Abstract
Sepsis, a critical condition marked by systemic inflammation, profoundly impacts both innate and adaptive immunity, often resulting in lymphopenia. This immune alteration can spare regulatory T cells (Tregs) but significantly affects other lymphocyte subsets, leading to diminished effector functions, altered cytokine profiles, and metabolic changes. The complexity of sepsis stems not only from its pathophysiology but also from the heterogeneity of patient responses, posing significant challenges in developing universally effective therapies. This review emphasizes the importance of phenotyping in sepsis to enhance patient-specific diagnostic and therapeutic strategies. Phenotyping immune cells, which categorizes patients based on clinical and immunological characteristics, is pivotal for tailoring treatment approaches. Flow cytometry emerges as a crucial tool in this endeavor, offering rapid, low cost and detailed analysis of immune cell populations and their functional states. Indeed, this technology facilitates the understanding of immune dysfunctions in sepsis and contributes to the identification of novel biomarkers. Our review underscores the potential of integrating flow cytometry with omics data, machine learning and clinical observations to refine sepsis management, highlighting the shift towards personalized medicine in critical care. This approach could lead to more precise interventions, improving outcomes in this heterogeneously affected patient population.
Keywords: sepsis, immune response, lymphopenia, phenotyping, biomarkers, flow cytometry, personalized medicine
1. Introduction
As critical care medicine continues to evolve, significant updates in the understanding and management of sepsis have emerged. The 2023 guidelines have notably redefined sepsis as “life-threatening organ dysfunction resulting from a dysregulated host response to infection”, a pivotal shift that has reformed the clinical and conceptual approach to this condition [1,2]. This redefinition emphasizes the acute severity and potential lethality of sepsis, highlighting the urgency for rapid diagnosis and effective therapeutic intervention. Further underscoring its significance, the World Health Organization has recognized sepsis as a global health priority [3].
The current treatment regimen, primarily consisting of antibiotics, fluid therapy, and organ support, has yet to significantly improve patient outcomes, contributing to reduce the persistently high mortality rates, which range from 38.6% to 80%, depending on the patient population and study [4]. Despite advances in treatment, sepsis remains a formidable challenge in clinical care, remaining a leading cause of mortality worldwide, as evidenced by high in-hospital mortality rates. It is estimated to account for about 20% of all global mortality, making it one of the most life-threatening conditions encountered in emergency departments [5,6,7]. In 2017 alone, sepsis accounted for nearly 49 million cases and 11 million deaths, reflecting its widespread impact [5,8,9].
Systemic inflammation is a characteristic feature of sepsis. Clinical symptoms include changes in body temperature (fever or hypothermia), leukocytosis and leukopenia, tachycardia, hypotension, and hyperventilation. It is possible, however, that they may also originate from non-infectious sources such as trauma or so-called sterile inflammation and are neither specific nor sensitive in detecting sepsis. Consequently, it is necessary to use markers that, by enabling early detection of sepsis and organ dysfunction, allow for early and targeted interventions. C-reactive protein (CRP) is a sensitive parameter for diagnosing non-systemic infections, whereas procalcitonin (PCT) appears to be a useful parameter for improving the diagnosis and monitoring of therapy in patients with sepsis and septic shock [10]. Also, tools like the Sequential sepsis-related Organ Failure Assessment (SOFA) score have been instrumental in assessing the severity of organ dysfunction and predicting mortality risk, aiding in early diagnosis and management [2,11].
A key challenge in sepsis management is the heterogeneity of patient populations, encompassing diverse underlying conditions and varying immune responses. This diversity complicates the development of universally effective treatment strategies [12]. This complexity is further compounded by recent research which brings to light the nuanced interplay between pre-sepsis host factors, the evolution of lymphopenia, and persistent changes in immune function following sepsis recovery. Such insights reveal the intricate and dynamic nature of sepsis immunology, underlining the importance of a more detailed and personalized approach to its understanding and management. This shift in perspective, advocating for comprehensive immunological profiling and considering the microbiome’s influence, aims to refine our strategies in sepsis prevention, diagnosis, and treatment [13].
The rising trend in sepsis cases is attributed to factors such as an aging population, increased use of invasive surgical procedures, widespread use of immunosuppressive medications and chemotherapy, and the escalating challenge of antibiotic resistance [14]. Particularly at risk are individuals with compromised immune systems, such as those with HIV/AIDS, cirrhosis, or those undergoing cancer treatments. A French ICU study indicated a nearly threefold (Odds Ratio 2.8) increased chance of sepsis in these groups [15].
Approximately a third of sepsis or septic shock patients described in a 1997–2011 study was overtly immunocompromised [16]. Cancer patients, in particular, face a quadrupled risk of sepsis and higher mortality rates [17]. Genetic predispositions also contribute to sepsis susceptibility. A Danish study highlighted an increased risk of infection-related death before age 50 if a biological parent had died of an infectious cause [18]. Lifestyle factors, such as alcohol consumption and smoking, have been associated with increased sepsis risk and complications [19]. Intriguingly, vitamin D deficiency has been linked to a higher sepsis risk, though the effectiveness of supplementation is unclear [20].
Notably, systemic inflammation is not always caused by infections. Conditions like pancreatitis, trauma, or severe allergic reactions can mimic septic states. The EPIC II study provided valuable insights into the infectious causes of sepsis, revealing the lung as the most common infection site among patients from 75 countries [21]. While these statistics and trends underscore the clinical urgency and complexity of sepsis management, they also point towards the necessity of a deeper understanding of its underlying mechanisms. Sepsis, far from being a singular pathologic entity, manifests through diverse physiological disruptions at various levels of human biology. This intricate web of pathophysiological changes, stemming from and extending beyond the infection site, necessitates a thorough exploration of its pathogenesis and cellular mechanisms. Such an understanding is crucial not only for enhancing diagnostic accuracy and treatment efficacy but also for paving the way towards personalized medicine in sepsis care.
2. Sepsis Pathophysiology: A Multi-Level Perspective
Sepsis presents a unique challenge due to its multi-level pathogenesis involving molecular, cellular, and organ systems. Understanding these layers is crucial for developing new effective diagnostic and therapeutic strategies.
2.1. Molecular and Immune Mechanisms in Sepsis
The pathogenesis of sepsis extends beyond the infection type, encompassing a spectrum of biological processes, including inflammation, coagulation, endothelial activation, and alterations in the microbiome [22,23].
Central to the pathogenesis of sepsis is an intricate interplay between the immune system and the relevant pathogens, ranging from bacteria to viruses and fungi. The immune response, initially protective, can become unbalanced due to the persistent stimulation of pattern recognition receptors (PRRs) like Toll-like receptors (TLRs), nucleotide-binding oligomerization domain-like receptors (NLRs), and C-type lectin receptors (CLRs). These receptors detect pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), ideally leading to pathogen elimination and restoration of homeostasis. However, in sepsis, this balanced response is often disrupted, leading to excessive inflammation and tissue injury, a state termed “hyperinflammation” [23,24,25]. The gut microbiome, vital in maintaining immune homeostasis and protecting against pathogenic invasion, undergoes significant disruption in sepsis. Dysbiosis in sepsis patients is marked by decreased microbial diversity and overgrowth of opportunistic pathogens such as Enterobacter, Enterococcus, and Staphylococcus. This dysbiosis contributes to increased gut permeability and systemic infection, affecting distant organ functions and systemic immunity [23,26,27].
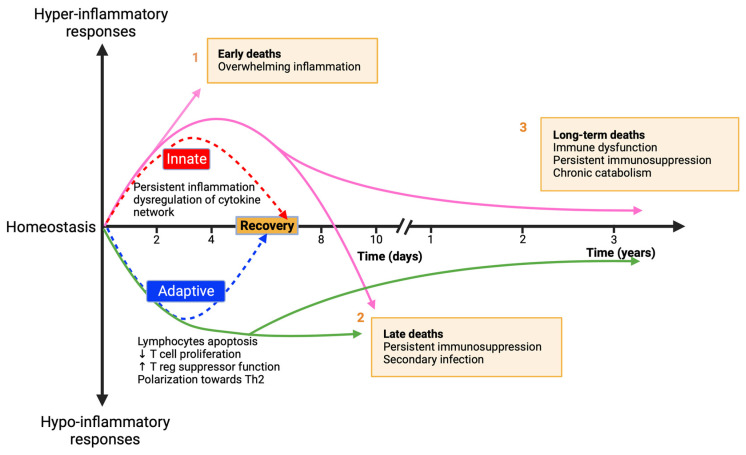
In clinical settings, sepsis patients often exhibit concurrent hyperinflammation and immune suppression, resulting in variable different clinical outcomes, including persistent inflammation, immunosuppression, and catabolism syndrome (PICS) in critically ill patients. This dichotomous response highlights the complexity and variability in sepsis pathogenesis, necessitating personalized therapeutic approaches [28,29]. As shown in Figure 1 (adapted from Hotchkiss et al. [30]), the proinflammatory phase in sepsis, i.e., the hyper-inflammatory response, often termed the “cytokine storm”, is characterized by intense systemic release of cytokines and other inflammatory mediators, leading to tissue damage and organ dysfunction. This phase aligns with the concept of Systemic Inflammatory Response Syndrome (SIRS) but also acknowledges the potential harm of unchecked inflammatory responses. Neutrophils play a vital role in this phase, contributing to hyperinflammation through the release of proteases and reactive oxygen species and forming neutrophil extracellular traps (NETs). While NETs are essential for antibacterial defense, excessive NETosis can lead to tissue damage, thrombosis, and organ failure [31,32].
Figure 1.
Sepsis Immune Response Timeline. 1: Early phase: intense immune activation causing high initial mortality due to “cytokine storm”. 2: Late phase: prolonged immunosuppression leading to secondary infections and organ failure. 3: Advances in ICU care have improved outcomes, yet late-phase mortality remains a challenge.
Inflammasomes, high molecular weight protein complexes central to the immune system, play a crucial role in sepsis. These machineries activate inflammatory caspases and cytokines of the IL-1 family. Their activation involves various components, such as plasma membrane receptors (e.g., P2 × 7R, panx-1), cytoplasmic mediators (ROS, TXNIP), and intrinsic elements like NLRPs and AIM2. The detection of PAMPs and DAMPs triggers these inflammasomes, initiating a critical immune response against sepsis [33]. The regulation of inflammasome activity is vital for modulating the immune response in sepsis. Novel therapeutic strategies focus on mitigating overactivation of inflammasomes. This includes blocking specific receptors and inhibiting mediators involved in their activation, as well as targeting the cytokines they process, such as IL-1β and IL-18 [34].
The anti-inflammatory phase, i.e., the hypo-inflammatory response (Figure 1), also referred to as “Immuno-paralysis”, is marked by reduced immune responsiveness, leaving patients susceptible to secondary infections, and complicating the recovery process. This phase involves the suppression of various immune cells, including T cells and B cells, their exhaustion, and their reprogramming through epigenetic changes, contributing to the susceptibility to secondary infections and viral reactivation [30,35]. Monocytes and macrophages in sepsis frequently exhibit a “tolerant” state, with diminished proinflammatory cytokine production and altered gene expression due to epigenetic modifications [36,37]. This state contributes to the immune suppressive environment observed in sepsis patients.
The central nervous system (CNS) also plays a role in sepsis, particularly through the neuro-immune inflammatory reflex. However, the contribution of disrupted neuro-immune interactions in sepsis-induced immune suppression remains to be fully understood [38]. The intricacy of sepsis, coupled with individual variability in immune responses influenced by health status, genetic predisposition, and infecting organisms, highlights the need for personalized therapeutic approaches in managing sepsis [30,39].
2.2. Sepsis Pathophysiology across Organ Systems
Sepsis pathophysiology extends to various organ systems, each affected differently:
Central to this process is the cardiovascular system, which undergoes significant changes during the progression of sepsis from localized infection to severe systemic inflammation and septic shock. Despite normal or increased cardiac output, patients with sepsis often experience acute biventricular dysfunction and elevated lactate levels, indicating a critical imbalance in tissue oxygenation and metabolic dysfunction [40,41,42,43,44].
At the endothelial level, sepsis induces profound alterations, such as increased leukocyte adhesion, a shift to a procoagulant state, and compromised barrier function, leading to tissue oedema and microvascular disturbances [19,45,46]. These changes, collectively described as endothelial dysfunction, coupled with widespread tissue factor expression and impaired anticoagulant mechanisms, can culminate in disseminated intravascular coagulation (DIC), further exacerbating organ dysfunction, and increasing mortality risk [47].
In the liver, sepsis impairs crucial functions, including the clearance of bilirubin and processing of pathogen lipids, which intensifies systemic inflammation [48]. Septic acute kidney injury (AKI) involves cytokine and immune-mediated microvascular and tubular dysfunction, rather than mere hypoperfusion or tubular necrosis [49,50,51,52,53].
This brief overview of sepsis pathophysiology, illustrating its extensive impact on various organ systems, underscores the necessity for tailored therapeutic strategies. As we move forward, the focus shifts towards individualized care, recognizing the unique ways in which sepsis manifests in each patient. This understanding forms the basis for the next crucial step in sepsis management: the development of phenotyping and personalized approaches in treatment.
3. Biomarkers in Sepsis
The complexity of sepsis and difficulty of its therapy require innovative approaches, particularly considering the limitations of current treatments. Regardless of the use of broad-spectrum antibiotics, patients often struggle to clear primary infections and are susceptible to secondary infections during hospitalization. Enhancing immune competence could be pivotal in resolving primary infections and preventing fatal secondary complications [54].
Recent therapies and treatment protocols have led to prolonged illnesses with a prevalence of the immunosuppressive phase in sepsis. This condition is increasingly affecting the elderly, with a significant percentage of sepsis cases and related deaths occurring in individuals over 65 years of age in advanced healthcare systems. The diminished efficiency of the immune system in the elderly, a phenomenon known as immunosenescence, along with comorbidities, heightens the risk of sepsis incidence and mortality [55,56].
The immune response in sepsis varies and depends on individual variables such as age, cytokine profiles, immune competence, and comorbidities. Understanding the patient’s phenotype is crucial in developing a tailored therapeutic approach, considering both the disease phase and individual patient factors.
The diversity of sepsis complicates the identification of high-risk patients, early diagnosis, and disease-specific treatments. Delay in appropriate treatment, especially in administering potent antibiotic regimens, substantially increases mortality risk [57] (p. 202).
Flow cytometry, a powerful technology for detecting and monitoring multiple markers at the cellular level, is effective for assessing cell heterogeneity (Figure 2) and is a quicker, less expensive method for studying immune cells. This technique, known as immunophenotyping, is pivotal in understanding the roles of innate and adaptive immune cells in sepsis [54,58].
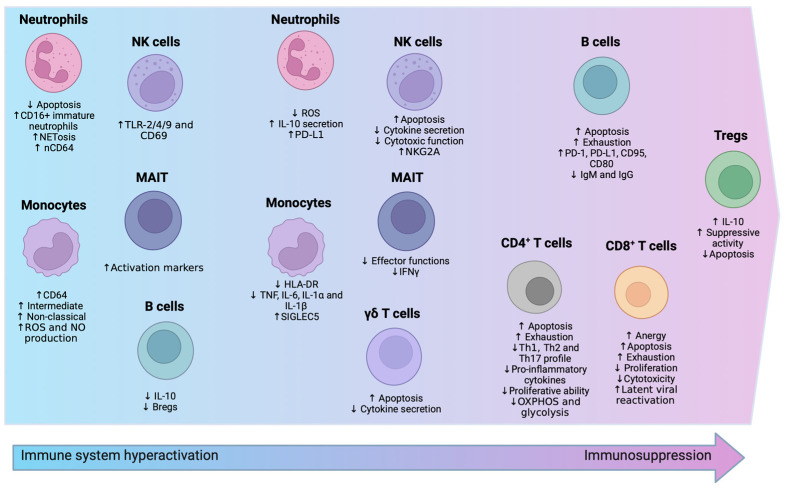
Figure 2.
Immune Cell Modulations in Sepsis. The image provides a comparative overview of immune responses by different cell types during sepsis. The initial hyperactivation with reduced apoptosis in neutrophils and elevated activation in NK cells is depicted, along with increased apoptosis in γδ T cells and MAIT cells. Subsequently, it shows the transition to immunosuppression, with Tregs enhancing suppressive functions and B cells becoming exhausted. Both CD4+ and CD8+ T cells are characterized by increased anergy and apoptosis, signaling a state of immune exhaustion, which collectively portrays the biphasic immune landscape in sepsis.
3.1. Innate Immunity
3.1.1. Neutrophils
Neutrophils are the most abundant innate cell type and the first responders to sites of microbial infection [59]. They play a crucial role in the eradication of microbes as well as the survival of patients suffering from sepsis [60].
Delayed neutrophil apoptosis is one of the most prominent innate immune changes in sepsis, compared to physiological conditions in which neutrophils are constitutively pro-apoptotic. This protracted apoptotic process leads to an accumulation of neutrophils in circulation, which are of varying maturity, and to persistent neutrophil dysfunction [61,62]. In addition, the bone marrow produces and releases immature neutrophils, which can worsen delayed cell death [63]. Immature neutrophils are identified by low expression of CD10 and CD16 (CD10lowCD16low) as well as their immunosuppressive functions and their presence correlates with early mortality after sepsis [64,65].
Furthermore, pro-inflammatory mediators can induce the activation of extracellular signal-regulated kinase (ERK) 2 and phosphoinositide 3-Kinase (PI3K) pathways which eventually lead to upregulation of anti-apoptotic Bcl-xL protein and downregulation of Bim [66,67]. Also, activation of Akt pathway results in phosphorylation of Bad which prevents apoptosome formation and therefore inhibits neutrophil apoptosis [68].
Moreover, neutrophils in sepsis display deficits in oxidative burst, with a reduced production of ROS, in cellular migration patterns, complement activation ability, and in bacterial clearance, along with decreased recruitment to infected tissues [59,69,70]. This persistent neutrophil dysfunction has been implicated in the development of hospital-acquired infections in several human studies [71]. In addition, patients with the most pronounced neutrophil dysfunction after sepsis are the most likely to develop ICU complications such as ventilator-associated pneumonia and other nosocomial infections [72].
Despite typically producing low cytokine amount, neutrophils in sepsis are notable for increased interleukin (IL)-10 production [73]. Also, apoptosis-induced lymphopenia may be caused by neutrophils secreting excessive amounts of IL-10 during sepsis, which hinders T lymphocyte proliferation [73].
Neutrophils can attack a wide range of microorganisms by forming NETs [31]. Studies suggest that NETs can prevent the spread of bacteria, especially during the early phase of infection [74,75]. The release of NETs is crucial in the pathogenesis of diseases such as sepsis, atherosclerosis, and autoimmunity [76,77]. Septic patients exhibited higher levels of NETs compared to healthy controls which have been linked to sepsis severity and organ dysfunction [32]. In sepsis and similarly to neutrophils in COVID-19, which lead to chronic basal inflammation, the system may be defined as refractory, resulting in enhanced glycolysis and glycogenolysis, which may explain why NETosis activity may have increased [78]. Additionally, NETs have been suggested to contribute to coagulation impairment in sepsis [79,80]. In vitro studies have shown that NETs promote fibrin formation and deposition, and they colocalize with fibrin in blood clots [80,81,82].
Neutrophil CD64 (nCD64) is considered a reliable and effective marker for identifying systemic infection, sepsis and tissue injury with high sensitivity and specificity [83]. nCD64 serves as a valid marker for detecting an early step immune response to bacterial infection, typically characterized by a >10-fold, rapid increase in its expression upon activation by pro-inflammatory cytokines [84,85]. As a diagnostic and prognostic biomarker, nCD64 is associated with ICU survival, mortality, and impending clinical deterioration. It could be used to differentiate sepsis severity stages and to guide antibiotic usage. Early increased percentages of CD64+ neutrophils and CD16+ monocytes are thus associated with diagnosis of sepsis [86]. The amount of plasma membrane expression of CD64, measured by flow cytometry evaluating its mean fluorescence index (MFI), can be used as a neonatal sepsis biomarker [87] and, indeed, it demonstrated a high sensitivity and specificity as a specific biomarker for systemic sepsis in adults [88]. Furthermore, its expression remains stable in EDTA-anticoagulated blood for at least 24 h at room temperature, facilitating its quantification for clinical applications [89].
Also, in septic patients, neutrophils showing elevated programmed death ligand (PD-L) 1 (a ligand that binds to the inhibitory co-receptor PD1 found on T cells) expression can induce apoptosis in CD4+ T lymphocytes, contributing to lymphocyte depletion, except for regulatory T cells (Tregs) [58]. Finally, it has been proposed that a subset of CD16hiCD62low immune suppressive neutrophils possess the ability to hinder T lymphocyte proliferation [90], although possessing poor ability to kill bacteria like Staphylococcus aureus [91].
3.1.2. Monocytes
Simply speaking, monocytes can be categorized in three main subpopulations: classical (CD14+CD16−), intermediate (CD14+CD16+), and non-classical (CD14lowCD16+) monocytes. Different monocyte subsets are expanded or reduced in specific pathologies, and indeed, CD16+ monocytes represent up to 50% of all circulating monocytes during sepsis [92]. An important rise in both the intermediate and non-classical subsets was noted among patients with sepsis. Interestingly, after treatment, non-classical inflammatory monocytes returned to normal levels [93]. This suggests that the expansion of CD16+ monocytes could be used to identify the inflammatory condition.
