having indirect influences on neuronal function, development, and integrity, all of which influence cognitive function.27 Additionally, some fatty acids are actively transported across the blood-brain barrier, such as DHA by MFSD2A, which is expressed exclusively in the endothelium of the blood-brain barrier of micro-vessels,30 thus impacting cognitive function in people with MS. A cross-sectional analysis from 11 cohorts assessing metabolites linked to general cognitive function and dementia found that DHA levels in the blood were associated with higher cognitive function.31 Therefore, the association between serum fatty acid profiles and SDMT-O in the present study provides compelling evidence of the role that fatty acids play in cognitive function in RRMS and merits further investigation.
The link between dietary fatty acids and cognitive function also may be mitigated by fatty acid–specific inflammatory responses. ω-3 fatty acids are shown to have antioxidant, anti-inflammatory, and neuroprotective effects, while also influencing multiple inflammatory response mechanisms, including gene expression and signaling cascades.29 A recent meta-analysis of 13 prospective cohort studies, including a study of 1353 participants exploring ω-3 PUFA consumption and inflammatory gene expression in MS, found that higher levels of DHA were significantly associated with lower EDSS scores.29 This protective association between serum DHA and EDSS scores has been observed in several studies among people with MS.21,26,32 Additionally, this meta-analysis found that ω-3 fatty acids showed notable influence on inflammatory response gene expression, including upregulation of peroxisome proliferator-activated receptor gamma (PPAR-γ) gene expression, with downregulation of tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) gene expression.29 Inflammation and neurodegeneration are pathological features of MS that impact cognitive impairment.33 Consequently, the improvement of cognitive function may be influenced by the anti-inflammatory properties of ω-3 PUFAs.
Besides cognitive function, dietary and supplementary PUFA intake is linked to reduced risk of MS. A multicenter incident case-control study showed that a higher intake of ω-3 PUFA, specifically derived from fish rather than from plants, was associated with a decreased risk of first clinical diagnosis of CNS demyelination.26 An Australian multicenter case-control study (with sex, age, and study region matched) found that fish oil supplementation was a major contributor to the ω-3 index, with higher ω-3 index values associated with lower risk of the first clinical diagnosis of CNS demyelination.34 Similarly, in a double-blind, randomized 12-month study, participants with RRMS observed a decreased relapse rate in groups either treated with ω-3 PUFAs and olive oil with daily dietary fat consumption limited to 15% energy or an olive oil-only group, whose daily dietary fat intake was limited to 30% energy.21 Thus, these studies suggest that either PUFA supplementation and/or dietary interventions improve MS-associated outcomes and, in the case of this study, improve cognitive function.
Although one of the strengths of the present study included faithful adherence to the assigned diet (≥ 80%) among participants in both groups,6 there were several limitations. The generalizability of the present findings are limited, given the extensive exclusion criteria and the lack of diversity in the ethnicity, race, and gender in the study sample. The lack of a “typical diet” comparison arm and disease activity evaluation further limits these findings. Given that fatty acids did not mediate the effect of diet on cognition, further exploration is needed to investigate the potential mechanism by which fatty acids impact cognition. Since only 1 version of the SDMT-O was used in the present study, the practice effect also may have contributed to the improvement in participant performance over time.35 Future studies should strongly consider incorporating varying SDMT-O forms, as well as objective, comprehensive cognitive assessments that include tests across domains to reveal more robust associations between fatty acids and cognition and remedy the practice effect, since SDMT-O was the only objective measure used to assess cognition and was the only measure associated with fatty acid changes. Additionally, all participants were asked to follow a supplement regimen that included cod liver oil, which is a rich source of ω-3 fatty acids; thus, changes in serum fatty acid profiles cannot solely be attributed to diet, as the dietary modification cannot be distinguished from the supplement use. Given the short follow-up period, there is potential for a ceiling effect for fatty acid biomarkers, which may not have been captured in the present study; thus, longer follow-up studies would be highly recommended.
