ABSTRACT
Background:
The human skin, with a pH of 4 to 6, serves as a barrier against external pathogens. Alkaline handwash products (HWPs) can compromise this barrier and are widely used following the Coronavirus disease-2019 (COVID-19) pandemic. This study aims to determine the pH of a sample of HWPs in Riyadh, Saudi Arabia, and assess the effect of environmental factors on their pH.
Methods:
This is a cross-sectional, descriptive, observational study carried out in Riyadh, Saudi Arabia. The study involved a convenience sample of 33 liquid soaps, soap bars, and synthetic detergents (Syndets) from various brands. The pH of the handwash products was measured using laboratory-validated techniques. Data analysis was conducted using RStudio 2022 software.
Results:
Of the HWPs, 16 (48.5%) had a highly alkaline pH (≥10), while 14 (42.4%) had an acidic pH (4.0-6.9). Most liquid soaps were acidic (84.6%), with a mean pH of 5.9, whereas soap bars had a mean pH of 10.3. Syndets had a mean pH of 6.0.
Conclusions:
On average, liquid soaps and syndets offered a more physiological pH than soap bars. Liquid soaps were more affordable than synthetic detergents, making them a better value option among the three types of HWPs. Environmental factors such as exposure to hot weather did not have a significant impact on HWPs.
Keywords: Atopic dermatitis, COVID-19, eczema, handwash products
Background
The human skin serves as the immune system’s first line of defense and forms a barrier against external pathogens. It is well established in the medical literature that the normal potential of hydrogen (pH) of the skin ranges from 4 to 6.[1,2] This physiologically acidic pH plays a pivotal role in maintaining the epidermal barrier and the balance of the skin’s microbiota.[3]
Composition and types of handwash products
Nonetheless, manufacturers provide a vast selection of handwash products (HWPs), each with a composition tailored to a specific skin type. These HWPs are classified into two major groups: soap-based handwash products and synthetic detergents (Syndets). Irrespective of their classification, HWPs usually contain surfactants as a critical component. Surfactants are amphiphilic compounds with hydrophilic heads and hydrophobic tails, giving them effective cleansing and antimicrobial properties.[4] Furthermore, depending on the hydrophilic head charge, surfactants are classified as anionic, cationic, non-ionic, or amphoteric. Anionic and cationic surfactants are potent skin irritants that can damage proteins and lipids, contributing to epidermal barrier dysfunction.[4,5]
Soap-based handwash products
Soap-based handwash products are made through the process of saponification of a strong alkali with long-chain fatty acid-producing salt of fatty acid.[6] These soaps typically have a high pH ranging from nine to ten, far from the skin’s physiological pH.[5,7]
Synthetic detergents
On the other hand, synthetic detergents contain synthetic surfactants, which, unlike soaps, their production does not involve a strong base.[4,7] As a result, their pH is neutral or slightly acidic, ranging from 5.5 to 7.0, making them less irritating than true soaps.[4,5]
Impact of skin cleansing on skin pH
Hand washing with soap and water is the most effective way to prevent the spread of infectious diseases.[8] Skin cleansing is one of the many endogenous or exogenous factors that may alter skin pH.[9] The prevailing notion suggests that the alkaline properties of soaps may modify the skin’s surface pH, leading to a disruption of the acid mantle that plays a crucial role in maintaining the physiological barrier of the skin.[10,11] However, the fundamental purpose of these soaps is defeated when continuous use of highly alkaline soaps results in skin dryness and irritation, thereby damaging the skin and forming an entry point for pathogenic organisms.[12]
Impact of handwash products on different skin conditions
During the Coronavirus disease-2019 (COVID-19) pandemic, people became more aware and educated about the importance of hand hygiene in preventing the spread of infections.[13] The use of alcohol-based sanitizers drastically climbed, as did soap and water.[13] Nevertheless, when using any cleansing product, individuals with skin conditions such as atopic dermatitis, rosacea, acne vulgaris, or sensitive skin should exercise caution, as prior research has indicated that highly alkaline HWPs can worsen these conditions.[7] A notable decrease in quality of life has been associated with these dermatoses,[14,15] and the ongoing use of inappropriate HWPs may contribute to the persistence of these diseases.[7] Since the use of HWPs is a daily necessity not confined to a specific demographic, the findings of this study have the potential to benefit a large segment of the community.