Monocytes in sepsis exhibit significant alterations in the expression of other surface markers. Notably, the reduced expression of the HLA-DR correlates with a decreased capacity to mount a pro-inflammatory response and to present antigens effectively. Furthermore, in early deceased patients the expression of co-stimulatory molecule CD86 was decreased, while in intermediate and non-classical monocytes its expression was lowered when compared to that of those who survived [94].
The trend of HLA-DR surface expression over time, studied by Bodinier et al. [95], suggests distinct phenotypes with varied clinical outcomes with the change in HLA-DR expression as a putative prognostic marker of septic shock in ICU [96,97]. Reduced HLA-DR expression on monocytes was associated with poor outcomes in sepsis [98,99,100]. HLA-DR expression levels can be measured using flow cytometry, either as the percentage of CD14+ cells positive for the marker or as the mean fluorescence intensity on total monocytes. Also, the number of HLA-DR molecules can be determined with flow cytometry using specific fluorophore-labelled beads. In septic patients, an increased programmed death ligand (PD-L)1 expression [101,102,103] was found that was associated with HLA-DR downregulation [101].
Interestingly, an increased expression of CD64, a marker related to phagocytosis, was detected in monocytes from septic patients; indeed, a preserved phagocytic activity has been found in monocytes during sepsis [104] which indicates that monocytes are not anergic. Further, those who survived sepsis showed an enhanced expression of CD64 and higher levels of HLA-DR than non survivors [105].
In sepsis, monocytes show impaired functionality with a diminished capacity to release pro-inflammatory cytokines like tumor necrosis factor (TNF), IL-6, IL-12, and IL-1α. Conversely, the release of anti-inflammatory mediators like IL-10 and IL-1RA is enhanced or remains unimpaired [106,107]. Moreover, ROS and nitric oxide (NO) production by monocytes is enhanced in septic patients, at least in the first phase of sepsis (either during sepsis or septic shock) [104,108,109,110,111]. Cytokines like granulocyte-macrophage colony-stimulating factor (GM-CSF), which modulates monocyte function by upregulating HLA-DR expression, showed promise in small clinical trials, suggesting potential for sepsis therapy [54]. However, it should be noted that HLA-DR downregulation correlates with reduced TNF, IL-6, and IL-1β release post- lipopolysaccharide (LPS) stimulation, increasing the risk of adverse events and death in sepsis [112,113,114].
Transcriptomic analysis using high-throughput multiplex mRNA sequencing has shown an increased expression of genes related to anti-inflammatory cytokines and inhibitory signaling molecules in sepsis, contrasting with the downregulation of pro-inflammatory cytokines and mediators such as T TLRs [115,116]. Notably, less than 5% of monocytes in septic patients can produce cytokines post-stimulation, a striking contrast to the 15–20% in healthy controls [117].
Single-cell RNA sequencing (scRNA-seq), particularly of leukocytes, is a powerful tool to delineate immune cell responses in sepsis. This approach, distinct from bulk RNA sequencing, offers insights into individual cell states. Recent studies have utilized scRNA-seq of peripheral blood mononuclear cells (PBMCs) from sepsis patients, identifying four distinct monocyte states (MS1–4) [116]. MS1 cells (CD14−) are marked by high levels of resistin, arachidonate 5-lipoxygenase activating protein, and interleukin-1 receptor type 2 (IL1R2). MS2 cells express high levels of class II major histocompatibility complex (MHC-II), MS3 cells are non-classical CD16high monocytes, and MS4 cells exhibit low class II MHC and cytokine expression. Expanded MS1 cells, associated with sepsis, suggest a link between mitochondrial dysfunction and immune paralysis, as evidenced by their lower response to LPS compared to healthy controls [118].
3.1.3. Natural Killer (NK) Cells
The particular tissue distribution and the low number of CD56+CD16+/− NK cells in peripheral blood make studying this cell subset challenging, especially in the context of human sepsis, and can explain, at least in part, why such cells have not been deeply investigated [119,120]. However, they are abundant in some tissues like the lungs [121,122] which are particularly prone to dysfunctions in ICU patients.
The initial phase of sepsis is characterized by an increased number of activated NK cells expressing high levels of TLR-2/4/9 and CD69 [123], as well as high plasma concentration of granzyme A, interferon (IFN)-γ, and IL-12p40 [119,124,125]. Furthermore, in the hyperinflammatory stage, the enhancement of inflammatory factors leads to abnormal NK cell activation that can trigger a cytokine storm through a positive feedback loop resulting in severe organ damage [126,127]. However, prolonged hyperactivation leads to impaired NK cell function underlined by downregulation of cytotoxic genes [128] and overexpression of the inhibitory molecule NKG2A [129,130].
Nevertheless, in septic patients, the number of circulating NK cells was significantly decreased, especially in patients with infections due to gram-negative bacteria [131,132], and it was associated with increased mortality [133]. The mechanism at the basis of NK reduction in the bloodstream is not fully understood, but it is likely that an increased tendency to apoptosis might promote this phenomenon. However, animal studies revealed that there can be a rapid migration of NK cells to the site of infection within 4–6 h; thus, this might promote NK cell decline from blood circulation [134]. Therefore, NK dysfunction and defects may be a feature of the immunosuppressive state during the late phase of sepsis.
NK cells’ reduced potency of cytokine production (i.e., IFN-γ, IL-23) in polymicrobial sepsis suggest their tolerance to TLR agonists [120,135,136,137]. As NK cells play an integral role in antiviral defense, impaired NK cell function may lead to the reactivation of latent viruses, like CMV, that is commonly reported among ICU patients [119,138,139,140]. In COVID-19 patients, however, the absolute number of cytokine-producing CD56bright NK cells or cytotoxic CD56dim NK cells decreased with enhanced NK cell activation characterized by Ki-67, HLA-DR, and CD69 [141], which indicated sepsis [142].
The recent observations on NK cells suggest a potential application of NK cell therapy for the treatment of sepsis, but no current studies provide exhaustive mechanistic insights into their function during sepsis for different reasons. First, measuring different parameters in blood NK cells from patients with sepsis cannot provide a detailed picture of all tissues and compartments where NK cells alter their phenotypic or functional characteristics. Second, several clinical studies have small sample sizes. Third, few parameters have been measured in the early phases of sepsis. Fourth, the population of patients under analysis has high heterogeneity in terms of the severity of the infection and type and site of infection. All of these limitations make it difficult to determine the real role of NK cells in the pathogenesis of sepsis. Future studies have to address this challenge by taking into account the aforementioned limitations.
3.1.4. γδ. T Cells and MAIT Cells
Sepsis affects γδ T cells, which recognize lipid antigens from various pathogens and primarily reside in the intestinal mucosa. These cells respond rapidly to a stimulus through IFN-γ and IL-17 release [143,144]. It has been shown that IL-17+ γδ T cells play an important role in polymicrobial sepsis [145], especially in lung failure [146]. Moreover, γδ T cells in septic patients showed lower levels of CD69 and IFN-γ expression after ex vivo stimulation, more so in non-survivors than survivors [147].
Significant depletion of γδ T cells in septic patients, due to increased apoptosis especially in CD3−CD56+ γδ T cells subset [148], correlates with higher illness severity and mortality [149,150]; also, their loss in the intestinal mucosa can lead to pathogen invasion and secondary infections.
Mucosal-associated invariant T (MAIT) cells are characterized by the expression of a semi-invariant αβ T-cell receptor (TCR) that differs from other conventional T cells. MAIT cells recognize and respond to various bacterial and fungal metabolites presented on the major histocompatibility complex class 1-related protein (MR1) [151,152,153] and play a main role in the response to different tumors [154,155]. The TCR expressed by MAIT cells is constituted of an invariant domain of the α chain consisting of TRAV1, corresponding to Vα7.2. Beside the expression of the aforementioned TCR, MAIT cells can also be identified by high expression of CD161 in humans [151,156]. Effector functions of MAIT cells, as well as their number in the bloodstream, are impaired during sepsis [152], which may reflect a re-distribution into the inflamed tissue, as shown for human peritonitis [157], and the deficiency of MAIT cells increases sepsis-related mortality in mice models. Also, downregulation of either Ifng mRNA or IFN-γ production occurs in septic mice [158]. In septic patients, an upregulation of activation markers like CD69, CD38, CD137 occurred, which return to normality during recovery [158]. Also, during sepsis, HLA-DR is upregulated in MAIT cells, and survivors present higher levels of HLA-DR and PD-L1 in comparison to non-survivors [159].
3.2. Adaptive Immunity
3.2.1. T Lymphocytes
Sepsis has marked effects on tissue and circulating T lymphocytes, particularly CD4+ T cells. CD4+ T helper (Th) cells facilitate immunological processes in the body by assisting other cell types, such as B cells, during differentiation, activating cytotoxic T cells, and stimulating monocytes [97,160,161,162,163,164,165]. Based on the type of cytokines that mature CD4+ Th cells produce upon stimulation, mature Th cells have been categorized into Th1, Th2, and Th17 subsets. Upon stimulation, the aforementioned Th subsets elicit the production of characteristic effector cytokines: IFN-γ for Th1, IL-4 for Th2, and IL-17 for Th17 T cells. These cytokines confer protection against specific pathogens, such as intracellular viruses/bacteria, helminths, and fungi [166]. Each CD4+ T cell subset is typically characterized by the expression of subset-specific transcription factors (TFs), which facilitate lineage-specific gene expression and cytokine secretion. Unfortunately, purifying cells through flow cytometry methods based on the intracellular detection of TFs, which requires cell fixation and permeabilization, inhibits most downstream analyses. Therefore, researchers developed strategies using the combinatorial expression of surface chemokine receptors or other surface markers to identify various subsets of CD4+ T cells. The expression of T-bet and the chemokine receptor CXCR3 feature in the Th1 subset, while Th2 is characterized by the expression of GATA-3 and CCR4 chemokine receptors, and Th17 is identified by the expression of both CCR6 and FOXP3 [166].
One of the most damaging consequences of septicemia is the development of apoptosis that dramatically reduces CD4+ cell populations [167,168]. Patients who did not survive to sepsis had a significantly greater incidence of apoptosis in lymphocytes, particularly CD4+ cells, compared to those who survived [167]. Among CD4+ cells, the production of Th1- and Th2-related cytokines decreases both during and after sepsis [169]. Significant reductions in the transcription factors T-bet and GATA3, which regulate the Th1 and Th2 responses, respectively, provide evidence that the above-mentioned CD4+ subsets are suppressed in the course of sepsis [170]. The reduction of Th17 cytokine production in patients with sepsis was likely to have a harmful effect on long-term survival and may increase their vulnerability to fungal infections that are often seen in critically ill populations [171]. Additionally, the administration of IL-7 enhanced Th17 cell responsiveness, leading to a reduction in mortality associated with secondary fungal infections [172].
When T cells do not undergo apoptosis after sepsis, their phenotype and functions are greatly impaired [173]. CD4+ T cells in septic patients upregulate inhibitory markers like cell immunoglobulin and mucin domain-containing protein-1 (ICAM-1, or CD54), lymphocyte activation gene 3 (LAG3, or CD223), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4, or CD152) as well as exhaustion markers such as PD-1 (CD279) and its ligand PD-L1 (CD274) [10,174], decreased production of pro-inflammatory cytokines like IL-2 and IFN-γ, decreased STAT-5 phosphorylation upon TCR activation, decreased CD3 and CD28 expression, and alterations of Th subsets with a shift towards the Th2 profile of cytokine secretion [54,112,113,175]. Also, PD-1 upregulation seemed to be correlated with diminished T cell proliferation and increased nosocomial infections and mortality [102]. Indeed, in COVID-19 patients, T lymphocytes showed a reduced proliferative ability in vitro [176].
Sepsis also results in the dysfunction of CD4+ T cells due to metabolic reprogramming. T lymphocytes from patients with sepsis exhibit altered basal metabolic content due to reduced levels of ATP and inhibition of the mitochondrial oxidative phosphorylation system (OXPHOS) and glycolysis pathways [173].
Meanwhile, the CD8+ T cells are responsible for clearing an infection and producing memory CD8+ T cells as a result of infection or vaccination. Secretions of cytokines (including IFN-γ and TNF) and the ability to lyse cells are the main effector functions of CD8+ T lymphocytes [177]. Here, there are several dysfunctions associated with CD8+ T cells, including increased apoptosis, cell exhaustion, decreased proliferation, and cytotoxic activity [178]. Monocytes can induce CD8+ T cell anergy through the membrane expression of sialic acid-binding Ig-like lectin 5 (SIGLEC5, or CD170), which is an immune checkpoint that also exists in soluble form. SIGLEC5 plays a specific role against CD8+ but not CD4+ T cells. Thus, its expression on monocytes is higher in septic patients than in healthy volunteers after ex vivo LPS stimulation. In addition, SIGLEC5 restrained CD8+ T cell proliferation in vitro without inducing apoptosis [179].
There is also another phenotypic change in CD8+ T cells from septic patients. In a study, the number of naïve CD8+ T cells decreased and the number of effector/effector memory CD8+ T cells increased in septic patients in the first 24 h of ICU admission. Furthermore, the naïve CD8+ T cell compartment was also restored in the septic individuals when blood collection occurred 28 days after ICU admission. Sepsis, therefore, can significantly alter the naïve CD8+ T cell composition and directly affect the host’s ability to respond to new infections. These findings may have direct implications for therapeutic interventions to improve CD8+ T cell function in immunocompromised patients [180]. Immunocompromised individuals with sepsis are more likely to experience reactivation of latent viruses [181,182]. A study examining the activity of T cells in sepsis patients with human cytomegalovirus (HCMV) found that CD8+ T cells had impaired polyfunctionality [183] (p. 201). Additionally, patients who experienced HCMV reactivation had a significantly reduced frequency of CD8+ T cells [183]. Patients with viral reactivation showed increased PD-1 expression on their T cells compared to those without [183]. This is another example of T cell exhaustion. Polyfunctional CD8+ T cells were inversely proportional to the expression of PD-1 [183].
In adaptive immunity, T regulatory cells (Tregs) play a major role in maintaining self-tolerance and suppressing autoimmune disease by inhibiting the responses of other effector T-cell subsets. Treg cells exhibit greater resistance to sepsis-induced apoptosis when compared to other T cell subpopulations [184]. In cases of sepsis, critical illness, and inflammation, Tregs enhance the harmful suppression of effector T cells, resulting in extended recovery periods and heightened risk of complications [185]. T lymphocyte dysfunction after sepsis is characterized by an increased proportion of a CD4+CD25+CD127low regulatory T cell subpopulation among circulating patients’ lymphocytes due to a decrease of effector T cell numbers [186].
The potential implication of an augmented quantity of circulating Tregs, specifically CD4+CD25+Foxp3+ cells, may be involved in the promotion of lymphocyte anergy and immunoparalysis linked to sepsis [58]. In fact, although pro-inflammatory effector Th cells have lost their cytokine production capacity, the capacity of Tregs of producing IL-10 can remain constant [187]. Furthermore, in critically ill patients, an increased proportion of Treg is linked to a higher risk of subsequent nosocomial infections. Additionally, it is possible to include this parameter in a panel that can be useful for classifying patients in the context of a precision medicine strategy [188].
3.2.2. B Lymphocytes
There is limited information about the B lymphocyte response in septic patients. As observed for Tregs, there can be a decrease in the absolute number of circulating B cells [131] but an increase in their relative percentage among total lymphocytes [35,124,131,133,168,189,190,191,192,193,194,195,196] (p. 2). Even if the reduction in the number of B cells and their subsets in sepsis remains debatable, B cell count in non-survivors was significantly lower compared to survivors [35,189,195,196,197,198,199,200].
The mechanism at the basis of B cell depletion is not fully understood, although accelerated apoptosis has been hypothesized, since previous evidence has revealed that B cell apoptosis occurs in blood, intestine, and peripheral lymphoid organs in patients with sepsis and animal models [201,202,203,204].
B cell phenotypes change in sepsis, with a marked reduction of CD19+CD23+ activated regulatory B cells and CD19+CD5+ natural responder B1a cells, although the number of CD19+CD69+ early activated B cells remains unaffected [191]. B1a cells are able to secrete IgM [205,206] as well as immunomodulatory cytokines like IL-10, spontaneously or after infection [206,207,208], and GM-CSF, IL-6, IL-3, TNF [206,207]; therefore, there is evidence that B1a cells play a protective role in sepsis and survival benefits [209].
Whilst the number of total B cells decreases, the proportion of B cell among total lymphocytes increases and the proportion of circulating CD21lowCD95hi exhausted-like B cells augments [189,192] along with the upregulation of PD-1, PD-L1, CD95, and CD80, that not only impair the antigen-presenting activity of B cells but also affect prognosis [191,193,200] (p. 2). Unlike T cells, there is a decrease of IL-10-producing Breg cells [210] which is correlated to poor prognosis [211]. Also, the reduction of the percentage of CD19+CD24hiCD38hi Breg cells is associated with development of septic shock [211].
As well as their phenotype, B cell function is also impaired in sepsis, with a reduced ability of proliferation and decreased ability of antibody secretion because of insufficient IgM and IgG synthesis [192,195,212]; indeed, hypogammaglobulinemia is common in sepsis [213,214] (p. 202). In particular, a significant decrease of IgM levels is associated with worse outcome [197,198] since lower levels of IgM have been found in non-survivors compared to survivors [212]. However, IgM deficiency is probably not due to the impairment of Ig class switching, but instead occurs because of the depletion of CD27+CD21hi resting memory B cells [35,195]. In elderly people with sepsis, a reduction of immunocompetent B cells with the augment of CD21−/low exhausted B cells is developed along with insufficient IgM production, which may increase susceptibility to secondary infections [192]. In fact, memory B cells are more susceptible to sepsis-induced cell death [215] triggered by the extrinsic pathway induced by receptors like Fas (CD95) and by the intrinsic pathway mediated by mitochondria [202,204,216,217].
Furthermore, in sepsis, B cells can produce a variety of pro-inflammatory cytokines like IL-6, TNF, IL-3, and GM-CSF to reinforce the systemic inflammatory response [218,219,220]. These cytokines also play an important role in polarizing CD4+ T cells towards the Th1 and Th17 phenotypes and in activating innate immune cells like monocytes [221,222].
3.3. Metabolic Shifts in Immune Cells
For both their housekeeping functions and specialized activities, immune cells require substantial amounts of energy. Glucose is metabolized via glycolysis or oxidative phosphorylation (OXPHOS) to produce ATP. OXPHOS is an oxygen-dependent process that takes place in the mitochondria and is highly efficient, producing a total of 36 molecules of ATP from each molecule of glucose. Glycolysis, on the other hand, is oxygen-independent and takes place in the cytosol, where one molecule of glucose is broken down into two molecules of pyruvate; this pathway is less efficient but can be mobilized rapidly [223,224]. Sepsis is initially characterized by a hypermetabolic state, featuring increased ATP production, respiration, and release of different hormones. Subsequently, a hypometabolic state ensues, characterized by reduced OXPHOS, ATP production, and endocrine hormone release or resistance [225,226]. This transition forms the basis of the “hibernation theory”, which posits a protective mechanism allowing cellular recovery by reducing metabolic demands in an environment of impaired ATP production [227]. A shift towards glycolysis as the primary energy-producing pathway occurs, with lactate—a by-product of anaerobic metabolism—serving as a clinical marker for sepsis diagnosis, despite its controversial predictive value [228,229,230].
Sepsis-induced mitochondrial damage is the main cause of metabolism disorders in septic patients [231]. Even though the mechanism at the basis of mitochondrial dysfunction is not completely understood, several hypotheses were made, including a possible involvement of pro-inflammatory cytokines, ROS, and NO, whose production is upregulated in sepsis [232,233]. Indeed, ROS and NO might interfere with the mitochondrial electron transport chain (ETC), hence affecting cellular respiration [227,234,235]. Moreover, a reduced expression of genes encoding for proteins involved in ECT [232,235,236] as well as lower copies of mitochondrial DNA (mtDNA) and a decrease of metabolic rate can play a role [237]. Also, hormonal changes may contribute to the reduced ability to produce ATP through OXPHOS [238]. While ETC dysfunction reduced its efficacy, it enhances the possibility of ROS generation, so it has been hypothesized that a shift towards glycolysis is set up as a protective adaptive mechanism [239]. Thus, a 67% increase in glucose uptake and an almost twofold increase in GLUT1 expression have been observed during sepsis in animal models [240] (p. 199).
Glycolysis in immune cells leads to the production of pro-inflammatory factors, causing “inflammatory storms” and subsequent cellular damage. Patients may survive these storms but then deteriorate due to ensuing immunosuppression. The development of immunosuppressive conditions often involves reduced expression of genes for pro-inflammatory cytokines (e.g., TNF, IL-6, CCL2) and increased expression of anti-inflammatory cytokines (e.g., IL-4, IL-10), impeding effective combat against secondary infections [241,242].
