Abstract
The need for biomarkers for acute ischemic stroke (AIS) to understand the mechanisms implicated in pathological clot formation is critical. The levels of the brain natriuretic peptides known as brain natriuretic peptide (BNP) and NT-proBNP have been shown to be increased in patients suffering from heart failure and other heart conditions. We measured their expression in AIS clots of cardioembolic (CE) and large artery atherosclerosis (LAA) etiology, evaluating their location inside the clots, aiming to uncover their possible role in thrombosis. We analyzed 80 thrombi from 80 AIS patients in the RESTORE registry of AIS clots, 40 of which were of CE and 40 of LAA etiology. The localization of BNP and NT-BNP, quantified using immunohistochemistry and immunofluorescence, in AIS-associated white blood cell subtypes was also investigated. We found a statistically significant positive correlation between BNP and NT-proBNP expression levels (Spearman’s rho = 0.668 p < 0.0001 *). We did not observe any statistically significant difference between LAA and CE clots in BNP expression (0.66 [0.13–3.54]% vs. 0.53 [0.14–3.07]%, p = 0.923) or in NT-proBNP expression (0.29 [0.11–0.58]% vs. 0.18 [0.05–0.51]%, p = 0.119), although there was a trend of higher NT-proBNP expression in the LAA clots. It was noticeable that BNP was distributed throughout the thrombus and especially within platelet-rich regions. However, NT-proBNP colocalized with neutrophils, macrophages, and T-lymphocytes, suggesting its association with the thrombo-inflammatory process.
Keywords: brain natriuretic peptide, BNP, NT-proBNP, stroke biomarkers, thrombus, acute ischemic stroke, stroke etiology
1. Introduction
Investigating the mechanisms of pathological clot formation and the etiology of acute ischemic stroke (AIS) is crucial. There is still much to learn about the roles of thrombus components in thrombus formation and stability, although huge progress has been made in the last years [1,2,3,4]. The diagnosis of the correct stroke etiology is also extremely important, since it affects both prognosis [5,6] and outcomes [7], as well as the formulation of strategies for stroke treatment [8] and the prevention of stroke recurrence [9,10]. The diagnosis of stroke etiology commonly uses the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria, which are based on clinical features and patient medical history, including data collected by tests such as brain imaging (computed tomography, CT and magnetic resonance imaging, MRI), cardiac imaging, duplex imaging of extracranial arteries, arteriography, and laboratory assessments for a prothrombotic state. The two main determined etiologies in cases of large artery occlusion are large artery atherosclerosis (LAA) and cardioembolism (CE) [8]. For an estimated 15–40% of ischemic strokes [11,12,13], it is not possible to determine any etiology, even after an extensive clinical investigation [14]. Therefore, it is necessary to consider alternative ways for the determination of stroke etiology. In the last few years, there has been great interest and investment in looking for diagnostic biomarkers of stroke etiology [15,16,17].
The physiological roles of natriuretic peptides include the relaxation of the vasomotor tone, the inhibition of sympathetic activity, the reduction in cardiac preload, the increase in renal blood flow, and the increase in natriuresis and diuresis [18]. It was shown that the production of natriuretic peptides by cardiomyocytes is triggered by inflammation and hemodynamic stress, which are important pathophysiological contributors to atrial fibrillation and thrombus development in the left atrial appendage [19]. BNP is initially synthesized in cardiomyocytes as a reaction to stretch as pre-proBNP and is then cleaved into proBNP. When secreted, proBNP is split into BNP, which is biologically active, and the remaining N-terminal proBNP (NT-proBNP), which is thought to be biologically inactive [20,21]. Several studies showed that the serum and/or plasma levels of cardiac natriuretic peptides, such as BNP and NT-proBNP, are increased in patients with cardioembolic stroke [22,23,24]. Therefore, their investigation as possible biomarkers of cardioembolic stroke etiology is particularly interesting. There is also evidence that high plasma/serum levels of cardiac natriuretic peptides might be indicators of stroke recurrence [25] and stroke severity [26].
To explore the potential of BNP and NT-proBNP as useful ischemic stroke biomarkers, we investigated BNP and NT-proBNP expression in blood clots retrieved from 80 patients with AIS, comparing BNP and NT-proBNP expression in clots of LAA and CE etiology. Finally, to investigate a possible causative link with inflammation, we investigated their location within the AIS clots through colocalization studies, focusing on NT-proBNP which was expressed in nucleated cells.
2. Results
2.1. Characteristics of the Patients
In Table 1, the baseline patient characteristics are reported. We did not find any difference between the LAA and the CE cohorts in terms of admission NIHSS score (H1 = 0.53, p = 0.818), rtPA administration (X1 = 0.050, p = 0.823), or final mTICI score (H1 = 0.397, p = 0.529). The occluded vessel was significantly different in LAA and CE patients (H1 = 16.460, p < 0.0001 *). The main differences lay in the number of cases with M1 occlusions, which represented 70% and 32.5%, respectively, of CE and LAA patients, and in the number of tandem occlusions, which accounted for 2.5% of CE and 35% of LAA occlusions.
Table 1.
