Abstract
As the prevalence of diabetes is rapidly increasing, the use of continuous glucose monitoring, which is effective in improving glycemic control in type 2 diabetes, is increasing. Methods: Systematic review was performed according to PRISMA criteria. The search was conducted for articles published until 31 May 2023 in PubMed, CINAHL, Cochrane Library, EMBASE, ClinicalKey, etc. The meta-analysis involved the synthesis of effect size; tests of homogeneity and heterogeneity; trim and fill plot; Egger’s regression test; and Begg’s test for assessing publication bias. Results: 491 studies were searched, of which 17 studies that met the selection criteria were analyzed. The overall effect on HbA1c was −0.37 (95% CI, −0.63~−0.11, p < 0.001), with HbA1c decreasing significantly after CGM interventions. Sub-analyses showed that the study was statistically significant in those aged 60 years or older, when rt-CGM was used and when the study was performed in multiple centers. Conclusion: The results of this study showed that intervention using CGM was effective in reducing HbA1c in type 2 diabetes. The factors identified in this study can be used as guidelines for developing future CGM intervention programs.
Keywords: type 2 diabetes, continuous glucose monitoring, glycemic control
1. Introduction
Diabetes is an insidious, chronic disease, and its incidence is increasing rapidly worldwide. As indicated by the Korean Diabetes Fact Sheet, the number of diabetic patients in South Korea aged 30 or more reached around 6 million in 2020 and showed a consistent upward trend [1]. However, only 24.5% of patients had successfully managed their diabetes, as determined by the key indicator, HbA1c, which should ideally be below 6.5% [2].
A recent analysis conducted by Kaptoge et al. (2023) elucidated the significant impact of an early diabetes diagnosis on life expectancy, revealing a marked reduction of approximately 3 to 4 years for every decade of life. The study notably highlighted that the earliest age of diabetes diagnosis was predominantly associated with an increased prevalence of vascular diseases, including myocardial infarction and stroke, alongside other non-neoplastic causes of death, such as respiratory, neurological, and infectious diseases, and external causes [3]. Furthermore, it was found that approximately 28.6% of individuals with early-diagnosed diabetes develop major vascular complications, including cardiovascular, cerebrovascular, or peripheral artery diseases. Conversely, a substantial 67.2% of individuals encounter microvascular complications, notably retinopathy, nephropathy, or neuropathy [4]. Given that the prognoses of diabetic patients depend heavily on the presence of complications, the prevention of chronic diabetic complications by the diligent self-management of glycemic control is the foremost priority [5,6].
Self-monitoring of blood glucose (SMBG) is widely accepted to be the most effective means of achieving long-term blood sugar control in diabetic patients [7]. However, despite its effectiveness, SMBG is limited by its invasive nature and associated pain and inconvenience, which leads to reduced patient compliance, especially when patients are accompanied by others [8]. Furthermore, SMBG results provide limited understanding of specific blood glucose fluctuations, such as postprandial glucose spikes or asymptomatic hypoglycemia [9]. Consequently, continuous glucose monitoring systems (CGMs) have been increasingly utilized to address these limitations by providing real-time blood glucose readings to patients. In addition, CGM information can positively impact treatment planning, medication regimens, self-blood glucose monitoring schedules, and the adoption of appropriate lifestyle habits. In particular, CGM data are extremely useful for establishing more accurate diagnosis and treatment plans and enabling blood glycemic control [10].
Studies have demonstrated that CGM use leads to improved self-management behaviors, enhanced blood glycemic control, effective reductions in HbA1c levels, and hypoglycemic improvements in diabetic patients [11,12,13,14]. For these reasons, CGMs have been increasingly used, even by type 2 diabetic patients. However, the majority of investigative studies on the effects of CGM have focused on type 1 diabetes, and its effects on type 2 diabetes have received relatively little attention. Nevertheless, a recent large-scale retrospective cohort study on CGM reported that patients with type 2 diabetes taking insulin showed greater HbA1c improvements than patients with type 1 diabetes [15]. This observation suggests that CGM may be more effective in type 2 diabetic patients and prompts questions regarding whether the levels of glycemic control provided by CGM and SMBG differ in type 2 diabetic patients. Meta-analyses of CGM in type 2 diabetic patients conducted to date have only included a limited number of randomized controlled trials (RCTs) and primarily focused on the impact of CGM intervention on HbA1c levels without investigating whether CGM directly ameliorates hypoglycemia or influences psychological or physiological factors, such as weight, BMI, or cholesterol.
In this study, we aimed to enhance the evidence base for CGM interventions in type 2 diabetic patients by comprehensively evaluating the effects of interventions on glycemic control and physiological and psychological factors and providing a substantiated rationale for the use of CGM as an effective intervention in type 2 diabetic patients.
This study systematically reviews the characteristics and key findings of studies that validated the effectiveness of intervention programs utilizing CGM in type 2 diabetic patients.
2. Materials and Methods
2.1. Study Design
Systematic literature review and meta-analysis were utilized to analyze the impact of CGM intervention on glycemic control in type 2 diabetic patients.
2.2. Inclusion and Exclusion Criteria
The reviewed literature was analyzed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) [16]. The PRISMA 2020 Checklist is presented as Supplementary Materials. A systematic literature search based on PICO-SD (participants, intervention, comparison, outcomes, study design) was conducted to select literature for analysis. Participants (P) were type 2 diabetes patients aged over 18. Intervention (I) using a continuous glucose monitoring (CGM) were included, regardless of the CGM type. The control group (C) consisted of patients that received usual care and self-monitoring of blood glucose (SMBG). The outcome was glycemic control. Only randomized controlled trials (RCTs) were included to ensure objective evidence on intervention effectiveness. All studies compared two groups with HbA1c as the outcome variable and provided convertible statistical data (sample sizes, means, standard deviations, and effect sizes). Studies were published in Korean or English before 31 May 2023. Studies or theses not available as original text, survey research, and single-group comparative studies were excluded.
2.3. Literature Search Strategy
The literature search was conducted based on COSI (COre Standard, Ideal) provided by the National Library of Medicine (NLM) using the following core databases [17]: international databases such as PubMed, CINAHL, Cochrane Library, EMBASE, and ClinicalKey and domestic databases such as Research Information Sharing Service [RISS], KMbase, KISS, and KoreaMed. The search was conducted for studies published up to 31 May 2023. The primary search terms used were ‘diabetes mellitus, type 2’ [MeSH Terms], ‘continuous glucose monitoring’, and ‘glycemic control’ [MeSH Terms]. For domestic databases, the search was conducted using combinations of type 2 diabetes, continuous glucose monitoring, and glycemic control. In addition, manual searches were conducted for studies included as references, and the Google Scholar search engine was utilized for related research topics. This review protocol was registered with Prospero registration no. CRD42024505351 available at https://www.crd.york.ac.uk/prospero/#recordDetails (accessed on 3 February 2024).