Status of the current literature
There have been relatively few studies evaluating the pH levels of different HWPs. Recently, a Polish research team analyzed over 100 samples of soaps, shampoos, and creams. Their findings showed that the average pH of soaps was 5.04, falling within the ideal range for human skin.[16]
In 2016, Mendes et al.[17] carried out a study involving 90 handwash product samples, including soap-based products in both liquid and bar forms, as well as synthetic detergents. The researchers noted a significant pH difference between liquid soaps and synthetic detergents compared to soap bars, with the latter having higher pH values. Interestingly, only 2% of the evaluated samples provided pH information on their product labels. This observation aligns with another study that discovered more than 90% of the tested samples had alkaline pH values and did not include pH information on their labels.[18] Another important finding was that 4% of soap bars had pH values of less than nine, while 50% of liquid soaps had a pH of ≤6.9.[19]
Limitations of previous studies
While these studies offer valuable insights into the pH levels of various handwash products, they do have some limitations. For instance, some studies did not categorize handwash products based on their types, which could cause the reported averages to be skewed by outliers among soap bars, liquid soaps, or synthetic detergents.[16] Additionally, most studies did not consider the impact of environmental factors on pH levels.[16,17,18,19] Lastly, these investigations were conducted in specific countries and did not cover the Saudi market.
Consequently, the objective of this study was to analyze the pH values of a variety of HWPs offered in the market of Riyadh, Saudi Arabia, using laboratory-validated methods. In addition, the study investigated the effect of temperature and different types of water on the pH of HWPs. The aim is to provide both patients and physicians with the essential information needed for informed decision-making concerning the selection of HWPs.
Methods
Study setting, duration, and design
This is a descriptive, observational, cross-sectional study conducted in Riyadh, Saudi Arabia, over a period of six months, from July 2022 to December 2022.
Sample size and sampling
Handwash products of widely available brands were collected from conveniently selected local shops across Riyadh, Saudi Arabia. A total of 33 samples of liquid soaps, soap bars, and syndet bars were included for testing. All samples were coded prior to pH testing.
Data collection
Preparation of soap bars and syndet samples
The soap and syndet bars were unwrapped and grated into powder. Each bar’s powder was then used to create two samples weighing one and ten grams. Each sample was then mixed with 100 ml of distilled water without producing much lather. The outcome was a mixture concentration of 1% and 10% per bar (solute mass/solution volume x100). All mixtures were then left undisturbed for 24 hours or until maximum soap dissolution was reached.
Preparation of liquid soap samples
Liquid soap samples of 1 and 10 ml were put in a beaker glass, and distilled water was added to each sample to reach a volume of 100 ml (Solute volume/Solution volume x100 = 1% and 10%). Then, the solution was thoroughly mixed without forming a lather and left undisturbed for 30 minutes. The pH of each sample was then measured.
Measuring pH value
Each sample solution was made at 1% and 10% dilutions in distilled water. The pH of the distilled water used ranged from 6.9 to 7.0. The pH of the analytical samples was measured using STARTER 2100, OHAUS® pH meter (OHAUS® Corporation, USA). The pH meter was calibrated with buffer solutions at pH = 4, pH = 7, and pH = 9.
Assessment of the effect of temperature
Thirteen samples of HWPs were chosen. New samples of these HWPs were prepared using the methods described earlier. The effect of heat on pH value was measured by placing the samples in a drying oven at 60°C for two weeks. The effect of sunlight was examined by placing the HWP under direct sunlight for two weeks. Furthermore, the effect of hot water temperature was studied by heating the soap solution to 60°C on a hot plate. The pH was then measured using the method described earlier.
Assessment of the effect of different water zones
Handwash product samples having various pH ranges were selected. New samples of these HWPs were prepared using the method described earlier. Tap water was collected from the northern, southern, western, eastern, and central areas of Riyadh, Saudi Arabia. For comparison, a solution of soap samples was made at 1% and 10% dilutions in tap and bottled water. Then, the pH was measured for each.
Exclusion of 10% mixture sample category
As reporting more than one concentration may bring confusion, only the one-percent concentrations of only 1% were included in the results after finding no statistical difference between different dilutions.
Data analysis
Data analysis of the study was conducted using RStudio 2022.12.0+353. There were no missing data in the dataset. The Shapiro–Wilk test was used to test for normality. Continuous data were described using the mean (standard deviation), while categorical data were described using numbers and percentages (n - %). Spearman correlation was applied to examine the relationship between product pH and price. A paired t-test was also used to examine the statistical significance of pH changes in the different products. A P value ≤0.05 was considered statistically significant.