Baseline clinical characteristics of the overall cohort of patients and of patients grouped according to stroke etiology.
| Overall Cohort of Patients (N = 80) | LAA (N = 40) | CE (N = 40) | Statistical Analysis | |
|---|---|---|---|---|
| Admission NIHSS a | 14 [9–19] | 15 [9–19] | 14 [9–19] | H1 = 0.53, p = 0.818 |
| rtPA b administration | ||||
| rtPA yes | 39 | 20 | 19 | X1 = 0.050, p = 0.823 |
| rtPA no | 41 | 20 | 21 | |
| Occluded vessel(s) c | ||||
| MCA, M1 | 41 (51.2%) | 13 (32.5%) | 28 (70.0%) | H1 = 16.460, p < 0.0001 * |
| MCA, M2 | 5 (6.3%) | 2 (5.0%) | 3 (7.5%) | |
| MCA, M3 | 1 (1.3%) | 0 (0.0%) | 1 (2.5%) | |
| MCA, not specified | 2 (2.5) | 1 (2.5%) | 1 (2.5%) | |
| MCA (multiple branches/segments) | 1 (1.3%) | 0 (0.0%) | 1 (2.5%) | |
| ICA and ICA terminus | 6 (7.5%) | 4 (10.0%) | 2 (5.0%) | |
| ACA | 1 (1.3%) | 0 (0.0%) | 1 (2.5%) | |
| VB | 6 (7.5%) | 4 (10.0%) | 2 (5.0%) | |
| Tandem occlusion | 15 (18.8%) | 14 (35.0%) | 1 (2.5%) | |
| 3 Or more occluded vessels | 2 (2.5%) | 2 (5.0%) | 0 (0.0%) | |
| Final mTICI d score | ||||
| mTICI 0 | 2 (2.5%) | 1 (2.5%) | 1 (2.5%) | H1 = 0.397, p = 0.529 |
| mTICI 1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| mTICI 2a | 1 (1.3%) | 1 (2.5%) | 0 (0.0%) | |
| mTICI 2b | 12 (15.0%) | 7 (17.5%) | 5 (12.5%) | |
| mTICI 2c | 17 (21.3%) | 8 (20.0%) | 9 (22.5%) | |
| mTICI 3 | 48 (60.0%) | 23 (57.5%) | 25 (62.5%) |
a NIHSS: National Institutes of Health Stroke Scale, N = 77, 37 LAA and 40 CE patients. b rtPA: recombinant tissue plasminogen activator c MCA: middle cerebral artery; ICA: internal carotid artery; ACA: anterior cerebral artery; VB: vertebro-basilar. d mTICI: modified treatment in Cerebral Ischemia. Data shown as N (%) of cases or median [IQ1, IQ3]. * indicates a statistically significant difference.
2.2. BNP and NT-proBNP Expression Levels in AIS Clots Are Positively Correlated, and the Expression of Each Molecule Is Similar in LAA and CE Clots
We found a statistically significant positive correlation between BNP and NT-proBNP expression (Spearman’s rho = 0.668 p < 0.0001 *). However, we did not find any significant correlation between BNP expression and NIHSS score at admission (Spearman’s rho = 0.114, p = 0.323), rtPA administration (Spearman’s rho = 0.141, p = 0.211), occlusion location (Spearman’s rho = −0.188, p = 0.095), and final mTICI score (Spearman’s rho = 0.126, p = 0.267). We found similar results for NT-proBNP, whose expression was not correlated with NIHSS score at admission (Spearman’s rho = 0.165, p = 0.152), rtPA administration (Spearman’s rho = 0.045, p = 0.692), occlusion location (Spearman’s Rho = −0.036, p = 0.754), and final mTICI score (Spearman’s rho = 0.097, p = 0.390).
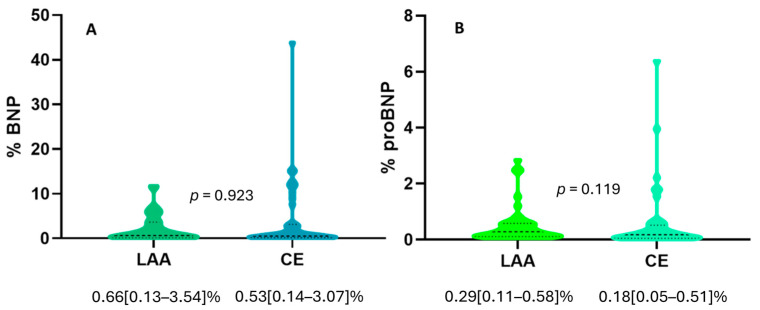
Also, we did not observe any significant difference in BNP expression between LAA and CE clots (0.66 [0.13–3.54]% vs. 0.53 [0.14–3.07]%, p = 0.923), as shown in Figure 1A. Similarly, we did not find any significant difference in NT-proBNP expression between LAA and CE clots (0.29 [0.11–0.58]% vs. 0.18 [0.05–0.51]%, p = 0.119), although there was a trend of higher expression of NT-proBNP in LAA clots, as shown in Figure 1B.
Figure 1.
(A) Violin plot showing no difference in BNP expression in clots from patients with LAA (N = 40) or CE stroke etiology (N = 40). (B) Violin plot showing no difference in NT-proBNP expression in clots from patients with LAA (N = 40) or CE stroke etiology (N = 40). Dashed lines represent the median, while dotted lines represent the interquartile ranges, Q1 (lower dotted lines) and Q3 (upper dotted lines). The p value from Mann–Whitney U-test analysis is indicated.