2.4. Quality Assessment of the Selected Studies
The quality of selected studies was assessed using the checklist for RCT studies included in Joanna Briggs Institute of Critical Appraisal Tools [18]. This checklist comprises 13 items and are as follows: random assignment, allocation concealment, treatment group similarity, blinding of participants, blinding of delivered treatment, blinding of outcome assessor, similar treatment, follow-up completion, intention-to-treat analysis, consistent method of assessing outcome measures in groups, reliability of outcome measures, appropriate statistical analysis, and appropriate trial design. Each item received a score of 0 (‘no’ or ‘unclear’) or 1 (‘yes’), and thus the maximum possible score was 13 points. One reviewer performed this assessment for each study, and a second reviewer confirmed the results. Discrepancies were resolved by discussion and consensus.
2.5. Selection Process for the Analyzed Literature
Two researchers independently reviewed the identified studies. A list of identified studies from domestic and international databases was compiled using Microsoft Excel 2016, and duplicate studies were removed. Subsequently, titles and abstracts were reviewed to determine whether studies met the selection criteria. Finally, full texts were reviewed, and studies were selected for analysis.
2.6. Data Coding
Author names, publication years, countries, number of research centers, funding, participant numbers and characteristics, study design, type of CGM, intervention period, comparator, outcomes, and quality assessment scores were recorded. The Libre had ‘flash’ CGM (fCGM) as the sensor and had to be scanned at least every 8 h to download the data to the reader. However, We coded Libre’s CGM as real-time CGM in this study because participants could receive results immediately without waiting for a doctor. The following outcome variables were subjected to effect size analysis: HbA1c, weight, BMI, SBP/DBP, hypoglycemia, hyperglycemia, and time in range, average blood glucose level, distress, QoL, satisfaction, and HDL-cholesterol. Two researchers conducted data coding independently, and disagreements were resolved by consensus based on the joint reviews of original texts.
2.7. Data Analysis
Data analysis was performed using MIX 2.0 Pro, version 2.015 (MIX Professional software for meta-analysis in Excel) [19]. For all study outcomes, Hedge’s g was utilized as the effect size, considering that many studies had a small sample size [20]. Hedge’s g values were interpreted as follows: an effect size of ≥0.2 but <0.5 was categorized as small, an effect size of ≥0.5 but <0.8 as medium, and an effect size of ≥0.8 as large [21]. The significance level for effect size was set at 0.05, and the confidence interval (CI) at 95%. The analysis was conducted using a random effects model because of the variances exhibited by study participants and study heterogeneity. Heterogeneity was assessed using the I-squared (I2) statistic and was deemed absent when I2 was 0%, medium at 50%, and high at 75% [22]. Egger’s regression and Begg’s tests and the trim and fill method were used to confirm publication bias [23,24].
3. Results
3.1. Literature Selection
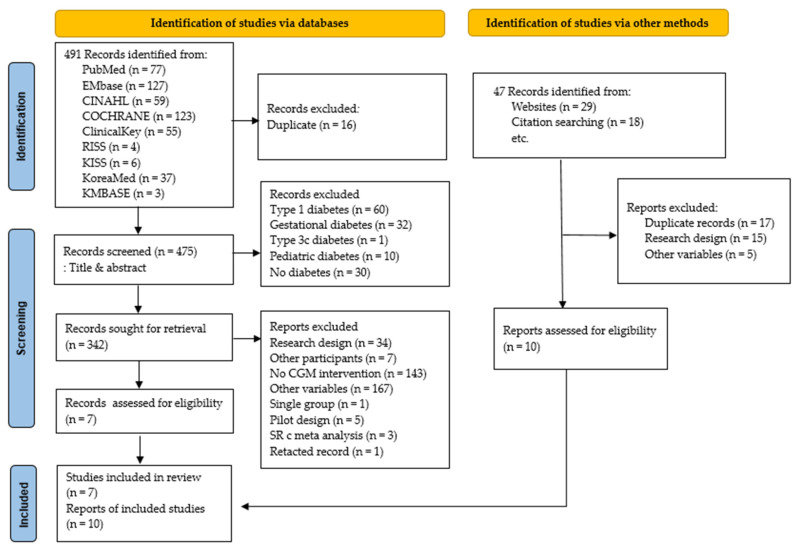
Overall, 491 studies were identified during the initial search, but only 7 were included after applying study selection and exclusion criteria. However, 10 additional studies were selected by reviewing references in these 7 papers and performing a search using the Google Scholar search engine. Thus, 17 studies were included in the analysis (Figure 1).
Figure 1.
PRISMA flow diagram.
3.2. Characteristics of the Included Studies
A total of 10 of the 17 studies included were published after 2015, and higher number of studies were conducted in the United States than in other countries (6 of the 17). Twelve studies were conducted across multiple centers. All 17 studies were funded, and all were RCTs. In total, 1619 patients were involved. The CGM devices used for interventions were 11 real-time CGM and 6 retrospective CGM. The most common intervention period was 12 weeks; six studies adopted this timeframe. In control groups, SMBG was performed in the normal manner. In all studies, HbA1c was used as the outcome variable. In 8 studies, CGM data and physiological variables were measured as follows: weight in 5 studies, BMI in 4, BP in 4, and HDL-cholesterol in 2. Distress and satisfaction were assessed in three studies and QoL in four (Table 1).
Table 1.
Characteristics of the included studies.