This immunometabolic shift from glucose to fatty acid oxidation is marked by increased levels of fatty acid transporters (like CD36) and CPT-1 in leukocytes [243]. SIRT1 and SIRT6 facilitate this metabolic transition and link metabolism with the acute inflammation response phases. Fatty acid oxidation (FAO) not only promotes an anti-inflammatory phenotype but also produces lactate during the pro-inflammatory phase, exerting a potent immunosuppressive effect [243].
4. Sepsis Therapy: Phenotyping and Personalized Approaches
Sepsis presents significant challenges in diagnosis and treatment due to its heterogeneity that often leads to difficulties in designing successful clinical trials and variable diagnoses among critical care experts, particularly in cases where symptoms are masked by comorbidities, or when laboratory evidence is unclear [11,19,244,245,246]. The concept of precision medicine is gaining traction in response to these challenges, focusing on tailored strategies based on individual patient characteristics. This approach is crucial given the varied manifestations of sepsis and the need to determine effective treatment strategies for different patient subgroups at the optimal times in the disease process [247,248]. In the ICU, the diversity in patients’ clinical histories and conditions, and their responses to treatments, especially in sepsis, highlights the need for a more nuanced understanding of individual characteristics. Recent guidelines aimed at standardizing care for sepsis and septic shock patients have been discussed for potentially oversimplifying the complexity of sepsis management, suggesting that a singular strategy may not be suitable for all patients [249,250,251,252]. This complex scenario underscores the emerging role of cell phenotyping in sepsis, which involves categorizing patients based on observable characteristics, such as clinical presentation and first outcomes. Understanding these phenotypes could aid in tailoring treatments more effectively and could potentially lead to the discovery of novel therapeutic targets and specific biomarkers that might respond differently to various treatments [253,254,255].
However, identifying these phenotypes is challenging, primarily due to the lack of empirical evidence on how to individualize treatment based solely on clinical variables. Artificial intelligence (AI) and machine learning have been making significant inroads in this area. These technologies analyze large data sets to identify patterns and patient groups, proving invaluable in recognizing different disease expressions in sepsis. Clustering analysis, a form of unsupervised machine learning, has been used to identify novel patient subgroups or phenotypes based on multiple variables [256,257]. Nevertheless, these methods have limitations, such as biases in training datasets and indeterminacy in the optimal number of clusters. Therefore, findings from machine learning need rigorous evaluation and external validation for real-world applicability [258,259,260,261,262]. Temperature phenotyping in sepsis, for instance, has shown that a significant portion of septic patients remain hypothermic, linked to increased mortality, whereas fever is associated with decreased mortality. This variation in body temperature was investigated in large cohorts, revealing additional phenotypic characteristics and associations [244,263]. Hemodynamic traits and multiorgan dysfunction have also been used for phenotyping septic patients, identifying distinct phenotypes based on factors like systolic blood pressure and organ dysfunction patterns. These phenotypes could guide more personalized treatment approaches in sepsis, such as tailored fluid responsiveness and vasodilator therapies [264,265,266,267,268,269,270]. Furthermore, the impact of fluid management on sepsis outcomes has been noted, with different phenotypes responding variably to fluid resuscitation. Identifying phenotypes prone to fluid overload could optimize fluid management strategies, potentially impacting patient outcomes [271,272,273]. Ongoing research in sepsis phenotyping is considering various factors like the site of infection, organ dysfunction, and specific diseases like COVID-19 and ARDS, aiming to correlate phenotypes with treatment responses and outcomes [274].
In conclusion, the path towards effectively managing sepsis lies in the implementation of precision medicine, where immune therapy is tailored to individual patient characteristics and pathophysiological changes. Despite centuries of understanding and decades of clinical definitions, sepsis remains a complex and ill-defined syndrome. The future of sepsis treatment will likely involve a nuanced approach, depicted in Figure 3 (adapted from Sinha et al. [115]), based upon a combination of data that considers both clinical phenotypes and immunological profiles, to guide therapy in a manner that is both time-sensitive and individualized [275,276,277].
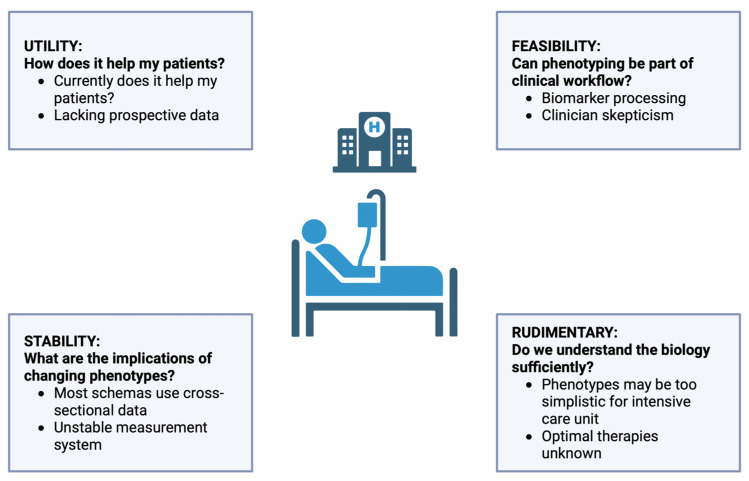
Figure 3.
Evaluating Sepsis Phenotyping. This infographic details key considerations in sepsis phenotyping, divided into three aspects: UTILITY, questioning the immediate benefits for patient care; FEASIBILITY, addressing the practicality of incorporating phenotyping into clinical routines; and STABILITY, discussing the consistency of phenotypic classifications. A final note on RUDIMENTARY aspects suggests a need for deeper biological understanding to support phenotype-based treatments in intensive care settings.
In the evolving landscape of sepsis therapy, the move towards personalized medicine reflects a deeper understanding of the condition’s complexity. Sepsis indeed underscores the inadequacy of one-size-fits-all treatment approaches. The push for individualized therapies is rooted in the recognition of sepsis’s diverse manifestations and the necessity to tailor treatments for different patient subgroups at the most effective stages of the disease process [248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270]. The heterogeneity among sepsis patients results in divergent outcomes despite similar interventions. This variation challenges the universal applicability of standardized care protocols like those proposed by the Surviving Sepsis Campaign by the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM) [249,250,251,252]. Consequently, there is an increasing call for a nuanced approach that considers each patient’s unique characteristics in sepsis management.
A crucial aspect of personalized sepsis therapy involves the dynamic balance between mitigating excessive inflammation and boosting the host’s immune response. This balance is pivotal and varies depending on several factors, including age, overall health status, and the stage of sepsis. For instance, older patients, who are more susceptible to developing immunosuppression (or to an increase in immunosuppression due to their age), may benefit from immune-boosting strategies, while those in the initial hyperinflammatory phase might need anti-inflammatory interventions. The timing of these treatments is critical, as patients often transition from a state of hyperinflammation to immunosuppression during the course of sepsis [163]. Recent advances in sepsis research have facilitated the identification of gene expression-based subphenotypes, aiding in the development of clinical classifiers and underscoring the molecular heterogeneity of the disease. Studies by Wong et al. [245,278], Scicluna et al. [116], and Sweeney et al. [279] have revealed various sepsis phenotypes such as “inflammopathic”, “adaptive”, and “coagulopathic”, paving the way for personalized treatment approaches [115].
Flow cytometry has become a valuable tool in sepsis research, particularly for quantifying HLA-DR on monocytes, a key marker of immunosuppression in critically ill patients. This technique not only correlates with the ability to mount an effective immune response but also aids in distinguishing sepsis from other conditions like SIRS and in understanding the chronic and treatment-resistant aspects of sepsis [30,280]. The journey towards effective molecular targeted therapies in sepsis has been challenging. Initial optimism based on preclinical studies targeting TNF was dampened by clinical trials that revealed increased mortality with anti-TNF antibodies [19,281]. Similarly, the role of corticosteroids in sepsis treatment remains contentious, with early reports of reduced mortality in septic shock not being consistently replicated in subsequent trials [282,283].
One of the major challenges in developing effective therapies is the inherent heterogeneity of sepsis patients. Researchers have noted that the broad clinical criteria used to define sepsis result in a patient group so diverse that finding universally effective therapies becomes a daunting task [284].
This heterogeneity stems from variations in patient factors such as age, sex, comorbidities, lifestyle, genetics, site of infection, and the specific pathogen involved [285]. To address this, strategies to reduce heterogeneity in clinical trials have been suggested, such as limiting enrollment based on specific criteria or utilizing biomarkers to target the underlying pathophysiology. While this approach increases the precision of patient selection, it also raises research costs and limits the generalizability of results [286]. The complexity of molecular pathways in sepsis further complicates therapeutic development. Even modest stimuli can elicit extensive and dynamic changes in genes and metabolites, suggesting that targeting a single pathway in sepsis may be insufficient for significant clinical improvement [287,288]. The use of preclinical models, typically involving young, healthy animals with minimal supportive care, also poses challenges for translating findings to human sepsis cases. Differences between species, such as mice’s resistance to endotoxin, contribute to the discrepancies between preclinical successes and clinical trial outcomes [289,290].
Emerging treatments for sepsis are increasingly focusing on optimizing fluid, hemodynamic, and sedative management. There is a shift towards boosting immunity during later stages of immunoparalysis and enhancing organ function through approaches such as cell-based therapies [291,292,293]. The development of new guidelines and definitions, such as the Sepsis-3 definition by the SCCM and the ESICM, reflects the evolving understanding of sepsis and aims to improve its identification and management [294].
In conclusion, the pathogenesis and treatment of sepsis demand a multifaceted approach. Despite the failure of many targeted therapies in clinical trials, advancements in supportive care have led to improved outcomes. Future directions in sepsis research should focus on novel therapeutic pathways targeting organ dysfunction and refining clinical and biological criteria for patient selection in clinical trials. The shift towards personalized medicine in sepsis treatment is crucial, considering the complexity and heterogeneity of the disease. This approach, combining clinical phenotypes with immunological profiles, holds promise for guiding more effective and individualized therapies (Figure 4).
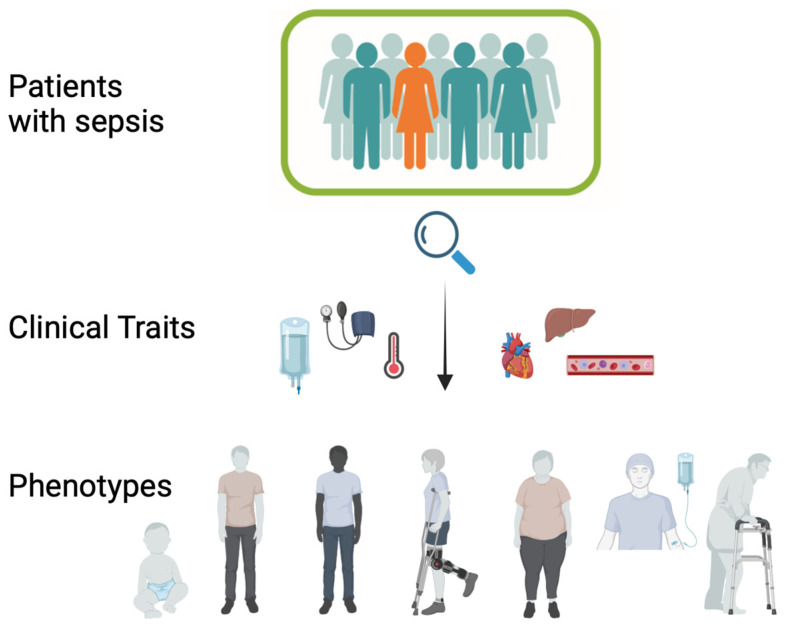
Figure 4.
Clinical Phenotyping in Sepsis. The figure illustrates the categorization of sepsis into distinct phenotypes based on clinical traits like temperature, hemodynamics, and multi-organ dysfunction. It underscores the shift towards personalized medicine, highlighting the potential of combining clinical and immunological data to inform targeted therapies and improve outcomes in sepsis care.
5. Omics Technologies
The evolving field of “omics” (genomics, transcriptomics, proteomics, metabolomics, cytomics) offers systems-biology-based approaches for developing new diagnostic tools in sepsis [295]. With increased computing power, deep phenotyping in critical care settings has become more feasible, aiming to identify homogeneous subgroups within otherwise heterogeneous populations using multidimensional data [115].
Phenotyping can categorize patients into subgroups of a clinical syndrome or disease, defined by a shared feature. Endotyping goes further, categorizing patients into subtypes associated with specific pathobiological or functional mechanisms [57]. Bulk transcriptomic approaches have been used to investigate phenotypes in adult sepsis patients, revealing two sepsis response signatures (SRS1 and SRS2) using unsupervised clustering algorithms. SRS1, associated with poorer outcomes, is enriched with transcripts related to cell death and immune exhaustion [296]. However, transcriptional phenotyping is challenged by variability in detected gene clusters across different methods and populations.
Epigenetics may also play a role in understanding the pathogenesis of sepsis-induced immune dysfunctions, particularly regarding DNA methylation and histone acetylation of inflammatory genes [297,298]. Micro-RNAs (miRNAs) and small non-coding RNAs (sncRNAs) modulate gene expression, with specific miRNAs, like miR-15a, miR-16, miR-122, miR-133, miR-193, miR-223, and miR-483–5p being potential biomarkers in sepsis [299,300,301].
Growing attention has recently been given to circulating extracellular vesicles (EVs), which are involved in cell-to-cell crosstalk through delivery of proteins, lipids, and genetic materials. EVs can be investigated in plasma through flow cytometry [302] and are also potential mediators of sepsis-induced endothelial injury and myocardial dysfunction [303,304]. The number of EVs increases during sepsis [305,306] and it is positively correlated with the severity of the disease [305]. In particular, in the early stage of sepsis, acute phase reactive proteins and Ig involved in inflammatory response are upregulated [307].
Also, levels of mRNA involved in antioxidant defense and oxidative stress are upregulated in the EVs of septic patients [308], that also show an upregulation of DNA methyltransferase (DNMT) 1, DNMT3A, and DNMT3B mRNA, which is correlated with poor prognosis [305]. Neutrophil-derived EVs mainly have anti-inflammatory effects [309,310,311,312], with upregulation of alpha-2-microglobulin and ceruloplasmin, which promote neutrophil adhesion, enhance bacterial clearance, reduce inflammatory response, and improve survival in septic mice [313,314]. On the other hand, host-derived EVs carry a variety of PAMPS and DAMPs like high mobility group box 1 (HMGB1), extracellular cold-inducible RNA-binding protein (eCIRP), mtDAMPs that promote inflammatory responses, neutrophil migration, and organ damage [315,316,317,318,319,320,321,322,323] (p. 20).
Thus, in sepsis, EVs play a range of regulatory roles in either the pathogenesis or treatment of sepsis, so they might serve as innovative biomarkers, together with all the alterations found in septic patients (summarized in Table 1), for predicting and monitoring sepsis progression. As omics technology has developed and been applied, more studies have shown that cargo carried by EVs in sepsis is expressed dynamically, and this correlates with specific clinical features of protein expression, RNA expression, and metabolic changes in EVs.
Table 1.
Main immune cells alterations, metabolic dysfunctions, and communication/cell-cell crosstalk that occur in septic patients.
| Alterations in Sepsis | Main Findings in Septic Patients | Refs. |
|---|---|---|
| Immune Cells Alterations | ||
| Neutrophils | reduced apoptosis and ROS production | [59,61,62,63,69,70] |
| upregulation of CD64 and PD-L1 expression | [58,83,84,85,87,88,90] | |
| upregulation of CD16+ immature neutrophils | [63,64,65] | |
| enhanced IL-10 production and NETosis | [32,73,76,77] | |
| Monocytes | enhanced CD64 expression | [104,105] |
| reduced expression of HLA-DR | [94,95,96,97,98,99,100] | |
| upregulation of intermediate and non-classical phenotypes | [92] | |
| enhanced ROS and NO production | [104,108,109,110,111] | |
| upregulation of SIGLEC5 expression | [180] | |
| downregulation of TNF, IL-6, IL-1β, and IL-1α production | [106,107,112,113,114] | |
| NK cells | enhanced apoptosis | [134] |
| upregulation of TLR-2/4/9 and CD69 in the initial phase of sepsis | [123] | |
| upregulation of NKG2A expression | [129,130] | |
| reduced cytokine secretion | [120,135,136,137] | |
| impaired cytotoxic function | [128] | |
| γδ T cells | enhanced apoptosis | [148] |
| reduced IFNγ secretion and CD69 expression | [147] | |
| MAIT cells | reduced effector functions | [152] |
| upregulation of HLA-DR and PD-L1 expression | [159] | |
| reduced IFNγ production | [158] | |
| upregulation of CD69, CD38 and CD137 expression | [158] | |
| CD4+ T cells | enhanced apoptosis | [167,168] |
| enhanced exhaustion | [10,174] | |
| reduced Th1, Th2, and Th17 populations | [169,170,171] | |
| reduced production of pro-inflammatory cytokines | [112,113,174,175] | |
| reduced proliferative ability | [176] | |
| reduced OXPHOS and glycolysis | [172] | |
| CD8+ T cells | augmented anergy | [179] |
| enhanced apoptosis | [178] | |
| enhanced exhaustion | [178] | |
| decreased naive CD8+ T cells and increased effector/effector memory CD8+ T cells in the first phase of sepsis | [180] | |
| reduced cell proliferation | [178] | |
| reduced cytotoxicity | [178] | |
| increased reactivation of latent viruses | [181,182,183] | |
| Tregs | augmented number of Tregs | [58,186] |
| constant IL-10 production | [187] | |
| reduced apoptosis | [184] | |
| B cells | enhanced apoptosis | [201,202,203,204] |
| enhanced exhaustion | [189,192] | |
| upregulation of PD-1, PD-L1, CD95, and CD80 expression | [191,193,200] (p. 2) | |
| reduced IL-10 production | [210] | |
| reduced IgM and IgG production | [35,192,195,212] | |
| reduced number of Bregs | [211] | |
| Metabolic Alterations | ||
| Mitochondrial dysfunction | reduced expression of genes encoding for proteins involved in electron transport chain (ECT) | [232,235,236] |
| lower copied of mtDNA | [237] | |
| decreased metabolic rate | [237] | |
| upregulated ROS production | [232,233,239] | |
| Glicolysis enhancement | augmented lactate production | [228,229,230] |
| increased GLUT1 expression | [240] (p. 199) | |
| Shift towards fatty acid oxidation (FAO) | increased levels of CD36 and CPT-1 | [243] |
| SIRT1 and SIRT6 cordinate the metabolic swith toward FAO | [243] | |
| Communication/Cell-Cell Crosstalk | ||
| miRNAs | upregulated levels of miR-15a, miR-16, miR-122, miR-133, miR-193, miR-223, and miR-483-5p | [299,300,301] |
| Extracellular vescicles (Evs) | increased number of EVs | [305,306] |
| EVs carrrying mRNA involved in antioxidand defence and oxidative stress | [308] | |
| upregulation of EVs carryng DNA methyltransferase (DNMT)1, DNMT3A, and DNMT3B mRNA | [305] | |
| neutrophil-derived EVs have anti-inflammatory effects | [309,310,311,312] | |
| pro-inflammatory response is enhanced by EVs carryng PAMPs, DAMPs like HMGB1, eCIRP, mtDAMPS | [315,316,317,318,319,320,321,322,323] (p. 20) | |
6. Future Directions
While omics technologies herald a new era of precision medicine in sepsis care, offering nuanced patient stratification, the immediate need for practical diagnostic and therapeutic tools in clinical settings is pressing. In this context, as illustrated in Figure 5 (which shows the phenotype and quantification of MAIT cells in septic patients and healthy donors), flow cytometry emerges as a crucial tool, enabling rapid and precise immunophenotyping. This technique’s ability to delineate specific immune cell populations and their activation states provides valuable insights into the immune dysfunctions characteristic of sepsis [30,324]. The utility of flow cytometry is contingent upon the standardization of protocols, ensuring consistent and reproducible results across different laboratories and clinical studies. This standardization is vital for the multi-center evaluation of immunological biomarkers and comparable patient stratification, which are essential for advancing personalized medicine in sepsis care [325].
Figure 5.