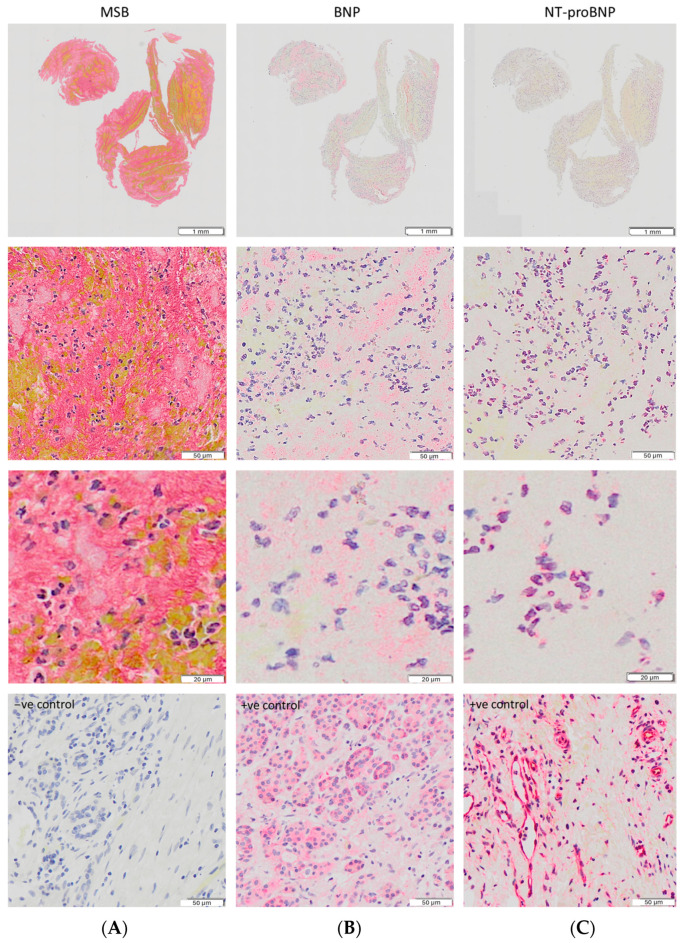
It was noticeable from the immunohistochemical staining (Figure 2) that while BNP was more diffuse in the clot and principally observed in platelet-rich regions, NT-proBNP expression colocalized with nucleated cells. This observation made us think that brain natriuretic peptides could be somehow involved in the mechanisms/pathways of pathological thrombosis, leading us to perform further colocalization experiments with markers of three WBC subtypes, i.e., CD3, a marker for T-lymphocytes, CD66b, a marker for neutrophils, and CD68, a marker for macrophages. In Supplementary Figure S1, IHC staining images of positive and negative controls for the examined white blood cell markers (CD3, CD66b, CD68, and a negative control) in human tonsils are provided.
Figure 2.
(A) MSB staining of the main clot components. (B,C) Expression of BNP and NT-proBNP in the same clot. BNP expression is located principally within platelet-rich regions, while NT-proBNP expression is only observed in nucleated cells. Higher magnification images are provided in the third row. In the fourth row, negative staining (A) and positive control staining images (pancreatic cancer tissue) for BNP (B) and proBNP (C) are shown. All images were captured using a 20× objective.
2.3. NT-ProBNP Expression in AIS Clots Is Associated with T-Lymphocytes, Neutrophils, and Macrophages
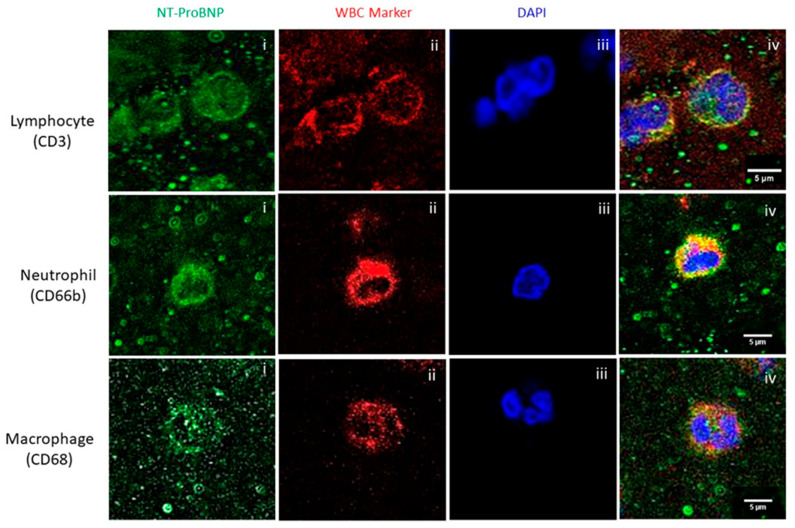
Immunofluorescence staining revealed the association and colocalization of NT-proBNP with the three WBC subtypes we studied, i.e., T-lymphocytes (CD3), neutrophils (CD66b), and macrophages (CD68), as shown in Figure 3.
Figure 3.
Colocalization analysis of NT-proBNP and WBC subtypes in AIS clots, showing individual signals (i–iii) and merged images (iv). Top row: T-lymphocytes (CD3); middle row: neutrophils (CD66b); bottom row: macrophages (CD68). All images were captured using a 60× objective (scale bar 5 μm).