| Study ID |
Author | Year | Country | Center | Fund | Participants | Characteristics of Participants |
Type of CGM |
Intervention Period (Weeks) |
Comparator | Outcome Variables |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ajjan et al. [25] |
2016 | UK | 9 | Yes | N = 45 (E:30, C:15) |
Age ≥ 18 years HbA1c 7.5–12.0% Receiving insulin therapy > 6 months |
FreeStyle Navigator (Abbott, Chicago, IL, USA) |
25 | SMBG | CGM data HbA1c Body weight (kg) Blood glucose testing Frequency (tests/day) |
| 2 | Allen et al. [12] |
2008 | US | 2 | Yes | N = 46 (E:21, C:25) |
Age > 20 years HbA1c > 7.5% Physical activity ≤2 days/week Not receiving insulin therapy |
Minimed (Medtronic, Northridge, CA, USA) | 8 | SMBG | HbA1c Physical activity Self-efficacy BP, BMI |
| 3 | Beck et al. [14] |
2017 | US | 25 | Yes | N = 158 (E:79, C:79) |
Age > 25 years HbA1c 7.5–10.0% Receiving insulin therapy > 1 year Stable medication regimen and weight >3 months SMBG ≥ 2/day Estimated glomerular filtration rate > 45 mL/min/1.73 m2 |
Dexcom G4 Platinum (Dexcom, San Diego, CA, USA) |
24 | SMBG | HbA1c Hypoglycemia QoL |
| 4 | Blackberry et al. [26] |
2014 | Australia | 22 | Yes | N = 88 (E:46, C:42) |
Age 18–80 years HbA1c ≥ 7.5% No previous experience with insulin therapy Stable OHA regimen > prior 3 months SMBG ≥ 2/day |
iPro2TM (Medtronic, Northridge, CA, USA) |
24 | SMBG | HbA1c QoL CGM satisfaction 36 Health survey questionnaire version 2 (SF-36 v2) |
| 5 | Cosson et al. [27] |
2009 | France | 5 | Yes | N = 25 (E:11, C:14) |
Age 40–70 years HbA1c 8.0–10.5% Stable OHA and insulin regimen prior to >3 months SMBG ≥ 4/week No previous experience with CGM |
The GlucoDay system (Menarini Diagnostics, Florence, Italy) |
12 | SMBG | HbA1c Glycemic control (Changes in 48 h CGM data) Hypoglycemia |
| 6 | Ehrhardt et al. [28] |
2011 | US | 1 | Yes | N = 100 (E:50, C:50) |
Age ≥ 18 years HbA1c 7.0–12.0% Diagnosis ≥ 3 months SMBG 4/day Treated with diet or exercise Not receiving prandial insulin |
DexComTM SEVEN (DexCom) |
12 | SMBG | HbA1c Glycemic control Weight BP Stress |
| 7 | Furler et al. [29] |
2020 | Australia | 25 | Yes | N = 299 (E:149, C:150) |
Age 18–80 years HbA1c ≥ 7.0% Diagnosis ≥ 1 year Receiving OHA or Insulin therapy |
FreeStyle Libre Pro (Abbott) |
52 | SMBG | HbA1c CGM data Distress |
| 8 | Haak et al. [30] |
2016 | European | 26 | Yes | N = 224 (E:149, C:75) |
Age ≥ 18 years HbA1c 7.5–12.0% Receiving insulin therapy ≥ 6 months (current regimen ≥ 3M SMBG ≥ 10/week at least 2 months |
FreeStyle LibreTM (Abbott) |
24 | SMBG | HbA1c CGM data QoL |
| 9 | Martens et al. [31] |
2021 | US | 15 | Yes | N = 156 (E:105, C:51) |
Age ≥ 30 years HbA1c 7.8–11.5% Diagnosis and insulin therapy ≥ 6 months SMBG ≥ 3/week |
Dexcom G6 (Dexcom) |
32 | SMBG | HbA1c Height Weight Cholesterol CGM satisfaction |
| 10 | Sato et al. [32] |
2016 | Japan | 1 | Yes | N = 34 (E:17, C:17) |
Age > 20 years HbA1c 6.9–11.0% Receiving insulin therapy |
iPro® 2 (Medtronic) |
32 | SMBG | HbA1c Diabetes Treatment Satisfaction (DTSQ) |
| 11 | Yoo et al. [33] |
2008 | Korea | 1 | Yes | N = 57 (E:29, C:28) |
Age 20–80 years HbA1c 8.0–10.0% Receiving OHA or insulin therapy ≥ 1 year Stable insulin or OHA regimen ≥ prior 2 months Stable OHA or lipid-lowering drugs ≥4 weeks |
Guardian RT (Medtronic) | 12 | SMBG | HbA1c FBS, PP2, Lipid profiles, Weight, Waist circumference BMI, Fat consumption Cholesterol intake (g/day) Exercise time (min/week) |
| 12 | Yeoh et al. [34] |
2018 | Singapore | 1 | Yes | N = 30 (E:14, C:16) |
Age ≥ 21 years HbA1c > 8% Type 2 diabetes with CKD stage 3 (eGFR 30–60 mL/min per 1.73 m2) Above (pre-dialysis) for >3 months Sustained for >6 months Receiving insulin and/or OHA |
iPro device (Medtronic) |
12 | SMBG | HbA1c CGM data |
| 13 | Ajjan et al. [35] |
2019 | England | 22 | No | N = 102 (E:50, C:52) |
Age ≥ 18 years HbA1c 7.5%–12.0% Receiving insulin therapy ≥ 6 month |
FreeStyle Libre ProTM (Abbott) |
28 | SMBG | HbA1c CGM data Treatment satisfaction (DTSQ) |
| 14 | Wada et al. [36] |
2020 | Japan | 5 | Yes | N = 93 (E:48, C:45) |
Age 20–70 years HbA1c 7.5–8.5% |
Free Style Libre (Abbott) |
24 | SMBG | HbA1c Weight, BP Diabetes medication change (DTSQ) |
| 15 | Moon et al. [37] |
2022 | Korea | 3 | Yes | N = 30 (E:15, C:15) |
Aged 30 to 65 years HbA1c 7.5–10.0% Receiving OHA Treated without insulin ≥ 3 months |
Guardian 3 (Medtronic MiniMed, Northridge, CA, USA) |
24 | SMBG | HbA1c CGM data, BP Lipid variables, Weight, Satisfaction K-DMSES, ADS-K, SDSCA-K |
| 16 | Price et al. [38] |
2021 | US | 8 | Yes | N = 68 (E:45, C:23) |
Age ≥ 30 years HbA1c 7.8–10.5% Treated with two or more noninsulin antidiabetic drugs Stable body weight over the past 3 months |
Dexcom G6 (Dexcom) |
12 | SMBG | CGM data HbA1c Adverse Events |
| 17 | Vigersky et al. [39] |
2012 | US | 1 | Yes | N = 100 (E:50, C:50) |
Age ≥ 18 years HbA1c 7.0–12.0% Diagnosis ≥ 3 months Not receiving prandial insulin SMGB 4/days |
DexCom SEVEN (DexCom) |
12 | SMBG | HbA1c Weight BP Stress |
Notes. E: experimental group; C: control group; CGM: continuous glucose monitoring; SMBG: self-monitoring of blood glucose; MDI: multiple daily injection; OHA: oral hypoglycemia agent; HbA1c: glycosylated hemoglobin; BP: blood pressure; and BMI: body mass index.
3.3. Quality Assessment
The average quality assessment score for the 17 studies was 8 points (range: 6–9). All 17 had a suitable RCT design and clearly described the random assignment procedure used. Participants were not blinded in any study, and information on assessor measurement reliability was not provided; thus, it was assessed as unclear. The mediator or measurer was blinded in one study apiece (Table 2).
Table 2.