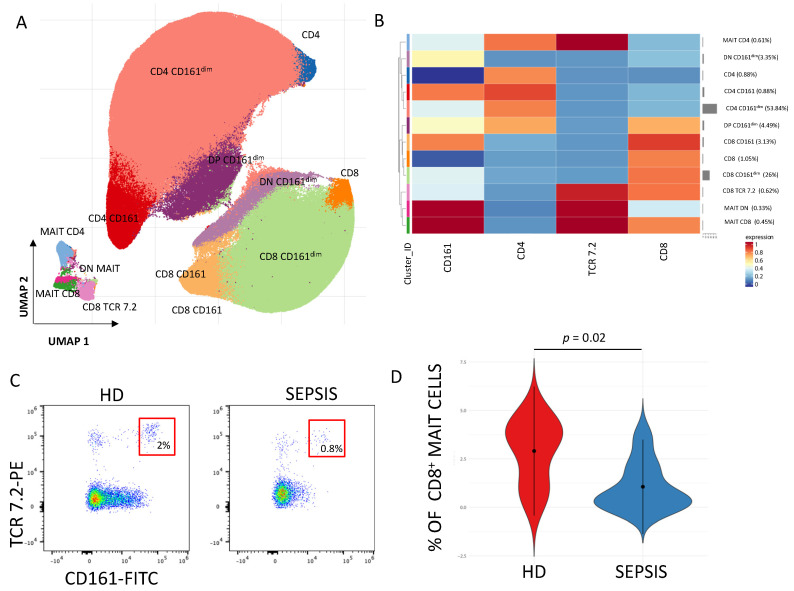
Quantification and phenotypic characterization of MAIT cells in septic patients. (A) The UMAP plot illustrates the 2D spatial distribution of CD3+ T cells across 15 samples, integrated with FlowSOM-based clusters. The color of the different clusters is reported on the left side of the heatmap. (B) Heatmap of the median marker intensities of the 4 lineage markers across the 12 cell populations obtained with FlowSOM algorithm (meta-20). The colors of cluster_id column correspond to the colors used to label the UMAP plot clusters. The color in the heatmap is referred to the median of the arcsinh marker expression (0–1 scaled) calculated over cells from all the samples. Blue represents lower expression, while red represents higher expression. The light gray line along the rows (clusters) indicates the relative sizes of the clusters. DP = double positive; DN = double negative. (C) Representative dot plot showing the percentages of CD8+ MAIT cells (within CD3+ T cells) in healthy donors (HD) and septic patients (SEPSIS). (D) Violin plot representing the percentage of CD8+MAIT cells in healthy donors (HD) and septic patients (SEPSIS) at time of admission in Intensive Care. Non-parametric student t-test; exact p-value is shown in the figure. [data produced by the Laboratory of Immunology, directed by Prof. Andrea Cossarizza, University of Modena and Reggio Emilia].
The integration of advanced machine learning techniques with multi-omics datasets, including genomics, proteomics, and metabolomics, also offers substantial progress in sepsis research. Support Vector Machines (SVMs) and ensemble methods such as Random Forests have proven effectiveness in identifying novel biomarkers and immune profiles from the complex, high-dimensional omics data, which are critical for early diagnosis and the development of personalized therapeutic strategies [326]. These algorithms excel in classifying patients based on subtle, multidimensional patterns detected within omics data, that may not be detectable using traditional statistical methods.
In the field of Artificial Intelligence, deep learning methods like Convolutional Neural Networks and Recurrent Neural Networks have gained traction for their ability to uncover and interpret intricate spatial and temporal patterns within omics datasets. A prediction tool named PENCIL, which utilizes the Learning with Rejection (LWR) strategy, has been developed to select informative features and identify cell subpopulations from single-cell data efficiently. PENCIL is capable of analyzing one million cells in one hour and has been used to identify cancer patients responding to immune checkpoint blockade therapy on the basis of immune cell subpopulations and clinical variables [327]. For instance, this method could be applied to predict sepsis outcome.
Another recent innovation is Stabl, a machine learning approach that provides a unified framework for supervised learning to identify a sparse, reliable set of biomarkers across various single-omics and multi-omics datasets. Starting from 1400–35,000 features in a dataset, it extrapolates 4–34 putative biomarkers. Hence, Stabl has been applied to integrates multi-omics data of pre-term birth and a pre-operative immune signature of post-surgical infections and to predict labor onset [328]. Thus, it might be exploited in sepsis settings to predict sepsis outcomes, as well as biomarkers associated with dysfunctional immune systems. Finally, NAVOY is a real-time sepsis prediction algorithm that uses 4 h of routinely collected laboratory values and clinical parameters as input to identify patients with risk of developing sepsis three hours before sepsis onset [329].
By analyzing variations in patient-specific immune responses, these models can suggest personalized and combined treatment strategies that are optimized for efficacy while minimizing adverse effects. This tailored approach to sepsis treatment represents a significant move towards precision medicine, harnessing the full potential of AI and omics data in clinical settings.
In conclusion, the integration of clinical data, advanced laboratory results, and the use of machine learning algorithms is now paving the way to personalized and targeted therapy.
7. Conclusions
Clinical phenotypes of septic patients are heterogeneous due to various factors such as age, gender, comorbidities, site of infection, and the specific pathogen involved, and are difficult to identify and classify. Thus, even if the treatment of sepsis, based on standardized clinical procedures characterized by the administration of broad-spectrum antibiotics, fluid therapy, and vasopressors, significantly reduces mortality, an improvement in its efficacy is still required.
In the era of multi-omics techniques, the identification of key changes in septic patients is critical to help clinicians determine the most effective diagnostic and therapeutic approaches for each individual, moving beyond one-size-fits-all solutions. The fine analysis of immune system alterations is now feasible by using different single-cell methods such as flow cytometry, which can simultaneously detect up to 50 proteins and scRNA-seq. Furthermore, the study of metabolic changes and of the cell–cell crosstalk is helping to provide a clearer scenario of sepsis phenotypes and its immune phase.
In last years, AI and machine learning made significant inroads in this area, enabling the analysis of large datasets to identify patterns and patients’ groups, through algorithms like Stabl and NAVOY and prediction frameworks like PENCIL.
The synergy of omics data and machine learning opens new perspectives in sepsis, not only supporting a better characterization of patients’ phenotypes, but also promoting a tailored approach to its treatment and towards personalized and targeted therapy.
Acknowledgments
S.B. and L.G. are Marylou Ingram Scholar of the International Society for Advancement of Cytometry (ISAC) for the period 2015–2020 and 2020–2025, respectively. All authors have consented to the acknowledgements.
Author Contributions
E.S., M.D., A.L.C., L.G., A.C., and S.D.B. wrote the manuscript. D.L.T., L.M., I.C., S.B., I.R., M.M., E.F., C.M. and M.G. prepared the figures. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by grant from Bando MIUR “Dipartimenti di Eccellenza 2023/2027”, Area CUN 06 Scienze Mediche to A.C.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Guarino M., Perna B., Cesaro A.E., Maritati M., Spampinato M.D., Contini C., De Giorgio R. 2023 Update on Sepsis and Septic Shock in Adult Patients: Management in the Emergency Department. J. Clin. Med. 2023;12:3188. doi: 10.3390/jcm12093188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavaillon J.-M., Singer M., Skirecki T. Sepsis Therapies: Learning from 30 Years of Failure of Translational Research to Propose New Leads. EMBO Mol. Med. 2020;12:e10128. doi: 10.15252/emmm.201810128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Grooth H.-J., Postema J., Loer S.A., Parienti J.-J., Oudemans-van Straaten H.M., Girbes A.R. Unexplained Mortality Differences between Septic Shock Trials: A Systematic Analysis of Population Characteristics and Control-Group Mortality Rates. Intensive Care Med. 2018;44:311–322. doi: 10.1007/s00134-018-5134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Report on the Epidemiology and Burden of Sepsis: Current Evidence, Identifying Gaps and Future Directions—World|ReliefWeb. [(accessed on 9 January 2024)]. Available online: https://reliefweb.int/report/world/global-report-epidemiology-and-burden-sepsis-current-evidence-identifying-gaps-and.
- 6.Schlapbach L.J., Kissoon N., Alhawsawi A., Aljuaid M.H., Daniels R., Gorordo-Delsol L.A., Machado F., Malik I., Nsutebu E.F., Finfer S., et al. World Sepsis Day: A Global Agenda to Target a Leading Cause of Morbidity and Mortality. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020;319:L518–L522. doi: 10.1152/ajplung.00369.2020. [DOI] [PubMed] [Google Scholar]
- 7.Seymour C.W., Rea T.D., Kahn J.M., Walkey A.J., Yealy D.M., Angus D.C. Severe Sepsis in Pre-Hospital Emergency Care: Analysis of Incidence, Care, and Outcome. Am. J. Respir. Crit. Care Med. 2012;186:1264–1271. doi: 10.1164/rccm.201204-0713OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu C., Legrand M. Epidemiology of Sepsis and Septic Shock. Curr. Opin. Anaesthesiol. 2021;34:71–76. doi: 10.1097/ACO.0000000000000958. [DOI] [PubMed] [Google Scholar]
- 9.Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunkhorst F.M., Reinhart K. Diagnostic Approach to Sepsis—State of the Art. In: Baue A.E., Berlot G., Gullo A., Vincent J.-L., editors. Sepsis and Organ Dysfunction. Springer; Milano, Italy: 2002. pp. 151–167. [DOI] [Google Scholar]
- 11.Suarez-de-la-Rica A., Maseda E. Precision Medicine in Sepsis and Septic Shock. J. Clin. Med. 2022;11:5332. doi: 10.3390/jcm11185332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fohner A.E., Greene J.D., Lawson B.L., Chen J.H., Kipnis P., Escobar G.J., Liu V.X. Assessing Clinical Heterogeneity in Sepsis through Treatment Patterns and Machine Learning. J. Am. Med. Inform. Assoc. 2019;26:1466–1477. doi: 10.1093/jamia/ocz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubio I., Osuchowski M.F., Shankar-Hari M., Skirecki T., Winkler M.S., Lachmann G., La Rosée P., Monneret G., Venet F., Bauer M., et al. Current Gaps in Sepsis Immunology: New Opportunities for Translational Research. Lancet Infect. Dis. 2019;19:e422–e436. doi: 10.1016/S1473-3099(19)30567-5. [DOI] [PubMed] [Google Scholar]
- 14.Vakkalanka J.P., Harland K.K., Swanson M.B., Mohr N.M. Clinical and Epidemiological Variability in Severe Sepsis: An Ecological Study. J. Epidemiol. Community Health. 2018;72:741–745. doi: 10.1136/jech-2018-210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brun-Buisson C., Doyon F., Carlet J., Dellamonica P., Gouin F., Lepoutre A., Mercier J.C., Offenstadt G., Régnier B. Incidence, Risk Factors, and Outcome of Severe Sepsis and Septic Shock in Adults. A Multicenter Prospective Study in Intensive Care Units. French ICU Group for Severe Sepsis. JAMA. 1995;274:968–974. doi: 10.1001/jama.1995.03530120060042. [DOI] [PubMed] [Google Scholar]
- 16.Tolsma V., Schwebel C., Azoulay E., Darmon M., Souweine B., Vesin A., Goldgran-Toledano D., Lugosi M., Jamali S., Cheval C., et al. Sepsis Severe or Septic Shock: Outcome According to Immune Status and Immunodeficiency Profile. Chest. 2014;146:1205–1213. doi: 10.1378/chest.13-2618. [DOI] [PubMed] [Google Scholar]
- 17.Williams M.D., Braun L.A., Cooper L.M., Johnston J., Weiss R.V., Qualy R.L., Linde-Zwirble W. Hospitalized Cancer Patients with Severe Sepsis: Analysis of Incidence, Mortality, and Associated Costs of Care. Crit. Care. 2004;8:R291–R298. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sørensen T.I., Nielsen G.G., Andersen P.K., Teasdale T.W. Genetic and Environmental Influences on Premature Death in Adult Adoptees. N. Engl. J. Med. 1988;318:727–732. doi: 10.1056/NEJM198803243181202. [DOI] [PubMed] [Google Scholar]
- 19.Gotts J.E., Matthay M.A. Sepsis: Pathophysiology and Clinical Management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 20.de Haan K., Groeneveld A.B.J., de Geus H.R.H., Egal M., Struijs A. Vitamin D Deficiency as a Risk Factor for Infection, Sepsis and Mortality in the Critically Ill: Systematic Review and Meta-Analysis. Crit. Care. 2014;18:660. doi: 10.1186/s13054-014-0660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent J.-L., Rello J., Marshall J., Silva E., Anzueto A., Martin C.D., Moreno R., Lipman J., Gomersall C., Sakr Y., et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 22.Wiersinga W.J., van der Poll T. Immunopathophysiology of Human Sepsis. EBioMedicine. 2022;86:104363. doi: 10.1016/j.ebiom.2022.104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Poll T., Shankar-Hari M., Wiersinga W.J. The Immunology of Sepsis. Immunity. 2021;54:2450–2464. doi: 10.1016/j.immuni.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 24.van der Poll T., van de Veerdonk F.L., Scicluna B.P., Netea M.G. The Immunopathology of Sepsis and Potential Therapeutic Targets. Nat. Rev. Immunol. 2017;17:407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S., Ingle H., Prasad D.V.R., Kumar H. Recognition of Bacterial Infection by Innate Immune Sensors. Crit. Rev. Microbiol. 2013;39:229–246. doi: 10.3109/1040841X.2012.706249. [DOI] [PubMed] [Google Scholar]
- 26.Haak B.W., Wiersinga W.J. The Role of the Gut Microbiota in Sepsis. Lancet Gastroenterol. Hepatol. 2017;2:135–143. doi: 10.1016/S2468-1253(16)30119-4. [DOI] [PubMed] [Google Scholar]
- 27.Adelman M.W., Woodworth M.H., Langelier C., Busch L.M., Kempker J.A., Kraft C.S., Martin G.S. The Gut Microbiome’s Role in the Development, Maintenance, and Outcomes of Sepsis. Crit. Care. 2020;24:278. doi: 10.1186/s13054-020-02989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gentile L.F., Cuenca A.G., Efron P.A., Ang D., Bihorac A., McKinley B.A., Moldawer L.L., Moore F.A. Persistent Inflammation and Immunosuppression: A Common Syndrome and New Horizon for Surgical Intensive Care. J. Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darden D.B., Kelly L.S., Fenner B.P., Moldawer L.L., Mohr A.M., Efron P.A. Dysregulated Immunity and Immunotherapy after Sepsis. J. Clin. Med. 2021;10:1742. doi: 10.3390/jcm10081742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotchkiss R.S., Monneret G., Payen D. Sepsis-Induced Immunosuppression: From Cellular Dysfunctions to Immunotherapy. Nat. Rev. Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil Extracellular Traps Kill Bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 32.Czaikoski P.G., Mota J.M.S.C., Nascimento D.C., Sônego F., Castanheira F.V.e.S., Melo P.H., Scortegagna G.T., Silva R.L., Barroso-Sousa R., Souto F.O., et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE. 2016;11:e0148142. doi: 10.1371/journal.pone.0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virgilio F.D. The Therapeutic Potential of Modifying Inflammasomes and NOD-Like Receptors. Pharmacol. Rev. 2013;65:872–905. doi: 10.1124/pr.112.006171. [DOI] [PubMed] [Google Scholar]
- 34.Dinarello C.A., Simon A., van der Meer J.W.M. Treating Inflammation by Blocking Interleukin-1 in a Broad Spectrum of Diseases. Nat. Rev. Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shankar-Hari M., Singer M., Spencer J. Can Concurrent Abnormalities in Free Light Chains and Immunoglobulin Concentrations Identify a Target Population for Immunoglobulin Trials in Sepsis? Crit. Care Med. 2017;45:1829–1836. doi: 10.1097/CCM.0000000000002627. [DOI] [PubMed] [Google Scholar]
- 36.Döcke W.D., Randow F., Syrbe U., Krausch D., Asadullah K., Reinke P., Volk H.D., Kox W. Monocyte Deactivation in Septic Patients: Restoration by IFN-γ Treatment. Nat. Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 37.Vachharajani V., Liu T., McCall C.E. Epigenetic Coordination of Acute Systemic Inflammation: Potential Therapeutic Targets. Expert Rev. Clin. Immunol. 2014;10:1141–1150. doi: 10.1586/1744666X.2014.943192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chavan S.S., Pavlov V.A., Tracey K.J. Mechanisms and Therapeutic Relevance of Neuro-Immune Communication. Immunity. 2017;46:927–942. doi: 10.1016/j.immuni.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yende S., Iwashyna T.J., Angus D.C. Interplay between Sepsis and Chronic Health. Trends Mol. Med. 2014;20:234–238. doi: 10.1016/j.molmed.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabuel C., Mebazaa A. Septic Shock: A Heart Story since the 1960s. Intensive Care Med. 2006;32:799–807. doi: 10.1007/s00134-006-0142-5. [DOI] [PubMed] [Google Scholar]
- 41.Kimchi A., Ellrodt A.G., Berman D.S., Riedinger M.S., Swan H.J., Murata G.H. Right Ventricular Performance in Septic Shock: A Combined Radionuclide and Hemodynamic Study. J. Am. Coll. Cardiol. 1984;4:945–951. doi: 10.1016/S0735-1097(84)80055-8. [DOI] [PubMed] [Google Scholar]
- 42.Parker M.M., Shelhamer J.H., Bacharach S.L., Green M.V., Natanson C., Frederick T.M., Damske B.A., Parrillo J.E. Profound but Reversible Myocardial Depression in Patients with Septic Shock. Ann. Intern. Med. 1984;100:483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 43.Shapiro N.I., Howell M.D., Talmor D., Nathanson L.A., Lisbon A., Wolfe R.E., Weiss J.W. Serum Lactate as a Predictor of Mortality in Emergency Department Patients with Infection. Ann. Emerg. Med. 2005;45:524–528. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Kraut J.A., Madias N.E. Lactic Acidosis. N. Engl. J. Med. 2014;371:2309–2319. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 45.Wolinsky H. A Proposal Linking Clearance of Circulating Lipoproteins to Tissue Metabolic Activity as a Basis for Understanding Atherogenesis. Circ. Res. 1980;47:301–311. doi: 10.1161/01.RES.47.3.301. [DOI] [PubMed] [Google Scholar]
- 46.Aird W.C. The Role of the Endothelium in Severe Sepsis and Multiple Organ Dysfunction Syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 47.Levi M. Pathogenesis and Treatment of Disseminated Intravascular Coagulation in the Septic Patient. J. Crit. Care. 2001;16:167–177. doi: 10.1053/jcrc.2001.30666. [DOI] [PubMed] [Google Scholar]
- 48.Walley K.R., Francis G.A., Opal S.M., Stein E.A., Russell J.A., Boyd J.H. The Central Role of Proprotein Convertase Subtilisin/Kexin Type 9 in Septic Pathogen Lipid Transport and Clearance. Am. J. Respir. Crit. Care Med. 2015;192:1275–1286. doi: 10.1164/rccm.201505-0876CI. [DOI] [PubMed] [Google Scholar]
- 49.Alobaidi R., Basu R.K., Goldstein S.L., Bagshaw S.M. Sepsis-Associated Acute Kidney Injury. Semin. Nephrol. 2015;35:2–11. doi: 10.1016/j.semnephrol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wan L., Bagshaw S.M., Langenberg C., Saotome T., May C., Bellomo R. Pathophysiology of Septic Acute Kidney Injury: What Do We Really Know? Crit. Care Med. 2008;36((Suppl. S4)):S198–S203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 51.Ishikawa K., May C.N., Gobe G., Langenberg C., Bellomo R. Cardiorenal Syndromes in Critical Care. Volume 165. S.Karger AG; Basel, Switzerland: 2010. Pathophysiology of Septic Acute Kidney Injury: A Different View of Tubular Injury; pp. 18–27. [DOI] [PubMed] [Google Scholar]
- 52.Takasu O., Gaut J.P., Watanabe E., To K., Fagley R.E., Sato B., Jarman S., Efimov I.R., Janks D.L., Srivastava A., et al. Mechanisms of Cardiac and Renal Dysfunction in Patients Dying of Sepsis. Am. J. Respir. Crit. Care Med. 2013;187:509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prowle J.R., Bellomo R. Sepsis-Associated Acute Kidney Injury: Macrohemodynamic and Microhemodynamic Alterations in the Renal Circulation. Semin. Nephrol. 2015;35:64–74. doi: 10.1016/j.semnephrol.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Hotchkiss R.S., Monneret G., Payen D. Immunosuppression in Sepsis: A Novel Understanding of the Disorder and a New Therapeutic Approach. Lancet Infect. Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin G.S., Mannino D.M., Moss M. The Effect of Age on the Development and Outcome of Adult Sepsis. Crit. Care Med. 2006;34:15–21. doi: 10.1097/01.CCM.0000194535.82812.BA. [DOI] [PubMed] [Google Scholar]