3. Discussion
Natriuretic peptides, including BNP and NT-proBNP, are biochemical markers included in international guidelines for the diagnosis and prognosis of heart failure [27]. Several studies showed that the serum and/or plasma levels of BNP and NT-proBNP are increased in patients with cardioembolic stroke [22,23,24], suggesting that they may have potential as possible biomarkers for stroke of CE etiology. We found no significant difference in the levels of either brain natriuretic peptide in CE and LAA AIS clots. However, BNP and NT-proBNP have also been proposed as biomarkers for atherosclerosis in acute coronary syndrome [28,29]. As coronary heart disease is the most common form of heart disease [30] and is of atherosclerotic origin, this may explain why we did not find any significant difference in BNP and NT-proBNP expression in AIS clots of LAA and CE etiology. In line with this, several recent studies associated high BNP/NT-pro BNP plasma levels with a higher risk of thrombotic events, although not only of cardioembolic etiology [31,32,33,34].
BNP and NT-proBNP are continuously released and are detectable in plasma. Their levels are increased upon ventricular myocardial wall stress, which is the main stimulus for their secretion [21]. As the plasma levels of NT-proBNP are higher than those of BNP, we expected that we might see higher expression of NT-proBNP than of BNP in AIS clots if their levels in clots are reflective of circulating proteins trapped within clots. However, we detected a 2–3-fold higher BNP expression than NT-proBNP expression. NT-proBNP expression in the AIS clots was predominantly located within nucleated cells. In contrast, BNP was principally distributed throughout platelet-rich regions of the thrombus, perhaps indicating a potential role in platelet activity. Interestingly, a correlation between BNP expression and mean platelet volume and a positive association of both features with heart failure have previously been shown [35]. This suggests that the bioactive BNP molecule may become trapped or stabilized within the clot structure perhaps through platelet association, while the biologically inert NT-proBNP molecule may not. Further research into the involvement of NT-proBNP and BNP in thrombosis is needed to explain this phenomenon.
The synthesis and secretion of natriuretic peptides can be induced by several circumstances, such as mechanical stress, systemic ischemia, hypoxia, and neurohumoral factors. Although the exact mechanisms of their regulation remain unclear, it is now generally accepted that mechanical stretch is the main cause of BNP expression rise in the myocardium [36]. However, several stimuli may act on BNP promoter elements through a variety of signaling pathways and affect their activity. For example, it was reported that activated T-lymphocytes produce inflammatory factors such as tumor necrosis factor, IL-1, and IL-6, which also selectively upregulate brain natriuretic peptide secretion [37]. This is in line with our findings that show colocalization between NT-proBNP and T-lymphocytes. Similarly, it is known that the recruitment of proinflammatory macrophages and granulocytes is one of the leading inflammatory processes causing cardiac depression and either new-onset heart failure or acute decompensation of chronic heart failure [38]. Further research is needed to understand the possible causative links with inflammation and the involvement of brain natriuretic peptides.
Our study has limitations. First, we acknowledge that our sample size was relatively small. Further studies including a higher number of patients would be useful to validate our findings. Furthermore, we only studied the LAA and CE etiologies. It would be useful to also study cryptogenic cases in future work. We acknowledge that the differences in occlusion location might represent a possible source of bias in our study, as there were significantly more M1 occlusions in the CE cases than in the LAA cases. Furthermore, we only included cases where all thrombus material was removed in the first pass, which may have introduced a bias. Although some studies showed that the BNP levels in the plasma of AIS patients correlated with stroke severity and outcome [26,39], we did not find any correlation between BNP or NT-proBNP levels and NIHSS score at admission or recanalization outcome (final mTICI score) in AIS clots. However, we acknowledge that we did not evaluate the correlation with other parameters of stroke severity and clinical outcome, such as cerebral infarct size, NIHSS score at discharge, or mRS scores, which were reported in a previous study [40]. It would also be useful to correlate the plasma BNP/NT-pro BNP levels with their expression levels in the clots in future studies. There is some evidence that the timing of BNP measurement is important, with the acute-phase levels being most indicative of the outcome. The BNP levels were shown to negatively correlate with a favorable outcome within 24 h of stroke onset [41]. Recent studies suggested that BNP and NT-proBNP may have use in predicting stroke risk in patients with heart failure with preserved ejection fraction [42], prognostic potential for functional outcome after AIS [43,44], and use in optimizing the management of stroke associated to heart disease [45]. Further work correlating BNP and NT-proBNP expression in AIS clots with longer-term patient outcome measures of stroke severity could yield further interesting insights. Future research investigating further the involvement of BNP and NT-proBNP in thrombo-inflammatory processes could lead to valuable advances in our understanding of the pathophysiology of ischemic stroke.
4. Materials and Methods
4.1. Patient Cohort
We selected 80 patients from the RESTORE registry of AIS clots, of which 40 patients with LAA stroke etiology, and 40 patients with CE stroke etiology. The RESTORE registry is a registry of thrombotic material extracted via mechanical thrombectomy from 1000 AIS patients during the period from February 2018 to December 2019 in four specialized stroke centers in Europe. This multi-center prospective study was performed in accordance with the ethical standards of the Declaration of Helsinki and its amendments [46] and was approved by the regional hospital ethics committees and the University of Galway research ethics committees (16-SEPT-08). We included only patients >18 years, having been treated with mechanical thrombectomy for AIS and whose thrombus material was available for analysis. Patients who recanalized without the need of performing mechanical thrombectomy or without thrombus material extracted were excluded from the Registry. For each patient, we collected an anonymized data abstraction form containing pertinent procedural data, such as previous rtPA administration, NIHSS score at admission, occlusion location, stroke etiology, and final mTICI score. Stroke etiology was reported according to the TOAST classification system [8]. The subset of 80 patients in this study were selected at random from cases of stroke with confirmed LAA or CE etiology, where the clot was removed in the first pass.