Quality assessment of the included studies.
| Joanna Briggs Institute of Critical Appraisal Tools Checklist for Randomized Controlled Trials | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Total Score |
| 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| 2 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| 3 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| 4 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 |
| 5 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| 6 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 6 |
| 7 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 9 |
| 8 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| 9 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| 10 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| 11 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 6 |
| 13 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 |
| 14 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| 15 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| 16 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| 17 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 6 |
| Total | 13 | 12 | 11 | 0 | 1 | 1 | 17 | 17 | 17 | 17 | 0 | 13 | 17 | 8 |
3.4. Effect of CGM Intervention on HbA1c
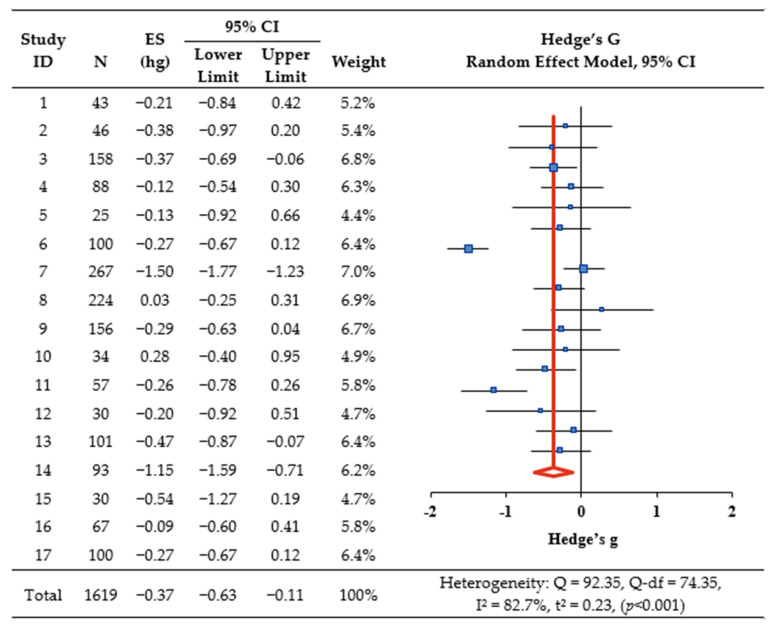
Overall, CGM intervention significantly decreased HbA1c, as indicated by Hedge’s g = −0.37 (95% confidence interval [CI]: −0.63, −0.11, p < 0.001) (Figure 2). The I2 was 82.7% (Q = 92.35, Q−df = 74.35, p < 0.001), indicating a high level of heterogeneity, thereby suggesting a need for exploratory explanations of the heterogeneity in effect sizes (Figure 2). Sub-analysis was conducted based on study characteristics, such as country, number of participants, number of centers, CGM intervention types, intervention period, quality assessment scores, and insulin therapy. Studies that targeted participants aged 60 or older, studies conducted at multiple centers, studies utilizing real-time CGM for interventions, and studies with reported quality assessment scores of ≤8 (Table 3).
Figure 2.
The effect of CGM on HbA1c. Notes. ES: effect size; CI: confidence interval.
Table 3.
Subgroup analysis regarding HbA1c based on study characteristics.
| Characteristics | Subgroup | K | Study ID | N | Overall ES |
95% CI | Z (p) | |
|---|---|---|---|---|---|---|---|---|
| Lower Limit |
Upper Limit |
|||||||
| Location (country of publication) |
US | 6 | 2,3,6,9,16,17 | 627 | −0.29 | −0.45 | −0.13 | −3.57 (<0.001) |
| others | 11 | 1,4,5,7,8,10, 11,12,13,14,15 |
992 | −0.41 | −0.82 | 0.00 | −1.98 (0.048) | |
| Participants | <60 | 7 | 1,2,5,10, 11,12,15 |
265 | −0.22 | −0.46 | 0.03 | −1.74 (0.082) |
| ≥60 | 10 | 3,4,6,7,8,9, 13,14,16,17 |
1354 | −0.46 | −0.81 | −0.11 | −2.56 (0.011) | |
| Study centers | 1 | 5 | 6,10,11,12,17 | 321 | −0.21 | −0.43 | 0.01 | −1.83 (0.067) |
| multiple | 12 | 1,2,3,4,5,7,8, 9,13,14,15,16 |
1298 | −0.45 | −0.78 | −0.12 | −2.65 (0.008) | |
| Intervention | r-CGM | 6 | 1,2,5,8,10,30 | 402 | −0.05 | −0.25 | 0.15 | −0.49 (0.621) |
| rt-CGM | 11 | 3,4,6,7,9,11, 13,14,15,16,17 |
1217 | −0.50 | −0.81 | −0.18 | −3.04 (0.002) | |
| Intervention period (week) | ≤24 | 7 | 2,5,6,11, 12,16,17 |
425 | −0.24 | −0.44 | −0.05 | −2.47 (0.013) |
| >24 | 10 | 1,3,4,7,8,9, 10,13,14,15 |
1194 | −0.45 | −0.84 | −0.07 | −2.30 (0.022) | |
| Quality score | ≤8 | 10 | 1,3,4,6,8,12,13, 14,16,17 |
1004 | −0.31 | −0.52 | −0.10 | −2.94 (0.003) |
| >8 | 7 | 2,5,7,9,10,11,15 | 615 | −0.43 | −0.98 | 0.12 | −1.54 (0.124) | |
| Insulin therapy |
Yes | 11 | 1,3,5,7,8,9,10, 11,12,13,14 |
1188 | −0.42 | −0.79 | −0.05 | −2.21 (0.027) |
| No | 6 | 2,4,6,15,16,17 | 431 | −0.25 | −0.44 | −0.05 | −2.51 (0.012) | |
Notes. ES: effect size; CI: confidence interval; r-CGM: retrospective continuous glucose monitoring; rt-CGM: real-time continuous glucose monitoring; and US: The United States.
Meta-regression analysis was also conducted to investigate heterogeneity potentially arising from differences between studies and participants. The moderators used in the meta-regression analysis to explain heterogeneity were country, number of participants, number of research centers, CGM type, intervention period, quality assessment scores, and insulin therapy. A significant reduction in HbA1c was observed in studies that enrolled participants ≥ 60 (Z = −2.06, p = 0.039), studies using real-time CGM (Z = −4.45, p < 0.001), studies with quality assessment scores of ≤8 (Z = −4.15, p < 0.001), and studies receiving insulin therapy (Z = −2.49, p = 0.013) (Table 4).
Table 4.
Meta-regression analysis evaluating HbA1c.
| Covariate (Ref.) | Estimate | SE | Z | p |
|---|---|---|---|---|
| Location (country of publication; Ref.: others) US | 0.26 | 0.11 | 2.49 | 0.013 |
| Participants (Ref.: <60) ≥ 60 | −0.28 | 0.14 | −2.06 | 0.039 |
| Study centers (Ref.: multicenter) one | 0.31 | 0.13 | 2.47 | 0.013 |
| Intervention (Ref.: r-CGM) rt-CGM | −0.53 | 0.12 | −4.45 | <0.001 |
| Intervention period (Ref.: week > 24) ≤ 24 | 0.29 | 0.12 | 2.49 | 0.013 |
| Quality assessment (Ref.: >8) ≤ 8 | −0.45 | 0.11 | −4.15 | <0.001 |
| Receiving insulin therapy (Ref.: not receiving) | −0.29 | 0.12 | −2.49 | 0.013 |
Notes. r-CGM: retrospective continuous glucose monitoring; rt-CGM: real-time continuous glucose monitoring; US: The United States; SE: standard error; and Ref.: reference.