- 56.Reber A.J., Chirkova T., Kim J.H., Cao W., Biber R., Shay D.K., Sambhara S. Immunosenescence and Challenges of Vaccination against Influenza in the Aging Population. Aging Dis. 2012;3:68–90. [PMC free article] [PubMed] [Google Scholar]
- 57.Baghela A., Pena O.M., Lee A.H., Baquir B., Falsafi R., An A., Farmer S.W., Hurlburt A., Mondragon-Cardona A., Rivera J.D., et al. Predicting Sepsis Severity at First Clinical Presentation: The Role of Endotypes and Mechanistic Signatures. EBioMedicine. 2022;75:103776. doi: 10.1016/j.ebiom.2021.103776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rimmelé T., Payen D., Cantaluppi V., Marshall J., Gomez H., Gomez A., Murray P., Kellum J.A., ADQI XIV Workgroup Immune Cell Phenotype and Function in Sepsis. Shock. 2016;45:282–291. doi: 10.1097/SHK.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delano M.J., Ward P.A. The Immune System’s Role in Sepsis Progression, Resolution, and Long-Term Outcome. Immunol. Rev. 2016;274:330–353. doi: 10.1111/imr.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nathan C. Neutrophils and Immunity: Challenges and Opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 61.Tamayo E., Gómez E., Bustamante J., Gómez-Herreras J.I., Fonteriz R., Bobillo F., Bermejo-Martín J.F., Castrodeza J., Heredia M., Fierro I., et al. Evolution of Neutrophil Apoptosis in Septic Shock Survivors and Nonsurvivors. J. Crit. Care. 2012;27:415.e1. doi: 10.1016/j.jcrc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Drifte G., Dunn-Siegrist I., Tissières P., Pugin J. Innate Immune Functions of Immature Neutrophils in Patients with Sepsis and Severe Systemic Inflammatory Response Syndrome. Crit. Care Med. 2013;41:820–832. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- 63.Hotchkiss R.S., Nicholson D.W. Apoptosis and Caspases Regulate Death and Inflammation in Sepsis. Nat. Rev. Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 64.Daix T., Guerin E., Tavernier E., Mercier E., Gissot V., Hérault O., Mira J.-P., Dumas F., Chapuis N., Guitton C., et al. Multicentric Standardized Flow Cytometry Routine Assessment of Patients With Sepsis to Predict Clinical Worsening. Chest. 2018;154:617–627. doi: 10.1016/j.chest.2018.03.058. [DOI] [PubMed] [Google Scholar]
- 65.Guérin E., Orabona M., Raquil M.-A., Giraudeau B., Bellier R., Gibot S., Béné M.-C., Lacombe F., Droin N., Solary E., et al. Circulating Immature Granulocytes with T-Cell Killing Functions Predict Sepsis Deterioration. Crit. Care Med. 2014;42:2007–2018. doi: 10.1097/CCM.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 66.Guo R.-F., Sun L., Gao H., Shi K.X., Rittirsch D., Sarma V.J., Zetoune F.S., Ward P.A. In Vivo Regulation of Neutrophil Apoptosis by C5a during Sepsis. J. Leukoc. Biol. 2006;80:1575–1583. doi: 10.1189/jlb.0106065. [DOI] [PubMed] [Google Scholar]
- 67.Perianayagam M.C., Balakrishnan V.S., Pereira B.J.G., Jaber B.L. C5a Delays Apoptosis of Human Neutrophils via an Extracellular Signal-Regulated Kinase and Bad-Mediated Signalling Pathway. Eur. J. Clin. Investig. 2004;34:50–56. doi: 10.1111/j.1365-2362.2004.01273.x. [DOI] [PubMed] [Google Scholar]
- 68.Simon H.-U. Neutrophil Apoptosis Pathways and Their Modifications in Inflammation. Immunol. Rev. 2003;193:101–110. doi: 10.1034/j.1600-065X.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 69.Alves-Filho J.C., Spiller F., Cunha F.Q. Neutrophil Paralysis in Sepsis. Shock. 2010;34((Suppl. S1)):15–21. doi: 10.1097/SHK.0b013e3181e7e61b. [DOI] [PubMed] [Google Scholar]
- 70.Kovach M.A., Standiford T.J. The Function of Neutrophils in Sepsis. Curr. Opin. Infect. Dis. 2012;25:321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 71.Morris A.C., Brittan M., Wilkinson T.S., McAuley D.F., Antonelli J., McCulloch C., Barr L.C., McDonald N.A., Dhaliwal K., Jones R.O., et al. C5a-Mediated Neutrophil Dysfunction Is RhoA-Dependent and Predicts Infection in Critically Ill Patients. Blood. 2011;117:5178–5188. doi: 10.1182/blood-2010-08-304667. [DOI] [PubMed] [Google Scholar]
- 72.Wilkinson T.S., Conway Morris A., Kefala K., O’Kane C.M., Moore N.R., Booth N.A., McAuley D.F., Dhaliwal K., Walsh T.S., Haslett C., et al. Ventilator-Associated Pneumonia Is Characterized by Excessive Release of Neutrophil Proteases in the Lung. Chest. 2012;142:1425–1432. doi: 10.1378/chest.11-3273. [DOI] [PubMed] [Google Scholar]
- 73.Kasten K.R., Muenzer J.T., Caldwell C.C. Neutrophils Are Significant Producers of IL-10 during Sepsis. Biochem. Biophys. Res. Commun. 2010;393:28–31. doi: 10.1016/j.bbrc.2010.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McDonald B., Urrutia R., Yipp B.G., Jenne C.N., Kubes P. Intravascular Neutrophil Extracellular Traps Capture Bacteria from the Bloodstream during Sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Meng W., Paunel-Görgülü A., Flohé S., Hoffmann A., Witte I., MacKenzie C., Baldus S.E., Windolf J., Lögters T.T. Depletion of Neutrophil Extracellular Traps in Vivo Results in Hypersusceptibility to Polymicrobial Sepsis in Mice. Crit. Care. 2012;16:R137. doi: 10.1186/cc11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sørensen O.E., Borregaard N. Neutrophil Extracellular Traps—The Dark Side of Neutrophils. J. Clin. Investig. 2016;126:1612–1620. doi: 10.1172/JCI84538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Warnatsch A., Ioannou M., Wang Q., Papayannopoulos V. Neutrophil Extracellular Traps License Macrophages for Cytokine Production in Atherosclerosis. Science. 2015;349:316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borella R., De Biasi S., Paolini A., Boraldi F., Lo Tartaro D., Mattioli M., Fidanza L., Neroni A., Caro-Maldonado A., Meschiari M., et al. Metabolic Reprograming Shapes Neutrophil Functions in Severe COVID-19. Eur. J. Immunol. 2022;52:484–502. doi: 10.1002/eji.202149481. [DOI] [PubMed] [Google Scholar]
- 79.Delabranche X., Stiel L., Severac F., Galoisy A.-C., Mauvieux L., Zobairi F., Lavigne T., Toti F., Anglès-Cano E., Meziani F., et al. Evidence of Netosis in Septic Shock-Induced Disseminated Intravascular Coagulation. Shock. 2017;47:313–317. doi: 10.1097/SHK.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 80.von Brühl M.-L., Stark K., Steinhart A., Chandraratne S., Konrad I., Lorenz M., Khandoga A., Tirniceriu A., Coletti R., Köllnberger M., et al. Monocytes, Neutrophils, and Platelets Cooperate to Initiate and Propagate Venous Thrombosis in Mice in Vivo. J. Exp. Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., Wrobleski S.K., Wakefield T.W., Hartwig J.H., Wagner D.D. Extracellular DNA Traps Promote Thrombosis. Proc. Natl. Acad. Sci. USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Massberg S., Grahl L., von Bruehl M.-L., Manukyan D., Pfeiler S., Goosmann C., Brinkmann V., Lorenz M., Bidzhekov K., Khandagale A.B., et al. Reciprocal Coupling of Coagulation and Innate Immunity via Neutrophil Serine Proteases. Nat. Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 83.Shang Y.X., Zheng Z., Wang M., Guo H.X., Chen Y.J., Wu Y., Li X., Li Q., Cui J.Y., Ren X.X., et al. Diagnostic performance of Neutrophil CD64 index, procalcitonin, and C-reactive protein for early sepsis in hematological patients. World J. Clin. Cases. 2022;10:2127–2137. doi: 10.12998/wjcc.v10.i7.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davis B.H. Improved Diagnostic Approaches to Infection/Sepsis Detection. Expert Rev. Mol. Diagn. 2005;5:193–207. doi: 10.1586/14737159.5.2.193. [DOI] [PubMed] [Google Scholar]
- 85.Song S.H., Kim H.K., Park M.H., Cho H.-I. Neutrophil CD64 Expression Is Associated with Severity and Prognosis of Disseminated Intravascular Coagulation. Thromb. Res. 2008;121:499–507. doi: 10.1016/j.thromres.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 86.Patnaik R., Azim A., Agarwal V. Neutrophil CD64 a Diagnostic and Prognostic Marker of Sepsis in Adult Critically Ill Patients: A Brief Review. Indian J. Crit. Care Med. 2020;24:1242–1250. doi: 10.5005/jp-journals-10071-23558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hashem H.E., El Masry S.A., Mokhtar A.M., Ismail E.A., Abdelaal N.M. Valuable Role of Neutrophil CD64 and Highly Sensitive CRP Biomarkers for Diagnostic, Monitoring, and Prognostic Evaluations of Sepsis Patients in Neonatal ICUs. BioMed Res. Int. 2020;2020:6214363. doi: 10.1155/2020/6214363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dal Ponte S.T., Alegretti A.P., Pilger D.A., Rezende G.P., Andrioli G., Ludwig H.C., Diogo L., Goldani L.Z., Loreto M., Machado P.S., et al. Diagnostic Accuracy of CD64 for Sepsis in Emergency Department. J. Glob. Infect. Dis. 2018;10:42–46. doi: 10.4103/jgid.jgid_130_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Meer W., van Dun L., Gunnewiek J.K., Roemer B., Scott C.S. Simultaneous Determination of Membrane CD64 and HLA-DR Expression by Blood Neutrophils and Monocytes Using the Monoclonal Antibody Fluorescence Capability of a Routine Haematology Analyser. J. Immunol. Methods. 2006;311:207–219. doi: 10.1016/j.jim.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 90.Pillay J., Kamp V.M., van Hoffen E., Visser T., Tak T., Lammers J.-W., Ulfman L.H., Leenen L.P., Pickkers P., Koenderman L. A Subset of Neutrophils in Human Systemic Inflammation Inhibits T Cell Responses through Mac-1. J. Clin. Investig. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leliefeld P.H.C., Pillay J., Vrisekoop N., Heeres M., Tak T., Kox M., Rooijakkers S.H.M., Kuijpers T.W., Pickkers P., Leenen L.P.H., et al. Differential Antibacterial Control by Neutrophil Subsets. Blood Adv. 2018;2:1344–1355. doi: 10.1182/bloodadvances.2017015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fingerle G., Pforte A., Passlick B., Blumenstein M., Ströbel M., Ziegler-Heitbrock H.W. The Novel Subset of CD14+/CD16+ Blood Monocytes Is Expanded in Sepsis Patients. Blood. 1993;82:3170–3176. doi: 10.1182/blood.V82.10.3170.3170. [DOI] [PubMed] [Google Scholar]
- 93.Mukherjee R., Kanti Barman P., Kumar Thatoi P., Tripathy R., Kumar Das B., Ravindran B. Non-Classical Monocytes Display Inflammatory Features: Validation in Sepsis and Systemic Lupus Erythematous. Sci. Rep. 2015;5:13886. doi: 10.1038/srep13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hortová-Kohoutková M., Lázničková P., Bendíčková K., De Zuani M., Andrejčinová I., Tomášková V., Suk P., Šrámek V., Helán M., Frič J. Differences in Monocyte Subsets Are Associated with Short-Term Survival in Patients with Septic Shock. J. Cell. Mol. Med. 2020;24:12504–12512. doi: 10.1111/jcmm.15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bodinier M., Peronnet E., Brengel-Pesce K., Conti F., Rimmelé T., Textoris J., Vedrine C., Quemeneur L., Griffiths A.D., Tan L.K., et al. Monocyte Trajectories Endotypes Are Associated With Worsening in Septic Patients. Front. Immunol. 2021;12:795052. doi: 10.3389/fimmu.2021.795052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leijte G.P., Rimmelé T., Kox M., Bruse N., Monard C., Gossez M., Monneret G., Pickkers P., Venet F. Monocytic HLA-DR Expression Kinetics in Septic Shock Patients with Different Pathogens, Sites of Infection and Adverse Outcomes. Crit. Care. 2020;24:110. doi: 10.1186/s13054-020-2830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gouel-Chéron A., Venet F., Allaouchiche B., Monneret G. CD4+ T-Lymphocyte Alterations in Trauma Patients. Crit. Care. 2012;16:432. doi: 10.1186/cc11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Dissel J.T., van Langevelde P., Westendorp R.G., Kwappenberg K., Frölich M. Anti-Inflammatory Cytokine Profile and Mortality in Febrile Patients. Lancet. 1998;351:950–953. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 99.Monneret G., Finck M.-E., Venet F., Debard A.-L., Bohé J., Bienvenu J., Lepape A. The Anti-Inflammatory Response Dominates after Septic Shock: Association of Low Monocyte HLA-DR Expression and High Interleukin-10 Concentration. Immunol. Lett. 2004;95:193–198. doi: 10.1016/j.imlet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 100.Osuchowski M.F., Winkler M.S., Skirecki T., Cajander S., Shankar-Hari M., Lachmann G., Monneret G., Venet F., Bauer M., Brunkhorst F.M., et al. The COVID-19 Puzzle: Deciphering Pathophysiology and Phenotypes of a New Disease Entity. Lancet Respir. Med. 2021;9:622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang K., Svabek C., Vazquez-Guillamet C., Sato B., Rasche D., Wilson S., Robbins P., Ulbrandt N., Suzich J., Green J., et al. Targeting the Programmed Cell Death 1: Programmed Cell Death Ligand 1 Pathway Reverses T Cell Exhaustion in Patients with Sepsis. Crit. Care. 2014;18:R3. doi: 10.1186/cc13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guignant C., Lepape A., Huang X., Kherouf H., Denis L., Poitevin F., Malcus C., Chéron A., Allaouchiche B., Gueyffier F., et al. Programmed Death-1 Levels Correlate with Increased Mortality, Nosocomial Infection and Immune Dysfunctions in Septic Shock Patients. Crit. Care. 2011;15:R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y., Li J., Lou J., Zhou Y., Bo L., Zhu J., Zhu K., Wan X., Cai Z., Deng X. Upregulation of Programmed Death-1 on T Cells and Programmed Death Ligand-1 on Monocytes in Septic Shock Patients. Crit. Care. 2011;15:R70. doi: 10.1186/cc10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Santos S.S., Carmo A.M., Brunialti M.K.C., Machado F.R., Azevedo L.C., Assunção M., Trevelin S.C., Cunha F.Q., Salomao R. Modulation of Monocytes in Septic Patients: Preserved Phagocytic Activity, Increased ROS and NO Generation, and Decreased Production of Inflammatory Cytokines. Intensive Care Med. Exp. 2016;4:5. doi: 10.1186/s40635-016-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferreira da Mota N.V., Brunialti M.K.C., Santos S.S., Machado F.R., Assuncao M., Azevedo L.C.P., Salomao R. Immunophenotyping of Monocytes During Human Sepsis Shows Impairment in Antigen Presentation: A Shift Toward Nonclassical Differentiation and Upregulation of FCγRi-Receptor. Shock. 2018;50:293–300. doi: 10.1097/SHK.0000000000001078. [DOI] [PubMed] [Google Scholar]
- 106.Cavaillon J.-M., Adib-Conquy M. Bench-to-Bedside Review: Endotoxin Tolerance as a Model of Leukocyte Reprogramming in Sepsis. Crit. Care. 2006;10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Biswas S.K., Lopez-Collazo E. Endotoxin Tolerance: New Mechanisms, Molecules and Clinical Significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 108.Brunialti M.K.C., Martins P.S., Barbosa de Carvalho H., Machado F.R., Barbosa L.M., Salomao R. TLR2, TLR4, CD14, CD11B, and CD11C Expressions on Monocytes Surface and Cytokine Production in Patients with Sepsis, Severe Sepsis, and Septic Shock. Shock. 2006;25:351–357. doi: 10.1097/01.shk.0000217815.57727.29. [DOI] [PubMed] [Google Scholar]
- 109.Salomao R., Brunialti M.K.C., Kallás E.G., Martins P.S., Rigato O., Freudenberg M. Lipopolysaccharide-Cell Interaction and Induced Cellular Activation in Whole Blood of Septic Patients. J. Endotoxin Res. 2002;8:371–379. doi: 10.1179/096805102125000704. [DOI] [PubMed] [Google Scholar]
- 110.Martins P.S., Brunialti M.K.C., Martos L.S.W., Machado F.R., Assunçao M.S., Blecher S., Salomao R. Expression of Cell Surface Receptors and Oxidative Metabolism Modulation in the Clinical Continuum of Sepsis. Crit. Care. 2008;12:R25. doi: 10.1186/cc6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Santos S.S., Brunialti M.K.C., Rigato O., Machado F.R., Silva E., Salomao R. Generation of Nitric Oxide and Reactive Oxygen Species by Neutrophils and Monocytes from Septic Patients and Association with Outcomes. Shock. 2012;38:18–23. doi: 10.1097/SHK.0b013e318257114e. [DOI] [PubMed] [Google Scholar]
- 112.Monneret G., Venet F., Pachot A., Lepape A. Monitoring Immune Dysfunctions in the Septic Patient: A New Skin for the Old Ceremony. Mol. Med. 2008;14:64–78. doi: 10.2119/2007-00102.Monneret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Venet F., Lukaszewicz A.-C., Payen D., Hotchkiss R., Monneret G. Monitoring the Immune Response in Sepsis: A Rational Approach to Administration of Immunoadjuvant Therapies. Curr. Opin. Immunol. 2013;25:477–483. doi: 10.1016/j.coi.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu S., Luo W., Szatmary P., Zhang X., Lin J.W., Chen L., Liu D., Sutton R., Xia Q., Jin T., et al. Monocytic HLA-DR Expression in Immune Responses of Acute Pancreatitis and COVID-19. Int. J. Mol. Sci. 2023;24:3246. doi: 10.3390/ijms24043246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sinha P., Meyer N.J., Calfee C.S. Biological Phenotyping in Sepsis and Acute Respiratory Distress Syndrome. Annu. Rev. Med. 2023;74:457–471. doi: 10.1146/annurev-med-043021-014005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scicluna B.P., van Vught L.A., Zwinderman A.H., Wiewel M.A., Davenport E.E., Burnham K.L., Nürnberg P., Schultz M.J., Horn J., Cremer O.L., et al. Classification of Patients with Sepsis According to Blood Genomic Endotype: A Prospective Cohort Study. Lancet Respir. Med. 2017;5:816–826. doi: 10.1016/S2213-2600(17)30294-1. [DOI] [PubMed] [Google Scholar]
- 117.Sinistro A., Almerighi C., Ciaprini C., Natoli S., Sussarello E., Di Fino S., Calò-Carducci F., Rocchi G., Bergamini A. Downregulation of CD40 Ligand Response in Monocytes from Sepsis Patients. Clin. Vaccine Immunol. 2008;15:1851–1858. doi: 10.1128/CVI.00184-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reyes M., Filbin M.R., Bhattacharyya R.P., Billman K., Eisenhaure T., Hung D.T., Levy B.D., Baron R.M., Blainey P.C., Goldberg M.B., et al. An Immune-Cell Signature of Bacterial Sepsis. Nat. Med. 2020;26:333–340. doi: 10.1038/s41591-020-0752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chiche L., Forel J.-M., Papazian L. The Role of Viruses in Nosocomial Pneumonia. Curr. Opin. Infect. Dis. 2011;24:152–156. doi: 10.1097/QCO.0b013e328343b6e4. [DOI] [PubMed] [Google Scholar]
- 120.Souza-Fonseca-Guimaraes F., Parlato M., Philippart F., Misset B., Cavaillon J.-M., Adib-Conquy M., Captain Study Group Toll-like Receptors Expression and Interferon-γ Production by NK Cells in Human Sepsis. Crit. Care. 2012;16:R206. doi: 10.1186/cc11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weissler J.C., Nicod L.P., Lipscomb M.F., Toews G.B. Natural Killer Cell Function in Human Lung Is Compartmentalized. Am. Rev. Respir. Dis. 1987;135:941–949. doi: 10.1164/arrd.1987.135.4.941. [DOI] [PubMed] [Google Scholar]