4.2. AIS Clot Collection and Processing
The blood clots extracted via mechanical thrombectomy were shipped to the University of Galway in pots containing 10% formalin. Upon arrival, a gross photo of each thrombus was taken with a Canon EOS 1300D camera (Canon Inc., Tokyo, Japan). Following this, the thrombi were placed in histological cassettes for tissue processing and paraffin embedding, maximizing longitudinal distal to proximal orientation, thereby ensuring that the tissue sections for analysis were representative of the whole clot length.
4.3. Immunohistochemistry Staining
We cut 3 µm sections from each paraffin-embedded block with a microtome, and BNP and NT-proBNP staining was performed by immunohistochemistry (IHC) on a Leica Bond-III autostainer using the BOND Polymer Refine Red Detection kit (Leica Biosystems, Wetzlar, Germany #DS9390). Antigen retrieval with tris-EDTA (Leica Biosystems, Wetzlar, Germany #AR9640) was performed for 20 min. the incubation time for the primary antibody rabbit anti-BNP (Abcam, Cambridge, UK, ab236101, 1:100 dilution) or rabbit anti-NT-proBNP (My BioSource Inc., San Diego, CA, USA, MBS2015959, 1:50 dilution) was 15 min, followed by 30 min of incubation with an anti-rabbit secondary antibody. Then, counterstaining of the tissue using hematoxylin was performed for 5 min. Finally, the sections were washed with a washing solution (Leica Biosystems, Wetzlar, Germany #AR9590), rinsed in distilled water, dehydrated in alcohol, cleared in xylene, and mounted with DPX. The negative controls were performed by omission of the primary antibody. Pancreatic cancer tissue (BioIVT, Westbury, NY, USA) was used as positive control tissue for BNP and NT-proBNP expression.
4.4. Slide Scanning and Quantification
The stained slides were scanned using an Olympus vs120 slide scanner at 20× magnification, generating digital whole slide scan images. The digital slides were then quantified using Orbit Image Analysis Software (www.orbit.bio), version 3.64 (9.6.2020) [47], as previously described [2]. In summary, we created two different models (exclusion and inclusion) to distinguish regions to be excluded (e.g., background and artefact) from regions containing the component of interest (BNP or NT-proBNP), enabling the molecules’ quantitative assessment.
4.5. Immunofluorescence Staining
Immunofluorescence staining was performed on a subset of samples in order to evaluate the colocalization of NT-proBNP with three WBC markers. We used the CD3 marker for T-lymphocytes, the CD68 marker for macrophages, and the CD66b marker for neutrophils. The primary antibodies used were as follows: rabbit anti-NT-proBNP (My BioSource, Inc., San Diego, CA, USA, MBS2015959, 1:50); mouse anti-CD3 (Abcam Cambridge, UK, ab17143, 1:10); mouse anti-CD68 (Abcam, Cambridge, UK, ab955, 1:50); and mouse anti-CD66b (Novus Biologicals, Centennial, CO, USA, NB100-77808, 1:100). Alexa Fluor 594-labelled goat anti-mouse IgG H&L (Abcam Cambridge, UK, ab150116, 1:200) and Alexa Fluor 488-labelled goat anti-rabbit IgG H&L (Abcam, Cambridge, UK ab150077, 1:200) were used as secondary antibodies.
Slides with 3 µm sections of thrombus tissue were deparaffinized with xylene, and the tissue was then rehydrated with serial 100%, 95%, 70%, and 50% v/v ethanol solutions. The slides were then incubated for 20 min in Tris-EDTA buffer in a microwave at 98 °C and washed with phosphate-buffered saline (PBS), followed by a wash with PBS containing 0.2% Tween 20 (PBS-Tx). The sections were then incubated with blocking buffer (3% normal goat serum, in PBS-Tx) for 1 h at room temperature under agitation. Incubation with two primary antibodies (anti-NT-proBNP and one of the anti-WBC primary antibodies per slide) followed at 37 °C for 1 h and then overnight at 4 °C. After washing, the sections were incubated with the secondary antibodies for 1 h at 37 °C and then coverslipped in the presence of the 4′,6-diamidino-2-phenylindole (DAPI) mounting medium for nucleic acid staining.
A FV3000 confocal laser scanning microscope (Olympus, Tokyo, Japan) was used to capture immunostaining images by using a 60× objective, and FIJI software (ImageJ, version 1.54h, 15 December 2023) was used to analyze the captured images.
4.6. Statistical Analysis
IBM SPSS-25 software was used for the statistical analyses, and GraphPad Prism 9.2.0 for graph creation. The quantitative variables did not follow a standard normal distribution, as indicated by the Kolmogorov–Smirnov test and the Shapiro–Wilk test. Therefore, the non-parametric Mann–Whitney U-test or the Kruskal–Wallis test with Dunn’s correction for multiple comparisons was used to assess the significance of the differences between the groups for the continuous variables. Chi-square analysis was used to assess the significance of the differences between the groups for the nominal variables. The non-parametric Spearman’s rho was used for the correlation analysis, specifically to measure the strength of a positive or negative association between two variables. The level of statistical significance was set at p < 0.05 (two-sided). The results are reported as median [IQ1–IQ3] or number of cases (%).