3.5. Effect of CGM Intervention on Secondary Outcomes
In addition to HbA1c (the primary outcome variable), various secondary outcomes such as CGM data, physiological factors (weight, BMI, cholesterol), and psychological factors (distress, satisfaction, and quality of life (QoL)) were also measured. However, results showed that CGM intervention had no overall effect on secondary outcomes (Table 5).
Table 5.
The effect of CGM intervention on secondary variables.
| Variables | Number of Studies | N | Hedge’s G | 95% CI | Z (p) | I2 (%) | |
|---|---|---|---|---|---|---|---|
| Lower Limit |
Upper Limit |
||||||
| Weight | 6 (1,3,6,9,11,17) | 593 | −0.52 | −1.22 | 0.18 | −1.46 (0.145) | 93.7 |
| BMI | 4 (2,9,11,17) | 330 | −0.04 | −0.27 | 0.20 | −0.30 (0.764) | 10.1 |
| Glucose | 6 (3,5,8,9,10,13) | 688 | −0.14 | −0.40 | 0.11 | −1.12 (0.263) | 56.3 |
| SBP | 4 (2,6,9,17) | 374 | −0.12 | −0.34 | 0.10 | −1.09 (0.274) | 4.8 |
| DBP | 4 (2,6,9,17) | 341 | 0.07 | −0.15 | 0.29 | 0.63 (0.527) | 0 |
| TIR | 10 (3,4,5,7,8,9,10,13,15,16) | 1110 | 0.31 | −0.14 | 0.75 | 1.35 (0.177) | 91.3 |
| Hyperglycemia | 10 (1,3,4,5,8,9,10,13,15,16) | 908 | −0.20 | −0.49 | 0.09 | −1.35 (0.178) | 74.6 |
| Hypoglycemia | 10 (1,3,4,5,8,9,10,13,15,16) | 898 | −0.19 | −0.52 | 0.13 | −1.16 (0.246) | 79.7 |
| HDL-cholesterol | 2 (9,11) | 194 | −0.33 | −0.68 | 0.03 | −1.78 (0.075) | 25.9 |
| Distress | 3 (3,7,17) | 510 | −0.08 | −0.36 | 0.20 | −0.56 (0.574) | 57.8 |
| QoL | 3 (3,4,8) | 462 | −1.29 | −3.87 | 1.29 | −0.98 (0.326) | 99.2 |
| Satisfaction | 3 (8,10,13) | 359 | 2.77 | −1.18 | 6.72 | 1.38 (0.169) | 99.2 |
Notes. BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; TIR: time in range; QoL: quality of life; HDL: high-density lipoprotein cholesterol; and CI: confidence interval.
3.6. Publication Bias Analysis
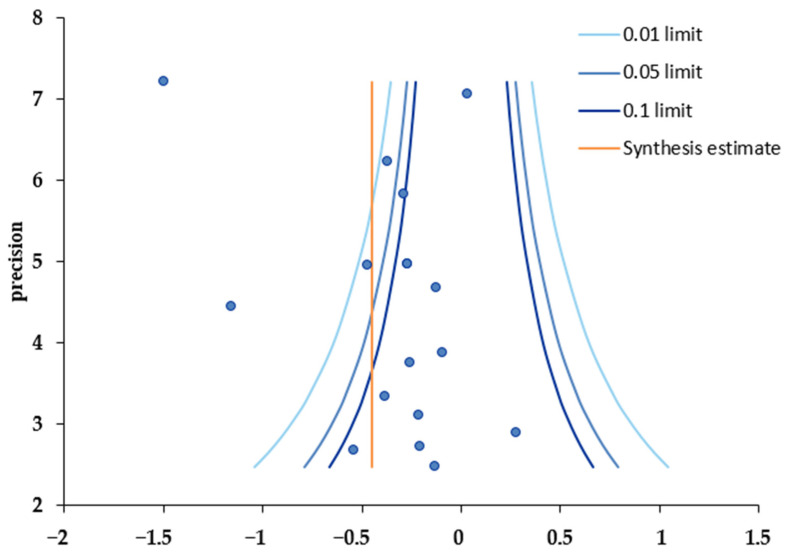
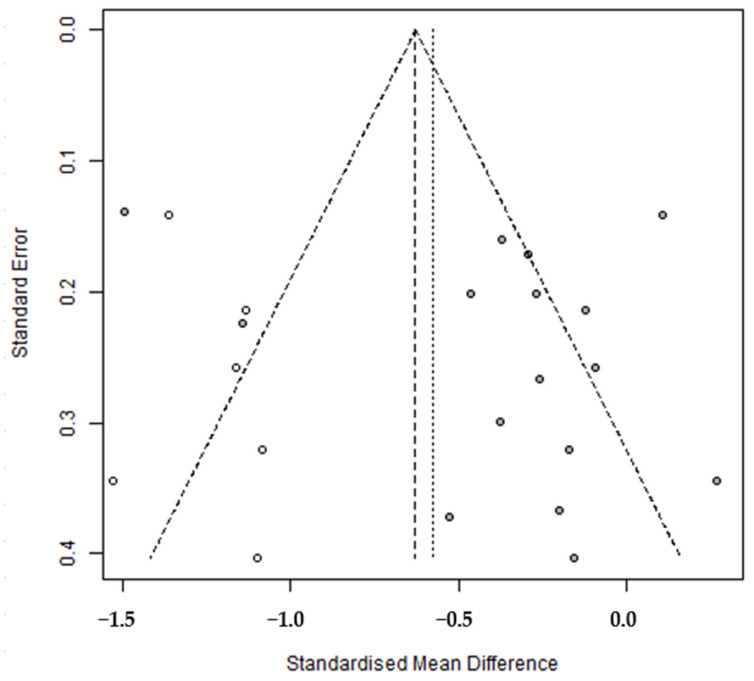
A funnel plot was used to verify the validity of the analyzed results and to assess publication bias. The plot showed that effect sizes were not symmetrically distributed around the central dotted line (Figure 3). Egger’s regression test, Begg’s test, and the trim and fill method were used to determine whether the degree of asymmetry was significant. The results indicated no publication bias (Table 6). Trim and fill analysis showed that the original combined effect size of CGM intervention was −0.36, and the adjusted overall effect size was −0.58 (95% CI: −0.83, −0.33), which resulted in an effect size increase from a small to an intermediate level (Table 6). Furthermore, when the six studies indicated by white circles in the plot were added to the left of the filled synthetic line, it appeared that publication bias had been corrected (Figure 4).
Figure 3.
Funnel plot.