Ethical issues
The required ethical approval was obtained from the institutional review board of King Saud University.
Guidelines for reporting
The manuscript adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational studies.
Results
A total of 33 HWP samples were collected from the Saudi market; these fell under three main categories: liquid soaps (39.4%), soap bars (48.4%), and syndets (12.1%). The mean pH value for these samples was 8.11. Most HWP samples (48.5%) had a highly alkaline pH of ≥10, whereas 42.4% of samples had an acidic pH ranging from 4.0-6.9 [Figure 1].
Figure 1.
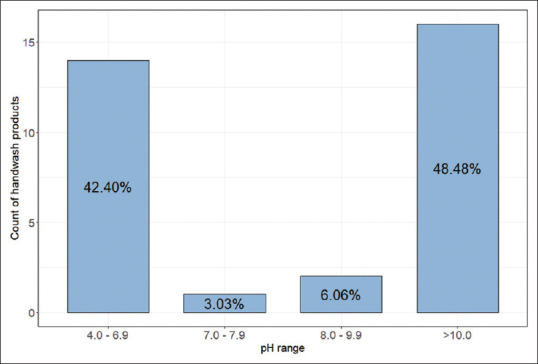
Frequency of handwash products in different pH ranges (n = 33)
The average pH level of each HWP category is provided in Table 1. In Figure 2, a significant correlation between price and pH was observed only in liquid soaps (r = 0.55, P = 0.05). Other HWP types did not demonstrate significant correlation.
Table 1.
Correlation between mean pH and mean price of different handwash product categories
| Handwash product category | Mean pH | Mean price (SAR) | Correlation coefficient (r) | P |
|---|---|---|---|---|
| Liquid Soaps | 6.00 (1.32) | 14.14 (7.79) | -0.55৳ | 0.05* |
| Soap bars | 10.35 (0.16) | 7.00 (6.29) | -0.38৳ | 0.145 |
| Syndet bars | 6.00 (0.80) | 25.45 (18.92) | -0.63৳ | 0.367 |
All data are presented in mean (SD), ৳ indicates Pearson correlation, *statistically significant result. SAR: Saudi Arabian Riyals
Figure 2.
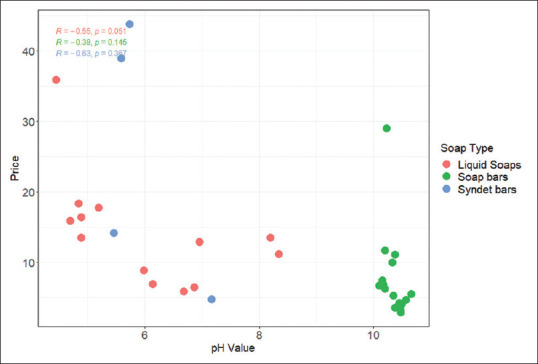
Correlation between price and pH values of handwash product samples (n = 33)
In Table 2, the impact of different environmental factors on the pH levels of HWPs are examined. For both soap bars and syndet bars, no noticeable differences in pH values were observed after a month of opening, exposure to sunlight, or heat exposure. However, liquid soaps experienced a meaningful shift in pH after being subjected to sunlight for two weeks (P = 0.02).
Table 2.
Effect of different environmental factors on mean pH
| Handwash product category | Initial mean pH | Mean pH after 1 month of product unseal | P | Mean pH after sunlight exposure for 2 weeks | P | Mean pH after exposure to 60°C of dry heat | P |
|---|---|---|---|---|---|---|---|
| Liquid Soaps | 6.46 (1.28) | 6.40 (1.19) | 0.33 ৳ | 5.9 (1.06) | 0.02 ৳* | 6.37 (1.37) | 0.114 ৳ |
| Soap bars | 10.40 (0.2) | 10.63 (0.166) | 0.07 ৳ | 10.42 (0.145) | 0.77 ৳ | 10.49 (0.199) | 0.21 ৳ |
| Syndet bars | 6.31 (1.21) | 6.51 (1.26) | 0.11 ৳ | 6.32 (1.032) | 0.97 ৳ | 6.46 (1.11) | 0.27 ৳ |
All data are presented in mean (SD), ৳ indicates paired t-test, *statistically significant result
Figure 3 highlights the influence of water temperature on the pH values of a selection of samples, accounting for 33.3% of the total and spanning a variety of pH ranges. Curiously, almost all samples exhibited a decrease in pH upon exposure to hot water, except for sample 3. Nevertheless, the pH changes for all samples were not considered significant (P = 0.11).