5. Conclusions
Further work is needed to determine whether the quantification of BNP and NT-BNP in LAA- and CE-derived AIS clots can serve as a predictive or prognostic biomarker or help to better understand the pathophysiology of AIS clots. From our observations, we can conclude that there is no significant difference in BNP and NT-proBNP expression in LAA and CE AIS clots. Therefore, we did not find any evidence that the brain natriuretic peptide levels in extracted clots can be used as specific biomarkers of cardioembolic stroke etiology, despite their utility as serum biomarkers of heart failure. We observed no correlation with the baseline clinical characteristics assessed. However, further work correlating BNP and NT-proBNP expression with longer-term patient outcome measures is needed. Nonetheless, as previously observed for other possible stroke biomarkers [48], our observation of the presence and localization of BNP and NT-proBNP in clots indicates that they might be involved in thrombosis, uncovering novel features of the thrombo-inflammatory pathway leading to pathological thrombus formation, which is certainly worth of further study.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25052999/s1.
Author Contributions
Conceptualization, R.R., T.T., J.J. and K.M.D.; data curation, D.J., A.D. and J.P.; formal analysis, R.R., D.J., A.D. and J.P.; investigation, R.R., J.J. and K.M.D.; methodology, R.R., T.T. and K.M.D.; project administration, K.M.D.; resources, M.G., R.M., P.R., A.N., E.C., D.D., J.C., I.S., G.T., K.P., J.T., A.R. and K.J.; supervision, K.M.D.; visualization, D.J., A.D., J.P., A.P., M.G., R.M., P.R., A.N., E.C., D.D., J.C., I.S., G.T., K.P., J.T., A.R., K.J., J.J. and K.M.D.; writing—original draft, R.R.; writing—review and editing, R.R., D.J., A.D., A.P., M.G., R.M., P.R., A.N., T.T., E.C., D.D., J.C., I.S., G.T., K.P., J.T., A.R., K.J., J.J. and K.M.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This multi-center prospective study was performed in accordance with the ethical standards of the Declaration of Helsinki and its amendments [46], with approval of the regional hospital ethics committees and the University of Galway research ethics committees (16-SEPT-08).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
Authors M.G. and R.M. were employed by the company Cerenovus. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This publication has emanated from research conducted with the financial support of Science Foundation Ireland (SFI) and is co-funded by the European Regional Development Fund under Grant Number 13/RC/2073_2. Furthermore, it was supported by the industrial partner Cerenovus.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Alkarithi G., Duval C., Shi Y., Macrae F.L., Ariëns R.A.S. Thrombus Structural Composition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021;41:2370–2383. doi: 10.1161/ATVBAHA.120.315754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas A., Fitzgerald S., Mereuta O.M., Rossi R., O’Leary S., Pandit A., McCarthy R., Gilvarry M., Holmegaard L., Abrahamsson M., et al. Platelet-rich emboli are associated with von Willebrand factor levels and have poorer revascularization outcomes. J. Neurointerv. Surg. 2020;12:557–562. doi: 10.1136/neurintsurg-2019-015410. [DOI] [PubMed] [Google Scholar]
- 3.Rossi R., Molina S., Mereuta O.M., Douglas A., Fitzgerald S., Tierney C., Pandit A., Brennan P., Power S., O’Hare A., et al. Does prior administration of rtPA influence acute ischemic stroke clot composition? Findings from the analysis of clots retrieved with mechanical thrombectomy from the RESTORE registry. J. Neurol. 2022;269:1913–1920. doi: 10.1007/s00415-021-10758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald S., Rossi R., Mereuta O.M., Jabrah D., Okolo A., Douglas A., Gil S.M., Pandit A., McCarthy R., Gilvarry M., et al. Per-pass analysis of acute ischemic stroke clots: Impact of stroke etiology on extracted clot area and histological composition. J. Neurointerv. Surg. 2021;13:1111–1116. doi: 10.1136/neurintsurg-2020-016966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ntaios G., Papavasileiou V., Makaritsis K., Milionis H., Michel P., Vemmos K. Association of ischaemic stroke subtype with long-term cardiovascular events. Eur. J. Neurol. 2014;21:1108–1114. doi: 10.1111/ene.12438. [DOI] [PubMed] [Google Scholar]
- 6.Varona J.F., Bermejo F., Guerra J.M., Molina J.A. Long-term prognosis of ischemic stroke in young adults. Study of 272 cases. J. Neurol. 2004;251:1507–1514. doi: 10.1007/s00415-004-0583-0. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald S., Rossi R., Mereuta O.M., Molina S., Okolo A., Douglas A., Jabrah D., Pandit A., McCarthy R., Gilvarry M., et al. Large Artery Atherosclerotic Clots are Larger than Clots of other Stroke Etiologies and have Poorer Recanalization rates. J. Stroke Cerebrovasc. Dis. 2021;30:105463. doi: 10.1016/j.jstrokecerebrovasdis.2020.105463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams H.P., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., Marsh E.E., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Lovett J.K., Coull A.J., Rothwell P.M. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–573. doi: 10.1212/01.WNL.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 10.Jones S.B., Sen S., Lakshminarayan K., Rosamond W.D. Poststroke outcomes vary by pathogenic stroke subtype in the Atherosclerosis Risk in Communities Study. Stroke. 2013;44:2307–2310. doi: 10.1161/STROKEAHA.113.000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart R.G., Catanese L., Perera K.S., Ntaios G., Connolly S.J. Embolic Stroke of Undetermined Source. Stroke. 2017;48:867–872. doi: 10.1161/STROKEAHA.116.016414. [DOI] [PubMed] [Google Scholar]