Table 6.
Publication bias analysis.
| Publication Bias Test | Coefficient | SE | 95% CI | Z | p | ||
|---|---|---|---|---|---|---|---|
| Lower Limit |
Upper Limit |
||||||
| Egger’s regression test | intercept | 2.23 | 1.83 | −1.35 | 5.81 | 1.22 | 0.222 |
| slope | −0.90 | 0.39 | −1.67 | −0.14 | −2.31 | 0.021 | |
| tau-b | ties | Z | p | ||||
| Begg’s test | standard | −0.05 | 4 | −0.29 | 0.771 | ||
| corrected | −0.04 | 4 | −0.25 | 0.805 | |||
| Hedge’s | 95% CI | ||||||
| Lower Limit | Upper Limit | ||||||
| Trim and fill | original | −0.36 | −0.62 | −0.10 | |||
| corrected | −0.58 | −0.83 | −0.33 | ||||
Notes. SE: standard error; CI: confidence interval.
Figure 4.
Trim and fill.
4. Discussion
Strategies designed to improve blood glucose management in diabetic patients are attracting considerable interest because of the rapidly increasing prevalence of diabetes. In particular, CGM are now viewed as crucial for blood glucose measurements, and their use is increasing among type 2 diabetes patients [40]. This study aimed to systematically review the characteristics of CGM interventions conducted in type 2 diabetic patients and integrate their effects to provide a foundation for developing effective CGM interventions.
All 17 RCT studies included in the meta-analysis were performed on patients over 18 years old. These studies were conducted in various regions, including the United States, Europe, and Asia. Most studies were conducted across multiple centers, and the predominant study period was 12 weeks. The control group received normal diabetes care based on SMBG. HbA1c was used as the primary effect variable, and physiological factors (CGM data, weight, BMI, BP, cholesterol) and psychological factors (distress, satisfaction, and QoL) were used as secondary effect variables.
The quality assessment revealed that participant allocation was random in all studies. However, the blinding of participants, interveners, and assessors was not achieved, and there was insufficient information regarding the reliability of assessor measurements, which resulted in an unclear rating. This lack of information is believed to be due to the inherent bias associated with studies involving the insertion of CGM devices into patients’ bodies. Future research should focus on devising methods to minimize potential biases related to the blinding of researchers, interveners, and assessors to facilitate the more rigorous evaluation of the effects of CGM.
In this study, the primary effect variable for glycemic control was HbA1c, and the overall effect of CGM intervention on HbA1c was −0.37, indicating a significant reduction in HbA1c levels. This result was greater than the −0.25 reported in a meta-analysis conducted by Janapala et al. (2019) [41] and similar to the −0.35 reported by Ida et al. [42]. The intervention methods used in this study were categorized as retrospective or real-time CGM. The patients who underwent retrospective CGM analyzed their blood glucose patterns retrospectively, unaware of their results at time of measurement, whereas those who underwent real-time CGM viewed CGM data in real-time [40]; eleven studies used real-time CGM intervention and six used retrospective CGM intervention. Meta-analysis showed real-time CGM intervention reduced HbA1c, which concurs with the findings of Ida et al. [42] and suggests that access to real-time blood glucose values and trends enables patients to reduce hyperglycemia, extend time in target blood glucose ranges, and prevent hypoglycemia [43,44]. These findings suggest that real-time CGM-based interventions are more effective at reducing HbA1c levels in type 2 diabetes patients than retrospective CGM-based interventions.
Furthermore, HbA1c reductions were more pronounced in individuals aged ≥60, which aligns with the findings of previous domestic and international studies [45,46] and indicates that younger age is associated with more difficult blood glycemic control. Individuals aged under 60 tend to be office workers with self-management challenges due to factors such as sleep deprivation caused by work-related stress, lack of exercise due to long working hours, and a social drinking culture [47]. Therefore, there is a need to develop an efficient way to manage blood sugar levels using CGM, which can be used by office workers without restrictions on time and place.
Finally, meta-regression analysis was conducted by entering study and participant characteristics to identify the sources of systematic heterogeneity across studies. This analysis showed that the following moderators explained heterogeneity, i.e., country, number of participants, number of research centers, CGM type, intervention period, quality assessment scores, and insulin therapy, which cautions that these characteristics might influence the heterogeneity of results. The results of the meta-regression analysis of this study showed that CGM was effective in reducing HbA1c when the intervention period was 24 weeks or more compared to when the intervention period was less than 24 weeks. These results show that studies with relatively long intervention periods are effective in reducing glycated hemoglobin [48]. However, the study by Furler et al. (2020) [29], which was conducted for 52 weeks, the longest period in this study, showed no HbA1c reduction effect, and this result was due to the use of a professional-mode flash glucose monitoring sensor device that did not allow patients to check glucose data. Therefore, to determine the intervention effect of diabetes management on changes in glycated hemoglobin in future long-term studies, the primary outcome should be measured at 12 weeks using an rt-CGM device to confirm changes in HbA1c and confirm whether the effect lasts for more than 24 weeks.
5. Conclusions and Recommendations
In this study, we aimed to elucidate the effects of CGM interventions on glycemic control in type 2 diabetes patients by meta-analysis. Our goal was to improve the level of evidence for intervention methods and offer suggestions for future research. A total of 17 RCT studies were analyzed, and CGM intervention was found to diminish HbA1c levels. Furthermore, real-time CGM effectively reduced HbA1c levels at multiple centers when an Intervention period of >24 weeks was used. The factors identified in this study might serve as basic guidelines for setting the direction of future CGM intervention research and developing and implementing effective intervention programs. Unfortunately, most of the studies included did not provide details of education during intervention. In previous studies, when no systematic education was provided during CGM usage, no HbA1c decrease was observed [48]. Improved glycemic control was only evident when specialized education was provided [30]. Thus, we suggest that studies should be undertaken to confirm the effects reported by studies conducted with a systematic education program. The limitations of this study include the possibility that some relevant literature may have been omitted during the literature search. Furthermore, caution is required when interpreting our results due to the presence of heterogeneity. Our analysis did not encompass the rate of hypoglycemia or the time spent in the glycemic target range, which are important outcomes for evaluating the effectiveness and safety of CGM interventions. We suggest that future research should include these various variables to provide a more holistic view of CGM’s impact on diabetes management. The inclusion criteria for the literature in our study were to merge the first measured value after the end of the program. In future research, we suggest an analysis that subdivides the types of CGM in glycemic control and considers the long-term effects and frequency of application of CGM. Additionally, we suggest that future studies should be undertaken to validate the effectiveness of CGM, focusing on devising methods that minimize these limitations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare12050571/s1. Reference [49] is cited in the Supplementary Materials.