- 122.Grégoire C., Chasson L., Luci C., Tomasello E., Geissmann F., Vivier E., Walzer T. The Trafficking of Natural Killer Cells. Immunol. Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Pablo R., Monserrat J., Torrijos C., Martín M., Prieto A., Alvarez-Mon M. The Predictive Role of Early Activation of Natural Killer Cells in Septic Shock. Crit. Care. 2012;16:413. doi: 10.1186/cc11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gogos C., Kotsaki A., Pelekanou A., Giannikopoulos G., Vaki I., Maravitsa P., Adamis S., Alexiou Z., Andrianopoulos G., Antonopoulou A., et al. Early Alterations of the Innate and Adaptive Immune Statuses in Sepsis According to the Type of Underlying Infection. Crit. Care. 2010;14:R96. doi: 10.1186/cc9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Giannikopoulos G., Antonopoulou A., Kalpakou G., Makaritsis K., Panou C., Papadomichelakis E., Sinapidis D., Theodotou A., Tzagkaraki A., Giamarellos-Bourboulis E.J. The Functional Role of Natural Killer Cells Early in Clinical Sepsis. Acta Pathol. Microbiol. Immunol. Scand. 2013;121:329–336. doi: 10.1111/apm.12002. [DOI] [PubMed] [Google Scholar]
- 126.Elemam N.M., Ramakrishnan R.K., Hundt J.E., Halwani R., Maghazachi A.A., Hamid Q. Innate Lymphoid Cells and Natural Killer Cells in Bacterial Infections: Function, Dysregulation, and Therapeutic Targets. Front. Cell. Infect. Microbiol. 2021;11:733564. doi: 10.3389/fcimb.2021.733564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nomura T., Kawamura I., Tsuchiya K., Kohda C., Baba H., Ito Y., Kimoto T., Watanabe I., Mitsuyama M. Essential Role of Interleukin-12 (IL-12) and IL-18 for Gamma Interferon Production Induced by Listeriolysin O in Mouse Spleen Cells. Infect. Immun. 2002;70:1049–1055. doi: 10.1128/IAI.70.3.1049-1055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yao C., Bora S.A., Parimon T., Zaman T., Friedman O.A., Palatinus J.A., Surapaneni N.S., Matusov Y.P., Cerro Chiang G., Kassar A.G., et al. Cell-Type-Specific Immune Dysregulation in Severely Ill COVID-19 Patients. Cell Rep. 2021;34:108590. doi: 10.1016/j.celrep.2020.108590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional Exhaustion of Antiviral Lymphocytes in COVID-19 Patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yaqinuddin A., Kashir J. Innate Immunity in COVID-19 Patients Mediated by NKG2A Receptors, and Potential Treatment Using Monalizumab, Cholroquine, and Antiviral Agents. Med. Hypotheses. 2020;140:109777. doi: 10.1016/j.mehy.2020.109777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Venet F., Davin F., Guignant C., Larue A., Cazalis M.-A., Darbon R., Allombert C., Mougin B., Malcus C., Poitevin-Later F., et al. Early Assessment of Leukocyte Alterations at Diagnosis of Septic Shock. Shock. 2010;34:358–363. doi: 10.1097/SHK.0b013e3181dc0977. [DOI] [PubMed] [Google Scholar]
- 132.Forel J.-M., Chiche L., Thomas G., Mancini J., Farnarier C., Cognet C., Guervilly C., Daumas A., Vély F., Xéridat F., et al. Phenotype and Functions of Natural Killer Cells in Critically-Ill Septic Patients. PLoS ONE. 2012;7:e50446. doi: 10.1371/journal.pone.0050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holub M., Klučková Z., Helcl M., Příhodov J., Rokyta R., Beran O. Lymphocyte Subset Numbers Depend on the Bacterial Origin of Sepsis. Clin. Microbiol. Infect. 2003;9:202–211. doi: 10.1046/j.1469-0691.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- 134.Guo Y., Patil N.K., Luan L., Bohannon J.K., Sherwood E.R. The Biology of Natural Killer Cells during Sepsis. Immunology. 2018;153:190–202. doi: 10.1111/imm.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Souza-Fonseca-Guimaraes F., Adib-Conquy M., Cavaillon J.-M. Natural Killer (NK) Cells in Antibacterial Innate Immunity: Angels or Devils? Mol. Med. 2012;18:270–285. doi: 10.2119/molmed.2011.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Souza-Fonseca-Guimaraes F., Parlato M., Fitting C., Cavaillon J.-M., Adib-Conquy M. NK Cell Tolerance to TLR Agonists Mediated by Regulatory T Cells after Polymicrobial Sepsis. J. Immunol. 2012;188:5850–5858. doi: 10.4049/jimmunol.1103616. [DOI] [PubMed] [Google Scholar]
- 137.Chiche L., Forel J.-M., Thomas G., Farnarier C., Cognet C., Guervilly C., Zandotti C., Vély F., Roch A., Vivier E., et al. Interferon-γ Production by Natural Killer Cells and Cytomegalovirus in Critically Ill Patients. Crit. Care Med. 2012;40:3162–3169. doi: 10.1097/CCM.0b013e318260c90e. [DOI] [PubMed] [Google Scholar]
- 138.Luyt C.-E., Combes A., Deback C., Aubriot-Lorton M.-H., Nieszkowska A., Trouillet J.-L., Capron F., Agut H., Gibert C., Chastre J. Herpes Simplex Virus Lung Infection in Patients Undergoing Prolonged Mechanical Ventilation. Am. J. Respir. Crit. Care Med. 2007;175:935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 139.Limaye A.P., Kirby K.A., Rubenfeld G.D., Leisenring W.M., Bulger E.M., Neff M.J., Gibran N.S., Huang M.-L., Santo Hayes T.K., Corey L., et al. Cytomegalovirus Reactivation in Critically Ill Immunocompetent Patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.2008.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cook C.H., Trgovcich J. Cytomegalovirus Reactivation in Critically Ill Immunocompetent Hosts: A Decade of Progress and Remaining Challenges. Antivir. Res. 2011;90:151–159. doi: 10.1016/j.antiviral.2011.03.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maucourant C., Filipovic I., Ponzetta A., Aleman S., Cornillet M., Hertwig L., Strunz B., Lentini A., Reinius B., Brownlie D., et al. Natural Killer Cell Immunotypes Related to COVID-19 Disease Severity. Sci. Immunol. 2020;5:eabd6832. doi: 10.1126/sciimmunol.abd6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jämsä J., Syrjälä H., Huotari V., Savolainen E.-R., Ala-Kokko T. Monocyte and Lymphocyte Surface Molecules in Severe Sepsis and Non-Septic Critically Ill Patients. Acta Pathol. Microbiol. Immunol. Scand. 2017;125:536–543. doi: 10.1111/apm.12670. [DOI] [PubMed] [Google Scholar]
- 143.Vantourout P., Hayday A. Six-of-the-Best: Unique Contributions of Γδ T Cells to Immunology. Nat. Rev. Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee H.W., Chung Y.S., Kim T.J. Heterogeneity of Human Γδ T Cells and Their Role in Cancer Immunity. Immune Netw. 2020;20:e5. doi: 10.4110/in.2020.20.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ogiku M., Kono H., Hara M., Tsuchiya M., Fujii H. Interleukin-17A Plays a Pivotal Role in Polymicrobial Sepsis According to Studies Using IL-17A Knockout Mice. J. Surg. Res. 2012;174:142–149. doi: 10.1016/j.jss.2010.11.901. [DOI] [PubMed] [Google Scholar]
- 146.de Souza Costa M.F., Bastos Trigo de Negreiros C., Ugarte Bornstein V., Hemmi Valente R., Mengel J., Henriques M.d.G., Farias Benjamim C., Penido C. Murine IL-17+ Vγ4 T Lymphocytes Accumulate in the Lungs and Play a Protective Role during Severe Sepsis. BMC Immunol. 2015;16:36. doi: 10.1186/s12865-015-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liao X.-L., Feng T., Zhang J.-Q., Cao X., Wu Q.-H., Xie Z.-C., Kang Y., Li H. Phenotypic Changes and Impaired Function of Peripheral Γδ T Cells in Patients With Sepsis. Shock. 2017;48:321–328. doi: 10.1097/SHK.0000000000000857. [DOI] [PubMed] [Google Scholar]
- 148.Andreu-Ballester J.C., Arribas M.A., Rico M., García-Ballesteros C., Galindo-Regal L., Sorando-Serra R., Albert L., Navarro A., López-Chuliá F., Peydró F., et al. Changes of CD3+CD56+ Γδ T Cell Number and Apoptosis during Hospital Admission Are Related to Mortality in Septic Patients. Clin. Immunol. 2022;236:108956. doi: 10.1016/j.clim.2022.108956. [DOI] [PubMed] [Google Scholar]
- 149.Cheng Z., Abrams S.T., Toh J., Wang S.S., Wang Z., Yu Q., Yu W., Toh C.H., Wang G. The Critical Roles and Mechanisms of Immune Cell Death in Sepsis. Front. Immunol. 2020;11:1918. doi: 10.3389/fimmu.2020.01918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Andreu-Ballester J.C., Tormo-Calandín C., Garcia-Ballesteros C., Pérez-Griera J., Amigó V., Almela-Quilis A., Ruiz del Castillo J., Peñarroja-Otero C., Ballester F. Association of Γδ T Cells with Disease Severity and Mortality in Septic Patients. Clin. Vaccine Immunol. 2013;20:738–746. doi: 10.1128/CVI.00752-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Treiner E., Duban L., Bahram S., Radosavljevic M., Wanner V., Tilloy F., Affaticati P., Gilfillan S., Lantz O. Selection of Evolutionarily Conserved Mucosal-Associated Invariant T Cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 152.Kjer-Nielsen L., Patel O., Corbett A.J., Le Nours J., Meehan B., Liu L., Bhati M., Chen Z., Kostenko L., Reantragoon R., et al. MR1 Presents Microbial Vitamin B Metabolites to MAIT Cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 153.Corbett A.J., Eckle S.B.G., Birkinshaw R.W., Liu L., Patel O., Mahony J., Chen Z., Reantragoon R., Meehan B., Cao H., et al. T-Cell Activation by Transitory Neo-Antigens Derived from Distinct Microbial Pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 154.De Biasi S., Gibellini L., Lo Tartaro D., Puccio S., Rabacchi C., Mazza E.M.C., Brummelman J., Williams B., Kaihara K., Forcato M., et al. Circulating Mucosal-Associated Invariant T Cells Identify Patients Responding to Anti-PD-1 Therapy. Nat. Commun. 2021;12:1669. doi: 10.1038/s41467-021-21928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Godfrey D.I., Koay H.-F., McCluskey J., Gherardin N.A. The Biology and Functional Importance of MAIT Cells. Nat. Immunol. 2019;20:1110–1128. doi: 10.1038/s41590-019-0444-8. [DOI] [PubMed] [Google Scholar]
- 156.Martin E., Treiner E., Duban L., Guerri L., Laude H., Toly C., Premel V., Devys A., Moura I.C., Tilloy F., et al. Stepwise Development of MAIT Cells in Mouse and Human. PLoS Biol. 2009;7:e1000054. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ibidapo-Obe O., Stengel S., Köse-Vogel N., Quickert S., Reuken P.A., Busch M., Bauer M., Stallmach A., Bruns T. Mucosal-Associated Invariant T Cells Redistribute to the Peritoneal Cavity During Spontaneous Bacterial Peritonitis and Contribute to Peritoneal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2020;9:661–677. doi: 10.1016/j.jcmgh.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Trivedi S., Labuz D., Anderson C.P., Araujo C.V., Blair A., Middleton E.A., Jensen O., Tran A., Mulvey M.A., Campbell R.A., et al. Mucosal-Associated Invariant T (MAIT) Cells Mediate Protective Host Responses in Sepsis. eLife. 2020;9:e55615. doi: 10.7554/eLife.55615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tian L., Xu J., Chen C., Lin J., Ju L., Chen L., Zhang Y., Han X., Liu L. HLA-DR+ Mucosal-Associated Invariant T Cells Predict Poor Prognosis in Patients with Sepsis: A Prospective Observational Study. Scand. J. Immunol. 2023;98:e13286. doi: 10.1111/sji.13286. [DOI] [PubMed] [Google Scholar]
- 160.Gutcher I., Becher B. APC-Derived Cytokines and T Cell Polarization in Autoimmune Inflammation. J. Clin. Investig. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cheadle W.G., Pemberton R.M., Robinson D., Livingston D.H., Rodriguez J.L., Polk H.C. Lymphocyte Subset Responses to Trauma and Sepsis. J. Trauma. 1993;35:844–849. doi: 10.1097/00005373-199312000-00007. [DOI] [PubMed] [Google Scholar]
- 162.Heffernan D.S., Monaghan S.F., Thakkar R.K., Machan J.T., Cioffi W.G., Ayala A. Failure to Normalize Lymphopenia Following Trauma Is Associated with Increased Mortality, Independent of the Leukocytosis Pattern. Crit. Care. 2012;16:R12. doi: 10.1186/cc11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hotchkiss R.S., Sherwood E.R. Getting Sepsis Therapy Right. Science. 2015;347:1201–1202. doi: 10.1126/science.aaa8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Inoue S., Suzuki-Utsunomiya K., Okada Y., Taira T., Iida Y., Miura N., Tsuji T., Yamagiwa T., Morita S., Chiba T., et al. Reduction of Immunocompetent T Cells Followed by Prolonged Lymphopenia in Severe Sepsis in the Elderly. Crit. Care Med. 2013;41:810–819. doi: 10.1097/CCM.0b013e318274645f. [DOI] [PubMed] [Google Scholar]
- 165.Le Tulzo Y., Pangault C., Gacouin A., Guilloux V., Tribut O., Amiot L., Tattevin P., Thomas R., Fauchet R., Drénou B. Early Circulating Lymphocyte Apoptosis in Human Septic Shock Is Associated with Poor Outcome. Shock. 2002;18:487–494. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 166.Brummelman J., Pilipow K., Lugli E. International Review of Cell and Molecular Biology. Volume 341. Elsevier; Amsterdam, The Netherlands: 2018. The Single-Cell Phenotypic Identity of Human CD8+ and CD4+ T Cells; pp. 63–124. [DOI] [PubMed] [Google Scholar]
- 167.Boomer J.S., To K., Chang K.C., Takasu O., Osborne D.F., Walton A.H., Bricker T.L., Jarman S.D., Kreisel D., Krupnick A.S., et al. Immunosuppression in Patients Who Die of Sepsis and Multiple Organ Failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hotchkiss R.S., Tinsley K.W., Swanson P.E., Schmieg R.E., Hui J.J., Chang K.C., Osborne D.F., Freeman B.D., Cobb J.P., Buchman T.G., et al. Sepsis-Induced Apoptosis Causes Progressive Profound Depletion of B and CD4+ T Lymphocytes in Humans. J. Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 169.O’Sullivan S.T., Lederer J.A., Horgan A.F., Chin D.H., Mannick J.A., Rodrick M.L. Major Injury Leads to Predominance of the T Helper-2 Lymphocyte Phenotype and Diminished Interleukin-12 Production Associated with Decreased Resistance to Infection. Ann. Surg. 1995;222:482–490; discussion 490–492. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pachot A., Monneret G., Voirin N., Leissner P., Venet F., Bohé J., Payen D., Bienvenu J., Mougin B., Lepape A. Longitudinal Study of Cytokine and Immune Transcription Factor mRNA Expression in Septic Shock. Clin. Immunol. 2005;114:61–69. doi: 10.1016/j.clim.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 171.Smeekens S.P., Ng A., Kumar V., Johnson M.D., Plantinga T.S., van Diemen C., Arts P., Verwiel E.T.P., Gresnigt M.S., Fransen K., et al. Functional Genomics Identifies Type I Interferon Pathway as Central for Host Defense against Candida albicans. Nat. Commun. 2013;4:1342. doi: 10.1038/ncomms2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Unsinger J., McGlynn M., Kasten K.R., Hoekzema A.S., Watanabe E., Muenzer J.T., McDonough J.S., Tschoep J., Ferguson T.A., McDunn J.E., et al. IL-7 Promotes T Cell Viability, Trafficking, and Functionality and Improves Survival in Sepsis. J. Immunol. 2010;184:3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Venet F., Monneret G. Advances in the Understanding and Treatment of Sepsis-Induced Immunosuppression. Nat. Rev. Nephrol. 2018;14:121–137. doi: 10.1038/nrneph.2017.165. [DOI] [PubMed] [Google Scholar]
- 174.Boomer J.S., Shuherk-Shaffer J., Hotchkiss R.S., Green J.M. A Prospective Analysis of Lymphocyte Phenotype and Function over the Course of Acute Sepsis. Crit. Care. 2012;16:R112. doi: 10.1186/cc11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takahama M., Patil A., Richey G., Cipurko D., Johnson K., Carbonetto P., Plaster M., Pandey S., Cheronis K., Ueda T., et al. A pairwise cytokine code explains the organism-wide response to sepsis. Nat. Immunol. 2024;25:226–239. doi: 10.1038/s41590-023-01722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Avendaño-Ortiz J., Lozano-Rodríguez R., Martín-Quirós A., Maroun-Eid C., Terrón V., Valentín J., Montalbán-Hernández K., Ruiz de la Bastida F., García-Garrido M.A., Cubillos-Zapata C., et al. Proteins from SARS-CoV-2 Reduce T Cell Proliferation: A Mirror Image of Sepsis. Heliyon. 2020;6:e05635. doi: 10.1016/j.heliyon.2020.e05635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kaech S.M., Hemby S., Kersh E., Ahmed R. Molecular and Functional Profiling of Memory CD8 T Cell Differentiation. Cell. 2002;111:837–851. doi: 10.1016/S0092-8674(02)01139-X. [DOI] [PubMed] [Google Scholar]
- 178.Brady J., Horie S., Laffey J.G. Role of the Adaptive Immune Response in Sepsis. Intensive Care Med. Exp. 2020;8:20. doi: 10.1186/s40635-020-00309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lozano-Rodríguez R., Avendaño-Ortíz J., Montalbán-Hernández K., Ruiz-Rodríguez J.C., Ferrer R., Martín-Quirós A., Maroun-Eid C., González-López J.J., Fàbrega A., Terrón-Arcos V., et al. The Prognostic Impact of SIGLEC5-Induced Impairment of CD8+ T Cell Activation in Sepsis. eBioMedicine. 2023;97:104841. doi: 10.1016/j.ebiom.2023.104841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Berton R.R., McGonagil P.W., Jensen I.J., Ybarra T.K., Bishop G.A., Harty J.T., Griffith T.S., Badovinac V.P. Sepsis Leads to Lasting Changes in Phenotype and Function of Naïve CD8 T Cells. PLoS Pathog. 2023;19:e1011720. doi: 10.1371/journal.ppat.1011720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Walton A.H., Muenzer J.T., Rasche D., Boomer J.S., Sato B., Brownstein B.H., Pachot A., Brooks T.L., Deych E., Shannon W.D., et al. Reactivation of Multiple Viruses in Patients with Sepsis. PLoS ONE. 2014;9:e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gatto I., Biagioni E., Coloretti I., Farinelli C., Avoni C., Caciagli V., Busani S., Sarti M., Pecorari M., Gennari W., et al. Cytomegalovirus Blood Reactivation in COVID-19 Critically Ill Patients: Risk Factors and Impact on Mortality. Intensive Care Med. 2022;48:706–713. doi: 10.1007/s00134-022-06716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Choi Y.J., Kim S.B., Kim J.H., Park S.-H., Park M.S., Kim J.M., Han S.H., Shin E.-C. Impaired Polyfunctionality of CD8+ T Cells in Severe Sepsis Patients with Human Cytomegalovirus Reactivation. Exp. Mol. Med. 2017;49:e382. doi: 10.1038/emm.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cao C., Chai Y., Shou S., Wang J., Huang Y., Ma T. Toll-like Receptor 4 Deficiency Increases Resistance in Sepsis-Induced Immune Dysfunction. Int. Immunopharmacol. 2018;54:169–176. doi: 10.1016/j.intimp.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 185.Venet F., Chung C.-S., Kherouf H., Geeraert A., Malcus C., Poitevin F., Bohé J., Lepape A., Ayala A., Monneret G. Increased Circulating Regulatory T Cells (CD4+CD25+CD127−) Contribute to Lymphocyte Anergy in Septic Shock Patients. Intensive Care Med. 2009;35:678–686. doi: 10.1007/s00134-008-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Venet F., Pachot A., Debard A.-L., Bohé J., Bienvenu J., Lepape A., Monneret G. Increased Percentage of CD4+CD25+ Regulatory T Cells during Septic Shock Is Due to the Decrease of CD4+CD25− Lymphocytes. Crit. Care Med. 2004;32:2329–2331. doi: 10.1097/01.CCM.0000145999.42971.4B. [DOI] [PubMed] [Google Scholar]
- 187.Brinkhoff A., Sieberichs A., Engler H., Dolff S., Benson S., Korth J., Schedlowski M., Kribben A., Witzke O., Wilde B. Pro-Inflammatory Th1 and Th17 Cells Are Suppressed During Human Experimental Endotoxemia Whereas Anti-Inflammatory IL-10 Producing T-Cells Are Unaffected. Front. Immunol. 2018;9:1133. doi: 10.3389/fimmu.2018.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Conway Morris A., Datta D., Shankar-Hari M., Stephen J., Weir C.J., Rennie J., Antonelli J., Bateman A., Warner N., Judge K., et al. Cell-Surface Signatures of Immune Dysfunction Risk-Stratify Critically Ill Patients: INFECT Study. Intensive Care Med. 2018;44:627–635. doi: 10.1007/s00134-018-5247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gustave C.-A., Gossez M., Demaret J., Rimmelé T., Lepape A., Malcus C., Poitevin-Later F., Jallades L., Textoris J., Monneret G., et al. Septic Shock Shapes B Cell Response toward an Exhausted-like/Immunoregulatory Profile in Patients. J. Immunol. 2018;200:2418–2425. doi: 10.4049/jimmunol.1700929. [DOI] [PubMed] [Google Scholar]