- 12.Ntaios G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020;75:333–340. doi: 10.1016/j.jacc.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Yaghi S., Bernstein R.A., Passman R., Okin P.M., Furie K.L. Cryptogenic Stroke. Circ. Res. 2017;120:527–540. doi: 10.1161/CIRCRESAHA.116.308447. [DOI] [PubMed] [Google Scholar]
- 14.Marini C., De Santis F., Sacco S., Russo T., Olivieri L., Totaro R., Carolei A. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: Results from a population-based study. Stroke. 2005;36:1115–1119. doi: 10.1161/01.STR.0000166053.83476.4a. [DOI] [PubMed] [Google Scholar]
- 15.Whiteley W., Tian Y., Jickling G.C. Blood biomarkers in stroke: Research and clinical practice. Int. J. Stroke. 2012;7:435–439. doi: 10.1111/j.1747-4949.2012.00784.x. [DOI] [PubMed] [Google Scholar]
- 16.Dagonnier M., Donnan G.A., Davis S.M., Dewey H.M., Howells D.W. Acute Stroke Biomarkers: Are We There Yet? Front. Neurol. 2021;12:619721. doi: 10.3389/fneur.2021.619721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi R., Mereuta O.M., Barbachan e Silva M., Molina Gil S., Douglas A., Pandit A., Gilvarry M., McCarthy R., O’Connell S., Tierney C., et al. Potential Biomarkers of Acute Ischemic Stroke Etiology Revealed by Mass Spectrometry-Based Proteomic Characterization of Formalin-Fixed Paraffin-Embedded Blood Clots. Front. Neurol. 2022;13:854846. doi: 10.3389/fneur.2022.854846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potter L.R., Yoder A.R., Flora D.R., Antos L.K., Dickey D.M. Handbook of Experimental Pharmacology. Springer; Berlin/Heidelberg, Germany: 2009. Natriuretic Peptides: Their Structures, Receptors, Physiologic Functions and Therapeutic Applications; pp. 341–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamel H., Okin P.M., Elkind M.S., Iadecola C. Atrial Fibrillation and Mechanisms of Stroke: Time for a New Model. Stroke. 2016;47:895–900. doi: 10.1161/STROKEAHA.115.012004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin E.R., Gardner D.G., Samson W.K. Natriuretic peptides. N. Engl. J. Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 21.Weber M., Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006;92:843–849. doi: 10.1136/hrt.2005.071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai J., Sun H., Xie L., Zhu Y., Feng Y. Detection of cardioembolic stroke with B-type natriuretic peptide or N-terminal pro-BNP: A comparative diagnostic meta-analysis. Int. J. Neurosci. 2018;128:1100–1108. doi: 10.1080/00207454.2017.1408612. [DOI] [PubMed] [Google Scholar]
- 23.Llombart V., Antolin-Fontes A., Bustamante A., Giralt D., Rost N.S., Furie K., Shibazaki K., Biteker M., Castillo J., Rodriguez-Yanez M., et al. B-type natriuretic peptides help in cardioembolic stroke diagnosis: Pooled data meta-analysis. Stroke. 2015;46:1187–1195. doi: 10.1161/STROKEAHA.114.008311. [DOI] [PubMed] [Google Scholar]
- 24.Nigro N., Wildi K., Mueller C., Schuetz P., Mueller B., Fluri F., Christ-Crain M., Katan M. BNP but Not s-cTnln is associated with cardioembolic aetiology and predicts short and long term prognosis after cerebrovascular events. PLoS ONE. 2014;9:e102704. doi: 10.1371/journal.pone.0102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longstreth W.T., Jr., Kronmal R.A., Thompson J.L., Christenson R.H., Levine S.R., Gross R., Brey R.L., Buchsbaum R., Elkind M.S.V., David L., et al. Amino terminal pro-B-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke. 2013;44:714–719. doi: 10.1161/STROKEAHA.112.675942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomita H., Metoki N., Saitoh G., Ashitate T., Echizen T., Katoh C., Fukuda M., Yasujima M., Osanai T., Okumura K. Elevated plasma brain natriuretic peptide levels independent of heart disease in acute ischemic stroke: Correlation with stroke severity. Hypertens. Res. 2008;31:1695–1702. doi: 10.1291/hypres.31.1695. [DOI] [PubMed] [Google Scholar]
- 27.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.-P., Jankowska E.A., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 28.Ashley K.E., Galla J.M., Nicholls S.J. Brain Natriuretic Peptides as Biomarkers for Atherosclerosis. Prev. Cardiol. 2008;11:172–176. doi: 10.1111/j.1751-7141.2008.08578.x. [DOI] [PubMed] [Google Scholar]