Author Contributions
Conceptualization, M.-K.C.; Data curation, S.-Y.K.; Formal analysis, M.-K.C.; Investigation, S.-Y.K.; Methodology, M.-K.C.; Resources, S.-Y.K. and M.-K.C.; Software, S.-Y.K. and M.-K.C.; Supervision, M.-K.C.; Validation, S.-Y.K. and M.-K.C.; Visualization, S.-Y.K.; Writing—original draft, S.-Y.K.; Writing—review and editing, M.-K.C.; Funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kwon H.-S. Prevalence and treatment status of diabetes mellitus in Korea. J. Korean Med. Assoc. 2023;66:404–407. doi: 10.5124/jkma.2023.66.7.404. [DOI] [Google Scholar]
- 2.Korean Diabetes Association . Diabetes Fact Sheet in Korea 2020. Korean Diabetes Association; Seoul, Republic of Korea: 2022. pp. 16–29. [Google Scholar]
- 3.Kaptoge S., Seshasai S.R.K., Sun L., Walker M., Bolton T., Spackman S., Ataklte F., Willeit P., Bell S., Burgess S., et al. Life expectancy associated with different ages at diagnosis of type 2 diabetes in high-income countries: 23 million person-years of observation. Lancet Diabetes Endocrinol. 2023;11:731–742. doi: 10.1016/S2213-8587(23)00223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghandour R., Mikki N., Abu Rmeileh N.M.E., Jerdén L., Norberg M., Eriksson J.W., Husseini A. Complications of type 2 diabetes mellitus in Ramallah and al-Bireh: The Palestinian diabetes complications and control study (PDCCS) Prim. Care Diabetes. 2018;12:547–557. doi: 10.1016/j.pcd.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Korean Diabetes Association. [(accessed on 1 June 2023)]. Available online: https://www.diabetes.or.kr/bbs/?code=guide&category=2023.
- 6.International Diabetes Federation. [(accessed on 1 June 2023)]. Available online: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html.
- 7.Schnell O., Alawi H., Battelino T., Ceriello A., Diem P., Felton A.-M., Grzeszczak W., Harno K., Kempler P., Satman I., et al. Self-monitoring of blood glucose in type 2 diabetes: Recent studies. J. Diabetes Sci. Technol. 2013;7:478–488. doi: 10.1177/193229681300700225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J.H. The occurrence and management of adverse skin events due to continuous glucose monitoring. J. Korean Diabetes. 2022;23:43–49. doi: 10.4093/jkd.2022.23.1.43. [DOI] [Google Scholar]
- 9.Boland E., Monsod T., Delucia M., Brandt C.A., Fernando S., Tamborlane W.V. Limitations of conventional methods of self-monitoring of blood glucose. Diabetes Care. 2001;24:1858–1862. doi: 10.2337/diacare.24.11.1858. [DOI] [PubMed] [Google Scholar]
- 10.Bae S.K. Master’s Thesis. Korea University Graduate School; Seoul, Republic of Korea: 2021. Development of Artificial Skin Phantom for Continuous Glucose Monitoring System. [Google Scholar]
- 11.Rivera-Ávila D.A., Esquivel-Lu A.I., Salazar-Lozano C.R., Jones K., Doubova S.V. The effects of professional continuous glucose monitoring as an adjuvant educational tool for improving glycemic control in patients with type 2 diabetes. BMC Endocr. Disord. 2021;21:79. doi: 10.1186/s12902-021-00742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen N.A., Fain J.A., Braun B., Chipkin S.R. Continuous glucose monitoring in non–insulin-using individuals with type 2 diabetes: Acceptability, feasibility, and teaching opportunities. Diabetes Technol. Ther. 2009;11:151–158. doi: 10.1089/dia.2008.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poolsup N., Suksomboon N., Kyaw A.M. Systematic Review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glycemic control in diabetes. Diabetol Metab. Syndr. 2013;5:39. doi: 10.1186/1758-5996-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck R.W., Riddlesworth T.D., Ruedy K., Ahmann A., Haller S., Kruger D., McGill J.B., Polonsky W., Price D., Aronoff S., et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections. Ann. Intern. Med. 2017;167:365. doi: 10.7326/M16-2855. [DOI] [PubMed] [Google Scholar]
- 15.Karter A.J., Parker M.M., Moffet H.H., Gilliam L.K., Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325:2273. doi: 10.1001/jama.2021.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. Prisma 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bidwell S., Jensen M.F. Using a search protocol to identify sources of information: The COSI model. In: Topfer L.A., Auston I., editors. Etext on Health Technology Assessment (HTA) Information Resources [Internet] National Library of Medicine; Washington, DC, USA: 2003. [(accessed on 1 June 2023)]. Available online: https://www.nlm.nih.gov/archive/20060905/nichsr/ehta/chapter3.html#COSI. [Google Scholar]
- 18.Joanna Briggs Institute. [(accessed on 30 June 2023)]. Available online: https://jbi.global/critical-appraisal-tools.
- 19.Bax L., Yu L.-M., Ikeda N., Tsuruta H., Moons K.G. Development and validation of MIX: Comprehensive Free Software for meta-analysis of causal research data. BMC Med. Res. Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim S.R., Kim S.-J. Intervention meta-analysis: Application and practice using R software. Epidemiol. Health. 2019;41:e2019008. doi: 10.4178/epih.e2019008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedges L.V., Olkin I. Statistical Methods for Meta-Analysis. Academic Press; San Diego, CA, USA: 2002. [Google Scholar]
- 22.Higgins J.P. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Littell J.H., Corcoran J., Pillai V.K. Systematic Reviews and Meta-Analysis. Oxford University Press; New York, NY, USA: 2008. [Google Scholar]
- 24.Mavridis D., Salanti G. How to assess publication bias: Funnel Plot, trim-and-fill method and selection models. BMJ Mental Health. 2014;17:30. doi: 10.1136/eb-2013-101699. [DOI] [PubMed] [Google Scholar]
- 25.Ajjan R.A., Abougila K., Bellary S., Collier A., Franke B., Jude E.B., Rayman G., Robinson A., Singh B.M. Sensor and software use for the glycaemic management of insulin-treated type 1 and type 2 diabetes patients. Diabetes. Vasc. Dis. Res. 2016;13:211–219. doi: 10.1177/1479164115624680. [DOI] [PubMed] [Google Scholar]