Figure 3.
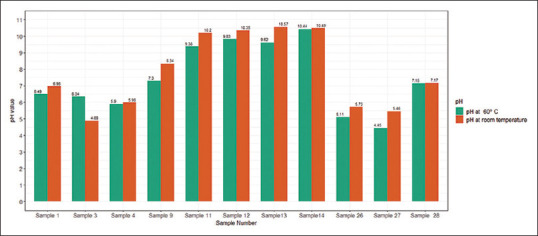
Comparison of pH values at room temperature versus 60°C of a randomly selected sample of handwash products (P value = 0.112)
Discussion
Handwash products with alkaline pH can cause considerable skin irritation due to their effect on the epidermal barrier of the skin.[10,11] This study analyzed a diverse range of 33 HWP samples. Unfortunately, most of our samples had a pH of ≥10 [Figure 1]. Such predominantly high pH of HWPs can cause a significant impact on the skin when utilized by individuals with atopic dermatitis, rosacea, or acne vulgaris, among many others, and this impact can extend to healthy individuals as well.[7] Furthermore, our data showed that liquid soaps and syndet bars were significantly more skin-friendly than soap bars, with the latter having an average pH of 10.3. Our results align with those of a similar study where syndets showed a pH closer to the physiological range.[17] This is also consistent with the results of Volochtchuk et al.,[19] as most soap bars had a pH between 9 and 10 and were more alkaline than liquid soaps. In terms of cost-effectiveness, liquid soaps offer the greatest value among the three types of HWPs. Although syndet bars are skin-friendly, their higher cost makes them less accessible. This is an important factor to consider by both patients and the prescribing physician, as lower-priced HWPs options are generally more frequently preferred.[20]
This study also evaluated the effects of heat and sunlight on the pH level of HWPs during storage. As shown in Table 2, these variables may not influence the quality of HWPs as much as one might assume. Therefore, it may be acceptable to conclude that consumers should not choose one product over another based solely on its storage methods.
Similar to two other studies,[17,18] more than 75% of the tested sample lacked labels identifying their pH level or the skin types for which the product is appropriate, thus making the identification of suitable products more challenging for consumers and healthcare professionals alike. Notably, one product marketed as ideal for sensitive skin types had a pH of about ten. Such findings, which were also observed in another study,[11] may imply that when in doubt, consumers should choose their HWPs based on the labeled pH level rather than the skin type specified by the manufacturer.
In this study, a diverse range of HWP samples were analyzed. Additionally, the study assessed the influence of temperature, sunlight, and water types on pH levels, offering information about how these factors might affect the pH of different HWPs.
Nonetheless, this study did have a few limitations. The sample size consisted of only 33 HWP samples, which were conveniently selected from local shops in Riyadh, Saudi Arabia. This may not accurately represent the entire range of HWPs available in the Saudi market. A more extensive and randomized sample would enhance the study’s generalizability. Moreover, while the study employed laboratory-validated methods to analyze the pH values of handwash products, it remains uncertain how closely these methods resemble real-world usage. Factors such as lather production, handwashing duration, and individual skin type could cause pH levels to fluctuate during actual use. Finally, this study did not specifically examine the effects of pH on people with different dermatoses. Further research is necessary to understand how the pH of HWPs impact people with different skin conditions and identify the most appropriate products for each specific condition.
Based on the findings, it is suggested that physicians generally recommend liquid soaps to their patients over other types of handwash products. Furthermore, consumers are encouraged to consider the pH levels of handwash products rather than relying solely on skin type claims made by manufacturers.
Conclusion
On average, liquid soaps and syndets offered a more physiological pH than soap bars. Liquid soaps were more affordable than synthetic detergents, making them a better value option among the three types of HWPs. Environmental factors such as exposure to hot weather did not significantly impact the pH of the tested HWPs.