The results from this secondary analysis suggest that diet and supplement interventions that enrich serum ω-3 fatty acid profiles may also improve cognitive function among individuals with RRMS. Future studies should include cognitive assessment as a component of monitoring MS progression.2 Exploring methods for improving cognitive function, such as through dietary and supplement interventions, will likely improve QOL and daily functioning for individuals with MS. Diet and supplements can play a vital role as adjuncts and/or alternative therapies for people with MS. The implications of this secondary analysis illustrate that diet and/or supplement regimens rich in ω-3 fatty acids may improve cognitive function among individuals with RRMS. This study provides evidence for future studies to assess the intersection between diet (including supplements), serum ω-3 fatty acids, and cognitive function in MS.
PRACTICE POINTS
Both the Swank and Wahls diets, with cod liver oil supplementation, have favorable impacts on serum levels of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), arachidonic acid (ARA), ω-3 index (EPA + DHA), ω-6/ω-3 ratio, and the EPA/ARA ratio, as well as cognitive function, among individuals with relapsing-remitting multiple sclerosis.
Improvement of serum ω-3 fatty acid profiles and its effect on cognitive function warrants further research.
Supplementary Material
ACKNOWLEDGMENTS:
The authors would like to acknowledge the following contributors: Mary Ehlinger for her role in study recruitment and as the primary study coordinator; Karen Smith for her role in nutrition data acquisition and as an intervention dietitian; Lisa Brooks for her role as an intervention dietitian; Michaella Edwards for her role in study coordination and data acquisition; Zaidoon Al-Share for his role in data acquisition; and the students at the University of Iowa who supported recruitment and data acquisition.
Funding Statement
FUNDING/SUPPORT: This study was supported in part by the National Multiple Sclerosis Society grant RG-1506-04312 (TLW), the Institute for Clinical and Translational Science (ICTS) at the University of Iowa, and University of Iowa institutional funds. The ICTS is supported by the National Institutes of Health Clinical and Translational Science Award program (grant UL1TR002537). Dr Saxby is a research trainee of the University of Iowa Fraternal Order of Eagles Diabetes Research Center (T32DK112751-06). Dr Saxby, Dr Titcomb, and Dr Shemirani are supported by the Carter Chapman Shreve Family Foundation and the Carter Chapman Shreve Fellowship Fund for diet and lifestyle research conducted by the Wahls Research team at the University of Iowa. In-kind support was provided by the University of Iowa College of Public Health Preventive Intervention Center.
Footnotes
FINANCIAL DISCLOSURES: Dr Wahls personally follows and promotes the Wahls diet. She has equity interest in the following companies: Terry Wahls LLC; TZ Press LLC; The Wahls Institute, PLC; FBB Biomed Inc; Levels Health Inc, Foogal Inc, and the website http://www.terrywahls.com. She also owns the copyright to the books Minding My Mitochondria, The Wahls Protocol, and The Wahls Protocol Cooking for Life, and the trademarks The Wahls Protocol and Wahls diet, Wahls Paleo diet, Wahls Paleo Plus diets, and Wahls Behavior Change. She has completed grant funding from the National Multiple Sclerosis Society for the Dietary Approaches to Treating Multiple Sclerosis-Related Fatigue Study. She has financial relationships with BioCeuticals Ltd, Genova Diagnostics Inc, Institute for Functional Medicine, MCG Health LLC, MasterHealth Technologies Inc, Standard Process Inc, and Vibrant America LLC. She receives royalty payments from Penguin Random House. Dr Wahls has conflict of interest management plans in place with the University of Iowa and the Iowa City Veteran’s Affairs Medical Center. All other authors report no personal or financial conflicts of interest.
PRIOR PRESENTATION: Aspects of this work were presented at the Americas Committee for Treatment and Research in Multiple Sclerosis Forum 2020; February 27-29; West Palm Beach, Florida; and the Consortium of Multiple Sclerosis Centers annual meeting; October 25-28, 2021; Orlando, Florida.