- 190.Andaluz-Ojeda D., Iglesias V., Bobillo F., Almansa R., Rico L., Gandía F., Loma A.M., Nieto C., Diego R., Ramos E., et al. Early Natural Killer Cell Counts in Blood Predict Mortality in Severe Sepsis. Crit. Care. 2011;15:R243. doi: 10.1186/cc10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Monserrat J., de Pablo R., Diaz-Martín D., Rodríguez-Zapata M., de la Hera A., Prieto A., Alvarez-Mon M. Early Alterations of B Cells in Patients with Septic Shock. Crit. Care. 2013;17:R105. doi: 10.1186/cc12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Suzuki K., Inoue S., Kametani Y., Komori Y., Chiba S., Sato T., Inokuchi S., Ogura S. Reduced Immunocompetent B Cells and Increased Secondary Infection in Elderly Patients With Severe Sepsis. Shock. 2016;46:270–278. doi: 10.1097/SHK.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 193.Wilson J.K., Zhao Y., Singer M., Spencer J., Shankar-Hari M. Lymphocyte Subset Expression and Serum Concentrations of PD-1/PD-L1 in Sepsis—Pilot Study. Crit. Care. 2018;22:95. doi: 10.1186/s13054-018-2020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Brinkhoff A., Zeng Y., Sieberichs A., Dolff S., Shilei X., Sun M., Engler H., Benson S., Korth J., Schedlowski M., et al. B-Cell Dynamics during Experimental Endotoxemia in Humans. Biosci. Rep. 2019;39:BSR20182347. doi: 10.1042/BSR20182347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dong X., Liu Q., Zheng Q., Liu X., Wang Y., Xie Z., Liu T., Yang F., Gao W., Bai X., et al. Alterations of B Cells in Immunosuppressive Phase of Septic Shock Patients. Crit. Care Med. 2020;48:815–821. doi: 10.1097/CCM.0000000000004309. [DOI] [PubMed] [Google Scholar]
- 196.Duan S., Jiao Y., Wang J., Tang D., Xu S., Wang R., Jiang T., Shao J., He Z., Yu W. Impaired B-Cell Maturation Contributes to Reduced B Cell Numbers and Poor Prognosis in Sepsis. Shock. 2020;54:70–77. doi: 10.1097/SHK.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 197.Tian L., Zhu J., Jin J., Tong C., Zeng W., Deng S., Zou S. Prognostic Value of Circulating Lymphocyte B and Plasma Immunoglobulin M on Septic Shock and Sepsis: A Systematic Review and Meta-Analysis. Am. J. Transl. Res. 2019;11:7223–7232. [PMC free article] [PubMed] [Google Scholar]
- 198.Krautz C., Maier S.L., Brunner M., Langheinrich M., Giamarellos-Bourboulis E.J., Gogos C., Armaganidis A., Kunath F., Grützmann R., Weber G.F. Reduced Circulating B Cells and Plasma IgM Levels Are Associated with Decreased Survival in Sepsis—A Meta-Analysis. J. Crit. Care. 2018;45:71–75. doi: 10.1016/j.jcrc.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 199.Hohlstein P., Gussen H., Bartneck M., Warzecha K.T., Roderburg C., Buendgens L., Trautwein C., Koch A., Tacke F. Prognostic Relevance of Altered Lymphocyte Subpopulations in Critical Illness and Sepsis. J. Clin. Med. 2019;8:353. doi: 10.3390/jcm8030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schenz J., Tamulyte S., Nusshag C., Brenner T., Poschet G., Weigand M.A., Uhle F. Population-Specific Metabolic Alterations in Professional Antigen-Presenting Cells Contribute to Sepsis-Associated Immunosuppression. Shock. 2020;53:5–15. doi: 10.1097/SHK.0000000000001337. [DOI] [PubMed] [Google Scholar]
- 201.Hotchkiss R.S., Swanson P.E., Cobb J.P., Jacobson A., Buchman T.G., Karl I.E. Apoptosis in Lymphoid and Parenchymal Cells during Sepsis: Findings in Normal and T- and B-Cell-Deficient Mice. Crit. Care Med. 1997;25:1298–1307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 202.Ayala A., Xin Xu Y., Ayala C.A., Sonefeld D.E., Karr S.M., Evans T.A., Chaudry I.H. Increased Mucosal B-Lymphocyte Apoptosis during Polymicrobial Sepsis Is a Fas Ligand but Not an Endotoxin-Mediated Process. Blood. 1998;91:1362–1372. doi: 10.1182/blood.V91.4.1362. [DOI] [PubMed] [Google Scholar]
- 203.Hotchkiss R.S., Swanson P.E., Freeman B.D., Tinsley K.W., Cobb J.P., Matuschak G.M., Buchman T.G., Karl I.E. Apoptotic Cell Death in Patients with Sepsis, Shock, and Multiple Organ Dysfunction. Crit. Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 204.Hotchkiss R.S., Osmon S.B., Chang K.C., Wagner T.H., Coopersmith C.M., Karl I.E. Accelerated Lymphocyte Death in Sepsis Occurs by Both the Death Receptor and Mitochondrial Pathways. J. Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 205.Ordoñez C., Savage H.P., Tarajia M., Rivera R., Weeks-Galindo C., Sambrano D., Riley L., Fernandez P.L., Baumgarth N., Goodridge A. Both B-1a and B-1b Cells Exposed to Mycobacterium tuberculosis Lipids Differentiate into IgM Antibody-Secreting Cells. Immunology. 2018;154:613–623. doi: 10.1111/imm.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Smith F.L., Baumgarth N. B-1 Cell Responses to Infections. Curr. Opin. Immunol. 2019;57:23–31. doi: 10.1016/j.coi.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Aziz M., Holodick N.E., Rothstein T.L., Wang P. The Role of B-1 Cells in Inflammation. Immunol. Res. 2015;63:153–166. doi: 10.1007/s12026-015-8708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Girardis M., Cossarizza A. Early Alterations of B Cells in Patients with Septic Shock: Another Piece in the Complex Puzzle of the Immune Response in Sepsis. Crit. Care. 2013;17:162. doi: 10.1186/cc12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ma C., Liu H., Yang S., Li H., Liao X., Kang Y. The Emerging Roles and Therapeutic Potential of B Cells in Sepsis. Front. Pharmacol. 2022;13:1034667. doi: 10.3389/fphar.2022.1034667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Knippenberg S., Peelen E., Smolders J., Thewissen M., Menheere P., Cohen Tervaert J.W., Hupperts R., Damoiseaux J. Reduction in IL-10 Producing B Cells (Breg) in Multiple Sclerosis Is Accompanied by a Reduced Naïve/Memory Breg Ratio during a Relapse but Not in Remission. J. Neuroimmunol. 2011;239:80–86. doi: 10.1016/j.jneuroim.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 211.Wang C., Xu H., Gao R., Leng F., Huo F., Li Y., Liu S., Xu M., Bai J. CD19+CD24hiCD38hi Regulatory B Cells Deficiency Revealed Severity and Poor Prognosis in Patients with Sepsis. BMC Immunol. 2022;23:54. doi: 10.1186/s12865-022-00528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Giamarellos-Bourboulis E.J., Apostolidou E., Lada M., Perdios I., Gatselis N.K., Tsangaris I., Georgitsi M., Bristianou M., Kanni T., Sereti K., et al. Kinetics of Circulating Immunoglobulin M in Sepsis: Relationship with Final Outcome. Crit. Care. 2013;17:R247. doi: 10.1186/cc13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Akatsuka M., Tatsumi H., Sonoda T., Masuda Y. Low Immunoglobulin G Level Is Associated with Poor Outcomes in Patients with Sepsis and Septic Shock. J. Microbiol. Immunol. Infect. 2021;54:728–732. doi: 10.1016/j.jmii.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 214.Alagna L., Meessen J.M.T.A., Bellani G., Albiero D., Caironi P., Principale I., Vivona L., Grasselli G., Motta F., Agnelli N.M., et al. Higher Levels of IgA and IgG at Sepsis Onset Are Associated with Higher Mortality: Results from the Albumin Italian Outcome Sepsis (ALBIOS) Trial. Ann. Intensive Care. 2021;11:161. doi: 10.1186/s13613-021-00952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chang L.-Y., Li Y., Kaplan D.E. Endotoxemia Contributes to CD27+ Memory B-Cell Apoptosis via Enhanced Sensitivity to Fas Ligation in Patients with Cirrhosis. Sci. Rep. 2016;6:36862. doi: 10.1038/srep36862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chung C.-S., Xu Y.X., Wang W., Chaudry I.H., Ayala A. Is Fas Ligand or Endotoxin Responsible for Mucosal Lymphocyte Apoptosis in Sepsis? Arch. Surg. 1998;133:1213–1220. doi: 10.1001/archsurg.133.11.1213. [DOI] [PubMed] [Google Scholar]
- 217.Chung C.S., Wang W., Chaudry I.H., Ayala A. Increased Apoptosis in Lamina Propria B Cells during Polymicrobial Sepsis Is FasL but Not Endotoxin Mediated. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G812–G818. doi: 10.1152/ajpgi.2001.280.5.G812. [DOI] [PubMed] [Google Scholar]
- 218.Rauch P.J., Chudnovskiy A., Robbins C.S., Weber G.F., Etzrodt M., Hilgendorf I., Tiglao E., Figueiredo J.-L., Iwamoto Y., Theurl I., et al. Innate Response Activator B Cells Protect against Microbial Sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Weber G.F., Chousterman B.G., He S., Fenn A.M., Nairz M., Anzai A., Brenner T., Uhle F., Iwamoto Y., Robbins C.S., et al. Interleukin-3 Amplifies Acute Inflammation and Is a Potential Therapeutic Target in Sepsis. Science. 2015;347:1260–1265. doi: 10.1126/science.aaa4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Honda S.-I., Sato K., Totsuka N., Fujiyama S., Fujimoto M., Miyake K., Nakahashi-Oda C., Tahara-Hanaoka S., Shibuya K., Shibuya A. Marginal Zone B Cells Exacerbate Endotoxic Shock via Interleukin-6 Secretion Induced by Fcα/μR-Coupled TLR4 Signalling. Nat. Commun. 2016;7:11498. doi: 10.1038/ncomms11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tsay G.J., Zouali M. The Interplay Between Innate-Like B Cells and Other Cell Types in Autoimmunity. Front. Immunol. 2018;9:1064. doi: 10.3389/fimmu.2018.01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lo L.-W., Chang C.-W., Chiang M.-F., Lin I.-Y., Lin K.-I. Marginal Zone B Cells Assist With Neutrophil Accumulation to Fight Against Systemic Staphylococcus aureus Infection. Front. Immunol. 2021;12:636818. doi: 10.3389/fimmu.2021.636818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.MacIver N.J., Michalek R.D., Rathmell J.C. Metabolic Regulation of T Lymphocytes. Annu. Rev. Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Stienstra R., Netea-Maier R.T., Riksen N.P., Joosten L.A.B., Netea M.G. Specific and Complex Reprogramming of Cellular Metabolism in Myeloid Cells during Innate Immune Responses. Cell Metab. 2017;26:142–156. doi: 10.1016/j.cmet.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 225.Singer M., Brealey D. Mitochondrial Dysfunction in Sepsis. Biochem. Soc. Symp. 1999;66:149–166. doi: 10.1042/bss0660149. [DOI] [PubMed] [Google Scholar]
- 226.Van den Berghe G., de Zegher F., Bouillon R. Acute and Prolonged Critical Illness as Different Neuroendocrine Paradigms. J. Clin. Endocrinol. Metab. 1998;83:1827–1834. doi: 10.1210/jc.83.6.1827. [DOI] [PubMed] [Google Scholar]
- 227.Singer M. Mitochondrial Function in Sepsis: Acute Phase versus Multiple Organ Failure. Crit. Care Med. 2007;35((Suppl. S9)):S441–S448. doi: 10.1097/01.CCM.0000278049.48333.78. [DOI] [PubMed] [Google Scholar]
- 228.Kushimoto S., Akaishi S., Sato T., Nomura R., Fujita M., Kudo D., Kawazoe Y., Yoshida Y., Miyagawa N. Lactate, a Useful Marker for Disease Mortality and Severity but an Unreliable Marker of Tissue Hypoxia/Hypoperfusion in Critically Ill Patients. Acute Med. Surg. 2016;3:293–297. doi: 10.1002/ams2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Moran J.L., Santamaria J. Reconsidering Lactate as a Sepsis Risk Biomarker. PLoS ONE. 2017;12:e0185320. doi: 10.1371/journal.pone.0185320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sauer C.M., Gómez J., Botella M.R., Ziehr D.R., Oldham W.M., Gavidia G., Rodríguez A., Elbers P., Girbes A., Bodi M., et al. Understanding Critically Ill Sepsis Patients with Normal Serum Lactate Levels: Results from U.S. and European ICU Cohorts. Sci. Rep. 2021;11:20076. doi: 10.1038/s41598-021-99581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang H., Feng Y.-W., Yao Y.-M. Potential Therapy Strategy: Targeting Mitochondrial Dysfunction in Sepsis. Mil. Med. Res. 2018;5:41. doi: 10.1186/s40779-018-0187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Singer M. The Role of Mitochondrial Dysfunction in Sepsis-Induced Multi-Organ Failure. Virulence. 2014;5:66–72. doi: 10.4161/viru.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Correa T., Jakob S., Takala J. Mitochondrial Function in Sepsis. Crit. Care Horiz. 2015;1:31. [Google Scholar]
- 234.Fink M.P. Bench-to-Bedside Review: Cytopathic Hypoxia. Crit. Care. 2002;6:491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Larsen F.J., Schiffer T.A., Weitzberg E., Lundberg J.O. Regulation of Mitochondrial Function and Energetics by Reactive Nitrogen Oxides. Free Radic. Biol. Med. 2012;53:1919–1928. doi: 10.1016/j.freeradbiomed.2012.08.580. [DOI] [PubMed] [Google Scholar]
- 236.Carré J.E., Orban J.-C., Re L., Felsmann K., Iffert W., Bauer M., Suliman H.B., Piantadosi C.A., Mayhew T.M., Breen P., et al. Survival in Critical Illness Is Associated with Early Activation of Mitochondrial Biogenesis. Am. J. Respir. Crit. Care Med. 2010;182:745–751. doi: 10.1164/rccm.201003-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Haden D.W., Suliman H.B., Carraway M.S., Welty-Wolf K.E., Ali A.S., Shitara H., Yonekawa H., Piantadosi C.A. Mitochondrial Biogenesis Restores Oxidative Metabolism during Staphylococcus aureus Sepsis. Am. J. Respir. Crit. Care Med. 2007;176:768–777. doi: 10.1164/rccm.200701-161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Singer M., De Santis V., Vitale D., Jeffcoate W. Multiorgan Failure Is an Adaptive, Endocrine-Mediated, Metabolic Response to Overwhelming Systemic Inflammation. Lancet. 2004;364:545–548. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 239.Arulkumaran N., Deutschman C.S., Pinsky M.R., Zuckerbraun B., Schumacker P.T., Gomez H., Gomez A., Murray P., Kellum J.A. Mitochondrial Function in Sepsis. Shock. 2016;45:271–281. doi: 10.1097/SHK.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Vary T.C., Drnevich D., Jurasinski C., Brennan W.A. Mechanisms Regulating Skeletal Muscle Glucose Metabolism in Sepsis. Shock. 1995;3:403–410. [PubMed] [Google Scholar]
- 241.Zhang Q., Hu Y., Zhang J., Deng C. iTRAQ-Based Proteomic Analysis of Endotoxin Tolerance Induced by Lipopolysaccharide. Mol. Med. Rep. 2019;20:584–592. doi: 10.3892/mmr.2019.10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Morris M., Li L. Molecular Mechanisms and Pathological Consequences of Endotoxin Tolerance and Priming. Arch. Immunol. Ther. Exp. 2012;60:13–18. doi: 10.1007/s00005-011-0155-9. [DOI] [PubMed] [Google Scholar]
- 243.Liu T.F., Vachharajani V.T., Yoza B.K., McCall C.E. NAD+-Dependent Sirtuin 1 and 6 Proteins Coordinate a Switch from Glucose to Fatty Acid Oxidation during the Acute Inflammatory Response. J. Biol. Chem. 2012;287:25758–25769. doi: 10.1074/jbc.M112.362343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Papathanakos G., Andrianopoulos I., Xenikakis M., Papathanasiou A., Koulenti D., Blot S., Koulouras V. Clinical Sepsis Phenotypes in Critically Ill Patients. Microorganisms. 2023;11:2165. doi: 10.3390/microorganisms11092165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wong H.R. Personalized Medicine, Endotypes, and Intensive Care Medicine. Intensive Care Med. 2015;41:1138–1140. doi: 10.1007/s00134-015-3812-3. [DOI] [PubMed] [Google Scholar]
- 246.Rhee C., Kadri S.S., Danner R.L., Suffredini A.F., Massaro A.F., Kitch B.T., Lee G., Klompas M. Diagnosing Sepsis Is Subjective and Highly Variable: A Survey of Intensivists Using Case Vignettes. Crit. Care. 2016;20:89. doi: 10.1186/s13054-016-1266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kellum J.A., Formeck C.L., Kernan K.F., Gómez H., Carcillo J.A. Subtypes and Mimics of Sepsis. Crit. Care Clin. 2022;38:195–211. doi: 10.1016/j.ccc.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shah F.A., Meyer N.J., Angus D.C., Awdish R., Azoulay É., Calfee C.S., Clermont G., Gordon A.C., Kwizera A., Leligdowicz A., et al. A Research Agenda for Precision Medicine in Sepsis and Acute Respiratory Distress Syndrome: An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2021;204:891–901. doi: 10.1164/rccm.202108-1908ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., Machado F.R., Mcintyre L., Ostermann M., Prescott H.C., et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Castela Forte J., Perner A., van der Horst I.C.C. The Use of Clustering Algorithms in Critical Care Research to Unravel Patient Heterogeneity. Intensive Care Med. 2019;45:1025–1028. doi: 10.1007/s00134-019-05631-z. [DOI] [PubMed] [Google Scholar]
- 251.Vincent J.-L., Singer M., Einav S., Moreno R., Wendon J., Teboul J.-L., Bakker J., Hernandez G., Annane D., de Man A.M.E., et al. Equilibrating SSC Guidelines with Individualized Care. Crit. Care. 2021;25:397. doi: 10.1186/s13054-021-03813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., Machado F.R., Mcintyre L., Ostermann M., Prescott H.C., et al. Executive Summary: Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021;49:1974–1982. doi: 10.1097/CCM.0000000000005357. [DOI] [PubMed] [Google Scholar]
- 253.Seymour C.W., Gomez H., Chang C.-C.H., Clermont G., Kellum J.A., Kennedy J., Yende S., Angus D.C. Precision Medicine for All? Challenges and Opportunities for a Precision Medicine Approach to Critical Illness. Crit. Care. 2017;21:257. doi: 10.1186/s13054-017-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Beutler B., Du X., Hoebe K. From Phenomenon to Phenotype and from Phenotype to Gene: Forward Genetics and the Problem of Sepsis. J. Infect. Dis. 2003;187((Suppl. S2)):S321–S326. doi: 10.1086/374757. [DOI] [PubMed] [Google Scholar]
- 255.Ward N., Levy M. Sepsis: Definitions, Pathophysiology and the Challenge of Bedside Management. Humana; Cham, Switzerland: 2017. [DOI] [Google Scholar]
- 256.Ke X., Zhang F., Huang G., Wang A. Interpretable Machine Learning to Optimize Early In-Hospital Mortality Prediction for Elderly Patients with Sepsis: A Discovery Study. Comput. Math. Methods Med. 2022;2022:4820464. doi: 10.1155/2022/4820464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vranas K.C., Jopling J.K., Sweeney T.E., Ramsey M.C., Milstein A.S., Slatore C.G., Escobar G.J., Liu V.X. Identifying Distinct Subgroups of ICU Patients: A Machine Learning Approach. Crit. Care Med. 2017;45:1607–1615. doi: 10.1097/CCM.0000000000002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Qin Y., Caldino Bohn R.I., Sriram A., Kernan K.F., Carcillo J.A., Kim S., Park H.J. Refining Empiric Subgroups of Pediatric Sepsis Using Machine-Learning Techniques on Observational Data. Front. Pediatr. 2023;11:1035576. doi: 10.3389/fped.2023.1035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Komorowski M., Green A., Tatham K.C., Seymour C., Antcliffe D. Sepsis Biomarkers and Diagnostic Tools with a Focus on Machine Learning. EBioMedicine. 2022;86:104394. doi: 10.1016/j.ebiom.2022.104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Li H., Markal A., Balch J.A., Loftus T.J., Efron P.A., Ozrazgat-Baslanti T., Bihorac A. Methods for Phenotyping Adult Patients in Sepsis and Septic Shock: A Scoping Review. Crit. Care Explor. 2022;4:e0672. doi: 10.1097/CCE.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sharma M., Taweesedt P.T., Surani S. Utilizing Artificial Intelligence in Critical Care: Adding A Handy Tool to Our Armamentarium. Cureus. 2021;13:e15531. doi: 10.7759/cureus.15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Loftus T.J., Shickel B., Balch J.A., Tighe P.J., Abbott K.L., Fazzone B., Anderson E.M., Rozowsky J., Ozrazgat-Baslanti T., Ren Y., et al. Phenotype Clustering in Health Care: A Narrative Review for Clinicians. Front. Artif. Intell. 2022;5:842306. doi: 10.3389/frai.2022.842306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Baek M.S., Kim J.H., Kwon Y.S. Cluster Analysis Integrating Age and Body Temperature for Mortality in Patients with Sepsis: A Multicenter Retrospective Study. Sci. Rep. 2022;12:1090. doi: 10.1038/s41598-022-05088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhu J.-L., Yuan S.-Q., Huang T., Zhang L.-M., Xu X.-M., Yin H.-Y., Wei J.-R., Lyu J. Influence of Systolic Blood Pressure Trajectory on In-Hospital Mortality in Patients with Sepsis. BMC Infect. Dis. 2023;23:90. doi: 10.1186/s12879-023-08054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Daulasim A., Vieillard-Baron A., Geri G. Hemodynamic Clinical Phenotyping in Septic Shock. Curr. Opin. Crit. Care. 2021;27:290–297. doi: 10.1097/MCC.0000000000000834. [DOI] [PubMed] [Google Scholar]