- 26.Blackberry I.D., Furler J.S., Ginnivan L.E., Manski-Nankervis J.-A., Jenkins A., Cohen N., Best J.D., Young D., Liew D., Ward G., et al. An exploratory trial of basal and prandial insulin initiation and titration for type 2 diabetes in primary care with adjunct retrospective continuous glucose monitoring: Initiation Study. Diabetes Res. Clin. Pract. 2014;106:247–255. doi: 10.1016/j.diabres.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Cosson E., Hamo-Tchatchouang E., Dufaitre-Patouraux L., Attali J.-R., Pariès J., Schaepelynck-Bélicar P. Multicentre, randomised, controlled study of the impact of continuous sub-cutaneous glucose monitoring (GlucoDay®) on glycaemic control in type 1 and type 2 diabetes patients. Diabetes Metab. 2009;35:312–318. doi: 10.1016/j.diabet.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Ehrhardt N.M., Chellappa M., Walker M.S., Fonda S.J., Vigersky R.A. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J. Diabetes Sci. Technol. 2011;5:668–675. doi: 10.1177/193229681100500320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furler J., O’Neal D., Speight J., Blackberry I., Manski-Nankervis J.-A., Thuraisingam S., de La Rue K., Ginnivan L., Doyle R., Holmes-Truscott E., et al. Use of professional-mode flash glucose monitoring, at 3-month intervals, in adults with type 2 diabetes in general practice (GP-osmotic): A Pragmatic, open-label, 12-month, randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8:17–26. doi: 10.1016/S2213-8587(19)30385-7. [DOI] [PubMed] [Google Scholar]
- 30.Haak T., Hanaire H., Ajjan R., Hermanns N., Riveline J.-P., Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: A Multicenter, open-label Randomized Controlled Trial. Diabetes Ther. 2016;8:55–73. doi: 10.1007/s13300-016-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martens T., Beck R.W., Bailey R., Ruedy K.J., Calhoun P., Peters A.L., Pop-Busui R., Philis-Tsimikas A., Bao S., Umpierrez G., et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin. JAMA. 2021;325:2262–2272. doi: 10.1001/jama.2021.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato J., Kanazawa A., Ikeda F., Shigihara N., Kawaguchi M., Komiya K., Uchida T., Ogihara T., Mita T., Shimizu T., et al. Effect of treatment guidance using a retrospective continuous glucose monitoring system on glycaemic control in outpatients with type 2 diabetes mellitus: A randomized controlled trial. J. Int. Med. Res. 2015;44:109–121. doi: 10.1177/0300060515600190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo H.J., An H.G., Park S.Y., Ryu O.H., Kim H.Y., Seo J.A., Hong E.G., Shin D.H., Kim Y.H., Kim S.G., et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res. Clin. Pract. 2008;82:73–79. doi: 10.1016/j.diabres.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Yeoh E., Lim B.K., Fun S., Tong J., Yeoh L.Y., Sum C.F., Subramaniam T., Lim S.C. Efficacy of self-monitoring of blood glucose versus retrospective continuous glucose monitoring in improving glycaemic control in diabetic kidney disease patients. Nephrology. 2018;23:264–268. doi: 10.1111/nep.12978. [DOI] [PubMed] [Google Scholar]
- 35.Ajjan R.A., Jackson N., Thomson S.A. Reduction in hba1c using professional flash glucose monitoring in insulin-treated type 2 diabetes patients managed in primary and secondary care settings: A pilot, multicentre, randomised controlled trial. Diabetes Vasc. Dis. Res. 2019;16:385–395. doi: 10.1177/1479164119827456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wada E., Onoue T., Kobayashi T., Handa T., Hayase A., Ito M., Furukawa M., Okuji T., Okada N., Iwama S., et al. Flash glucose monitoring helps achieve better glycemic control than conventional self-monitoring of blood glucose in non-insulin-treated type 2 diabetes: A randomized controlled trial. BMJ Open Diabetes Res. Care. 2020;8:e001115. doi: 10.1136/bmjdrc-2019-001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon S.J., Kim K., Lee W.J., Lee M.Y., Vigersky R., Park C. Efficacy of intermittent short-term use of a real-time continuous glucose monitoring system in non-insulin–treated patients with type 2 diabetes: A randomized controlled trial. Diabetes Obes Metab. 2022;25:110–120. doi: 10.1111/dom.14852. [DOI] [PubMed] [Google Scholar]
- 38.Price D.A., Deng Q., Kipnes M., Beck S.E. Episodic real-time CGM use in adults with type 2 diabetes: Results of a pilot randomized controlled trial. Diabetes Ther. 2021;12:2089–2099. doi: 10.1007/s13300-021-01086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vigersky R.A., Fonda S.J., Chellappa M., Walker M.S., Ehrhardt N.M. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2011;35:32–38. doi: 10.2337/dc11-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim W.J., Kim J.H., Yoo H.J., Son J.W., Khang A.R., Kwon S.K., Kim J.H., Kim T.H., Ryu O.H., Park K.H., et al. A position statement of the utilization and support status of continuous glucose monitoring in Korea. J. Korean Diabetes. 2021;22:225–237. doi: 10.4093/jkd.2021.22.4.225. [DOI] [Google Scholar]
- 41.Janapala R.N., Jayaraj J.S., Fathima N., Kashif T., Usman N., Dasari A., Jahan N., Sachmechi I. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: A systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ida S., Kaneko R., Murata K. Utility of real-time and retrospective continuous glucose monitoring in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. J. Diabetes Res. 2019;2019:4684815. doi: 10.1155/2019/4684815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edelman S.V., Argento N.B., Pettus J., Hirsch I.B. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care. 2018;41:2265–2274. doi: 10.2337/dc18-1150. [DOI] [PubMed] [Google Scholar]
- 44.Petrie J.R., Peters A.L., Bergenstal R.M., Holl R.W., Fleming G.A., Heinemann L. Improving the clinical value and utility of CGM systems: Issues and recommendations. Diabetes Care. 2017;40:1614–1621. doi: 10.2337/dci17-0043. [DOI] [PubMed] [Google Scholar]
- 45.Kim K. The influencing factors associated with glycemic control among adult diabetes patients. J. Korea Acad.-Ind. Coop. Soc. 2015;16:3284–3292. doi: 10.5762/KAIS.2015.16.5.3284. [DOI] [Google Scholar]
- 46.Harrabi I., Harbi F.A., Ghamdi S.A. Predictors of glycemic control among patients with type 2 diabetes in Najran Armed Forces Hospital: A pilot study. J. Diabetes Mellit. 2014;04:141–147. doi: 10.4236/jdm.2014.42021. [DOI] [Google Scholar]
- 47.Inolopú J., Hilario-Huapaya N., Tantaleán-Del-Águila M.A., Hurtado-Roca Y., Ugarte-GilI C. Interventions for the prevention of risk factors and incidence of type 2 diabetes in the work environment: A systematic review. Rev. Saúde Pública. 2019;53:101. doi: 10.11606/s1518-8787.2019053001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sequeira P.A., Montoya L., Ruelas V., Xing D., Chen V., Beck R., Peters A.L. Continuous glucose monitoring pilot in low-income type 1 diabetes patients. Diabetes Technol. Therap. 2013;15:855–858. doi: 10.1089/dia.2013.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.PRISMA Transparent Reporting of Systematic Reviews and Meta-Analyses. PRISMA 2020 Checklist. [(accessed on 23 January 2024)]. Available online: http://www.prisma-statement.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.