REFERENCES
- 1.Romero-Pinel L, Bau L, Matas E, et al. The age at onset of relapsing-remitting multiple sclerosis has increased over the last five decades. Mult Scler Relat Disord. 2022;68:104103–104103. doi: 10.1016/j.msard.2022.104103. [DOI] [PubMed] [Google Scholar]
- 2.Sumowski JF, Benedict R, Enzinger C, et al. Cognition in multiple sclerosis: state of the field and priorities for the future. Neurology. 2018;90(6):278–288. doi: 10.1212/wnl.0000000000004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hakim EA, Bakheit AM, Bryant TN, et al. The social impact of multiple sclerosis—a study of 305 patients and their relatives. Disabil Rehabil. 2000;22(6):288–293. doi: 10.1080/096382800296755. [DOI] [PubMed] [Google Scholar]
- 4.Yu H, Bai S, Hao Y, Guan Y. Fatty acids role in multiple sclerosis as “metabokines. J Neuroinflammation. 2022;19(1):157. doi: 10.1186/s12974-022-02502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahls TL, Chenard CA, Snetselaar LG. Review of two popular eating plans within the multiple sclerosis community: low saturated fat and modified paleolithic. Nutrients. 2019;11(2):352. doi: 10.3390/nu11020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahls TL, Titcomb TJ, Bisht B, et al. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: the WAVES randomized parallel-arm clinical trial. Mult Scler J Exp Transl Clin. 2021;7(3):20552173211035399. doi: 10.1177/20552173211035399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chenard CA, Rubenstein LM, Snetselaar LG, Wahls TL. Nutrient composition comparison between a modified paleolithic diet for multiple sclerosis and the recommended healthy U.S.-style eating pattern. Nutrients. 2019;11(3):537. doi: 10.3390/nu11030537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chenard CA, Rubenstein LM, Snetselaar LG, Wahls TL. Nutrient composition comparison between the low saturated fat Swank Diet for multiple sclerosis and healthy U.S.-style eating pattern. Nutrients. 2019;11(3):616. doi: 10.3390/nu11030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braley TJ, Chervin RD. Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. Sleep. 2010;33(8):1061–1067. doi: 10.1093/sleep/33.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 13.Wahls T, Scott MO, Alshare Z, et al. Dietary approaches to treat MS-related fatigue: comparing the modified paleolithic (Wahls Elimination) and low saturated fat (Swank) diets on perceived fatigue in persons with relapsing-remitting multiple sclerosis: study protocol for a randomized controlled trial. Trials. 2018;19(1):309. doi: 10.1186/s13063-018-2680-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Titcomb TJ, Brooks L, Smith KL, et al. Change in micronutrient intake among people with relapsing-remitting multiple sclerosis adapting the Swank and Wahls Diets: an analysis of weighed food records. Nutrients. 2021;13(10):3507. doi: 10.3390/nu13103507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strober L, Luca J, Benedict RH, et al. Symbol Digit Modalities Test: a valid clinical trial endpoint for measuring cognition in multiple sclerosis. Mult Scler. 2019;25(13):1781–1790. doi: 10.1177/1352458518808204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strober LB, Binder A, Nikelshpur OM, Chiaravalloti N, DeLuca J. The Perceived Deficits Questionnaire: perception, deficit, or distress? Int J MS Care. 2016;18(4):183–190. doi: 10.7224/1537-2073.2015-028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCulloch CE. Generalized linear mixed models. NSF-CBMS Regional Conference Series in Probability and Statistics . 2003;7:i–84. Accessed February 26, 2024. http://www.jstor.org/stable/4153190 . [Google Scholar]