List of abbreviations
| Abbreviation | Definition |
|---|---|
| pH | Potential of Hydrogen |
| HWP(s) | Handwash product(s) |
| Syndets | Synthetic detergents |
| COVID-19 | Coronavirus disease-2019 |
Ethical policy and Institutional Review board statement
The required ethical approval was obtained from the institutional review board of King Saud University.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28:359–70. doi: 10.1111/j.1467-2494.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 2.Ali SM, Yosipovitch G. Skin pH: From basic science to basic skin care. Acta Derm Venereol. 2013;93:261–7. doi: 10.2340/00015555-1531. [DOI] [PubMed] [Google Scholar]
- 3.Stalder JF, Fluhr JW, Foster T, Glatz M, Proksch E. The emerging role of skin microbiome in atopic dermatitis and its clinical implication. J Dermatolog Treat. 2019;30:357–64. doi: 10.1080/09546634.2018.1516030. [DOI] [PubMed] [Google Scholar]
- 4.Mijaljica D, Spada F, Harrison IP. Skin cleansing without or with compromise: Soaps and syndets. Molecules. 2022;27:2010. doi: 10.3390/molecules27062010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Draelos ZD. The science behind skin care: Cleansers. J Cosmet Dermatol. 2018;17:8–14. doi: 10.1111/jocd.12469. [DOI] [PubMed] [Google Scholar]
- 6.Coiffard L, Couteau C. Soap and syndets: Differences and analogies, sources of great confusion. Eur Rev Med Pharmacol Sci. 2020;24:11432–9. doi: 10.26355/eurrev_202011_23637. [DOI] [PubMed] [Google Scholar]
- 7.Mukhopadhyay P. Cleansers and their role in various dermatological disorders. Indian J Dermatol. 2011;56:2. doi: 10.4103/0019-5154.77542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf R, Parish LC. Effect of soaps and detergents on epidermal barrier function. Clin Dermatol. 2012;30:297–300. doi: 10.1016/j.clindermatol.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19:296–302. doi: 10.1159/000094670. [DOI] [PubMed] [Google Scholar]
- 10.Castanedo-Cázares JP, Cortés-García JD, Cornejo-Guerrero MF, Torres-Álvarez B, Hernández-Blanco D. Study of the cytotoxic and irritant effects of skin cleansing soaps. Gac Med Mex. 2020;156:418–23. doi: 10.24875/GMM.M20000430. [DOI] [PubMed] [Google Scholar]
- 11.Baranda L, González-Amaro R, Torres-Alvarez B, Alvarez C, Ramírez V. Correlation between pH and irritant effect of cleansers marketed for dry skin. Int J Dermatol. 2002;41:494–9. doi: 10.1046/j.1365-4362.2002.01555.x. [DOI] [PubMed] [Google Scholar]
- 12.Wolf R, Parish LC. Effect of soaps and detergents on epidermal barrier function. Clin Dermatol. 2012;30:297–300. doi: 10.1016/j.clindermatol.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Zakout YM, Khatoon F, Bealy MA, Khalil NAR, Alhazimi AM. Role of the Coronavirus Disease 2019 (COVID-19) pandemic in the upgrading of personal hygiene. A cross-sectional study in Saudi Arabia. Saudi Med J. 2020;41:1263–9. doi: 10.15537/smj.2020.11.25402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blome C, Radtke MA, Eissing L, Augustin M. Quality of life in patients with atopic dermatitis: Disease burden, measurement, and treatment benefit. Am J Clin Dermatol. 2016;17:163–9. doi: 10.1007/s40257-015-0171-3. [DOI] [PubMed] [Google Scholar]
- 15.Lasek RJ, Chren MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134:454–8. doi: 10.1001/archderm.134.4.454. [DOI] [PubMed] [Google Scholar]
- 16.Nieradko-Iwanicka B, Dąbrowska K, Chodun W. The pH of soaps, skin care products and cosmetics used in the period of COVID-19 pandemic. Pol J Public Health. 2020;130:57–60. [Google Scholar]
- 17.Mendes BR, Shimabukuro DM, Uber M, Abagge KT. Critical assessment of the pH of children's soap. J Pediatr (Rio J) 2016;92:290–5. doi: 10.1016/j.jped.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Tarun J, Susan J, Suria J, Susan VJ, Criton S. Evaluation of pH of bathing soaps and shampoos for skin and hair care. Indian J Dermatol. 2014;59:442–4. doi: 10.4103/0019-5154.139861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volochtchuk OM, Fadel AP, Almeida T, Fujita EM, Auada MP, Marinoni LP. Variations in the pH of soaps and indications for its use in normal and diseased skin. An Bras Dermatol. 2000;75:697–703. [Google Scholar]
- 20.Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892–908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]