- 266.Geri G., Vignon P., Aubry A., Fedou A.-L., Charron C., Silva S., Repessé X., Vieillard-Baron A. Cardiovascular Clusters in Septic Shock Combining Clinical and Echocardiographic Parameters: A Post Hoc Analysis. Intensive Care Med. 2019;45:657–667. doi: 10.1007/s00134-019-05596-z. [DOI] [PubMed] [Google Scholar]
- 267.Knox D.B., Lanspa M.J., Kuttler K.G., Brewer S.C., Brown S.M. Phenotypic Clusters within Sepsis-Associated Multiple Organ Dysfunction Syndrome. Intensive Care Med. 2015;41:814–822. doi: 10.1007/s00134-015-3764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ibrahim Z.M., Wu H., Hamoud A., Stappen L., Dobson R.J.B., Agarossi A. On Classifying Sepsis Heterogeneity in the ICU: Insight Using Machine Learning. J. Am. Med. Inform. Assoc. 2020;27:437–443. doi: 10.1093/jamia/ocz211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhang Z., Zhang G., Goyal H., Mo L., Hong Y. Identification of Subclasses of Sepsis That Showed Different Clinical Outcomes and Responses to Amount of Fluid Resuscitation: A Latent Profile Analysis. Crit. Care. 2018;22:347. doi: 10.1186/s13054-018-2279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Seymour C.W., Kennedy J.N., Wang S., Chang C.-C.H., Elliott C.F., Xu Z., Berry S., Clermont G., Cooper G., Gomez H., et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA. 2019;321:2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Shald E.A., Erdman M.J., Ferreira J.A. Impact of Clinical Sepsis Phenotypes on Mortality and Fluid Status in Critically Ill Patients. Shock. 2022;57:57–62. doi: 10.1097/SHK.0000000000001864. [DOI] [PubMed] [Google Scholar]
- 272.Wang M., Zhu B., Jiang L., Luo X., Wang N., Zhu Y., Xi X. Association between Latent Trajectories of Fluid Balance and Clinical Outcomes in Critically Ill Patients with Acute Kidney Injury: A Prospective Multicenter Observational Study. Kidney Dis. 2022;8:82–92. doi: 10.1159/000515533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ma P., Liu J., Shen F., Liao X., Xiu M., Zhao H., Zhao M., Xie J., Wang P., Huang M., et al. Individualized Resuscitation Strategy for Septic Shock Formalized by Finite Mixture Modeling and Dynamic Treatment Regimen. Crit. Care. 2021;25:243. doi: 10.1186/s13054-021-03682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Taylor S.P., Bray B.C., Chou S.-H., Burns R., Kowalkowski M.A. Clinical Subtypes of Sepsis Survivors Predict Readmission and Mortality after Hospital Discharge. Ann. Am. Thorac. Soc. 2022;19:1355–1363. doi: 10.1513/AnnalsATS.202109-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yang R., Han D., Zhang L., Huang T., Xu F., Zheng S., Yin H., Lyu J. Analysis of the Correlation between the Longitudinal Trajectory of SOFA Scores and Prognosis in Patients with Sepsis at 72 Hour after Admission Based on Group Trajectory Modeling. J. Intensive Med. 2022;2:39–49. doi: 10.1016/j.jointm.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Soussi S., Sharma D., Jüni P., Lebovic G., Brochard L., Marshall J.C., Lawler P.R., Herridge M., Ferguson N., Del Sorbo L., et al. Identifying Clinical Subtypes in Sepsis-Survivors with Different One-Year Outcomes: A Secondary Latent Class Analysis of the FROG-ICU Cohort. Crit. Care. 2022;26:114. doi: 10.1186/s13054-022-03972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Cajander S., Kox M., Scicluna B.P., Weigand M.A., Mora R.A., Flohé S.B., Martin-Loeches I., Lachmann G., Girardis M., Garcia-Salido A., et al. Profiling the Dysregulated Immune Response in Sepsis: Overcoming Challenges to Achieve the Goal of Precision Medicine. Lancet Respir. Med. 2023 doi: 10.1016/S2213-2600(23)00330-2. [DOI] [PubMed] [Google Scholar]
- 278.Wong H.R., Salisbury S., Xiao Q., Cvijanovich N.Z., Hall M., Allen G.L., Thomas N.J., Freishtat R.J., Anas N., Meyer K., et al. The Pediatric Sepsis Biomarker Risk Model. Crit. Care. 2012;16:R174. doi: 10.1186/cc11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sweeney T.E., Azad T.D., Donato M., Haynes W.A., Perumal T.M., Henao R., Bermejo-Martin J.F., Almansa R., Tamayo E., Howrylak J.A., et al. Unsupervised Analysis of Transcriptomics in Bacterial Sepsis Across Multiple Datasets Reveals Three Robust Clusters. Crit. Care Med. 2018;46:915–925. doi: 10.1097/CCM.0000000000003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Marutescu L.G. Current and Future Flow Cytometry Applications Contributing to Antimicrobial Resistance Control. Microorganisms. 2023;11:1300. doi: 10.3390/microorganisms11051300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Fisher C.J., Agosti J.M., Opal S.M., Lowry S.F., Balk R.A., Sadoff J.C., Abraham E., Schein R.M., Benjamin E. Treatment of Septic Shock with the Tumor Necrosis Factor Receptor:Fc Fusion Protein. N. Engl. J. Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 282.Patel G.P., Balk R.A. Systemic Steroids in Severe Sepsis and Septic Shock. Am. J. Respir. Crit. Care Med. 2012;185:133–139. doi: 10.1164/rccm.201011-1897CI. [DOI] [PubMed] [Google Scholar]
- 283.Schumer W. Steroids in the Treatment of Clinical Septic Shock. Ann. Surg. 1976;184:333–341. doi: 10.1097/00000658-197609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Vincent J.-L., Martinez E.O., Silva E. Evolving Concepts in Sepsis Definitions. Crit. Care Nurs. Clin. N. Am. 2011;23:29–39. doi: 10.1016/j.ccell.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 285.Munford R.S. Severe Sepsis and Septic Shock: The Role of Gram-Negative Bacteremia. Annu. Rev. Pathol. 2006;1:467–496. doi: 10.1146/annurev.pathol.1.110304.100200. [DOI] [PubMed] [Google Scholar]
- 286.Drazen J.M. Transparency for Clinical Trials--the TEST Act. N. Engl. J. Med. 2012;367:863–864. doi: 10.1056/NEJMe1209433. [DOI] [PubMed] [Google Scholar]
- 287.Calvano S.E., Xiao W., Richards D.R., Felciano R.M., Baker H.V., Cho R.J., Chen R.O., Brownstein B.H., Cobb J.P., Tschoeke S.K., et al. A Network-Based Analysis of Systemic Inflammation in Humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 288.Kamisoglu K., Sleight K.E., Calvano S.E., Coyle S.M., Corbett S.A., Androulakis I.P. Temporal Metabolic Profiling of Plasma during Endotoxemia in Humans. Shock. 2013;40:519–526. doi: 10.1097/SHK.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Seok J., Warren H.S., Cuenca A.G., Mindrinos M.N., Baker H.V., Xu W., Richards D.R., McDonald-Smith G.P., Gao H., Hennessy L., et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Osuchowski M.F., Remick D.G., Lederer J.A., Lang C.H., Aasen A.O., Aibiki M., Azevedo L.C., Bahrami S., Boros M., Cooney R., et al. Abandon the Mouse Research Ship? Not Just Yet! Shock. 2014;41:463–475. doi: 10.1097/SHK.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bode C., Weis S., Sauer A., Wendel-Garcia P., David S. Targeting the host response in sepsis: Current approaches and future evidence. Crit. Care. 2023;27:478. doi: 10.1186/s13054-023-04762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lombardo E., van der Poll T., DelaRosa O., Dalemans W. Mesenchymal Stem Cells as a Therapeutic Tool to Treat Sepsis. World J. Stem Cells. 2015;7:368–379. doi: 10.4252/wjsc.v7.i2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Liu K.D., Wilson J.G., Zhuo H., Caballero L., McMillan M.L., Fang X., Cosgrove K., Calfee C.S., Lee J.-W., Kangelaris K.N., et al. Design and Implementation of the START (STem Cells for ARDS Treatment) Trial, a Phase 1/2 Trial of Human Mesenchymal Stem/Stromal Cells for the Treatment of Moderate-Severe Acute Respiratory Distress Syndrome. Ann. Intensive Care. 2014;4:22. doi: 10.1186/s13613-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Abraham E. New Definitions for Sepsis and Septic Shock: Continuing Evolution but With Much Still to Be Done. JAMA. 2016;315:757–759. doi: 10.1001/jama.2016.0290. [DOI] [PubMed] [Google Scholar]
- 295.Loupy A., Lefaucheur C., Vernerey D., Chang J., Hidalgo L.G., Beuscart T., Verine J., Aubert O., Dubleumortier S., Duong van Huyen J.-P., et al. Molecular Microscope Strategy to Improve Risk Stratification in Early Antibody-Mediated Kidney Allograft Rejection. J. Am. Soc. Nephrol. 2014;25:2267–2277. doi: 10.1681/ASN.2013111149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Davenport E.E., Burnham K.L., Radhakrishnan J., Humburg P., Hutton P., Mills T.C., Rautanen A., Gordon A.C., Garrard C., Hill A.V.S., et al. Genomic Landscape of the Individual Host Response and Outcomes in Sepsis: A Prospective Cohort Study. Lancet Respir. Med. 2016;4:259–271. doi: 10.1016/S2213-2600(16)00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Semmler A., Prost J.-C., Smulders Y., Smith D., Blom H., Bigler L., Linnebank M. Methylation Metabolism in Sepsis and Systemic Inflammatory Response Syndrome. Scand. J. Clin. Lab. Investig. 2013;73:368–372. doi: 10.3109/00365513.2013.785587. [DOI] [PubMed] [Google Scholar]
- 298.Nicodeme E., Jeffrey K.L., Schaefer U., Beinke S., Dewell S., Chung C.-W., Chandwani R., Marazzi I., Wilson P., Coste H., et al. Suppression of Inflammation by a Synthetic Histone Mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Tacke F., Roderburg C., Benz F., Cardenas D.V., Luedde M., Hippe H.-J., Frey N., Vucur M., Gautheron J., Koch A., et al. Levels of Circulating miR-133a Are Elevated in Sepsis and Predict Mortality in Critically Ill Patients. Crit. Care Med. 2014;42:1096–1104. doi: 10.1097/CCM.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 300.Ma Y., Vilanova D., Atalar K., Delfour O., Edgeworth J., Ostermann M., Hernandez-Fuentes M., Razafimahatratra S., Michot B., Persing D.H., et al. Genome-Wide Sequencing of Cellular microRNAs Identifies a Combinatorial Expression Signature Diagnostic of Sepsis. PLoS ONE. 2013;8:e75918. doi: 10.1371/journal.pone.0075918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wang H., Zhang P., Chen W., Feng D., Jia Y., Xie L. Serum microRNA Signatures Identified by Solexa Sequencing Predict Sepsis Patients’ Mortality: A Prospective Observational Study. PLoS ONE. 2012;7:e38885. doi: 10.1371/journal.pone.0038885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Camussi G., Deregibus M.C., Bruno S., Cantaluppi V., Biancone L. Exosomes/Microvesicles as a Mechanism of Cell-to-Cell Communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 303.Gambim M.H., do Carmo A.d.O., Marti L., Veríssimo-Filho S., Lopes L.R., Janiszewski M. Platelet-Derived Exosomes Induce Endothelial Cell Apoptosis through Peroxynitrite Generation: Experimental Evidence for a Novel Mechanism of Septic Vascular Dysfunction. Crit. Care. 2007;11:R107. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Azevedo L.C.P., Janiszewski M., Pontieri V., Pedro M.d.A., Bassi E., Tucci P.J.F., Laurindo F.R.M. Platelet-Derived Exosomes from Septic Shock Patients Induce Myocardial Dysfunction. Crit. Care. 2007;11:R120. doi: 10.1186/cc6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Dakhlallah D.A., Wisler J., Gencheva M., Brown C.M., Leatherman E.R., Singh K., Brundage K., Karsies T., Dakhlallah A., Witwer K.W., et al. Circulating Extracellular Vesicle Content Reveals de Novo DNA Methyltransferase Expression as a Molecular Method to Predict Septic Shock. J. Extracell. Vesicles. 2019;8:1669881. doi: 10.1080/20013078.2019.1669881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Timár C.I., Lorincz A.M., Csépányi-Kömi R., Vályi-Nagy A., Nagy G., Buzás E.I., Iványi Z., Kittel A., Powell D.W., McLeish K.R., et al. Antibacterial Effect of Microvesicles Released from Human Neutrophilic Granulocytes. Blood. 2013;121:510–518. doi: 10.1182/blood-2012-05-431114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Morris D.C., Jaehne A.K., Chopp M., Zhang Z., Poisson L., Chen Y., Datta I., Rivers E.P. Proteomic Profiles of Exosomes of Septic Patients Presenting to the Emergency Department Compared to Healthy Controls. J. Clin. Med. 2020;9:2930. doi: 10.3390/jcm9092930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Real J.M., Ferreira L.R.P., Esteves G.H., Koyama F.C., Dias M.V.S., Bezerra-Neto J.E., Cunha-Neto E., Machado F.R., Salomão R., Azevedo L.C.P. Exosomes from Patients with Septic Shock Convey miRNAs Related to Inflammation and Cell Cycle Regulation: New Signaling Pathways in Sepsis? Crit. Care. 2018;22:68. doi: 10.1186/s13054-018-2003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Eken C., Gasser O., Zenhaeusern G., Oehri I., Hess C., Schifferli A. Polymorphonuclear Neutrophil-Derived Ectosomes Interfere with the Maturation of Monocyte-Derived Dendritic Cells. J. Immunol. 2008;180:817–824. doi: 10.4049/jimmunol.180.2.817. [DOI] [PubMed] [Google Scholar]
- 310.Gasser O., Schifferli J.A. Activated Polymorphonuclear Neutrophils Disseminate Anti-Inflammatory Microparticles by Ectocytosis. Blood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 311.Dalli J., Norling L.V., Renshaw D., Cooper D., Leung K.-Y., Perretti M. Annexin 1 Mediates the Rapid Anti-Inflammatory Effects of Neutrophil-Derived Microparticles. Blood. 2008;112:2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 312.Eken C., Martin P.J., Sadallah S., Treves S., Schaller M., Schifferli J.A. Ectosomes Released by Polymorphonuclear Neutrophils Induce a MerTK-Dependent Anti-Inflammatory Pathway in Macrophages. J. Biol. Chem. 2010;285:39914–39921. doi: 10.1074/jbc.M110.126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Dalli J., Montero-Melendez T., Norling L.V., Yin X., Hinds C., Haskard D., Mayr M., Perretti M. Heterogeneity in Neutrophil Microparticles Reveals Distinct Proteome and Functional Properties. Mol. Cell. Proteom. 2013;12:2205–2219. doi: 10.1074/mcp.M113.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Dalli J., Norling L.V., Montero-Melendez T., Canova D.F., Lashin H., Pavlov A.M., Sukhorukov G.B., Hinds C.J., Perretti M. Microparticle Alpha-2-Macroglobulin Enhances pro-Resolving Responses and Promotes Survival in Sepsis. EMBO Mol. Med. 2014;6:27–42. doi: 10.1002/emmm.201303503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wisler J.R., Singh K., Mccarty A.R., Abouhashem A.S.E., Christman J.W., Sen C.K. Proteomic Pathway Analysis of Monocyte-Derived Exosomes during Surgical Sepsis Identifies Immunoregulatory Functions. Surg. Infect. 2020;21:101–111. doi: 10.1089/sur.2019.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wang E., Liu Y., Qiu X., Tang Y., Wang H., Xiao X., Chen F., Lu B. Bacteria-Released Outer Membrane Vesicles Promote Disseminated Intravascular Coagulation. Thromb. Res. 2019;178:26–33. doi: 10.1016/j.thromres.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 317.Sui X., Liu W., Liu Z. Exosomes Derived from LPS-Induced MHs Cells Prompted an Inflammatory Response in Sepsis-Induced Acute Lung Injury. Respir. Physiol. Neurobiol. 2021;292:103711. doi: 10.1016/j.resp.2021.103711. [DOI] [PubMed] [Google Scholar]
- 318.Nair R.R., Mazza D., Brambilla F., Gorzanelli A., Agresti A., Bianchi M.E. LPS-Challenged Macrophages Release Microvesicles Coated With Histones. Front. Immunol. 2018;9:1463. doi: 10.3389/fimmu.2018.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Wang G., Huang W., Wang S., Wang J., Cui W., Zhang W., Lou A., Geng S., Li X. Macrophagic Extracellular Vesicle CXCL2 Recruits and Activates the Neutrophil CXCR2/PKC/NOX4 Axis in Sepsis. J. Immunol. 2021;207:2118–2128. doi: 10.4049/jimmunol.2100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Murao A., Tan C., Jha A., Wang P., Aziz M. Exosome-Mediated eCIRP Release From Macrophages to Induce Inflammation in Sepsis. Front. Pharmacol. 2021;12:791648. doi: 10.3389/fphar.2021.791648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Barbora K., Jinbong P., Woon-Yong K., Barbora V., Quanzhi Z., Wei H., Hyo In K., Michael B.Y., Leo E.O., Kiyoshi I., et al. Monocyte Exocytosis of Mitochondrial Danger-Associated Molecular Patterns in Sepsis Suppresses Neutrophil Chemotaxis. J. Trauma Acute Care Surg. 2021;90:46–53. doi: 10.1097/TA.0000000000002973. [DOI] [PubMed] [Google Scholar]
- 322.Yang K., Fan M., Wang X., Xu J., Wang Y., Tu F., Gill P.S., Ha T., Liu L., Williams D.L., et al. Lactate Promotes Macrophage HMGB1 Lactylation, Acetylation, and Exosomal Release in Polymicrobial Sepsis. Cell Death Differ. 2022;29:133–146. doi: 10.1038/s41418-021-00841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kumari P., Vasudevan S.O., Russo A.J., Wright S.S., Fraile-Ágreda V., Krajewski D., Jellison E.R., Rubio I., Bauer M., Shimoyama A., et al. Host Extracellular Vesicles Confer Cytosolic Access to Systemic LPS Licensing Non-Canonical Inflammasome Sensing and Pyroptosis. Nat. Cell Biol. 2023;25:1860–1872. doi: 10.1038/s41556-023-01269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Monneret G., Gossez M., Aghaeepour N., Gaudilliere B., Venet F. How Clinical Flow Cytometry Rebooted Sepsis Immunology. Cytom. Part A J. Int. Soc. Anal. Cytol. 2019;95:431–441. doi: 10.1002/cyto.a.23749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ruiz-Rodriguez J.C., Plata-Menchaca E.P., Chiscano-Camón L., Ruiz-Sanmartin A., Pérez-Carrasco M., Palmada C., Ribas V., Martínez-Gallo M., Hernández-González M., Gonzalez-Lopez J.J., et al. Precision Medicine in Sepsis and Septic Shock: From Omics to Clinical Tools. World J. Crit. Care Med. 2022;11:1–21. doi: 10.5492/wjccm.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Yoon J.H., Pinsky M.R., Clermont G. Artificial Intelligence in Critical Care Medicine. Crit. Care. 2022;26:75. doi: 10.1186/s13054-022-03915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ren T., Chen C., Danilov A.V., Liu S., Guan X., Du S., Wu X., Sherman M.H., Spellman P.T., Coussens L.M., et al. Supervised learning of high-confidence phenotypic subpopulations from single-cell data. Nat. Mach. Intell. 2023;5:528–541. doi: 10.1038/s42256-023-00656-y. [DOI] [Google Scholar]
- 328.Hédou J., Marić I., Bellan G., Einhaus J., Gaudillière D.K., Ladant F.-X., Verdonk F., Stelzer I.A., Feyaerts D., Tsai A.S., et al. Discovery of sparse, reliable omic biomarkers with Stabl. Nat. Biotechnol. 2024 doi: 10.1038/s41587-023-02033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Persson I., Macura A., Becedas D., Sjövall F. Early prediction of sepsis in intensive care patients using the machine learning algorithm NAVOY® Sepsis, a prospective randomized clinical validation study. J. Crit. Care. 2024;80:154400. doi: 10.1016/j.jcrc.2023.154400. [DOI] [PubMed] [Google Scholar]