- 18.Lövdén M, Fratiglioni L, Glymour MM, Lindenberger U, Tucker-Drob EM. Education and cognitive functioning across the life span. Psychol Sci Public Interest. 2020;21(1):6–41. doi: 10.1177/1529100620920576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akaike H. A new look at the statistical model identification. IEEE Transactions on Control Systems Technology. 1974;19(6):716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 20.Jung SJ. Introduction to mediation analysis and examples of its application to real-world data. J Prev Med Public Health. 2021;54(3):166–172. doi: 10.3961/jpmph.21.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinstock-Guttman B, Baier M, Park Y, et al. Low fat dietary intervention with omega-3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins Leukot Essent Fatty Acids. 2005;73(5):397–404. doi: 10.1016/j.plefa.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Lourida I, Soni M, Thompson-Coon J, et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24(4):479–489. doi: 10.1097/EDE.0b013e3182944410. [DOI] [PubMed] [Google Scholar]
- 23.Tangney CC. DASH and Mediterranean-type dietary patterns to maintain cognitive health. Curr Nutr Rep. 2014;3(1):51–61. doi: 10.1007/s13668-013-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallböök T, Ji S, Maudsley S, Martin B. The effects of the ketogenic diet on behavior and cognition. Epilepsy Res. 2012;100(3):304–309. doi: 10.1016/j.eplepsyres.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JE, Bisht B, Hall MJ, et al. A multimodal, nonpharmacologic intervention improves mood and cognitive function in people with multiple sclerosis. J Am Coll Nutr. 2017;36(3):150–168. doi: 10.1080/07315724.2016.1255160. [DOI] [PubMed] [Google Scholar]
- 26.Hoare S, Lithander F, van der Mei I, Ponsonby AL, Lucas R, Ausimmune Investigator Group Higher intake of omega-3 polyunsaturated fatty acids is associated with a decreased risk of a first clinical diagnosis of central nervous system demyelination: results from the Ausimmune Study. Mult Scler. 2016;22(7):884–92. doi: 10.1177/1352458515604380. [DOI] [PubMed] [Google Scholar]
- 27.Bogie JFJ, Haidar M, Kooij G, Hendriks JJA. Fatty acid metabolism in the progression and resolution of CNS disorders. Adv Drug Deliv Rev. 2020;159:198–213. doi: 10.1016/j.addr.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Trofimiuk E, Braszko JJ. Long-term administration of cod liver oil ameliorates cognitive impairment induced by chronic stress in rats. Lipids. 2011;46(5):417–423. doi: 10.1007/s11745-011-3551-3. [DOI] [PubMed] [Google Scholar]
- 29.Darestani NG, Bahrami A, Mozafarian MR, et al. Association of polyunsaturated fatty acid intake on inflammatory gene expression and multiple sclerosis: a systematic review and meta-analysis. Nutrients. 2022;14(21):4627. doi: 10.3390/nu14214627. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Nguyen LN, Ma D, Shui G, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509(7501):503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 31.van der Lee SJ, Teunissen CE, Pool R, et al. Circulating metabolites and general cognitive ability and dementia: evidence from 11 cohort studies. Alzheimers Dement. 2018;14(6):707–722. doi: 10.1016/j.jalz.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Nordvik I, Myhr KM, Nyland H, Bjerve KS. Effect of dietary advice and n-3 supplementation in newly diagnosed MS patients. Acta Neurol Scand. 2000;102(3):143–149. doi: 10.1034/j.1600-0404.2000.102003143.x. [DOI] [PubMed] [Google Scholar]
- 33.Rocca MA, Amato MP, De Stefano N, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302–317. doi: 10.1016/S1474-4422(14)70250-9. [DOI] [PubMed] [Google Scholar]
- 34.Daly A, Martin C, Sherriff J, et al. Omega-3 Index, fish consumption, use of fish oil supplements and first clinical diagnosis of central nervous system demyelination. Mult Scler Relat Disord. 2021;55:103210. doi: 10.1016/j.msard.2021.103210. [DOI] [PubMed] [Google Scholar]
- 35.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