- 29.Gan L., Feng C., Liu C., Tian S., Song X., Yang L. Association between serum N-terminal pro-B-type natriuretic peptide levels and characteristics of coronary atherosclerotic plaque detected by coronary computed tomography angiography. Exp. Ther. Med. 2016;12:667–675. doi: 10.3892/etm.2016.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khunti K., Stone M., Paul S., Baines J., Gisborne L., Farooqi A., Luan X., Squire I. Disease management programme for secondary prevention of coronary heart disease and heart failure in primary care: A cluster randomised controlled trial. Heart. 2007;93:1398–1405. doi: 10.1136/hrt.2006.106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Huang J., Gu W., Hao X., Li G., Yuan Y., Lu Y. Relationship between Brain Natriuretic Peptide and Thromboembolic Events in Elderly Patients with Nonvalvular Atrial Fibrillation. Cardiol. Res. Pract. 2024;2024:5594637. doi: 10.1155/2024/5594637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bayram Z., Gündüz S., Güner A., Kalçik M., Doğan C., Gürsoy M.O., Ozane M., Süleymanf K., Mehmeta Ö. The value of brain natriuretic peptide in the prosthetic valve thrombosis. Blood Coagul. Fibrinolysis. 2020;31:445–451. doi: 10.1097/MBC.0000000000000943. [DOI] [PubMed] [Google Scholar]
- 33.Luo X., Fan Q., Afeku K., Wang Z.Q., Zheng Z. The BNP to Albumin Ratio and Heart Rate in Stroke Complicating Atrial Fibrillation. Curr. Probl. Cardiol. 2023;48:101556. doi: 10.1016/j.cpcardiol.2022.101556. [DOI] [PubMed] [Google Scholar]
- 34.Lu W.T., Du W.T., Lu D.S., You J., Li H.Y. Predictive value of serum initial brain natriuretic peptide and troponin on functional prognosis in noncardiogenic patients with anterior and posterior circulation cerebral infarction. Arq. Neuropsiquiatr. 2022;80:985–993. doi: 10.1055/s-0042-1755270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budak Y.U., Huysal K., Demirci H. Correlation between mean platelet volume and B-type natriuretic peptide concentration in emergency patients with heart failure. Biochem. Med. 2015;25:97–102. doi: 10.11613/BM.2015.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Z., Jia Y., Zhu B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019;20:1820. doi: 10.3390/ijms20081820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sergeeva I.A., Christoffels V.M. Regulation of expression of atrial and brain natriuretic peptide, biomarkers for heart development and disease. Biochim. Biophys. Acta. 2013;1832:2403–2413. doi: 10.1016/j.bbadis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Court O., Kumar A., Parrillo J.E., Kumar A. Clinical review: Myocardial depression in sepsis and septic shock. Crit. Care. 2002;6:500–508. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuwashiro T., Yasaka M., Ito Y., Tokunaga K., Takaguchi G., Nakamura A., Gotoh S., Okada Y. Significance of BNP for Neurological Severity in Acute Ischemic Stroke (I2.009) Neurology. 2016;86((Suppl. S16)):I2.009. doi: 10.1212/WNL.86.16_supplement.I2.009. [DOI] [Google Scholar]
- 40.Maruyama K., Shiga T., Iijima M., Moriya S., Mizuno S., Toi S., Arai K., Ashihara K., Abe K., Uchiyama S. Brain natriuretic peptide in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014;23:967–972. doi: 10.1016/j.jstrokecerebrovasdis.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Fukuhara K., Ogata T., Takeshita S., Tsuboi Y. Serum B-type natriuretic peptide level and timing of its measurement as a predictor of acute ischemic stroke outcome. eNeurologicalSci. 2020;18:100217. doi: 10.1016/j.ensci.2019.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X., Abudukeremu A., Yu P., Cao Z., Sun R., Wu M., Chen Z., Ma J., Zhu W., Chen Y., et al. Usefulness of B-Type Natriuretic Peptide for Predicting the Risk of Stroke in Patients with Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2022;11:e024302. doi: 10.1161/JAHA.121.024302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srisujikul P., Thiankhaw K., Tanprawate S., Soontornpun A., Wantaneeyawong C., Teekaput C., Sirimaharaj N., Nudsasarn A. Serum NT-proBNP level for predicting functional outcomes after acute ischemic stroke. Sci. Rep. 2023;13:13903. doi: 10.1038/s41598-023-41233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen X., Liao J., Jiang Y., Xu Y., Liu M., Zhang X., Dong N., Yu L., Cheng Q., Fang Q. Elevated NT-proBNP levels are associated with CTP ischemic volume and 90-day functional outcomes in acute ischemic stroke: A retrospective cohort study. BMC Cardiovasc. Disord. 2022;22:431. doi: 10.1186/s12872-022-02861-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lip G.Y.H., Lane D.A., Lenarczyk R., Boriani G., Doehner W., Benjamin L.A., Fisher M., Lowe D., Sacco R.L., Schnabel R., et al. Integrated care for optimizing the management of stroke and associated heart disease: A position paper of the European Society of Cardiology Council on Stroke. Eur. Heart J. 2022;43:2442–2460. doi: 10.1093/eurheartj/ehac245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 47.Stritt M., Stalder A.K., Vezzali E. Orbit Image Analysis: An open-source whole slide image analysis tool. PLoS Comput. Biol. 2020;16:e1007313. doi: 10.1371/journal.pcbi.1007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossi R., Douglas A., Gil S.M., Jabrah D., Pandit A., Gilvarry M., McCarthy R., Prendergast J., Jood K., Redfors P., et al. S100b in acute ischemic stroke clots is a biomarker for post-thrombectomy intracranial hemorrhages. Front. Neurol. 2023;13:1067215. doi: 10.3389/fneur.2022.1067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.